Abstract
Since the beginning of the 21st century, the treatment of lung cancer has changed dramatically. New treatments are improving survival outcomes for patients but have led to dramatic increases in cost. In a value-based payment system, patients should have access to comprehensive outcome measurements, including survival rates, quality of life, and cost. High value in cancer care will optimize the outcomes that matter to patients relative to cost.
Health care continues to be responsible for an ever greater percentage of the gross domestic product of the U.S. Cancer care costs are expected to reach $206 billion in 2020, with lung cancer care costs responsible for $18.8 billion of this estimate [1]. Currently, most of the cancer care costs are incurred at the initial diagnosis and during the last year of life. Over the past decade, new drugs for cancer have been approved by the Food and Drug Administration (FDA) at a pace not seen in the previous 30 years. Between 2002 and 2014, a total of 71 therapies for cancer were approved by the FDA. The median survival benefit of these drugs in the trials that led to their approval was 2.1 months [2]. Whether the magnitude of this benefit is meaningful to patients and justifies the costs depends on individual priorities and preferences.
Value, as perceived by those who consume, provide, and pay for health care is a moving target and is generally considered through the prism of a defined point of view [3]. The traditional fee-for-service model does not serve to align the interests of patients, providers, and payers. In order to successfully migrate beyond a system in which fee-for-service is no longer the dominant method of payment, it will be important to create a value-based payment system that successfully represents these three distinct points of view [4, 5].
From the patient’s perspective, a diagnosis of lung cancer is anxiety-provoking and life-altering. Patient education, shared decision-making, and systematic measurement of quality of life using validated tools such as patient-reported outcomes surveys are designed to ensure adequate engagement of the patient and caregiver [6]. This ensures that the model of care delivery is truly designed to provide these three distinct elements of care following the diagnosis.
From the provider’s perspective, enhanced measurement capability and improved access to cost data are useful. In selecting and recommending the best, most appropriate patient care, the prescriber can benefit from reliance on clinical pathways that incorporate evidence-based guidelines and cost data that provide the necessary support for effective clinical decision-making. The addition of these items will complement the current exclusive reliance on a single aspect of efficacy data: survival.
From the payer’s perspective, value is generally focused on cost savings. Public and private payer entities are seeking methods to reduce cancer-related expenditures. Concurrently, cancer drug costs and cancer-related hospitalization charges increasingly represent a growing proportion of expenditures in cancer care [7]. Risk sharing models, in which the provider, hospital, and payer agree to a pre-established corridor of acceptable usage and charges, sharing the gains and losses of a predefined patient population, are popular approaches to addressing the increasing costs.
Using a Value-Based Framework to Improve the Care of Lung Cancer Patients
In order to successfully use the value-based reimbursement framework, strategies for the measurement of outcomes are needed. Comprehensive outcomes measurements are important indicators of which efforts will improve value and which will not. However, in the U.S. to date, interest in measuring quality of care has been relatively low. Also, reimbursement has generally been uncoupled from the quality of care. Recently, however, the Centers for Medicare and Medicaid Services (which determines the reimbursement policies for much of U.S. health care) has set ambitious goals around value-based reimbursement, with a plan to have 90% of payments linked to quality by 2018 [8]. Current models under consideration include the patient-centered oncology medical home, shared savings models, and bundled or episode-based payments [9–11].
In a care delivery system that aligns quality and value with reimbursement, effective outcomes measurement and reporting are imperative. At present, the best approach for measuring quality of care remains an open issue. Most current metrics from the National Quality Forum and other entities have focused on so-called process measures (e.g., the number of lymph nodes sampled and the timely delivery of adjuvant therapy), rather than patient-level outcomes such as survival, quality of life, and functional status [12]. Efforts to create standards to measure the “outcomes that matter to patients” have been initiated by the International Consortium for Health Outcomes Measurement for a number of conditions, including lung cancer [13]. The goal of these efforts is to create tools that would support a common standard for reporting outcomes and identify important factors for risk-adjustment of outcomes. These measures will include survival, quality of life (measured using patient-reported outcomes), and quality of death measures.
The National Comprehensive Cancer Network (NCCN) assembles expert panels to develop guidelines regarding treatment for most cancers based on available evidence and expert opinion [14]. The NCCN guidelines, however, offer many choices for a given clinical scenario and, in the absence of clear efficacy differences, offer minimal guidance regarding which option to choose. Clinical pathways have been developed by a number of organizations (i.e., providers, commercial insurers) to help clinicians choose among the numerous therapeutic options in a given clinical situation [15, 16]. Pathways can also help to reduce the costs associated with advanced imaging. At our institution, pathways for thoracic malignancies have been created by a consensus process using tiered criteria, first around efficacy, second around toxicities, and finally, around cost.
Pathways have the potential to reduce unnecessary variation, which can be a driver of excess costs. It is understood that pathway-directed care is not appropriate for all patients at all times, and clinical judgment will dictate that a subset of patients should not be treated on pathway because of comorbidities, unusual clinical presentations, and other factors. The benefit of early palliative care in advanced lung cancer is another evidence-based intervention that creates value by improving mood, quality of life, and quality of death and reducing aggressive care at the end of life [17]. Value-based reimbursement has the potential to improve the outcomes for patients with innovative care models and improve provider satisfaction by reducing reliance on payers for preauthorization and other cost-containing measures.
Value in Cancer Care: The Case of Non-Small Cell Lung Cancer
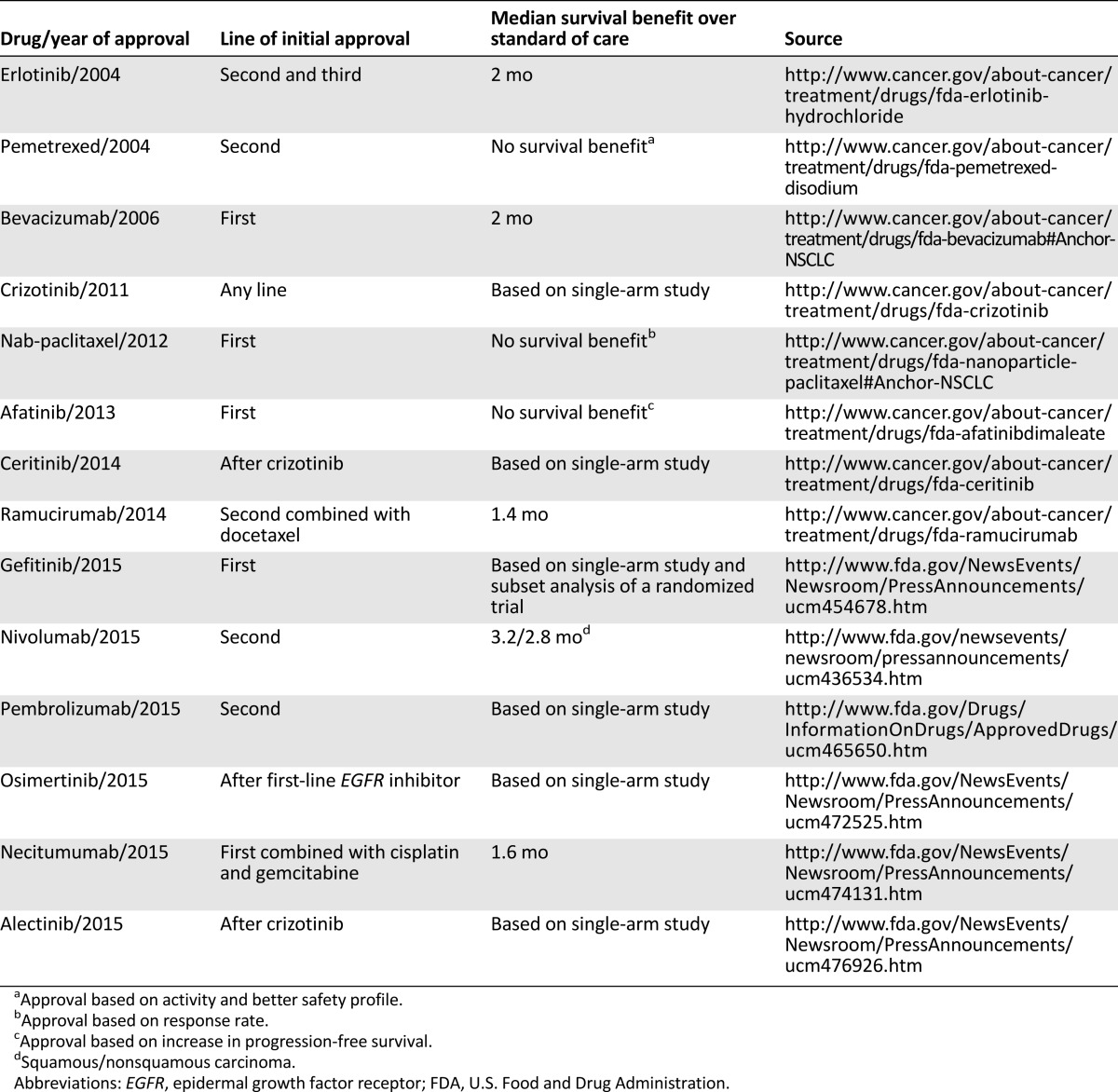
Lung cancer causes more deaths than breast, colon, and prostate cancer combined [18]. Non-small cell lung cancer accounts for approximately 85% of lung cancer cases. Since 2004, 14 new drugs have been approved by the FDA for non-small cell lung cancer (Table 1). Although some of the drugs have a significant impact on survival and quality of life, others have marginal benefits. Recently, the NCCN non-small cell lung cancer guideline panel decided, for the first time in its existence, not to incorporate an FDA-approved therapy (necitumumab). Based on a review of the data in the noted references, the panel consensus was to add the combination regimen of cisplatin/gemcitabine/necitumumab as a first‐line therapy option for patients with squamous cell carcinoma as a category 3 recommendation. The panel noted concerns over toxicity versus benefit [19].
Table 1.
Drugs approved by the FDA for non-small cell lung cancer from 2004 to 2015
As cancer care costs continue to increase at an unsustainable rate, it has become increasingly clear that medical oncologists need to focus on delivering value-centered care. When faced with which treatment options to offer patients, providers must rely on their knowledge of lung cancer and clinical trial findings to discern between which medical interventions will improve the outcomes that matter to patients relative to cost and which will not. Although exceptions exist for every rule, there are three such examples of interventions that are not supported by the randomized data and should not be performed: (a) intense radiological follow-up after curative-intended therapy; (b) the use of positron emission tomography (PET) scans to evaluate therapy given to patients with metastatic disease; and (c) the use of more than two lines of conventional chemotherapy in the palliative setting.
Intense Radiological Follow-Up After Curative-Intended Therapy
There are two main reasons to perform radiological follow-up of patients treated with curative intent. The first is to detect a new primary lung cancer for which curative therapy can be offered. The rationale behind this strategy was based on clinical trial data indicating a survival benefit for lung cancer screening with computed tomography. Although it is true that patients with a history of lung cancer have a high risk of developing a new primary, they were not included in the National Lung Screening Trial [20]. Consequently, the rationale for radiological follow-up to search for a new primary is an extrapolation of the screening data and would only justify performing scans once yearly.
When scans are performed more frequently than yearly, the goal is to find the recurrence before symptoms develop. Two theoretical benefits exist for this strategy. First, the initiation of therapy before patients become symptomatic could prevent symptoms of recurrence from developing and maintain patients’ quality of life. Second, early initiation of palliative chemotherapy, while the recurrence is still asymptomatic, might improve survival. However, no evidence has shown that the early initiation of treatment can achieve either. The few data that do exist include studies of patients with the highest risk of recurrence and those treated with chemoradiotherapy, suggesting that most recurrences will become symptomatic between the predefined radiological follow-up points and that although patients are more often offered potentially curative therapy, the strategy has not been associated with better outcomes [21]. Furthermore, a recent study using Surveillance, Epidemiology, and End Results and Medicare combined data evaluated the survival of patients with lung and esophageal cancer according to the number of PET scans performed to detect recurrence [22]. The lowest usage quintile hospitals performed 0.05 scan per person per year for lung cancer and the highest usage institutions performed 0.7. No difference was found in survival between the patients in these groups. The adjusted 2-year survival was 65.1% for the lowest PET usage quintile and 65.5% for the highest.
Use of PET Scans to Evaluate Response in the Metastatic Setting
Evidence has shown that a PET scan is better for evaluating metastatic disease to the bone [23]. It has also been shown that a PET scan performed after one cycle of chemotherapy can predict the response to conventional chemotherapy in the metastatic setting [24]. However, no evidence has shown that treatment decisions based on PET scan results can lead to better outcomes. The trial data that serve as the guidance to our current therapeutic choices have used tumor growth, as determined by size change or the appearance of new measurable lesions, to guide treatment decisions. The use of maintenance chemotherapy for patients without tumor progression, using these criteria, improves survival [25]. Labeling a therapy as ineffective because of an increase in standardized uptake values or a new area of uptake without a measurable lesion could lead to faster transitions among a limited number of treatments, negating the full benefit described in clinical trials and, ultimately, producing worse outcomes.
Use of More Than Two Lines of Conventional Chemotherapy
The number of therapeutic options for non-small cell lung cancer is quickly expanding. For example, patients with metastatic disease harboring an activating EGFR mutation have two lines of tyrosine kinase inhibitors (assuming the presence of a T790M mutation at disease progression). Immunotherapy is now approved after first-line chemotherapy for all non-small cell histologic features. These are impressive developments, considering that in the beginning of the past decade, patients only had two lines of therapy that were considered the standard of care: doublet chemotherapy and second-line docetaxel. As expectations of therapeutic benefit change, it might become more difficult for patients and oncologists to have a discussion about the lack of options to control the disease. Furthermore, these emotionally charged and time-consuming discussions have not received appropriate financial compensation, another reason for them not to occur [26]. In parallel, the early initiation of palliative care improves the outcomes of patients with metastatic disease [17]. No evidence has shown that more than two lines of conventional chemotherapy improves survival, symptom control, or quality of life. It can be argued that if chemotherapy is used instead of palliative care, in the context of hospice, all these outcomes could be worsened and with higher costs. Attempts to limit the use of expensive, unsupported, strategies have been based on guidelines indicating what should be done and, recently, the specification of interventions that should not be done.
Conclusion
Since the beginning of the 21st century, the treatment of lung cancer has changed dramatically. Screening has and will continue to increase cure rates. Molecular profiling, coupled with targeted therapies and the expanding role of immunotherapy, is extending the lives and quality of life of patients with advanced disease. These treatments are so effective that, justifiably, they have frequently been approved based on phase I or II data. Patients are living longer and, although this is desired, improvements in survival outcome have also contributed to unsustainable increases in health care costs. It is clear that providers should not continue recommending services (e.g., therapies or diagnostic tests) that have not demonstrated improvements in the outcomes that matter to patients.
We should aim for a future in which consumers (i.e., patients) will have the information they need to choose where they want to receive care. For example, patients with each subtype of advanced lung cancer should have access to 1-, 2-, and 3-year survival rates; a percentage of time with good functional capacity (performance status); patient-reported quality of life; and cost. This focus on reporting has the potential to improve outcomes and is our best hope to control the increase in costs or at least to document that this increase improves the quality of care.
A value-based reimbursement system, as proposed by Porter [27], has the ability to align care delivery, ultimately increasing access and providing better care to patients. Simply put, high value in cancer care will optimize the outcomes that matter to patients relative to the cost. Value-based purchasing should not simply be defined as a method for improving quality while reducing or managing costs. Rather, developing incentive models that align the imperatives for patients, providers, and payers offers the opportunity to put patient goals and choices at the center of the decision-making paradigm, ensuring that the care that is delivered is the right care, at the right time, for the right reason.
Author Contributions
Conception/Design: Keith D. Eaton, Barbara Jagels, Renato G. Martins
Collection and/or assembly of data: Keith D. Eaton, Barbara Jagels, Renato G. Martins
Data analysis and interpretation: Keith D. Eaton, Barbara Jagels, Renato G. Martins
Manuscript writing: Keith D. Eaton, Barbara Jagels, Renato G. Martins
Final approval of manuscript: Keith D. Eaton
Disclosures
The authors indicated no financial relationships.
References
- 1.National Cancer Institute. National Expenditures for Cancer Care. Available at http://costprojections.cancer.gov/expenditures.html. Accessed March 15, 2016.
- 2.Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics—The pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: The John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 2014;140:1225–1236. doi: 10.1001/jamaoto.2014.1570. [DOI] [PubMed] [Google Scholar]
- 3.Yong PL, Olsen LA, McGinnis JM.(Institute of Medicine [U.S.] Roundtable on Value & Science-Driven Health Care). Value in Health Care: Accounting for Cost, Quality, Safety, Outcomes, and Innovation Washington, DC: National Academies Press,201029–38. Available from: http://www.ncbi.nlm.nih.gov/books/NBK50926/. [PubMed] [Google Scholar]
- 4.VanLare JM, Conway PH. Value-based purchasing—National programs to move from volume to value. N Engl J Med. 2012;367:292–295. doi: 10.1056/NEJMp1204939. [DOI] [PubMed] [Google Scholar]
- 5.Gruessner V. CMS innovation center’s role in improving value-based care. Available at http://healthpayerintelligence.com/news/cms-innovation-centers-role-in-improving-value-based-care. Accessed March 15, 2016.
- 6.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 7.American Society of Clinical Oncology. Focus on Cost. Available at http://www.asco.org/practice-research/cancer-care-america-2015/focus-cost. Accessed March 15, 2016.
- 8.Centers for Medicare & Medicaid Services. Better Care. Smarter Spending. Healthier People: Paying Providers for Value, Not Volume. Available at http://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed March 15, 2016.
- 9.Page RD, Newcomer LN, Sprandio JD, et al. The patient-centered medical home in oncology: From concept to reality. Am Soc Clin Oncol Educ Book. 2015:e82–e89. doi: 10.14694/EdBook_AM.2015.35.e82. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer LN. Changing physician incentives for cancer care to reward better patient outcomes instead of use of more costly drugs. Health Aff (Millwood) 2012;31:780–785. doi: 10.1377/hlthaff.2012.0002. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services. Bundled Payments for Care Improvement Initiative (BPCI) Fact Sheet. Available at http://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-08-13-2.html. Accessed March 15, 2016.
- 12.National Quality Forum. Endorsement Summary: Cancer Measures. Available at http://www.qualityforum.org/News_And_Resources/Endorsement_Summaries/Endorsement_Summaries.aspx. Accessed March 15, 2016.
- 13.International Consortium for Health Outcomes Measurement. Lung Cancer. Available at http://www.ichom.org/medical-conditions/lung-cancer/. Accessed March 15, 2016.
- 14.National Comprehensive Cancer Network. About the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Available at http://www.nccn.org/professionals/default.aspx. Accessed March 15, 2016.
- 15.Feinberg BA, Lang J, Grzegorczyk J, et al. Implementation of cancer clinical care pathways: A successful model of collaboration between payers and providers. J Oncol Pract. 2012;8(suppl):e38s–e43s. doi: 10.1200/JOP.2012.000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7:54–56. doi: 10.1200/JOP.2010.000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Common Cancer Types. Available at http://www.cancer.gov/types/common-cancers. Accessed March 15, 2016.
- 19.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 20.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benamore R, Shepherd FA, Leighl N, et al. Does intensive follow-up alter outcome in patients with advanced lung cancer? J Thorac Oncol. 2007;2:273–281. doi: 10.1097/01.JTO.0000263708.08332.76. [DOI] [PubMed] [Google Scholar]
- 22.Healy MA, Yin H, Reddy RM, et al. Use of positron emission tomography to detect recurrence and associations with survival in patients with lung and esophageal cancers. J Natl Cancer Inst. 2016;108:djv429. doi: 10.1093/jnci/djv429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheran SK, Herndon JE, II, Patz EF., Jr Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004;44:317–325. doi: 10.1016/j.lungcan.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: Prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–2657. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 26.Martins RG, Reynolds CH, Riely GJ. Beyond “second-line” in non-small cell lung cancer: Therapy and supportive care. Am Soc Clin Oncol Educ Book. 2015:e414–e418. doi: 10.14694/EdBook_AM.2015.35.e414. [DOI] [PubMed] [Google Scholar]
- 27.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]