SUMMARY
The rapid spread of the Zika virus (ZIKV) in the Americas and its potential association with thousands of suspected cases of microcephaly in Brazil and higher rates of Guillain-Barré syndrome meet the conditions for a Public Health Emergency of International Concern, as stated by the World Health Organization in February 2016. Two months later, the Centers for Disease Control and Prevention (CDC) announced that the current available evidence supports the existence of a causal relationship between prenatal Zika virus infection and microcephaly and other serious brain anomalies. Microcephaly can be caused by several factors, and its clinical course and prognosis are difficult to predict. Other pathogens with proven teratogenicity have been identified long before the current ZIKV epidemic. Despite the growing number of cases with maternal signs of infection and/or presence of ZIKV in tissues of affected newborns or fetuses, it is currently difficult to assess the magnitude of increase of microcephaly prevalence in Brazil, as well as the role of other factors in the development of congenital neurological conditions. Meanwhile, health agencies and medical organizations have issued cautious guidelines advising health care practitioners and expectant couples traveling to, returning from, or living in affected areas. Analogous to dengue virus (DENV) epidemics, ZIKV has the potential to become endemic in all countries infested by Aedes mosquitoes, while new mutations could impact viral replication in humans, leading to increased virulence and consequently heightened chances of viral transmission to additional naive mosquito vectors. Studies are urgently needed to answer the questions surrounding ZIKV and its role in congenital neurological conditions.
INTRODUCTION
Early in 2015, an outbreak of Zika virus (ZIKV), a Flavivirus transmitted by Aedes mosquitoes, was observed in northeast Brazil (1–4). By September, reports of higher numbers of infants born with microcephaly in ZIKV-affected areas began to emerge (5). Prior to this, the prevalence of microcephaly at birth averaged 1 to 2 cases per 10,000 live births (6), although the exact rate is unknown. In Brazil, more than 4,000 suspected cases of microcephaly have been reported to the Ministry of Health since September 2015 through two special notification protocols, leading to an estimated prevalence of approximately 10 cases per 10,000 live births as of December 2015 (3, 7, 8).
At the beginning of February 2016, the World Health Organization (WHO) declared that the health threat severity associated with the continuing spread of ZIKV disease in Latin America and the Caribbean constituted a Public Health Emergency of International Concern (9). The rapid spread of ZIKV in the Americas, composed of countries with a low level of population immunity, its possible association with thousands of suspected cases of infant microcephaly, and higher rates of Guillain-Barré syndrome (GBS) meet the conditions for a Public Health Emergency of International Concern (i.e., a public health risk to other states through the international spread of disease potentially requiring a coordinated international response) (10).
According to the available circumstantial evidence, a causal relationship between ZIKV infection in pregnant women and microcephaly is highly suspected by experts and health organizations. In addition to the temporal associations, the virus has been detected in cases of diagnosed microcephaly in the amniotic fluid obtained by ultrasound-guided amniocentesis (11), as well as in tissues of newborns that died shortly after birth or following termination of pregnancy (8, 12). French Polynesia, which experienced a ZIKV outbreak in 2013 and 2014, declared retrospectively that more than a dozen newborns with neural defects were identified (8, 13). Since then, two studies, a retrospective study from French Polynesia and a prospective study from Brazil, have provided the first attempts to quantify the risk of microcephaly (14, 15). In April 2016, the Centers for Disease Control and Prevention (CDC) announced that the available evidence supports the existence of a causal relationship between prenatal Zika virus infection and microcephaly and other serious brain anomalies (16).
Since 2007, locally transmitted cases of ZIKV from 62 countries or territories, mostly located in the Americas, have been reported to the Pan American Health Organization (PAHO) (17, 18). “Further spread to other countries, the lack of vaccines and rapid, reliable diagnostic tests, as well as the absence of population immunity in newly affected countries” have been cited by PAHO as further cause for concern.
In this review, we will explore the increased rate of microcephaly reported in Brazil that coincided with the ZIKV outbreak in the Americas, address the possible reasons for ZIKV's shift in pathogenicity and spread, and discuss the evidence supporting a causal link.
MICROCEPHALY
Microcephaly is a rare pediatric condition, with potentially significant complications for the child and his or her family. In Europe, the European Surveillance of Congenital Anomaly Network (EUROCAT) reports a microcephaly rate of 1.87/10,000 births (6). In 2009, approximately 25,000 children in the United States were diagnosed with microcephaly (19), with no evidence of ZIKV involvement, due to its absence in the Americas at that time.
Definition and Diagnosis
Most current guidelines define microcephaly as an occipito-frontal circumference (OFC) below the third percentile (<2 standard deviations [SD]), and the term “severe microcephaly” is used for an OFC of <3 SD (19, 20). OFC measurements are considered to correlate to brain size and reflect brain development. Distinction should be made between primary microcephaly, in which abnormal OFC is observed at birth, and secondary microcephaly, which develops later (21).
The diagnosis of microcephaly is dependent on an arbitrary cutoff limit defined by an acceptable deviation from the average on reference charts. The dividing line between normal and abnormal may therefore not reflect a true clinical diagnosis. Several considerations need to be taken into account when discussing the diagnosis of microcephaly. First, OFC is a clinical sign and adequate measurement techniques are required. Second, the choice of reference chart used can have a significant impact on the number of cases identified. This is illustrated by the significant change in the estimated number of annual suspected cases of microcephaly in Brazil when using different diagnostic criteria. A total of 46,000 cases per year would be identified using the updated Brazil Ministry of Health recommendations of an OFC of <32 cm or 2 SD below the Fenton reference (22) versus 18,000 cases using 2 SD below the InterGrowth standards (20, 23). The authors of this analysis recommended using the InterGrowth criteria to diagnose microcephaly, as these charts were obtained using a standardized multicenter prospective study and take additional factors into account, such as gestational age (GA) (20, 23). The WHO recommends a cutoff of OFC below the third percentile, and the WHO child growth charts are an additional standardized reference that can be used after adjustment for gestational age (24, 25). Third, OFC measurements may not be reliable during the first days of life due to the presence of delivery sequelae, such as caput succedanum or cephalohematoma; therefore, the OFC measurement should be confirmed 24 h after birth and diagnosis only made thereafter (25). Finally, in utero predictions for microcephaly at birth using the head circumference (HC) measurement may not be accurate; an abnormal HC in utero should be interpreted in its clinical context (26, 27).
Etiology
Microcephaly can be caused by several factors leading to brain injury. The majority of neurons are generated prior to 21 weeks gestation; nevertheless, significant brain development occurs afterwards through myelination and dendritic connections, explaining why both pre- and postnatal brain injuries can lead to microcephaly (28). The most common etiologies of microcephaly are listed in Table 1. In a study evaluating 680 children with microcephaly, the prevalence rates of different etiologies were as follows: 28.5% genetic (including inborn errors of metabolism), 26.7% perinatal brain injury (such as maternal disease [3.8%], birth injury [17.3%], and exposure to teratogenic substances [4.4%]), 13% cryptogenic (suspected genetic cause but no diagnosis identified), 2.1% craniosynostosis, 1.9% postnatal brain injury (such as encephalitis, child abuse, concussion, and infarct), and the remaining 40.7% with no specific etiology found (21). It is important to emphasize that although congenital infections have been identified as a cause of microcephaly, genetic anomalies are more frequently the cause. The number of genetic anomalies associated with microcephaly has increased recently due to significant improvements in genetic testing (29). Additional genetic anomalies associated with microcephaly might be identified through the generalization of next-generation sequencing techniques (21). Therefore, a genetic cause should be ruled out for each case of proven microcephaly as part of the differential diagnosis for suspected cases of congenital ZIKV infection (12). Figure 1 shows the sagittal magnetic resonance imaging (MRI) analysis of an infant with an OFC below the third percentile and currently undergoing investigations to determine etiology.
TABLE 1.
Main etiologies of microcephaly and associated examples
| Microcephaly etiology category and example(s) |
|---|
| Genetic |
| Syndromic |
| Trisomy (21, 18, 13) |
| Continuous gene deletion |
| 5p− deletion (Cri-du-chat syndrome) |
| Monogenic syndromes |
| Rett |
| Cornelia de Lange |
| Rubinstein-Taybi |
| Smith-Lemli-Opitz |
| Isolated |
| Autosomal dominant |
| Familial (autosomal recessive) |
| X-linked |
| Microdeletions/duplications |
| Inborn errors of metabolism |
| Perinatal brain injury |
| Congenital infections |
| CMV |
| Rubella |
| Toxoplasmosis |
| Teratogen exposure |
| Fetal alcohol syndrome |
| Hydantoin |
| Radiation |
| Hypoxic-ischemic encephalopathy |
| Maternal disease |
| Hyperphenylalaninemia |
| Poorly controlled diabetes |
| Severe maternal hypothyroidism |
| Folate deficiency |
| Placental insufficiency |
| Postnatal brain injury |
| Severe malnutrition |
| Meningitis/encephalitis |
| Trauma |
| Severe chronic disease |
| Hypothyroidism |
| Chronic renal insufficiency |
| Toxin exposure |
| Lead poisoning |
| Craniosynostosis |
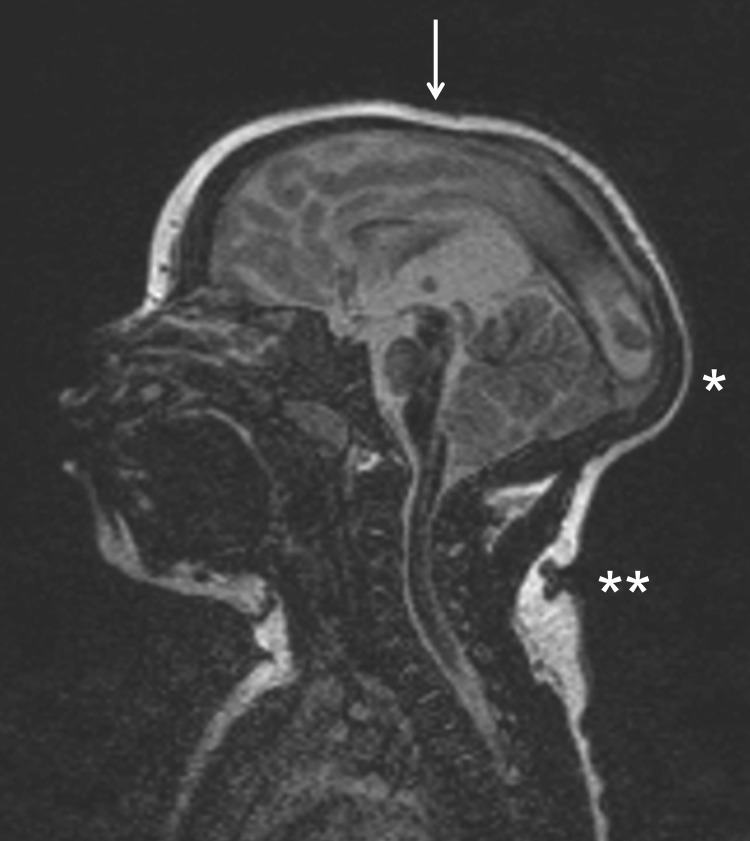
FIG 1.
Sagittal MRI image of the head of an infant who was born with a head circumference below the third percentile (microcephaly), under investigation. The arrow shows a collapse of the skull, inducing cranio-facial disruption, an exuberant external occipital protuberance (*), and redundant scalp skin (**). (Courtesy of Anita Truttmann, Lausanne-CHUV, Switzerland; reproduced with permission.)
Clinical Course and Prognosis
The clinical course and prognosis of microcephaly are difficult to predict, as they depend on the etiology and presence of additional lesions. The prognosis is therefore worse in children in whom microcephaly is part of a syndrome or resulting from a congenital infection (30). In the same cohort of 680 cases of microcephaly described above, 65% presented with either intellectual disability or neurodevelopmental delay and 43% suffered from epilepsy (21). These results are congruent with findings of another study in which only half of the children with microcephaly had a normal intelligence quotient (IQ) (31). The severity of the neurological impairment seems to be associated with the severity of microcephaly (32). Given the above, the prognosis may be poor for suspected ZIKV microcephaly cases due to a potential early insult to brain development and systemic involvement, depending on the timing of infection. Long-term studies are urgently needed to characterize the prognosis.
TERATOGENIC INFECTIOUS DISEASES
Infections during pregnancy have been known to have a significant impact on neonatal morbidity and mortality, as well as pregnancy outcome, long before the current ZIKV epidemic. Complications and outcomes associated with the most common teratogenic infectious agents have been well described, and screening as well as treatment strategies have been developed. As evidenced by the ZIKV outbreak in Brazil, additional unknown teratogenic pathogens may exist. Known pathogens, however, should still be considered first as part of the differential diagnosis. The most common pathogens associated with congenital manifestations are summarized by the acronym TORCH (toxoplasmosis, others [including parvovirus B19, syphilis, varicella-zoster virus [VZV], and HIV], rubella, cytomegalovirus [CMV], and herpes simplex virus [HSV]). This list is not exhaustive and may be subject to variations among authors. To help clinicians develop their differential diagnosis, we reviewed the most common pathogens associated with congenital infections, with a special focus on toxoplasmosis, CMV, and rubella, as well as the recently associated lymphocytic choriomeningitis virus (LCMV), which all show significant similarities with the ZIKV congenital manifestations by its specific neurotropic aspect. A deep understanding of the TORCH congenital infections will help to better understand emerging ZIKV infections (33). Our findings are summarized in Table 2 (34–46).
TABLE 2.
Infectious agents associated with materno-fetal transmission and their potential effectsa
| Associated infection |
Transmission route |
Maternal rash | Fetal consequences | Fetal ultrasound findings | Microcephaly | Basis of diagnosis | Prenatal screening | Treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Infectious agent or disease | Organism or description | To mother | Mother to fetus | |||||||
| Viral | ||||||||||
| CMV | Herpesviridae double-stranded DNA virus | Contact with body fluids | Transplacental | Rare | IUGR, cerebral, ocular, or auditive lesions, hematological anomalies, hepatic dysfunction | IUGR, ventriculomegaly, microcephaly, cerebral calcifications, ascites, hydrops fetalis, hepatic calcifications, oligoamnios | Yes | Maternal serology, amniocentesis at >21 WG/6 wks after infection, PCR of amniotic fluid | Not routine, but varies according to nations' policies | Controversial: hyperimmune immunoglobulins, valacyclovir |
| Rubella | Togaviridae single-stranded positive-sense RNA virus | Aerosols | Transplacental | Yes, maculo-papular | Miscarriage, stillbirth, cardiac, ocular, or cerebral anomalies, IUGR | Cardiac defects, ocular anomalies (two most common); IUGR, hepatosplenomegaly, microcephaly | Yes | Maternal serology, amniocentesis at >21 WG/6 wks after infection, PCR of amniotic fluid | Performed in most developed countries | No specific antiviral treatment |
| LCMV | Arenaviridae bisegmented single-stranded negative-sense RNA virus | Zoonosis (rodents) | Transplacental | No | Neurological or ocular lesions (hydrops fetalis) | Microcephaly, intracranial calcification, ventriculomegaly, ocular anomalies, hydrops fetalis | Yes | Maternal serology, amniocentesis | No | No specific antiviral treatment |
| Parvovirus B19 | Parvoviridae single-stranded DNA virus | Contact (respiratory, fomites, blood) | Transplacental | Yes, maculo-papular | Miscarriage, stillbirth, anemia, hydrops fetalis, cardiomyopathy | Hydrops fetalis, ascites, polyhydramnios, increased middle cerebral artery peak systolic velocity | No | Maternal serology | No | No specific antiviral treatment, fetal transfusion |
| HIV | Retroviridae, single-stranded positive-sense RNA virus | STD, blood | Intrapartum, breastfeeding, transplacental | May occur with prime infection | No | Maternal serology, antigen p24, PCR | Generally proposed | Tritherapy | ||
| HSV | Herpesviridae double-stranded DNA virus | STD, direct contact | Transplacental (rare), intrapartum | No | Skin lesions, ocular or neurological anomalies | Limb hypoplasia, skeletal anomalies, cerebral anomalies (calcifications, ventriculomegaly, microcephaly), ocular anomalies (microophthalmia) | Some rare cases | Maternal serology, amniocentesis | Medical history, physical examination | Valacyclovir, acyclovir |
| Varicella | Herpesviridae double-stranded DNA virus | Direct contact | Transplacental, contact | Yes; papulo-vesicular | IUGR, skin lesions in a dermatome distribution, osteoarticular anomalies, neurological or ocular anomalies | Limb hypoplasia, skeletal anomalies, cerebral anomalies (calcifications, ventriculomegaly, microcephaly), ocular anomalies (microophthalmia), systemic calcifications, hydramnios, IUGR | Some rare cases | Maternal serology, amniocentesis | Medical history, maternal serology | Immunoglobulins, acyclovir |
| Bacterial | ||||||||||
| Syphilis | Treponema pallidum | STD | Transplacental | Yes, in secondary syphilis | Hepatic dysfunction, hematologic anomalies, hydrops fetalis IUGR, stillbirth, miscarriage | Hydrops fetalis, hepatosplenomegaly, placentomegaly, polyhydramnios, ascites, increased middle cerebral artery peak systolic velocity | No | Maternal blood tests, Amniocentesis | VDRL at first prenatal visit followed by specific treponemal antibody tests (FTA-ABS, TPHA, TPPA) to confirm positive results | Penicillin |
| Parasitic | ||||||||||
| Toxoplasmosis | Toxoplasma gondii | Cats, vegetables, fruits, meat | Transplacental | No | Cerebral or ocular lesions, hepatic dysfunction, hematologic anomalies | Intracranial calcifications, ventriculomegaly, hepatosplenomegaly, placentomegaly, ascites | Not present at birth, may develop later | Maternal serology, amniocentesis at >18 WG, PCR of amniotic fluid | Not routine, but varies according to national policies | Controversial: spiramycin, pyrimethamine-sulfadiazine |
Abbreviations: STD, sexually transmitted disease; WG, weeks gestation; VDRL, Venereal Disease Research Laboratory test; FTA-ABS, fluorescent treponemal antibody absorption test; TPHA, T. pallidum hemagglutination assay; TPPA, T. pallidum particle agglutination assay.
General Considerations
The consequences associated with infections during pregnancy include (i) increased maternal morbidity, demonstrated by the higher rate of complications and mortality in pregnant women infected with the influenza virus (47); (ii) adverse pregnancy outcome, such as premature preterm rupture of membranes (PPROM), preterm labor (PL), and miscarriage, which have been associated with bacterial vaginosis (48) and Chlamydia trachomatis infection (49, 50); (iii) congenital infections, discussed below; (iv) severe fetal disease, such as parvovirus B19 infection-associated anemia (37); and finally (v) neonatal infections, such as group B Streptococcus meningitis. Materno-fetal transmission can occur through transplacental infection, ascending infection from the genital tract after membrane rupture, vaginal delivery, and breastfeeding.
Toxoplasmosis
Toxoplasmosis is caused by the strict intracellular parasite Toxoplasma gondii. Humans can be infected through contact with cat feces containing oocysts, either directly or through contaminated soil, vegetables, and fruits or through consumption of undercooked meat from infected animals (51). Previously considered a widespread parasite, recent reports suggest a decrease in the prevalence of T. gondii, to approximately 30% in Western populations (52, 53). Higher seroprevalence rates, however, are still observed in developing countries and some specific populations, such as farmers. Primary infection in immunocompetent patients is generally asymptomatic, and public health considerations are focused on the potential complications during pregnancy or reactivation in immunosuppressed patients (retinitis or encephalitis). In cases of a primary infection during pregnancy or reactivation in immunocompromised patients, there is a risk of transplacental infection of the fetus that is proportional to the gestational age. The most high-risk period is considered to be between 4 and 28 weeks gestational age.
Although placental transmission of T. gondii also occurs at later stages, fetal consequences are less marked at later stages. The risk of congenital toxoplasmosis is 59%, of which 10 to 12% of cases are clinically apparent when infection occurs during the third trimester, versus 9% of cases when infection occurs during the first trimester but from which 75% will present with severe disabilities (52). Fetal infection is associated with cerebral, ocular, and hepatic anomalies, which can be diagnosed via ultrasound. The most specific signs are cerebral calcifications and ventricular enlargement, which mimic ZIKV infection effects, as illustrated in Fig. 2A and B (54, 55). Despite significant cerebral lesions, microcephaly has not been described at birth (primary microcephaly) (54, 56), but it can be observed later (secondary microcephaly). The presence of a high maternal antibody titer to Toxoplasma has been associated with an increase in secondary microcephaly (60%) and a lower IQ (<70, in 30%) (56). Stillbirth, intrauterine growth restriction (IUGR), and premature labor are rarely observed (57). Manifestations during the first days of life range from asymptomatic to severe chorioretinitis, neurological impairments, and hematological manifestations. For paucisymptomatic infants, the most common complication is the development of chorioretinitis later in life. Despite the significant morbidity associated with infection during pregnancy, most current guidelines no longer recommend general prenatal screening (58–60), due to the lack of evidence for effective treatment and standardized diagnostic criteria as well as the risk of unnecessary amniocentesis procedures. Screening might still be offered to high-risk women. Some European countries, such as France, still perform monthly screening in seronegative pregnant women (61). Due to the similar clinical presentation of fetal ZIKV infection and the difficulty of making a definitive diagnosis, reintroduction of toxoplasmosis screening at the first prenatal visit might be considered during the ZIKV epidemic in order to document initial serological status. Diagnosis of an acute infection relies on the presence of IgM, which appears within 2 weeks following infection in a seronegative patient and can stay positive for a year following infection. Specific IgG then appears, the levels peak at 6 weeks, and those infected remain positive for life. Some screened women will present with both IgG and IgM, making the dating of the infection difficult. IgG avidity testing does not provide reliable information, as a low avidity can be observed for many years, especially in pregnant women receiving treatment (62). In cases of suspected infection, amniocentesis should be discussed at least 4 weeks after the suspected time infection and after 18 weeks GA to increase sensitivity. Amniotic fluid is evaluated by specific PCR, and these results are integrated with gestational age at the time of seroconversion to estimate the risk of congenital toxoplasmosis (52, 63). Even in cases of a negative amniocentesis, monthly ultrasound monitoring is recommended, as cases of late materno-fetal transmission have been described (64, 65). Current guidelines recommend treatment with spiramycin as soon as maternal seroconversion is observed, which can eventually be switched to pyrimethamine-sulfonamides once fetal infection is confirmed. Such regimens, however, have been shown to have only a weak effect on materno-fetal transmission when started within 3 weeks following maternal infection, and controversy regarding the benefit of treatment exists (66, 67). Treatment might still be beneficial, as treated infants have been observed to have a reduction in neurological lesions (68, 69).
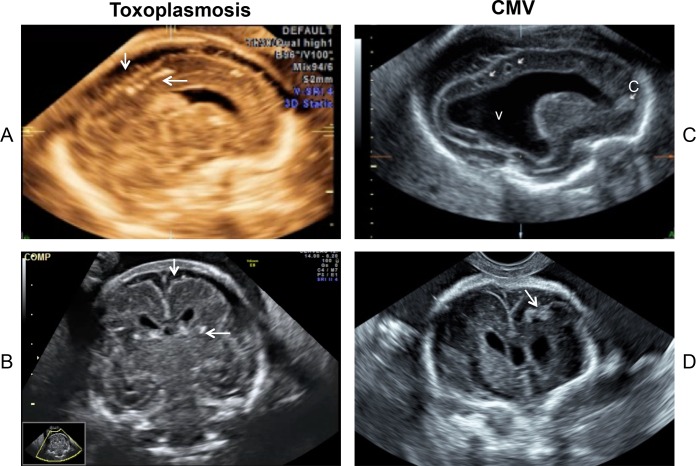
FIG 2.
Second-trimester ultrasound images of congenital infections: toxoplasmosis (A and B) and cytomegalovirus (C and D). (A and C) Sagittal plane; (B and D) coronal plane. (A) Dystrophic calcifications in the junction between cortical and subcortical white matter (horizontal arrow), with the enlargement of the subarachnoid space (vertical arrows). (B) Image for the same patient as in panel A. (C) Severe fetal cytomegalic inclusion disease (arrows at top) with ventriculomegaly (v) and calcification (c). There is a noticeable reduction of the brain parenchyma thickness. (D) Image for the same patient as in panel C, showing calcifications (arrow). (Courtesy of Yvan Vial, Lausanne-CHUV, Switzerland; reproduced with permission.)
Cytomegalovirus
Cytomegalovirus infection is caused by human cytomegalovirus (also known as human herpesvirus 5), a member of the Herpesvirideae family, and is the most common congenital infection, affecting approximately 1 in 150 babies (70). It is the leading cause of developmental delay and sensorineural hearing loss. CMV infection is clearly associated with microcephaly, as one of the first isolations of the virus was performed in a 3-month-old child with microcephaly in 1957 (71). Prevalence in pregnant women ranges between 40 and 85% and is higher in low-socio-economic populations (72). Infection occurs through contact with body fluids (saliva, urine, sperm, mucus) of an infected person.
Both primary and secondary infections (i.e., reactivation or infection with another strain) are associated with materno-fetal transmission, although the lesions are less severe in cases of secondary infections, due to partial protection via maternal immunoglobulins (73). In primary maternal infections during pregnancy, CMV crosses the placental barrier in 30% of cases. Only 15% of those newborns, however, will be symptomatic at birth, and 5 to 15% of asymptomatic newborns will develop complications later in life, mostly sensorineural hearing loss (72, 74, 75). Analogous to CMV, it is unlikely that 100% of the fetuses will be symptomatic in cases of ZIKV congenital infection. The impact of secondary CMV infection on fetal outcomes is still highly debated; it was commonly thought that clinical manifestations were seen in less than 1% of newborns, but recent studies have shown that the incidence of symptomatic cases following secondary infection might be higher, especially in case of infection with another strain (74, 76, 77). Therefore, evidence of past infection does not rule out the possibility of a congenital CMV infection.
Similar to toxoplasmosis, the severity of the lesions from congenital CMV infection seems to be inversely correlated with gestational age (74). The most common manifestations at birth are IUGR, microcephaly, hepatosplenomegaly, and petechia due to severe thrombocytopenia. Later complications include developmental delay, sensorineural hearing loss, and chorioretinitis.
Serological screening at the first prenatal encounter is controversial due to the difficulty of interpreting the results. The presence of specific IgM, low IgG avidity, and low IgG titers evokes a recent primary infection, and rising titers of IgG, high-avidity IgG, and the possible reappearance of IgM suggest a reactivation (74, 78). The diagnosis of congenital CMV infection should be excluded in the presence of compatible fetal anomalies on ultrasound, as summarized in Table 2. An example of a cerebral ultrasound of a CMV-infected fetus is shown in Fig. 2C and D. Congenital CMV infection is confirmed through amniocentesis and subsequent PCR performed after 21 weeks gestation and 6 to 7 weeks after the suspected time of maternal infection. When performed at this gestational age, sensitivity and specificity are 90% and 100%, respectively (74, 75).
Hyperimmune immunoglobulins have been evaluated as a potential treatment to prevent materno-fetal transmission. Though early observational studies showed positive results (79), a recent randomized trial performed on 123 women was unable to confirm this (80); larger studies are needed. Similarly, maternal treatment with valacyclovir has been shown to be associated with a reduction of neonatal complications without maternal toxicity in cases of confirmed fetal infection (81), but confirmation of these promising results is required. Finally, early postnatal treatment of symptomatic newborns with either intravenous gancyclovir or oral valgancyclovir has been shown to improve hearing function and neurodevelopmental outcomes (82–84). These treatment options give a perspective on putative treatment strategies to prevent ZIKV congenital infections and improve neonatal outcomes. They also emphasize, however, that even after more than 50 years of research on congenital CMV, no definitive strategies have been established.
Lymphocytic Choriomeningitis Virus
LCMV is a member of the Arenaviridae family that emerged in the 1990s as a teratogenic agent (85), although the first case of congenital infection was described in England in 1955 (86). Since then, approximately 100 cases have been described, though the prevalence might be higher, as LCMV infection may be misdiagnosed due to lack of knowledge (87, 88). LCMV is a rodent-borne zoonotic infection. Humans become infected through direct or indirect contact with rodents, such as mice, guinea pigs, and hamsters (85, 89). No human-to-human transmission has been reported except for vertical transmission and via organ transplantation (85, 90). Infections in immunocompetent adults usually cause mild symptoms, though severe neurological complications, such as aseptic meningitis, encephalitis, and Guillain-Barré syndrome, occur in approximately 1/3 of patients (70).
Similar to ZIKV, LCMV seems to have a strict neurotropism when contracted in utero, and congenital infections result in severe neurological lesions (88, 91). Chorioretinitis is the most common finding, and it is observed in almost all infected cases (85); funduscopic examination often shows peripheral scarring, while macular scarring is observed in ZIKV, toxoplasmosis, or CMV infection (85, 92–96). Microcephaly, congenital hydrocephaly resulting in macrocephaly, and periventricular calcifications are often observed at birth (85). Unlike other TORCH infections and similar to ZIKV infection, signs of systemic infection, such as skin rash, thrombocytopenia, or hepatosplenomegaly, seem to be rarely observed (85, 88), although some fetuses present with nonimmune hydrops fetalis (88, 97). Late complications include seizures, cerebral palsy, mental retardation, and loss of vision (85). An association with sensorineural hearing loss has been described; however, it seems to be less frequent than in cases of CMV infection (3/44 cases) (85, 88).
Currently, most agencies, including the American Congress of Obstetricians and Gynecologists (ACOG) and the Royal College of Obstetricians and Gynaecologists (RCOG), do not have specific guidelines for prevention of LCMV congenital infections, which is likely secondary to the low number of cases. Data from animal models, however, strongly support a causative role of LCMV in fetal brain damage (91). Therefore, it seems rational to consider LCMV as a differential diagnosis in cases of suspected congenital infection. Diagnosis mostly relies on serological testing, and while a specific PCR has been developed, it has not been widely used. An immunofluorescence assay with adequate sensitivity and specificity is commercially available and should be used as a screening tool in suspected cases (70, 85, 87). No specific treatment to prevent congenital infection in cases of maternal viremia currently exists; specific antiviral therapies that have been shown to be efficient against RNA viruses, such as ribavirin or favipiravir, might be potential therapies in the future (87).
Rubella
Congenital rubella has become rare due to efficient vaccination campaigns in Western countries (rubella is part of the MMR vaccine, which includes protection against measles, mumps, and rubella). Rubella is still a significant cause of disabilities in developing countries. Additionally, a decrease in immunity is currently observed in Western countries, and the absence of immunity is observed in approximately 9 to 14% of pregnant women at first prenatal visit in the United States (98).
The rubella virus belongs to the Togaviridae family. Like ZIKV, rubella virus is an enveloped single-stranded positive RNA virus (99) that normally causes a mild disease in immunocompetent patients that is associated with a maculo-papular rash (99). Humans are the only reservoir, and infections occur through aerosolization when in contact with an infected person and through vertical transmission. Infectivity typically ranges from 7 days before the onset of rash until up to 7 to 10 days after (100, 101). As with toxoplasmosis and CMV, the severity of fetal damage is inversely correlated to gestational age; birth defects are rarely observed when infection occurs after 16 weeks gestation, except for sensorineural hearing loss that, as with CMV infection, can still be observed in asymptomatic newborns (102). The risk is further decreased if infection occurs after 20 weeks gestation (101). Similar to CMV, toxoplasmosis, LCMV, and potentially ZIKV, congenital rubella is associated with severe brain damage. The most common defects at birth are IUGR, ocular lesions (glaucoma, cataract, microophthalmia, and pigment retinopathy), sensorineural hearing loss, purpura and petechia (Blueberry muffin baby) caused by severe thrombocytopenia and dermal hematopoiesis, hepatosplenomegaly, and cardiac malformations (101, 103). Microcephaly is clearly associated with congenital rubella (101). The most common cardiac malformations include patent ductus arteriosus and/or peripheral pulmonary artery stenosis, but other heart defects are possible. Cardiac defects are quite typical of congenital rubella and may also be a unique feature compared to other congenital infections, potentially including those caused by ZIKV. Long-term complications are similar to those due to toxoplasmosis and CMV, including hearing and/or vision loss and developmental delay. Interestingly, congenital rubella is associated with some endocrine complications, especially type I diabetes and thyroid dysfunction (101).
Most countries perform a maternal serological screening at the first prenatal visit (104), and IgG titers of >10 IU are considered to be the sign of immunity. Though reinfections can occur, especially after vaccine-gained immunity, fetal risks are most likely very low (less than <5%) (100, 101).
Diagnosis at birth relies on the presence of specific IgM and molecular detection of the virus in the newborn, which has been shown to be detectable for months after birth. Interpretation of mild disease detected later (i.e., only hearing deficit) should be done cautiously, as children may have already received the MMR vaccine (101). No specific treatment exists, and prevention relies on vaccination that should only be performed in nonpregnant women.
Syphilis, HIV, Parvovirus B19, Herpes Simplex Viruses, and Varicella-Zoster Virus
Epidemiology, fetal and neonatal outcomes associated with the remaining TORCH teratogenic infections are presented in Table 2. As these infections present differently from ZIKV congenital infection, they will not be discussed in detail here. Nevertheless, it is important to emphasize that, similar to CMV, HSV, and VZV, which are also members of the Herpesviridae family, have also been associated with microcephaly in case of in utero infection. This phenomenon, however, is extremely rare, as a transplacental infection is scarcely observed. When they occur, HSV and VZV fetal infections are usually characterized by cutaneous and osteoarticular lesions, which have not been observed thus far in ZIKV infection. Complications associated with these infectious diseases are mostly related to early neonatal infections, which are not associated with fetal malformations (43–46, 105–110).
POSSIBLE EXPLANATION FOR ZIKV'S SHIFT IN PATHOGENICITY AND SPREAD
Zika Outbreaks: New Mutations and Host Adaptations?
ZIKV is a Flavivirus, a member of the Flaviviridae family, which encompasses other emerging viruses, such as DENV and West Nile virus (WNV) (111). Of note, Chikungunya virus (CHIKV), often mentioned with ZIKV, is an Alphavirus. Due to its transmission by an arthropod vector, ZIKV is also classified as an arbovirus (arthropod-borne) (112). In the case of ZIKV, the main arthropod vector is the Aedes aegypti mosquito (113). The main aspects of ZIKV biology, including its description, history, emergence, and phylogeny, have been extensively reviewed by Musso and Gubler (114).
Until recently, although sporadic cases of human infection in areas where ZIKV is endemic were described, the first of which was reported in Nigeria in 1954 (115), ZIKV was regarded as a zoonotic pathogen with limited danger for humans. In fact, humans were not considered part of the viral cycle, which was thought to involve mosquitoes and nonhuman primates, and humans were described as dead-end hosts secondary to a low viremia that prevents reinfection of the vector (116).
In the last decade, however, a geographic expansion of the Asian lineage was observed (117, 118). Since the first febrile outbreak in Yap Island (Federated States of Micronesia) in 2007 (119), ZIKV has spread through the Pacific Islands and more recently into the Americas, especially in Brazil (see Introduction) (1, 2, 9, 120, 121). Humans are now considered amplifying hosts in the urban cycle of ZIKV, as evidenced by epidemics in the Pacific that occurred on islands where nonhuman primates are absent (122).
Despite the lack of experimental evidence, it is plausible that the recent increased ability of ZIKV to spread among humans and cause outbreaks may have a genetic basis that results in the appearance of new viral types that might have an enhanced ability to infect and replicate within the vector and/or humans. Such adaptation to novel hosts could cause devastating epidemics in which the virus may spread into an immunologically naive population that has never encountered the pathogen before (123). As with the other Flavivirus members, however, ZIKV must efficiently infect two hosts, mosquitoes and primates, and consequently there are major constraints on its genomic evolution (124). It has been suggested that appearance of novel ZIKV variants could occur through recombination events (120). Currently, however, there is no experimental evidence of such events, which are rather infrequent among Flavivirus members (125). Although the potential of a recombination event has been observed experimentally in the case of DENV, based on phylogenetic analyses and genetic confirmation of identical crossover breakpoints (126), a viable clonal recombinant Flavivirus has not been observed in nature or under experimental conditions (127). Therefore, it is unlikely that recombination plays an important role in the generation of novel ZIKV variants.
On the other hand, novel variants might occur through point mutations at different levels in the ZIKV genome, which might improve adaptation of the virus. First, as previously proposed, mutations at the amino acid level might change the glycosylation pattern of viral proteins. Second, mutations might occur in the envelope protein (E protein), the main surface protein of ZIKV that mediates specific recognition of host receptors and fusion to the host membrane. In particular, gains and losses of putative N-linked glycosylation sites may impact infectivity, viral release, and neuroinvasiveness; these have been previously studied in WNV and DENV. In a mouse model of infection, subcutaneous injection of WNV carrying glycosylated E protein demonstrated higher rates of neurological disease than did viruses in which the site was absent (128). Interestingly, in the majority of isolates causing human outbreaks, the glycosylation site was present (129, 130). In contrast, absence of the N-linked glycosylation site was associated with an enhanced infectivity of C6/36 mosquito cells and, to a lesser extent, mammalian cells (131). This was also observed in DENV, where the ablation of the glycosylation site resulted in a 100-fold increase in infectivity of C6/36 mosquito cells, although release of viral particles was highly reduced in these mutants in both mosquito and mammalian cell lines (132). In all sequenced ZIKV isolates from recent outbreaks in Micronesia, French Polynesia, and Brazil, the N-linked glycosylation site was present at position 154 of the E protein (114). In contrast, most of the African lineage isolates lack this site. The biological significance of N-linked glycosylation of the E protein in ZIKV requires further investigation and sequencing of additional isolates. Particular attention must be dedicated to the choice of ZIKV isolates for investigation, as the deletion of the N-linked glycosylation site at position 154 of the E protein has been linked to extensive passage of the virus in mouse brain and cell culture (117, 120). Alternatively, as suggested for other Flavivirus members, glycosylation of other viral proteins in ZIKV, especially the nonstructural protein 1 (NS1), may play an important role in viral replication and evasion of the host immune response (133, 134). In the case of ZIKV, analysis of newly sequenced isolates and experimental evidence using adequate animal models are required to confirm the link between genetic mutations and increased ability to infect and replicate in humans and/or vectors.
Adaptation to new hosts can be observed not only at the protein level, but also in the nucleic acid composition of the genome, as in the case of the codon usage bias with respect to human or vector hosts. Again, evaluation of additional genomes is of great importance to confirm these results and perform analyses to assess the impact of other fine-tuning mutations involved in the adaptation to the new host. This should include examination of the untranslated regions (UTRs) of the ZIKV genome that flank the polyprotein gene, as these UTRs play an important role in virus replication and cyclization (135). Studies focused on DENV showed that specific sequences in the 3′ UTR were essential for viral replication in mosquito cells but not for replication in mammalian cells (136). An increased ability of the virus to infect and replicate in mammalian cells was associated with variation of the 3′ UTR, including duplication of specific sites, without interfering with replication in vector cells (137). Such mutations could be of great importance in the ZIKV adaptation process to efficiently replicate in humans, without interfering with the viral fitness in the vector host. In the case of ZIKV, the previously observed ability to sporadically infect humans suggests that recent mutations allowed fine-tuning of the interaction with the new host, thus optimizing viral replication. Similarly, increasing viral titers were observed for WNV, CHIKV, and DENV, in which humans became the amplifying host (138).
Analysis of newly sequenced ZIKV isolates is required to confirm the link between genetic mutations and an increased ability to infect and replicate in humans and/or vectors. More importantly, adequate animal models will be required to experimentally assess the impact of E protein glycosylation, and mutations in general, to the ability of ZIKV to infect the host. Mouse models of infection have been successfully used to characterize ZIKV infection in the past (139–141) and more recently (142). Nevertheless, future models of infection should focus on nonhuman primates, which are part of the natural sylvatic cycle of ZIKV infection, thus representing a more adequate model to study adverse fetal outcomes (143).
Conditions Favoring Outbreaks: Climate Change, Role of the Vector, and Travel
The recent exceptional ZIKV epidemic spread in South America might be linked to extraordinary climate conditions observed during recent years. The 2015 El Niño-South Oscillation (ENSO) caused an uncommonly warm winter and spring in the northeastern part of South America (144) and a very favorable condition for the spread of mosquitoes. Global climate changes are increasingly linked to the emergence and spread of infectious diseases (145–147). Although in the case of ZIKV this hypothesis needs to be further explored, it has been speculated that ENSO influenced the spread of DENV in the region (148–150). Congruently, recent ZIKV epidemic foci in South America overlap regions that experienced the most marked effects on climate caused by ENSO (144).
It is well known that the development and reproduction of Aedes mosquitoes, including the A. aegypti vector, are greatly influenced by environmental factors (151–153). Due to their short life cycle (approximately 10 days), mosquito populations are highly dynamic in changing environments. In particular, temperature fluctuations not only affect reproduction rates and mosquito behavior (154, 155), but also viral interactions and replication within the vector (156, 157).
Water availability is another key factor for mosquito development, as larvae require water, preferably fresh, for development, and in normal situations water is supplied in the form of precipitation water.
As an example, expansion of the Aedes mosquito distribution was linked to the increase of container-stored water in households in which vector mosquitoes could breed (158). As a result, the increase in vector-human contact raises the risk of epidemics.
Recent estimations indicate that 40 to 50% of the human population is exposed to Aedes mosquitoes (159, 160) and potentially to arbovirus infections, including ZIKV. In several cases, importation of arboviruses into naive geographic areas has been linked to human activities, such as traveling for tourism or commerce (138, 161). The number of travelers is constantly increasing in the current globalization era and has more than doubled in the last 20 years, reaching a total of 3.3 billion in 2014 (162). ZIKV infections have been diagnosed in several cases in travelers returning from regions of the epidemic (163–168). This situation is a major international health concern, especially in cases of global events organized in regions where the virus is epidemic. Preventive measures are urgently required.
ZIKV AS A POTENTIAL TERATOGENIC AGENT
Confirmed Infection during Pregnancy
Confirmed cases of ZIKV infection during pregnancy or the neonatal period have been reported by different agencies (CDC, European Center for Disease Prevention and Control [ECDC], Brasil Ministério da Saúde, Latin American Network of Congenital Malformations, WHO); however, detailed case descriptions are often lacking. Additionally, single cases may be reported multiple times by different sources (publication, web, national agencies), making the identification and distinction between each individual case difficult. There is a concern that the positive cases described below have been counted multiple times, thus overestimating the true number of infected mothers and newborns. Table 3 summarizes the patients presented in this section.
TABLE 3.
Confirmed cases of ZIKV infection during pregnancya
| Study author(s) (reference) | No. of cases | Timing of symptoms during pregnancy | Clinical findings |
Neonatal outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Maternal |
Fetal |
||||||||
| TORCH | ZIKV RT-PCR | Other PCR/serology findings | Karyotype | Amniocentesis | Ultrasound | ||||
| Besnard et al. (169) | 2 | Peripartum | ? | Pos serum, milk, saliva | DENV Neg | ? | ? | Asymptomatic | |
| Day 3 postpartum | ? | Pos serum, milk, urine | DENV Neg | ? | ? | Hypotrophy, thrombopenia, transient rash | |||
| Oliveira Melo et al. (11) | 2 | 18–19 WG | Neg | Neg blood | ? | ? | 28 WG, ZIKV Pos | Abnormal, 29–31 WG | Ongoing pregnancies |
| Calvet et al. (178) | 2 | 10 and 18 WG | Neg | Neg | DENV, CHIKV Neg | ? | 28 WG, CHIKV IgM | Abnormal 21 and 22 WG | Ongoing pregnancies |
| WHO/PAHO (180) | 1 | ? | ? | ? | ? | ? | ? | Neonatal death within 5 min | |
| CDC (181) | 4 | Yes | ? | ? | ? | ? | ? | 2 miscarriages, 2 neonatal deaths | |
| Victora et al. (23) | 14 | ? | ? | ? | ? | ? | ? | All with microcephaly (2 deaths) | |
| Hawaii Dept. of Health (184)* | 1 | ? | ? | ? | ? | ? | ? | ? | |
| Colombian Ministry of Health (183) | 459 | ? | ? | Pos | ? | ? | ? | Ongoing pregnancies | |
| Mlakar J. et al. (12)* | 1 | 13 WG | ? | ? | TORCH, DENV, CHIKV Neg | Normal | Normal at 14 and 20 WG, abnormal at 28 WG | TOP, ZIKV+ brain | |
| Jouannic et al. (197) | 6 | 3 WG (all 1st Trim) | Neg | ND | ? | Normal | 4 cases ZIKV Pos | Abnormal at 21–29 WG | All with microcephaly, abnormal corpus callosum |
| Brasil et al. (15) | 88 | Inclusion criteria: rash (24%, 53%, and 23% in 1st, 2nd, 3rd Trim, respectively); only 28% had fever | Neg (CMV, rubella, syphilis) | 72/88 (82%) Pos in blood/urine (60 serum sample, 46 urine sample, 34 both sample types) | 88% DENV IgG Pos | Normal (n = 1) | 42 ZIKV Pos by accepted US; abnormal in 12/42 (29%); 7 Doppler/liquids, 5 IUGR, 7 CNS anomalies, 2 miscarriages (1st Trim) | 8/42 delivered, 6 alive; 2 IUFD (36 and 38 WG), 2 normal children, 2 IUGR, 1 with macular lesions, 1 normal growth but abnormal EEG, 1 microcephaly, calcifications, macular lesions, 2 early miscarriages | |
| Meaney-Delman et al. (184) | 9 | All had at least a rash (6 in 1st, 2 in 2nd, and 1 in 3rd Trim) | ? | Molecular or serological confirmation (no details) | ? | ? | 1 case ZIKV Pos (20 WG) | Abnormal 20 WG, 1 case | 2 early miscarriages, 2 TOP (reason not described); 3 live births, 2 healthy, 1 with microcephaly; 2 ongoing pregnancies |
| Sarno et al. (188) | 1 | No symptoms | Neg | ? | ? | ? | ? | Normal at 14 WG, IUGR at 18 WG, then fetal hydrops | IUFD at 32 WG, ZIKV+ brain and amniotic fluid, ZIKV− liver, placenta, heart, and lung |
| Villamil-Gomez et al. (189) | 28 | 75% symptomatic (4%, 43%, and 54% in 1st, 2nd, and 3rd Trim, respectively) | Neg | All Pos, but samples not described | DENV, CHIKV Neg | ? | Expected for abnormal cases | Brain calcifications in 2 cases | All ongoing pregnancies |
| Driggers et al. (190) | 1 | 12 WG | Neg | ZIKV RNA positive (16–21 WG) | DENV IgM− IgG+ | ? | ZIKV Pos at TOP | Normal at 13, 16, and 17 WG | TOP, 21 WG |
| ZIKV IgM+ IgG+ | CHIKV Neg | Abnormal from 19 WG | High ZIKV load in fetal brain | ||||||
| Yellow fever vaccine | Absence of TORCH DNA in fetal brain | ||||||||
Abbreviations: WG, week of gestation; TOP, termination of pregnancy; Pos, positive; Neg, negative; Doppler/liquids, abnormal Doppler blood flows; Trim, trimester; IUFD, intrauterine fetal death; IUGR, intrauterine growth restriction; US, ultrasound. A question mark indicates data that were not known or were not reported. *, the patient traveled in an area(s) where ZIKV is endemic.
In late 2013, Besnard et al. were the first researchers to report a perinatal transmission of ZIKV (169) during the French Polynesia outbreak, in which 11% of the population was estimated to have been potentially infected (30,000 people) (122). The authors presented two cases in which infection was suspected and then confirmed at birth (38 weeks gestation for both patients). The first case was described in a symptomatic mother (pruritic rash without fever 2 days before delivery lasting 4 days in total) who gave birth to an asymptomatic newborn delivered vaginally. In the second case, both mother (mild fever, pruritic rash, and myalgia 3 days post-Cesarean delivery) and her child were symptomatic (isolated diffuse rash and thrombocytopenia 24 h after maternal symptoms). The pregnancy for the second case had been complicated since the second trimester with gestational diabetes and intrauterine growth restriction. The authors did not report the details of either of these complications (diet or insulin treatment, Doppler signs of placental insufficiency). While both pathologies are likely unrelated to ZIKV infection, they may have weakened the neonate (intrauterine growth restriction, hypoglycemia, neonatal jaundice) and/or increased his susceptibility to infection. Both mothers and children evolved favorably. At least one sample per patient (maternal and neonatal) tested positive via a specific ZIKV reverse transcription-PCR (RT-PCR). Both mothers were likely viremic at the time of delivery, since the first had a rash 2 days prior to delivery and the second had a positive RT-PCR on day 1 postdelivery. The authors stated that transplacental infection was unlikely, as the second newborn remained ZIKV negative until day 4 postpartum. Infection during the late stages of pregnancy, delivery, or postnatally through close contact with the mother is more probable. RT-PCR was also positive in saliva from the mother and newborn in the first case, in the urine of the second newborn, and in the milk of both mothers. Interestingly, ZIKV RNA was detectable in neonatal urine at a higher load and for a longer duration than foudn in serum, as reported by others for ZIKV (165, 170) and for other flaviviruses (171, 172). Of note, milk cultures on Vero cells remained negative for ZIKV in both cases (121, 173). All samples tested negative for DENV by RT-PCR. No other viral causes of rashes (coxsackievirus, Epstein-Barr virus, varicella-zoster virus, parvovirus B19) (174, 175), and viral serologies (including ZIKV) were documented. The long-term outcomes of these neonates, especially neurological, are unknown.
Oliveira Melo et al. (11) and the Brazil Ministry of Health (176) published the first description of intrauterine transmission of the virus. These authors reported 2 cases of fetal microcephaly in fetuses from women who suffered from symptoms compatible with ZIKV infection at 18 and 19 weeks gestation, respectively (121). Both pregnant patients originated from a state of Brazil (Paraiba) considered part of the “microcephaly cluster.” Maternal blood testing was negative for ZIKV, but time elapsed between initial symptoms and testing and investigation of urine/saliva samples were not documented. ZIKV RNA (Asian genotype) was present in the amniotic fluid of both patients (with a viral load 10,000 times higher than what is normally found in blood from adults with acute infection [121]), whereas all other TORCH pathogen serologies were negative. Fetal sonographic markers are described here and can be found on the Phenotip database website (177). The same publication briefly described six other microcephalic children from the same state, all positive for ZIKV, and born to mothers who were symptomatic during pregnancy. Their neonatal outcomes were not described, except for one child with severe arthrogryposis.
Two similar cases were reported by the same research team (178). Both mothers, also originating from the state of Paraiba in Brazil, experienced ZIKV-like symptoms at 10 and 18 weeks gestation, respectively. ZIKV genome was detected at 28 weeks gestation in the amniotic fluid of both pregnant women, whose fetuses had been diagnosed in utero with microcephaly and brain calcifications (at 21 and 22 weeks, respectively). All maternal samples were negative for ZIKV, CHIKV, and DENV molecular detection as well as TORCH serologies. Both patients were still pregnant at the time of the publication. Despite a viral metagenomics approach used in addition to the ZIKV RT-PCR, the presence of other viruses was not reported. ZIKV IgM antibodies were detected in the amniotic fluid but were not confirmed by plaque reduction neutralization tests (PRNT) (179).
More recently, the WHO and PAHO (180) described the first ZIKV-related neonatal death. The newborn, from the state of Pará in Brazil, presented with microcephaly and other congenital anomalies (not currently described) and died within 5 min after birth. ZIKV RNA was identified in multiple fetal tissues, including the brain and blood. Confirmation of presence of the viral genome was provided by the Evandro Chagas Institute, the national reference laboratory for arboviruses in Belém, Pará, Brazil (180). More details on the pregnancy, potential maternal symptoms, and the nature of the other congenital anomalies are not yet available.
Four other cases from Brazil have been described by the CDC (5, 181), but full reports of parts of these cases are still pending. All four mothers reported having experienced a febrile rash illness during their pregnancies. Two pregnancies ended in miscarriage and two resulted in full-term infants with microcephaly who died shortly after birth. Samples from all four pregnancies, including brain samples from the infants, tested positive for ZIKV infection, and genetic sequence analyses confirmed that the virus was similar to the ZIKV strain currently circulating in Brazil. Again, details about the type of samples tested, pregnancy history (gestational age at miscarriage), and time of maternal symptoms have not been fully described to date.
ZIKV was also detected in 14 cases (including 2 fetal losses) among 387 babies with confirmed microcephaly and brain anomalies (23). Reports of details for these 14 cases are pending.
Mlakar et al. described the case of an expectant mother who had ZIKV-like symptoms at 13 weeks gestation (12). She had worked as a volunteer in Rio Grande do Norte state of Brazil. Ultrasounds performed at 14 and 20 weeks gestation were normal. Microcephaly with brain and placental calcifications were identified at 29 weeks gestation upon her return to Europe. This was again confirmed 3 weeks later along with the new identification of intrauterine growth restriction with normal blood flow on Doppler imaging. ZIKV infection during pregnancy was not confirmed. The patient opted for termination of pregnancy due to the dismal prognosis. Birthweight and head circumference were at the 10th and 1st percentile, respectively. Fetal autopsy confirmed an abnormally small brain (4 SD below average), almost complete agyria, hydrocephalus, calcifications in the cortex, and subcortical white matter, with associated cortical displacement and mild focal inflammation. All other organs were normal. RT-PCR confirmed ZIKV only in the fetal brain tissue, with consistent findings on electron microscopy. The viral load (6.5 × 107) detected in the fetal brain was higher than those previously reported in the serum of ZIKV-infected patients, but similar to those reported in semen samples (182). All autopsy samples were negative for other Flavivirus species, CHIKV, and TORCH PCRs, and karyotype by microarray technology was normal. The complete ZIKV genome was recovered from the fetal brain and showed 99.7% identity with the ZIKV strains isolated in French Polynesia (2013) and in Sao Paolo (2015). The authors stated that the presence of two major amino acid substitutions in nonstructural proteins NS1 and NS4B might indicate a process of eventual adaptation of the virus to a new environment (12).
Some national authorities have described additional cases, but information on diagnostic and clinical outcomes is not yet available. The Colombian Ministry of Health is currently monitoring 5,013 pregnant women, among whom 633 are suspected ZIKV cases, 3,921 are clinical cases, and 459 are laboratory-confirmed cases by RT-PCR (183). Details regarding pregnant patients with confirmed ZIKV infection are not available. The Hawaii State Department of Health reported one case of microcephaly in a baby born with ZIKV infection. The mother was likely infected with ZIKV while living in Brazil in May 2015 (184). In the French Caribbean area, 23 pregnant women with ZIKV infection are currently benefiting from enhanced monitoring in Martinique (n = 13), French Guiana (n = 8), and Guadeloupe (n = 2) (185). At the time of this publication, no congenital abnormalities have been detected.
More recently, 88 pregnant patients from Rio de Janeiro were prospectively enrolled in a cohort study that took place from September 2015 through February 2016 (15). Inclusion criteria were a rash that developed within the 5 days prior to prenatal consultation. Of note, only 28% reported having fever. Among 88 pregnant patients, 82% had a positive ZIKV RT-PCR in blood and/or urine samples; gestational age at the time of diagnosis ranged from 5 to 38 weeks gestation. Positive DENV serologies were reported in 88% of the patients, whereas ZIKV serologies were not reported. Twenty-eight ZIKV-positive women declined fetal imaging studies secondary either to distance from the obstetrical facility or fear of possible fetal abnormalities related to ZIKV infection. Two ZIKV-positive women miscarried during the first trimester. Among the 42 ZIKV-positive women who had further fetal ultrasonography, 12 (29%) showed fetal abnormalities (Table 3). Intrauterine fetal death occurred in 2 fetuses whose mothers were infected at 25 and 32 weeks gestation, respectively (autopsy not mentioned). Other adverse findings included in utero growth restriction with or without microcephaly (5 fetuses), central nervous system lesions or calcifications (7 fetuses), and abnormal amniotic fluid volume or cerebral or umbilical artery blood flow (7 fetuses). These findings were confirmed in 6 of the 42 babies delivered at the time of publication. Lesions were noted in the fetuses independent of gestational age at the time of exposure to ZIKV, and brain anomalies were also seen in fetuses potentially infected as late as 27 weeks of gestation. Mothers infected in late gestation were more prone to develop placental insufficiency, fetuses with growth restriction, or fetal death. Isolated microcephaly was observed in only one case. Since only symptomatic patients were included in this study (15), it is not possible to know whether the high rate (29%) of fetal/neonatal anomalies observed would be similar in asymptomatic ZIKV-positive mothers.
Meaney-Delman et al. (186) briefly described CDC reports of 9 pregnant travelers with laboratory-confirmed ZIKV infections (an additional 10 pregnant travelers are still under investigation). Both ZIKV RT-PCR and IgM-specific serologies were used to confirm infection, but details of each case are not available. All 9 travelers reported at least one symptom compatible with ZIKV infection. Outcomes included 2 miscarriages, with intrauterine transmission confirmed in both cases by molecular detection of ZIKV in the products of conception (one case was described earlier [187]), 2 elective pregnancy terminations (only one described, see below), and 3 live births (2 apparently healthy infants and 1 infant with severe microcephaly). In the 2 miscarriage cases previously described by Martines et al. (187), serologic testing confirmed recent ZIKV infection and products of conception were ZIKV positive by RT-PCR and immunohistochemistry. The last two pregnancies are still ongoing (currently approximately 18 weeks and 34 weeks gestation) without any complications described so far. In this report, adverse outcomes were identified only in patients in whom infection occurred during the first trimester of pregnancy and compared to during the second or third trimester of pregnancies for the two uneventful pregnancies.
Sarno et al. (188) reported a case of a fetal demise at 32 weeks gestation in a 20-year-old asymptomatic pregnant woman from the city of Salvador, Brazil, that was positive for ZIKV. Examinations in the second and third trimesters demonstrated progressive hydrops (see below). Extracts of cerebral cortex, medulla oblongata, and cerebrospinal and amniotic fluids were all positive for ZIKV by RT-PCR, while it was not detected in extracts from the heart, lung, liver, vitreous body of the eye, or placenta. The association between ZIKV infection and hydrops fetalis suggests that the virus may not be limited to the central nervous system but may also cause damage to other organs.
Villamil-Gomez et al. (189) reported 2 cases of brain calcifications in an ongoing cohort of 28 ZIKV-positive pregnant patients from Colombia. Since all these patients were still pregnant at the time of the publication, more details are pending.
Finally, Driggers et al. (190) recently described a case of a pregnant woman infected with ZIKV at 11 weeks gestation with subsequent prolonged maternal viremia. Indeed, ZIKV RNA was identified in maternal serum from 16 weeks until termination of pregnancy at 21 weeks gestation due to major brain anomalies. In addition, serological testing demonstrated evidence of a recent infection, with ZIKV IgM detection at a titer of >1:2,560 in the serum on a plaque-reduction neutralization test (PRNT). ZIKV DNA was detected in fetal tissues. The highest ZIKV viral loads were found in fetal brain, followed by the placenta, fetal membranes, and umbilical cord. Smaller amounts were detected in fetal muscle, liver, lung, spleen, and amniotic fluid. On postmortem analysis, the brain showed diffuse cortical thinning and abundant apoptosis, extensive axonal rarefaction, and macrophage infiltrates by microscopic analysis. Viral particles were detected, and ZIKV was subsequently isolated. Since the patient remained viremic 10 weeks after the clinical onset of ZIKV infection, but not after delivery, those authors suspected that the persistent ZIKV viremia was a consequence of viral replication in the fetus or placenta. Indeed, prolonged maternal viremia might reflect feto-placental shedding in severely infected fetuses. This study highlights the possible importance of testing pregnant women beyond the first week after symptom onset.
In summary, evidence of ZIKV infections during pregnancy has been documented. However, many details regarding patient history, diagnostic method used, or full evaluation of the differential diagnosis are lacking, as suggested by others (191). There is now an urgent need to move from case reports to strong and well-designed case-control or cohort studies to better understand the true role of ZIKV during pregnancy (179), as was done with Guillain-Barré syndrome (192). Even if the severity of fetal damage is likely related to gestational age at the time of maternal infection, the cases described above demonstrate a huge range in fetal outcomes. Similar to other intrauterine infections, such as cytomegalovirus infection or toxoplasmosis, where not all infected fetuses are symptomatic, the reported cases of microcephaly and brain damage might represent only the more severely affected children. Moreover, fetal damage likely occurs weeks after infection, and the latter is thus difficult to confirm retrospectively. Indeed, by the time of testing, the virus has disappeared and serological tests may cross-react with other flaviviruses, especially dengue virus (193). Despite the neurotropic nature of ZIKV, it is possible that newborns with less severe disease and other affected organs have not yet been diagnosed.
Suspected Cases under Ongoing Investigation
At this time, many cases of microcephaly are considered “suspect” because they have been linked to maternal symptoms reported during pregnancy. The only reliable method available to diagnose ZIKV infection, however, is RT-PCR, which is only useful for viral detection during the acute phase of illness, which lasts a few days (a “hit-and-go” virus). This means that definitive diagnosis of ZIKV infection will not be possible in many cases, since microcephaly is only diagnosed during the third trimester or at birth, potentially weeks after the acute phase. Moreover, since approximately 80% of patients with infections are asymptomatic (119, 194, 195), the lack of symptoms does not rule out ZIKV infection.
The health authorities of French Polynesia retrospectively reported an unusual increase of at least 18 cases of brain malformations in fetuses and newborns, coinciding with the ZIKV outbreaks in the islands (September 2014 to March 2015) (13, 121, 196). In 15 cases (88%), the first two trimesters of the pregnancies coincided with the French Polynesian ZIKV outbreaks, leading to an estimated prevalence rate of 6/1,000 births for microcephaly (13). This prevalence is extremely high and comparable to that observed in the “microcephaly cluster” of Brazil. None of the pregnant women experienced clinical signs of infection. Flavivirus-positive IgG serologies were found in 4 tested mothers who were also negative for dengue virus, suggesting a possible asymptomatic ZIKV infection. Of the 18 malformations registered, brain malformations or syndromes with brain lesions were identified in 13, of which 10 women opted to terminate the pregnancy and 3 delivered babies with microcephaly. All karyotypes (n = 10) were normal and CMV PCR was negative (n = 7). The five remaining cases were infants with brainstem dysfunction and absence of swallowing. More data regarding these cases was later published by Jouannic et al. (197). Following the Brazilian alert on possible fetal brain damage secondary to ZIKV, 6 available stored amniotic fluid samples were retrospectively tested using RT-PCR. Among those, 4 were positive ZIKV and viral symptoms during the first trimester of pregnancy were reported retrospectively in 3 of the 4 cases (197). Microcephaly, severe abnormalities of midline structures and the cerebellum, as well as abnormal gyration were observed. The same group of authors published another series of 19 cases that were similar (198), without any mention of their previous study (197).
Cauchemez et al. also discussed 8 microcephaly cases in French Polynesia from September 2013 to July 2015 among 66% of the general population infected by ZIKV (199). Five cases that were diagnosed during pregnancy opted for termination, and three children were born alive. These cases have not been further described. From this publication, Cauchemez et al. provided a quantitative estimate of the prevalence of microcephaly associated with ZIKV infection (95 cases per 10,000 women infected in the first trimester; CI 95% confidence interval [CI], 34 to 191).
In their weekly report in January 2016, Schuler-Faccini et al. (5) described 37 infants with microcephaly (including 25 infants with a head circumference of <3 SD and 11 infants with excessive scalp skin). Two infants were excluded due to the identification of an autosomal recessive microcephaly and a confirmed CMV infection. Rash during the first (n = 21, 57%) or second (n = 5, 14%) trimester was reported by 26 (74%) mothers, and all of them were living in or had traveled to known regions of ZIKV endemicity. All infants tested negative for syphilis, toxoplasmosis, rubella, CMV, and HSV infections. Details of the postnatal neuroimaging are described later. Additionally, 5 infants had talipes, 4 had arthrogryposis, and 1 had microphthalmia. On neurologic examination, abnormalities were reported in 49% of the cases, including hypertonia/spasticity (37%), hyperreflexia (20%), irritability (20%), tremors (11%), and seizures (9%). The same series of 35 cases of suspected congenital ZIKV syndrome was further described in a second report by Miranda-Filho et al. (200). The infants were described to have microcephaly, facial disproportionality (face appears large in comparison to a smaller head), and cutis girata (skin scalp folds caused by the continued growth of the skin as brain development slows down). Ventura et al. (95) described 13 other term babies with microcephaly, brain calcifications seen on computed tomography (CT) scan, and ocular manifestations (see details below). All were born during the ZIKV infection outbreak in Brazil, and eight (62%; one described in reference 95 and seven described in reference 96) of the mothers had malaise, rash, and arthralgia during pregnancy, of which seven (87.5%) were in the first trimester. Intrauterine ZIKV infection is highly suspected, as serologies for toxoplasmosis, rubella, CMV, and HIV were negative.
Werner et al. (201) presented a case of a 27-year-old patient with ZIKV-like symptoms at 12 weeks gestation. Ultrasounds at 12 and 21 weeks gestation were normal; however, microcephaly was suspected at 32 weeks. Pre- and postnatal MRIs confirmed microcephaly, brain calcifications, reduced gyration, corpus callosum dysgenesis, and premature closure of the sutures. Since maternal TORCH, DENV, and CHIKV testing was negative, the authors suspected a possible congenital ZIKV infection.
Two cases of women that live in Barcelona but traveled in areas where ZIKV is endemic at the end of 2015 and have tested positive for ZIKV have been reported (202). These pregnancies are ongoing and no fetal anomalies have been detected to date, but the final outcomes of these pregnancies are yet to be determined.
Prenatal Sonographic Markers
As mentioned above, Oliveira Melo et al. (11) described the first two prenatal cases of ZIKV infection. Microcephaly of 3.1 and 2.6 SD below the norm was identified at 29.2 and 30.1 weeks gestation, respectively, and lesions were limited to the brain. Both fetuses had otherwise-normal growth (19th and 21st percentiles) and no signs of fetal anemia (normal cerebral Dopplers). In one case, cerebral lesions consisted of calcifications located around the lateral and fourth ventricles and severe unilateral ventriculomegaly causing displacement of the midline, asymmetric cerebral hemispheres, thinning of the parenchyma on the dilated side, failure to visualize or disappearance of the corpus callosum and thalami, thin pons and brainstem, and a nonhomogeneous small mass in the area of the basal ganglia. The second case demonstrated even coarser calcifications involving the white matter of the frontal lobe and cerebellum, corpus callosum, and vermian dysgenesis and enlarged cisterna magna. Among the 6 other cases described in the same study (11), fetal neurosonograms showed 2 cases with cerebellar involvement and 3 with brain calcifications.
Prenatal sonographic features were also well described in the paper of Mlakar et al. (see description of the case above) (12). Whereas ultrasounds at 14 and 20 weeks gestation were normal, the 28-week ultrasound showed reduced fetal movements, numerous calcifications in the placenta and the brain, and blurred brain structures, as well as a dilated occipital horn of the lateral ventricles.
In a cohort study by Brasil et al. (15), 42 ZIKV-positive pregnant patients accepted prenatal ultrasonographic examinations. A total of 12 fetuses demonstrated anomalies. Signs of placental insufficiency were frequently reported and included intrauterine growth restriction (n = 5), abnormal Doppler studies (n = 4), low amniotic fluid volume (n = 2), and increased placental thickness (n = 2). Brain anomalies were reported in 8 fetuses and included microcephaly (n = 4), cerebral calcifications (n = 6), ventriculomegaly (n = 5), mega cisterna magna (n = 4), and other brain anomalies (agenesis of the vermis, Blake's pouch cyst, cerebellar atrophy). Clubfoot or arthorgryposis, which are signs of potential brain anomalies, were reported in 3 fetuses. Cerebral calcifications were seen in fetuses of women infected as late as 27 weeks, whereas intrauterine growth restriction was present in fetuses of women infected during any trimester. Of note, 3 of 4 fetuses diagnosed with microcephaly in utero were born at the time of the publication, 1 with isolated microcephaly and 2 identified as small for gestational age (nonisolated microcephaly).
In 1 of the 9 infected patients described by Meaney-Delman et al., absence of the corpus callosum, ventriculomegaly, and brain atrophy were observed on ultrasound at 20 weeks and via magnetic resonance imaging (186). ZIKV RNA was detected in amniotic fluid, and the patient opted for termination of her pregnancy.
Sarno et al. (188) reported the first case of hydrops fetalis linked to ZIKV infection. Indeed, ultrasound at 26 and 30 weeks gestation demonstrated intrauterine growth restriction, severe microcephaly, hydranencephaly, intracranial calcifications, and destructive lesions of the posterior fossa, in addition to hydrothorax, ascites, and subcutaneous edema.
In the case presented by Driggers et al. (190), fetal ultrasonography showed no evidence of microcephaly or intracranial calcifications until 5 weeks post-ZIKV infection. There was a progressive decrease in the fetal head circumference, however, with abnormal intracranial anatomy at 19 weeks gestation. Intraventricular hemorrhage was suspected due to the presence of echogenic material in the frontal horns and the dilated ventricles. Anomalies of the corpus callosum were also suspected. No parenchymal calcifications were seen.
Postnatal Imaging and Findings
Schuler-Faccini et al. published a detailed report on postnatal brain imaging findings (5). CT scans and brain ultrasounds performed on 35 infants suspected of intrauterine ZIKV infection (see previous section) showed a pattern similar to the prenatal findings described above. The authors described widespread brain calcifications (74%), mainly localized to the periventricular, parenchymal, and thalamic areas and in the basal ganglia. Ventriculomegaly secondary to cortical/subcortical atrophy was reported in 44% of cases, and 33% of infants also had evidence of cell migration abnormalities (e.g., lissencephaly, pachygyria).
At the time of the publication by Brasil et al. (15), six live births to mothers who presented with a rash during their pregnancy and tested positive for ZIKV by RT-PCR occurred (see above). Two newborns of mothers infected at 30 and 31 weeks gestation had normal biometrics and normal examinations at birth. One infant, whose mother was infected at 8 weeks gestation, had severe microcephaly, global cerebral atrophy, and calcifications confirmed by CT scan as identified prenatally. Two infants with maternal infection at 22 and 26 weeks gestation were reported to have growth restriction in utero, which was confirmed as small for gestational age at delivery with proportionally small heads. Finally, one infant with anhydramnios whose mother was infected at 35 weeks gestation was found to have normal measurements at birth but poor sucking reflex and electroencephalogram abnormalities.
Meaney-Delman et al. (186) described the case of a mother who had ZIKV-like symptoms during her first trimester of pregnancy while she was living in Brazil. She delivered a term infant with severe microcephaly. Molecular and pathological evaluation of the placenta demonstrated ZIKV by RT-PCR and immunohistochemistry, respectively. The infant exhibited seizures, difficulty swallowing, and hypertonia. A CT scan demonstrated multiple scattered and periventricular brain calcifications. Fundoscopic examination revealed a pale optic nerve and mild macular chorioretinitis.
Other Fetal and Neonatal Anomalies
In addition to the brain anomalies described above, ZIKV infections may be involved with specific ocular tropism. One case described by Oliveira Melo et al. (11) showed bilateral cataracts and intraocular calcifications as well as a size discrepancy between eyes.
Ventura et al. presented 2 reports discussing adverse ocular outcomes in children born with microcephaly after the ZIKV outbreak in Brazil (95). In the first publication, outcomes for 1 male and 2 female babies were described, and only one mother reported symptoms of rash and arthralgia in the first trimester. None of the mothers had ocular lesions on biomicroscopy and fundoscopy. The pregnancies and prenatal follow-up ultrasounds were not described. All infants were born between 37 and 38 weeks gestation, and two of them were below the 10th percentile for weight. These infants had microcephaly and cerebral calcifications detected by CT scan. Although the presence of ZIKV infection was not evaluated by RT-PCR, intrauterine infection is highly suspected since toxoplasmosis, rubella, CMV, HSV, syphilis, and HIV were ruled out in all cases (mothers and infants). On examination at 56 to 88 days of life, the three infants had unilateral ocular findings restricted to the macular region (gross macular pigment mottling and fovea reflex loss) (95). A well-defined macular neuroretinal atrophy was also observed in one infant.
In another publication (96), the same authors reported 10 other infants with ocular anomalies, microcephaly, and brain calcifications. Pregnancy details are described above in the section “Suspected cases under ongoing investigation.” The mothers' ocular examinations were all normal. The infants had normal anterior segment structures, normal reactive pupils with no afferent pupillary defect, no retinal detachment or vessels anomalies. One infant presented with horizontal nystagmus and six had strabismus (4 exophoria and 2 esophoria). Optic nerve hypoplasia (9 eyes) and macular alterations (15 eyes) were present in 85% of the cases. Macular alterations consisted of foveal reflex loss, mild to gross pigment mottling, and sharply demarcated circular areas of chorioretinal atrophy (96).
An additional 29 cases have been described by Freitas et al. (92, 94). Microcephalic infants with a presumed diagnosis of congenital ZIKV infection were recruited through an active search in December 2015 in Salvador and Sao Paulo, Brazil (203). All other infections were ruled out (TORCH, HIV). ZIKV-like symptoms were reported by 79.3% of the mothers (18, 4, and 1 in the first, second, and third trimesters, respectively). Ocular abnormalities, mainly bilateral (n = 7), were present in 10 children (34.5%). Anomalies identified were focal pigment mottling of the retina and chorioretinal atrophy (64.7%), optic nerve abnormalities (47.1%), and bilateral iris coloboma and lens subluxation (1 patient each).
Among the 6 live births described by Brasil et al. (15), fundoscopy demonstrated macular hypoplasia and/or scarring in 2 newborns of mothers who presented with a rash during their pregnancy and were confirmed to be ZIKV positive at 8 and 22 weeks gestation, respectively.
Other Adverse Obstetrical Outcomes Associated with ZIKV Infection
Through the confirmed and suspected cases described above, ZIKV infection has been associated with miscarriage or adverse neonatal outcomes. The pathophysiology of the brain lesions may be related to the virus itself or a toxin leading to an inflammatory reaction. This could result in findings of severe cerebral anomalies (abnormal development leading to microcephaly, cerebral calcification, and ocular lesions), especially when the infection occurs in the first or second trimester of pregnancy. This is similar to other vertically transmitted infections, such as toxoplasmosis, rubella, and CMV. Moreover, other unknown factors, such as the immunologic responses of mother and fetus or the amount of viral circulation, may play an important role in the abnormalities observed in newborns.
The rate of preterm births does not seem to increase in cases of ZIKV infection. Among the 34 cases reported by Schuler-Faccini (5), 9% were delivered prematurely, which does not differ from the normal rate of preterm birth outside a ZIKV outbreak (204, 205).
Differences from Other Teratogenic Infections
The classical group of teratogenic pathogens is referred to as “TORCH” and is recognized to cause multiple fetal anomalies (206). In contrast, mainly brain and ocular malformations have been identified after ZIKV infection during pregnancy. This potential “pure” neurotropic effect of a virus is surprising, but it has previously been described for other viruses (see the section on LCMV, above). Future reports, however, may describe other fetal organ involvement.
The severe damage of the cerebellum, brainstem, and thalami that is seen after congenital ZIKV infection is rarely associated with other intrauterine infections (11). The 2 fetal cases described by Oliveira-Melo et al. showed some similarities to CMV and toxoplasmosis cases (11), but a more severe and destructive pattern was noted, with a lack of nodules.
Miranda-Filho et al. (200) described the differences from other congenital infections, based on 35 cases with presumed congenital ZIKV syndrome. Congenital ZIKV cases have no hepatosplenomegaly, petechiae, purpura rash, or other skin lesions. In contrast to congenital toxoplasmosis, parvovirus B19 infection, and syphilis, hearing abnormalities can be present after ZIKV infection. Calcifications may be more severe with ZIKV than with toxoplasmosis or CMV infections. But like in all congenital TORCH infections, ocular abnormalities may be present with ZIKV.
As described by Rasmussen et al. (16), fetuses and infants with congenital ZIKV infection have a typical pattern, including microcephaly and intracranial calcifications, sometimes accompanied by other brain anomalies, eye findings, redundant scalp skin, and arthrogryposis. Moreover, some infants present features consistent with “fetal brain disruption sequence” (16), characterized by prominent occipital bone, overlapping cranial sutures, and redundant scalp skin, findings that are not typically seen in other forms of microcephaly. This phenotype suggests a growth of the scalp skin in parallel with an interruption of cerebral growth after an injury that occurred after the initial formation of brain structures, followed by partial collapse of the skull (16).
INVESTIGATION OF THE CAUSAL LINK
Evidence of cell line susceptibility to ZIKV, as well as human and animal intrauterine transmission in genetically related viruses (DENV, WNV, JEV) or in viruses with similar epidemiology (CHIKV), is reviewed below.
Cell Lines
With mosquitoes as the vector for transmission and replication, it is expected that ZIKV can infect and replicate in several mosquito cell lines, such as C6/36 (Aedes albopictus) and AP-61 (Aedes pseudoscutellaris) (207, 208). In addition, several mammalian cells lines are susceptible to ZIKV as well, including PS-C1 cells (porcine kidney), LLC-MK2 cells (Macaca mulatta, rhesus monkey, kidney), and Vero cells (Chlorocebus aethiops, African green monkey, kidney) (139, 209). Recently, Hamel et al. (210) investigated the ability of ZIKV to infect human skin cells, as these are thought to be the target cells for ZIKV inoculation during the mosquito blood-feeding process. They identified that human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells are susceptible to ZIKV. The virus activates the innate immune response, including type I and type II interferons (IFNs), in infected primary human fibroblasts. In addition, Toll-like receptor 3 plays a role in the recognition of ZIKV (210). Skin fibroblasts infected with ZIKV resulted in the formation of an autophagosome, which is associated with enhanced viral replication (210). Although autophagy has not been described in ZIKV-infected neural cells so far, it could potentially play a role in the development of microcephaly. It has been reported that one potential cause of microcephaly involves abnormal centrosome function (211). The amplification of the centrosome number has been revealed to be an inducer of microcephaly (212). Several proteins, such as the UV irradiation resistance-associated gene (UVRAG) product and Beclin-1, have a dual role in autophagy as well as centrosome stability (213, 214). Specifically, Beclin-1, which induces an increase of centrosomes in mice, results in a delay in mitosis, an increase in apoptosis, improper neural stem cell orientation, premature neuronal differentiation, and a decrease in progenitor cells in mice. Overall, the reduction in brain matter formation leads to a decrease in brain size indicative of microcephaly (212). Studies investigating the role of ZIKV-infected neurons, autophagy, and the possible effect on microcephaly development need to be further addressed.
Other groups have shown that ZIKV grows efficiently in A549 human lung epithelial cells (215) and human neural stem cells (216). ZIKV targets human brain cells and reduces their viability and their growth into neurospheres and brain organoids. Those authors concluded that ZIKV abrogates neurogenesis during human brain development (216). Bayer et al. (217) discovered that primary human trophoblasts from full-term placentas are refractory to ZIKV infection. In addition, medium from uninfected trophoblast cells protects nonplacental cells from ZIKV infection, possibly secondary to the constitutive release of IFN-λ.
Mice
Despite limited studies of ZIKV in mice, ZIKV has been shown to be highly neurotropic (140, 218, 219). Dick et al. (218) demonstrated that mice were susceptible to infection after intracerebral inoculation with ZIKV, independent of their age. In contrast, intraperitoneal inoculation with ZIKV only led to disease in mice who were 2 weeks or younger. Mice of 2 weeks and older could rarely be infected with ZIKV via intraperitoneal inoculation (218). Following intracerebral inoculation, mice showed evidence of neuronal degeneration especially in the region of the hippocampus, cellular infiltration, nuclear swelling with margination, and pycnosis of the chromatin, followed by karyrrhexis and softening of brain tissue. Cowdry type A inclusion bodies were observed especially in brains of young mice showing extensive lesions (218, 219). Infection of young mice (between 1 and 5 days old) was also associated with skeletal myositis and myocarditis (219). In another study, 1-day-old mice and 5-week-old mice were infected intracerebrally with ZIKV (140). The most prominent changes were found in Ammon's horn. In newborn animals, localized segments of necrosis in the band of pyriform cells, large amounts of hyperchromatic debris, and astrocyte hypertrophy were noted. In the 5-week-old mice, remarkable changes in astroglial cells were observed throughout the whole cortex. Electron microscopy revealed that ZIKV was able to replicate in astroglial cells and neurons (140). It is important to note that in these studies the mice were inoculated intracerebrally with ZIKV, and in order to confirm the neurotrophism of ZIKV, other inoculation routes should be evaluated.
To our knowledge, no studies in which pregnant mice have been infected with ZIKV have been performed. Several other studies concerning related viruses, such as WNV and Japanese encephalitis virus (JEV, another Flavivirus), however, have shown transplacental transmission and susceptibility of embryos. WNV infection in pregnant mice (usual length of gestation of 20 to 21 days), for example, resulted in extremely high mortality, independent of the infecting dose or the week of gestation (220, 221). Several mouse studies demonstrated intrauterine transmission of WNV, which was more efficient in mice infected during the second week of gestation than during the third week of gestation (P < 0.0001) (220, 222, 223). Mouse embryos are susceptible to WNV infection after the formation of the trophectoderm around 3.5 days postcoitus (dpc) through the formation of the functional placenta around 10.5 dpc (223).
The transplacental transmission of JEV was investigated using a mouse model, in which pregnant mice were infected by intraperitoneal inoculation on the first (day 5), second (day 8), or third (day 15) week of gestation (224). Although no clinical symptoms were observed in the inoculated mice, a brief viremia was followed by replication of the virus in the spleen, liver, kidney, and placenta. Infection of pregnant mice with JEV during the first week of gestation resulted in more fetal and neonatal deaths (66%) than in mice infected during the last 2 weeks of gestation. JEV was isolated from brain tissue from stillborn, newborn, and/or infant mice independent of the time of maternal inoculation during the pregnancy (224).
Pigs
To date, no studies have been performed on ZIKV infection in pigs; however, the closely related JEV is known to be a common cause of reproductive diseases in pigs and has been associated with brain damage. While adult pigs show no clinical symptoms upon JEV infection, infection of sows in early pregnancy results in stillbirths, abnormal young, and JEV isolation from brain tissue (225). Another study in pigs identified JEV in the brains of 3 piglets, which died shortly after birth (226). More recently, Yamada et al. (227) infected 3-week-old piglets with JEV, and this resulted in encephalitis and lesions in the cerebrum, midbrain, and cerebellum. Perivascular cuffing, neurophagia, neuronal necrosis, and glial nodules were observed in the infected piglets. In addition, they showed that JEV was able to reach the central nervous system 3 days after infection by crossing the blood-brain barrier (227). Weaning piglets infected with WNV demonstrated similar lesions in the central nervous system, as mentioned above (228). JEV and WNV infections in pigs is reviewed in detail in the chapter on flaviviruses in the book Diseases of Swine by Zimmerman et al. (229).
In contrast to the adverse effects of JEV and WNV infection in pregnant animals, the effects of JEV and WNV infection during human pregnancy (described below) are limited. The observed differences in disease outcomes between animal and human hosts could be due to the experimental setup (unnatural dose of infection and infection route in animals), the immune response of the host against the virus, or the virulence of the strain used for experimental infection. The fact that JEV and WNV are able to cause reproductive complications and induce brain damage in newborn mice and pigs, however, suggests that these viruses have the potential to cause serious effects in humans as well.
Humans
Reports of human intrauterine transmission of related viruses are summarized in Table 4.
TABLE 4.
Case reports concerning flaviviruses and perinatal transmission
| Virus | Country and yr (reference[s]) | Study type (n); case no. | Maternal clinical manifestations | Maternal diagnosis basis | Gestational age at onseta | Transmission route | Clinical manifestations of fetus/newborn | Diagnosis for fetus |
|---|---|---|---|---|---|---|---|---|
| DENV | Malaysia 1996 (234) | Case report (2); Case 1 | “Pre-eclampsia-like” symptoms | IgM-positive serum | 36 WG | Intrauterine (suspected) | Severe acute renal failure, hypotension, seizures, hypoglycemia, abnormal liver function, and coagulopathy; died on day 6 of life | Positive PCR blood sample |
| Malaysia 1996 (234) | Case report (2); Case 2 | Fever and mild dysuria, petechiae with severe thrombocytopenia | IgM-positive serum, PCR-positive blood sample | 38 WG | Perinatal | No clinical symptoms | IgM-positive serum (days 6 and 11) | |
| New Caledonia 2013 (342) | Case report (1) | Persistent fever, anemia, and thrombocytopenia | PCR-positive blood and breast milk | 30 WG | Breastfeeding | Low-grade fever and thrombocytopenia | Positive PCR blood sample | |
| WNV | USA 2002 (230, 231) | Case report (1) | Flu-like symptoms developed into paraparesis, which slowly resolved | IgM-positive serum and CSF | 27 WG and 38 WG (delivery) | Intrauterine | Bilateral chorioretinitis, dramatic cerebral abnormalities | IgM-positive cord blood, heel stick blood, and CSF |
| USA 2002 (343) | Case report (1) | WNV meningoencephalitis after receiving WNV-contaminated blood | IgM-positive CSF | NR | Breastfeeding | No clinical symptoms | IgM-positive serum | |
| JEV | India 1978 (242) | Case report (5)b | Unconscious state, history of pyrexia of abrupt onset, convulsions, neck rigidity, extensor plantar reflex | IgM-positive serum | 22 WG | Intrauterine | Aborted | JEV isolation from placenta, brain, and liver |
| YFV | Brazil 2009 (243) | Case report (1) | Fever, jaundice, and conjunctival suffusion (day 7 postpartum) | IgM-positive serum | Late pregnancy | Intrauterine | Liver and renal failure, intravascular coagulation, seizures, resulting in coma and death; massive liver necrosis, pulmonary hemorrhage, and acute tubular necrosis found | IgM-positive serum, PCR-positive serum |
| Brazil 2009 (244) | Case report (1) | Headache, malaise, and low fever (5 days after receiving 17DD YFV vaccine 20 days postpartum) | No serum or breast milk tested | 39 WG (delivery) | Breastfeeding | Starting at 23 days of life: fever, convulsions, perioral cyanosis, meningoencephalitis | IgM-positive serum and CSF, PCR-positive CSF for 17DD YFV vaccine |
WG, weeks gestation; NR, not reported.
Cases 2 to 5 in the study were excluded due to unknown pregnancy outcome or no JEV diagnosis in the newborn.
West Nile virus.
The first and only case of intrauterine transmission of WNV was documented in 2002 when a 20-year-old woman was diagnosed with WNV 2 months prior to delivery (230, 231). She was hospitalized with flu-like symptoms that developed into paraparesis, which subsequently slowly resolved. The physical examination of the female newborn was completely normal (head circumference of 31.5 cm), and no rash or other skin findings were present (231). Ophthalmologic examination revealed bilateral chorioretinitis, and MRI of the brain showed dramatic cerebral abnormalities. The brain demonstrated general lissencephaly as well as a rectangular-shaped 2-cm cyst involving the left posterior temporal lobe and projecting beyond the cortex, likely at a site of more severe brain damage (231). As the cord blood, heel stick blood, and cerebrospinal fluid (CSF) of the newborn were positive for WNV-specific IgM and neutralizing antibodies and intrauterine transmission, and infection of the infant was confirmed. Perinatal transmission of WNV has also been investigated in a larger study of pregnant women in the United States (232). Cord blood samples from 22 of 547 deliveries tested positive for IgG antibodies against WNV. No cord blood samples, however, tested positive for IgM antibodies to WNV, providing no evidence of congenital WNV infections (232). The majority of these newborns were healthy, although a few infants were born with malformations, such as chorioretinitis, severe cerebral abnormalities, and neonatal respiratory distress, though no definite association to WNV infection could be established (232). A second study, which included 72 infants, indicated similar findings (233). Most of the newborns had no physical abnormalities, while seven infants had large malformations. Major birth defects followed first-trimester maternal WNV infection in 1 case (polydactyly), second-trimester infection in 3 cases (2 with microcephaly and 1 with Down syndrome), and third-trimester infection in 3 cases (1 aortic coarctation, 1 cleft palate, and 1 lissencephaly). The two newborns diagnosed with microcephaly did not have specific anti-WNV IgM antibodies in their serum or CSF, and no WNV RNA was detected by PCR in cord blood, cord tissue, or placenta (233).
Dengue virus.
Several case reports have suggested vertical transmission of DENV from mother to newborn, although none has been definitively confirmed (234–237). All documented cases of perinatal transmission of DENV thus far are linked to third-trimester maternal infection. A 25-year-old Malay woman was admitted to the hospital at 36 weeks gestation for stabilization of preeclampsia. Two days later, she developed an acute fever. At days 4, 7, and 10 of her illness, DENV infection was diagnosed through IgM-specific antibodies (234). On the fifth day of hospitalization, she delivered a male infant vaginally, who developed respiratory distress syndrome as well as a large uncontrollable left intracerebral hemorrhage. DENV type 2 was isolated from his blood, and the infant died of multiorgan failure on day 6 (234). The illness of the infant at time of birth suggests intrauterine transmission of DENV. DENV-specific IgM antibodies, however, were not detected in blood samples taken at days 2 and 6, which could be explained by the possible incubation time required to achieve detectable levels of IgM. During a retrospective study of 53 pregnant women in French Guiana who contracted dengue fever during their pregnancy, 20 blood samples were taken from the umbilical cord at birth (237). In three samples (15%), perinatal transmission was confirmed. DENV-specific IgM antibodies were detected in two of these cases, and DENV serotype 2 was isolated from the newborn of the third case. Another example is the case of a 22-year-old woman who was hospitalized at 40 weeks gestation for fever, uterine contractions, and flu-like symptoms. The infant was born via Caesarean section and monitored carefully at the hospital, as vertical transmission was suspected. Five days after birth, the infant developed fever and worsening thrombocytopenia. Both the mother and the infant tested positive (serology and PCR) for DENV serotype 2 (237). Ocular manifestations, such as bilateral chorioretinitis, are part of the characterized symptoms in adults infected with DENV. In most of the cases, these symptoms are self-limiting or treated with antibiotics and/or steroids; however, they can eventually lead to permanent visual impairment (238–240). To our knowledge, no reports have been published regarding ocular manifestations in newborns born from DENV-infected mothers, manifestations which are present in TORCH and WNV infections (231, 241).
Japanese encephalitis virus.
In 1978, during an extensive epidemic of JEV in Uttar Pradesh (India), a woman was admitted to the hospital at 22 weeks gestation with typical signs and symptoms of JEV (242). Her JEV-specific IgM serology was positive, and she aborted 4 days after disease onset. Specimens taken from brain, liver, and placenta of the fetus were all positive for JEV, indicating intrauterine transmission of the virus (242). So far, this is the only documented case of intrauterine transmission of JEV in humans; however, experimental JEV infections in animal models confirm the possibility of intrauterine transmission.
Yellow fever virus.
The first evidence of vertical transmission of yellow fever was observed in 2009 during an outbreak of sylvatic yellow fever in São Paulo State (Brazil) (243). A 30-year-old woman presented with complaints of fever, headache, and jaundice in late pregnancy. She delivered a female infant 3 days later via vaginal delivery in a local hospital. Seven days postpartum, the mother was admitted to the hospital with fever, jaundice, and conjunctivitis. She had elevated liver enzymes and mild anemia. On the 11th day of disease, a yellow fever IgM serology was positive (243). Her infant daughter was asymptomatic at birth and discharged from the hospital after 2 days of exclusive breastfeeding. She was admitted to the hospital on the third day of life, however, with fever and cyanosis, which progressed to hematemesis, melena, hypoglycemia, and oliguria by day 8 of life. Despite extensive therapy, the newborn had livery and kidney failure followed by disseminated intravascular coagulation, seizures, and finally coma. Death occurred on day 12, and autopsy samples showed massive liver necrosis, pulmonary hemorrhage, and acute tubular necrosis (243). Serum samples taken at 5 days after disease onset were positive for YFV-specific IgM antibodies, and the presence of YFV was confirmed by PCR. It is not possible to rule out transmission of yellow fever virus via breastfeeding, although this would suggest an unusually short incubation period of the virus. Transmission via breastfeeding has been recorded for the yellow fever vaccine virus (attenuated 17DD substrain; approved by WHO) (244). Several studies have investigated the effect of yellow fever vaccination during early pregnancy (245–247). None of these studies indicated that in utero exposure to yellow fever vaccine resulted in an increased risk of major malformations. IgM antibodies against the vaccine strains, however, were detected in cord blood of a newborn, indicating the possibility of intrauterine transmission (245).
Chikungunya virus.
Most of the reports concerning the effect of CHIKV infection during pregnancy describe no major effect on pregnancy outcomes or newborns if the mother was infected before the peripartum period (248–250). However, CHIKV-specific IgM antibodies were detected in the cord blood of the infants, providing evidence for intrauterine transmission of the virus (249, 250). In contrast, another study identified that in the case of a symptomatic mother (2 days before and 2 days following delivery), the neonate also developed CHIKV symptoms (251). A study by Geraldin et al. (252) examined 33 CHIKV-infected children at around 2 years of age and compared them with 135 uninfected peers. CHIKV perinatal transmission was identified for the infants of mothers infected during pregnancy with a positive PCR and/or presence of CHIKV-specific IgM antibodies. After adjustment for maternal social situation, IUGR, and head circumference, CHIKV infection was found to be associated with global neurodevelopmental delay (incidence rate ratio, 2.79; 95% CI, 1.45 to 5.34). Twelve infants presented with CHIKV neonatal encephalopathy, and five developed microcephaly (252).
CLINICAL MANAGEMENT
Prenatal Care
Screening strategies and general pregnancy monitoring.
To date, few agencies have expressed detailed recommendations with regard to ZIKV. Nevertheless, everyone agrees that specific attention is required. Obstetricians should actively evaluate for signs of active/past ZIKV infections in regions where it is endemic or for those with history of travel to countries where ZIKV is circulating (25, 253–257). Several agencies emphasize the importance of regular prenatal visits to complete recommended biological screening as well as ongoing general recommendations, such as avoidance of alcohol and other teratogenic substances (255, 258). Recently, the U.S. Centers for Disease Control and Prevention has published detailed guidelines on screening strategies (256, 259). The CDC proposes to offer serological screening to pregnant women (i) upon return from travel to a country where ZIKV virus is circulating and (ii) at the first prenatal visit, in combination with the recommended routine infectious disease screening (generally including HIV, CMV, rubella, HBV, HCV, and possibly toxoplasmosis) for pregnant women living in countries where ZIKV is endemic. Seronegative women continuously exposed to ZIKV can be offered follow-up screening during the second trimester (256). At first, the CDC and ACOG limited screening to returning pregnant travelers demonstrating symptoms during or within 2 weeks of travel, i.e., a mild fever, maculopapular rash, arthritis, or conjunctivitis, but they rapidly extended it to all returning pregnant women (253, 256). Due to the high proportion of asymptomatic infections (122), general screening may be more appropriate in areas where ZIKV is not endemic, as proposed by Baud et al., who recently published detailed recommendations on the management of suspected ZIKV infection in pregnant women (260). Challenges associated with general screening, especially in areas of endemicity, include the high rate of cross-reactivity with other flaviviruses and the risk of false-positive results. Positive serologies need to be confirmed by a PRNT, which is only performed in highly specialized laboratories (179). Findings from such a test may still be associated with cross-reactions in cases of secondary Flavivirus infections (i.e., another Flavivirus infection that occurs after a previous Flavivirus infection) (114, 179). Additionally, in countries where ZIKV is endemic, a general screening strategy might overwhelm laboratory capacity, as suggested by Samarasekera et al., who discussed the limited number of RT-PCRs that could be performed per day (i.e., 100) in Brazil (261). Therefore, some agencies (258) have not made any specific recommendations regarding laboratory screening, while others (254) suggest laboratory screening of pregnant women with ongoing symptoms, including RT-PCR of a urine or plasma sample as well as serological testing (254). These agencies also propose freezing of a clotted plasma sample in asymptomatic women with a history of exposure to ZIKV for potential retrospective serological diagnosis for cases of suspected fetal ZIKV infection. This strategy may be more cost-beneficial, as even in the absence of specific IgM 2 to 12 weeks following exposure, maternal infection cannot necessarily be ruled out, and ultrasound monitoring may still be utilized, as discussed below (253, 256, 257, 260). Furthermore, the risk of fetal sequelae in the case of a past or recent maternal infection is not known. On the other hand, general screening could provide extensive epidemiological data that are currently needed to describe epidemics. A discussion on the performance of the different diagnostic tools for ZIKV infection has been offered by Musso et al. (114).
Ultrasound monitoring.
As expressed by all major agencies and through analogy to toxoplasmosis and CMV infections, ultrasound monitoring provides more information than maternal serological status and therefore represents the main screening strategy for pregnant women exposed to ZIKV (253–256, 258). In most countries, routine prenatal care includes two ultrasounds, one performed at the end of the first trimester for pregnancy dating and a second performed between 18 and 22 weeks gestation to detect major fetal anomalies (51). The course of fetal ZIKV infection, however, is not currently known, and specific lesions may develop late in pregnancy. In addition, the correlation between head circumference in utero and OFC at birth is stronger when measurements are performed during the third trimester (i.e., after 28 weeks gestation) (27, 262). It should be emphasized that while prenatal ultrasound may suggest microcephaly, the definitive diagnosis can only be made at birth. The presence of additional cerebral anomalies may increase the predictive value in utero (27). Baud et al. (260) and several agencies (256–258, 263) have suggested that at least one additional ultrasound should be performed in the third trimester in pregnant women exposed to ZIKV, independent of maternal serological status, even in the absence of fetal anomalies on previous ultrasounds. In higher-income countries where ultrasound may be more easily accessible, additional serial ultrasounds could be proposed. Baud et al., as well as the major agencies (Collège National des Gynécologues et Obstétriciens de Frances [CNGOF], RCOG, SOGC, CDC, and ACOG), recommend obtaining a baseline ultrasound 3 to 4 weeks after maternal exposure or at 16 to 18 weeks gestation and then every 3 to 4 weeks for the remainder of the pregnancy (253–257, 260). Less frequent monitoring might be proposed for seronegative pregnant women (253, 256, 257, 260). In the presence of fetal anomalies, an MRI may be beneficial at 30 to 34 weeks gestation to allow a better description of the lesions (254, 255).
Amniocentesis.
Amniocentesis and subsequent molecular analysis is the gold standard to detect fetal CMV, toxoplasmosis, and LCMV infection (see above). The specific ZIKV RT-PCR may be performed on amniotic fluid samples, and therefore amniocentesis may be considered for diagnosis of a fetal ZIKV infection (11, 178, 264).
Amniocentesis carries a significant risk of spontaneous abortion (0.1 to 1%), especially when performed early in pregnancy (265), and unnecessary amniocenteses should be strictly avoided (33). Our group has recently suggested several considerations that need to be taken into account when discussing amniocentesis recommendations (33). First, the sensitivity of the ZIKV RT-PCR in amniotic fluid is not known. It is known, however, for T. gondii and CMV that optimal detection in amniotic fluid can only be performed once the fetal kidney produces a sufficient amount of urine to allow for pathogen shedding. This is achieved around 20 weeks gestation, or once the pathogens have breached the placental barrier, which may occur 6 to 8 weeks after maternal infection at the earliest (75). It is likely that ZIKV detection is limited by the same factors, and therefore amniocentesis should not be performed earlier than 21 weeks gestation and 6 to 8 weeks after suspected maternal infection. Second, no data exist on the relationship between a positive amniocentesis and the development of brain anomalies, and there is currently no treatment available. Similarly to toxoplasmosis and CMV, it is unlikely that 100% of fetuses will develop symptoms, and the severity of the damage might be related to the gestational age at the time of infection. In addition, a normal test may not provide reassurance. Infection could still occur later in gestation, as described for toxoplasmosis (65). Therefore, independent of the amniocentesis results, close ultrasound monitoring (every 3 to 4 weeks) would still be required. Similar to CMV, factors that could help predict prognosis include viral load and the severity of the lesions observed on ultrasound; however, data are lacking regarding ZIKV infection to make any statement at this point (74).
Given the above, it may be prudent to offer amniocentesis only in the presence of fetal evidence of infection to prevent amniocentesis-related miscarriage or termination of asymptomatic fetuses (33), as suggested by most gynecologic and obstetrical agencies and the recently published recommendations by Baud et al. (253, 254, 257, 260, 263, 266). Of note, the CDC initially proposed to offer amniocentesis to every pregnant woman with (i) positive or inconclusive testing or (ii) ultrasound findings compatible with ZIKV infection starting from 15 weeks gestational age (256), but the CDC rapidly updated this recommendation to acknowledge the above-mentioned considerations (263, 267). In any case, adequate parental counseling is required regarding the risks and benefits of amniocentesis. Furthermore, prognosis of infected fetuses showing a concomitant brain anomaly is difficult to predict. Long-term follow-up studies, however, are not available for congenital ZIKV infections, and parents should be counseled by a multidisciplinary medical team with expertise in the infectious disease field. The presence of fetal anomalies is considered to be an acceptable indication for pregnancy termination in cases of confirmed toxoplasmosis or CMV infection (268), but pregnancy termination remains a highly debated subject (269). The RCOG mentions that in the case of severe brain anomalies, termination of pregnancy should be considered at any gestational age (254). Nevertheless, as reminded by the WHO/PAHO, the decision to interrupt the pregnancy can only be made by the mother-to-be, a decision that will be highly influenced by her social and legal context (258). As mentioned by Roa in a recent comment, abortion is illegal in most ZIKV-circulating countries. Even in countries that allow pregnancy termination in cases of serious risk to maternal or fetal health, such as Colombia, adequate information is rarely given to mothers, partly due to significant social and religious pressures (269). In that context, the benefit of amniocentesis is questionable.
Additional recommendations for pregnant women.
Pregnant women may require a blood transfusion in cases involving significant blood loss, such as placenta previa or severe hemolysis, which can occur in sickle cell disease, for example (270, 271). Caution should be taken when transfusing pregnant women. The presence of ZIKV has been detected in asymptomatic blood donors (272). Therefore, pregnant women should only receive blood products from an area without active transmission. If only locally collected products are available, they should be ZIKV negative or pathogen reduced (273). Many blood bank agencies have also now declined blood donations from recent travelers to areas where ZIKV is endemic.
Management of acute infection in pregnancy.
The clinical course of acute ZIKV infection is similar to that in nonpregnant patients, and symptoms, if present, are similar to those in nonpregnant patients (i.e., maculo-papular rash [an example of which is presented in Fig. 3], conjunctivitis, low-grade fever, and arthralgia); an increase in the rate of complications, such as Guillain-Barré syndrome, or of hospitalization rates has not been reported so far (258). Clinical management relies on symptomatic treatment after having excluded an alternative diagnosis (119, 255, 258). Suspicion of ZIKV infection should not postpone antibiotic treatment in the presence of high fever if chorioamnionitis or pyelonephritis are suspected. Paracetamol, also called acetaminophen, as well as antihistamine medications in case of a pruritic rash can be safely administered during pregnancy.
FIG 3.
Example of a pruritic maculo-papular rash on a traveler coming back from New Caledonia (2014).
Postnatal Care
Asymptomatic newborn.
In asymptomatic newborns, clinical evaluation and complementary examinations should focus on early detection and prevention of latent and long-term symptoms. As for any newborn, a full clinical examination should be performed to look for any anomalies; biometric parameters, including adequate OFC measurements, the presence of a skin rash, petechia, hepatosplenomagaly, and neurological symptoms should be clearly documented. All current recommendations suggest that further testing should only be advised for asymptomatic newborns from mothers with a confirmed ZIKV infection (254, 255, 260, 267, 274). For exposed mothers whose serological status is unknown at the time of delivery, the CDC additionally recommends screening mothers who have presented symptoms compatible with a ZIKV infection during pregnancy (275).
For newborns at risk, serum samples should be obtained, ideally from the umbilical cord, within 2 days, for RT-PCR and specific IgM detection and confirmation by PRNT if positive. In regions where ZIKV is endemic, testing for other arboviruses, especially DENV, is recommended (254, 255, 274). Extrapolating from the current recommendations for diagnosis of a congenital CMV infection (276), RT-PCR for urine and saliva samples could also be performed, as molecular detection of ZIKV has been shown to be highly efficient for both sample types (170, 260, 277). RT-PCR can eventually be performed in cerebrospinal fluid if a sample is available, but lumbar puncture should not be performed without an additional indication (274). The placenta should be carefully examined and sent for histopathological analysis as well as tested for the presence of ZIKV by RT-PCR or immunohistochemistry (254, 255, 274, 278). Similar analyses on fetal tissues, placenta, and maternal serum should also be performed in cases of fetal loss (254, 255).
As suggested by Baud et al., active monitoring for latent sequelae should be performed for infected asymptomatic newborns (260). Additional examinations should include complete blood count, liver function tests, fundoscopy, and hearing evaluation through evoked autoemission acoustic or brainstem stimulation, as well as cerebral ultrasound, except if a recent prenatal one is available (260, 274).
Newborns presenting with microcephaly or prenatal cerebral calcifications.
In infants presenting with anomalies, clinical evaluation and complementary examinations should not only aim at detection of early and long-term complications but also evaluate the possible differential diagnosis (260). Clinical management requires the participation of neurologists, geneticists, obstetricians, and pediatricians and should take into account recommendations for management of microcephaly (reviewed in reference 21). At the first clinical evaluation, signs of dysmorphism, congenital infections, and inborn errors of metabolism should be actively evaluated (21, 260, 274). The newborn and placenta should be tested for ZIKV as described above, and maternal status for ZIKV infection should be confirmed (255, 260, 274). In addition, other congenital infections should be excluded. A CMV PCR for either a urine or saliva sample needs to be rapidly performed (21, 260), and maternal serological status for all other congenital TORCH infections should be obtained (260). Detailed family history, as well as pregnancy and birth history are required. Results of uniform newborn screening, which in most countries includes congenital hypothyroidism, phenylketonuria, congenital adrenal hyperplasia, hemoglobinopathies, galactosemia, or maple syrup urine disease, among others, is useful (260, 279). The severity of the brain lesions should be evaluated appropriately (i.e., cerebral imaging, electroencephalogram) (260), and fundoscopy as well as a hearing evaluation should be rapidly performed (260, 274). Finally, appropriate genetic testing should be discussed with a geneticist (260); it seems reasonable to complete at least karyotype and comparative genomic hybridization (CGH) array testing (21).
Long-term follow up.
Long-term monitoring of a child infected congenitally has yet to be defined, but in addition to medical management, close monitoring is required in order to gather precious information. Using CMV, toxoplasmosis, and LCMV congenital infections as a model, expected long-term complications include developmental delay, cerebral palsy, seizures, decreased visual acuity, and sensorineural hearing loss, which should be closely monitored (260). The CDC proposes monitoring of developmental milestones and OFC measurements as well as at least one follow-up hearing evaluation at 6 months for asymptomatic newborns (274). This seems to be a minimum, and further regular hearing evaluations may be proposed as delayed-onset and progressive hearing loss have described for congenital CMV infections (280). Similarly, regular ophthalmologic exams may be useful, since the course of ZIKV ocular lesions is currently not known (260). Management of symptomatic patients needs to be based on the severity of the lesions (260).
COUNSELING OF WOMEN OF REPRODUCTIVE AGE AND PREGNANT PATIENTS
Knowledge regarding interplay between ZIKV infection and pregnancy is limited and still evolving. The following section is based on current recommendations that are likely to be updated periodically to reflect emerging evidence. A summary of the described recommendations is available in Table 5.
TABLE 5.
Recommendations issued from health agencies and medical organizations related to Zika virus (as of 24 March 2016)
| Organizationa | Recommendations for women of childbearing age and pregnant women for areas where ZIKV is endemic |
||
|---|---|---|---|
| Traveling to such areas | Returning from such areas | Living in such areas | |
| CDC | (1) Consider postponing travel to affected areas (pregnant women); (2) Use appropriate protective measures against mosquito bites (pregnant women and women trying to get pregnant) | (3) Mention travel during antenatal doctor visits regardless of the presence of symptoms (pregnant women); (4) Abstain from sexual activity or use condoms for duration of the pregnancy to prevent infection through sex (for pregnant women whose male partner resides in or has traveled to an area of active Zika virus transmission) | Recommendation 4 also applies to nonpregnant women; (5) Evaluate pregnancy intentions and timing with women of reproductive age; (6) Prevent unintended pregnancy for women who do not want to become pregnant by using the most effective contraceptive method; (7) Prevent mosquito bites (pregnant women) |
| WHO/PAHO | Similar to CDC recommendations 1 and 2; abstain from sexual activity or use condoms (women and partner traveling to affected areas) | Similar to CDC recommendations 3 and 4; abstain from sexual activity or use condoms during 28 days (for women at risk of getting pregnant or planning pregnancy whose male partner resides in or has traveled to area of active Zika virus transmission) | Identify and eliminate potential mosquito breeding sites; recommendations similar to CDC recommendations 4 and 7, also for nonpregnant women; infected breastfeeding women should continue breastfeeding |
| ACOG | Similar to CDC recommendation 1; similar to CDC recommendation 2 but only for pregnant women | Similar to CDC recommendation 3 | Similar to CDC recommendations 5, 6, and 7; infected breastfeeding women should continue breastfeeding |
| RCOG | Similar to CDC recommendation 1; similar to CDC recommendation 2 but only for pregnant women; avoid becoming pregnant while traveling and within 28 days upon return, or 28 days after the abatement of ZIKV-like symptoms | Similar to CDC recommendations 3 and 4; abstain from sexual activity or use condoms during 28 days if male partner is without symptoms and 6 mo after woman's recovery from symptoms; for women at risk of getting pregnant or planning pregnancy whose male partner resides in or has traveled to area of active Zika virus transmission | Infected breastfeeding women should continue breastfeeding |
| CNGOF | Similar to CDC recommendation 1; similar to CDC recommendation 2 but only for pregnant women; abstain from sexual activity or use condoms (women and their partner traveling to affected areas) | Similar to CDC recommendation 3; abstain from sexual activity or use condoms during 28 days or more (for pregnant women and women at risk of getting pregnant or planning pregnancy whose male partner resides in or has traveled to area of active Zika virus transmission) | Similar to CDC recommendations 6 and 7; women planning pregnancy should consider postponing pregnancy during outbreak; pregnant women should abstain from sexual activity or use condoms for the duration of the pregnancy; identify and eliminate potential mosquito breeding sites |
| ECDC | Similar to CDC recommendation 1, but also includes women who are planning to become pregnant; similar to CDC recommendation 2, but only for pregnant women | Similar to CDC recommendations 3 and 4 | |
| Other health agencies or teratology information services surveyedb | Some surveyed agencies/services: Consider postponing travel to affected areas (pregnant women); avoid becoming pregnant while traveling and for 28 days upon return, or if woman develops symptoms compatible with ZIKV infection on return avoid becoming pregnant for a further 28 days following recovery Every surveyed agency/service: Pregnant women should use appropriate protection measures against mosquito bites | Some surveyed agencies/services: Abstain from sexual activity or use condoms during 28 days (for women at risk of getting pregnant or planning pregnancy and whose male partner resides in or has traveled to an area of active Zika virus transmission) | Jamaican Ministry of Health: Delay becoming pregnant for the next 6 to 12 mo; Large majority of health ministries surveyed: Use appropriate protection measures against mosquito bites; identify and eliminate potential mosquito breeding sites |
CDC, U.S. Centers for Disease Control and Prevention; WHO, World Health Organization; PAHO, Pan American Health Organization; ACOG, American Congress of Obstetricians and Gynecologists; RCOG, Royal College of Obstetricians and Gynaecologists; CNGOF, Collège National des Gynécologues et Obstétriciens Français; ECDC, European Centre for Disease Prevention and Control.
The recommendations from other agencies or services included French or English websites from health ministries in countries or territories of the Americas and the Caribbean islands reporting active Zika virus, or elsewhere if recommendations on Zika were available (Australia, Canada, Commonwealth of Dominica, France, Haiti, Israel, Jamaica, Mexico, New Zealand, Samoa, Singapore, St. Maarten), the MotherToBaby service of the Organization of Teratology Information Specialists, the UK Teratology Information Service, and Centre de Référence sur les Agents Tératogènes (Paris).
Women of Childbearing Age and Pregnant Patients Traveling to Areas where ZIKV Is Endemic
It is not known if pregnant women are at higher risk of infection, but it can be assumed that they have at least the same risk as the rest of the population of being infected with ZIKV. The WHO, as well as the large majority of surveyed health agencies and medical organizations, recommend that pregnant women in any trimester discuss travel plans with their health care providers and consider postponing their travel to affected areas, especially to areas with increasing or widespread transmission (254, 259, 281–293). The English and French Colleges for Obstetrics and Gynecology interim guidelines add that women of childbearing age should be advised to avoid becoming pregnant while traveling. The RCOG suggests a 28-day waiting period upon return or following the abatement of ZIKV-compatible symptoms (254), and the Committee To Advise on Tropical Medicine and Travel from Canada advises a 2-month waiting period upon return before trying to conceive (293). In an effort to develop more precise guidance for travelers, the CDC issued a revision to their ZIKV Travel Notice on 18 March 2016, stating that travel entirely limited to elevations of >2,000 m is considered to pose minimal threat for mosquito-borne Zika virus transmission (294), as spatial analyses indicate that because of unsuitable ecologic factors, A. aegypti is unlikely to be found at elevations of >2,000 m (294).
Pregnant women traveling to regions where ZIKV is endemic should be advised to stay informed about ZIKV (295). As there is neither a vaccine nor prophylactic or therapeutic medications available to prevent or cure ZIKV infection, the large majority of the guidelines issued from health agencies or medical organizations recommend that pregnant women or women trying to get pregnant traveling to such regions of endemicity be advised to take measures to avoid being bitten by mosquitoes (254, 259, 266, 281–290, 293, 296). The CDC's mosquito prevention strategies include wearing long-sleeved shirts and long pants, using registered insect repellents, permethrin-treated clothing and gear, and staying and sleeping in screened-in or air-conditioned rooms (297). Several of the health agencies and medical organizations surveyed add that since the Aedes mosquitoes (the primary vector for transmission) are day-biting mosquitoes, it is recommended that those who sleep during the daytime should stay under mosquito nets (bed nets), with or without insecticide treatment (254, 286, 298).
The CDC suggests the use of U.S. Environmental Protection Agency (EPA)-registered insect repellents, such as diethyl-m-toluamide (DEET), picaridin, lemon eucalyptus oil or para-menthane-diol (PMD), and IR3535 (ethyl butylacetylaminopropionate), as well as permethrin-treated clothing and gear to prevent mosquito bites (299). The EPA, CDC, and the ACOG do not recommend any additional precautions for repellents used by pregnant or nursing women (286). According to the CDC, products containing lemon eucalyptus oil should not to be used on children under the age of 3 years (299), and the ECDC recommends avoiding the use of DEET-based repellents in children under 3 months of age (196). Among the above products, higher concentrations of DEET-based products (30% and more), as well as PMD in one of the studies, have been shown to provide the highest level of protection for the longest duration under laboratory conditions (300–303), as well as under standardized field conditions across the Americas for formulated products (304). Its effectiveness in a noncontrolled setting in an area of endemicity, however, is still being debated (305). Pregnant women may be concerned about using DEET or the other suggested compounds. The available data on toxic effects in humans and animals are reassuring for DEET (306–312), with the exception of the results of one animal study that assessed much higher doses than the normal human dose (313) and a retrospective human study that noted an association between hypospadias and insect repellent use during the first trimester of pregnancy (314). Interpretation of the latter study should be done with caution, as the methodology used cannot establish a causal relationship. Reassuring data are also available for permethrin (18, 315). Thus, even if the available safety data for pregnancy are still limited, it is widely assumed that the benefits of DEET and permethrin protection against mosquito-driven infectious diseases outweigh the remaining unknown potential risks for the unborn child. DEET is currently recommended by several teratogen information specialists and medical organizations for use by all pregnant women who are traveling to areas where mosquito-borne diseases are present (254, 311, 312). To prevent direct infant exposure, breastfeeding women should be advised to wash their hands before putting the infant to the breast (266). With regard to other EPA-listed insect repellents, available data on toxic effects in humans and animals are scarce (311, 316, 317).
Prenatal Counseling for Pregnant Patients or Their Partner with a History of Travel to an Area where ZIKV Is Endemic
Symptoms of ZIKV infection, such as fever, skin rashes, joint and muscle pains, headaches, and red eyes are expected to occur within 14 days upon return from affected areas to regions where ZIKV is not endemic. As only one in four humans infected with ZIKV develops symptoms, many women may remain unaware that they have been infected, as they may not develop any symptoms. Several health agencies and medical organizations surveyed recommend advising pregnant women who have traveled to areas known for ZIKV transmission to discuss their travel during antenatal visits in order to be assessed and monitored appropriately regardless of the absence of symptoms (196, 254, 259, 266, 286, 296). In symptomatic patients, this includes the exclusion of dengue, which has a similar clinical presentation and geographic distribution but has an improved outcome after proper clinical management (266, 318).
Several cases of sexual transmission from males to their partners have been reported (207, 319–326). Male-to-male sexual transmission of ZIKV has also been described (327). The interval between onset of symptoms in a man and his partner has been described to range between 4 and 19 days (207, 324, 328). Additional studies are needed to better characterize the risk for sexual transmission of ZIKV. In the meantime, several health agencies and medical organizations have recommended that pregnant women with a male partner that resides in or has traveled to an area of active ZIKV transmission refrain from sexual activity or use condoms during sexual activity (i.e., vaginal or anal intercourse or fellatio) (254, 266, 329). According to the CDC, ECDC, RCOG, and WHO, these precautions should be followed for the duration of the pregnancy (329). Furthermore, according to the RCOG, for women at risk of becoming pregnant, or planning pregnancy, these precautions should be exercised for 28 days following potential exposure for asymptomatic male partners and 6 months following recovery in symptomatic male partners. The WHO and CNGOF recommend exercising precautions for 4 weeks regardless of the presence of symptoms (254, 290). Pregnant women are advised to discuss this issue with their health care provider (329). The ZIKV genome has also been detected in saliva during the acute phase of the disease, but there is no information about the presence of viable virus, viral load, and duration. The ECDC concludes that the risk of transmission via saliva cannot be further assessed at this time (196).
Counseling for Women of Childbearing Age and Pregnant Patients Living in an Area where ZIKV Is Endemic
The CDC's updated interim guideline from 5 February 2016 expand its guidance to women who reside in areas with ongoing ZIKV transmission (256). One of the pillars of the CDC's recommendations is the evaluation of pregnancy intention and timing for women of reproductive age residing in areas of ongoing ZIKV transmission (256). Referring to the rate of unintended pregnancies in the Unite States of 1 in 2 pregnancies (330), the CDC guidelines emphasize strategies to prevent unintended pregnancy (e.g., family planning and use of contraceptive methods) (256). Patients should be counseled to use the most effective contraceptive method that can be used correctly and consistently. Long-acting reversible contraception (e.g., contraceptive implants and intrauterine devices) are cited as the best choice for women desiring highly effective contraception (331). The issue of the access to this type of birth control can be raised, however, due to the inequality in contraceptive access described in areas where ZIKV is endemic (332–334).
Some health authorities, such as the Jamaican Ministry of Health, are proposing delaying conception and are advising women to delay becoming pregnant for the next 6 to 12 months (281, 335, 336).
Another pillar of the CDC's February 5 updated interim guidelines as well as those of other health agencies or medical organizations is based on strategies to prevent mosquito bites (256, 292, 337, 338). Again, these strategies may have little impact on women with limited resources (305). Some of the health agencies and medical organizations highlight the importance of eliminating potential mosquito breeding sites by emptying, cleaning, or covering containers that can hold water from every home and its surroundings (266, 281, 288, 289, 292, 296, 337–339).
The ZIKV viremia is expected to last approximately 1 week in patients with clinical illness (193, 277), although the actual duration of viremia is not yet well known (340). There is no current evidence to suggest that a fetus would be at risk for infection if he or she was conceived after maternal viremia had resolved. Thus, women of reproductive age with current or previous laboratory-confirmed ZIKV infection should be counseled that there is no evidence that prior ZIKV infection poses a risk for birth defects in future pregnancies, according to the CDC (256). The interval between the onset of symptoms and the time it becomes safe for women to conceive, however, remains difficult to determine precisely.
Evidence of excretion of ZIKV in human breast milk has been reported (169, 341). ZIKV cultures in breast milk, however, remain negative. The risk of transmission through oral ingestion is unknown. Breast milk transmission has been reported for DENV (342) and WNV (343). Neonatal infection is likely to be mild and of short-term consequence (286). The ACOG, RCOG, and WHO recommend that women continue breastfeeding, as the benefits of breastfeeding likely outweigh the potential neonatal risks (286).
CONCLUSION AND PERSPECTIVES
At the beginning of February, the WHO declared that the severity of the health threat associated with the continuing spread of the ZIKV infection in Latin America and the Caribbean constituted a Public Health Emergency of International Concern (9). The WHO Director-General explained that “declaring an emergency would allow for a coordinate global effort to get under way, enabling surveillance for microcephaly to be standardized and research to be intensified” (261). With the rapidly evolving concern among the public, health care providers, and the media, as well as the existing gender and social inequalities in most of the areas where ZIKV is endemic, there is an urgent need to assess the magnitude of the prevalence of microcephaly in Brazil related to ZIKV, as well as the presence of other factors that could play a significant role in the development of these congenital neurological conditions and modify the risks.
Standard criteria for proving causation adapted from Robert Koch (344) (i.e., isolation of the causative organism, inoculation of a susceptible person who develops the disease, followed by a reisolation of the organism) would not be applicable in this situation, mainly for ethical reasons. Therefore, the required assessment relies on a combination of scientific and epidemiologic evidence (345). Yet, despite the growing scientific evidence supporting the causal relationship (e.g., neurotropic virus, other proven teratogen pathogens, microcephaly cases described with CHIKV, and an increase in the number of cases with observed presence of ZIKV in tissues of affected newborn or fetuses) (12) and the CDC's announcement on the established causal relationship between prenatal Zika virus infection and microcephaly and other serious brain anomalies (16), current epidemiologic evidence is still scarce (Table 6). In March 2016, a cohort study reported a risk of 29% ultrasound-detected abnormalities (12 cases), including intrauterine growth restriction, CNS findings, and fetal death, in fetuses of women with PCR-positive ZIKV infection (15). The study conducted in Rio de Janeiro enrolled 88 symptomatic pregnant women (i.e., presenting with a rash that had developed within the previous 5 days). Among them, 72 (82%) had positive results for ZIKV via PCR of blood, urine, or both sample types, 42 (60%) had prenatal ultrasonographic examinations, and 28 declined imaging studies (15). Later, a retrospective study using a modeling approach provided a quantitative estimate of the prevalence of microcephaly associated with ZIKV infection in French Polynesia (95 cases per 10,000 women infected in the first trimester; 95% CI, 34 to 191) (199). These two studies quoted different risk estimates, suggesting that the risk could depend on other factors, such as the presence of clinical symptoms (inclusion criteria in Rio de Janeiro's study but not in the French Polynesia study) or other coinfections (Table 6) (14).
TABLE 6.
Gaps in knowledge concerning ZIKV infection in pregnant women and women of reproductive age
| Knowledge gaps impacting clinical management and counseling of pregnant women and women of reproductive age |
|---|
| Benefits of universal screening in countries where ZIKV is endemic, as well as development of an adequate screening diagnostic tool(s) |
| Treatments to prevent transplacental transmission (i.e., hyperimmune immunoglobulin, antiviral treatments, vaccine) |
| Clinical course of congenital ZIKV infection |
| • Spectrum of disease (including extraneurological lesions, late-onset symptoms) |
| • Severity of disease and prognosis |
| • Benefit of early antiviral treatment |
| Risk assessment for transplacental transmission and congenital lesions |
| • Quantitative estimate of the risk of microcephaly and other ZIKV-associated birth defects in symptomatic and asymptomatic patients |
| • Gestational ages with highest transmission rate, highest risk of severe fetal lesions, and risk-free period (if such exists) |
| • Transmission rates in primary vs secondary infections |
| • Additional risk factors (e.g., maternal IgG titer, maternal viremia, viral load in amniotic fluid, specific ultrasound markers, coinfections) |
| Prevention measures |
| • Interval between the onset of symptoms and time it becomes safe for the woman to conceive |
| • Period during which ZIKV-infected patients are contagious to their sexual partner(s) |
| • Other transmission modes (e.g., saliva, blood, breast milk) |
| • Effectiveness of mosquito control strategies and other measures to avoid ZIKV infection in areas where ZIKV is endemic |
We have seen that the diagnostic criterion for microcephaly is an arbitrary cutoff based on an acceptable deviation from the average on reference charts. In Brazil, microcephaly was initially defined as a head circumference below 33 cm, and this was later corrected to 32 cm (261). Thus, some experts suggest that the observed number of cases reported in Brazil could be partly due to an overestimation by overdiagnosis, as well as an active search derived from the mandatory reporting of cases of microcephaly (346). Furthermore, several diseases or factors can cause microcephaly. For example, the Latin American Collaborative Study of Congenital Malformations (ECLAMC) estimates that 38% of cases of congenital microcephaly reported in their database are caused by chromosomal and single-gene errors (346). To evaluate the causal relationship with ZIKV, microcephaly cases with another known cause (environmental or genetic) should be excluded. Also, to evaluate the magnitude of the increase in prevalence, the baseline rate should be well defined. Yet, in Brazil, the historical reported prevalence for microcephaly is low, approximately 0.5/10,000 live births (5), raising doubts about the veracity of the baseline rate. A prospective cohort study with standardized evaluation of the outcome could probably clarify the situation. Finally, similar to other congenital infections (e.g., CMV or toxoplasmosis), diagnosis of microcephaly might only represent the more severely affected children. Thus, other outcomes should be considered, as it is possible that newborns with less severe disease and other affected organs have not yet been diagnosed (Table 6) (347, 348).
Another issue in evaluating the causal relationship is the lack of diagnostic tools and laboratory capacity to confirm exposure to ZIKV. Infection with ZIKV is difficult to confirm retrospectively, because serological tests cross-react with other flaviviruses, especially DENV, which cocirculates in the Brazilian population. Therefore, retrospective reporting of symptoms is used as a proxy for ZIKV exposure. Moreover, ZIKV genome identification by RT-PCR is hindered by the short period in which the virus is present in the blood or other tissues. Thus, the exposure remains difficult to confirm without more specific diagnostic tools, at least retrospectively. Additionally, the window of exposure that may constitute the period at risk is still unclear. What is the impact of the gestational age at the time of maternal infection on the severity of fetal complications? For example, the burden of disease is inversely correlated to gestational age with Toxoplasma gondii and CMV. Are we facing a teratogenic effect limited to first-trimester exposure or are pregnant women at risk during the entire pregnancy (Table 6)? Patricia Brasil and colleagues suggested that the teratogenic effect may not be limited to the first trimester, as abnormalities were observed after infection occurred at any week of gestation (15). In contrast, Simon Cauchemez and colleagues suggested that the highest risk for microcephaly is associated with infection in the first trimester of pregnancy (199), as previously suspected (349). Again, a prospective cohort study looking at different exposure timing could likely clarify the situation (179). Finally, we do not yet know if asymptomatic ZIKV-infected pregnant women can be considered “unexposed” (Table 6). Other pathogens have shown teratogenic effects even in asymptomatic patients (e.g., Toxoplasma gondii, CMV), as well as in cases of secondary infection (e.g., CMV). Even though studies could expand their inclusion criteria to include asymptomatic patients, without more specific diagnostic tools, differentiating asymptomatic patients from uninfected patients will result in misclassification issues.
To date, there is no available vaccine or treatment for ZIKV (350), although a team of vaccine developers seemed optimistic that a vaccine could already be available in 2016, at least for a subset of the population (351). As vaccines exist for several of ZIKV's relatives, including dengue virus, yellow fever virus, and Japanese encephalitis virus, and because ZIKV has only one serotype, compared to four dengue virus serotypes, experts estimate that a ZIKV vaccine should follow a straightforward developmental pathway (352). Large sections of the targeted population, however, have neutralizing antibodies to other flaviviruses, and it is unknown if this will affect people who receive the vaccine (i.e., antibody-dependent enhancement or cross-immunity) (353). Furthermore, issues linked to practical and ethical considerations when testing a vaccine in a targeted population, pregnant women, may slow down its access for those who would benefit most (354). An inactivated vaccine has the best chance of getting regulatory approval for the targeted population of pregnant women (352). Antiviral drugs could be a treatment option to investigate, even if the few results available in other teratogenic infections remain inconclusive in terms of preventing congenital infections and improving neonatal outcomes (e.g., valacyclovir in CMV infection). Meanwhile, strategies to fight mosquito vectors are being tested, including the utilization of Wolbachia bacterium, a novel and promising form of control for mosquito-transmitted diseases (355, 356).
Until more is known (Table 6), health agencies and medical organizations have issued very cautious guidance for health care practitioners and pregnant patients or their male partners traveling to, returning from, or living in affected areas. Guidelines for women living in areas where ZIKV is endemic do not always take into consideration poor access to contraception and the cultural or legal barriers that make it difficult for a large group of women to negotiate the use of contraception, or to even have access to pregnancy termination (269). Furthermore, several of the suggested prenatal screening strategies (e.g., risks and benefits, amniocentesis in pregnant women with suspected infection) are linked to poor predictive values and clinical benefits because of the absence of studies (Table 6). Questions can be raised about risk-benefit and cost-benefit ratios linked to these screening strategies, as well as the amount of unnecessary anxiety generated in case of positive results (Table 6).
In analogy to the DENV epidemics, ZIKV has the potential to become endemic in more than 100 countries. New mutations could impact viral replication in humans (i.e., leading to increased virulence and consequently increased chances of virus transmission to a naive mosquito vector), as well as replication and transmission in Aedes mosquitoes. Furthermore, the impact of human activity might also impact ZIKV spread, directly (mass tourism and high population densities in urban areas) and/or indirectly (climate change). Further studies are urgently needed to assess the impact of mutations on virulence, as well as on the magnitude of the causal relationship between microcephaly and ZIKV outbreaks and the roles of other factors that might modify the risk.
ACKNOWLEDGMENTS
We certify that we have no financial interest or nonfinancial interest for the subject matter or materials discussed in the manuscript.
We thank Patrick Hohlfeld for critical review of the manuscript. We thank Karine Lepigeon for her significant help in manuscript handling.
The contribution of Alice Panchaud was supported by a grant of the Swiss National Science Foundation (SNSF P3SMP3-158808/1). Manon Vouga is funded through an M.D.-Ph.D. grant from the SNSF (323530_158123). David Baud is supported by the Department Femme-Mère-Enfant, by the Fondation Leenaards through the Bourse pour la relève académique, and by the SNSF (310030_156169/1).
Biographies

Alice Panchaud, Ph.D., is a certified clinical pharmacist and pharmacologist trained as a teratogen information service counselor at the University Hospital of Lausanne and at the Hospital for Sick Children in Toronto. She is a lecturer on drug therapy optimization in pregnant and lactating women at the School of Pharmacy of Geneva and Lausanne. Her research interests focus on safety and efficacy of drugs during pregnancy and lactation, using various methodological approaches. She has been the recipient of several national and international research awards. She is currently doing a research fellowship at the Department of Epidemiology, Harvard T. H. Chan School of Public Health in Boston, working with large administrative databases to pursue her work in the neglected field of drug therapy during pregnancy. Her fellowship is currently supported by the Swiss National Research Foundation.

Miloš Stojanov, Ph.D., is a postdoctoral researcher currently working at the University Hospital of Lausanne, Switzerland. He is interested in medical microbiology, with a particular emphasis on molecular determinants of pathogenesis and antibiotic resistance. He received his Ph.D. at the University of Lausanne, where he worked on the transmission of antibiotic resistance in staphylococci. He later focused on epidemiology of major hospital-acquired pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium difficile, as a postdoctoral fellow at the University Hospital of Lausanne. His current research at the Department of Gynecology and Obstetrics includes development of vaccination strategies against Chlamydia trachomatis and investigation of the role of Waddlia chondrophila in human infertility and pathogenesis.

Anne Ammerdorffer, Ph.D., is a trained biologist focused on infectious diseases and immunology. At the moment, she is a postdoctoral researcher working at the Department of Gynecology and Obstetrics and the Institute of Microbiology at the University Hospital (CHUV) in Lausanne, Switzerland. Her research involves the immunopathogenesis of Waddlia chondrophila, an intracellular bacterium associated with adverse pregnancy outcomes. Her Ph.D. studies were a collaboration between the Radboud UMC (Nijmegen, the Netherlands) and the Central Veterinary Institute (Lelystad, the Netherlands) and investigated the innate immune responses against Coxiella burnetii, the causative agent of Q fever, in humans and goats. Her interests involve the investigation of the immune responses against zoonotic intracellular bacteria.

Manon Vouga, M.D., is a medical fellow in Gynecology and Obstetrics at the University Hospital in Lausanne. She is currently pursuing an M.D.-Ph.D. and her thesis is on infectious diseases and adverse pregnancy outcomes in collaboration with the Microbiology Institute of the University Hospital, Lausanne, for which she received a grant from the National Swiss Science Foundation. Her research specifically focuses on emerging bacteria, such as Chlamydia-related bacteria, and their impact on pregnancy.

David Baud, M.D., Ph.D., is a specialist in materno-fetal medicine (MFM) and is presently working as staff at the University Hospital in Lausanne, Switzerland. He is board certified in the specialties of obstetrics and gynecology and of operative gynecology. After his Ph.D. from the Institute of Microbiology—Lausanne, he trained at various hospitals worldwide, including Mount Sinai Hospital in Toronto (2010 to 2012), Hopital Necker in Paris (2009 to 2010), and St. Mary's Hospital in London, United Kingdom (2004 to 2005). He continues active research and clinical collaborations. His current research is focused on emerging infectious causes of adverse pregnancy outcomes.
REFERENCES
- 1.Campos GS, Bandeira AC, Sardi SI. 2015. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. 2015. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2015. Zika virus outbreaks in the Americas. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, Quintana Mde S, Mendonca MC, Lupi O, de Souza RV, Romero C, Zogbi H, Bressan Cda S, Alves SS, Lourenco-de-Oliveira R, Nogueira RM, Carvalho MS, de Filippis AM, Jaenisch T. 2016. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis 10:e0004636. doi: 10.1371/journal.pntd.0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian Medical Genetics Society-Zika Embryopathy Task Force. 2016. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 6.EUROCAT. 2016. Cases and prevalence (per 10,000 births) for all full member registries from 2008 to 2012. http://www.eurocat-network.eu/accessprevalencedata/prevalencetables Accessed 3 February 2016.
- 7.World Health Organization. 2015. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8.European Centre for Disease Prevention and Control. 2015. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. ECDC, Solna, Sweden. [Google Scholar]
- 9.World Health Organization. 2016. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/ Accessed 3 February 2016.
- 10.World Health Organization. 2016. IHR procedures concerning public health emergencies of international concern (PHEIC). http://www.who.int/ihr/procedures/pheic/en/ Accessed 3 February 2016.
- 11.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. 2016. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 12.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. 2016. Zika virus associated with microcephaly. N Engl J Med 374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 13.Direction de la Santé, Centre d'hygiène et de salubrité publique. 2 December 2015. Note sur les investigations autour des malformations cérébrales congénitales ayant suivi l'épidémie de zika de 2013–2014. http://www.hygiene-publique.gov.pf/IMG/pdf/note_malformations_congenitales_cerebrales.pdf.
- 14.Rodrigues LC. 15 March 2016. Microcephaly and Zika virus infection. Lancet doi: 10.1016/S0140-6736(16)00742-X. [DOI] [PubMed] [Google Scholar]
- 15.Brasil P, Pereira JP Jr, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, Calvet GA, Neves ES, Moreira ME, Rodrigues Baiao AE, Nassar de Carvalho PR, Janzen C, Valderramos SG, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 4 March 2016. Zika virus infection in pregnant women in Rio de Janeiro: preliminary report. N Engl J Med doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 13 April 2016. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 17.Hennessey M, Fischer M, Staples JE. 2016. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 65:55–58. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 18.Horton MK, Rundle A, Camann DE, Boyd Barr D, Rauh VA, Whyatt RM. 2011. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics 127:e699–e706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashwal S, Michelson D, Plawner L, Dobyns WB, Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. 2009. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 73:887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, Gravett MG, Purwar M, Frederick IO, Noble AJ, Pang R, Barros FC, Chumlea C, Bhutta ZA, Kennedy SH, International Fetal and Newborn Growth Consortium for the 21st Century. 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 21.von der Hagen M, Pivarcsi M, Liebe J, von Bernuth H, Didonato N, Hennermann JB, Bührer C, Wieczorek D, Kaindl AM. 2014. Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev Med Child Neurol 56:732–741. doi: 10.1111/dmcn.12425. [DOI] [PubMed] [Google Scholar]
- 22.Fenton TR, Kim JH. 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victora CG, Schuler-Faccini L, Matijasevich A, Ribeiro E, Pessoa A, Barros FC. 2016. Microcephaly in Brazil: how to interpret reported numbers? Lancet 387:621–624. doi: 10.1016/S0140-6736(16)00273-7. [DOI] [PubMed] [Google Scholar]
- 24.Borghi E, de Onis M, Garza C, Van den Broeck J, Frongillo EA, Grummer-Strawn L, Van Buuren S, Pan H, Molinari L, Martorell R, Onyango AW, Martines JC, WHO Multicentre Growth Reference Study Group. 2006. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med 25:247–265. doi: 10.1002/sim.2227. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization-Pan American Health Organisation. 2016. Preliminary guidelines for the surveillance of microcephaly in newborns in settings with risk of Zika virus circulation. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33000&lang=en Accessed 13 February 2016.
- 26.Deloison B, Chalouhi GE, Bernard J-P, Ville Y, Salomon LJ. 2012. Outcomes of fetuses with small head circumference on second-trimester ultrasonography. Prenat Diagn 32:869–874. doi: 10.1002/pd.3923. [DOI] [PubMed] [Google Scholar]
- 27.Leibovitz Z, Daniel-Spiegel E, Malinger G, Haratz K, Tamarkin M, Gindes L, Ben-Sira L, Lev D, Shapiro I, Bakry H, Weizman B, Zreik A, Egenburg S, Arad A, Tepper R, Kidron D, Lerman-Sagie T. 29 October 2015. Microcephaly at birth: the accuracy of three references for fetal head circumference. How can we improve prediction? Ultrasound Obstet Gynecol doi: 10.1002/uog.15801. [DOI] [PubMed] [Google Scholar]
- 28.Woods CG. 2004. Human microcephaly. Curr Opin Neurobiol 14:112–117. doi: 10.1016/j.conb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Mochida GH, Walsh CA. 2001. Molecular genetics of human microcephaly. Curr Opin Neurol 14:151–156. doi: 10.1097/00019052-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Rios A. 1996. Microcephaly. Pediatr Rev 17:386–387. [PubMed] [Google Scholar]
- 31.Watemberg N, Silver S, Harel S, Lerman-Sagie T. 2002. Significance of microcephaly among children with developmental disabilities. J Child Neurol 17:117–122. doi: 10.1177/088307380201700205. [DOI] [PubMed] [Google Scholar]
- 32.Dolk H. 1991. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol 33:974–983. [DOI] [PubMed] [Google Scholar]
- 33.Vouga M, Musso D, Van Mieghem T, Baud D. 2016. CDC guidelines for pregnant women during the Zika virus outbreak. Lancet 387:843–844. doi: 10.1016/S0140-6736(16)00383-4. [DOI] [PubMed] [Google Scholar]
- 34.Hohlfeld P. 2012. Le livre de l'interne Obstétrique, 4th ed Lavoisier, Paris, France. [Google Scholar]
- 35.Public Health England. 2016. Guidance on viral rash in pregnancy. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322688/Viral_rash_in_pregnancy_guidance.pdf Accessed 13 February 2016.
- 36.Ergaz Z, Ornoy A. 2006. Parvovirus B19 in pregnancy. Reprod Toxicol 21:421–435. doi: 10.1016/j.reprotox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Lamont RF, Sobel JD, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Kim SK, Uldbjerg N, Romero R. 2011. Parvovirus B19 infection in human pregnancy. BJOG 118:175–186. doi: 10.1111/j.1471-0528.2010.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauerbrei A, Wutzler P. 2000. The congenital varicella syndrome. J Perinatol 20:548–554. doi: 10.1038/sj.jp.7200457. [DOI] [PubMed] [Google Scholar]
- 39.Sauerbrei A, Wutzler P. 2007. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 1: herpes simplex virus infections. Med Microbiol Immunol 196:89–94. doi: 10.1007/s00430-006-0031-0. [DOI] [PubMed] [Google Scholar]
- 40.Tan MP, Koren G. 2006. Chickenpox in pregnancy: revisited. Reprod Toxicol 21:410–420. doi: 10.1016/j.reprotox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Scheffer IE, Baraitser M, Brett EM. 1991. Severe microcephaly associated with congenital varicella infection. Dev Med Child Neurol 33:916–920. [DOI] [PubMed] [Google Scholar]
- 42.Mandelbrot L. 2012. Fetal varicella: diagnosis, management, and outcome. Prenat Diagn 32:511–518. doi: 10.1002/pd.3843. [DOI] [PubMed] [Google Scholar]
- 43.Fiumara NJ. 1975. Syphilis in newborn children. Clin Obstet Gynecol 18:183–189. [DOI] [PubMed] [Google Scholar]
- 44.Fiumara NJ, Lessell S. 1970. Manifestations of late congenital syphilis. An analysis of 271 patients. Arch Dermatol 102:78–83. [PubMed] [Google Scholar]
- 45.Rashid U, Yaqoob U, Bibi N, Bari A. 2015. Symptomatic early congenital syphilis: a common but forgotten disease. J Coll Physicians Surg Pak 25(Suppl 2):S137–S139. doi: 10.2015/JCPSP.S137139. [DOI] [PubMed] [Google Scholar]
- 46.Rac MW, Bryant SN, McIntire DD, Cantey JB, Twickler DM, Wendel GD, Sheffield JS. 2014. Progression of ultrasound findings of fetal syphilis after maternal treatment. Am J Obstet Gynecol 211:426.e1–426.e6. doi: 10.1016/j.ajog.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 47.Callaghan WM, Creanga AA, Jamieson DJ. 2015. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol 126:486–490. doi: 10.1097/AOG.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, Rao AV. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 333:1737–1742. [DOI] [PubMed] [Google Scholar]
- 49.Andrews WW, Goldenberg RL, Mercer B, Iams J, Meis P, Moawad A, Das A, Vandorsten JP, Caritis SN, Thurnau G, Miodovnik M, Roberts J, McNellis D. 2000. The Preterm Prediction Study: association of second-trimester genitourinary Chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol 183:662–668. doi: 10.1067/mob.2000.106556. [DOI] [PubMed] [Google Scholar]
- 50.Baud D, Goy G, Vasilevsky S, Osterheld MC, Roth-Kleiner M, Croxatto A, Greub G. 2015. Roles of bovine Waddlia chondrophila and Chlamydia trachomatis in human preterm birth. New Microbes New Infect 3:41–45. doi: 10.1016/j.nmni.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones JL, Dubey JP. 2012. Foodborne toxoplasmosis. Clin Infect Dis 55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- 52.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 53.Mosti M, Pinto B, Giromella A, Fabiani S, Cristofani R, Panichi M, Bruschi F. 2013. A 4-year evaluation of toxoplasmosis seroprevalence in the general population and in women of reproductive age in central Italy. Epidemiol Infect 141:2192–2195. doi: 10.1017/S0950268812002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hohlfeld P, MacAleese J, Capella-Pavlovski M, Giovangrandi Y, Thulliez P, Forestier F, Daffos F. 1991. Fetal toxoplasmosis: ultrasonographic signs. Ultrasound Obstet Gynecol 1:241–244. doi: 10.1046/j.1469-0705.1991.01040241.x. [DOI] [PubMed] [Google Scholar]
- 55.Malinger G, Werner H, Rodriguez Leonel JC, Rebolledo M, Duque M, Mizyrycki S, Lerman-Sagie T, Herrera M. 2011. Prenatal brain imaging in congenital toxoplasmosis. Prenat Diagn 31:881–886. doi: 10.1002/pd.2795. [DOI] [PubMed] [Google Scholar]
- 56.Sever JL, Ellenberg JH, Ley AC, Madden DL, Fuccillo DA, Tzan NR, Edmonds DM. 1988. Toxoplasmosis: maternal and pediatric findings in 23,000 pregnancies. Pediatrics 82:181–192. [PubMed] [Google Scholar]
- 57.Freeman K, Oakley L, Pollak A, Buffolano W, Petersen E, Semprini AE, Salt A, Gilbert R, European Multicentre Study on Congenital Toxoplasmosis. 2005. Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG 112:31–37. doi: 10.1111/j.1471-0528.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 58.American Congress of Obstetricians and Gynecologists. 2015. Practice bulletin no. 151: cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 125:1510–1525. doi: 10.1097/01.AOG.0000466430.19823.53. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert RE, Peckham CS. 2002. Congenital toxoplasmosis in the United Kingdom: to screen or not to screen? J Med Screen 9:135–141. doi: 10.1136/jms.9.3.135. [DOI] [PubMed] [Google Scholar]
- 60.Boubaker K, Raeber PA, Vaudaux B, Bucher HC, Garweg JG, Hoesli I, Kind C, Hohlfeld P, Swiss Working Group on Congenital Toxoplasmosis. 2008. Toxoplasmosis during pregnancy and infancy. A new approach for Switzerland. Swiss Med Wkly 138:1–8. [DOI] [PubMed] [Google Scholar]
- 61.Haute Autorité de Santé. 2009. Surveillance sérologique et prévention de la toxoplasmose et de la rubéole au cours de la grossesse. Haute Autorité de Santé, Saint-Denis, France. [Google Scholar]
- 62.Meroni V, Genco F, Tinelli C, Lanzarini P, Bollani L, Stronati M, Petersen E. 2009. Spiramycin treatment of Toxoplasma gondii infection in pregnant women impairs the production and the avidity maturation of T. gondii-specific immunoglobulin G antibodies. Clin Vaccine Immunol 16:1517–1520. doi: 10.1128/CVI.00253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thalib L, Gras L, Romand S, Prusa A, Bessieres MH, Petersen E, Gilbert RE. 2005. Prediction of congenital toxoplasmosis by polymerase chain reaction analysis of amniotic fluid. BJOG 112:567–574. doi: 10.1111/j.1471-0528.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 64.Gay-Andrieu F, Marty P, Pialat J, Sournies G, Drier de Laforte T, Peyron F. 2003. Fetal toxoplasmosis and negative amniocentesis: necessity of an ultrasound follow-up. Prenat Diagn 23:558–560. doi: 10.1002/pd.632. [DOI] [PubMed] [Google Scholar]
- 65.Villena I, Bory J-P, Chemla C, Hornoy P, Pinon J-M. 2003. Congenital toxoplasmosis: necessity of clinical and ultrasound follow-up despite negative amniocentesis. Prenat Diagn 23:1098–1099. doi: 10.1002/pd.754. [DOI] [PubMed] [Google Scholar]
- 66.SYROCOT Study Group, Thiebaut R, Leproust S, Chene G, Gilbert R. 2007. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients' data. Lancet 369:115–122. doi: 10.1016/S0140-6736(07)60072-5. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert R, Gras L, European Multicentre Study on Congenital Toxoplasmosis. 2003. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 110:112–120. doi: 10.1046/j.1471-0528.2003.02325.x. [DOI] [PubMed] [Google Scholar]
- 68.Foulon W, Villena I, Stray-Pedersen B, Decoster A, Lappalainen M, Pinon JM, Jenum PA, Hedman K, Naessens A. 1999. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Am J Obstet Gynecol 180:410–415. doi: 10.1016/S0002-9378(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 69.Cortina-Borja M, Tan HK, Wallon M, Paul M, Prusa A, Buffolano W, Malm G, Salt A, Freeman K, Petersen E, Gilbert RE, European Multicentre Study on Congenital Toxoplasmosis (EMSCOT). 2010. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med 7:e1000351. doi: 10.1371/journal.pmed.1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease Control Prevention. 2016. Lymphocytic choriomeningitis (LCM). CDC, Atlanta, GA: http://www.cdc.gov/vhf/lcm/ Accessed 10 February 2016. [Google Scholar]
- 71.Craig JM, Macauley JC, Weller TH, Wirth P. 1957. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med 94:4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- 72.Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 74.Yinon Y, Farine D, Yudin MH. 2010. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstet Gynecol Surv 65:736–743. doi: 10.1097/OGX.0b013e31821102b4. [DOI] [PubMed] [Google Scholar]
- 75.Benoist G, Leruez-Ville M, Magny JF, Jacquemard F, Salomon LJ, Ville Y. 2013. Management of pregnancies with confirmed cytomegalovirus fetal infection. Fetal Diagn Ther 33:203–214. doi: 10.1159/000342752. [DOI] [PubMed] [Google Scholar]
- 76.Gaytant MA, Rours GIJG, Steegers EAP, Galama JMD, Semmekrot BA. 2003. Congenital cytomegalovirus infection after recurrent infection: case reports and review of the literature. Eur J Pediatr 162:248–253. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliveira PdF, Duarte G, Britt WJ. 2010. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 202:297.e1–e8. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ville Y, Leruez-Ville M. 2014. Managing infections in pregnancy. Curr Opin Infect Dis 27:251–257. doi: 10.1097/QCO.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 79.Nigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 80.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, CHIP Study Group. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 81.Jacquemard F, Yamamoto M, Costa JM, Romand S, Jaqz-Aigrain E, Dejean A, Daffos F, Ville Y. 2007. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 114:1113–1121. doi: 10.1111/j.1471-0528.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- 82.Oliver SE, Cloud GA, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Soong S-J, Whitley RJ, Kimberlin DW. 2009. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 46(Suppl 4):S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kimberlin DW, Lin C-Y, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Kiell JM, Soong S-J, Whitley RJ. 2003. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Pediatr 143:16–25. doi: 10.1016/S0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 84.Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, Ashouri N, Englund JA, Estrada B, Jacobs RF, Romero JR, Sood SK, Whitworth MS, Abzug MJ, Caserta MT, Fowler S, Lujan-Zilbermann J, Storch GA, DeBiasi RL, Han J-Y, Palmer A, Weiner LB, Bocchini JA, Dennehy PH, Finn A, Griffiths PD, Luck S, Gutierrez K, Halasa N, Homans J, Shane AL, Sharland M, Simonsen K, Vanchiere JA, Woods CR, Sabo DL, Aban I, Kuo H, James SH, Prichard MN, Griffin J, Giles D, Acosta EP, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2015. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 372:933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barton LL, Mets MB. 2001. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin Infect Dis 33:370–374. doi: 10.1086/321897. [DOI] [PubMed] [Google Scholar]
- 86.Komrower GM, Williams BL, Stones PB. 1955. Lymphocytic choriomeningitis in the newborn: probable transplacental infection. Lancet 265:697–698. doi: 10.1016/S0140-6736(55)91066-7. [DOI] [PubMed] [Google Scholar]
- 87.Bonthius DJ. 2012. Lymphocytic choriomeningitis virus: an under-recognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol 19:89–95. doi: 10.1016/j.spen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson JL, Levy PT, Leonard KB, Smyser CD, Tychsen L, Cole FS. 2014. Congenital lymphocytic choriomeningitis virus: when to consider the diagnosis. J Child Neurol 29:837–842. doi: 10.1177/0883073813486295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skinner HH, Knight EH, Buckley LS. 1976. The hamster as a secondary reservoir host of lymphocytic choriomeningitis virus. J Hyg 76:299–306. doi: 10.1017/S0022172400055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Macneil A, Ströher U, Farnon E, Campbell S, Cannon D, Paddock CD, Drew CP, Kuehnert M, Knust B, Gruenenfelder R, Zaki SR, Rollin PE, Nichol ST, LCMV Transplant Investigation Team. 2012. Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg Infect Dis 18:1256–1262. doi: 10.3201/eid1808.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonthius DJ, Perlman S. 2007. Congenital viral infections of the brain: lessons learned from lymphocytic choriomeningitis virus in the neonatal rat. PLoS Pathog 3:e149. doi: 10.1371/journal.ppat.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R Jr. 9 February 2016. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol doi: 10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jampol LM, Goldstein DA. 9 February 2016. Zika virus infection and the eye. JAMA Ophthalmol doi: 10.1001/jamaophthalmol.2016.0284. [DOI] [PubMed] [Google Scholar]
- 94.McCarthy M. 2016. Severe eye damage in infants with microcephaly is presumed to be due to Zika virus. BMJ 352:i855. doi: 10.1136/bmj.i855. [DOI] [PubMed] [Google Scholar]
- 95.Ventura CV, Maia M, Bravo-Filho V, Gois AL, Belfort R Jr. 2016. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 96.Ventura CV, Maia M, Ventura BV, Linden VV, Araujo EB, Ramos RC, Rocha MA, Carvalho MD, Belfort R Jr, Ventura LO. 2016. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol 79:1–3. doi: 10.5935/0004-2749.20160002. [DOI] [PubMed] [Google Scholar]
- 97.Meritet JF, Krivine A, Lewin F, Poissonnier MH, Poizat R, Loget P, Rozenberg F, Lebon P. 2009. A case of congenital lymphocytic choriomeningitis virus (LCMV) infection revealed by hydrops fetalis. Prenat Diagn 29:626–627. doi: 10.1002/pd.2240. [DOI] [PubMed] [Google Scholar]
- 98.Hutton J, Rowan P, Greisinger A, Mouzoon M. 2014. Rubella monitoring in pregnancy as a means for evaluating a possible reemergence of rubella. Am J Obstet Gynecol 211:534.e1–534.e4. doi: 10.1016/j.ajog.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 99.Frey TK. 1994. Molecular biology of rubella virus. Adv Virus Res 44:69–160. doi: 10.1016/S0065-3527(08)60328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bouthry E, Picone O, Hamdi G, Grangeot-Keros L, Ayoubi J-M, Vauloup-Fellous C. 2014. Rubella and pregnancy: diagnosis, management and outcomes. Prenat Diagn 34:1246–1253. doi: 10.1002/pd.4467. [DOI] [PubMed] [Google Scholar]
- 101.Banatvala JE, Brown DWG. 2004. Rubella. Lancet 363:1127–1137. doi: 10.1016/S0140-6736(04)15897-2. [DOI] [PubMed] [Google Scholar]
- 102.Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. 2015. Rubella. Lancet 385:2297–2307. doi: 10.1016/S0140-6736(14)60539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehta V, Balachandran C, Lonikar V. 2008. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J 14:8. [PubMed] [Google Scholar]
- 104.Public Health England. 2016. NHS Infectious Diseases in Pregnancy Screening (IDPS) Programme. https://www.gov.uk/topic/population-screening-programmes/infectious-diseases-in-pregnancy Accessed 12 February 2016.
- 105.Wolff T, Shelton E, Sessions C, Miller T. 2009. Screening for syphilis infection in pregnant women: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 150:710–716. doi: 10.7326/0003-4819-150-10-200905190. [DOI] [PubMed] [Google Scholar]
- 106.Bowen V, Su J, Torrone E, Kidd S, Weinstock H. 2015. Increase in incidence of congenital syphilis—United States, 2012-2014. MMWR Morb Mortal Wkly Rep 64:1241–1245. doi: 10.15585/mmwr.mm6444a3. [DOI] [PubMed] [Google Scholar]
- 107.Walker DG, Walker GJA. 2002. Forgotten but not gone: the continuing scourge of congenital syphilis. Lancet Infect Dis 2:432–436. doi: 10.1016/S1473-3099(02)00319-5. [DOI] [PubMed] [Google Scholar]
- 108.Hollier LM, Harstad TW, Sanchez PJ, Twickler DM, Wendel GD. 2001. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol 97:947–953. doi: 10.1016/S0029-7844(01)01367-9. [DOI] [PubMed] [Google Scholar]
- 109.U.S. Preventive Services Task Force. 2009. Screening for syphilis infection in pregnancy: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 150:705–709. doi: 10.7326/0003-4819-150-10-200905190-00008. [DOI] [PubMed] [Google Scholar]
- 110.Centers for Disease Control and Prevention. 2008. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005–2006. MMWR Morb Mort Wkly Rep 57:872–875. [PubMed] [Google Scholar]
- 111.International Committee on Taxonomy of Viruses. 2015. Virus taxonomy: 2014 release. http://ictvonline.org/virusTaxonomy.asp Accessed 08 February 2016.
- 112.Dick GW. 1953. Epidemiological notes on some viruses isolated in Uganda; Yellow fever, Rift Valley fever, Bwamba fever, West Nile, Mengo, Semliki forest, Bunyamwera, Ntaya, Uganda S and Zika viruses. Trans R Soc Trop Med Hyg 47:13–48. doi: 10.1016/0035-9203(53)90021-2. [DOI] [PubMed] [Google Scholar]
- 113.Weaver SC, Barrett AD. 2004. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol 2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simpson DI. 1964. Zika virus infection in man. Trans R Soc Trop Med Hyg 58:335–338. doi: 10.1016/0035-9203(64)90200-7. [DOI] [PubMed] [Google Scholar]
- 116.Gubler DJ. 2001. Human arbovirus infections worldwide. Ann N Y Acad Sci 951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 117.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. 2012. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. 2014. Concurrent outbreaks of dengue, Chikungunya and Zika virus infections: an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill 19:pii=20929 http://dx.doi.org/10.2087/1560-7917.ES2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 119.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 120.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. 2014. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.European Centre for Disease Prevention and Control. 10 December 2015. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. ECDC, Solna, Sweden: http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf. [Google Scholar]
- 122.Musso D, Nilles EJ, Cao-Lormeau VM. 2014. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 20:O595–O596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 123.Patz JA, Reisen WK. 2001. Immunology, climate change and vector-borne diseases. Trends Immunol 22:171–172. doi: 10.1016/S1471-4906(01)01867-1. [DOI] [PubMed] [Google Scholar]
- 124.Lobo FP, Mota BE, Pena SD, Azevedo V, Macedo AM, Tauch A, Machado CR, Franco GR. 2009. Virus-host coevolution: common patterns of nucleotide motif usage in Flaviviridae and their hosts. PLoS One 4:e6282. doi: 10.1371/journal.pone.0006282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simon-Loriere E, Rossolillo P, Negroni M. 2011. RNA structures, genomic organization and selection of recombinant HIV. RNA Biol 8:280–286. doi: 10.4161/rna.8.2.15193. [DOI] [PubMed] [Google Scholar]
- 126.Aaskov J, Buzacott K, Field E, Lowry K, Berlioz-Arthaud A, Holmes EC. 2007. Multiple recombinant dengue type 1 viruses in an isolate from a dengue patient. J Gen Virol 88:3334–3340. doi: 10.1099/vir.0.83122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McGee CE, Tsetsarkin KA, Guy B, Lang J, Plante K, Vanlandingham DL, Higgs S. 2011. Stability of yellow fever virus under recombinatory pressure as compared with Chikungunya virus. PLoS One 6:e23247. doi: 10.1371/journal.pone.0023247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beasley DW, Whiteman MC, Zhang S, Huang CY, Schneider BS, Smith DR, Gromowski GD, Higgs S, Kinney RM, Barrett AD. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hindiyeh M, Shulman LM, Mendelson E, Weiss L, Grossman Z, Bin H. 2001. Isolation and characterization of West Nile virus from the blood of viremic patients during the 2000 outbreak in Israel. Emerg Infect Dis 7:748–750. doi: 10.3201/eid0704.017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 131.Hanna SL, Pierson TC, Sanchez MD, Ahmed AA, Murtadha MM, Doms RW. 2005. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J Virol 79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee E, Leang SK, Davidson A, Lobigs M. 2010. Both E protein glycans adversely affect dengue virus infectivity but are beneficial for virion release. J Virol 84:5171–5180. doi: 10.1128/JVI.01900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Youn S, Cho H, Fremont DH, Diamond MS. 2010. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J Virol 84:9516–9532. doi: 10.1128/JVI.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Somnuke P, Hauhart RE, Atkinson JP, Diamond MS, Avirutnan P. 2011. N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement. Virology 413:253–264. doi: 10.1016/j.virol.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gritsun TS, Gould EA. 2007. Origin and evolution of 3′UTR of flaviviruses: long direct repeats as a basis for the formation of secondary structures and their significance for virus transmission. Adv Virus Res 69:203–248. doi: 10.1016/S0065-3527(06)69005-2. [DOI] [PubMed] [Google Scholar]
- 136.Villordo SM, Gamarnik AV. 2013. Differential RNA sequence requirement for dengue virus replication in mosquito and mammalian cells. J Virol 87:9365–9372. doi: 10.1128/JVI.00567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Villordo SM, Filomatori CV, Sanchez-Vargas I, Blair CD, Gamarnik AV. 2015. Dengue virus RNA structure specialization facilitates host adaptation. PLoS Pathog 11:e1004604. doi: 10.1371/journal.ppat.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cleton N, Koopmans M, Reimerink J, Godeke GJ, Reusken C. 2012. Come fly with me: review of clinically important arboviruses for global travelers. J Clin Virol 55:191–203. doi: 10.1016/j.jcv.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 139.Way JH, Bowen ET, Platt GS. 1976. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol 30:123–130. doi: 10.1099/0022-1317-30-1-123. [DOI] [PubMed] [Google Scholar]
- 140.Bell TM, Field EJ, Narang HK. 1971. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch 35:183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- 141.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 5 April 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. 28 March 2016. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Becker R. 2016. Missing link: animal models to study whether Zika causes birth defects. Nat Med 22:225–227. doi: 10.1038/nm0316-225. [DOI] [PubMed] [Google Scholar]
- 144.Paz S, Semenza JC. 2016. El Nino and climate change-contributing factors in the dispersal of Zika virus in the Americas? Lancet 387:745. doi: 10.1016/S0140-6736(16)00256-7. [DOI] [PubMed] [Google Scholar]
- 145.Chretien JP, Anyamba A, Small J, Britch S, Sanchez JL, Halbach AC, Tucker C, Linthicum KJ. 26 January 2015. Global climate anomalies and potential infectious disease risks: 2014-2015. PLoS Curr doi: 10.1371/currents.outbreaks.95fbs4a8fb4695e049baabf2fc8289f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heffernan C. 2015. Climate change and infectious disease: time for a new normal? Lancet Infect Dis 15:143–144. doi: 10.1016/S1473-3099(14)71077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parham PE, Waldock J, Christophides GK, Michael E. 2015. Climate change and vector-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci 370:20140377. doi: 10.1098/rstb.2014.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Banu S, Guo Y, Hu W, Dale P, Mackenzie JS, Mengersen K, Tong S. 2015. Impacts of El Nino Southern Oscillation and Indian Ocean Dipole on dengue incidence in Bangladesh. Sci Rep 5:16105. doi: 10.1038/srep16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferreira MC. 2014. Geographical distribution of the association between El Nino South Oscillation and dengue fever in the Americas: a continental analysis using geographical information system-based techniques. Geospat Health 9:141–151. doi: 10.4081/gh.2014.12. [DOI] [PubMed] [Google Scholar]
- 150.Huang X, Clements AC, Williams G, Devine G, Tong S, Hu W. 2015. El Nino-Southern Oscillation, local weather and occurrences of dengue virus serotypes. Sci Rep 5:16806. doi: 10.1038/srep16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. 2015. Climate change influences on global distributions of dengue and Chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci 370:20140135. doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Crans WJ. 2004. A classification system for mosquito life cycles: life cycle types for mosquitoes of the northeastern United States. J Vector Ecol 29:1–10. [PubMed] [Google Scholar]
- 153.Monaghan AJ, Morin CW, Steinhoff DF, Wilhelmi O, Hayden M, Quattrochi DA, Reiskind M, Lloyd AL, Smith K, Schmidt CA, Scalf PE, Ernst K. 26 March 2016. On the seasonal occurrence and abundance of the Zika virus vector mosquito Aedes aegypti in the contiguous United States. PLoS Curr doi: 10.1371/currents.outbreaks.50dfc7f46798675fc63e7d7da563a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rueda LM, Patel KJ, Axtell RC, Stinner RE. 1990. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J Med Entomol 27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 155.Tun-Lin W, Burkot TR, Kay BH. 2000. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol 14:31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 156.Alto BW, Bettinardi D. 2013. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am J Trop Med Hyg 88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pontes RJ, Freeman J, Oliveira-Lima JW, Hodgson JC, Spielman A. 2000. Vector densities that potentiate dengue outbreaks in a Brazilian city. Am J Trop Med Hyg 62:378–383. [DOI] [PubMed] [Google Scholar]
- 159.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IR, Teng HJ, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, Wint GR, Golding N, Hay SI. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nah K, Mizumoto K, Miyamatsu Y, Yasuda Y, Kinoshita R, Nishiura H. 2016. Estimating risks of importation and local transmission of Zika virus infection. PeerJ 4:e1904. doi: 10.7717/peerj.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.International Air Transport Association. 26 November 2015. IATA air passenger forecast shows dip in long-term demand, press release no. 55. IATA, Montreal, Canada: http://www.iata.org/pressroom/pr/Pages/2015-11-26-01.aspx. [Google Scholar]
- 163.Fonseca K, Meatherall B, Zarra D, Drebot M, MacDonald J, Pabbaraju K, Wong S, Webster P, Lindsay R, Tellier R. 2014. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg 91:1035–1038. doi: 10.4269/ajtmh.14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Korhonen EM, Huhtamo E, Smura T, Kallio-Kokko H, Raassina M, Vapalahti O. 2016. Zika virus infection in a traveller returning from the Maldives, June 2015. Euro Surveill doi: 10.2807/1560-7917.ES.2016.21.2.30107. [DOI] [PubMed] [Google Scholar]
- 165.Kutsuna S, Kato Y, Takasaki T, Moi M, Kotaki A, Uemura H, Matono T, Fujiya Y, Mawatari M, Takeshita N, Hayakawa K, Kanagawa S, Ohmagari N. 2014. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014 [corrected]. Euro Surveill 19:pii=20683 http://dx.doi.org/10.2807/1560-7917.ES2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 166.Tappe D, Rissland J, Gabriel M, Emmerich P, Gunther S, Held G, Smola S, Schmidt-Chanasit J. 2014. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill 19:pii=20865 http://dx.doi.org/10.2807/1560-7917.ES2014.19.4.20685. [DOI] [PubMed] [Google Scholar]
- 167.Waehre T, Maagard A, Tappe D, Cadar D, Schmidt-Chanasit J. 2014. Zika virus infection after travel to Tahiti, December 2013. Emerg Infect Dis 20:1412–1414. doi: 10.3201/eid2008.140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zammarchi L, Tappe D, Fortuna C, Remoli ME, Gunther S, Venturi G, Bartoloni A, Schmidt-Chanasit J. 2015. Zika virus infection in a traveller returning to Europe from Brazil, March 2015. Euro Surveill 20:pii=21153 http://dx.doi.org/10.2807/1560-7917.ES2015.20.23.21153. [DOI] [PubMed] [Google Scholar]
- 169.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. 2014. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 19:20751 http://dx.doi.org/10.2807/1560-7917.ES2014.19.13.20751. [PubMed] [Google Scholar]
- 170.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. 2015. Detection of Zika virus in urine. Emerg Infect Dis 21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hirayama T, Mizuno Y, Takeshita N, Kotaki A, Tajima S, Omatsu T, Sano K, Kurane I, Takasaki T. 2012. Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: a laboratory diagnostic method useful after disappearance of the genome in serum. J Clin Microbiol 50:2047–2052. doi: 10.1128/JCM.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, Cattai M, Cusinato R, Palu G. 2013. Excretion of West Nile virus in urine during acute infection. J Infect Dis 208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 173.Instituto Fernandes Figueira da Fiocruz and Centro de Referência da Rede de Banco de Leite Humano. 2015. Comunicação e informação. Rio de Janeiro Rede Nacional de Bancos de Leite Humano. http://www.redeblh.fiocruz.br/cgi/cgilua.exe/sys/start.htm?infoid=1847&sid=368. [Google Scholar]
- 174.Biesbroeck L, Sidbury R. 2013. Viral exanthems: an update. Dermatol Ther 26:433–438. doi: 10.1111/dth.12107. [DOI] [PubMed] [Google Scholar]
- 175.Carneiro SC, Cestari T, Allen SH, Ramos e-Silva M. 2007. Viral exanthems in the tropics. Clin Dermatol 25:212–220. doi: 10.1016/j.clindermatol.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 176.Brazil Ministry of Health. 2015. Microcephaly. Ministry of Health releases epidemiological bulletin. http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/20805-ministerio-da-saude-divulga-boletim-epidemiologico. [Google Scholar]
- 177.Porat S, de Rham M, Giamboni D, Van Mieghem T, Baud D. 2014. Phenotip: a web-based instrument to help diagnosing fetal syndromes antenatally. Orphanet J Rare Dis 9:204. doi: 10.1186/s13023-014-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonca MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RM, Tanuri A, de Filippis AM. 17 February 2016. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 179.Musso D, Baud D. 17 February 2016. Zika virus: time to move from case reports to case control. Lancet Infect Dis doi: 10.1016/S1473-3099(16)00096-7. [DOI] [PubMed] [Google Scholar]
- 180.World Health Organization-Pan American Health Organisation. 2015. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas—epidemiological alert. WHO, Geneva, Switzerland. [Google Scholar]
- 181.Centers for Disease Control and Prevention. 2016. Transcript for CDC telebriefing: Zika virus travel alert. CDC, Atlanta, GA. [Google Scholar]
- 182.Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. 2016. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis 16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- 183.Instituto Nacional de Salud Colombia. 2016. Semana epidemiológica número 03 de 2016 (17 ene. al 23 ene.). Boletín epidemiológico semanal. http://www.ins.gov.co/boletin-epidemiologico/Boletn%20Epidemiolgico/2016%20Boletin%20epidemiologico%20semana%205.pdf.
- 184.Hawaii Department of Health. 2016. News release: Hawaii Department of health receives confirmation of Zika infection in baby born with microcephaly. http://health.hawaii.gov/news/files/2013/05/HAWAII-DEPARTMENT-OF-HEALTH-RECEIVES-CONFIRMATION-OF-ZIKA-INFECTION-IN-BABY-BORN-WITH-MICROCEPHALY.pdf.
- 185.Cire Antilles Guyane. 2016. Emergence du virus Zika aux Antilles Guyane. Point épidémiologique du 4 février 2016. Le point épidémio. http://www.invs.sante.fr/fr/content/download/122087/431231/version/99/file/pe_zika_antilles_guyane_040216.pdf.
- 186.Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Hennenfent AK, Rabe IB, Panella A, Oduyebo T, Honein MA, Zaki S, Lindsey N, Lehman JA, Kwit N, Bertolli J, Ellington S, Igbinosa I, Minta AA, Petersen EE, Mead P, Rasmussen SA, Jamieson DJ. 2016. Zika virus infection among U.S. pregnant travelers—August 2015–February 2016. MMWR Morb Mortal Wkly Rep 65:211–214. doi: 10.15585/mmwr.mm6508e1. [DOI] [PubMed] [Google Scholar]
- 187.Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, Shieh WJ, Luz KG, Ramos AM, Davi HP, Kleber de Oliveria W, Lanciotti R, Lambert A, Zaki S. 2016. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:159–160. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- 188.Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, Santos LA, Nery N Jr, Vasilakis N, Ko AI, de Almeida AR. 2016. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis 10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Villamil-Gomez WE, Mendoza-Guete A, Villalobos E, Gonzalez-Arismendy E, Uribe-Garcia AM, Castellanos JE, Rodriguez-Morales AJ. 2016. Diagnosis, management and follow-up of pregnant women with Zika virus infection: a preliminary report of the ZIKERNCOL cohort study on Sincelejo, Colombia. Travel Med Infect Dis 14:155–158. doi: 10.1016/j.tmaid.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 190.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O. 30 March 2016. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 191.Alvarado-Socarras JL, Rodriguez-Morales AJ. 2016. Etiological agents of microcephaly: implications for diagnosis during the current Zika virus epidemic. Ultrasound Obstet Gynecol 47:525–526. doi: 10.1002/uog.15885. [DOI] [PubMed] [Google Scholar]
- 192.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. 2016. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hayes EB. 2009. Zika virus outside Africa. Emerg Infect Dis 15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cerbino-Neto J, Mesquita EC, Souza TM, Parreira V, Wittlin BB, Durovni B, Lemos MC, Vizzoni A, Bispo de Filippis AM, Sampaio SA, Goncalves BS, Bozza FA. 15 July 2016. Clinical manifestations of Zika virus infection, Rio de Janeiro, Brazil, 2015. Emerg Infect Dis doi: 10.3201/eid2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.European Centre for Disease Prevention and Control. 2016. Rapid risk assessment. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome. Second update. http://ecdc.europa.eu/en/publications/Publications/zika-virus-rapid-risk-assessment-8-february-2016.pdf Accessed 8 February 2016.
- 197.Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. 2016. Zika virus infection in French Polynesia. Lancet 387:1051–1053. doi: 10.1016/S0140-6736(16)00625-5. [DOI] [PubMed] [Google Scholar]
- 198.Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastere S, Bost-Bezeaud F, Marcelis L, Abadie V, Garel C, Moutard ML, Jouannic JM, Rozenberg F, Leparc-Goffart I, Mallet HP. 31 March 2016. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill doi: 10.2807/1560-7917.ES.2016.21.13.30181. [DOI] [PubMed] [Google Scholar]
- 199.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. 15 March 2016. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miranda-Filho DDB, Martelli CM, Ximenes RA, Araujo TV, Rocha MA, Ramos RC, Dhalia R, Franca RF, Marques Junior ET, Rodrigues LC. 2016. Initial description of the presumed congenital Zika syndrome. Am J Public Health 106:598–600. doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Werner H, Fazecas T, Guedes B, Dos Santos JL, Daltro P, Tonni G, Campbell S, Araujo Junior E. 2016. Intrauterine Zika virus infection and microcephaly: perinatal imaging correlations with 3D virtual physical models. Ultrasound Obstet Gynecol 47:657–660. doi: 10.1002/uog.15901. [DOI] [PubMed] [Google Scholar]
- 202.Bocanegra C. 5 April 2016. Zika virus infection in pregnant women in Barcelona, Spain. Clin Microbiol Infect doi: 10.1016/j.cmi.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 203.Giovanetti M, Faria NR, Nunes MR, de Vasconcelos JM, Lourenco J, Rodrigues SG, Vianez JL Jr da Silva SP, Lemos PS, Tavares FN, Martin DP, do Rosario MS, Siqueira I, Ciccozzi M, Pybus OG, de Oliveira T, Alcantara LCJ. 2016. Zika virus complete genome from Salvador, Bahia, Brazil. Infect Genet Evol 41:142–145. doi: 10.1016/j.meegid.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 204.Souza RT, Cecatti JG, Passini R Jr, Tedesco RP, Lajos GJ, Nomura ML, Rehder PM, Dias TZ, Haddad SM, Pacagnella RC, Costa ML, Brazilian Multicenter Study on Preterm Birth Study Group. 2016. The burden of provider-initiated preterm birth and associated factors: evidence from the Brazilian Multicenter Study on Preterm Birth (EMIP). PLoS One 11:e0148244. doi: 10.1371/journal.pone.0148244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oliveira RR, Melo EC, Falavina LP, Mathias TA. 2015. The growing trend of moderate preterm births: an ecological study in one region of Brazil. PLoS One 10:e0141852. doi: 10.1371/journal.pone.0141852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adams Waldorf KM, McAdams RM. 2013. Influence of infection during pregnancy on fetal development. Reproduction 146:R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. 2013. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 10:311. doi: 10.1186/1743-422X-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Buckley A, Gould EA. 1988. Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with Zika or Langat virus. J Gen Virol 69:1913–1920. doi: 10.1099/0022-1317-69-8-1913. [DOI] [PubMed] [Google Scholar]
- 210.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Thornton GK, Woods CG. 2009. Primary microcephaly: do all roads lead to Rome? Trends Genet 25:501–510. doi: 10.1016/j.tig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Marthiens V, Rujano MA, Pennetier C, Tessier S, Paul-Gilloteaux P, Basto R. 2013. Centrosome amplification causes microcephaly. Nat Cell Biol 15:731–740. doi: 10.1038/ncb2746. [DOI] [PubMed] [Google Scholar]
- 213.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. 2008. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tetro JA. 2016. Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect 18:167–168. doi: 10.1016/j.micinf.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 215.Frumence E, Roche M, Krejbich-Trotot P, El-Kalamouni C, Nativel B, Rondeau P, Misse D, Gadea G, Viranaicken W, Despres P. 2016. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-beta production and apoptosis induction. Virology 493:217–226. doi: 10.1016/j.virol.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 216.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. 10 April 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 217.Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y, Coyne CB. 5 April 2016. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dick GW. 1952. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 219.Weinbren MP, Williams MC. 1958. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 52:263–268. doi: 10.1016/0035-9203(58)90085-3. [DOI] [PubMed] [Google Scholar]
- 220.Blazquez AB, Saiz JC. 2010. West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res 151:240–243. doi: 10.1016/j.virusres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 221.Cordoba L, Escribano-Romero E, Garmendia A, Saiz JC. 2007. Pregnancy increases the risk of mortality in West Nile virus-infected mice. J Gen Virol 88:476–480. doi: 10.1099/vir.0.82439-0. [DOI] [PubMed] [Google Scholar]
- 222.Julander JG, Winger QA, Olsen AL, Day CW, Sidwell RW, Morrey JD. 2005. Treatment of West Nile virus-infected mice with reactive immunoglobulin reduces fetal titers and increases dam survival. Antiviral Res 65:79–85. doi: 10.1016/j.antiviral.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 223.Julander JG, Winger QA, Rickords LF, Shi PY, Tilgner M, Binduga-Gajewska I, Sidwell RW, Morrey JD. 2006. West Nile virus infection of the placenta. Virology 347:175–182. doi: 10.1016/j.virol.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 224.Mathur A, Arora KL, Chaturvedi UC. 1981. Congenital infection of mice with Japanese encephalitis virus. Infect Immun 34:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shimizu T, Kawakami Y, Fukuhara S, Matumoto M. 1954. Experimental stillbirth in pregnant swine infected with Japanese encephalitis virus. Jpn J Exp Med 24:363–375. [PubMed] [Google Scholar]
- 226.Burns KF. 1950. Congenital Japanese B encephalitis infection of swine. Proc Soc Exp Biol Med 75:621–625. doi: 10.3181/00379727-75-18285. [DOI] [PubMed] [Google Scholar]
- 227.Yamada M, Nakamura K, Yoshii M, Kaku Y. 2004. Nonsuppurative encephalitis in piglets after experimental inoculation of Japanese encephalitis flavivirus isolated from pigs. Vet Pathol 41:62–67. doi: 10.1354/vp.41-1-62. [DOI] [PubMed] [Google Scholar]
- 228.Platt KB. 2004. Characterization of West Nile virus infection in swine. Final report, project 02-118. National Pork Board, Clive, IA. [Google Scholar]
- 229.Zimmerman JJ. 2012. Diseases of swine, 10th ed Wiley-Blackwell, Chichester, West Sussex, United Kingdom. [Google Scholar]
- 230.Centers for Disease Control and Prevention. 2002. Intrauterine West Nile Virus infection—New York, 2002. MMWR Morb Mortal Wkly Rep 51:1135–1136. [PubMed] [Google Scholar]
- 231.Alpert SG, Fergerson J, Noel LP. 2003. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol 136:733–735. doi: 10.1016/S0002-9394(03)00452-5. [DOI] [PubMed] [Google Scholar]
- 232.Paisley JE, Hinckley AF, O'Leary DR, Kramer WC, Lanciotti RS, Campbell GL, Hayes EB. 2006. West Nile virus infection among pregnant women in a northern Colorado community, 2003 to 2004. Pediatrics 117:814–820. doi: 10.1542/peds.2005-1187. [DOI] [PubMed] [Google Scholar]
- 233.O'Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ, Kightlinger LK, Beecham BD, Miller TK, Neitzel DF, Michaels SR, Campbell GL, Lanciotti RS, Hayes EB. 2006. Birth outcomes following West Nile virus infection of pregnant women in the United States: 2003-2004. Pediatrics 117:e537–e545. doi: 10.1542/peds.2005-2024. [DOI] [PubMed] [Google Scholar]
- 234.Chye JK, Lim CT, Ng KB, Lim JM, George R, Lam SK. 1997. Vertical transmission of dengue. Clin Infect Dis 25:1374–1377. doi: 10.1086/516126. [DOI] [PubMed] [Google Scholar]
- 235.Thaithumyanon P, Thisyakorn U, Deerojnawong J, Innis BL. 1994. Dengue infection complicated by severe hemorrhage and vertical transmission in a parturient woman. Clin Infect Dis 18:248–249. doi: 10.1093/clinids/18.2.248. [DOI] [PubMed] [Google Scholar]
- 236.Boussemart T, Babe P, Sibille G, Neyret C, Berchel C. 2001. Prenatal transmission of dengue: two new cases. J Perinatol 21:255–257. doi: 10.1038/sj.jp.7200530. [DOI] [PubMed] [Google Scholar]
- 237.Basurko C, Carles G, Youssef M, Guindi WE. 2009. Maternal and fetal consequences of dengue fever during pregnancy. Eur J Obstet Gynecol Reprod Biol 147:29–32. doi: 10.1016/j.ejogrb.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 238.Khairallah M, Kahloun R. 2013. Ocular manifestations of emerging infectious diseases. Curr Opin Ophthalmol 24:574–580. doi: 10.1097/ICU.0b013e3283654e09. [DOI] [PubMed] [Google Scholar]
- 239.Lim WK, Mathur R, Koh A, Yeoh R, Chee SP. 2004. Ocular manifestations of dengue fever. Ophthalmology 111:2057–2064. doi: 10.1016/j.ophtha.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 240.Beral L, Merle H, David T. 2008. Ocular complications of dengue fever. Ophthalmology 115:1100–1101. doi: 10.1016/j.ophtha.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 241.Mets MB, Chhabra MS. 2008. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol 53:95–111. doi: 10.1016/j.survophthal.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 242.Chaturvedi UC, Mathur A, Chandra A, Das SK, Tandon HO, Singh UK. 1980. Transplacental infection with Japanese encephalitis virus. J Infect Dis 141:712–715. doi: 10.1093/infdis/141.6.712. [DOI] [PubMed] [Google Scholar]
- 243.Bentlin MR, de Barros Almeida RA, Coelho KI, Ribeiro AF, Siciliano MM, Suzuki A, Fortaleza CM. 2011. Perinatal transmission of yellow fever, Brazil, 2009. Emerg Infect Dis 17:1779–1780. doi: 10.3201/eid1709.110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Centers for Disease Control and Prevention. 2010. Transmission of yellow fever vaccine virus through breast-feeding—Brazil, 2009. MMWR Morb Mortal Wkly Rep 59:130–132. [PubMed] [Google Scholar]
- 245.Tsai TF, Paul R, Lynberg MC, Letson GW. 1993. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis 168:1520–1523. doi: 10.1093/infdis/168.6.1520. [DOI] [PubMed] [Google Scholar]
- 246.Cavalcanti DP, Salomao MA, Lopez-Camelo J, Pessoto MA, Campinas Group of Yellow Fever Immunization during Pregnancy. 2007. Early exposure to yellow fever vaccine during pregnancy. Trop Med Int Health 12:833–837. doi: 10.1111/j.1365-3156.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 247.Robert E, Vial T, Schaefer C, Arnon J, Reuvers M. 1999. Exposure to yellow fever vaccine in early pregnancy. Vaccine 17:283–285. doi: 10.1016/S0264-410X(98)00051-6. [DOI] [PubMed] [Google Scholar]
- 248.Laoprasopwattana K, Suntharasaj T, Petmanee P, Suddeaugrai O, Geater A. 2016. Chikungunya and dengue virus infections during pregnancy: seroprevalence, seroincidence and maternal-fetal transmission, southern Thailand, 2009-2010. Epidemiol Infect 144:381–388. doi: 10.1017/S0950268815001065. [DOI] [PubMed] [Google Scholar]
- 249.Fritel X, Rollot O, Gerardin P, Gauzere BA, Bideault J, Lagarde L, Dhuime B, Orvain E, Cuillier F, Ramful D, Samperiz S, Jaffar-Bandjee MC, Michault A, Cotte L, Kaminski M, Fourmaintraux A, Chikungunya-Mere-Enfant Team. 2010. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis 16:418–425. doi: 10.3201/eid1604.091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ramful D, Samperiz S, Fritel X, Michault A, Jaffar-Bandjee MC, Rollot O, Boumahni B, Gerardin P. 2014. Antibody kinetics in infants exposed to Chikungunya virus infection during pregnancy reveals absence of congenital infection. J Infect Dis 209:1726–1730. doi: 10.1093/infdis/jit814. [DOI] [PubMed] [Google Scholar]
- 251.Gerardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, Lenglet Y, Touret Y, Bouveret A, Grivard P, Le Roux K, Blanc S, Schuffenecker I, Couderc T, Arenzana-Seisdedos F, Lecuit M, Robillard PY. 2008. Multidisciplinary prospective study of mother-to-child Chikungunya virus infections on the island of La Reunion. PLoS Med 5:e60. doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gerardin P, Samperiz S, Ramful D, Boumahni B, Bintner M, Alessandri JL, Carbonnier M, Tiran-Rajaoefera I, Beullier G, Boya I, Noormahomed T, Okoi J, Rollot O, Cotte L, Jaffar-Bandjee MC, Michault A, Favier F, Kaminski M, Fourmaintraux A, Fritel X. 2014. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis 8:e2996. doi: 10.1371/journal.pntd.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.American Congress of Obstetricians and Gynecologists. 2016. Practice advisory: updated interim guidance for care of obstetric patients and women of reproductive age during a Zika virus outbreak. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients-During-a-Zika-Virus-Outbreak Accessed 13 February 2016.
- 254.Royal College of Obstetricians and Gynaecologists. 2016. Interim clinical guidelines on Zika virus infection and pregnancy. https://www.rcog.org.uk/en/news/interim-clinical-guidelines-on-zika-virus-infection-and-pregnancy/ Accessed 13 February 2016.
- 255.Conseil National Professionnel de Gynécologie et Obstétrique. 2016. Virus ZIKA Et femme enceinte ou en âge de procréer. http://www.cnpgo.org/1/upload/virus_zika_et_grossesse_cnpgo_version_1.3_recos.pdf Accessed 13 February 2016.
- 256.Oduyebo T, Petersen EE, Rasmussen SA, Mead PS, Meaney-Delman D, Renquist CM, Ellington SR, Fischer M, Staples JE, Powers AM, Villanueva J, Galang RR, Dieke A, Munoz JL, Honein MA, Jamieson DJ. 2016. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 65:122–127. doi: 10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- 257.Canada Society of Obstetricians and Gynecologists. 2016. SOGC recommendation on ZIKA virus exposure for clinicians caring for pregnant women and those who intend to get pregnant. http://sogc.org/wp-content/uploads/2016/02/SOGC-Update-on-Zika-.pdf Accessed 30 March 2016.
- 258.World Health Organization-Pan American Health Organisation. 2016. Provisional remarks on Zika virus infection in pregnant women. Document for health care professionals. WHO, Geneva, Switzerland. [Google Scholar]
- 259.Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. 2016. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 260.Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. 4 April 2016. Clinical management of pregnant women exposed to Zika virus. Lancet Infect Dis doi: 10.1016/S1473-3099(16)30008-1. [DOI] [PubMed] [Google Scholar]
- 261.Samarasekera U, Triunfol M. 2016. Concern over Zika virus grips the world. Lancet 287:521–524. doi: 10.1016/S0140-6736(16)00257-9. [DOI] [PubMed] [Google Scholar]
- 262.Gambotto M. 2011. Fetology: diagnosis and management of the fetal patient. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 263.Petersen EE, Polen KN, Meaney-Delman D, Ellington SR, Oduyebo T, Cohn A, Oster AM, Russell K, Kawwass JF, Karwowski MP, Powers AM, Bertolli J, Brooks JT, Kissin D, Villanueva J, Munoz-Jordan J, Kuehnert M, Olson CK, Honein MA, Rivera M, Jamieson DJ, Rasmussen SA. 2016. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 65:315–322. doi: 10.15585/mmwr.mm6512e2. [DOI] [PubMed] [Google Scholar]
- 264.Calvet G, Aguiar RS, Melo ASO, Sampaio SA, Filippis Id Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, dos Santos FB, Nogueira RM, Tanuri A, de Filippis AMB. 17 February 2016. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 265.Tabor A, Philip J, Madsen M, Bang J, Obel EB, Nørgaard-Pedersen B. 1986. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet i:1287–1293. [DOI] [PubMed] [Google Scholar]
- 266.Collège National des Gynécologues et Obstétriciens Français. 2016. Virus Zika et femme enceinte ou en âge de procréer. http://www.cngof.fr/actualites/456-virus-zika-et-femme-enceinte-ou-en-age-de-procreer Accessed 5 February 2016.
- 267.Vouga M, Musso D, Van Mieghem T, Baud D. 2016. CDC guidelines for pregnant women during the Zika virus outbreak. Lancet 387:843–844. doi: 10.1016/S0140-6736(16)00383-4. [DOI] [PubMed] [Google Scholar]
- 268.Guerra B, Simonazzi G, Banfi A, Lazzarotto T, Farina A, Lanari M, Rizzo N. 2007. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol 196:221.e221–221.e226 http://dx.doi.org/10.1016/j.ajog.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 269.Roa M. 2016. Zika virus outbreak: reproductive health and rights in Latin America. Lancet 387:843. doi: 10.1016/S0140-6736(16)00331-7. [DOI] [PubMed] [Google Scholar]
- 270.Vasquez AM, Sapiano MR, Basavaraju SV, Kuehnert MJ, Rivera-Garcia B. 2016. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus infection—Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep 65:375–378. doi: 10.15585/mmwr.mm6514e1. [DOI] [PubMed] [Google Scholar]
- 271.Musso D, Stramer SL, Busch MP. 14 May 2016. Zika virus: a new challenge for blood transfusion. Lancet 387:1993–1994. doi: 10.1016/S0140-6736(16)30428-7. [DOI] [PubMed] [Google Scholar]
- 272.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. 2014. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 19:pii=20761 htttp://dx.doi.org/10.2807/1560-7917.ES2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 273.FDA Center for Biologics Evaluation and Research. 2016. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion transmission of Zika virus: guidance for industry. FDA, Rockville, MD: Accessed 1 April 2016. [Google Scholar]
- 274.Staples JE, Dziuban EJ, Fischer M, Cragan JD, Rasmussen SA, Cannon MJ, Frey MT, Renquist CM, Lanciotti RS, Munoz JL, Powers AM, Honein MA, Moore CA. 2016. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 65:63–67. doi: 10.15585/mmwr.mm6503e3. [DOI] [PubMed] [Google Scholar]
- 275.Staples JE, Gershman M, Fischer M, Centers for Disease Control and Prevention. 2010. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 59(RR-7):1–27. [PubMed] [Google Scholar]
- 276.Ross SA, Ahmed A, Palmer AL, Michaels MG, Sánchez PJ, Bernstein DI, Tolan RW, Novak Z, Chowdhury N, Fowler KB, Boppana SB, National Institute on Deafness and Other Communication Disorders CHIMES Study. 2014. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 210:1415–1418. doi: 10.1093/infdis/jiu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. 2015. Detection of Zika virus in saliva. J Clin Virol 68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 278.Musso D, Baud D. 17 February 2016. Zika virus: time to move from case reports to case control studies. Lancet Infect Dis doi: 10.1016/S1473-3099(16)00096-7. [DOI] [PubMed] [Google Scholar]
- 279.Wilcken B, Wiley V. 2015. Fifty years of newborn screening: newborn screening. J Paediatr Child Health 51:103–107. doi: 10.1111/jpc.12817. [DOI] [PubMed] [Google Scholar]
- 280.American Academy of Pediatrics Joint Committee on Infant Hearing. 2007. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 281.Ministry of Health of Jamaica. 2016. Notes for Minister of Health Hon. Horace Dalley. http://moh.gov.jm/presentation/notes-for-minister-of-health-hon-horace-dalley-post-cabinet-press-briefing-january-20-2016-at-11a-m-office-of-the-prime-minister/ Accessed 5 February 2016.
- 282.Ministry of health of New Zealand. 2016. Zika virus. http://www.health.govt.nz/news-media/news-items/ministry-health-advice-zika-virus-0.
- 283.Public Health Agency of Canada. 2016. Zika virus infection in the Americas. http://www.phac-aspc.gc.ca/tmp-pmv/notices-avis/notices-avis-eng.php?id=143 Accessed 5 February 2016.
- 284.Department of Health of Australia. 2016. Zika virus: information for clinicians and public health practitioners. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-zika-health-practitioners.htm Accessed 5 February 2016.
- 285.Ministry of Health of Singapore. 2016. Zika virus. https://www.moh.gov.sg/content/moh_web/home/pressRoom/Current_Issues/2016/zika-virus.html Accessed 5 February 2016.
- 286.American Congress of Obstetricians and Gynecologists. 2016. Interim guidance for care of obstetric patients during Zika virus outbreak. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients-During-a-Zika-Virus-Outbreak Accessed 5 February 2016.
- 287.MotherToBaby: a service of the Organization of Teratology Information Specialists. 2016. Zika virus and pregnancy answers. http://mothertobaby.org/news-press/zika-virus-pregnancy-answers/ Accessed 5 February 2016.
- 288.Ministère Français des affaires sociales de la santé et des droit des femmes. 2016. Virus Zika: début d'épidémie en Martinique et Guyane. http://social-sante.gouv.fr/actualites/presse/communiques-de-presse/article/debut-d-epidemie-d-infections-a-virus-zika-dans-les-departements-francais-d Accessed 5 February 2016.
- 289.Ministère de la Santé Publique et de la Population de Haiti. 2016. Fiche d'information sur le virus Zika. http://mspp.gouv.ht/site/downloads/Fiche%20Information%20ZIKA%20MSPP.pdf.
- 290.European Centre for Disease Prevention and Control. 2016. Updated rapid risk assessment on Zika virus in the Americas and potential complications. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133-18ff4cb1b568&ID=1348&ContentTypeId=0x010082EE625D0C434588A3E95C31FC12D7A70104000C92BA0F0E932049B9C0FB633C874119.
- 291.Ministry of Health, State of Israel. 2015. Recommendations for persons travelling abroad in view of morbidity caused by Zika virus. http://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/31122015.aspx Accessed 7 February 2016.
- 292.Ministry of Health of Mexico. 2016. Zika virus: a rising global heathcare concern. http://mexicosalud.com/zika-virus-a-rising-global-healthcare-concern/ Accessed 7 February 2016.
- 293.Government of Canada. 2016. Canadian Recommendations on the precention and treatment of Zika virus. http://healthycanadians.gc.ca/publications/diseases-conditions-maladies-affections/committee-statement-treatment-prevention-zika-declaration-comite-traitement-prevention/index-eng.php?id=zika_virus_16_hcdns Accessed 9 February 2016.
- 294.Cetron M. 2016. Revision to CDC's Zika travel notices: minimal likelihood for mosquito-borne Zika virus transmission at elevations above 2,000 meters. MMWR Morb Mortal Wkly Rep 65:267–268. doi: 10.15585/mmwr.mm6510e1. [DOI] [PubMed] [Google Scholar]
- 295.Jin J. 13 April 2016. Zika virus disease. JAMA doi: 10.1001/jama.2016.4741. [DOI] [PubMed] [Google Scholar]
- 296.World Health Organisation. 2016. Zika virus. http://www.who.int/mediacentre/factsheets/zika/en/ Accessed 8 February 2016.
- 297.Centers for Disease Control Prevention. 2016. Avoid bug bites. http://wwwnc.cdc.gov/travel/page/avoid-bug-bites Accessed 5 February 2016.
- 298.World Health Organization. 2016. Zika virus infection, United States of America, United States Virgin Islands: http://www.who.int/csr/don/29-january-2016-zika-usa/en/. [Google Scholar]
- 299.Centers for Disease Control Prevention. 2015. Insect repellent use and safety. http://www.cdc.gov/westnile/faq/repellent.html Accessed 5 February 2016.
- 300.Yoon JK, Kim KC, Cho Y, Gwon YD, Cho HS, Heo Y, Park K, Lee YW, Kim M, Oh YK, Kim YB. 2015. Comparison of repellency effect of mosquito repellents for DEET, citronella, and fennel oil. J Parasitol Res 2015:361021. doi: 10.1155/2015/361021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rodriguez SD, Drake LL, Price DP, Hammond JI, Hansen IA. 2015. The efficacy of some commercially available insect repellents for Aedes aegypti (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae). J Insect Sci 15:140. doi: 10.1093/jisesa/iev125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fradin MS, Day JF. 2002. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 303.Lupi E, Hatz C, Schlagenhauf P. 2013. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.: a literature review. Travel Med Infect Dis 11:374–411. doi: 10.1016/j.tmaid.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 304.Lawrence KL, Achee NL, Bernier UR, Mundal KD, Benante JP. 2014. Field evaluations of topical arthropod repellents in North, Central, and South America. J Med Entomol 51:980–988. doi: 10.1603/ME14075. [DOI] [PubMed] [Google Scholar]
- 305.Wilson AL, Chen-Hussey V, Logan JG, Lindsay SW. 2014. Are topical insect repellents effective against malaria in endemic populations? A systematic review and meta-analysis. Malar J 13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Roth-Harer A, Lilienthal H, Bubser M, Kronthaler U, R Mundy W, R Ward T, Schmidt W, Winterhoff H, Winneke G. 2001. Neurotransmitter concentrations and binding at dopamine receptors in rats after maternal exposure to 3,4,3′,4′-tetrachlorobiphenyl: the role of reduced thyroid hormone concentrations. Environ Toxicol Pharmacol 9:103–115. doi: 10.1016/S1382-6689(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 307.Barr DB, Ananth CV, Yan X, Lashley S, Smulian JC, Ledoux TA, Hore P, Robson MG. 2010. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Sci Total Environ 408:790–795. doi: 10.1016/j.scitotenv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 308.Wickerham EL, Lozoff B, Shao J, Kaciroti N, Xia Y, Meeker JD. 2012. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int 47:80–85. doi: 10.1016/j.envint.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.McGready R, Simpson JA, Htway M, White NJ, Nosten F, Lindsay SW. 2001. A double-blind randomized therapeutic trial of insect repellents for the prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg 95:137–138. doi: 10.1016/S0035-9203(01)90137-3. [DOI] [PubMed] [Google Scholar]
- 310.McGready R, Hamilton KA, Simpson JA, Cho T, Luxemburger C, Edwards R, Looareesuwan S, White NJ, Nosten F, Lindsay SW. 2001. Safety of the insect repellent N,N-diethyl-M-toluamide (DEET) in pregnancy. Am J Trop Med Hyg 65:285–289. [DOI] [PubMed] [Google Scholar]
- 311.Centre de Référence sur les Agents Tératogènes. 2016. Répulsifs: grossesse et allaitement. http://lecrat.fr/spip.php?page=article&id_article=444 Accessed 5 February 2016.
- 312.Teratology Information Service UK. 2014. Chemical insect repellents. http://www.medicinesinpregnancy.org/Medicine-pregnancy/Insect-repellents/ Accessed 7 February 2016.
- 313.Schoenig GP, Neeper-Bradley TL, Fisher LC, Hartnagel RE Jr. 1994. Teratologic evaluations of N,N-diethyl-m-toluamide (DEET) in rats and rabbits. Fundam Appl Toxicol 23:63–69. doi: 10.1006/faat.1994.1079. [DOI] [PubMed] [Google Scholar]
- 314.Dugas J, Nieuwenhuijsen MJ, Martinez D, Iszatt N, Nelson P, Elliott P. 2010. Use of biocides and insect repellents and risk of hypospadias. Occup Environ Med 67:196–200. doi: 10.1136/oem.2009.047373. [DOI] [PubMed] [Google Scholar]
- 315.Mytton OT, McGready R, Lee SJ, Roberts CH, Ashley EA, Carrara VI, Thwai KL, Jay MP, Wiangambun T, Singhasivanon P, Nosten F. 2007. Safety of benzyl benzoate lotion and permethrin in pregnancy: a retrospective matched cohort study. BJOG 114:582–587. doi: 10.1111/j.1471-0528.2007.01290.x. [DOI] [PubMed] [Google Scholar]
- 316.Astroff AB, Young AD, Holzum B, Sangha GK, Thyssen JH. 2000. Conduct and interpretation of a dermal developmental toxicity study with KBR 3023 (a prospective insect repellent) in the Sprague-Dawley rat and Himalayan rabbit. Teratology 61:222–230. doi:. [DOI] [PubMed] [Google Scholar]
- 317.Astroff AB, Freshwater KJ, Young AD, Stuart BP, Sangha GK, Thyssen JH. 1999. The conduct of a two-generation reproductive toxicity study via dermal exposure in the Sprague-Dawley rat: a case study with KBR 3023 (a prospective insect repellent). Reprod Toxicol 13:223–232. doi: 10.1016/S0890-6238(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 318.World Health Organization. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 319.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.McCarthy M. 2016. CDC updates Zika virus guidance to protect pregnant women. BMJ 352:i786. doi: 10.1136/bmj.i786. [DOI] [PubMed] [Google Scholar]
- 321.McCarthy M. 2016. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ 352:i720. doi: 10.1136/bmj.i720. [DOI] [PubMed] [Google Scholar]
- 322.D'Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Yazdanpanah Y, Leparc-Goffart I. 13 April 2016. Evidence of sexual transmission of Zika virus. N Engl J Med doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 323.McCarthy M. 2016. US health officials investigate sexually transmitted Zika virus infections. BMJ 352:i1180. doi: 10.1136/bmj.i1180. [DOI] [PubMed] [Google Scholar]
- 324.Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G, Bartoloni A. 2016. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- 325.Rowland A, Washington CI, Sheffield JS, Pardo-Villamizar CA, Segars JH. 2016. Zika virus infection in semen: a call to action and research. J Assist Reprod Genet 33:435–437. doi: 10.1007/s10815-016-0684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Oster AM, Russell K, Stryker JE, Friedman A, Kachur RE, Petersen EE, Jamieson DJ, Cohn AC, Brooks JT. 2016. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 65:323–325. doi: 10.15585/mmwr.mm6512e3. [DOI] [PubMed] [Google Scholar]
- 327.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. 2016. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep 65:372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- 328.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. 2016. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 329.Oster AM, Brooks JT, Stryker JE, Kachur RE, Mead P, Pesik NT, Petersen LR. 2016. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 65:120–121. doi: 10.15585/mmwr.mm6505e1. [DOI] [PubMed] [Google Scholar]
- 330.Finer LB, Zolna MR. 2014. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health 104(Suppl 1):S43–S48. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Centers for Disease Control Prevention. 2015. Contraception. How effective are birth control methods? CDC, Atlanta, GA: http://www.cdc.gov/reproductivehealth/unintendedpregnancy/contraception.htm Accessed 5 February 2016. [Google Scholar]
- 332.Gakidou E, Vayena E. 2007. Use of modern contraception by the poor is falling behind. PLoS Med 4:e31. doi: 10.1371/journal.pmed.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mbizvo MT, Phillips SJ. 2014. Family planning: choices and challenges for developing countries. Best Pract Res Clin Obstet Gynaecol 28:931–943. doi: 10.1016/j.bpobgyn.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 334.Hodge JG, Corbett A, Repka A, Judd PJ. 9 March 2016. Zika virus and global implications for reproductive health reforms. Disaster Med Public Health Prep 34:1–3. doi: 10.1017/dmp2016. [DOI] [PubMed] [Google Scholar]
- 335.Dyer O. 2016. Jamaica advises women to avoid pregnancy as Zika virus approaches. BMJ 352:i383. doi: 10.1136/bmj.i383. [DOI] [PubMed] [Google Scholar]
- 336.McCarthy M. 2016. Couples at risk from exposure to Zika virus should consider delaying pregnancy, says CDC. BMJ 352:i1813. doi: 10.1136/bmj.i1813. [DOI] [PubMed] [Google Scholar]
- 337.Ministry of Health of Samoa. 2016. Situational report Zika virus. http://www.health.gov.ws/index.php/for-consumers/disease-surveillance Accessed 7 February 2016.
- 338.Government of the Commonwealth of Dominica. 2016. Zika virus threat to Dominica. http://www.dominica.gov.dm/notices/540-zika-virus-threat-to-dominica Accessed 8 February 2016.
- 339.Sint Maarten Ministry of Public Health, Social Development and Labour. 2016. Multi-disciplinary group meets in preparation for Zika virus. http://www.sintmaartengov.org/PressReleases/Pages/Multi-disciplinary-group-meets-in-preparation-for-Zika-virus.aspx Accessed 8 February 2016.
- 340.Gostin LO, Hodge JG Jr. 13 April 2016. Is the United States prepared for a major Zika virus outbreak? JAMA doi: 10.1001/jama.2016.4919. [DOI] [PubMed] [Google Scholar]
- 341.Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E. 2016. Infectious Zika viral particles in breastmilk. Lancet 387:1051. doi: 10.1016/S0140-6736(16)00624-3. [DOI] [PubMed] [Google Scholar]
- 342.Barthel A, Gourinat AC, Cazorla C, Joubert C, Dupont-Rouzeyrol M, Descloux E. 2013. Breast milk as a possible route of vertical transmission of dengue virus? Clin Infect Dis 57:415–417. doi: 10.1093/cid/cit227. [DOI] [PubMed] [Google Scholar]
- 343.Centers for Disease Control Prevention. 2002. Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. MMWR Morb Mortal Wkly Rep 51:877–878. [PubMed] [Google Scholar]
- 344.Fredricks DN, Relman DA. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev 9:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rubin EJ, Greene MF, Baden LR. 2016. Zika virus and microcephaly. N Engl J Med 374:984–985. doi: 10.1056/NEJMe1601862. [DOI] [PubMed] [Google Scholar]
- 346.Latin American Colaborative Study of Congenital Malformations. 2015. ECLAMC final document. http://www.eclamc.org/eng/index.php Accessed 10 February 2016.
- 347.Mayor S. 2016. Zika infection in pregnancy is linked to range of fetal abnormalities, data indicate. BMJ 352:i1362. doi: 10.1136/bmj.i1362. [DOI] [PubMed] [Google Scholar]
- 348.Gulland A. 2016. Zika virus may be linked to several birth defects, expert warns. BMJ 352:i1322. doi: 10.1136/bmj.i1322. [DOI] [PubMed] [Google Scholar]
- 349.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, Araujo de Franca GV. 2016. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 350.Anonymous. 2016. The next steps on Zika. Nature 530:5. doi: 10.1038/530005a. [DOI] [PubMed] [Google Scholar]
- 351.Dyer O. 2016. Zika vaccine could be in production by year's end, says maker. BMJ 352:i630. doi: 10.1136/bmj.i1630. [DOI] [PubMed] [Google Scholar]
- 352.Cohen J. 2016. The race for a Zika vaccine is on. Science 351:543–544. doi: 10.1126/science.351.6273.543. [DOI] [PubMed] [Google Scholar]
- 353.Pulla P. What it will take to make a Zika vaccine. The Wire. http://thewire.in/2016/02/21/what-it-will-take-to-make-a-zika-vaccine-22149/. [Google Scholar]
- 354.Omer SB, Beigi RH. 2016. Pregnancy in the time of Zika: addressing barriers for developing vaccines and other measures for pregnant women. JAMA 315:1227–1228. doi: 10.1001/jama.2016.2237. [DOI] [PubMed] [Google Scholar]
- 355.Caragata EP, Dutra HL, Moreira LA. 2015. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol 32:207–218. doi: 10.1016/j.pt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 356.Yakob L, Walker T. 2016. Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health 4:e148–e149. doi: 10.1016/S2214-109X(16)00048-6. [DOI] [PubMed] [Google Scholar]