Abstract
Woody encroachment due to changes in climate or in the disturbance regimes (fire and herbivory) has been observed throughout the savannah biome over the last century with ecological, hydrological and socioeconomic consequences. We assessed changes in tree density and basal area and estimated changes in rain interception by the canopies across a 5-year period over a biomass gradient in Cerrado vegetation protected from fire. We modelled throughfall, stemflow and net rainfall on the basis of tree basal area (TBA). Tree density increased by an average annual rate of 6.7%, basal area at 5.7% and rain interception by the canopies at 0.6% of the gross rainfall. Independent of the vegetation structure, we found a robust relationship of 0.9% less rainfall reaching the ground as TBA increases by 1 m2 ha−1. Increases in tree biomass with woody encroachment may potentially result in less water available for uptake by plants and to recharge rivers and groundwater reserves. Given that water is a seasonally scarce resource in all savannahs, woody encroachment may threaten the ecosystem services related to water resources.
This article is part of the themed issue ‘Tropical grassy biomes: linking ecology, human use and conservation’.
Keywords: ecohydrology, Cerrado, tree biomass, fire suppression, rain partitioning, rain interception
1. Introduction
Increases in biomass, stem densities or cover of woody plants in arid and semi-arid environments have been documented over the past decades in various regions around the planet [1–6], with a number of proposed abiotic and biotic mechanisms. Among the possible factors behind woody encroachment are changes in rainfall regime [2,4], elevation of atmospheric CO2 concentration [5,7], invasion by exotic trees [8], changes in disturbance regimes such as suppression of fire [3,7,9] and decline in browsers or overgrazing [3,7,9]. Increases in woody cover or density can result in altered habitat for specialized fauna, changes in plant species composition [10,11] and also biogeochemical cycles and ecosystem services, such as carbon storage and water provisioning [1,8,12–15].
Vegetation structure is closely linked to hydrological processes, and several studies have shown that reduction in forest cover causes increases in water yield and increasing tree cover on low biomass vegetation causes a decrease in water yield [8,12,13,16–19]. Therefore, woody encroachment can have direct consequences on the water cycle [20]. Increases in tree biomass lead to higher evaporative losses [18,21–26], but the vapour returned to the atmosphere does not become rain in the same location where it evaporates [27]. Consequently, at the scale of a watershed, the biomass increase has been demonstrated to result in lower production of water [8,13,16,18] in spite of models predicting that this vapour may have the effect of increasing rainfall at the global scale [28]. The increase in carbon storage due to woody encroachment or through forest plantations (in regions where the historical vegetation was not forest) may come at the cost of water [13–15], aggravating the water crisis already experienced by countries in savannah regions. Changes in the water cycle due to woody encroachment or afforestation on grassy biomes have not received public attention, while carbon sequestration initiatives have been stimulated worldwide, under the assumption that ‘the more trees the better’ [14,15]. The trade-off between water and carbon is also related to scale, because the benefits of carbon storage in the biomass are global, while the subsequent decrease in provision of water is local.
Woody encroachment has been recorded in many non-forest ecosystems around the world including the Cerrado, a savannah vegetation covering the central plateau of Brazil. Woody biomass increases and forest expansion in Cerrado vegetation has been recorded in the core area of the biome [29], and also at the edges, close to the Amazonian forest [30], the Atlantic forest [31,32] and the dry Caatinga [33]. Woody encroachment in the Cerrado has been related to a decrease in fire frequency or total fire suppression, with estimated rates ranging from a 1 [33] to 4% [30] increase in total basal area (TBA) per year. The Cerrado biome is a dynamic mosaic of vegetation types with varying biomass that can be related to environmental conditions [34], but has been maintained by fire at different frequencies [35,36]. In addition to natural fires caused by lightning [37], the whole Cerrado biome was historically burned by Indians once every 5–10 years and in the last three centuries it was burned every 1–2 years by ranchers [38]. In recent decades, however, fire frequency in the Cerrado remnants has been reduced, leading to woody encroachment where no abiotic limitations exist for biomass accumulation. Inside protected areas, fire has been suppressed over the past decades as a national conservation policy, even if the ecosystems are fire dependent. In the whole state of São Paulo, the use of fire on native vegetation has been prohibited by law since the year 2000. On private properties, farmers also protect their land from fire when used for crops, silviculture or pasture with exotic grasses [39]. Under these land uses, fire is negative, as it destroys what has been cultivated, including the dry biomass of exotic grass that is eaten by cattle in the winter.
Occupying the highest altitudes of central Brazil, the Cerrado comprises a series of vegetation types with variable structure and biomass, ranging from open grasslands to forest vegetation. Soils in the Cerrado are mostly oxisols or entisols (Quartzipsament), distrophic, acidic with low fertility, very deep and well drained (89%), with high infiltration and therefore efficient groundwater recharge [40]. The Cerrado is the moistest savannah in the world [41], with annual precipitation in 86% of the biome ranging from 1000 to 2000 mm [40] and evapotranspiration estimated between 700–1900 mm [24,42,43]. Such climate conditions permit savannah and forest as alternative stable states [44], with woody encroachment occurring after fire suppression. The favourable climate and soil conditions in the majority of the Cerrado biome have provided perennial rivers that supply eight of the 12 largest watersheds of Brazil, in spite of the long dry season, which ranges from three to seven months [40].
The Cerrado biome covered 22% of Brazil up to very recently, because climate and soils were not suitable for traditional cultivation, constraining agriculture expansion. This barrier has been surpassed in the last decades by technological advances, resulting in a rapid land conversion and certainly huge biodiversity losses [45,46]. Land conversion for pasture, agriculture or forestry, the most common land uses in the Cerrado biome [45], has probably influenced many ecological processes, among which are those related to water resources. Distinct land uses can have opposite influences on hydrological processes, such as annual river discharge increasing after forest conversion for agriculture or pasture [47,48] or decreasing after afforestation [13,49]. Besides land-use changes, woody encroachment has the potential to modify the ecohydrology of watersheds covered by native Cerrado vegetation. Across the natural mosaic of tree biomass in the Cerrado vegetation, it is generally considered that the proportion of rainfall lost as evapotranspiration or recharging groundwater reserves and providing streamflow vary across the structural gradient as has been demonstrated worldwide [8,21,22,25,26]. If biomass increases due to woody encroachment, changes are also expected in the partitioning of rainfall in interception, stemflow and throughfall; this has never been assessed in the Cerrado vegetation where previous hydrological studies have mostly focused on evapotranspiration only [23,24,43].
Despite rain interception often accounting for more than 20% of annual rainfall, and up to 45%, depending on vegetation characteristics and climate [50], rain interception by tree canopies as a separate process has often been neglected, particularly when the consequences of changes in land use, in climate or in vegetation cover are analysed [51]. Water intercepted by the canopies has, however, a completely distinct fate from the water reaching the ground and infiltrating, because the first is not available for use by plants and does not contribute to groundwater recharge or streamflow. Given the increase in woody cover, and particularly due to altered fire regimes, our aim was to determine how woody encroachment may affect the hydrological cycle in the Cerrado, focusing particularly on rain partitioning—the relative amounts of rainfall retained by the canopies (interception), running down the stems (stemflow) or falling on the ground (throughfall).
Specifically, we quantified throughfall and stemflow across a biomass gradient in the Cerrado vegetation, to elucidate the relationships between tree biomass and the proportion of rain water lost to the atmosphere due to interception by the canopies. We expected a decrease in the amount of rainfall reaching the ground as the woody encroachment progresses based on the premise from past hydrological studies that higher aerial biomass results in higher rain interception by the canopies. We searched for robust relationships between the hydrological processes and vegetation structure to make predictions about canopy rain interception, a highly relevant hydrological process that is often neglected by ecologists due to the difficulty in quantifying it. We briefly discuss some possible implications of woody encroachment on ground water recharge and water supply and also management interventions to optimize this ecosystem service.
2. Material and methods
(a). Study area
We conducted our study at the Ecological Station of Assis (EEcA), in southwestern São Paulo state, Brazil (22°35′ S and 50°23′ W). The regional land relief is relatively flat and the soils in the whole study site are very deep, well drained, with no apparent stoniness or rockiness, and no mechanical restriction to the expansion of root systems [34]. In the study plots, soil was classified as loamy Rhodic Hapludox and Quartzipsaments, slightly differing among plots in the soil water holding capacity [34]. According to the Köppen classification, the climate is Cfa—warm temperate, humid with a hot summer. The mean annual temperature is 22°C, with a mean maximum of 28.7°C and a mean minimum of 15.4°C. Annual rainfall is 1441 mm, concentrated in the summer (October–March), with a dry season in the winter (April–September) [34] and the potential evapotranspiration is estimated to be 1100 mm [52]. The rainfall regime, in the study region, is characterized by a large proportion of rain events with very low volume, a regime that is similar to the whole Cerrado biome, where rain events with volume above 30 mm are relatively rare [53].
The EEcA preserves 1312 ha of Cerrado vegetation, which was previously used for cattle grazing for about a century (historical records of EEcA) and burned every 1–3 years, as the traditional livestock system in the whole Cerrado biome [38]. Since 1959, cattle were excluded and the vegetation has been protected from fire [29]. The protected vegetation has changed in structure over the last few decades, with a remarkable increase in biomass after fire suppression, detected by aerial photographs and satellite images from 1962 to 2006 [31]. Grasslands with no trees covered 21.3% of the area in 1962, but did not exist in 2006, while the cerradão (forest-type vegetation) expanded from 53 to 91.4% of the protected area in the same time period. In the last survey [54], a tree biomass gradient ranging from cerrado stricto sensu (savannah) to the cerradão was still apparent, with the tree community varying in density (from 860 to 1800 individuals ha−1), basal area (5.2 to 23.4 m2 ha−1), mean height (2.9 to 12.6 m) and canopy cover (42.8 to 90.0%). The ground is covered by a sparse layer of small shrubs, forbs and grasses in the lower biomass vegetation, which has been gradually replaced by a litter layer as biomass increases (electronic supplementary material, figure S1). This replacement has been observed, although not quantified, over the 5-year period of monitoring.
(b). Vegetation sampling
Changes in the vegetation in the EEcA have been monitored on the ground since 2006 by means of a network of 30 permanent plots. In this study, the biomass gradient from open canopied cerrado stricto sensu to closed canopied cerradão was represented by 14 plots (20 × 50 m each, subdivided into 10 subplots of 10 × 10 m), that were randomly distributed over the whole protected area. The maximum and minimum distances between plots were 3 km and 50 m, respectively. In each plot, all living trees with diameter at breast height—dbh ≥ 5 cm were measured between January and April 2006, and again between January and April 2011. From these data, the community attributes, tree density and tree basal area (TBA), were obtained on the two occasions (electronic supplementary material, table S1), to quantify the encroachment over the 5-year period in each plot.
(c). Rainfall partitioning
The rainfall regime over the study period was characterized on the basis of data obtained from the nearest climatological station [55], located at distances ranging from 2 to 4 km from the sampled plots. Throughfall and stemflow in each sample unit were quantified as proportions related to the gross precipitation, simultaneously collected. We recorded the gross precipitation from the automatic station and, additionally, in nine funnel precipitation gauges (pluviometers), positioned around the sampling plots, following rules recommended by Helvey & Patric [56]. We installed the aluminium funnels, each with a catchment area of 165 cm2 and an 8 cm high sloping edge, at 1 m above the ground surface (electronic supplementary material, figure S2). From September 2010 to January 2012, we sampled on 61 occasions. Each sampling occasion, as defined by Ziegler [57], comprised one or more consecutive rain events. A rainfall event was defined as a measurable amount of rainfall separated from the previous or the next rainfall input by the time required for the foliage and trunks to dry [58]. For the purpose of this study, after field observations, we established this interval to be 5 h. On many occasions, the rain volume varied considerably and randomly among the pluviometers in open areas. To avoid the influence of this variation on the analyses, we selected 26 occasions when gross precipitation was spatially homogeneous (coefficient of variation <15%) and represented the local variation in rain intensities (distribution of rain volumes and intensities at electronic supplementary material, figure S3). The gross precipitation used in the analysis was obtained by the sum of the 26 average values (volumes transformed in millimetres) from the 10 pluviometers in open areas (electronic supplementary material, table S2). When one or more data points were missed, the average for one occasion was calculated among the other pluviometers.
To quantify the throughfall in each 1000 m2 plot, we installed 10 pluviometers, one in each subplot of 100 m2, moving them periodically at random [56,59,60]. The pluviometers used to collect the throughfall were installed at least 15 cm from the closest stem, to avoid heavy run-off from the trunks and branches [59]. The total volume of throughfall collected in each plot was quantified by the sum of the 26 average values (volumes transformed in millimetres) among the 10 pluviometers [60] (electronic supplementary material, table S2). When one or more pluviometer data points were missed, the average for one event was calculated among the other pluviometers in the same plot. The proportion of throughfall in each plot (Ptf%) was obtained by dividing the total throughfall in the plot (sum of 26 average values) by the average value of total gross precipitation obtained from the 10 pluviometers at open areas on the 26 occasions (following the method in [60]).
Stemflow was quantified at the community level (see [61]) in eight subplots (100 m2 each), one inside each plot, representing the biomass gradient (on the basis of TBA). All trees within each subplot were sampled from March 2011 to January 2012, on 17 occasions (electronic supplementary material, table S3), totalling 102 trees. A polyurethane collector (see [62]) was fixed to each stem at a height of 1.3 m above ground (electronic supplementary material, figure S2), channelling the stemflow to a container. On the occasions when the volume of stemflow exceeded the capacity of the container, we estimated the missing values as follows: we obtained a linear regression for each individual tree using all data collected for that tree, where the stemflow was the response variable and the gross rainfall was the predictor variable [58]. The total volume of stemflow collected from all trees within the subplot was transformed in millimetres, considering that this volume corresponded to the amount of rainfall running down the stems over an area of 100 m2 in that period [61]. From the division of this value by the gross precipitation in the same period, we obtained the proportion of rain over a plot reaching the ground running down the stems in each subplot—stemflow (Psf%).
The net rainfall in this study was represented by the sum of throughfall and stemflow, expressed as percentages of gross rainfall. The difference between the gross rainfall and the net rainfall corresponds to the rain interception by the canopies [56,58,60].
(d). Data analyses
Tree density and TBA were calculated for each plot (and subplot) in 2006 and 2011. The encroachment over the 5-year period was quantified as the difference between the two surveys in each plot, presented as histograms. The annual rate of encroachment for each variable was calculated by transforming the absolute increase in a percentage related to the value recorded in 2006, divided by 5 years. The average value and standard deviation for the whole study area are presented.
The sampling unit for modelling throughfall was the 1000 m2 plot and for stemflow was a 100 m2 subplot. For modelling the hydrological processes, we used the community data from 2011, the year that we started sampling rainfall. Tree density and TBA were assessed as possible predictor variables for the hydrological processes, representing the vegetation structure and biomass. The highest correlation coefficients with the response variables were obtained when using TBA, the predictor variable in this study. We used linear regression to predict the throughfall (Ptf%) and the stemflow (Psf%) as a function of TBA. Analyses were performed using the software Statistica v. 7.0 [63].
3. Results
(a). Changes in the vegetation structure
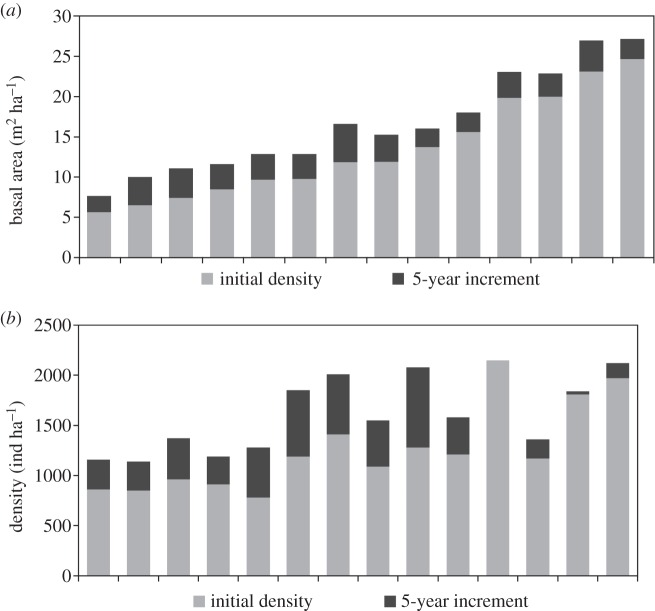
TBA increased in the study site at an average rate of 5.7% (±2.7%) per year. TBA increased in all plots with absolute values of change ranging from 2.04 to 4.77 m2 ha−1 over a 5-year period (figure 1a). No relationship was found between the initial basal area and the extent of change in basal area, indicating that the biomass accumulation over 5 years was not influenced by the initial biomass. Density increased in 13 from the 14 plots (figure 1b), at an average rate for the whole study site of 6.7% (±4.0%) per year. Density did not change in the plot with the highest density in the first survey. The greatest increase was 800 trees ha−1 in 5 years (12.8% per year) in a plot with an initial TBA of 9.8 m2 ha−1 and initial density of 1280 ind ha−1.
Figure 1.
Changes over a 5-year period in the Cerrado vegetation protected from fire: tree basal area (a) and density of trees from 5 cm dbh (b). Bars correspond to fourteen 1000 m2 plots of native vegetation sampled for this study in 2006 and 2011, ordered from the left in increasing plot basal area.
(b). Relationships between hydrological processes and tree basal area
Throughfall estimation was based on the total gross rainfall of 908.5 mm, collected on 26 occasions and corresponding to 73 rain events. Stemflow was quantified as a proportion of the total gross rainfall of 583.1 mm recorded on 17 occasions, corresponding to 41 rain events.
Throughfall (Ptf%) varied across the biomass gradient from 75.4% of the gross rainfall in the plot covered by the closed canopy vegetation to 97.2% in the plot covered by the more open canopy vegetation, therefore being linearly and negatively correlated with the tree biomass (figure 2a). The higher the TBA, the lower the proportion of rainfall falling below the canopy. TBA explained 75% of the variation in the proportion of throughfall across the biomass gradient in the Cerrado vegetation (Ptf% = 102.82–0.94 TBA; r2 = 0.75, p < 0.001).
Figure 2.
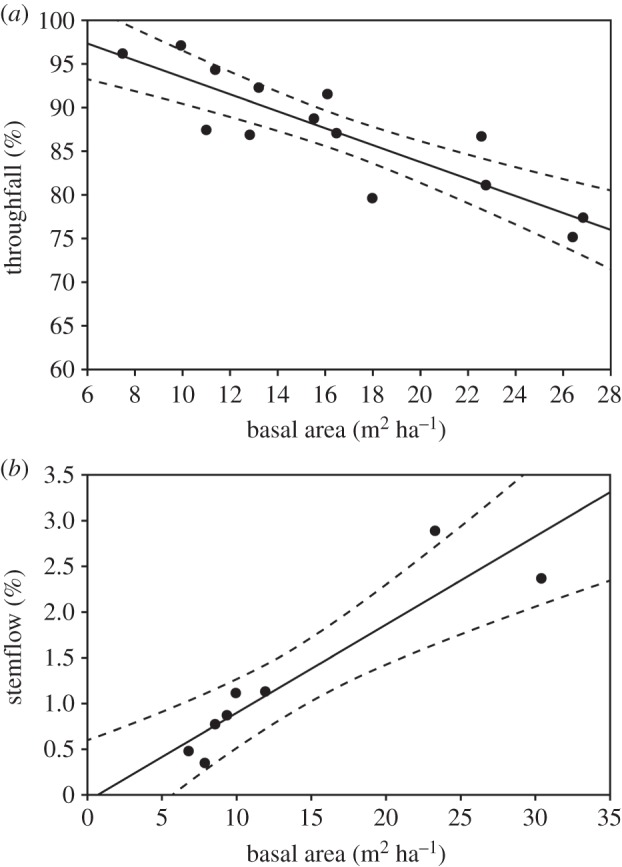
Linear regressions representing changes in throughfall (a) and stemflow (b) as tree basal area increases across the biomass gradient of the Cerrado vegetation. Dashed lines represent the 95% confidence interval.
Stemflow (Psf%) ranged from 0.3% of the gross rainfall in the subplot covered by the more open canopy vegetation to 2.9% in the subplot with the more closed canopy vegetation, showing a linear and positive correlation with tree biomass (figure 2b). The higher the biomass the higher the proportion of rainfall running down the stems; TBA explained 84% of the variation in Psf% across the biomass gradient in the Cerrado vegetation (Psf% = −0.07 + 0.10 TBA; r2 = 0.84, p = 0.002).
Throughfall and stemflow presented opposite tendencies. As stemflow corresponded to a very small proportion of gross rainfall, it had a negligible influence on the estimation of net rainfall. Therefore, the model obtained from the sum of the two previous regression equations indicates a negative influence of TBA on the net rainfall Pn% (Pn% = 102.73–0.85 TBA).
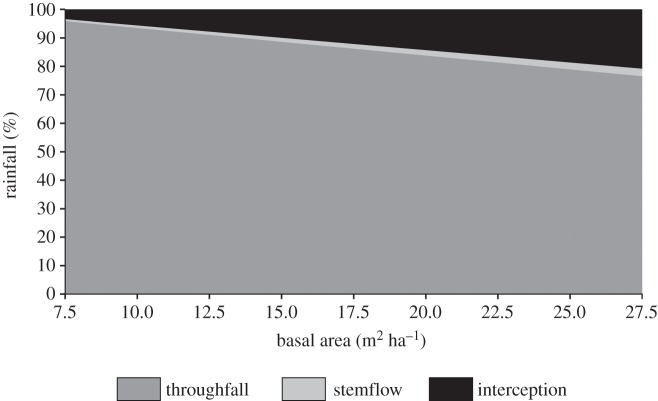
We found the partitioning of rainfall gradually changed as tree biomass increased (figure 3). The proportion of rain reaching the ground (throughfall + stemflow) under the more open cerrado stricto sensu vegetation in the sampled area (TBA of 7.5 m2 ha−1) corresponded to 96.6% of gross rainfall, while this proportion was 79.7% for the densest cerradão (TBA of 27 m2 ha−1). Interception by the canopies, therefore, was 3.4% of rain in the cerrado stricto sensu and 20.3% in the cerradão. Considering the rate of woody encroachment recorded, we estimated an annual increase in rain interception for the whole study site of 0.6% (±0.1%) of the gross rainfall per year.
Figure 3.
Partitioning of rainfall (throughfall, stemflow and interception by the canopies) across the biomass gradient of Cerrado vegetation, represented by tree basal area.
4. Discussion
An increase in tree density and biomass has been observed in savannahs in different regions and continents. The causes of woody encroachment vary among regions and ecosystems, but some major factors are already known to favour colonization of grassy biomes by woody species. Even though encroachment can have multiple causes that are not clearly understood, at least some of the effects are clear and predictable, including a direct increase in carbon storage and decrease in water production at the level of watersheds [13–15,49]. In this study, we demonstrated that the amount of rainfall intercepted by the canopies is also predictable. We documented the woody encroachment of Cerrado vegetation due to fire protection and quantified the direct effects of the encroachment on rain interception by the canopies. We modelled throughfall, stemflow, net rainfall and rain interception by the canopies on the basis of TBA across the biomass gradient from open savannah to closed savannah–forest vegetation. We also assessed tree density as a predictor of hydrological changes, but this community attribute did not relate well to throughfall or stemflow, while basal area, a precise and easily obtainable variable, provided robust models. We found a remarkable increase in the proportion of rain intercepted as a consequence of woody encroachment. This ‘missing’ rain is substantial given that the potential evapotranspiration in the region is estimated at 77% of annual rainfall [52] and only the excess water will recharge groundwater reserves and provide streamflow. Classical studies on forest management have demonstrated the strong relationship between tree biomass and hydrological processes [16], and an increase in tree biomass in periodically dry ecosystems has been reported to cause remarkable decrease in streamflow [8,13,16,18]. Rain interception by the canopies, therefore, has the potential to exert a strong influence on the ground water recharge and streamflow at the scale of a watershed.
(a). Woody encroachment
Tree density and also TBA are rapidly increasing at the study site, at rates slightly above those mentioned in other studies documenting woody encroachment in the Cerrado (e.g. [30,33]). Fire suppression or decreasing fire frequency have been reported as the main explanation for woody encroachment in Cerrado vegetation across Brazil [29–33,64], with frequent fires, regardless of season, been shown to reduce woody biomass [65]. Changes over time in density and TBA in our study differed: TBA increased in the cerradão plots at similar rates as those of the cerrado stricto sensu, but density stabilized around 2000 ind ha−1, corresponding to a basal area around 23 m2 ha−1, and after that, mortality equalled recruitment, resulting in no significant changes in density over 5 years. The larger trees kept growing, accumulating carbon and probably increasing the roughness of the canopy surface, which leads to increase in rain interception [58]. Eventually, the community structure instead resembles a tropical forest, and the floristic composition switches from endemic Cerrado species to generalists or shade-tolerant forest species, as demonstrated by previous studies [30,33,54]. In the long term, due to competition, the community will probably be composed of even larger trees at lower densities. We document that changes in the vegetation have been followed by hydrological changes, as previously observed in other regions [8,13,18].
(b). Hydrological processes in the biomass gradient
The stemflow ranged from 0.3 to 2.9% of the total rainfall and increased with gains in woody biomass, due to increase in number and size of tree stems channelling water to the ground per area unit. The amount of water running down the stems was five-times higher in the high biomass vegetation compared with the low biomass vegetation. The proportions of stemflow recorded in this study are among the lowest already documented for different vegetation types around the world [22,25,58–61]. The low efficiency of Cerrado trees in channelling the rain falling over their canopies to the ground through their stems is linked to functional traits such as stem tortuosity and inclination or thick bark, common attributes of Cerrado trees that are negatively correlated with stemflow efficiency [66].
Within the biomass gradient analysed, throughfall varied from low values similar to those recorded in temperate ecosystems up to values as high as those observed in tropical regions [67]. Even though net rainfall encompasses both throughfall and stemflow, the low contribution of the latter, as observed in numerous ecosystems around the world [67] does not change the trend for decreasing water reaching the ground as tree biomass increases. The expected compensatory effect between the two processes that could result in similar values of net rainfall independent of the biomass [58,59] was not observed in this study. The net rainfall in the Cerrado vegetation is, therefore, almost entirely determined by the throughfall.
Rain interception by the canopies in this study ranged from 3.4% of the gross rainfall in the open canopied to 20.3% in the closed canopied savannah. We did not quantify ground interception. In addition to water retained by canopy, additional rain is probably retained by small plants or litter across the biomass gradient in the Cerrado vegetation, which would result in an even lower proportion of rain reaching the soil. Rain interception by the canopies in this study was lower than values recorded in different forest ecosystems around the world, which were mostly above 20% [50]. If these values, however, are analysed under the encroachment perspective, when an open Cerrado vegetation turns into a forest after fire suppression (or due to other causes), the amount of water retained by the canopy is six-times higher than the historical value, increasing at a rate of 0.6% of total rainfall per year. Most hydrological studies do not separate this arrested water from that used by plants [8,16,18,47], which returns to the atmosphere as transpiration, because it neither recharges groundwater reserves or becomes streamflow. Transpiration and rain interception have, however, completely different ecological meanings [51]. While transpiration is a function performed by trees taking up water from the soil and has ecophysiological constraints, rain interception is a physical process of the water evaporating from the leaf surface without being used by plants, dependent only on environmental conditions [17]. The amount of rain intercepted by the canopies over a year, thus, can be considered as a direct decrease in annual rainfall, with consequences for the ecological processes and hydrological fluxes in a watershed. Given that water returning to the atmosphere as vapour generally does not become rain in the same location where it evaporates [27], both increasing rain interception and transpiration result in reduced streamflow [8,13,18].
(c). The robust relationship between long-term rain interception and tree basal area
Within the range of the TBA analysed in this study, we estimated that as TBA increases by 1 m2 ha−1, there will be an increase in 0.9% in the proportion of rainfall retained by the canopies of Cerrado vegetation. Studies have demonstrated a consistent increase in throughfall as woody biomass decrease. For example, thinning of Pinus halepensis plantations in Valencia, Spain, resulted in an increase of throughfall in 1%, as TBA decreases by 1 m2 ha−1 [25], and data from Indonesia show an increase of about 0.8% in throughfall, as TBA decreased by 1 m2 ha−1 [22]. Despite most studies [22,26] being wary about making predictions in other systems, we noted a remarkable consistency of the relationship between increase in TBA and the corresponding decrease in throughfall in different studies and across vegetation types: a narrow range of 0.8 to 1.0% less net rainfall as biomass of a particular vegetation type increases in 1 m2 ha−1. This pattern may be very informative when predicting the hydrological consequences of changes in vegetation biomass across the world. The strong and consistent relationship between rain interception and tree biomass supports the recommendation [25] of using dendrometrical variables to inform forest management aiming at water production, and TBA arises as a good predictor from this and other studies [22,25,26].
Easily obtained, globally comprehensible, precise and not dependent on destructive sampling, TBA can be a robust predictor of throughfall, stemflow and rain interception at the community level, all crucial processes when elucidating and quantifying the influences of vegetation on rainfall partition. The recommendation of TBA as a predictor variable for hydrological processes arises after decades of leaf area index (LAI) being almost universally used as the community attribute to predict rainfall partition, because this is, undeniably, the variable most clearly related to rain interception and transpiration [8,22,25,58]. However, even though LAI can be essential for studies focused on hydrological processes in individual trees, it is influenced by many different factors, such as variability in space, time [22–24,58,68] and species composition [22,58,68]. These factors reduce the predictive power of LAI for a community as a whole or for long periods (see, for example, [22]). Most criticisms of LAI as a predictor variable for hydrological processes are valid also for canopy cover. This descriptor of vegetation also varies with phenological seasonality, and has low precision, because the different methods of estimation (e.g. line interception, densitometers, hemispherical photographs) result in different values for the same plant community.
(d). Consequences of woody encroachment on the water cycle
The inverse relationship between tree biomass and streamflow is well known, on local [13,18] or regional scales [47]. Among the consequences of woody encroachment in savannahs and grasslands is, therefore, the decrease in water productivity [8,20]. A decrease in water production as tree biomass increases is a direct consequence of increasing rain interception and transpiration.
Understanding the relationships between tree biomass, transpiration, interception and streamflow has provided opportunities to predict change in water production in response to management interventions [12,19]. Conflicts related to water use due to trade-offs among different demands (human or cattle consumption, industry, hydroelectricity and irrigation) can be aggravated if woody encroachment or afforestation cause biomass increases, probably culminating in total suppression of water supply in drier regions where the average annual precipitation does not surpass 1000 mm [13]. Hydrological responses to management interventions to avoid woody encroachment or to reduce TBA (e.g. prescribed fires or thinning) are, however, more relevant in regions under mesic conditions, where rainfall surpasses evapotranspiration only in part of the year. Management interventions are likely to be more effective if soils are deep and well drained, capable of storing the excess of water falling during the rainy season that supplies perennial springs and rivers in the dry season [12,19]. These are the dominant environmental conditions in the Cerrado biome.
The shift from savannah or grassland to forest vegetation poses a trade-off between two highly relevant ecosystem services—carbon sequestration and water supply [13–15]. While there is a carbon market, because carbon sequestration benefits the whole world, there is no value attached to water provisioning, a critical ecosystem service that has a much higher local value than carbon sequestration [13–15]. Besides the effects on water provisioning, woody encroachment has indirect consequences on ecosystem functioning [1,15] and biodiversity [10,35,36]. Changes in the amount of rain reaching the ground have the potential to cause habitat changes by decreasing soil water availability for plants, soil fauna and microorganisms.
Re-establishing disturbance regimes to maintain the historical vegetation structure and avoiding afforestation are urgent and strongly recommended strategies to prevent the negative consequences of woody biomass increase on the water resources in the savannahs. As fire is the most relevant disturbance conditioning the existence of savannahs and grasslands in different regions of the world [3,41,44,69], prescribed burning is among the strategies to maintain the hydrological regimes in watersheds occupied by savannahs and grasslands, especially when and where water supply is the first priority among ecosystem services.
Supplementary Material
Acknowledgements
We are grateful to field assistants E. A. Berto, E. Damasceno and L. C. Malícia, and to interns L. M. T. Aparecido, D. B. Borges, C. B. Jardim, K. D. Spera, D. Schievenin and D. Narita for their help during our extensive fieldwork, and D. Dias for improving the quality of the figures. We thank also the editors of this special issue, Kate Parr and Caroline Lehmann, the four anonymous reviewers, and Adam Pellegrini for their constructive comments on the manuscript.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
G.D. and E.A.H. designed research, collected and analysed data and wrote the paper.
Competing interests
We have no competing interests.
Funding
G.D. thanks the Conselho Nacional de Desnvolvimento Científico e Tecnológico—CNPq, for the productivity grant (≠303402/2012-1).
References
- 1.Archer S, Boutton TW, Hibbard KA. 2001. Trees in grasslands: biogeochemical consequences of woody plant expansion. In Global biogeochemical cycles in the climate system (eds Schulze ED, Harrison S, Heiman M, Holland E, Lloyd J, Prentice I, Schimel D), pp. 115–133. San Diego, CA: Academic Press. [Google Scholar]
- 2.Bowman DMJS, Walsh A, Milne DJ. 2001. Forest expansion and grassland contraction within a Eucalyptus savanna matrix between 1941 and 1994 at Litchfield National Park in the Australian monsoon tropics. Glob. Ecol. Biogeogr. 10, 535–548. ( 10.1046/j.1466-822X.2001.00252.x) [DOI] [Google Scholar]
- 3.Roques KG, O'Connor TG, Watkinson AR. 2001. Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 38, 268–280. ( 10.1046/j.1365-2664.2001.00567.x) [DOI] [Google Scholar]
- 4.Kulmatiski A, Beard KH. 2013. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Change 3, 833–837. ( 10.1038/nclimate1904) [DOI] [Google Scholar]
- 5.Tng DY, Murphy BP, Weber E, Sanders G, Williamson GJ, Kemp J, Bowman DM. 2011. Humid tropical rain forest has expanded into eucalypt forest and savanna over the last 50 years. Ecol. Evol. 2, 34–45. ( 10.1002/ece3.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchard ETA, Flintrop CM. 2013. Woody encroachment and forest degradation in sub-Saharan Africa's woodlands and savannas 1982–2006. Phil. Trans. R. Soc. B 368, 20120406 ( 10.1098/rstb.2012.0406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond WJ, Midgley GF. 2012. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Phil. Trans. R. Soc. B 367, 601–612. ( 10.1098/rstb.2011.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Maitre DC, Gush MB, Dzikiti S. 2015. Impacts of invading alien plant species on water flows at stand and catchment scales. AoB Plants 7, plv043. ( 10.1093/aobpla/plv043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankaran M, Ratnam J, Hanan N. 2008. Woody cover in African savannas: the role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 17, 236–245. ( 10.1111/j.1466-8238.2007.00360.x) [DOI] [Google Scholar]
- 10.Andersen AN, Hertog T, Woinarski JC. 2006. Long-term fire exclusion and ant community structure in an Australian tropical savanna: congruence with vegetation succession. J. Biogeogr. 33, 823–832. ( 10.1111/j.1365-2699.2006.01463.x) [DOI] [Google Scholar]
- 11.Ratajczak Z, Nippert JB, Collins SL. 2012. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93, 697–703. ( 10.1890/11-1199.1) [DOI] [PubMed] [Google Scholar]
- 12.Huxman T, et al. 2005. Ecohydrological implications of woody plant encroachment. Ecology 86, 308–319. ( 10.1890/03-0583) [DOI] [Google Scholar]
- 13.Jackson RB, et al. 2005. Trading water for carbon with biological carbon sequestration. Science 30, 1944–1947. ( 10.1126/science.1119282) [DOI] [PubMed] [Google Scholar]
- 14.Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN. 2014. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 1–9. ( 10.1016/j.tree.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 15.Veldman JW, et al. 2015. Where tree planting and forest expansion are bad for biodiversity and ecosystem services. BioScience 65, biv118. ( 10.1093/biosci/biv118) [DOI] [Google Scholar]
- 16.Bosch JM, Hewlett JD. 1982. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J. Hydrol. 55, 3–23. ( 10.1016/0022-1694(82)90117-2) [DOI] [Google Scholar]
- 17.Calder IR. 1998. Water use by forests, limits and controls. Tree Physiol. 18, 625–631. ( 10.1093/treephys/18.8-9.625) [DOI] [PubMed] [Google Scholar]
- 18.Brown AE, Zhang L, McMahon TA, Western AW, Vertessy RA. 2005. A Review of paired catchment studies for determining changes in water yield resulting from alterations in vegetation. J. Hydrol. 310, 28–61. ( 10.1016/j.jhydrol.2004.12.010) [DOI] [Google Scholar]
- 19.Doody TM, Nagler PL, Glenn EP, Moore GW, Morino K, Hultine KR, Benyon RG. 2011. Potential for water salvage by removal of non-native woody vegetation from dryland river systems. Hydrol. Process. 25, 4117–4131. ( 10.1002/hyp.8395) [DOI] [Google Scholar]
- 20.Zou CB, Turton DJ, Will RE, Engle DM, Fuhlendorf SD. 2014. Alteration of hydrological processes and streamflow with juniper (Juniperus virginiana) encroachment in a mesic grassland catchment. Hydrol. Process. 28, 6173–6182. ( 10.1002/hyp.10102) [DOI] [Google Scholar]
- 21.Zhang L, Dawes WR, Walker GR. 2001. Response of mean annual evapotranspiration to vegetation changes at catchment scale. Water Resour. Res. 37, 701–708. ( 10.1029/2000WR900325) [DOI] [Google Scholar]
- 22.Dietz J, Hölscher D, Leushner C, Hendrayanto H. 2006. Rainfall partitioning in relation to forest structure in differently managed montane forest stands in Central Sulawesi, Indonesia. Forest Ecol. Manage. 237, 170–178. ( 10.1016/j.foreco.2006.09.044) [DOI] [Google Scholar]
- 23.Bucci SJ, Scholz FG, Goldstein G, Hoffmann WA, Meinzer FC, Franco AC, Giambellucca T, Miralles-Wolhelm F. 2008. Controls on stand transpiration and soil water utilization along a tree density gradient in a Neotropical savanna. Agric. Forest Meteorol. 148, 839–849. ( 10.1016/j.agrformet.2007.11.013) [DOI] [Google Scholar]
- 24.Giambelluca TW, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Hoffmann WA, Franco A, Buchert M. 2009. Evapotranspiration and energy balance of Brazilian savannas with contrasting tree density. Agric. Forest Meteorol. 149, 1365–1376. ( 10.1016/j.agrformet.2009.03.006) [DOI] [Google Scholar]
- 25.Molina AJ, Del Campo AD. 2012. The effects of experimental thinning on throughfall and stemflow: a contribution towards hydrology-oriented silviculture in Aleppo pine plantations. Forest Ecol. Manage. 269, 206–213. ( 10.1016/j.foreco.2011.12.037) [DOI] [Google Scholar]
- 26.Del Campo AD, Fernandes TJ, Molina AJ. 2014. Hydrology-oriented (adaptive) silviculture in a semiarid pine plantation: how much can be modified the water cycle through forest management? Eur. J. Forest Res. 133, 879–894. ( 10.1007/s10342-014-0805-7) [DOI] [Google Scholar]
- 27.Eltahir EAB, Bras RL. 1996. Precipitation recycling. Rev. Geophys. 34, 367–378. ( 10.1029/96RG01927) [DOI] [Google Scholar]
- 28.Hoffmann WA, Jackson RB. 2000. Vegetation–climate feedbacks in the conversion of tropical savanna to grassland. J. Clim. 13, 1593–1602. ( 10.1175/1520-442(2000)013%3C1593:VCFITC%3E2.0.CO;2) [DOI] [Google Scholar]
- 29.Moreira AG. 2000. Effects of fire protection on savanna structure in Central Brazil. J. Biogeogr. 27, 1021–1029. ( 10.1046/j.1365-2699.2000.00422.x) [DOI] [Google Scholar]
- 30.Mews HA, Marimon BS, Maracahipes L, Franczak DD, Marimon-Junior BH. 2011. Dinâmica da comunidade lenhosa de um Cerrado Típico na região Nordeste do Estado de Mato Grosso, Brasil. Biota Neotrop. 11, 73–82. ( 10.1590/S1676-06032011000100007) [DOI] [Google Scholar]
- 31.Pinheiro ES, Durigan G. 2009. Dinâmica espaço-temporal (1962–2006) das fitofisionomias em unidade de conservação do Cerrado no sudeste do Brasil. Rev. Bras. Bot. 32, 441–454. ( 10.1590/S0100-84042009000300005) [DOI] [Google Scholar]
- 32.Oliveira AP, Schiavini I, Vale VS, Lopes SF, Arantes CS, Gusson AE, Prado Junior JA, Dias-Neto OC. 2014. Mortality, recruitment and growth of the tree communities in three forest formations at the Panga Ecological Station over ten years (1997–2007). Acta Bot. Bras. 28, 234–248. ( 10.1590/S0102-33062014000200010) [DOI] [Google Scholar]
- 33.Roitman I, Felfili JM, Rezende AV. 2008. Tree dynamics of a fire-protected cerrado sensu stricto surrounded by forest plantations, over a 13-year period (1991–2004) in Bahia, Brazil. Plant Ecol. 197, 255–267. ( 10.1007/s11258-007-9375-9) [DOI] [Google Scholar]
- 34.Assis ACC, Coelho RM, Da Silva Pinheiro E, Durigan G. 2011. Water availability determines physiognomic gradient in an area of low-fertility soils under Cerrado vegetation. Plant Ecol. 212, 1135–1147. ( 10.1007/s11258-010-9893-8) [DOI] [Google Scholar]
- 35.Coutinho LM. 1990. Fire in the ecology of the Brazilian Cerrado. In Fire in the tropical biota—ecosystem processes and global challenges (ed. Goldammer JG.), pp. 82–105. Berlin, Germany: Springer. [Google Scholar]
- 36.Durigan G, Ratter JA. 2016. The need for a consistent fire policy for Cerrado conservation. J. Appl. Ecol. 53, 11–15. ( 10.1111/1365-2664.12559) [DOI] [Google Scholar]
- 37.Ramos-Neto MB, Pivello VR. 2000. Lightning fires in a Brazilian savanna national park: rethinking management strategies. Environ. Manage. 26, 675–684. ( 10.1007/s002670010124) [DOI] [PubMed] [Google Scholar]
- 38.Dias BFS. 2006. Degradação ambiental: os impactos do fogo sobre a biodiversidade do Cerrado. In Dimensões humanas da biodiversidade: o desafio de novas relações sociedade natureza no século XXI (eds Garay I, Becker B), pp. 187–188. Petrópolis, Brazil: Vozes. [Google Scholar]
- 39.Durigan G, Siqueira MF, Franco GADC. 2007. Threats to the Cerrado remnants of the state of São Paulo, Brazil. Sci. Agric. 64, 355–363. ( 10.1590/S0103-90162007000400006) [DOI] [Google Scholar]
- 40.Adámoli J, Macêdo J, Azevedo LG, Madeira Netto J. 1986. Caracterização da região dos Cerrados. In Solos dos Cerrados—Tecnologias e estratégias de manejo (ed. Goedert WJ.), pp. 33–74. Brasília, Brazil: EMBRAPA–CEPAC. [Google Scholar]
- 41.Lehmann CE, et al. 2014. Savanna vegetation–fire–climate relationships differ among continents. Science 343, 548–552. ( 10.1126/science.1247355) [DOI] [PubMed] [Google Scholar]
- 42.Oliveira PTS, Nearing MA, Moran MS, Goodrich DC, Wendland E, Gupta HV. 2014. Trends in water balance components across the Brazilian Cerrado. Water Resour. Res. 50, 7100–7114. ( 10.1002/2013WR015202) [DOI] [Google Scholar]
- 43.Cabral OM, da Rocha HR, Gash JH, Freitas HC, Ligo MA. 2015. Water and energy fluxes from a woodland savanna (Cerrado) in southeast Brazil. J. Hydrol: Reg. Stud. 4, 22–40. ( 10.1016/j.ejrh.2015.04.010) [DOI] [Google Scholar]
- 44.Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232. ( 10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 45.Sano EE, Rosa R, Brito JL, Ferreira LG. 2010. Land cover mapping of the tropical savanna region in Brazil. Environ. Monit. Assess. 166, 113–124. ( 10.1007/s10661-009-0988-4) [DOI] [PubMed] [Google Scholar]
- 46.Overbeck GE, et al. 2015. Conservation in Brazil needs to include non-forest ecosystems. Divers. Distrib. 21, 1455–1460. ( 10.1111/ddi.12380) [DOI] [Google Scholar]
- 47.Costa MH, Botta A, Cardille JA. 2003. Effects of large-scale changes in land cover on the discharge of the Tocantins River, Amazonia. J. Hydrol. 283, 206–217. ( 10.1016/S0022-1694(03)00267-1) [DOI] [Google Scholar]
- 48.Hayhoe SJ, Neill C, Porder S, McHorney R, LeFebvre P, Coe MT, Eisenbeer H, Krusche AV. 2011. Conversion to soy on the Amazonian agricultural frontier increases streamflow without affecting stormflow dynamics. Glob. Change Biol. 17, 1821–1833. ( 10.1111/j.1365-2486.2011.02392.x) [DOI] [Google Scholar]
- 49.Nosetto M, Jobbagy EG, Paruelo J. 2005. Land-use change and water losses: the case of grassland afforestation across a soil textural gradient in central Argentina. Glob. Change Biol. 11, 1101–1117. ( 10.1111/j.1365-2486.2005.00975.x) [DOI] [Google Scholar]
- 50.Carlyle-Moses DE, Gash JH. 2011. Rainfall interception loss by forest canopies. In Forest hydrology and biogeochemistry (eds Levia DF, Carlyle-Moses D, Ttanaka T), pp. 407–423. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 51.Savenije HHG. 2004. The importance of interception and why we should delete the term evapotranspiration from our vocabulary. Hydrol. Process. 18, 1507–1511. ( 10.1002/hyp.5563) [DOI] [Google Scholar]
- 52.Sentelhas PC, Marin FR, Ferreira AS, Sá EJS. 2003. Banco de Dados Climáticos do Brasil. See http://www.bdclima.cnpm.embrapa.br (accessed 26 March 2016).
- 53.Assad ED, Evangelista BA. 1994. Análise frequencial da precipitação pluviométrica. In Chuva nos Cerrados: análise e espacialização (coord. Assad ED.), pp. 25–42. Brasilia, Brazil: EMBRAPA-CPAC. [Google Scholar]
- 54.Pinheiro ES, Durigan G. 2012. Diferenças florísticas e estruturais entre fitofisionomias do Cerrado em Assis, SP, Brasil. Rev. Árvore 36, 181–193. ( 10.1590/S0100-67622012000100019) [DOI] [Google Scholar]
- 55.CIIAGRO-Centro Integrado de Informações Agrometeorológicas. Dados diários de chuva no período de 01/01/1991 a 31/08/2014. See http://www.ciiagro.sp.gov.br (accessed 9 September 2014).
- 56.Helvey J, Patric JH. 1965. Canopy and litter interception of rainfall by hardwoods of Eastern United States. Water Resour. Res. 1, 193–206. ( 10.1029/WR001i002p00193) [DOI] [Google Scholar]
- 57.Ziegler AD, Giambelluca TW, Nullet MA, Sutherland RA, Tantasarin C, Vogler JB, Negishi JN. 2009. Throughfall in an evergreen-dominated forest stand in northern Thailand: comparison of mobile and stationary methods. Agric. Forest. Meteorol. 149, 373–384. ( 10.1016/j.agrformet.2008.09.002) [DOI] [Google Scholar]
- 58.Krämer I, Hölscher D. 2009. Rainfall partitioning along a tree diversity gradient in a deciduous old-growth forest in Central Germany. Ecohydrology 2, 102–114. ( 10.1002/eco.44) [DOI] [Google Scholar]
- 59.Rutter AJ. 1963. Studies in the water relations of Pinus sylvestris in plantation conditions I. Measurements of rainfall and interception. J. Ecol. 51, 191–203. ( 10.2307/2257513) [DOI] [Google Scholar]
- 60.Lloyd CR, Marques Filho AO. 1988. Spatial variability of throughfall and stemflow measurements in Amazonian rainforest. Agric. Forest. Meteorol. 42, 63–73. ( 10.1016/0168-1923(88)90067-6) [DOI] [Google Scholar]
- 61.Hanchi A, Rapp M. 1997. Stemflow determination in forest stands. Forest Ecol. Manage. 97, 231–235. ( 10.1016/S0378-1127(97)00066-2) [DOI] [Google Scholar]
- 62.Likens GE, Eaton JS. 1970. A polyurethane stemflow collector for trees and shrubs. Ecology 51, 938–939. ( 10.2307/1933996) [DOI] [Google Scholar]
- 63.STATSOFT INC. 2004. Statistica: data analysis software system-version 7.0.61.Tulsa.
- 64.Durigan G, Ratter JA. 2006. Successional changes in Cerrado and Cerrado/forest ecotonal vegetation in Western São Paulo State, Brazil, 1962–2000. Edinb. J. Bot. 63, 119–130. ( 10.1017/S0960428606000357) [DOI] [Google Scholar]
- 65.Sato MN, Miranda HS, Maia JMF. 2010. O fogo e o estrato arbóreo do Cerrado: efeitos imediatos e de longo prazo. In Efeitos do regime do fogo sobre a estrutura de comunidades de Cerrado: resultados do projeto fogo (org Miranda HS.), pp. 77–91. Brasília, Brazil: Ibama. [Google Scholar]
- 66.Honda EA, Mendonça AH, Durigan G. 2014. Factors affecting the stemflow of trees in the Brazilian Cerrado. Ecohydrology 8, 1351–1362. ( 10.1002/eco.1587) [DOI] [Google Scholar]
- 67.Levia DF, Frost EE. 2006. Variability of throughfall volume and solute inputs in wooded ecosystems. Prog. Phys. Geogr. 30, 605–632. ( 10.1177/0309133306071145) [DOI] [Google Scholar]
- 68.Šraj M, Brilly M, Mikoš M. 2008. Rainfall interception by two deciduous Mediterranean forests of contrasting stature in Slovenia. Agric. Forest Meteol. 148, 121–134. ( 10.1016/j.agrformet.2007.09.007) [DOI] [Google Scholar]
- 69.Bond WJ, Woodward FI, Midgley GF. 2005. The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–538. ( 10.1111/j.1469-8137.2004.01252.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.