Abstract
The interplay between adjacent transcription units can result in transcription-dependent alterations in chromatin structure or recruitment of factors that determine transcription outcomes, including the generation of intragenic or other cryptic transcripts derived from cryptic promoters. Mutations in a number of genes in Saccharomyces cerevisiae confer both cryptic intragenic transcription and the Suppressor of Ty (Spt-) phenotype for the lys2-128∂ allele of the LYS2 gene. Mutants that suppress lys2-128∂ allow transcription from a normally inactive Ty1 ∂ promoter, conferring a LYS+ phenotype. The arrangement of transcription units at lys2-128∂ is reminiscent of genes containing cryptic promoters within their open reading frames. We set out to examine the relationship between RNA Polymerase II (Pol II) activity, functions of Spt elongation factors, and cryptic transcription because of the previous observation that increased-activity Pol II alleles confer an Spt- phenotype. We identify both cooperating and antagonistic genetic interactions between Pol II alleles and alleles of elongation factors SPT4, SPT5, and SPT6. We find that cryptic transcription at FLO8 and STE11 is distinct from that at lys2-128∂, though all show sensitivity to reduction in Pol II activity, especially the expression of lys2-128∂ found in Spt- mutants. We determine that the lys2-128∂ Spt- phenotypes for spt6-1004 and increased activity rpo21/rpb1 alleles each require transcription from the LYS2 promoter. Furthermore, we identify the Ty1 transcription start site (TSS) within the ∂ element as the position of Spt- transcription in tested Spt- mutants.
Keywords: cryptic transcription, Ty1 element, transcription initiation, gene expression, transcription elongation
It has become increasingly clear that eukaryotic genomes are extensively and pervasively transcribed outside of coding regions. Furthermore, beyond pervasive transcription, which in many cases is suppressed at the RNA level by RNA processing and decay pathways (Jensen et al. 2013; Libri 2015), genomes encode the potential for additional transcription in the form of cryptic promoters and transcription units (Hennig and Fischer 2013; Smolle and Workman 2013). Some cryptic transcription units may have the potential to be regulated (expressed under certain conditions) (Cheung et al. 2008), while others may become apparent upon disruption of chromatin structure or nuclear pathways that suppress them (Kaplan et al. 2003; Rando and Winston 2012). A complete understanding of gene regulation will require incorporation of knowledge about the relationships between adjacent transcription units and how they influence each other. To this end, Saccharomyces cerevisiae has been a powerful system for understanding the pervasive transcription, cryptic transcription, and transcriptional interference that can occur among proximally located transcription units.
Now-classic genetic screens in S. cerevisiae have identified a number of general or widely used transcription factors through alterations in locus-specific gene expression. A paradigm for these types of genetic analyses has been the Suppressor of Ty (Spt) screens, which leveraged transposable-element mediated changes in gene expression at particular auxotrophic gene loci for the identification of genetic suppressors of transcription defects (Winston 1992). Spt screens were able to identify a conserved and essential general transcription factor (SPT15, encoding TBP) (Eisenmann et al. 1989; Hahn et al. 1989), members of transcriptional coactivator complexes (a number of SAGA subunits, a subunit of Mediator) (Winston et al. 1984, 1987; Fassler and Winston 1988, 1989; Gansheroff et al. 1995; Marcus et al. 1996; Roberts and Winston 1996; Grant et al. 1997), histones and histone chaperones (HTA1-HTB1, SPT6, and SPT16)(Clark-Adams and Winston 1987; Clark-Adams et al. 1988; Fassler and Winston 1988; Malone et al. 1991; Rowley et al. 1991), conserved elongation factors (SPT4 and SPT5), and factors regulating histone gene expression (HIR2, SPT10, and SPT21)(Natsoulis et al. 1991; Sherwood and Osley 1991). While not found in Spt screens, mutations in a number of other factors have the ability to confer Spt- phenotypes, indicating both the sensitivity and possible complexity of these phenotypes.
The major shared characteristic of disrupted genetic loci used for Spt- screens is that a Ty1 retroelement insertion disrupts normal gene expression while creating a compound transcription unit (Winston et al. 1984, 1987; Winston 1992). Compound transcription units are created because Ty1 elements contain both Ty1 promoter and terminator regions within the inserted Ty1 LTR DNA (known as a ∂ element). For one particular Spt allele, lys2-128∂ (the allele relevant to the work presented here), the Ty1 ∂ element is inserted within the LYS2 ORF, causing premature termination of LYS2 transcription, presumably at the Ty1 terminator, but also placing a Ty1 ∂ promoter in the path of LYS2 transcription (Winston et al. 1987). Spt- mutants that suppress lys2-128∂ allow transcription from within the Ty1 ∂ element to produce a 5′ truncated but functional LYS2 mRNA (Clark-Adams and Winston 1987; Swanson et al. 1991; Malone et al. 1993). This suppression results in the Lys+ phenotype for Spt- suppressor mutants of lys2-128∂, whereas WT lys2-128∂ strains are Lys-. This arrangement likely sensitizes Ty1 ∂ initiation to LYS2 transcription and transcription-mediated chromatin control. Naturally occurring compound transcription units containing transcribed promoter elements have been found in the yeast genome and many of these exhibit modulation by many factors (Martens et al. 2004, 2005; Hainer et al. 2011; Bird et al. 2006). Furthermore, widespread antisense transcription is being revealed as a mechanism for shaping gene regulation in a number of ways, including transcription over promoters (Pelechano et al. 2013; Castelnuovo et al. 2014; Nguyen et al. 2014; Murray et al. 2015).
Extensive molecular characterization of intragenic cryptic transcription and control of the yeast gene SER3 repression by transcription and transcription-dependent chromatin control (by the SRG1 noncoding RNA that overlaps the SER3 promoter) have revealed principles by which transcription units may regulate other transcription units. In the cases of SER3 and intragenic cryptic promoters, transcription over these regions (from SRG1 for SER3 and from regular gene promoters for cryptic promoters) establishes a chromatin structure that is refractory to initiation of the transcribed promoter (Kaplan et al. 2003; Martens et al. 2004, 2005; Hainer et al. 2011; Kaplan 2013). In the case of SER3, transcription is required for establishment of the inhibitory chromatin structure, but for some cryptic promoters, transcription under aberrant conditions is required to disrupt chromatin structure and allow cryptic transcription (Cheung et al. 2008). These relationships allow factors to participate in repression of cryptic or transcribed promoters that otherwise function in activation for other promoters. Relatedly, Spt transcription elongation factors (e.g., Spt4/Spt5, Spt6, and Spt16) are recruited almost universally to transcribed chromatin via Pol II (Andrulis et al. 2000; Kaplan et al. 2000; Saunders et al. 2003; Mayer et al. 2010), and have many attributes of positive factors. In contrast, initial genetic analyses of their function at Spt reporter genes indicated negative functions, because wild-type (WT) alleles of these genes are required to suppress cryptic Spt promoters.
Alteration of Pol II activity levels through active site mutations can have wide ranging effects on gene expression, including transcription start site selection, mRNA processing, termination, and overall transcription levels (reviewed in Kaplan 2013). Interestingly, a class of Pol II active site mutant that exhibits increased catalytic activity confers the Spt- phenotype for lys2-128∂ (Kaplan et al. 2008, 2012). These results suggested that lys2-128∂ Spt- phenotypes might be sensitive to Pol II activity, and that altered Pol II activity might contribute to cryptic transcription. We also were led to wonder how the Spt- phenotypes for Spt elongation factors relate to their functions during Pol II transcription. A direct relationship between their functions during Pol II transcription and regulation of Pol II activity might predict that some Spt- phenotypes could be a manifestation of loss of negative roles in Pol II activity (because increased Pol II activity correlates with the Spt- phenotype). Alternatively, phenotypes for distinct Spt factors may have different origins. To address these questions, we examined genetic interactions between a panel of Pol II alleles with known consequences for Pol II activity and alleles in spt4, spt5, and spt6, while probing the relationship between Pol II and cryptic transcription for FLO8, STE11, and lys2-128∂.
Materials and Methods
Yeast strains, plasmids, and media
Yeast strains and plasmids used in this study are found in Supplemental Material, Table S1 and Table S2, respectively. Please refer to Table S2 or (Jin and Kaplan 2014) for a note on the rpb1-N1082S (+T1161R) plasmid used in these studies. Yeast media were prepared according to standard protocols (Amberg et al. 2005), with modifications described in Kaplan et al. (2012). All yeast strains are isogenic to a GAL2+ derivative of S288C (Winston et al. 1995). Yeast media for the growth of cultures for RNA isolation for examination of lys2-128∂ transcription were either YPD, SC-Leu, or “Synthetic Defined” (SD), supplemented with appropriate amino acids/nucleobases (including lysine), as noted. Strains for the examination of lys2-128∂ in minimal media were amplified overnight in YPD, washed in sterile H2O, and aliquots resuspended in SC-Leu or supplemented SD and grown for around three doublings prior to harvesting. All other media for plate phenotyping and growth are as described in Jin and Kaplan (2014).
Heat maps for genetic interaction phenotypes
Phenotyping was as described in Jin and Kaplan (2014). Heatmaps and semiquantitative phenotype scoring were done as in Jin and Kaplan (2014). Briefly, growth on each medium was quantified using a 0–5 scoring scale (0 = no growth, 5 = WT growth for media except YPRafGal and SC-Lys; 0 = WT growth, 5 = growth of the mutant with maximum growth on the relevant YPRafGal or SC-Lys plate). Synthetic growth defects in Pol II and Spt- mutants on YPD and SC-Leu were calculated by subtracting the mutant growth score by the WT growth score; growth differences on phenotype medium were calculated by normalizing the growth difference on the phenotype plate to the corresponding control medium to obtain the net difference caused by phenotypes. For determination of suppression of spt5-242 by Pol II mutants at different temperatures, all mutants on YPD at different temperatures were normalized to WT on each corresponding plate by subtraction. Calculated score difference tables were turned into heatmaps using GENE-E (http://www.broadinstitute.org/cancer/software/GENE-E/index.html).
RNA isolation and northern blotting
RNA isolation and northern blotting were as previously described (Kaplan et al. 2012). Briefly, RNA isolation followed the method of Schmitt et al. (1990). Initial northern blotting utilized formaldehyde-containing RNA gels as in Kaplan et al. (2012), while later gels were run using BPTE buffer with no formaldehyde (Sambrook and Russell 2001). For these later gels, RNA samples were treated with glyoxal loading dye (Ambion) following the manufacturer’s instructions. Blotting was as in Kaplan et al. (2012). Northern probes were labeled using random priming (Decaprime II Random Priming kit, Ambion) following the manufacturer’s instructions. The SED1 probe was first amplified from yeast genomic DNA by PCR using oligonucleotide primers CKO468 and CKO469 (oligo sequences provided in Table S3). The LYS2 5′ end probe was amplified from LYS2+ genomic DNA (i.e., not lys2-128∂) using primers CKO551 and CKO552 so that the probe might hybridize to transcripts arising from the LYS2 promoter and lys2-128∂, as long as the respective transcripts contained sequence derived from within ∼2200 bp from the 5′ end of LYS2. Since the ∂ element of lys2-128∂ is inserted after position +159 [Figure S2, reported as +158 in Fassler and Winston (1988)] from the LYS2 ATG, this probe will detect transcripts from upstream of the ∂ and those emanating from the ∂ into LYS2.
5′ rapid amplification of cDNA ends (RACE), 3′ RACE, and primer extension analysis of lys2-128∂ transcripts
Primer extension followed the method of Ranish and Hahn (1991) (http://research.fhcrc.org/content/dam/stripe/hahn/methods/mol_biol/Primer%20extension%20yeast%20RNA.pdf) exactly, with volume modifications described in Kaplan et al. (2012). CKO1569 was used to detect lys2-128∂ RNAs. The GeneRacer RACE kit (Life Technologies) was used for 5′ RACE following the manufacturer’s instructions, using oligo CKO1569 for initial amplification of lys2-128∂ cDNAs, and CKO1585 for nested amplification. 3′ RACE was performed using Illustra Ready-to-Go cDNA beads (GE Healthcare Life Sciences). Oligo-dT plus linker sequence (CKO099) was used for first strand synthesis per the manufacturer’s instructions. Initial 3′ RACE PCR was done with CKO843 LYS2 primer and CKO100 primer (to CKO099 linker) (35 cycles). Nested PCR was with primers CKO844 (LYS2 primer) and CKO101 primer (to CKO099 linker) (35 cycles). RACE cDNAs were cloned by TOPO-Blunt cloning (Life Technologies) and their inserts sequenced.
Data availability
Yeast strains and plasmids described in this work are stored in the Kaplan lab strain collections and are available upon request. Supplemental materials for this work are as follows: Figure S1, Figure S2, and Figure S3 and their associated legends, Table S1 of yeast strains used, Table S2 of plasmids, and Table S3 of oligonucleotide sequences (see also File S1).
Results
Extensive allele-specific genetic interactions between Pol II alleles and SPT elongation factor mutant alleles
We wished to understand the relationship between altered Pol II catalytic activity and the Spt- phenotype. To explore this relationship, we examined genetic interactions between Pol II alleles with altered catalytic properties and alleles of Spt elongation factors that also confer Spt- phenotypes. We assessed genetic interactions between a panel of rpo21/rpb1 alleles, most of which encode substitutions in the Pol II active site trigger loop, and alleles of Spt elongation factors SPT4, SPT5, and SPT6. Pol II trigger loop substitutions confer a range of catalytic activities in vitro and in many cases show allele-specific genetic interactions that correlate with the nature of the activity defects (Braberg et al. 2013). For example, genetic interactions can be positive/epistatic or negative depending on Pol II allele strength and whether the Pol II allele confers increased or decreased catalytic activity. We term mutants that increase Pol II catalytic activity (determined by in vitro elongation rate) as “gain of function” (GOF) and those that decrease catalytic activity as “loss of function” (LOF).
In order to assess genetic interactions, we utilized yeast strains containing a deletion in the largest subunit of Pol II, rpo21∆ (complemented by RPO21/RPB1 expressed from its native promoter on a CEN plasmid), and the following SPT gene alleles: spt4∆, spt5-194 [encoding E338K (Guo et al. 2008)], spt5-242 (encoding A268V, G. Hartzog, personal communication), and spt6-1004 [encoding a deletion of residues 931–994, replaced by a short linker (Kaplan 2003; Kaplan et al. 2005)]. We transformed each of these strains with a series of plasmids encoding Pol II alleles in RPO21/RPB1. In the course of yeast transformation experiments, we noted that the presence of certain rpo21/rpb1 mutant plasmids conferred extremely slow growth on transformants dependent on the presence of SPT gene alleles (most obvious for spt6-1004, Figure S1). For spt6-1004, this phenomenon occurs with both reduced activity (loss of function or LOF) and increased activity (gain of function or GOF) Pol II mutants, but is stronger for LOF alleles. Furthermore, this uncovering of dominant rpo21/rpb1 phenotypes extends to lethal rpo21/rpb1 alleles encoding presumed extreme LOF and GOF variants, suggesting that these variants are assembled into Pol II complexes that can functionally interfere with WT subunit function.
To reveal genetic interactions between Pol II alleles in the haploid state and SPT genes, we performed plasmid shuffling to select for strains having lost the WT RPO21/RPB1 plasmid while retaining an rpo21/rpb1 plasmid. Viability of strains upon plasmid shuffling was assayed by replica-plating to 5-FOA medium (Boeke et al. 1987) and is shown for spt4∆ and spt5 alleles (Figure 1) and spt6-1004 (Figure 2). Viable double mutant strains and single mutant and WT controls were further phenotyped for genetic interactions affecting growth under a number of conditions. These conditions included suppression or enhancement of growth phenotypes detected by reporter alleles, such as the Spt- reporter lys2-128∂ (Simchen et al. 1984) or the transcription interference reporter gal10∆56 (Kaplan et al. 2005), or those relating to gene-specific transcription defects, such as expression of IMD2, detected by sensitivity to mycophenolic acid (MPA) (Shaw and Reines 2000; Jenks et al. 2008; Kuehner and Brow 2008) [reviewed in Kaplan (2013)]. Results are shown for spt4∆ and spt5-194 (Figure 3), spt5-242 (Figure 4), and spt6-1004 (Figure 5). Genetic interaction results are summarized in the form of heat maps and compiled in Figure 6.
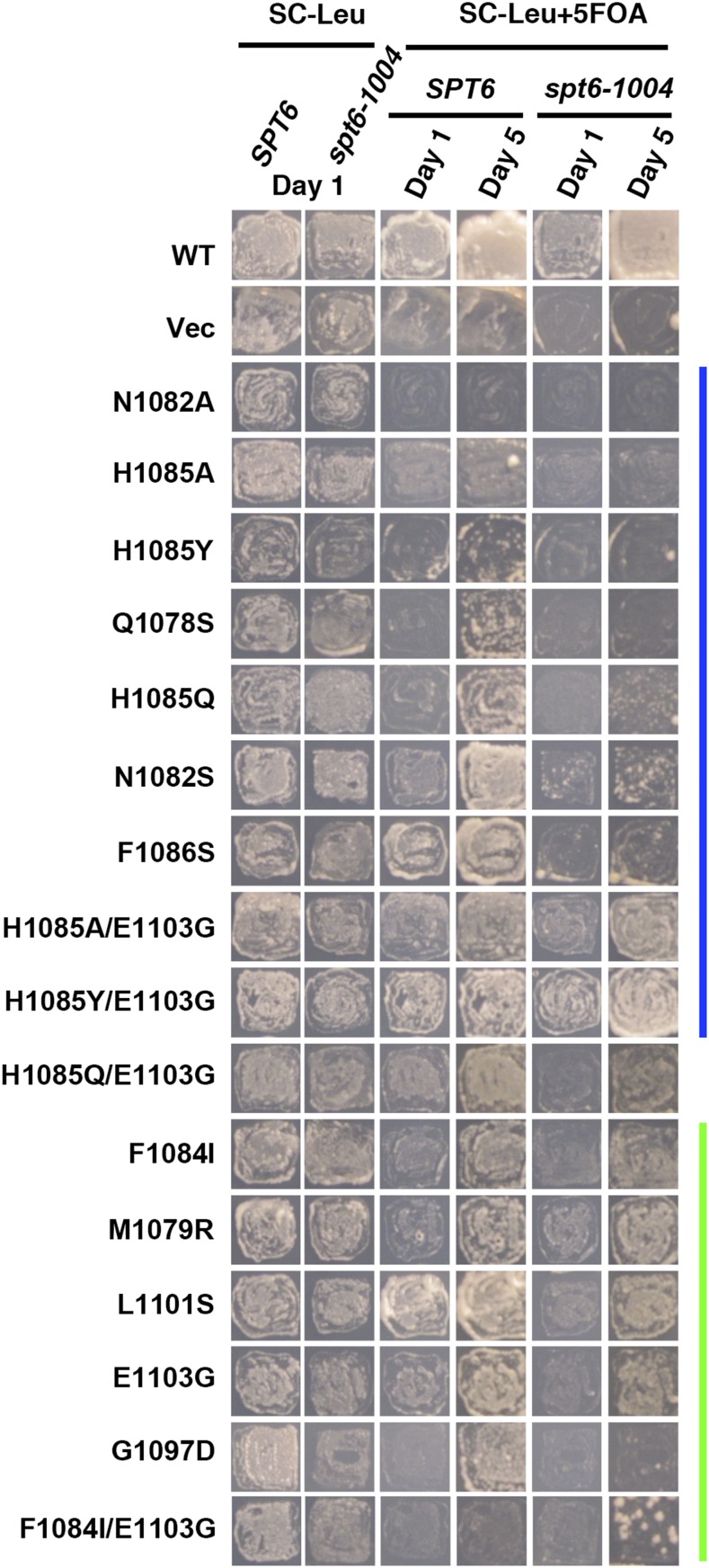
Figure 1.
Allele-specific synthetic lethality between Pol II activity mutants and alleles of SPT4 and SPT5. Yeast strains containing mutants of SPT4 or SPT5 and rpo21∆ were transformed with LEU2-marked CEN plasmids containing rpo21/rpb1 alleles. Transformants were patched and replica-plated to media allowing rpo21/rpb1 complementation by a URA3-marked CEN RPO21/RPB1 plasmid (SC-Leu) or media toxic to cells maintaining the URA3-marked CEN RPO21/RPB1 plasmid (SC-Leu+5FOA). Resulting growth of replica-plated patches was documented after 1 and 5 d of growth. Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. H1085Q/E1103G has mixed LOF/GOF properties based on genetic assays. GOF, gain of function; LOF, loss of function; Vec, vector only; WT, wild-type.
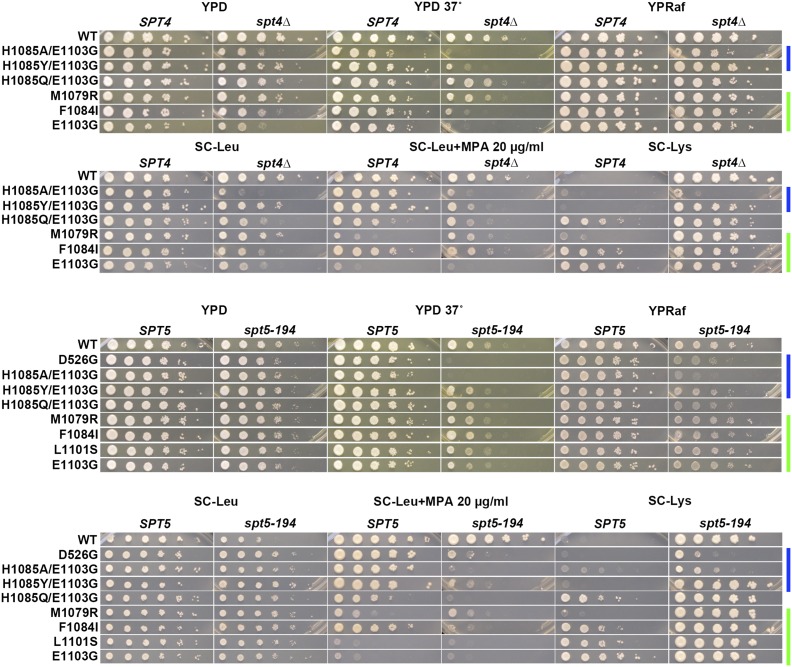
Figure 2.
Allele-specific synthetic lethality between Pol II activity mutants and the SPT6 allele, spt6-1004. Yeast strain containing spt6-1004 and rpo21∆ was transformed with LEU2-marked CEN plasmids containing rpo21/rpb1 alleles. Transformants were patched and replica-plated to media allowing rpo21/rpb1 complementation by a URA3-marked CEN RPO21/RPB1 plasmid (SC-Leu) or media toxic to cells maintaining the URA3-marked CEN RPO21/RPB1 plasmid (SC-Leu 5FOA). Resulting growth of replica-plated patches was documented after 1 and 5 d of growth. Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. Vec, vector only; WT, wild-type.
Figure 3.
Conditional genetic interactions between Pol II activity mutants and spt4∆ and spt5-194. Phenotypes of viable double mutants between spt4∆ or spt5-194 combined with rpo21/rpb1 alleles (see Materials and Methods). Serial dilutions (10-fold) of control or double mutant strains spotted on various media for phenotyping of genetic interactions (general growth, temperature sensitivity, Spt-, MPAS, and GalR phenotypes). Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. Gal, galactose; MPA, mycophenolic acid; WT, wild-type; YPD, yeast extract peptone dextrose.
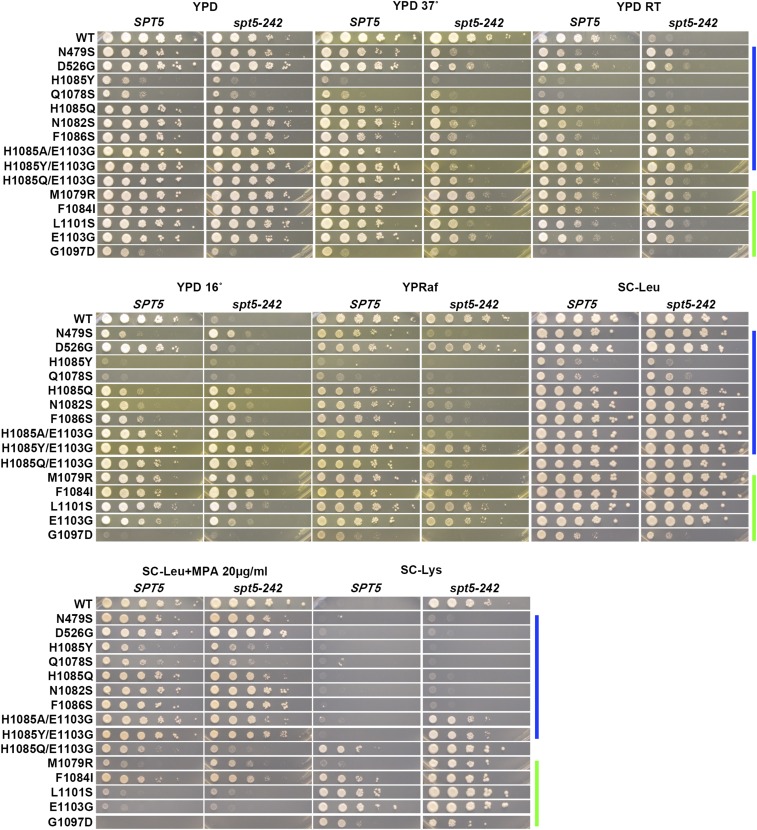
Figure 4.
Conditional genetic interactions between Pol II activity mutants and spt5-242. Phenotypes of viable double mutants between spt5-242 combined with rpo21/rpb1 alleles (see Materials and Methods). Serial dilutions (10-fold) of control or double mutant strains spotted on various medial for phenotyping of genetic interactions (general growth, temperature sensitivity, Spt-, MPAS, and GalR phenotypes). Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. Raf, raffinose; Gal, galactose; MPA, mycophenolic acid; WT, wild-type; YPD, yeast extract peptone dextrose.
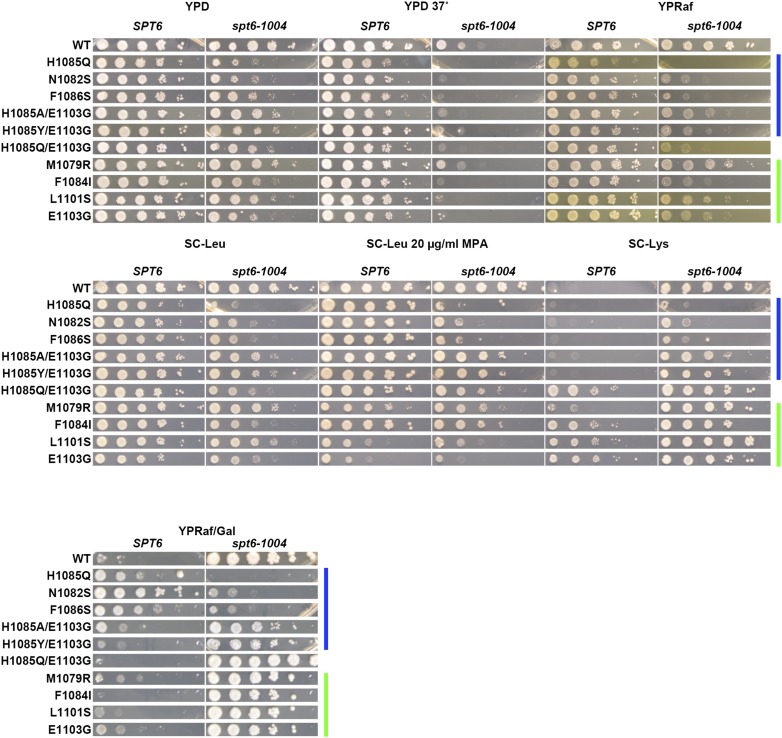
Figure 5.
Conditional genetic interactions between Pol II activity mutants and spt6-1004. Phenotypes of viable double mutants between spt6-1004 combined with rpo21/rpb1 alleles (see Materials and Methods). Serial dilutions (10-fold) of control or double mutant strains spotted on various medial for phenotyping of genetic interactions (general growth, temperature sensitivity, Spt-, MPAS, and GalR phenotypes). Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. Raf, raffinose; Gal, galactose; MPA, mycophenolic acid; WT, wild-type; YPD, yeast extract peptone dextrose.
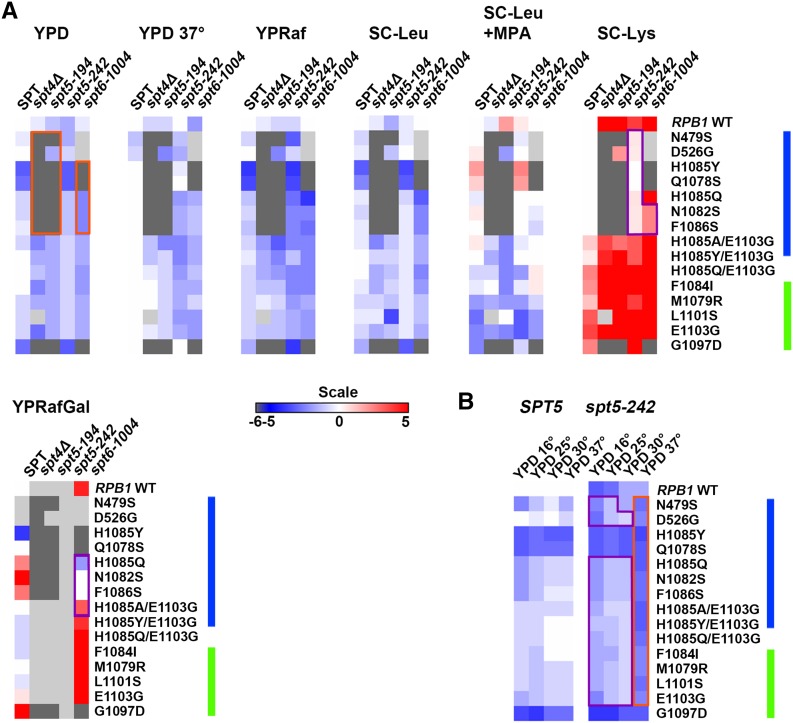
Figure 6.
Summary of genetic interactions between Pol II activity mutants and Spt elongation factor alleles. Phenotypes of viable Spt-Pol II double mutants are shown as a heatmap with qualitative determinations of growth defects for the relevant media. Dark gray indicates inviable double mutants. Light gray indicates conditions/strains not tested. For YPD, YPD 37°, YPRaf, and SC-Leu media, single and double mutant growth levels were normalized to WT. Decreased growth relative to WT is shown in shades of blue. Red indicates increased growth relative to WT. For SC-Leu+MPA, growth differences were normalized to those on SC-Leu to account for general effects on growth vs. those specific to MPA treatment. Similarly, YPRafGal was normalized to defects on YPD, and SC-Lys to those on SC-Leu. See Materials and Methods for additional explanation of heatmaps. (A) Interactions between Spt gene alleles and Pol II mutants. Orange and purple outlines indicate examples of clusters of allele-specific interactions. (B) Temperature-dependent genetic interactions between spt5-242 and Pol II alleles. Purple outline highlights suppression of spt5-242 at lower temperatures (but apparent at 30°). Orange outline highlights decreased growth at 37° for double mutants. Raf, raffinose; Gal, galactose; MPA, mycophenolic acid; WT, wild-type; YPD, yeast extract peptone dextrose.
We observed extensive allele-specific genetic interactions between Pol II alleles and SPT elongation factor alleles. First, we observed synthetic lethality and synthetic sickness much more commonly between Pol II LOF alleles and spt4∆, spt5-194, or spt6-1004 than between Pol II GOF alleles and the same (Figures 1, Figure 2, Figure 3, Figure 4, Figure 5, and Figure 6). The similar responses of Pol II alleles to spt4∆ and spt5-194 were expected based on previous experiments indicating defects in Spt4 association with Spt5 in spt5-194 (Guo et al. 2008). The overall strength of interactions with Pol II LOF alleles appears to be spt4∆ > spt5-194 > spt6-1004, judging by decreasing synthetic lethality (summarized in Figure 6). Conversely, spt4∆ and spt6-1004 show synthetic growth defects with a number of GOF Pol II alleles, as evidenced by reduced growth on rich (YPD) or defined (SC-Leu) media, that were not observed for combinations with spt5-194. For spt5-194, enhancement of MPA sensitivity was apparent for both presumed LOF Pol II alleles (D526G substitution outside the TL and compound TL alleles) and for presumed GOF alleles (M1079R, F1084I) (Figure 3 and Figure 6). Examination of conditional growth defects shows that spt5-194 and Pol II double mutants can become MPA sensitive, even for combinations where neither single mutant itself was strongly MPA sensitive (Figure 3 and Figure 6).
Interactions between Pol II mutants and spt5-242 were even more complex. spt5-242 was originally identified as a cold sensitive (Cs-) allele of SPT5 (Hartzog et al. 1998). The spt5-242 Cs- phenotype can be suppressed by a number of mutants or conditions, including alleles of genes encoding subunits of the Paf1C elongation complex (Squazzo et al. 2002), histone subunits (Quan and Hartzog 2010), chromatin remodelers (Simic et al. 2003), reduced function Pol II alleles (rpb2-10), or those with unknown effects on catalysis (rpb1-221 and rpb1-244) (Hartzog et al. 1998), or the nucleotide-depleting drug 6-azauracil (Hartzog et al. 1998). The molecular defects of spt5-242 in gene expression are poorly understood, though it confers apparent slow Pol II elongation in vivo (Quan and Hartzog 2010). The spt5-242 defect has been proposed to relate to kinetic uncoupling between transcription and other elongation events leading to cold-sensitive growth (Hartzog et al. 1998). Slowing Pol II or relaxation of transcribed chromatin structure, specifically by loss of H3K4me or H3K36me modifications or factors that promote them (Paf1C factors), or function of its effectors (Rpd3S complex, Chd1), suppresses spt5-242 cold sensitivity (Quan and Hartzog 2010). Similar to previous studies showing suppression of the spt5-242 Cs- phenotype by rpb2-10 (Hartzog et al. 1998), an allele of RPB2 causing elongation defects in vivo and in vitro, we observed suppression of spt5-242 broadly in essentially all LOF Pol II alleles tested. Intriguingly, we also observed suppression of spt5-242 by most GOF Pol II alleles tested (see Discussion). Furthermore, spt5-242 confers a sensitivity to 37° for almost all Pol II alleles, whether LOF or GOF (Figure 4 and Figure 6). These results are consistent with spt5-242 causing a continuum of defects from 15°–37° or having distinct defects at low and high temperatures that can be either suppressed or exacerbated by alteration in Pol II catalytic activity.
Sensitivity of lys2-128∂ suppression to Pol II activity
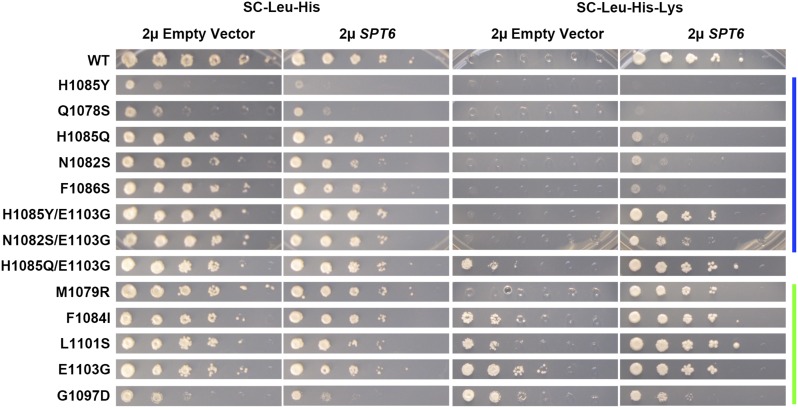
Examination of suppression of lys2-128∂ (Spt- phenotype) revealed complex relationships between Spt factors and Pol II alleles. A model that correlates Spt- phenotypes with other underlying defects of Spt- alleles predicts that Spt- alleles of SPT elongation factor genes and Spt- alleles of Pol II would show exacerbated phenotypes when combined, if their Spt- defects arose from similar causes. This is not supported by the observed interactions. First, GOF Pol II alleles confer the Spt- phenotype, yet there are much stronger genetic interactions between non-Spt- LOF Pol II alleles and alleles of SPT elongation factor genes (discussed above). Second, while exacerbating other growth defects, LOF Pol II alleles suppressed Spt- phenotypes of spt4∆ and spt5-194 where the double mutants were viable. LOF Pol II alleles also suppressed the Spt- phenotype of spt5-242, though both LOF and GOF Pol II alleles suppressed spt5-242 cold sensitivity. Distinct from spt4∆ and spt5 alleles, the Spt- phenotype of spt6-1004 was not strongly affected in spt6-1004/Pol II allele double mutants for GOF mutants. spt6-1004/LOF mutants were quite sick and appeared to be Spt- when considering their overall growth defects. To further examine the relationship between Pol II activity and the Spt- phenotype upon SPT6 alteration, we examined the Spt- phenotype conferred upon overexpression of SPT6 (Figure 7). We asked if the Spt- phenotype, due to expression of SPT6 from a high copy plasmid, was sensitive to Pol II activity, and found that this Spt- phenotype was also suppressed in LOF Pol II mutants. These results suggest that suppression of lys2-128∂ under a number of conditions is sensitive to Pol II activity.
Figure 7.
Pol II LOF alleles suppress the Spt- phenotype of high copy SPT6. Strains containing rpo21/rpb1 alleles were transformed by introduction of high copy (2µ) empty vector (pRS423) or SPT6 (pRS423 SPT6) and general growth (SC-Leu-His medium) or Spt- phenotypes (SC-Leu-His-Lys medium) were assessed. Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. LOF, loss of function; WT, wild-type.
Cryptic intragenic transcription is distinct from transcription at lys2-128∂
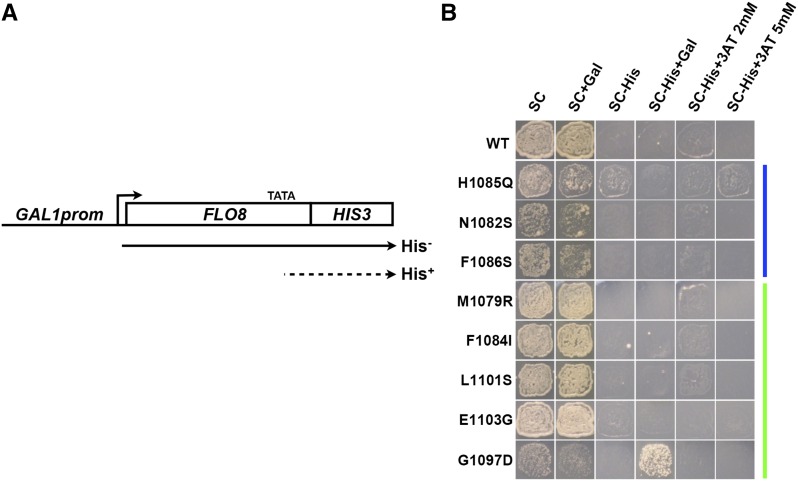
Given that Spt- phenotypes are conferred by mutations in a number of factors that also confer cryptic transcription (Kaplan et al. 2003; Cheung et al. 2008), we asked if Pol II mutants conferred cryptic transcription. We reasoned that increasing Pol II activity through trigger loop substitutions, which correlates with an Spt- phenotype, might allow suboptimal cryptic promoters to function. Supporting the idea that promoters might be sensitive to Pol II catalytic activity, transcription start site selection is altered upon alteration of the Pol II trigger loop, which directly influences Pol II catalysis (Kaplan et al. 2012; Braberg et al. 2013). Alternatively, altered Pol II elongation might confer chromatin defects that lead to both Spt- and cryptic transcription phenotypes, as cryptic promoters are derepressed by disruptions in transcription-coupled chromatin assembly (reviewed in Smolle and Workman (2013). To assess cryptic transcription, we performed genetic and molecular characterization of transcription from a FLO8 intragenic promoter reporter construct (Cheung et al. 2008) (Figure 8), as well as native FLO8 and STE11 cryptic promoters (Figure 9). We utilized a sensitive reporter for cryptic transcription developed by the Winston lab, wherein a FLO8 intragenic promoter is fused to HIS3 (FLO8-HIS3), with the native FLO8 promoter replaced by the GAL1 promoter (Cheung et al. 2008). This reporter allows cryptic transcription to be monitored by HIS3 levels in the presence or absence of high levels of transcription from the GAL1 promoter. A number of factors restrict expression from FLO8-HIS3, with many being revealed only under conditions of high GAL1p::FLO8-HIS3 expression (Cheung et al. 2008). For Pol II mutants, only our most extreme GOF substitution (G1097D) conferred detectable FLO8-HIS3 expression, as inferred through growth on medium lacking histidine, which requires HIS3 gene expression. G1097D derepression of the FLO8-HIS3 reporter also required galactose in the medium, presumably related to high-level expression of FLO8-HIS3. This result suggests that while it is possible for altered Pol II activity to promote cryptic expression, it is neither at a high level nor a widely distributed phenomenon for Pol II alleles when considering FLO8-HIS3. We then asked a converse question: is spt6-1004 cryptic transcription sensitive to Pol II activity, given that Spt- phenotypes of spt alleles can be suppressed by Pol II alleles. These observations raised the possibility that cryptic transcription might be sensitive to Pol II activity. Therefore, we examined native transcription for both FLO8 and STE11 in SPT6 and spt6-1004 backgrounds for Pol II catalytic mutants (Figure 9).
Figure 8.
Derepression of the FLO8 intragenic cryptic promoter occurs in an extreme Pol II allele, G1097D, and requires FLO8 transcription. (A) Schematic of GAL1promoter::FLO8::HIS3 construct allowing analysis of HIS3-dependent growth due to transcription from a cryptic FLO8 intragenic promoter (denoted by “TATA”) driving HIS3 expression. (B) Assay of rpo21/rpb1 allele activation of the FLO8 cryptic intragenic promoter dependent on activation of the GAL1 promoter by presence of galactose in growth medium (+Gal). Gal, galactose; WT, wild-type.
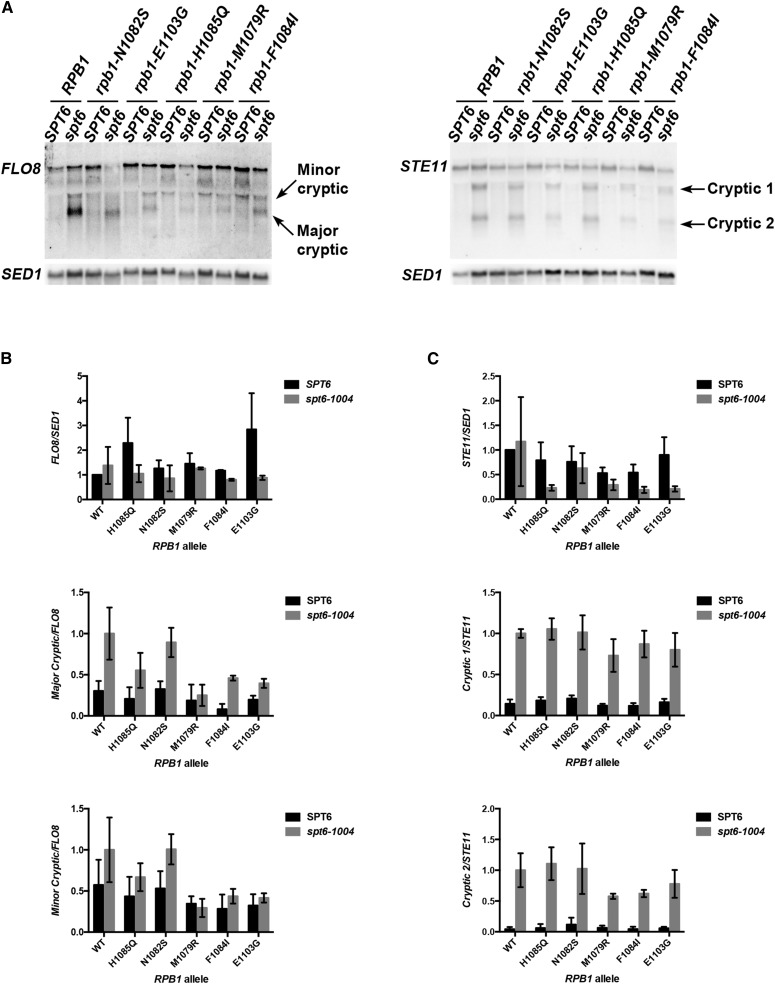
Figure 9.
Expression levels of FLO8 and STE11 cryptic transcripts are sensitive to reduced Pol II activity mutants. (A) Northern blotting for FLO8-derived RNAs (left) or STE11-derived RNAs (right) in various mutant strains. Northern blotting for SED1 provided a normalization control. spt6 indicates presence of spt6-1004 allele. (B) Quantification of mRNA levels for FLO8-derived transcripts (full-length, top; major cryptic, middle; and minor cryptic, bottom) normalized to SED1. Full-length FLO8 expression was normalized relative to the level in WT. Levels of cryptic transcripts were normalized to full-length and then relative to those in spt6-1004. Error bars represent standard deviation of the mean for at least three independent biological replicates. (C) Quantification of mRNA levels for STE11-derived transcripts (full-length, top; cryptic 1, middle; and cryptic 2, bottom) normalized to SED1. Full-length STE11 expression was normalized relative to the level in WT. Levels of cryptic transcripts were normalized relative to full-length then to those in spt6-1004. Error bars as in (B). WT, wild-type.
Northern blotting for FLO8 and STE11 showed previously identified cryptic transcripts in spt6-1004 cells at a permissive temperature of 30°. spt6-1004/Pol II double mutants showed cryptic transcripts for both FLO8 and STE11. Pol II mutants did not exhibit cryptic transcription on their own for either FLO8 and STE11. Full-length STE11, but not FLO8, was generally reduced in spt6-1004/Pol II double mutants regardless of LOF or GOF character of the Pol II substitution, but cryptic mRNA levels relative to full-length mRNA levels were not affected. For FLO8 cryptic transcripts, GOF Pol II alleles appear to reduce the ratio of cryptic transcripts relative to full-length in spt6-1004 strains. These results suggest that apparent cryptic transcription is not overly sensitive to Pol II catalytic mutants, and is distinct from the sensitivity to reduced Pol II activity mutants apparent in their suppression of lys2-128∂.
Transcription from the LYS2 promoter is both required for the Spt- phenotype and can modulate genetic requirements for it
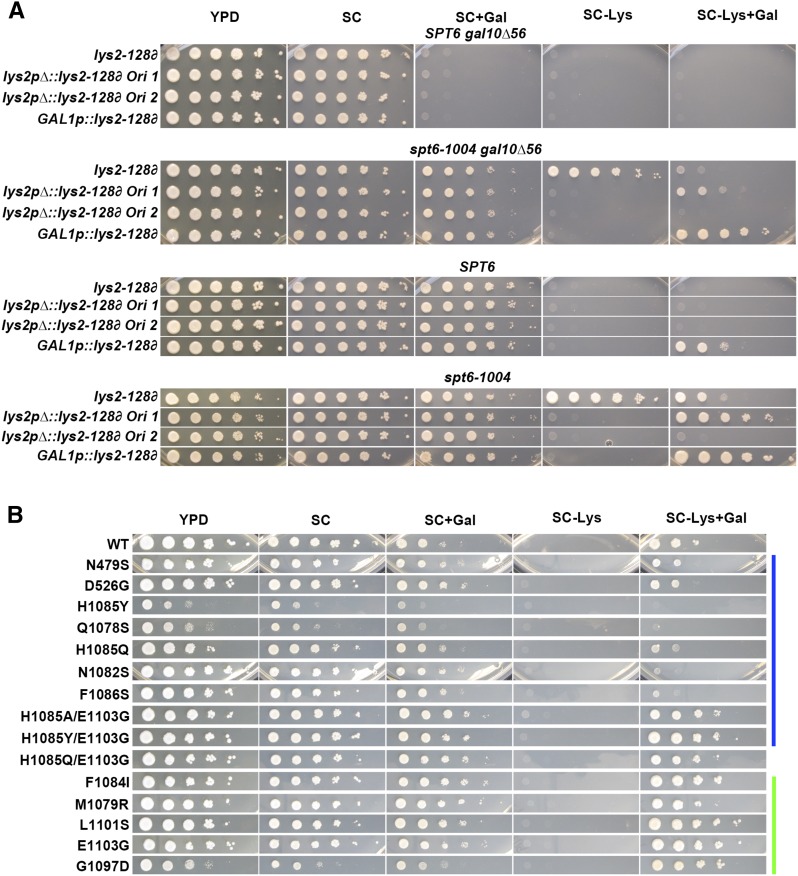
Experiments described above indicated that cryptic transcription and transcription leading to the Spt- phenotype for lys2-128∂ have distinct properties. Notwithstanding this conclusion, we further explored commonalities between the architectures of cryptic transcription units and lys2-128∂. Where examined, Spt- mutants that suppress lys2-128∂ allow generation of a truncated transcript that presumably arises from initiation within the Ty1 ∂ element downstream of the LYS2 promoter. Because the upstream LYS2 promoter is highly active at lys2-128∂, the TSS used in Spt- cells is located within transcribed chromatin, just as cryptic promoters are. For many cryptic transcription-allowing mutants, derepression of cryptic promoters requires disruption of chromatin by transcription (Cheung et al. 2008). In other compound transcription units, such as SRG1-SER3, transcription over a downstream promoter is required for repression of that promoter, and loss of transcription itself can be sufficient in otherwise wild-type cells to allow promoter activation (Martens et al. 2004). Additionally, the lys2-128∂ Ty ∂ element is distinct from cryptic promoters in that the ∂ element contains a bona fide promoter (Elder et al. 1983), which in a full-length Ty1 element would be transcribed. Therefore, we sought to determine the requirement for transcription from the LYS2 promoter for transcription originating within the Ty1 ∂. We deleted the LYS2 promoter in three conformations in SPT6 and spt6-1004 backgrounds to determine the requirements for LYS2 transcription in silencing the Ty1 ∂ element or as a condition for the Spt- phenotype in spt6-1004 or Pol II mutants (Figure 10).
Figure 10.
Suppression of lys2-128∂ by spt6-1004 or rpo21/rpb1 alleles requires upstream transcription and can be modulated by an ectopic promoter. (A) Analysis of spt6-1004 effects on lys2-128∂ promoter derivatives. lys2-128∂ was modified in the promoter region for LYS2 in SPT6 and spt6-1004 strains containing either GAL10 or gal10∆56. Modifications included deletion of the LYS2 promoter by homologous recombination-based insertion of a drug resistance cassette (in either orientation denoted “Ori 1” or “Ori 2”) or by replacement of the LYS2 promoter with the GAL1 promoter. Strains were assayed for the Spt- phenotype on media containing galactose (+Gal). All other media used glucose as carbon source. Ten-fold serial dilutions of each strain spotted onto appropriate medium. (B) Analysis of rpo21/rpb1 allele effects on lys2-128∂ promoter derivatives. The LYS2 promoter was replaced with the GAL1 promoter as in (B) for a strain allowing transformation with different rpo21/rpb1 alleles. Analysis and conditions as in (B). Color bars indicate genetically predicted/known reduction of function (blue) or increase of function (green) rpo21/rpb1 alleles. Gal, galactose; WT, wild-type; YPD, yeast extract peptone dextrose.
Deletion of the LYS2 promoter was coupled to the insertion of a marker cassette containing hphNT1 in either orientation by homologous recombination, or replacement of the LYS2 promoter with the GAL1 promoter linked to kamMX4 to allow ectopic activation in the presence of galactose (see Materials and Methods). We obtained the following results (Figure 10): first, deletion of the LYS2 promoter in multiple conformations does not itself allow activation of the Ty1 ∂ promoter in a fashion that confers a Lys+ phenotype, suggesting that LYS2 transcription does not silence the ∂ promoter in the lys2-128∂ context. This result is consistent with prior work suggesting that Ty1 expression requires sequences with the body of the Ty1 element, though transcription was not directly measured in that work (Yu and Elder 1989). Second, deletion of the LYS2 promoter blocks spt6-1004 suppression of lys2-128∂, suggesting that transcription over the ∂ from LYS2 is required for the Spt- phenotype. Pol II Spt- mutants were similarly blocked for lys2-128∂ suppression in the GAL1p::lys2-128∂ conformation when cells were grown in glucose. Third, ectopic transcription of lys2-128∂ by the GAL1 promoter in the presence of galactose allowed a weak Lys+ phenotype itself, which could be further augmented by either spt6-1004 or Pol II GOF Spt- mutants. These results suggest that lys2-128∂ remains sensitive to Spt--conferring mutants, even when presumed high-level transcription allows a Lys+ phenotype in an Spt+ background. The ectopic Lys+ phenotype for GAL1p::lys2-128∂ was similarly sensitive to Pol II activity (Figure 10B) as Pol II LOF mutants suppressed it. Thus, suppression of the lys2-128∂ transcription by Pol II LOF extends to both a number of genetic backgrounds and promoter configurations. These results suggest that transcription establishes a state of lys2-128∂ that sensitizes it to Spt- mutants for derepression, while remaining sensitive to reduced (LOF) Pol II mutants. Interestingly, we also observed that the Spt- phenotype of spt6-1004 is weakened in the presence of galactose, while one orientation of hphNT1 confers a galactose-dependent spt6-1004 Spt- phenotype. We note that the coding region of hphNT1 contains at least one possible Gal4 binding site (5′-CGG-N11-CCG-3′) and we speculate this provides ectopic galactose regulation to lys2-128∂ that still requires spt6-1004 for a Lys+ phenotype.
Molecular basis for Pol II mutant effects at lys2-128∂
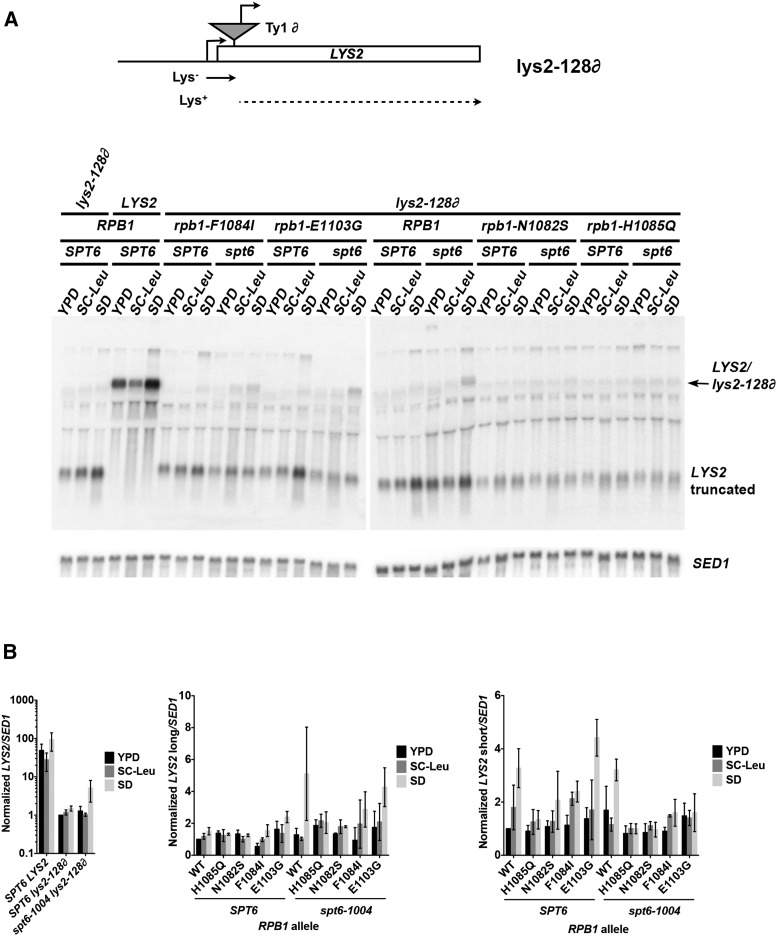
To understand how Spt- mutants suppress lys2-128∂ and are themselves suppressed by reduction in Pol II activity, we performed northern blotting for LYS2 (Figure 11), sequenced lys2-128∂ to confirm the location of the ∂ element in LYS2 and its sequence (Figure S2), performed primer extension and 5′ RACE to determine the transcription start sites for ∂-originating transcripts (Figure 12), and performed 3′ RACE to determine the 3′ termini of LYS2-originating transcripts (Figure S3). Northern blotting revealed a detectable increase in an almost full-length LYS2 transcript for spt6-1004 (Figure 11A), as previously observed for particular alleles of SPT4, SPT5, and SPT6 (Clark-Adams and Winston 1987; Swanson et al. 1991; Malone et al. 1993). This increase was, however, only a fraction of WT LYS2 levels (quantitated on the left, Figure 11B), consistent with only a very slight level of expression of truncated lys2-128∂ mRNA being needed for a Lys+ phenotype. GOF Pol II Spt- mutant E1103G shows a slight increase in lys2-128∂ mRNA above WT, however, the Spt- Pol II mutant F1084I does not to a significant extent (Figure 11B). LOF Pol II mutants reduce lys2-128∂ mRNA to WT levels in the spt6-1004 background and do not show the increase in minimal defined (SD) medium over minimal complete (SC-Leu) that was apparent in spt6-1004 alone, consistent with observations that they can suppress Spt- phenotypes of Spt mutants. When we quantified levels of transcription from the LYS2 promoter (right, Figure 11B), which terminates within the ∂ element (Figure S3), we found that the Pol II LOF mutant H1085Q blunted the induction of LYS2 seen in SD medium relative to YPD in WT cells. In the spt6-1004 background, all Pol II mutants appeared to reduce this induction relative to spt6-1004 alone, regardless of their Spt- phenotypes. These results suggest that reduction in LYS2 expression from the upstream LYS2 promoter alone does not confer reduced Lys+ phenotypes or reduced lys2-128∂ mRNA expression.
Figure 11.
Expression levels of LYS2 and lys2-128∂ transcripts in Spt- phenotype-modulating mutants. (A) Schematic of lys2-128∂ (top). Northern blotting for LYS2/lys2-128∂-derived RNAs under different media conditions in rpo21/rpb1 and/or spt6-1004 (spt6) mutant strains. SED1 mRNA provides a normalization control. (B) Quantification of LYS2/lys2-128∂-derived RNA levels under different media conditions in rpo21/rpb1 and/or spt6 mutant strains. Error bars indicate standard deviation of the mean of three independent biological replicates. Left: full- or near-full-length LYS2 mRNA levels normalized to level in WT strain grown in SD comparing LYS2 with lys2-128∂ and effects of spt6-1004. Note that scale of y-axis is logarithmic. Center: effects on full- or near-full-length LYS2 mRNA levels in rpo21/rpb1 and/or spt6 mutant strains. Right: Effects on truncated (short) LYS2 mRNA levels in rpo21/rpb1 and/or spt6 mutant strains. SD, synthetic defined; WT, wild-type; YPD, yeast extract peptone dextrose.
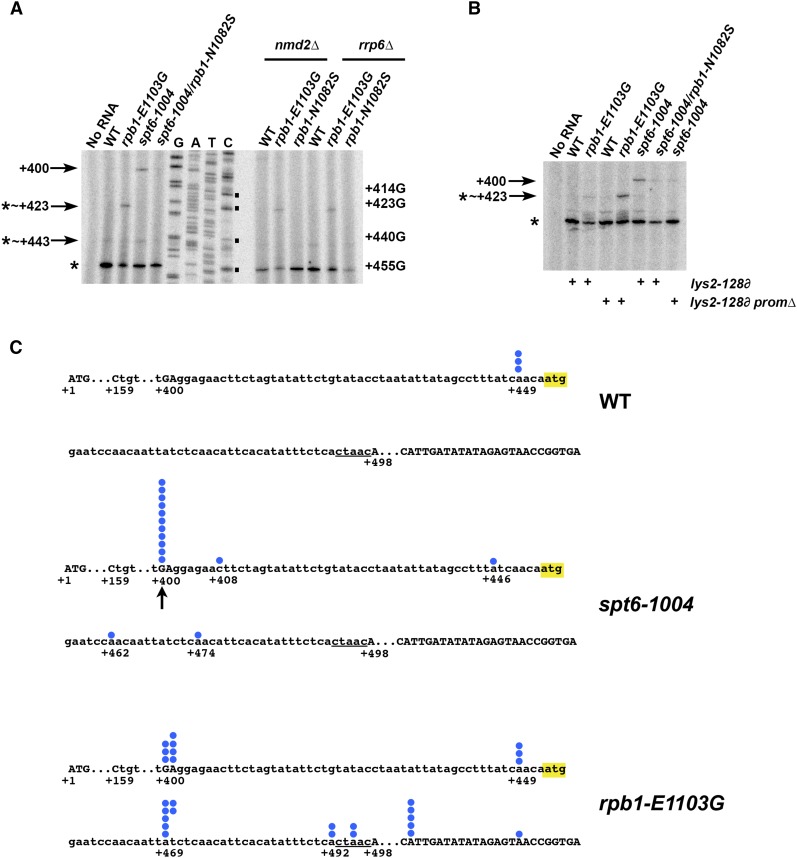
Figure 12.
Detection of lys2-128∂ transcript 5′ ends in an Spt- Pol II allele rpb1-E1103G and spt6-1004. (A) Primer extension analysis detecting transcripts emanating from the Ty1 ∂ element at lys2-128∂ for rpo21/rpb1 and spt6-1004 strains (left). Right side of panel shows nmd2∆ and rrp6∆ versions of strains for detection of possible unstable transcripts in the region targeted by nonsense-mediated decay (nmd2∆) or by the nuclear exosome (rrp6∆). Numbered asterisks are bands more apparent in mutant strains but are not specific to LYS2 (see B). Asterisk indicates nonspecific band in all strains, present even upon deletion of LYS2 (not shown) “G A T C” represents Sanger DNA sequencing reactions using same primer as primer extension reactions, for identification of putative lys2-128∂ transcripts. (B) Primer extension analysis for strains with or without the LYS2 promoter driving lys2-128∂. Deletion of the LYS2 promoter (spt6-1004 strain) or inhibition of transcription upstream of lys2-128∂ (GAL1p:lys2-128∂, rpb1-E1103G strain under glucose growth) suppresses the Spt- phenotypes of spt6-1004 and rpo21/rpb1 alleles (Figure 10) and is predicted to abolish transcripts conferring Lys2 function. (C) Schematic showing RNA 5′ ends emanating from the downstream end of the Ty1 ∂ element from lys2-128∂ detected by 5′ RACE in WT, spt6-1004, and rpo21/rpb1-E1103G strains. The +1 position is defined as the A in the LYS2 ATG. Lower case letters indicate ∂ element sequence. Yellow highlighting indicates ∂ ATG in-frame with downstream LYS2 sequence. Underlined sequence is a 5 bp duplication created upon Ty1 retrotransposition (Farabaugh and Fink 1980). Blue dots indicate number of clones detected by sequencing and the positions of their 5′ ends. RACE, rapid amplification of cDNA ends; WT, wild-type.
Primer extension analysis revealed that the spt6-1004 Spt- phenotype likely arises from activation of the normal Ty1 TSS (Elder et al. 1983), as primer extension and 5′ RACE (Figure 12, A and B) both identify transcripts consistent with the previously determined Ty1 TSS. This TSS is upstream of an ATG within the ∂ element that is in-frame with the LYS2 coding sequence. This particular ATG is the only nominal start codon for LYS2 downstream of the ∂ insertion for hundreds of nucleotides. No transcripts upstream of this ATG were identified for WT cells by primer extension (Figure 12A), while 5′ RACE identified three cDNA clones starting four nucleotides upstream of this ATG from a WT strain, unlikely to allow efficient translation (Figure 12B). Consistent with suppression of Spt- phenotypes by reduced Pol II activity, the lys2-128∂ transcript observed by primer extension for spt6-1004 was suppressed by Pol II N1082S (Figure 12A). For the Spt- Pol II allele rpb1-E1103G, we observed a transcript with a presumptive RNA 5′ end within the ∂ element by primer extension (Figure 12A). Given that primer extension can be prone to cross-priming of unrelated transcripts, we ascertained the requirement for the intact LYS2 promoter, which is required for the Spt- phenotype, of spt6-1004 and rpb1-E1103G transcripts observed by primer extension (Figure 12C). The presumptive ∂ TSS observed by primer extension for spt6-1004 is abolished upon deletion of the LYS2 promoter, while another 5′ end seen for all genotypes, and one 5′ end specific to rpb1-E1103G at ∼+423 from the LYS2 ATG, are not affected by deletion/repression of the LYS2 promoter. Consistent with these observations, we do not observe a presumptive TSS for rpb1-E1103G 5′ RACE matching the spurious transcript ∼+423 seen by PE. We conclude that the observed ∼+423 rpb1-E1103G transcript is derived from rpb1-E1103G effects on an unrelated promoter. However, 5′ RACE did identify transcripts originating from the normal Ty1 TSS and one nucleotide downstream within the ∂ for rpb1-E1103G, along with a number of additional putative TSSs unobserved in either spt6-1004 5′ RACE or WT 5′ RACE. We speculated that rpb1-E1103G might be causing upstream TSS shifts from a cryptic promoter that does not normally generate stable transcripts, just as it shifts TSS upstream at a number of promoters (Kaplan et al. 2012). Such transcripts initiating downstream of the ∂ ATG might be unstable due to a lack of in-frame ATG or other reasons. Therefore, we performed primer extension in nmd2∆ (defective for Nonsense Mediated Decay) and rrp6∆ backgrounds (Figure 12C). We were unable to detect cryptic, unstable transcription in the vicinity of the ∂, but interpretation was hampered by the apparent low level of RNA required for the Spt- phenotype. Finally, we also examined RNA 3′ ends terminating within the ∂ element, as LYS2 transcription impacts Spt- phenotypes, and it is possible that differential polymerase read-through from the upstream LYS2 promoter might underlie Pol II mutant effects at lys2-128∂, especially in light of known effects of Pol II or Spt mutants on 3′ end formation or termination (Cui and Denis 2003; Kaplan et al. 2005; Hazelbaker et al. 2013). We did not observe substantial changes in polyadenylation sites within the ∂ in the examined strains (Figure S3). We cannot rule out additional effects on terminating polymerases downstream of the polyadenylation sites.
Discussion
Determination of the molecular basis for alterations in gene expression brought about by genetic perturbation to transcription or chromatin regulators allows us to understand the orchestration of gene expression and its regulation by chromatin structure. We know now that transcription units can be complex, with promoters adjacent to one another, possibly driving overlapping or antisense transcription. Interestingly, loci in yeast harboring compound transcription units are the bases for a number of in vivo growth phenotypes, and these have successfully been exploited for the study of transcriptional processes. These loci share the attribute that transcription may be delicately poised between states allowing for sensitive detection of transcription defects, even for factors functioning in transcription genome-wide and not simply at the loci in question. One such locus in S. cerevisiae, lys2-128∂, has played an important role in identifying and characterizing conserved transcriptional regulator genes such as SPT4, SPT5, and SPT6, among others. In this work, we addressed genetic relationships between the Spt elongation factors Spt4, Spt5, and Spt6, Pol II catalytic activity, and the Lys+ Spt- phenotype conferred by Spt- alleles of Spt factors and Pol II.
We found extensive allele-specific genetic interactions between Spt factors and mutants known to alter Pol II activity. Similar to our previous genetic interaction studies with Pol II alleles and a large panel of gene deletions (Braberg et al. 2013), or with viable alleles of essential general transcription factor genes (Jin and Kaplan 2014), we found that genetic interactions were generally dependent on whether Pol II alleles were associated with decreased (LOF) or increased (GOF) Pol II activity. While both classes can have wide-ranging genetic interactions, interactions with particular genes/alleles tend to be stronger with one class or another. All Spt gene alleles tended to show stronger interactions with LOF Pol II alleles, consistent with defects in positive transcription functions showing synergy with a decrease in Pol II catalytic rate. The greater allele-specificity between spt4∆/spt5-194 and Pol II LOF alleles than for spt6-1004 are consistent with direct positive roles of the Spt4/Spt5 complex in elongation, stemming from its likely close association with the Pol II elongation complex (Wada et al. 1998; Bourgeois et al. 2002; Zhang et al. 2004; Hartzog and Kaplan 2011; Martinez-Rucobo et al. 2011).
Interactions between Pol II catalytic rate alleles and spt5-242 indicate complexities about which we might only speculate. Previously, suppressors of spt5-242 have been interpreted as being of two classes (Hartzog et al. 1998; Quan and Hartzog 2010). The first class alters Pol II function such that it is slower, while the second class alters chromatin structure such that transcribed chromatin is expected to be disrupted. The former is proposed to allow Pol II additional time to deal with obstacles to transcription in light of defective Spt5 function in spt5-242, while the latter class was proposed to reduce chromatin obstacles to Pol II elongation, alleviating some requirements for WT Spt5 function. Our observations here show that many Pol II mutants with bona fide catalytic defects suppress spt5-242 regardless of LOF or GOF status. Furthermore, most Pol II alleles, whether presumed fast or slow, enhance spt5-242 growth defects at 37°. These results are consistent with the previously proposed model that Pol II activity and Spt5 function need to be kinetically matched for proper transcription (Hartzog et al. 1998). Additionally, it is possible that increase in Pol II activity allows some elongation barriers to be bypassed, also alleviating spt5-242 defects. Further consideration of these issues will require direct evidence that elongation phenotypes of Pol II alleles are apparent in vivo as predicted from in vitro elongation rates (Malagon et al. 2006; Kaplan et al. 2008, 2012; Kireeva et al. 2008; Larson et al. 2012). Human analogs of some Pol II mutants assessed here (rpb1-E1103G and rpb1-H1085Y) have been shown to be fast or slow in a human cell line (Fong et al. 2014), as predicted from previous biochemical experiments on yeast Pol II mutant enzymes. One of the Pol II mutants analyzed here, rpb1-E1103G, was reported to elongate faster than WT in vivo as predicted (Hazelbaker et al. 2013); however, we are currently revisiting this conclusion.
From the results presented here, we hypothesize that Pol II alleles suppress lys2-128∂ for reasons distinct from Spt elongation factor alleles, or at a minimum distinct from spt6-1004. We find that lys2-128∂ has features common to cryptic intragenic transcription, including a requirement for transcription from the LYS2 promoter for the spt6-1004 Spt- phenotype at lys2-128∂. Coupled with previous work suggesting that chromatin proximal to Ty1 or Ty1 ∂ elements in the yeast genome is sensitive to spt6-1004 (Perales et al. 2013), spt6-1004 activation of lys2-128∂ likely proceeds through LYS2 promoter-dependent transcriptional disruption of nucleosomes that repress the lys2-128∂ promoter. We do not favor a similar mechanism for Pol II Spt alleles’ Spt- phenotypes, though our conclusions are necessarily tempered by the low level of lys2-128∂ expression underlying their phenotypes. We argue as follows: first, we would expect synthetic or enhancing interactions between Pol II Spt- alleles and Spt elongation factors if their respective defects were in similar functions. Second, we might expect to a greater modulation of cryptic transcription by Pol II alleles than we have observed, if altered Pol II activity suppressed lys2-128∂ through elongation-mediated chromatin disruption. Though activation of a cryptic transcription reporter can be observed for one of the most extreme Pol II mutants, rpb1-G1097D, suppression is weak. The identification of a number of 5′ RACE clones indicating potential TSSs in rpb1-E1103G downstream of the Ty1 ∂ TSS raises the possibility that rpb1-E1103G initiation and not elongation defects underlie its Spt- phenotype. For example, rpb1-E1103G might activate a cryptic promoter by allowing initiation from positions upstream of “frustrated” initiation sites where initiation is abortive or nonproductive. A prediction of this model is that mutants that generally shift TSSs upstream at promoters should confer the Spt- phenotype, and that mutants that can suppress upstream TSS shifts in rpb1-E1103G should suppress this phenotype. In support of these predictions, we showed previously that a tfg2 allele (in Pol II initiation factor TFIIF) confers a weak Spt- phenotype along with shifting TSSs upstream at analyzed promoters (e.g., ADH1) (Jin and Kaplan 2014). Furthermore, Pol II Spt- alleles’ Spt phenotype at lys2-128∂ was suppressed by alleles of SUA7, encoding yeast TFIIB. These sua7 alleles shift TSSs downstream on their own, and suppress upstream TSS shifts of Pol II alleles at ADH1 (Jin and Kaplan 2014). Taken together, these results suggest that the interplay of Pol II initiation and elongation can converge at lys2-128∂ to render transcription finely balanced between nonfunctional and functional LYS2 expression.
Supplementary Material
Acknowledgments
We thank Grant Hartzog (University of California, Santa Cruz) and Fred Winston (Harvard Medical School) for strains and plasmids. We thank Indranil Malik for help with northern blotting. We thank members of the Kaplan laboratory for helpful discussions and comments regarding the manuscript. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM097260, and a Welch Foundation grant A-1763. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Welch Foundation.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.030346/-/DC1
Communicating editor: C. S. Hoffman
Literature Cited
- Amberg D. C., Burke D. J., Strathern J. N., 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 Edition Cold Spring Harbor Press, Cold Spring Harbor, NY. [Google Scholar]
- Andrulis E. D., Guzman E., Doring P., Werner J., Lis J. T., 2000. High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev. 14: 2635–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. J., Gordon M., Eide D. J., Winge D. D. R., 2006. Repression of ADH1 and ADH3 during zinc deficiency by Zap1-induced intergenic RNA transcripts. EMBO J 25: 5726–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R., 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Bourgeois C. F., Kim Y. K., Churcher M. J., West M. J., Karn J., 2002. Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences. Mol. Cell. Biol. 22: 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braberg H., Jin H., Moehle E. A., Chan Y. A., Wang S., et al. , 2013. From structure to systems: high-resolution, quantitative genetic analysis of RNA polymerase II. Cell 154: 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnuovo M., Zaugg J. B., Guffanti E., Maffioletti A., Camblong J., et al. , 2014. Role of histone modifications and early termination in pervasive transcription and antisense-mediated gene silencing in yeast. Nucleic Acids Res. 42: 4348–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V., Chua G., Batada N. N., Landry C. R., Michnick S. W., et al. , 2008. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol. 6: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Adams C. D., Winston F., 1987. The SPT6 gene is essential for growth and is required for delta-mediated transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Adams C. D., Norris D., Osley M. A., Fassler J. S., Winston F., 1988. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 2: 150–159. [DOI] [PubMed] [Google Scholar]
- Cui Y., Denis C. L., 2003. In vivo evidence that defects in the transcriptional elongation factors RPB2, TFIIS, and SPT5 enhance upstream poly(A) site utilization. Mol. Cell. Biol. 23: 7887–7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann D. M., Dollard C., Winston F., 1989. SPT15, the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo. Cell 58: 1183–1191. [DOI] [PubMed] [Google Scholar]
- Elder R. T., Loh E. Y., Davis R. W., 1983. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc. Natl. Acad. Sci. USA 80: 2432–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R., 1980. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature 286: 352–356. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Winston F., 1988. Isolation and analysis of a novel class of suppressor of Ty insertion mutations in Saccharomyces cerevisiae. Genetics 118: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., Winston F., 1989. The Saccharomyces cerevisiae SPT13/GAL11 gene has both positive and negative regulatory roles in transcription. Mol. Cell. Biol. 9: 5602–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N., Kim H., Zhou Y., Ji X., Qiu J., et al. , 2014. Pre-mRNA splicing is facilitated by an optimal RNA polymerase II elongation rate. Genes Dev. 28: 2663–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansheroff L. J., Dollard C., Tan P., Winston F., 1995. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo. Genetics 139: 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. A., Duggan L., Cote J., Roberts S. M., Brownell J. E., et al. , 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- Guo M., Xu F., Yamada J., Egelhofer T., Gao Y., et al. , 2008. Core structure of the yeast spt4-spt5 complex: a conserved module for regulation of transcription elongation. Structure 16: 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L., 1989. Isolation of the gene encoding the yeast TATA binding protein TFIID: a gene identical to the SPT15 suppressor of Ty element insertions. Cell 58: 1173–1181. [DOI] [PubMed] [Google Scholar]
- Hainer S. J., Pruneski J. A., Mitchell R. D., Monteverde R. M., Martens J. A., 2011. Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev. 25: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzog G. A., Kaplan C. D., 2011. Competing for the clamp: promoting RNA polymerase processivity and managing the transition from initiation to elongation. Mol. Cell 43: 161–163. [DOI] [PubMed] [Google Scholar]
- Hartzog G. A., Wada T., Handa H., Winston F., 1998. Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev. 12: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbaker D. Z., Marquardt S., Wlotzka W., Buratowski S., 2013. Kinetic competition between RNA Polymerase II and Sen1-dependent transcription termination. Mol. Cell 49: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B. P., Fischer T., 2013. The great repression: chromatin and cryptic transcription. Transcription 4: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks M. H., O’Rourke T. W., Reines D., 2008. Properties of an intergenic terminator and start site switch that regulate IMD2 transcription in yeast. Mol. Cell. Biol. 28: 3883–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. H., Jacquier A., Libri D., 2013. Dealing with pervasive transcription. Mol. Cell 52: 473–484. [DOI] [PubMed] [Google Scholar]
- Jin H., Kaplan C. D., 2014. Relationships of RNA polymerase II genetic interactors to transcription start site usage defects and growth in Saccharomyces cerevisiae. G3 (Bethesda) 5: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, C. D., 2003 Spt6, a conserved, essential regulator of chromatin structure and transcription elongation by RNA polymerase II. Ph.D. Thesis, Harvard University, Cambridge. [Google Scholar]
- Kaplan C. D., 2013. Basic mechanisms of RNA polymerase II activity and alteration of gene expression in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1829: 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C. D., Morris J. R., Wu C., Winston F., 2000. Spt5 and spt6 are associated with active transcription and have characteristics of general elongation factors in D. melanogaster. Genes Dev. 14: 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C. D., Laprade L., Winston F., 2003. Transcription elongation factors repress transcription initiation from cryptic sites. Science 301: 1096–1099. [DOI] [PubMed] [Google Scholar]
- Kaplan C. D., Holland M. J., Winston F., 2005. Interaction between transcription elongation factors and mRNA 3′-end formation at the Saccharomyces cerevisiae GAL10–GAL7 locus. J. Biol. Chem. 280: 913–922. [DOI] [PubMed] [Google Scholar]
- Kaplan C. D., Larsson K. M., Kornberg R. D., 2008. The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Mol. Cell 30: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C. D., Jin H., Zhang I. L., Belyanin A., 2012. Dissection of Pol II trigger loop function and Pol II activity-dependent control of start site selection in vivo. PLoS Genet. 8: e1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva M. L., Nedialkov Y. A., Cremona G. H., Purtov Y. A., Lubkowska L., et al. , 2008. Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol. Cell 30: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner J. N., Brow D. A., 2008. Regulation of a eukaryotic gene by GTP-dependent start site selection and transcription attenuation. Mol. Cell 31: 201–211. [DOI] [PubMed] [Google Scholar]
- Larson M. H., Zhou J., Kaplan C. D., Palangat M., Kornberg R. D., et al. , 2012. Trigger loop dynamics mediate the balance between the transcriptional fidelity and speed of RNA polymerase II. Proc. Natl. Acad. Sci. USA 109: 6555–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri D., 2015. Sleeping Beauty and the Beast (of pervasive transcription). RNA 21: 678–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagon F., Kireeva M. L., Shafer B. K., Lubkowska L., Kashlev M., et al. , 2006. Mutations in the Saccharomyces cerevisiae RPB1 gene conferring hypersensitivity to 6-azauracil. Genetics 172: 2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. A., Clark C. D., Chiang A., Winston F., 1991. Mutations in SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 5710–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. A., Fassler J. S., Winston F., 1993. Molecular and genetic characterization of SPT4, a gene important for transcription initiation in Saccharomyces cerevisiae. Mol. Gen. Genet. 237: 449–459. [DOI] [PubMed] [Google Scholar]
- Marcus G. A., Horiuchi J., Silverman N., Guarente L., 1996. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol. Cell. Biol. 16: 3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens J. A., Laprade L., Winston F., 2004. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429: 571–574. [DOI] [PubMed] [Google Scholar]
- Martens J. A., Wu P. Y., Winston F., 2005. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 19: 2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rucobo F. W., Sainsbury S., Cheung A. C., Cramer P., 2011. Architecture of the RNA polymerase-Spt4/5 complex and basis of universal transcription processivity. EMBO J. 30: 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Lidschreiber M., Siebert M., Leike K., Soding J., et al. , 2010. Uniform transitions of the general RNA polymerase II transcription complex. Nat. Struct. Mol. Biol. 17: 1272–1278. [DOI] [PubMed] [Google Scholar]
- Murray S. C., Haenni S., Howe F. S., Fischl H., Chocian K., et al. , 2015. Sense and antisense transcription are associated with distinct chromatin architectures across genes. Nucleic Acids Res. 43: 7823–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsoulis G., Dollard C., Winston F., Boeke J. D., 1991. The products of the SPT10 and SPT21 genes of Saccharomyces cerevisiae increase the amplitude of transcriptional regulation at a large number of unlinked loci. New Biol. 3: 1249–1259. [PubMed] [Google Scholar]
- Nguyen T., Fischl H., Howe F. S., Woloszczuk R., Serra Barros A., et al. , 2014. Transcription mediated insulation and interference direct gene cluster expression switches. eLife 3: e03635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelechano V., Wei W., Steinmetz L. M., 2013. Extensive transcriptional heterogeneity revealed by isoform profiling. Nature 497: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales R., Erickson B., Zhang L., Kim H., Valiquett E., et al. , 2013. Gene promoters dictate histone occupancy within genes. EMBO J. 32: 2645–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T. K., Hartzog G. A., 2010. Histone H3K4 and K36 methylation, Chd1 and Rpd3S oppose the functions of Saccharomyces cerevisiae Spt4-Spt5 in transcription. Genetics 184: 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando O. J., Winston F., 2012. Chromatin and transcription in yeast. Genetics 190: 351–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranish J. A., Hahn S., 1991. The yeast general transcription factor TFIIA is composed of two polypeptide subunits. J. Biol. Chem. 266: 19320–19327. [PubMed] [Google Scholar]
- Roberts S. M., Winston F., 1996. SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 3206–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley A., Singer R. A., Johnston G. C., 1991. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol. Cell. Biol. 11: 5718–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W., 2001. Molecular cloning: a laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- Saunders A., Werner J., Andrulis E. D., Nakayama T., Hirose S., et al. , 2003. Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science 301: 1094–1096. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L., 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18: 3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Reines D., 2000. Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol. Cell. Biol. 20: 7427–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood P. W., Osley M. A., 1991. Histone regulatory (hir) mutations suppress delta insertion alleles in Saccharomyces cerevisiae. Genetics 128: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G., Winston F., Styles C. A., Fink G. R., 1984. Ty-mediated gene expression of the LYS2 and HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes. Proc. Natl. Acad. Sci. USA 81: 2431–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic R., Lindstrom D. L., Tran H. G., Roinick K. L., Costa P. J., et al. , 2003. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 22: 1846–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolle M., Workman J. L., 2013. Transcription-associated histone modifications and cryptic transcription. Biochim. Biophys. Acta 1829: 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squazzo S. L., Costa P. J., Lindstrom D. L., Kumer K. E., Simic R., et al. , 2002. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 21: 1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Malone E. A., Winston F., 1991. SPT5, an essential gene important for normal transcription in Saccharomyces cerevisiae, encodes an acidic nuclear protein with a carboxy-terminal repeat. Mol. Cell. Biol. 11: 3009–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Takagi T., Yamaguchi Y., Ferdous A., Imai T., et al. , 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, F., 1992 47 Analysis of SPT Genes: A Genetic Approach toward Analysis of TFIID, Histones, and Other Transcription Factors of Yeast. Cold Spring Harbor Monograph Archive 22B. Available at: https://cshmonographs.org/index.php/monographs/article/view/3464.
- Winston F., Chaleff D. T., Valent B., Fink G. R., 1984. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107: 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Dollard C., Malone E. A., Clare J., Kapakos J. G., et al. , 1987. Three genes are required for trans-activation of Ty transcription in yeast. Genetics 115: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Dollard C., Ricupero-Hovasse S. L., 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11: 53–55. [DOI] [PubMed] [Google Scholar]
- Yu K., Elder R. T., 1989. A region internal to the coding sequences is essential for transcription of the yeast Ty-D15 element. Mol. Cell. Biol. 9: 3667–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu C. H., Gilmour D. S., 2004. Analysis of polymerase II elongation complexes by native gel electrophoresis. Evidence for a novel carboxyl-terminal domain-mediated termination mechanism. J. Biol. Chem. 279: 23223–23228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Yeast strains and plasmids described in this work are stored in the Kaplan lab strain collections and are available upon request. Supplemental materials for this work are as follows: Figure S1, Figure S2, and Figure S3 and their associated legends, Table S1 of yeast strains used, Table S2 of plasmids, and Table S3 of oligonucleotide sequences (see also File S1).