Abstract
There is a significant need for an effective multivalent Shigella vaccine that targets the most prevalent serotypes. Most Shigella vaccines under development utilize serotype-specific lipopolysaccharides (LPSs) as a major component based on protection and epidemiological data. As vaccine formulations advance from monovalent to multivalent, assays and reagents need to be developed to accurately and reproducibly quantitate the amount of LPSs from multiple serotypes in the final product. To facilitate this effort, we produced 36 hybridomas that secrete monoclonal antibodies (MAbs) against the O antigen on the LPS from Shigella flexneri 2a, Shigella flexneri 3a, and Shigella sonnei. We used six of these monoclonal antibodies for an inhibition enzyme-linked immunosorbent assay (iELISA), measuring LPSs with high sensitivity and specificity. It was also demonstrated that the Shigella serotype-specific MAbs were useful for bacterial surface staining detected by flow cytometry. These MAbs are also useful for standardizing the serum bactericidal assay (SBA) for Shigella. Functional assays, such as the in vitro bactericidal assay, are necessary for vaccine evaluation and may serve as immunological correlates of immunity. An S. flexneri 2a-specific monoclonal antibody killed S. flexneri 2b isolates, suggesting that S. flexneri 2a LPS may induce cross-protection against S. flexneri 2b. Overall, the Shigella LPS-specific MAbs described have potential utility to the vaccine development community for assessing multivalent vaccine composition and as a reliable control for multiple immunoassays used to assess vaccine potency.
INTRODUCTION
Shigella is a genus of Gram-negative bacteria that is responsible for bacillary dysentery, or shigellosis, which is a major public health problem in the world, particularly in developing countries. It is estimated to cause about 90 million cases of severe diarrhea and about 100,000 deaths per year (1). In developed countries, Shigella causes a significant number of cases of diarrhea among children in day care settings, travelers, and military personnel (2). There are four species of Shigella: Shigella dysenteriae, Shigella flexneri, Shigella boydii, and Shigella sonnei. S. sonnei and S. flexneri are common throughout the world, but S. dysenteriae and S. boydii are rare in the United States (3).
A clear and well-defined correlate of immunity has not been fully established to guide the rational design of an effective Shigella vaccine (3). Although cross-reactions among different lipopolysaccharides (LPSs) have been reported (4, 5), protection against shigellosis appears to be serotype specific, highlighting the significant role of immune responses to the O-antigen (O-Ag) portion of the LPS (6, 7). Antibodies to O-Ag have been used to define at least 50 serotypes among Shigella isolates (3, 8). S. flexneri has 14 serologically unique serotypes, which have O-Ags with distinct molecular structures (9). The serotype-specific epitopes (except for type 6) are generated by lysogenic phages that add O-acetyl groups or glucose residues to the common S. flexneri O-Ag tetrasaccharide repeating units (3, 9, 10). The O-Ag of S. sonnei type I (phase I) has an O-Ag disaccharide repeating unit (8, 11), which is produced by genes in a large (180 to 200 kb) plasmid (pINV) (11, 12). When S. sonnei loses pINV, it produces LPSs without O-Ag (phase II) and thus is rough and becomes avirulent (11). The structures of LPS O-Ags from S. dysenteriae and S. boydii differ from those of S. flexneri and S. sonnei (9).
Antibodies to Shigella O-Ag can fix complement on the target bacteria and kill them in a serotype-specific manner. Thus, in the wake of successful multivalent pneumococcal conjugate vaccines, a leading approach for Shigella vaccines is to use LPSs conjugated to carrier proteins (13–16). LPS serotypes included in the vaccines are chosen based on their geographical prevalence and their induction of cross-reactivity (13–16). LPS conjugate vaccines often include LPSs from S. flexneri 2a, S. flexneri 3a, and S. sonnei since they are commonly isolated in many parts of the world (17). The development of Shigella LPS conjugate vaccines can be facilitated if one can easily identify the target bacterial strains for vaccine production, measure the quantity of LPSs in the vaccine lots, and determine vaccine-induced immunity. Thus, we have produced monoclonal antibodies (MAbs) to these three serotypes, and we demonstrate their utility in vaccine development assays, such as inhibition enzyme-linked immunosorbent assay (iELISA), bacterial surface staining by flow cytometry, and bactericidal assay (BCA).
MATERIALS AND METHODS
Bacterial isolates.
Eleven reference strains of Shigella were obtained from either the ATCC or the Walter Reed Army Institute of Research (WRAIR) in Washington, DC, as shown in Table 1. An additional 49 clinical isolates from Kenya were serotyped at WRAIR and were supplied by WRAIR to the University of Alabama at Birmingham (UAB), which confirmed the serotyping results using the Shigella antisera set 2 kit (product 294838; Denka Seiken Co. Ltd, Tokyo, Japan).
TABLE 1.
Reference strains of Shigella used in the study
| Strain and serotype | Reference strain name | Source |
|---|---|---|
| S. dysenteriae 1 | ATCC 9361 | ATCC |
| S. dysenteriae 2 | ATCC 9750 | ATCC |
| S. flexneri 2a | ATCC 700930 | ATCC |
| S. flexneri 2a | 2457T | WRAIR |
| S. flexneri 2b | ATCC 12022 | ATCC |
| S. flexneri 3a | J17B | WRAIR |
| S. flexneri 5a | M90T-Wash | WRAIR |
| S. boydii 1 | ATCC 9207 | ATCC |
| S. sonnei I | ATCC 9290 | ATCC |
| S. sonnei I | 53G | WRAIR |
| S. sonnei I | Moseley | WRAIR |
MAb production.
Female BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) at 4 to 6 weeks of age were used for immunization with S. flexneri 2a, S. flexneri 3a, or S. sonnei LPSs. All studies were carried out using a protocol (APN 10019) approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The mice were immunized intraperitoneally with 10 μg of LPS/mouse on days 0, 14, and 28. S. flexneri 2a and S. sonnei LPSs were obtained from the WRAIR and the Lanzhou Institute of Biological Products (LIBP; Lanzhou, China), and S. flexneri 3a LPS was from the WRAIR. LPS was extracted using the hot phenol method of Westphal and Jann (18). After the increased level of antibodies to LPSs was confirmed by ELISA on day 35, mice were boosted intraperitoneally with 10 μg of the same LPS/mouse on day 60. The spleen was removed on day 64, and splenocytes were fused with SP2/0-Ag14. Primary culture wells were screened with LPS ELISA (as described below), and ELISA-positive wells were cloned twice by limiting dilution. The MAb isotypes were determined using the IsoStrip mouse monoclonal antibody isotyping kit (product 11493027001; Sigma). Cloned hybridoma cell lines were kept frozen in liquid nitrogen. Hybridoma supernatant was collected after the hybridoma cell was allowed to grow to the terminal stage in the serum-free medium Hybridoma-SFM (product 12045-076; Gibco).
LPS ELISAs.
The reactivity of MAbs with LPSs isolated from different Shigella serotypes was determined by ELISA. S. flexneri 3a LPS was obtained from the WRAIR, and the LPSs of S. flexneri 2a and S. sonnei were obtained from LIBP. LPS from Escherichia coli 0111:B4 was purchased from Sigma Chemical (St. Louis, MO). Each well of an ELISA plate was coated with 100 μl of 1 μg/ml of LPS in phosphate-buffered saline (PBS) for 18 h at 4°C, washed, and blocked with 0.5% bovine serum albumin (BSA) in PBS. Undiluted samples were loaded into each well and incubated for 1 h. After unbound antibodies were washed away, alkaline phosphatase-conjugated anti-mouse Ig (product A0162; Sigma) was added to each well. After a 1-h incubation, the wells were washed and incubated with p-nitrophenyl phosphate (pNPP), and the optical densities of the plates were measured at 405 nm (OD405). All reactions were performed at room temperature.
For the LPS iELISA, hybridoma supernatants were diluted either 1:20 (Hflex2a1, Hsoni1, and Hsoni5) or 1:40 (Hflex2a4, Hflex3a2, and Hflex3a5) in buffer containing various concentrations of the competing LPS (shown in Fig. 1) and were added to LPS-coated ELISA plates. After incubation for 1 h, unbound antibodies were removed by washing, and the wells were incubated for 1 h with alkaline phosphatase-conjugated anti-mouse Ig (product A0162; Sigma). Then, the wells were washed and incubated with pNPP, and their optical densities were measured at 405 nm.
FIG 1.
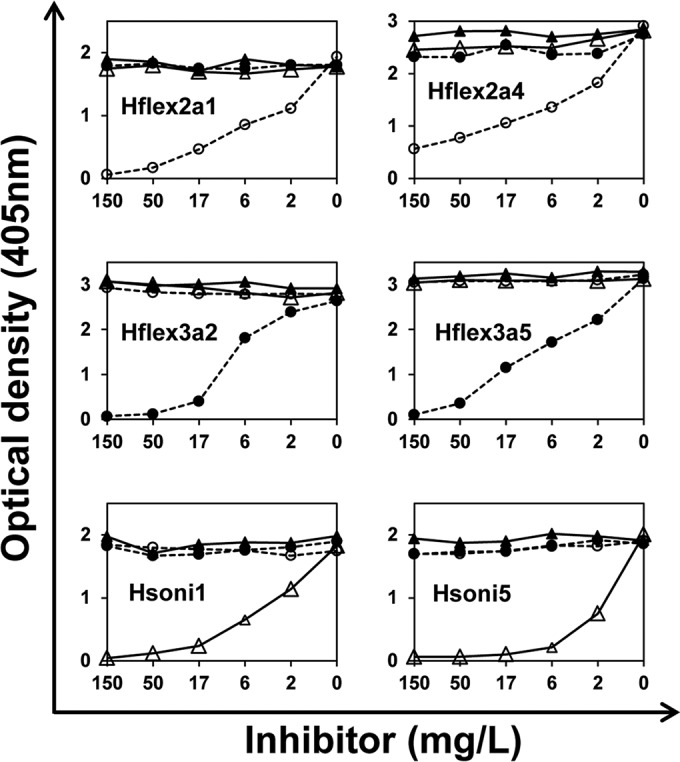
Amount of MAbs bound to the LPS-coated plate (y axis) at various inhibitor concentrations (x axis) in iELISA. MAbs used for each iELISA are shown in each graph. Inhibitors are LPSs from S. flexneri 2a (open circles), S. flexneri 3a (solid circles), S. sonnei (open triangles), and E. coli (solid triangles). Values of 4.7 mg/liter and 5.1 mg/liter of S. flexneri 2a LPSs are needed for 50% inhibition of Hflex2a1 and Hflex2a4, respectively. Values of 8.9 mg/liter and 8 mg/liter of S. flexneri 3a LPSs are needed to inhibit Hflex3a2 and Hflex3a5, respectively. Values of 3.4 mg/liter and 1.5 mg/liter of S. sonnei LPSs are necessary for Hsoni1 and Hsoni5, respectively. All of the experiments were performed more than two times, and one representative example is shown.
Bacterial surface staining by flow cytometry.
Flow cytometric analysis was performed as previously described (19). In brief, frozen bacterial aliquots were washed and resuspended in a flow cytometry buffer (phosphate-buffered saline [PBS; 140 mM NaCl, 3 mM KCl, 5 mM Na2HPO4, 2 mM KH2PO4, pH 7.4] with 3% fetal bovine serum and 0.01% sodium azide). Aliquots of 50 μl (containing ∼5 × 105 CFU) were incubated with 50 μl of hybridoma culture supernatant (diluted 1:2 to 1:250) at 4°C for 30 min. After incubation, bacteria were washed twice and incubated with goat anti-mouse Ig antibody conjugated with phycoerythrin (PE) (product 550589; BD Biosciences). After being washed, the bacteria were examined in a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). The data were analyzed with FCS Express versions 3.0 and 4.0 (De Novo Software, Los Angeles, CA).
Serotyping by agglutination.
The bacterial isolates were serotyped with undiluted hybridoma supernatants as described in the Shigella antisera set 2 kit (Denka Seiken Co. Ltd, Tokyo, Japan). Briefly, each strain was grown on a sheep blood agar plate (BAP) overnight at 37°C. A small portion of a bacterial colony was taken from the BAP and mixed with 10 μl of MAb supernatant on a microscope slide. After the slide was gently tilted back and forth for 1 min, agglutination was examined under indirect light. Only clear and strong agglutinations were scored as positive.
Bactericidal assay procedure.
The BCA was performed by mixing 20 μl of a diluted hybridoma supernatant, 10 μl of target bacteria (∼105 CFU/ml), and 50 μl of 16% baby rabbit serum (product 31061-3; Pel-Freez) as the source of complement in a 96-well microtiter plate. Strains 2457T, J17B, and Moseley were used as target bacteria for S. flexneri 2a, S. flexneri 3a, and S. sonnei, respectively. To simplify the assay, a target bacterial strain was grown in Luria-Bertani (LB) broth and frozen in aliquots at −70°C, and an aliquot was thawed and used for each BCA. After 2 h of incubation at 37°C, 10 μl of reaction mixture was applied to a small area of a square petri dish (12 cm by 12 cm) containing LB agar. Each petri dish was divided into 48 areas to accommodate reaction mixtures from 48 wells of the microtiter plate. The culture was incubated overnight at 29°C to limit the colony size. The agar plate was then overlaid with agar containing 0.1% NaN3 and 100 μg/ml of triphenyltetrazolium chloride (TTC), which is reduced by the growing bacteria to a red compound resulting in colored colonies. The petri dish was photographed with a digital camera, and the number of colonies was determined with a computer program called NICE (NIST's integrated colony enumerator) (20).
RESULTS
Screening MAbs for binding specificity with LPS ELISA and flow cytometry.
As shown in Table 2, the cell fusions yielded a large number of hybridoma clones secreting about 1 to 30 μg/ml of MAbs. To select the hybridoma clones that are useful for further studies, we first determined their immunoglobulin isotypes. Most clones secreting LPS-specific antibodies were IgM, but a few clones were IgG (Table 2). The IgG subclass secreted by the hybridomas was either IgG2 or IgG3, which is consistent with previous reports on MAbs binding T cell-independent type 1 antigens like LPS (21). Antibody-binding specificities of undiluted hybridoma supernatants were also determined by ELISA (summarized in Table 2). As expected, all MAbs bound to the homologous LPS used for immunization. In several cases, the S. flexneri MAbs reproducibly showed cross-reactivity with other S. flexneri serotypes. For instance, Hflex2a2, Hflex2a3, and Hflex2a10 cross-reacted with S. flexneri 3a. Conversely, Hflex3a3 and Hflex3a4 cross-reacted with S. flexneri 2a. Also, Hflex2a3 and Hflex3a4 cross-reacted with E. coli LPS, although the cross-reaction was weak.
TABLE 2.
Properties of MAbs to S. flexneri 2a, S. flexneri 3a, and S. sonnei
| Immunizing LPS serotype | MAb name | Isotype | Specificity by ELISA plates coated with LPS froma |
Specificity by flow cytometry with formalin-fixed target bacteriab |
|||||
|---|---|---|---|---|---|---|---|---|---|
| S. flexneri 2a | S. flexneri 3a | S. sonnei | E. coli | S. flexneri 2a | S. flexneri 3a | S. sonnei | |||
| S. flexneri 2a | Hflex2a1 | IgG3 | + | − | − | − | + | − | − |
| Hflex2a2 | IgG2b | + | + | − | − | + | − | − | |
| Hflex2a3 | IgM | + | ± | − | ± | ± | − | − | |
| Hflex2a4 | IgM | + | − | − | − | + | − | − | |
| Hflex2a5 | IgM | + | − | − | − | + | − | − | |
| Hflex2a6 | IgM | + | − | − | − | + | − | − | |
| Hflex2a7 | IgM | + | − | − | − | + | − | − | |
| Hflex2a8 | IgM | + | − | − | − | + | − | − | |
| Hflex2a9 | IgM | + | − | − | − | + | − | − | |
| Hflex2a10 | IgM | + | + | − | − | − | − | − | |
| S. flexneri 3a | Hflex3a1 | IgG2b | − | + | − | − | − | + | − |
| Hflex3a2 | IgG3 | − | + | − | − | − | + | − | |
| Hflex3a3 | IgG2b | + | + | − | − | − | − | − | |
| Hflex3a4 | IgG2b | + | + | − | ± | − | − | − | |
| Hflex3a5 | IgM | − | + | − | − | − | + | − | |
| Hflex3a6 | IgM | − | + | − | − | − | + | − | |
| Hflex3a7 | IgM | − | + | − | − | − | + | − | |
| Hflex3a8 | IgM | − | + | − | − | − | + | − | |
| Hflex3a9 | IgM | − | + | − | − | − | + | − | |
| Hflex3a10 | IgM | − | + | − | − | − | + | − | |
| Hflex3a11 | IgM | − | + | − | − | − | + | − | |
| Hflex3a12 | IgM | − | + | − | − | − | + | − | |
| Hflex3a13 | IgM | − | + | − | − | − | + | − | |
| Hflex3a14 | IgM | − | + | − | − | − | + | − | |
| Hflex3a15 | IgM | − | + | − | − | − | + | − | |
| Hflex3a16 | IgM | − | + | − | − | − | + | − | |
| Hflex3a17 | IgM | − | + | − | − | − | + | − | |
| Hflex3a18 | IgM | − | + | − | − | − | + | − | |
| S. sonnei | Hsoni1 | IgG3 | − | − | + | − | + | − | + |
| Hsoni2 | IgM | − | − | + | − | − | − | + | |
| Hsoni3 | IgM | − | − | + | − | − | − | + | |
| Hsoni4 | IgM | − | − | + | − | − | − | + | |
| Hsoni5 | IgM | − | − | + | − | − | − | + | |
| Hsoni6 | IgM | − | − | + | − | − | − | + | |
| Hsoni7 | IgM | − | − | + | − | − | − | + | |
| Hsoni8 | IgM | − | − | + | − | − | − | + | |
ELISA results were scored as “−” when the OD405 was less than 0.2, “±” when OD405 was greater than or equal to 0.2 but less than 0.5, and “+” when OD405 was greater than or equal to 0.5. The OD405 of wells with no supernatant was ∼0.1. Undiluted hybridoma supernatants were used for ELISA.
Target strains used for flow cytometric studies were ATCC 700930, J17B, and ATCC 9290, respectively, for S. flexneri 2a, S. flexneri 3a, and S. sonnei. The flow cytometric results are scored as “−” when real signal/background signal (S/N) is less than 2, “±” when S/N is greater than or equal to 2 but less than 5, and “+” when S/N is greater than or equal to 5. Background is the geometric mean fluorescence obtained with no MAb, and the signals are 20, 17, and 16 mean fluorescence units for S. flexneri 2a, S. flexneri 3a, and S. sonnei, respectively. Optimally diluted (ranging from 1:2 to 1:250) supernatants were used for flow cytometry.
To independently confirm the specificities of these MAbs, we tested their binding to formalin-fixed Shigella bacteria by flow cytometry (Table 2). Most of the flow cytometric data are consistent with the ELISA data, with a few exceptions, which primarily involved cross-reactive antibodies. Hflex3a3 and Hflex3a4 did not bind the target bacteria in flow cytometry. No MAbs against S. flexneri LPS cross-reacted with each other or S. sonnei, but the S. sonnei MAb Hsoni1 cross-reacted with S. flexneri 2a. Thus, the observed cross-reactivity may be assay dependent and may not reflect true biologically meaningful cross-reactivity. Taken together, our hybridomas produce MAbs binding the O-Ag and may not bind the more conserved inner core of LPS. Based on these results, we chose 6 out of 36 hybridomas—Hflex2a1, Hflex3a2, Hsoni1, Hflex2a4, Hflex3a5, and Hsoni5—as representative clones for further studies. Two hybridomas (one IgG and one IgM) were chosen for each serotype.
Ability of Shigella-specific MAbs to agglutinate clinical isolates and reference strains of Shigella.
The serotype specificities of the six selected hybridomas were investigated further for their ability to agglutinate clinical isolates representing all four different species of Shigella (Table 3). The clinical isolates were first serotyped with the conventional rabbit serotyping reagents. Undiluted supernatants of the six hybridomas expressing either IgG or IgM MAbs induced clumping. Furthermore, MAbs reacted only with the homologous serotypes and produced results identical to those obtained with the rabbit antisera. Thus, hybridomas produce MAbs specific to the O-Ag of LPS.
TABLE 3.
Slide agglutination test with six selected MAbsc
| Target bacterial serotype | Strain name | Hflex2a1 or Hflex2a4 | Hflex3a2 or Hflex3a5 | Hsoni1 or Hsoni5 |
|---|---|---|---|---|
| S. dysenteriaea | MHK03853 | − | − | − |
| MHK04764 | − | − | − | |
| NTS01572 | − | − | − | |
| MHK04372 | − | − | − | |
| S. dysenteriae 1 | ATCC 9361b | − | − | − |
| S. dysenteriae 2 | ATCC 9750b | − | − | − |
| S. flexneri 1a | NTS01435b | − | − | − |
| S. flexneri 1b | MHK03955b | − | − | − |
| S. flexneri 2a | MHK03875b | + | − | − |
| MHK03876 | + | − | − | |
| MHK04642 | + | − | − | |
| MHK04649 | + | − | − | |
| MHK04687 | + | − | − | |
| MHK04964 | + | − | − | |
| MHK04720 | + | − | − | |
| NTS01277 | + | − | − | |
| NTS01021 | + | − | − | |
| NTS01011 | + | − | − | |
| ATCC 700930 | + | − | − | |
| 2457Tb | + | − | − | |
| S. flexneri 2b | ATCC 12022b | − | − | − |
| S. flexneri 3a | MHK03936b | − | + | − |
| MHK03563 | − | + | − | |
| MHK05049 | − | + | − | |
| NTS01591 | − | + | − | |
| MHK03620 | − | + | − | |
| MHK04508 | − | + | − | |
| MHK04754 | − | + | − | |
| MHK03792 | − | + | − | |
| MHK01327 | − | + | − | |
| MHK03857 | − | + | − | |
| J17Bb | − | + | − | |
| S. flexneri IVa | MHK04643b | − | − | − |
| MHK04947 | − | − | − | |
| NTS01425b | − | − | − | |
| MHK03739 | − | − | − | |
| MHK04790 | − | − | − | |
| S. flexneri 5a | M90T-Washb | − | − | − |
| S. flexneri 6 | MHK04055 | − | − | − |
| NTS00780b | − | − | − | |
| NTS00731b | − | − | − | |
| NTS01352 | − | − | − | |
| MHK03789 | − | − | − | |
| S. boydiia | MHK03826 | − | − | − |
| NTS01627 | − | − | − | |
| MHK03680 | − | − | − | |
| S. boydii 1 | ATCC 9207b | − | − | − |
| S. sonnei I | MHK04042 | − | − | + |
| MHK04536 | − | − | + | |
| NTS01648b | − | − | + | |
| ATCC 9290 | − | − | + | |
| 53Gb | − | − | + | |
| Moseley | − | − | + | |
| S. sonnei II | MHK03809b | − | − | − |
| MHK03868 | − | − | − | |
| NTS00870b | − | − | − | |
| MHK04321 | − | − | − | |
| MHK04955 | − | − | − | |
| NTS01169 | − | − | − | |
| NTS00961 | − | − | − |
Subtypes are unknown.
These are the 19 strains used for the iELISA studies.
+, test results showed large and unambiguous clumps; −, results showed no evidence of clumps.
Studying the six MAbs for binding LPS with iELISA and flow cytometry.
A valuable assay for Shigella vaccine production would be able to measure the amount of LPSs from different serotypes contained within a multivalent formulation. As an iELISA based on MAbs can be used to measure LPS concentrations, we investigated the six selected hybridomas for an iELISA. As seen in Fig. 1, iELISA can be developed using hybridoma supernatants diluted 1:20 (Hflex2a1, Hsoni1, and Hsoni5) or 1:40 (Hflex2a4, Hflex3a2, and Hflex3a5), and the iELISA using the MAbs was sensitive (down to 1 to 10 mg/liter) and highly specific to the homologous serotype.
To investigate the usefulness of iELISA in serotyping Shigella isolates, we examined culture supernatants from 19 selected strains (indicated in Table 3) with iELISA based on MAbs Hflex2a4, Hflex3a5, and Hsoni5. The selected strains represent the four species of Shigella. Supernatants of strains expressing LPS homologous specificity of iELISA inhibited more than 40% at 1:3 dilutions; however, supernatants from strains expressing nonhomologous serotypes inhibited 20% or less at 1:3 dilutions (see Fig. S1 in the supplemental material). The findings suggest that S. flexneri 2a, S. flexneri 3a, and S. sonnei lysates have about 11 μg/ml, 38 μg/ml, and 5 μg/ml of LPS, respectively. Thus, the iELISA is also useful in typing bacterial isolates.
Inactivated whole bacteria are often investigated as Shigella vaccine candidates (22). To investigate whether MAbs from the six hybridomas can be used to characterize such whole-cell vaccines, we studied MAbs for their usefulness in the staining of individual bacteria using flow cytometry. MAbs demonstrated selective and unambiguous staining of bacteria when the MAbs were used at optimal dilutions (described in Fig. 2). Generally, IgM antibodies required higher dilutions than IgG antibodies, perhaps because IgM antibodies may clump the bacteria and prevent staining at lower dilutions.
FIG 2.
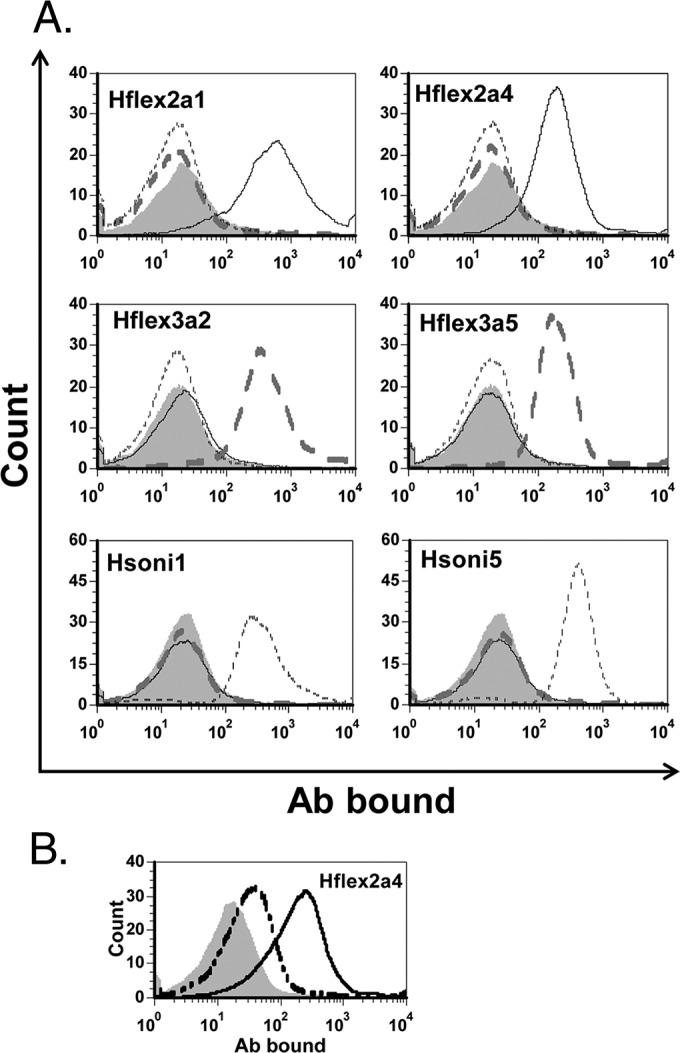
Flow cytometry results with different target bacteria and MAbs, which are identified in each graph. (A) The hybridoma supernatants were diluted 1:2 (Hflex2a1), 1:50 (Hsoni1, Hsoni5), or 1:250 (all others). Target bacteria are S. flexneri 2a (black solid line), S. flexneri 3a (gray dashed line), and S. sonnei (black dotted line). Gray filled areas show negative controls obtained with the bacteria (targeted by the MAb) in the absence of MAb. (B) Flow cytometric results with S. flexneri 2b target bacteria stained with Hflex2a4 diluted 1:10 (solid line) or 1:250 (dashed line) and with no MAb (gray filled area). All of the experiments were performed more than two times, and one representative example is shown here.
Bactericidal activity of Shigella-specific MAbs.
An important measure of vaccine-induced immunity is studying immune serum for its ability to kill bacteria in vitro. Since MAbs can be useful as controls or standards in bactericidal assays, we examined the supernatants from the six selected hybridomas for killing various Shigella strains. To obtain supernatants useful for BCA, hybridoma cells were grown in an antibiotic-free medium without animal sera (Hybridoma-SFM; Gibco) since animal sera often have bactericidal activity (data not shown). Monoclonal antibody Hflex2a1 killed S. flexneri 2a isolates specifically (Fig. 3), but Hflex2a4 killed S. flexneri 2b isolates in addition to S. flexneri 2a isolates (Fig. 3). Consistent with this observation, Hflex2a4 also bound S. flexneri 2b by flow cytometry (Fig. 2). Hflex3a2 and Hflex3a5 killed S. flexneri 3a isolates only, and Hsoni1 and Hsoni5 killed S. sonnei isolates only (Fig. 3). Interestingly, some survivors were noted with target strain Moseley (S. sonnei). The survivor may have lost the pINV plasmid spontaneously and escaped killing by antibodies. Also, we noted that high concentrations of IgM antibodies showed a prozone effect in killing (see Fig. S2 in the supplemental material). Thus, BCAs with IgM MAbs were performed after a predilution of 1:100 to 1:5,000. Interestingly, the MAbs that showed a cross-reaction with S. flexneri 2a LPS by ELISA (e.g., Hflex3a3 and Hflex3a4) did not cross-kill S. flexneri 2a isolates (see Fig. S3 in the supplemental material). None of our MAbs killed S. flexneri 5a, S. dysenteriae 1, S. dysenteriae 2, or S. boydii 1 target bacteria (see Fig. S4 in the supplemental material).
FIG 3.

Bactericidal assay results. The relative numbers of surviving target bacteria (y axis) at various additional dilutions of a MAb (x axis) are shown. Names of the MAbs are indicated in each graph. Serial dilution was performed for each MAb after a predilution, which was 1:5,000 for Hflex3a5 against target strain S. flexneri 3a; 1:1,000 for Hflex2a4 against target strains S. flexneri 2a, S. flexneri 2b, and S. flexneri 3a; or 1:100 for Hflex3a2 against S. flexneri 3a and for Hsoni5 against S. sonnei. Target strains were 2457T for S. flexneri 2a (solid square), ATCC 1202 for S. flexneri 2b (open square), J17B for S. flexneri 3a (solid triangle), and ATCC 9290 for S. sonnei (solid circle). All of the experiments were performed more than two times, and one representative example is shown here.
DISCUSSION
Previous studies have described MAbs to LPSs from S. flexneri 2a (23, 24), S. flexneri 3a (25), and S. sonnei (11, 26). However, the MAbs were not characterized for the assays, such as BCA and flow cytometry, that are useful for vaccine development. Herein, we describe the production of MAbs to three common serotypes of Shigella and demonstrate their usefulness in iELISA, bacterial surface labeling by flow cytometry, and BCA, which are important assays for Shigella vaccine development. As the MAbs are serotype specific, they likely target the O-Ag and not the more-conserved inner core of the LPS.
We found iELISA based on these MAbs to be very useful for measuring the concentrations of LPS or O-Ag in bacterial culture supernatants or the purified LPS that is used to make conjugate vaccines. At the moment, LPS concentrations are determined by chemical assays or bioassays that do not distinguish different serotypes of LPSs. For instance, the WRAIR uses Endosafe-PTS, manufactured by Charles River (R. W. Kaminski, personal communication). Inhibition ELISA is a very simple, efficient, and reliable assay that can be established only with hybridoma supernatant without the need to purify MAbs. Inhibition ELISA is also sensitive, as it can detect 1 to 10 mg/liter of LPS (Fig. 1). Inhibition ELISA is also highly specific. It can be used to determine the serotype and, with appropriate MAbs, also detect O-acetylation, which is immunologically important yet could be lost during O-Ag purification for vaccine production. In contrast, the O-acetylation may be difficult to monitor with bioassay or chemical assays. Moreover, although the conventional chemical assays or bioassays cannot be multiplexed, MAb-based iELISA can be easily multiplexed with bead array technology (e.g., Luminex), as we have shown with pneumococcal antibodies (27). Since future LPS conjugate vaccines will be multivalent, the MAb-based iELISAs should be very useful in vaccine development by measuring LPS concentrations or acetylations of multiple types of LPS during vaccine production.
Immunoassays for Shigella LPSs would be useful for nonvaccine studies as well. For instance, the multiplexed iELISA may be very useful for serotyping Shigella isolates as was done for pneumococci (27). Another important application may be a rapid, direct, and simple detection of LPSs in stool samples with dipstick assays for shigella LPS (24, 26). Such an assay would obviate stool culture and facilitate the epidemiologic studies necessary for developing LPS conjugate vaccines in resource-poor locations.
Flow cytometric assays have been infrequently used with bacterial targets until recently. With the maturation of instrumentation, flow cytometry permits one to easily examine the expression of LPSs or other virulence factors at a single-cell level. Flow cytometric assays with these MAbs may be used to monitor the loss of pINV and consequently O-Ag from S. sonnei cultures in fermentors. It can be used to study the mixtures of target bacteria, which will be used to make multivalent whole-cell vaccines. Also, we can simultaneously monitor other parameters. For instance, one can simultaneously monitor the expression of LPS as well as some other protein virulence factors that may elicit protective antibodies. We believe that flow cytometric assays should be generally useful in whole-cell vaccine development.
BCAs should play an important role in Shigella vaccine evaluations because of the lack of animal models that replicate human infections (development of gastrointestinal symptoms with low doses of infection) (4, 5). An important observation was that these MAbs can kill target bacteria in an in vitro BCA, and therefore, these MAbs can be useful in the additional development of BCAs and/or for their standardization. Interestingly, the BCA against S. sonnei showed some survivors (very small percentage) when Moseley was used as the target. While these survivors were too few to affect BCA results, the survivors may have lost the plasmid pINV and produce LPSs with no O-Ag.
Induction of cross-protection is an important consideration in designing multivalent Shigella vaccines. Although previous studies have demonstrated that vaccination with S. flexneri 2a or with a recombinant vaccine expressing S. flexneri 2a O-Ag generates cross-reactive antibodies in a variety of species (4, 5), these studies used binding assays or animal models that do not replicate human diseases. Carlin and Lindberg reported that ELISA cross-reactivity was not reproduced with agglutination assays (25). Also, we show that cross-reactivity of MAbs can be demonstrated with one assay and not the other (i.e., ELISA versus flow cytometry). Thus, cross-binding may not imply actual cross-protection. In our studies, Hflex2a4 was shown to bind both S. flexneri 2a and S. flexneri 2b by flow cytometry and to kill both S. flexneri 2a and S. flexneri 2b target bacteria. Perhaps, the S. flexneri 2a LPS may elicit antibodies that not only cross-react with the S. flexneri 2b LPS but also cross-protect against S. flexneri 2b.
Taken together, our hybridomas should be useful in Shigella vaccine development. Our MAbs can serve as standards for these important assays for vaccine development, and they can be used for epidemiologic studies. To make our MAbs available to the public and enhance their broad use, we plan to deposit the six selected hybridoma lines in a cell-line bank.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by a grant from PATH to M.H.N. This study was conducted as a Cooperative Research and Development Agreement between the University of Alabama at Birmingham and the Walter Reed Army Institute of Research.
A number of the authors are employees of the U.S. government (R.W.K. and M.A.S.), and as such the views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, or the U.S. Government.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00148-16.
REFERENCES
- 1.Das JK, Tripathi A, Ali A, Hassan A, Dojosoeandy C, Bhutta ZA. 2013. Vaccines for the prevention of diarrhea due to cholera, Shigella, ETEC and rotavirus. BMC Public Health 13(Suppl:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. 2007. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman AB, Van de Verg LL, Mainhart CR, Tall BD, Smith-Gill SJ. 1996. Specificity of monoclonal antibodies elicited by mucosal infection of BALB/c mice with virulent Shigella flexneri 2a. Clin Diagn Lab Immunol 3:584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van De Verg LL, Bendiuk NO, Kotloff K, Marsh MM, Ruckert JL, Puryear JL, Taylor DN, Hartman AB. 1996. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine 14:1062–1068. doi: 10.1016/0264-410X(96)00006-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. 1991. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol 134:614–627. [DOI] [PubMed] [Google Scholar]
- 7.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis 164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd JG, Wang L, Reeves PR. 2000. Comparison of O-antigen gene clusters of Escherichia coli (Shigella) Sonnei and Plesiomonas shigelloides O17: Sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect Immun 68:6056–6061. doi: 10.1128/IAI.68.10.6056-6061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perepelov AV, Shekht ME, Liu B, Shevelev SD, Ledov VA, Senchenkova SN, L'Vov VL, Shashkov AS, Feng L, Aparin PG, Wang L, Knirel YA. 2012. Shigella flexneri O-antigens revisited: final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol Med Microbiol 66:201–210. doi: 10.1111/j.1574-695X.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Wang Q, Reeves PR, Wang L. 2008. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev 32:627–653. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 11.Viret JF, Bruderer U, Lang AB. 1992. Characterization of the Shigella serotype D (S. sonnei) O polysaccharide and the enterobacterial R1 lipopolysaccharide core by use of mouse monoclonal antibodies. Infect Immun 60:2741–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopecko DJ, Washington O, Formal SB. 1980. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun 29:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor DN, Sadoff JC, Chu C, Shiloach J, Schneerson R, Robbins JB. 1996. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun 64:4074–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. 1999. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun 67:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins JB, Chu C, Schneerson R. 1992. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis 15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DN, Trofa AC, Sadoff J, Chu C, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan WSR, Robbins JB. 1993. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun 61:3678–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Faruque AS, Alonso PL, Breiman RF, Zaidi AK, Sur D, Sow SO, Berkeley LY, O'Reilly CE, Mintz ED, Biswas K, Cohen D, Farag TH, Nasrin D, Wu Y, Blackwelder WC, Kotloff KL, Nataro JP, Levine MM. 2014. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure, p 83–91. In Whistler R, Wolfan M (ed), Methods in carbohydrate chemistry. Academic Press, New York, NY. [Google Scholar]
- 19.Brady AM, Calix JJ, Yu J, Geno KA, Cutter GR, Nahm MH. 2014. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J Infect Dis 210:1155–1165. doi: 10.1093/infdis/jiu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke ML, Burton RL, Hill AN, Litorja M, Nahm MH, Hwang J. 2010. Low-cost, high-throughput, automated counting of bacterial colonies. Cytometry A 77:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slack J, Der-Balian GP, Nahm M, Davie JM. 1980. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med 151:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. 2014. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol 21:366–382. doi: 10.1128/CVI.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlin NI, Lindberg AA. 1983. Monoclonal antibodies specific for O-antigenic polysaccharides of Shigella flexneri: clones binding to II, II:3,4, and 7,8 epitopes. J Clin Microbiol 18:1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nato F, Phalipon A, Nguyen TL, Diep TT, Sansonetti P, Germani Y. 2007. Dipstick for rapid diagnosis of Shigella flexneri 2a in stool. PLoS One 2:e361. doi: 10.1371/journal.pone.0000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlin NI, Lindberg AA. 1986. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type I and type III:6,7,8 antigens, group 6 antigen, and a core epitope. Infect Immun 53:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duran C, Nato F, Dartevelle S, Thi Phuong LN, Taneja N, Ungeheuer MN, Soza G, Anderson L, Benadof D, Zamorano A, Diep TT, Nguyen TQ, Nguyen VH, Ottone C, Begaud E, Pahil S, Prado V, Sansonetti P, Germani Y. 2013. Rapid diagnosis of diarrhea caused by Shigella sonnei using dipsticks; comparison of rectal swabs, direct stool and stool culture. PLoS One 8:e80267. doi: 10.1371/journal.pone.0080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Lin J, Kim KH, Benjamin WH Jr, Nahm MH. 2011. Development of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin Vaccine Immunol 18:1900–1907. doi: 10.1128/CVI.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


