Abstract
Advances in the treatment of childhood cancers have resulted in part from the development of national and international collaborative initiatives that have defined biologic determinants and generated risk-adapted therapies that maximize cure while minimizing acute and long-term effects. Currently, more than 80% of children with cancer who are treated with modern multidisciplinary treatments in developed countries are cured; however, of the approximately 160,000 children and adolescents who are diagnosed with cancer every year worldwide, 80% live in low- and middle-income countries (LMICs), where access to quality care is limited and chances of cure are low. In addition, the disease burden is not fully known because of the lack of population-based cancer registries in low-resource countries. Regional and ethnic variations in the incidence of the different childhood cancers suggest unique interactions between genetic and environmental factors that could provide opportunities for etiologic research. Regional collaborative initiatives have been developed in Central and South America and the Caribbean, Africa, the Middle East, Asia, and Oceania. These initiatives integrate regional capacity building, education of health care providers, implementation of intensity-graduated treatments, and establishment of research programs that are adjusted to local capacity and local needs. Together, the existing consortia and regional networks operating in LMICs have the potential to reach out to almost 60% of all children with cancer worldwide. In summary, childhood cancer burden has been shifted toward LMICs and, for that reason, global initiatives directed at pediatric cancer care and control are needed. Regional networks aiming to build capacity while incorporating research on epidemiology, health services, and outcomes should be supported.
INTRODUCTION
Advances in clinical and biologic characterization, the development of risk-adapted therapies, and the optimization of supportive care have resulted in a dramatic increase in the cure rates of children with cancer over the last four decades.1,2 Collaborative work by North American and European pediatric oncology consortia have been a centerpiece in achieving these milestones. Prognostic clinical and biologic factors have been identified and treatments have been optimized, often through complex biology-based risk stratification algorithms. However, one indisputable truth defines pediatric oncology: the most important prognostic factor for a child with cancer is where he or she was born.3 Thus, the major challenge in pediatric oncology for the next few decades will be how to translate gains achieved in higher-income settings to all children worldwide. Carefully designed initiatives, development of national and regional frameworks for collaboration, and incorporation of clinical, biomedical, and health services research are crucial to meeting this challenge.4 We believe that regional consortia are an ideal platform and potential catalyst for development and coordination of these activities. In this article, we define why pediatric oncology is a global health problem, describe regional differences in pediatric cancer burden, and summarize the state of regional and international collaborative efforts in limited-resource settings.
PEDIATRIC ONCOLOGY AS A GLOBAL PROBLEM
As low- and middle-income countries (LMICs) continue to improve their health status, the need to develop and maintain cancer programs is becoming imperative. Globally, the number of new cancer cases in all age groups will increase from 12.7 million in 2008 to 22.2 million by 2030. An increasing proportion of this cancer burden falls on LMICs, not only because of demographic change, but also because of a transition in risk factors resulting from globalization of economies and behaviors.5,6 However, there is a dramatic inequity in the distribution of resources for cancer care and control worldwide. Although almost 80% of the disability-adjusted life-years lost worldwide to cancer are in LMICs, these countries have less than 5% of global resources for cancer care and control.7 The global economic cost of new cancer cases in 2009, including medical and nonmedical costs, productivity losses, and the cost of cancer research, was estimated to be at least US$286 billion.8 Although LMICs host more than two thirds of the world population and new cancer cases, they account for only 6.2% of the financial expenditures on cancer worldwide.9
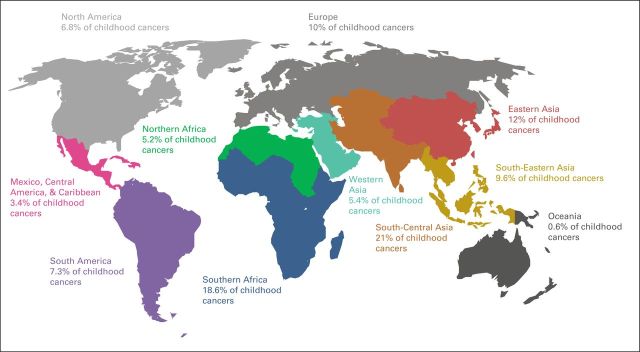
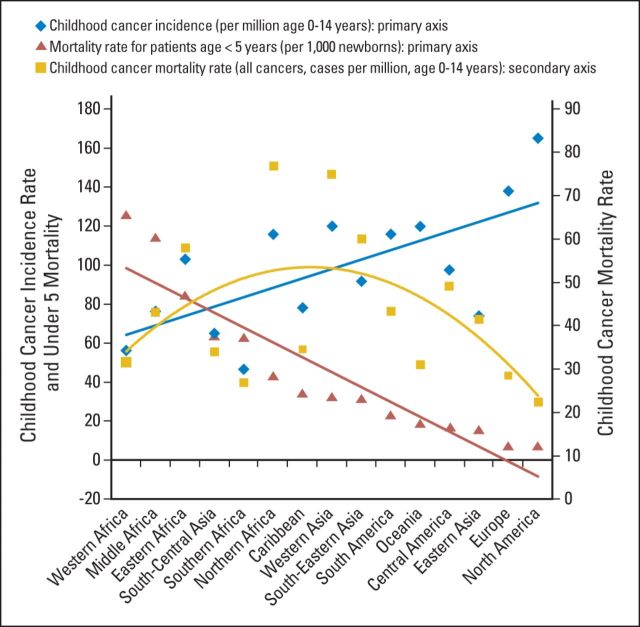
Collaborative efforts are needed to address these unique national and regional circumstances. Of the estimated more than 160,000 children and adolescents diagnosed with cancer every year, approximately 80% live in countries with limited resources (Fig 1). The crude numbers are sensitive to the reference population, incidence rates reported, and perspective used—economic (Table 1) versus regional (Table 2) —but the estimation of burden is consistent. The mortality rate for children younger than age 5 years (under-5 mortality rate [U5MR]) tends to be a good reflection of the strength of childhood health care delivery systems.13 Merged data from the World Bank and GLOBOCAN show that U5MR and childhood cancer incidence are inversely correlated (Fig 2); therefore, as child health care systems improve and competing causes of mortality decrease, the number of childhood cancer cases increases. In LMICs, the expectation is that pediatric cancer cases will increase by 30% by the end of this decade. Childhood cancer mortality, however, is polynomial: mortality rate estimates are low when cancer incidence and development are low, highest when development and cancer incidence are moderate, and low again when development and cancer incidence are high (Fig 2).
Fig 1.
Global distribution of childhood cancer by region. Source: World Bank Databank10 and GLOBOCAN 2012.11
Table 1.
Estimated Annual Cases of Childhood Cancer Worldwide by Income Level
| Income Category | Total Population* | Population Age 0-14 Years (as % of population)* | Population Age 0-14 Years | Incidence of Childhood Cancer (per million)† | Expected Childhood Cancer Cases | Percentage Living in LMIC |
|---|---|---|---|---|---|---|
| High | 1,306,000,000 | 17.3 | 226,068,600 | 148 | 22,458 | |
| Upper middle | 2,409,000,000 | 21.9 | 527,089,200 | 118 | 62,197 | |
| Lower middle | 2,561,000,000 | 32.0 | 818,751,700 | 73 | 59,769 | |
| Low | 848,700,000 | 39.3 | 333,199,620 | 76 | 25,323 | |
| Total | 7,124,700,000 | — | 1,905,109,120 | — | 180,747 | |
| Total for LMICs | 147,289 | 81.5 |
Abbreviation: LMIC, low- and middle-income country (combination of upper middle–, lower middle–, and low-income countries).
Source: World Bank Data by Country.10
GLOBOCAN does not report incidence by World Bank Atlas Method categories. Incidence for very high, high, medium, and low human development level was used as shown for high-, upper middle–, lower middle–, and low-income countries, respectively. The total number of cases for age 0-14 years reported in GLOBOCAN 2012 for the world is 163,282. Source: International Agency for Research on Cancer: GLOBOCAN 2012.11
Table 2.
Estimated Annual Cases of Childhood Cancer Worldwide by Region
| Region | Total Population* | U5MR* | Percentage of Total Population Age 0-14 Years* | Population Age 0-14 Years†‡ | Percentage of Global Population Age 0-14 Years† | Incidence of Childhood Cancer (per million)§ | Childhood Cancer Cases† |
|
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Europe | 743,008,000 | 7 | 15.6 | 115,857,187 | 6.3 | 139 | 16,104 | 9.9 |
| North America | 353,860,000 | 7 | 19.0 | 67,220,072 | 3.6 | 165 | 11,091 | 6.8 |
| Latin American and Caribbean | 611,122,000 | 21 | 26.0 | 158,891,720 | ||||
| Caribbean | 40,972,000 | 34 | 24.9 | 10,195,443 | 0.6 | 79 | 805 | 0.5 |
| Central America | 164,211,000 | 18 | 29.0 | 47,559,950 | 2.6 | 99 | 4,708 | 2.9 |
| South America | 405,938,000 | 22 | 25.0 | 101,351,072 | 5.5 | 117 | 11,858 | 7.3 |
| Africa | 1,150,795,000 | 96 | 40.0 | 460,318,000 | ||||
| Northern Africa | 223,477,000 | 42 | 32.2 | 71,942,482 | 3.9 | 117 | 8,417 | 5.2 |
| Eastern Africa | 363,289,000 | 84 | 43.9 | 159,659,240 | 8.6 | 103 | 16,445 | 10.1 |
| Middle Africa | 143,590,000 | 113 | 43.0 | 61,788,040 | 3.3 | 78 | 4,819 | 3.0 |
| Western Africa | 338,190,000 | 124 | 43.0 | 145,275,339 | 7.9 | 57 | 8,281 | 5.1 |
| Southern Africa | 56,091,000 | 61 | 29.0 | 16,239,477 | 0.9 | 46 | 747 | 0.5 |
| Asia | 4,306,686,000 | 44 | 24.3 | 1,046,524,698 | ||||
| Western | 247,120,000 | 32 | 29.5 | 72,910,480 | 3.9 | 120 | 8,749 | 5.4 |
| Eastern | 1,590,701,000 | 17 | 16.7 | 265,708,479 | 14.4 | 74 | 19,662 | 12.1 |
| South Central | 1,831,151,000 | 63 | 29.3 | 536,639,626 | 29.0 | 64 | 34,345 | 21.1 |
| South Eastern | 637,714,000 | 31 | 26.6 | 169,424,119 | 9.2 | 92 | 15,587 | 9.6 |
| Oceania | 36,709,000 | 20 | 22.3 | 8,202,179 | 0.4 | 120 | 984 | 0.6 |
| Summary | 7,176,023,000 | 53 | 40.0 | 1,849,909,185 | 28.6 | 88 | 162,603 | |
Abbreviation: U5MR, under-5 mortality rate (ie, mortality rate for children younger than age 5 years).
U5MR is the probability of dying between birth and exactly 5 years of age expressed per 1000 live births. See United States Census Bureau, International Programs, International Database12 for a list of countries included in each region.
Values obtained from calculations based on population and incidence data shown.
Values represent results of calculation of percentage indicated in Percentage of Total Population Age 0-14 Years column of total population included in Total Population column.
Source: International Agency for Research on Cancer: GLOBOCAN 2012.11
Fig 2.
The mortality rate for children younger than age 5 years (under-5 mortality rate [U5MR]), childhood cancer incidence, and childhood all-cancer mortality by region. U5MR and cancer incidence show opposite linear trends: as U5MR decreases, cancer incidence increases (r2 = −0.617). Sources: World Bank Databank for U5MR10 and GLOBOCAN 201211 for age-specific (0 to 14 years old) cancer incidence and mortality.
In LMICs, survival of children with cancer is directly proportional to several health indicators, including number of physicians and nurses per 1,000 population, annual government health care expenditure per capita, and several center-specific indicators such as human resources and level of supportive care.14,15 Strengthening of the health care systems as a whole is required for sustainability and scale-up health interventions.
CHILDREN WITH CANCER IN LMICS
Efforts aimed at improving outcomes for pediatric cancer in LMICs must consider the unique features of the problem—the host, the diseases, and the social, economic, and cultural contexts—and how these features may vary among and within regions.4 Examples from the perspectives of epidemiology, treatment delivery, and policy follow.
Epidemiology of Childhood Cancer
Little is known about the epidemiology of pediatric cancer in LMICs. Large portions of the world's population are not covered by cancer registries, especially those countries in which predictions indicate that the cancer burden is growing most rapidly.16 National and regional initiatives to develop or strengthen cancer registries to serve the pediatric population are critical for measuring cancer burden in specific populations and for generating information for etiologic research. This is particularly important, given the differences in incidence rates of childhood cancers between high- and low-income countries and also among the various ethnic and racial groups within a single country.4,17–19 Such differences may be the result of genomic determinants associated with race and ethnicity, early or delayed exposure to infectious diseases, and other environmental factors17,18,20–22; thus, comprehensive descriptive and molecular epidemiology studies are needed to elucidate these possible genome-environment interactions. The Global Initiative for Cancer Registry Development is addressing this gap through the establishment of six regional hubs that offer regional training, technical advice, and research support to registry staff; the focus is on empowering countries to develop cancer control plans by providing support and sharing knowledge.
Treatment of Children With Cancer in Resource-Limited Settings
Late presentation, abandonment of therapy, coexisting debilitating conditions such as malnutrition and infections, suboptimal supportive and palliative care, and inefficient health care delivery systems represent major limitations to pediatric cancer care in LMICs.4 However, curing children with cancer in a cost-effective manner is possible, even in the most deprived settings, as shown with the successful management of Burkitt lymphoma in sub-Saharan Africa, where long-term survival of approximately 50% has been achieved with treatment costs below US$100 per patient.23,24 Direct translation of effective protocols in high-income countries (HICs) to children in LMICs is not possible; adaptations are necessary to address inadequate health care capacity associated with limited resources, underdeveloped health care infrastructure, scarcity of pharmaceuticals, and cultural barriers.25 Treatment guidelines developed for countries with few resources are often based on low-intensity therapies in North America and Europe; intensity-adjusted treatment strategies are then increased gradually as safety and feasibility are documented and treatment-related mortality is decreased.26–31 These disease-specific and level-specific guidelines have provided a starting point for developing national and regional cancer control programs as well as collaborative clinical research initiatives. In addition, the development of these guidelines may give policy makers insights into how to plan resource-appropriate cancer care and control programs at both national and regional levels.25 Implementing these tailored approaches requires the development and validation of tier systems that accurately reflect the status of regions, countries, and pediatric oncology units of interest.27,31
Essential Medicines for Childhood Cancer
Among the myriad challenges to be overcome in LMICs are the availability, accessibility, and affordability of both antineoplastic and supportive care drugs.4 Thirty years ago, the WHO established a list of medications deemed essential for the general population.32 This list has been updated and is now reviewed every 2 years.33,34 The Model List includes only 14 antineoplastic drugs, including four corticosteroids (Appendix Table A1, online only).36 Independently, the Essential Medicines Working Group of the International Society of Pediatric Oncology (SIOP) has proposed a notable expansion of antineoplastic and supportive care drugs with a focus on LMICs. Agents included in the SIOP list are bleomycin, carboplatin, cisplatin, dacarbazine, etoposide, hydroxyurea, ifosfamide, and vinblastine, all of which are included in current standard treatment protocols. Within the past year, the Union for International Cancer Control has collaborated with the WHO to promote further additions to the Model List with input from the SIOP Working Group.
REGIONAL DIFFERENCES IN THE PEDIATRIC CANCER BURDEN AND DEVELOPMENT OF COLLABORATIVE EFFORTS IN LIMITED-RESOURCE SETTINGS
Although they are significant, the many challenges that LMICs face in the diagnosis and treatment of children with cancer represent a unique opportunity for the development of focused initiatives adjusted to the particularities of each region.
Africa and the Middle East
Africa, home to approximately 15% of the world's population and 25% of the world's children (500 million) is the most deprived continent, with notoriously deficient health care systems and competing health care needs that limit the development of cancer control programs (Table 2). The North Africa region is similar to the Middle East and/or Western Asia in terms of socioeconomic and cultural frameworks, and thus, they are discussed together. The young population in this region is expanding, with more than one third of the population consisting of individuals younger than age 15 years. North Africa and Western Asia account for approximately 10% of worldwide childhood cancer (Fig 1 and Table 2). Over the years, child health has improved and U5MR has decreased to its current level of below 40 per 1,000 live births. However, over the last two decades, unstable political situations, wars, and forced human displacements have had a deleterious impact on the health care systems of the region.37 Nevertheless, cooperative initiatives have established a new ground for regional support and development. The Middle East Cancer Consortium, an initiative sponsored by the US National Cancer Institute, was established in 1996 through an official agreement of the ministries of health of Cyprus, Egypt, Israel, Jordan, and the Palestinian Authority; Turkey joined the Consortium in 2004. The major activities of Middle East Cancer Consortium include the Cancer Registry Project and the Palliative Care Project, which notably includes cross-border collaborations between Arabs and Israelis.38 The pediatric-focused Middle East Childhood Cancer Alliance established in 2000 comprises member institutions from 16 countries in the Middle East and North Africa. The Middle East Childhood Cancer Alliance reported its first prospective study (CALLME1) to assess the feasibility of and establish mechanisms for collaborative data collection and management in the Middle East and to collect prospective data on childhood acute lymphoblastic leukemia (ALL).39 Pediatric oncologists from 22 countries in the Middle East and Mediterranean region established the Pediatric Oncology East and Mediterranean (POEM) Group (Fig 3 and Appendix Table A2, online only). Current priorities of POEM are to assess the needs in the different countries, establish robust registries and data management procedures, provide quality training to pediatric oncology professionals, install infection control and supportive care guidelines, secure access to care for all patients, and develop and apply resource-appropriate evidence-based guidelines. The group is also working on standardization for quality control and accreditation, public awareness and prevention, strategic public policy, and advocacy with local governments and communities.
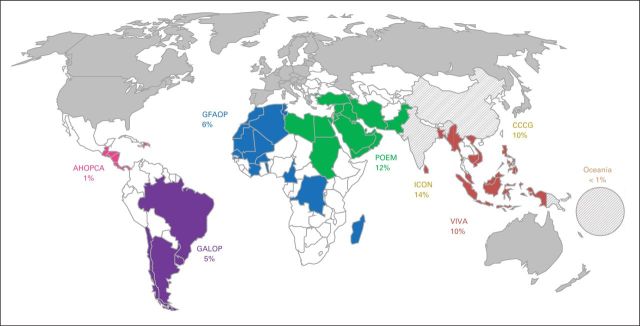
Fig 3.
Regional collaborative efforts identified the expected proportion of childhood cancer covered by each consortium (total, 58.5%). The Chinese Childhood Cancer Group (CCCG) and India's Indian Cooperative Oncology Network (ICON) are included, despite being national rather than international consortia, based on the size of the population they cover. India is also a member of Pediatric Oncology East and Mediterranean (POEM) Group, but case contribution is included only once (for ICON). Algeria, Morocco, and Tunisia are members of the Franco-African Group of Pediatric Oncology (GFAOP) and POEM; their case contribution is included only once (for POEM). Source: World Bank Databank10 and GLOBOCAN 2012.11 AHOPCA, Central American Association of Pediatric Hematology and Oncology; GALOP, Latin American Pediatric Oncology Group; VIVA, VIVA Foundation for Children with Cancer in Singapore.
The largest proportion of the African population lives in sub-Saharan countries where life expectancy is low. According to GLOBOCAN 2012, approximately 6% of the world's total new cancer cases at all ages and more than 20% of childhood cancer cases occur in Africa (Table 2).40 With current rates of population growth and improvements in the control of communicable diseases, the number of new cancer cases in Africa will increase by 70% between 2012 and 2030, yet less than half of sub-Saharan African countries have an operational policy, strategy, or action plan for cancer control.41 Several international organizations are collaborating to build capacity for sustainable cancer research programs in the region. For example, the International Network for Cancer Treatment and Research cancer registry program is coordinating an African Cancer Registry Network and working to improve the performance of existing registries. The African Cancer Registry Network is acting as a consortium that is building several regional hubs as part of the International Agency for Research on Cancer Global Initiative for Cancer Registry Development.42
More than 40% of the population in sub-Saharan Africa is younger than age 15 years; an estimated 40,000 new cases of pediatric cancers are expected to occur annually, representing approximately 20% of the world's total number (Fig 1 and Table 2). Although the region has the world's highest U5MR, with a regional average of 110 per 1000 live births per year, 90% of sub-Saharan countries have had faster decreases in child mortality from 2000 to 2013 than from 1990 to 2000, which will lead to a steady increase in the number of childhood cancers in the next decades.13 Thus, pediatric cancers constitute a larger fraction of the cancer burden in Africa than in many other parts of the globe; in some African regions, pediatric cancers account for 6% of all cases, whereas in developed countries this proportion is less than 1%.43
The Franco-African Group of Pediatric Oncology was founded in the year 2000 under the mentorship of the Institute Gustave-Roussy to advance pediatric oncology in Africa through twinning partnerships and the development of a cooperative group structure; this consortium comprises fifteen pilot units in 12 countries, and more than 1,000 children are treated annually in the Franco-African Group of Pediatric Oncology pilot units in sub-Saharan Africa, with a steady increase in patient numbers (Fig 3 and Appendix Table A2). An African School of Pediatric Oncology has recently been established in Marrakesh, Morocco, to increase the number of trained physicians.44 In most pilot units, one or two physicians have had training in pediatric oncology and all of them are involved in clinical research. Other collaborative regional efforts are ongoing in sub-Saharan Africa, including a prospective collaborative project on Wilms tumor.45 Finally, pediatric oncology programs in South Africa are engaged in developing regional capacity and in establishing new collaborative networks to improve cancer care and control.41,46
Asia
Asia, the most populous continent, has the largest share of the global cancer burden (48%), a proportion that is expected to increase in the next decade40; however, cancer control programs are still in their infancy in most of the region.47 Huge disparities in economy and infrastructure exist between countries and even among different regions in some large countries.27,48 However, significant progress has been made in the development of regional initiatives in cancer care and control. The resource-stratified guidelines for cancer care and control programs and disease-specific treatments developed by the Asian Oncology Summit series of workshops are the best example of regionally focused initiatives with the potential for developing cooperative clinical research programs.25,27,31
Approximately one fourth of the Asian population is younger than age 15 years, with 80,000 pediatric cancer cases expected to occur in the region annually, representing almost 50% of all childhood cancers worldwide (Fig 1 and Table 2). The regional U5MR ranges from less than three per 1,000 live births per year (high-income Asian Pacific countries) to 99 per 1,000 live births per year (Afghanistan), with rapidly declining rates over the last decade.13 Thus, the number of pediatric cancer cases is expected to increase significantly, further shifting the world's childhood cancer burden to this region. The status of pediatric oncology reflects the diversity of resources available. Although countries such as Japan, South Korea, and Singapore have well-established pediatric oncology programs, most programs in South-Central Asia and South-Eastern Asia are less than a decade old, and they focus on basic needs, education, and the application of simple protocols.27 In South-Eastern Asia, there are several initiatives to develop regional capacity and establish a framework for cooperative group structures. The VIVA Foundation for Children with Cancer in Singapore together with the National University of Singapore and St Jude Children's Research Hospital (SJCRH) started an annual workshop and forum in 2007 for pediatric oncologists from 14 countries in South and South-Eastern Asia. The workshops have focused on management of leukemia and solid tumors, supportive care in resource-limited settings, and presentation of local epidemiologic data, treatment results, and initiatives to combat common problems such as abandonment of therapy. A core group of oncologists from various countries in the region has developed a cohesive consortium that is currently planning common studies and region-wide initiatives in cancer registration, palliative care, ALL, and retinoblastoma (Fig 3 and Appendix Table A2). Collaborative efforts such as the Malaysia-Singapore ALL Study Group have demonstrated great potential in the region and are developing clinical and translational research efforts targeted at local populations and adjusted to existing resources.49,50
South-Central Asia and the Indian subcontinent present a slightly different scenario. The overall cancer mortality rate in India is close to 70%, which is likely similar to the rate in the pediatric population.51,52 Although the lack of resources to pay for treatment is one of the factors that result in poor cancer survival in India, initiatives such as the National Health Mission and the Rashtriya Swasthya Bima Yojna, which offer free treatment to economically challenged patients, are expected to ameliorate some of those limitations.51 In 2009, more than 50% of medical colleges in India did not have facilities or expertise for treating children with cancer.53 However, great efforts have been made to establish collaborative prospective studies, such as the modified MCP-841 ALL protocol, which resulted in an increase of survival rates from 20% to 60%.54 The recent development of the Indian Cooperative Oncology Network (ICON) has fostered regional medical and pediatric oncology initiatives. The Indian National Pediatric Oncology Group focuses on the development of cost-effective and logistically feasible protocols. The Jiv Daya Foundation recently launched the Indian Pediatric Oncology Initiative to support childhood cancer units across the country and the development of the Web-based India Pediatric Oncology Database, a secure database designed to create hospital-based registries and facilitate clinical research. The India Pediatric Oncology Database, in collaboration with ICON and Indian National Pediatric Oncology Group may serve as a platform for collaborative pediatric oncology trials in India.55 The pan-India Indian Childhood Collaborative Leukaemia Group protocol for childhood ALL is the first step in this direction, with an expected accrual of 2,200 children.51,52
In China, there are approximately 45,000 children with newly diagnosed cancer every year, including 10,000 to 12,000 with ALL. China has a largely privatized health care system, and the majority of rural residents are uninsured.48 Until recently, only approximately 10% of children with cancer received adequate treatment because of the lack of health insurance and inability to pay56; therefore, cancer has become a major cause of childhood death in China. In 2004, a standardized, cost-efficient protocol was developed jointly by the Shanghai Children's Medical Center, the Beijing Children's Hospital, and SJCRH to treat underprivileged children with low- and intermediate-risk ALL with the support of a charitable foundation. In 2009, the effectiveness and affordability of the clinical trial were reported,57 which drew the attention of China's leadership. In 2010, China's Ministry of Health initiated a pilot project that provided governmental funding for treatment of all children with ALL.58 This initiative has been extended to other cancers and thus has an impact on many more patients. With the drastic increase in the number of patients who have access to treatment and unprecedented opportunity for clinical and translational research, Shanghai Children's Medical Center in collaboration with SJCRH formed a national childhood ALL study group in 2014 with 20 major participating hospitals and medical centers across China.
Oceania
Oceania is characterized by its vast geography and unique, diverse mix of ethnic populations living in communities widely dispersed over large masses of land and sea. Of 18 countries and territories, only two are HICs (Australia and New Zealand); all other countries and territories are classified as small-island developing states. Australia and New Zealand are the most well-resourced countries in Oceania. Both have sophisticated publicly funded health care systems, so children and young people with cancer have benefited from the survival advances seen in Europe and North America over the last 40 years. Their 10 pediatric oncology centers are members of the Children's Oncology Group (COG) and the Australian and New Zealand Children's Hematology and Oncology Group. Membership in the COG, Australian and New Zealand Children's Hematology and Oncology Group, and various SIOP-associated collaborative clinical trials groups has ensured that children and young people in Australia and New Zealand have access to contemporary cancer care and novel emerging technology. However, the same is not true for pediatric cancer in the wider Pacific region. Across Pacific Island countries and Papua New Guinea, there is wide variation in resources and health care provision which, coupled with geographic barriers of distance and limited infrastructure, create great challenges in caring for children with cancer and underscore the need for locally adapted solutions. To address some of these issues, the New Zealand Children's Cancer Network, funded by New Zealand Aid, has developed an active twinning model that has adapted treatment regimens for selected cancers. By combining regular clinical outreach and teaching through weekly teleconferences with New Zealand, two child health services in Fiji treat most cases of childhood cancer in their own country. Fiji is now accepting selected referrals from other Pacific countries and is providing affordable regional cancer care. A similar but more limited twinning program was recently started between Papua New Guinea and Australia.
Latin America and the Caribbean
The Latin America and Caribbean region has approximately 8% of the world's population (Table 2). The economies of Latin America and the Caribbean are growing rapidly; however, Latin America is poorly equipped to deal with the alarming increase in cancer incidence and disproportionally high mortality rates compared with other world regions. Approximately 26% of the region's population is younger than age 15 years, and the region has 17,000 cases of childhood cancer annually (Fig 1 and Table 2). The annual U5MR ranges from 12 per 1,000 live births in the more developed southern countries to 18 in Central America, 28 in the Andean countries, and 34 in the Caribbean. The rate of decline in U5MR slowed down after 2000.13
One of the most successful models of pediatric oncology cooperative work and research in resource-limited settings has been the Central American Association of Pediatric Hematology and Oncology (AHOPCA).59 Twinning between ‘La Mascota’ Hospital in Managua, Nicaragua, and the Pediatric Clinic of the University of Milano-Bicocca in Monza, Italy, which was initiated in 1986, led to the establishment of the Monza International School of Pediatric Hematology-Oncology. The activities of Monza International School of Pediatric Hematology-Oncology culminated in the formation of AHOPCA in 1998 (Fig 3 and Appendix Table A2). The group initially focused on education and support for program building, but it has evolved into a true multidisciplinary group over the years. Support from institutions in North America (SJCRH, Children's Hospital of Colorado, Pediatric Oncology Group of Ontario, Hospital for Sick Children in Toronto, and Dana-Farber/Boston Children's Hospital) and Europe (Hospital San Gerardo, Monza, and Istituto Nazionale Tumori Milano) has helped optimize treatments and foster research capacity. AHOPCA now has a clinical research infrastructure with a shared Web-based database, data managers, and coordinators in all units. Multidisciplinary prospective protocols have been developed for most pediatric malignancies that generate evidence-based data to guide the development of programs in other resource-limited settings. The contributions to the field made by this cooperative group are highlighted by study results featured in nearly 50 peer-reviewed publications. To address knowledge deficits in pediatric specialists in the region, training programs in pediatric hematology and oncology and pediatric intensive care have been initiated in Guatemala. More recently, AHOPCA has organized a pediatric cancer epidemiology initiative with the support of Dana-Farber/Boston Children's Hospital, Santa Casa Medical School (Sao Paulo), Union for International Cancer Control, International Agency for Research on Cancer, and St. Baldrick's Foundation. Population-based pediatric cancer registries have been established in El Salvador and Guatemala in 2013, and expansion is planned to Nicaragua and Honduras in 2015, which will create a Central American network of registries and an infrastructure for epidemiology research. Following the steps that AHOPCA took, the Caribbean Pediatric Cancer and Blood program coordinated by the Hospital for Sick Children aims to develop a collaborative network among six Caribbean countries that focuses on cancer registration, program building, and education.
The heterogeneity of South America is reflected in disparities in human development and access to care. Although some countries have poor infrastructure and lack key components for successful treatment of children with cancer, others have a long-standing tradition of national cooperative groups and participation in international studies. Building on this experience and with the goal of fostering regional clinical research in pediatric oncology, those countries formed the Latin American Pediatric Oncology Group (GALOP). GALOP includes 12 pediatric cancer centers from Chile (as part of the National Program for Antineoplastic Drugs for Children in Chile), six from Argentina, 24 from Brazil, and the National Pediatric Oncology Center from Uruguay. GALOP has the support of the COG for organizing its clinical research infrastructure and strategic planning (Fig 3 and Appendix Table A2). Protocols have already been developed for retinoblastoma, osteosarcoma, and Ewing sarcoma and are in the planning stages for germ cell tumors and high-risk neuroblastoma. In addition, GALOP participates as a cooperative group in the ARET0321 (Combination Chemotherapy, Autologous Stem Cell Transplant, and/or Radiation Therapy in Treating Young Patients With Extraocular Retinoblastoma) protocol of the COG. The COG-GALOP partnership can be viewed as a model of collaboration at the cooperative group level, whereby experienced cooperative groups assist in the development and growth of similar structures in more resource-limited settings.
CLINICAL RESEARCH AND CLINICAL RESEARCH INFRASTRUCTURES IN LMICS
Collaborative research through consortia or large cooperative groups has created a pathway to the major advances experienced in pediatric oncology over the last few decades.1,2 However, less than 20% of children with cancer worldwide benefit from those large cooperative efforts, which are centered mostly in North America and Europe. By contrast, existing consortia and organized groups under development in LMICs may currently be covering more than 50% of the world's children with cancer (Fig 3 and Appendix Table A2). Strengthening those groups and fostering new regional collaborations has the potential to create new ground for collaborative research in low-resource settings. The infrastructure, organizational culture, systems, and expertise that develop as a result of sustained participation in cooperative clinical trials research may have a favorable impact on patient care and outcomes.60 Regional collaborations can further increase the level of expertise, increase regional capacity through shared resources, and enhance the spirit of collaboration that has been key to the successes made in pediatric cancer over the last four decades.61 Different levels of consortia could be identified, with step-wise increments in the level of complexity and range of initiatives developed (Table 3).
Table 3.
Proposed Components of Research Consortia by Their Longitudinal Experience and Research Resources
| Level | I | II | III | IV |
|---|---|---|---|---|
| Longitudinal experience | Young consortium | Young-experienced consortium | Experienced consortium | Maximally experienced consortium |
| Research resources | Lower resource setting | Medium resource setting | High resource setting | Maximal resource setting |
| Clinical trial type | Single-arm intervention study for common and highly curable disease (ALL, BL, WT) or target disease (OS, ES) | Single-arm intervention studies for most childhood cancers Biology studies for banking or focused etiologic research |
Incorporation of randomized clinical trials Few single- or multi-arm trials for relapse disease Biologic studies for expanded etiologic research |
Predominance of randomized clinical trials Focus on relapsed disease, phase I/II clinical trials, and effectiveness trials Biology studies for identification of new markers |
| No. of studies/trials | Few (1-5) | Several (> 5) | Multiple (> 10) | Multiple (> 20) |
| Research infrastructure | Strong emphasis on building local capacity by: Training data managers Establishing processes for data collection, quality, safety, and analysis Nurturing local researchers Establishing ethics committees |
Additional emphasis on: Increasing data quality checks, research staff, and oversight proportional to higher volume Support for development of secondary analysis and local projects |
Clinicians have dedicated time to be site-specific principal investigators Clinical research staff is more experienced and independent Stable financial support has been achieved |
Dedicated staff, time, and support has been secured Full set of research staff is available (CRC, CRA, CRN) Statistics core available Research has close ties to drug development and pharmaceutical companies (bench to bedside) |
| Informed consent | Relatively simple because studies likely reflect standard of care | Relatively simple because studies likely reflect standard of care | Increasing complexity | Increased complexity |
| Health systems | Intentional strengthening of health systems through: Drug procurement Access to care Quality checks Outcome assessment |
Additional emphasis on: Expanding the formulary (treatment and supportive) Improving early referral Building research teams |
Culture of research, quality, and safety has been achieved Clinical research is part of subspecialty training Nursing research |
Clinical research is embedded within health care systems Consortium influences health care policies |
| Health care delivery | Intentionally improving: Standardization of care for specific diseases Evaluation of barriers to implementation Monitoring of compliance |
Additional emphasis on: Standardization of care Processes and/or quality Integration and/or incorporation of subspecialists Increased access to expert care |
Care teams are supported by research teams Care is multidisciplinary and interdisciplinary More supportive staff allows for more outpatient care |
Cost-effective care Maximizing outpatient care Limiting impact on quality of life Accountability is high |
Abbreviations: ALL, acute lymphoblastic leukemia; BL, Burkitt lymphoma; CRA, clinical research assistant; CRC, clinical research coordinator; CRN, clinical research nurse; ES, Ewing sarcoma; OS, osteosarcoma; WT, Wilms tumor.
CONCLUSION
The shift in the pediatric cancer burden in the developing world highlights a pressing need for high-quality research to identify feasible and evidence-based therapies that are appropriate for low-resource settings.62 Cancer care in LMICs must not be limited to copying unrealistic strategies used in HICs—it demands innovation. Thinking beyond our present standards is mandatory for generating new constraint-adapted therapeutic strategies to treat patients with cancer who live in LMICs.63 The success of the regional initiatives suggests that enhancing regional research capacity in pediatric oncology in LMICs should be prioritized. Partnerships between institutions and cooperative groups in high- and low-resource settings provide successful models. As collaborations evolve, a clear research framework must be defined, and academic credit should be properly shared; research interventions are ethical only if the intervention under study has the potential to provide health benefits to the communities or countries in which the trials are conducted.62
Appendix
Table A1.
WHO Essential Medicines
| WHO Model List of Essential Medicines for Children (4th edition, April 2013; revised October 2013) |
|---|
| Asparaginase |
| Cyclophosphamide |
| Cytarabine |
| Dactinomycin |
| Daunorubicin |
| Dexamethasone |
| Doxorubicin |
| Hydrocortisone |
| Mercaptopurine |
| Methotrexate |
| Methylprednisolone |
| Prednisolone |
| Thioguanine |
| Vincristine |
NOTE. Data source contains a core list and a complementary list of antineoplastic agents.35
Table A2.
Proportion of Children With Cancer Covered by the International Consortia Identified and Evaluated
| Country | Country Income Level in 2013* | Population Total in 2013* | Population Age 0-14 Years in 2013 (%)* | Population Age 0-14 Years in 2013 | Incidence of Childhood Cancer (per million)†‡ | Annual Cases per Country | Coverage by Consortium (%) |
|---|---|---|---|---|---|---|---|
| Central America (AHOPCA) | |||||||
| Costa Rica | UMIC | 4,872,166 | 23.5 | 1,145,730 | 130 | 149 | |
| Dominican Republic | UMIC | 10,403,761 | 30.2 | 3,141,984 | 73 | 229 | |
| El Salvador | LMIC | 6,340,454 | 30.0 | 1,899,971 | 69 | 131 | |
| Guatemala | LMIC | 15,468,203 | 40.4 | 6,253,590 | 59 | 369 | |
| Haiti | LIC | 10,317,461 | 35.0 | 3,606,615 | 30 | 108 | |
| Honduras | LMIC | 8,097,688 | 35.2 | 2,853,268 | 60 | 171 | |
| Nicaragua | LMIC | 6,080,478 | 32.8 | 1,996,346 | 52 | 104 | |
| Panama | UMIC | 3,864,170 | 28.3 | 1,094,071 | 74 | 81 | |
| Total | 1,343 | 0.7 | |||||
| South America (GALOP) | |||||||
| Argentina | UMIC | 41,446,246 | 24.2 | 10,044,064 | 144 | 1,446 | |
| Brazil | UMIC | 200,361,925 | 24.1 | 48,256,432 | 124 | 5,984 | |
| Chile | HIC | 17,619,708 | 21.1 | 3,715,100 | 156 | 580 | |
| Uruguay | HIC | 3,407,062 | 21.8 | 744,385 | 115 | 86 | |
| Total | 8,095 | 4.5 | |||||
| North Africa (GFAOP) | |||||||
| Algeria | UMIC | 39,208,194 | 27.8 | 10,890,906 | 141 | 1,536 | |
| Burkina Faso | LIC | 16,934,839 | 45.5 | 7,712,086 | 55 | 424 | |
| Cameroon | LMIC | 22,253,959 | 43.0 | 9,559,669 | 184 | 1,759 | |
| Cote d'Ivoire | LMIC | 20,316,086 | 41.3 | 8,397,252 | 54 | 453 | |
| Democratic Republic of the Congo | LIC | 67,513,677 | 45.0 | 30,400,157 | 58 | 1,763 | |
| Madagascar | LIC | 22,924,851 | 42.4 | 9,718,059 | 75 | 729 | |
| Mali | LIC | 15,301,650 | 47.4 | 7,252,347 | 153 | 1,110 | |
| Mauritania | LIC | 3,889,880 | 40.1 | 1,560,022 | 57 | 89 | |
| Morocco | LMIC | 33,008,150 | 27.9 | 9,193,815 | 113 | 1,039 | |
| Senegal | LMIC | 14,133,280 | 43.5 | 6,148,984 | 84 | 517 | |
| Togo | LIC | 6,816,982 | 41.8 | 2,852,689 | 65 | 185 | |
| Tunisia | UMIC | 10,886,500 | 23.2 | 2,526,167 | 102 | 258 | |
| Total | 9,861 | 5.5 | |||||
| India (ICON)† | |||||||
| India | LMIC | 1,252,139,596 | 29.1 | 364,250,394 | 63 | 22,948 | |
| Total | 22,948 | 12.7 | |||||
| China (CCCG)† | |||||||
| China | UMIC | 1,357,380,000 | 18.0 | 244,746,591 | 69 | 16,888 | |
| Total | 16,888 | 9.3 | |||||
| Middle East (POEM)§ | |||||||
| Algeria | UMIC | 39,208,194 | 27.8 | 10,890,906 | 141 | — | |
| Armenia | LMIC | 2,976,566 | 20.2 | 602,719 | 100 | 60 | |
| Bahrain | HIC | 1,332,171 | 21.0 | 279,621 | 119 | 33 | |
| Egypt | LMIC | 82,056,378 | 31.1 | 25,555,143 | 138 | 3,527 | |
| India | LMIC | 1,252,139,596 | 29.1 | 364,250,394 | 63 | — | |
| Iran | UMIC | 77,447,168 | 23.8 | 18,440,404 | 100 | 1,844 | |
| Iraq | UMIC | 33,417,476 | 40.1 | 13,386,280 | 120 | 1,606 | |
| Jordan | UMIC | 6,459,000 | 34.0 | 2,198,516 | 109 | 240 | |
| Kuwait | HIC | 3,368,572 | 24.8 | 834,677 | 141 | 118 | |
| Lebanon | UMIC | 4467,390 | 20.8 | 930,182 | 167 | 155 | |
| Libya | HIC | 6,201,521 | 29.4 | 1,825,865 | 96 | 175 | |
| Morocco | LMIC | 33,008,150 | 27.9 | 9,193,815 | 113 | — | |
| Oman | HIC | 3,632,444 | 23.5 | 852,932 | 93 | 79 | |
| Pakistan | LMIC | 182,142,594 | 33.8 | 61,609,988 | 74 | 4,559 | |
| Saudi Arabia | HIC | 28,828,870 | 29.0 | 8,369,349 | 113 | 946 | |
| State of Palestine | LMIC | 4,169,506 | 40.1 | 1,672,057 | 125 | 209 | |
| Sudan | LMIC | 37,964,306 | 41.2 | 15,635,119 | 73 | 1,141 | |
| Syria | LMIC | 22,845,550 | 35.1 | 8,016,704 | 110 | 882 | |
| Tunisia | UMIC | 10,886,500 | 23.2 | 2,526,167 | 102 | — | |
| Turkey | UMIC | 74,932,641 | 25.7 | 19,224,026 | 143 | 2,749 | |
| United Arab Emirates | HIC | 9,346,129 | 15.3 | 1,429,356 | 105 | 150 | |
| Yemen | LMIC | 24,407,381 | 40.2 | 9,806,195 | 99 | 971 | |
| Total | 19,445 | 10.8 | |||||
| Asia (VIVA) | |||||||
| Bangladesh | LIC | 156,594,962 | 30.0 | 46,976,764 | 42 | 1,973 | |
| Cambodia | LIC | 15,135,169 | 31.1 | 4,704,539 | 151 | 710 | |
| Indonesia | LMIC | 249,865,631 | 28.9 | 72,185,545 | 107 | 7,724 | |
| Malaysia | LMIC | 29,716,965 | 26.1 | 7,758,045 | 103 | 799 | |
| Myanmar | LIC | 53,259,018 | 24.9 | 13,267,065 | 81 | 1,075 | |
| Philippines | LMIC | 98,393,574 | 34.1 | 33,592,525 | 65 | 2,184 | |
| Singapore | HIC | 5,399,200 | 16.1 | 867,208 | 140 | 121 | |
| Sri Lanka | LMIC | 20,483,000 | 25.2 | 5,154,515 | 52 | 268 | |
| Vietnam | LMIC | 89,708,900 | 22.7 | 20,365,733 | 69 | 1,405 | |
| Total | 16,259 | 9.0 | |||||
| Oceania‖ | |||||||
| Fiji | UMIC | 881,065 | 28.9 | 254,266 | 154 | 39 | |
| Papua New Guinea | LMIC | 7321,263 | 38.0 | 2,782,994 | 90 | 250 | |
| Total | 290 | 0.2 | |||||
| Total No. of children covered by these consortia | 95,128 | 58.5 | |||||
| Total No. of children with cancer in the world§ | 162,603 |
Abbreviations: AHOPCA, Central American Association of Pediatric Hematology and Oncology; CCCG, Chinese Childhood Cancer Group; GALOP, Latin American Pediatric Oncology Group; GFAOP, Franco-African Group of Pediatric Oncology; HIC, high-income country; ICON, Indian Cooperative Oncology Network; LIC, low-income country; LMIC, lower-middle-income country; POEM, Pediatric Oncology East and Mediterranean; UMIC, upper middle[en]income country; VIVA, VIVA Foundation for Children with Cancer in Singapore.
Country income level was determined by using gross national income per capita, Atlas method (current $) values, and cutoffs from 2013, except for Libya where most recent gross national income per capita was from 2009. Source: World Bank Data by Country.10
China's CCCG and India's ICON are included despite being national rather than international groups based on the size of the population they cover. India is also a member of POEM, but case contribution is included only once (for ICON).
All rates reported on the Web site were converted from cases/100,000 to cases/million. Source: International Agency for Research on Cancer: GLOBOCAN 2012.11
Algeria, Morocco, and Tunisia are members of GFAOP and POEM; their case contribution is included only once (for POEM). Palestine is reported as “State of Palestine” in GLOBOCAN11 and as “West Bank and Gaza” in World Bank Data.10
Source: International Agency for Research on Cancer: GLOBOCAN 2012.11
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Carlos Rodriguez-Galindo, Paola Friedrich, Ronald Barr, Thomas Gross
Financial support: Carlos Rodriguez-Galindo
Administrative support: Carlos Rodriguez-Galindo
Collection and assembly of data: Carlos Rodriguez-Galindo, Paola Friedrich, Patricia Alcasabas, Shripad Banavali, Trijn Israels, Mhammed Harif, Ching-Hon Pui, Luis Castillo, Michael J. Sullivan, Federico Antillon, Sima Jeha
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Carlos Rodriguez-Galindo
No relationship to disclose
Paola Friedrich
No relationship to disclose
Patricia Alcasabas
No relationship to disclose
Federico Antillon
No relationship to disclose
Shripad Banavali
No relationship to disclose
Luis Castillo
No relationship to disclose
Trijn Israels
No relationship to disclose
Sima Jeha
No relationship to disclose
Mhammed Harif
Travel, Accommodations, Expenses: Novartis
Michael J. Sullivan
Stock or Other Ownership: Pacific Edge Biotechnology
Thuan Chong Quah
No relationship to disclose
Catherine Patte
No relationship to disclose
Ching-Hon Pui
No relationship to disclose
Ronald D. Barr
No relationship to disclose
Thomas G. Gross
No relationship to disclose
REFERENCES
- 1.Hudson MM, Link MP, Simone JV. Milestones in the curability of pediatric cancers. J Clin Oncol. 2014;32:2391–2397. doi: 10.1200/JCO.2014.55.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard-Jones K, Pieters R, Reaman GH, et al. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. Lancet Oncol. 2013;14:e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan R, Kowalczyk JR, Agarwal B, et al. New policies to address the global burden of childhood cancers. Lancet Oncol. 2013;14:e125–e135. doi: 10.1016/S1470-2045(13)70007-X. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C, Friedrich P, Morrissey L, et al. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 5.Vineis P, Wild CP. Global cancer patterns: Causes and prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown M, Goldie S, Draisma G, et al. Chapter 29: Health service interventions for cancer control in developing countries. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries (ed 2) Washington, DC: World Bank; 2006. http://www.ncbi.nlm.nih.gov/books/NBK11728/ [PubMed] [Google Scholar]
- 7.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: A call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu N, Bloom D, Bloom R, et al. Breakaway: The global burden of cancer—Challenges and opportunities. A report from the Economist Intelligence Unit, 2009. http://graphics.eiu.com/marketing/pdf/EIU_LIVESTRONG_Global_Cancer_Burden.pdf.
- 9.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14:391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- 10.The World Bank. Data by Country: Countries and Economies. http://data.worldbank.org/country.
- 11.International Agency for Research on Cancer (IARC) GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/age-specific_table_sel.aspx.
- 12.United States Census Bureau. International Programs: International Database. http://www.census.gov/population/international/data/idb/informationGateway.php.
- 13.Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:957–979. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: A descriptive study. Lancet Oncol. 2008;9:721–729. doi: 10.1016/S1470-2045(08)70194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich P, Ortiz R, Fuentes S, et al. Barriers to effective treatment of pediatric solid tumors in middle-income countries: Can we make sense of the spectrum of nonbiologic factors that influence outcomes? Cancer. 2014;120:112–125. doi: 10.1002/cncr.28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valsecchi MG, Steliarova-Foucher E. Cancer registration in developing countries: Luxury or necessity? Lancet Oncol. 2008;9:159–167. doi: 10.1016/S1470-2045(08)70028-7. [DOI] [PubMed] [Google Scholar]
- 17.Valery PC, Moore SP, Meiklejohn J, et al. International variations in childhood cancer in indigenous populations: A systematic review. Lancet Oncol. 2014;15:e90–e103. doi: 10.1016/S1470-2045(13)70553-9. [DOI] [PubMed] [Google Scholar]
- 18.Chow EJ, Puumala SE, Mueller BA, et al. Childhood cancer in relation to parental race and ethnicity: A 5-state pooled analysis. Cancer. 2010;116:3045–3053. doi: 10.1002/cncr.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S. Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Cheng C, Devidas M, et al. ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2012;30:751–757. doi: 10.1200/JCO.2011.38.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamazon ER, Pinto N, Konkashbaev A, et al. Trans-population analysis of genetic mechanisms of ethnic disparities in neuroblastoma survival. J Natl Cancer Inst. 2013;105:302–309. doi: 10.1093/jnci/djs503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesseling P, Molyneux E, Kamiza S, et al. Endemic Burkitt lymphoma: A 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Ann Trop Paediatr. 2009;29:29–34. doi: 10.1179/146532809X402006. [DOI] [PubMed] [Google Scholar]
- 24.Molyneux EM, Rochford R, Griffin B, et al. Burkitt's lymphoma. Lancet. 2012;379:1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BO. Evidence-based methods to address disparities in global cancer control: The development of guidelines in Asia. Lancet Oncol. 2013;14:1154–1155. doi: 10.1016/S1470-2045(13)70496-0. [DOI] [PubMed] [Google Scholar]
- 26.Hunger SP, Sung L, Howard SC. Treatment strategies and regimens of graduated intensity for childhood acute lymphoblastic leukemia in low-income countries: A proposal. Pediatr Blood Cancer. 2009;52:559–565. doi: 10.1002/pbc.21889. [DOI] [PubMed] [Google Scholar]
- 27.Yeoh AE, Tan D, Li CK, et al. Management of adult and paediatric acute lymphoblastic leukaemia in Asia: Resource-stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol. 2013;14:e508–e523. doi: 10.1016/S1470-2045(13)70452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Israels T, Moreira C, Scanlan T, et al. SIOP PODC: Clinical guidelines for the management of children with Wilms tumour in a low income setting. Pediatr Blood Cancer. 2013;60:5–11. doi: 10.1002/pbc.24321. [DOI] [PubMed] [Google Scholar]
- 29.Hesseling P, Israels T, Harif M, et al. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource limited setting. Pediatr Blood Cancer. 2013;60:357–362. doi: 10.1002/pbc.24407. [DOI] [PubMed] [Google Scholar]
- 30.Chantada G, Luna-Fineman S, Sitorus RS, et al. SIOP-PODC recommendations for graduated-intensity treatment of retinoblastoma in developing countries. Pediatr Blood Cancer. 2013;60:719–727. doi: 10.1002/pbc.24468. [DOI] [PubMed] [Google Scholar]
- 31.Lewin J, Puri A, Quek R, et al. Management of sarcoma in the Asia-Pacific region: Resource-stratified guidelines. Lancet Oncol. 2013;14:e562–e570. doi: 10.1016/S1470-2045(13)70475-3. [DOI] [PubMed] [Google Scholar]
- 32.[No authors listed] Essential drugs for cancer chemotherapy. Memorandum from a WHO meeting. Bull WHO Health Organ. 1985;63:999–1002. [PMC free article] [PubMed] [Google Scholar]
- 33.[No authors listed] Essential drugs for cancer chemotherapy: WHO Consultation. Bull World Health Organ. 1994;72:693–698. [PMC free article] [PubMed] [Google Scholar]
- 34.Sikora K, Advani S, Koroltchouk V, et al. Essential drugs for cancer therapy: A World Health Organization consultation. Ann Oncol. 1999;10:385–390. doi: 10.1023/a:1008367822016. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Essential medicines and health products: WHO MODEL Lists of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/en/index.html.
- 36.Mehta PS, Wiernikowski JT, Petrilli JA, et al. Essential medicines for pediatric oncology in developing countries. Pediatr Blood Cancer. 2013;60:889–891. doi: 10.1002/pbc.24476. [DOI] [PubMed] [Google Scholar]
- 37.Al-Hadad SA, Al-Jadiry MF, Al-Darraji AF, et al. Reality of pediatric cancer in Iraq. J Pediatr Hematol Oncol. 2011;33:S154–S156. doi: 10.1097/MPH.0b013e318230e218. [DOI] [PubMed] [Google Scholar]
- 38.Silbermann M, Al-Hadad S, Ashraf S, et al. MECC regional initiative in pediatric palliative care: Middle Eastern course on pain management. J Pediatr Hematol Oncol. 2012;34:S1–S11. doi: 10.1097/MPH.0b013e318249aa98. [DOI] [PubMed] [Google Scholar]
- 39.Al-Mulla NA, Chandra P, Khattab M, et al. Childhood acute lymphoblastic leukemia in the Middle East and neighboring countries: A prospective multi-institutional international collaborative study (CALLME1) by the Middle East Childhood Cancer Alliance (MECCA) Pediatr Blood Cancer. 2014;61:1403–1410. doi: 10.1002/pbc.25031. [DOI] [PubMed] [Google Scholar]
- 40.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 41.Stefan DC, Elzawawy AM, Khaled HM, et al. Developing cancer control plans in Africa: Examples from five countries. Lancet Oncol. 2013;14:e189–e195. doi: 10.1016/S1470-2045(13)70100-1. [DOI] [PubMed] [Google Scholar]
- 42.Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–259. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkin DM, Bray F, Ferlay J, et al. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23:953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 44.Harif M, Traoré F, Hessissen L, et al. Challenges for paediatric oncology in Africa. Lancet Oncol. 2013;14:279–281. doi: 10.1016/S1470-2045(12)70569-7. [DOI] [PubMed] [Google Scholar]
- 45.Israëls T, Kambugu J, Kouya F, et al. Clinical trials to improve childhood cancer care and survival in sub-Saharan Africa. Nat Rev Clin Oncol. 2013;10:599–604. doi: 10.1038/nrclinonc.2013.137. [DOI] [PubMed] [Google Scholar]
- 46.Kruger M, Hendricks M, Davidson A, et al. Childhood cancer in Africa. Pediatr Blood Cancer. 2014;61:587–592. doi: 10.1002/pbc.24845. [DOI] [PubMed] [Google Scholar]
- 47.Moore MA. Cancer control programs in East Asia: Evidence from the international literature. J Prev Med Public Health. 2014;47:183–200. doi: 10.3961/jpmph.2014.47.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 49.Yeoh AE, Ariffin H, Chai EL, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: Results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 Study. J Clin Oncol. 2012;30:2384–2392. doi: 10.1200/JCO.2011.40.5936. [DOI] [PubMed] [Google Scholar]
- 50.Yeoh AE, Lu Y, Chan JY, et al. Genetic susceptibility to childhood acute lymphoblastic leukemia shows protection in Malay boys: Results from the Malaysia-Singapore ALL Study Group. Leuk Res. 2010;34:276–283. doi: 10.1016/j.leukres.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Mallath MK, Taylor DG, Badwe RA, et al. The growing burden of cancer in India: Epidemiology and social context. Lancet Oncol. 2014;15:e205–e212. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- 52.Patel V, Chatterji S, Chisholm D, et al. Chronic diseases and injuries in India. Lancet. 2011;377:413–428. doi: 10.1016/S0140-6736(10)61188-9. [DOI] [PubMed] [Google Scholar]
- 53.Arora B, Banavali SD. Pediatric oncology in India: Past, present and future. Indian J Med Paediatr Oncol. 2009;30:121–123. doi: 10.4103/0971-5851.65333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magrath I, Shanta V, Advani S, et al. Treatment of acute lymphoblastic leukaemia in countries with limited resources: Lessons from use of a single protocol in India over a twenty year period [corrected] Eur J Cancer. 2005;41:1570–1583. doi: 10.1016/j.ejca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Arora B, Kanwar V. Childhood cancers in India: Burden, barriers, and breakthroughs. Indian J Cancer. 2009;46:257–259. doi: 10.4103/0019-509X.55543. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro R, Pui CH. Saving the children: Improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352:2158–2160. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Chen J, Tang J, et al. Cost of childhood acute lymphoblastic leukemia care in Shanghai, China. Pediatr Blood Cancer. 2009;53:557–562. doi: 10.1002/pbc.22127. [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZR, Mi JQ, Gu LJ, et al. Using sound clinical paths and diagnosis-related groups (DRGs)-based payment reform to bring benefits to patient care: A case study of leukemia therapy. Front Med China. 2010;4:8–15. [Google Scholar]
- 59.Barr RD, Antillón Klussmann F, Baez F, et al. Asociación de Hemato-Oncología Pediátrica de Centro América (AHOPCA): A model for sustainable development in pediatric oncology. Pediatr Blood Cancer. 2014;61:345–354. doi: 10.1002/pbc.24802. [DOI] [PubMed] [Google Scholar]
- 60.Clarke M, Loudon K. Effects on patients of their healthcare practitioner's or institution's participation in clinical trials: A systematic review. Trials. 2011;12:16. doi: 10.1186/1745-6215-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denburg AE, Joffe S, Gupta S, et al. Pediatric oncology research in low income countries: Ethical concepts and challenges. Pediatr Blood Cancer. 2012;58:492–497. doi: 10.1002/pbc.23419. [DOI] [PubMed] [Google Scholar]
- 62.Joffe S, Miller FG. Ethics of cancer clinical trials in low-resource settings. J Clin Oncol. 2014;32:3192–3196. doi: 10.1200/JCO.2014.56.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.André N, Banavali S, Snihur Y, et al. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239–e248. doi: 10.1016/S1470-2045(13)70056-1. [DOI] [PubMed] [Google Scholar]