Abstract
Patients in the intensive care unit (ICU) typically present with decreased concentrations of plasma tri-iodothyronine, low thyroxine, and normal range or slightly decreased concentration of thyroid-stimulating hormone. This ensemble of changes is collectively known as non-thyroidal illness syndrome (NTIS). The extent of NTIS is associated with prognosis, but no proof exists for causality of this association. Initially, NTIS is a consequence of the acute phase response to systemic illness and macronutrient restriction, which might be beneficial. Pathogenesis of NTIS in long-term critical illness is more complex and includes suppression of hypothalamic thyrotropin-releasing hormone, accounting for persistently reduced secretion of thyroid-stimulating hormone despite low plasma thyroid hormone. In some cases distinguishing between NTIS and severe hypothyroidism, which is a rare primary cause for admission to the ICU, can be difficult. Infusion of hypothalamic-releasing factors can reactivate the thyroid axis in patients with NTIS, inducing an anabolic response. Whether this approach has a clinical benefit in terms of outcome is unknown. In this Series paper, we discuss diagnostic aspects, pathogenesis, and implications of NTIS as well as its distinction from severe, primary thyroid disorders in patients in the ICU.
Introduction
The hypothalamic–pituitary–thyroid (HPT) axis is controlled by a classic endocrine feedback loop. Thyrotropin-releasing hormone (TRH) is released at the level of the hypothalamus, which stimulates the anterior pituitary to secrete thyroid-stimulating hormone (TSH). In turn, TSH drives the thyroid gland to release thyroid hormones. The prohormone thyroxine (T4) is converted in peripheral tissues to the active hormone tri-iodothyronine (T3). Hypothalamic TRH neurons were identified as determinants of thyroid hormone setpoint regulation more than three decades ago, and this discovery was followed by thyroid hormone receptor (TR) β being shown to have a key role in thyroid hormone negative feedback at the level of both the hypothalamus and anterior pituitary. Thus, the HPT axis was assumed to have a fixed setpoint, aiming at individually determined serum concentrations of thyroid hormones.1 However, studies2 have shown that these serum concentrations can be variable and adaptive in response to environmental factors, including nutrient availability and inflammatory stimuli.
Substantial changes in plasma concentrations of thyroid hormones have been noted in a range of diseases, characterised by clearly decreased plasma T3, low plasma T4, and increased plasma reverse T3 (rT3) concentrations. Despite low T3 and T4, TSH is typically maintained within its normal range or is slightly decreased. This ensemble of changes in thyroid function tests is collectively known as the non-thyroidal illness syndrome (NTIS).3 In this Series paper we focus on the presentation, pathogenesis, metabolic consequences, and clinical management of thyroid dysfunction in critically ill patients. The distinction between NTIS and primary thyroid disorders in patients in the intensive care unit (ICU) can sometimes be difficult, which will also be briefly discussed.
Non-thyroidal illness
In both human beings and rodents, illness decreases serum concentrations of thyroid hormones without a concomitant rise in serum TSH. This effect represents a deviation from normal negative feedback regulation in the HPT axis. If a similar drop in serum T3 and T4 happened in the context of primary hypothyroidism, serum TSH would be substantially increased and the patient would need thyroid hormone replacement therapy. The combination of low serum T3 and serum TSH within the reference range in the context of illness is called NTIS.3,4 Several different names have been used to refer to NTIS, including sick euthyroid syndrome and low T3 syndrome5 (suggesting low plasma T3 as the most consistent and striking change); in this Series paper we will use the term NTIS.
Very rapidly after the onset of acute stress, such as from myocardial infarction or surgery, serum T3 concentrations decrease. In patients undergoing abdominal surgery, a fall in serum T3 was noted within 2 h after the start of surgery.6 NTIS has been reported in patients with acute and chronic illnesses including infectious diseases, cardiovascular and gastrointestinal diseases, cancer, burns, and trauma.4 Serum T3 further decreases as the severity of disease progresses. This change is shown in the reported correlations between the fall in serum T3 concentrations and the size of a myocardial infarction, the increase in serum creatinine in renal insufficiency, and also burn severity.4 Therefore, the scale of the decrease in plasma concentrations of thyroid hormone generally represents the severity of the disorder and as a result, is associated with prognosis. In a study7 with patients in the ICU, the sensitivity and specificity in predicting mortality were 75% and 80%, respectively, for serum T4 less than 40 nmol/L. For combined low serum T4 and high serum cortisol these numbers were even higher—ie, 100% and 81%. Likewise, low T3 was shown to be a strong predictor of mortality in patients with heart disease.8
NTIS is present in most, if not all, critically ill patients—ie, patients with any life-threatening disorder that requires support of vital organ function and without which death would be imminent.9 Thus, critically ill patients who need long-term treatment in the ICU typically show decreased plasma T3 and T4 concentrations. An absent TSH response in this context points to profoundly altered feedback regulation of the HPT axis.10 Although the normal TSH concentration in the presence of low plasma T3 has been interpreted as suggesting a euthyroid status, this assumption has not been sub stantiated by data. Common changes in plasma thyroid hormone ranges that can be easily assessed in the clinical setting are the result of changes in the central regulation of the thyroid axis, including decreased TSH pulsatility.10 Moreover, several changes take place in the peripheral components of the thyroid axis that vary according to the tissue and the severity of illness.11 Peripheral changes include, but are not limited to, changes in the concentrations of thyroid hormone binding proteins and transporters, in expression and activity of thyroid hormone deiodinases, and in expression of thyroid hormone receptors.3 Changes in thyroid characteristics that are similar but not identical to those reported during NTIS, occur in response to fasting in healthy individuals.12 NTIS in response to fasting in healthy people is regarded as adaptive and beneficial because it reduces energy expenditure to restrict catabolism via decreased thyroid hormone action.
Whether NTIS in response to critical illnesses should be seen as an adaptive mechanism, such as in starvation, or rather as a maladaptive mechanism is unknown. On the one hand, a seemingly logical assumption is that a reduction in serum T3 would decrease thyroid hormone action in important T3 target organs, such as the liver and muscles, thereby affecting metabolism that might be beneficial in critically ill patients. On the other hand, patients with extended critical illness show clear symptoms and signs that resemble those noted in patients with hypothyroidism including impaired con sciousness, myocardial function, hypothermia, neuropathy, muscle weakness, atrophy of the skin, and hair loss, which together might impede recovery.13,14 Although the hypothesis that NTIS in patients in the ICU could be maladaptive has been extensively discussed,9 surprisingly few clinical studies (some being randomised controlled trials [RCTs]) aimed at modulating NTIS to improve clinical outcome have been reported. With some clinical intervention studies, irrespective of their design, the risk–benefit ratio might seem favourable with most patients showing a benefit and only a few showing harm (table), but this ratio has not been formally analysed when only RCTs with clinically relevant outcome measures, such as mortality or morbidity, are taken into account.
Table.
Overview of clinical studies reporting interventions in patients with non-thyroidal illness syndrome
| RCT? | Participants | Intervention (duration) | Main outcomes | Beneficial or positive (endpoints) | Harmful? | |
|---|---|---|---|---|---|---|
| Children after cardiopulmonary bypass surgery, age range from 2 days to 10·4 years (n=40)15 | Yes | Children | 2 μg/kg IV of T3 given on day 1 after surgery and 1 μg/kg given on day 2 for 12 days | Cardiac function, ICU measures | Yes | No |
| Premature newborns, born after <37 days of gestation (n=100)16 | No | Children | LT4 (25 μg/day) plus T3 (5 μg/day) given orally | Mortality | Yes | No |
| Premature newborns, born after <32 weeks of gestation (n=49)17 | Yes | Children | LT4, 10 μg/kg (IV) or 20 μg/kg (through nasogastric tube) for 21 days | Chronic lung disease, death, CNS damage, sepsis | No | No |
| Children <18 years with cessation of neurological function during assessment for organ recovery (n=171)18 | No | Children | Weight-based LT4 bolus followed by infusion | Vasopressor score | Yes | No |
| Protracted critical illness (n=14)10 | Yes | Adults | TRH (1 μg/kg per h) IV for 5 days plus GHRP-2 (1 μg/kg per h) IV for 5 days | GH and TSH secretion; biochemical anabolism and catabolism variables | Yes | No |
| Critical illness (n=76)19 | Yes | Adults | GHRP-2 alone or in combination with TRH and GHRH (each peptide 1 μg/kg per h) | Synchrony among GH, TSH, and PRL release | Yes | No |
| Critical illness (n=40)20 | Yes | Adults | Combinations of GHRH, GHRP-2 (both 1 μg/kg), TRH (200 μg) given as bolus, time interval 6 h | Serum GH, TSH, T3, and T4 concentrations | Yes | No |
| Protracted critical illness (n=20)21 | Yes | Adults | Combinations of GHRH, GHRP-2, and TRH start with bolus (1 μg/kg) followed by 1 μg/kg per h (for two consecutive nights) | Serum hormone concentrations | Yes | No |
| Protracted critical illness (n=33)22 | Yes | Adults | Combinations of GHRH, GHRP-2, and TRH (1 μg/kg per h of each), GnRH (0·1 μg/kg per 90 min) for 5 days | Serum hormone and metabolic marker concentrations | Yes | No |
| Elective coronary bypass surgery (n=80)23 | Yes | Adults | T3 (125 μg/day) orally (7 days pre-operative until discharge) | Haemodynamic data, morbidity, mortality | Yes (haemodynamic values); No (morbidity, mortality) | No |
| Burn injury (n=36)24 | Yes | Adults | T3 (200 μg/day) orally or via nasogastric tube in four divided doses until wounds healed | Mortality, resting metabolic rate | No | No |
| Coronary bypass surgery (n=60)25 | Yes | Adults | T3 (0·8 μg/kg) IV for 6 h | Operative outcome, morbidity, mortality | No | No |
| Coronary bypass surgery (n=100)26 | Yes | Adults | T3 (20 μg/12 h) oral (48 h) | Serum T3, haemodynamic variables, morbidity | No | No |
| High risk valvular heart surgery (n=50)27 | Yes | Adults | T3 (20 μg/12 h) orally (24 h) | Vasopressor need | Yes | No |
| Patients in the ICU with low T4 concentrations (n=23)28 | Yes | Adults | 1·5 μg/kg bodyweight LT4 IV (2 weeks) | Mortality | No | No |
| Acute renal failure (n=59)29 | Yes | Adults | LT4 infusion, 150 μg/20 mL every 12 h for 48 h | Percentage dialysis, percentage recovery, mortality | No | Possibly (increased mortality) |
| Idiopathic dilated cardiomyopathy (n=20)30 | Yes | Adults | 100 μg/day of LT4 for 3 months | Cardiac performance | Yes | No |
| Congestive heart failure (n=86)31 | Yes | Adults | Thyroid hormone analogue DIPTA, two per day, maximum dose 360 mg/day | Composite congestive heart failure endpoints | No | Yes |
| Non-thyroidal illness syndrome after major trauma (n=31)32 | Yes | Adults | Selenium (500 μg/day) with or without vitamin E and zinc supplement vs placebo (5 days) | Serum selenium levels, T4 levels, duration of mechanical ventilation | Yes (T4 and selenium plasma concentrations); uncertain (ventilation) | No |
| Patients with sepsis in the ICU (n=40)17 | Yes | Adults | High dose selenium (158 μg/day for 3 days) followed by standard dose selenium-selenite (31·6 μg/day) | Serum selenium concentrations, oxidative damage values, need for renal replacement therapy | No | No |
Whether trial showed overall beneficial and positive outcomes or harmful effects was defined by the authors on the basis of results reported. RCT=randomised controlled trial. IV=intravenous. T3=tri-iodothyronine. ICU=intensive care unit. LT4=levothyroxine. TRH=thyrotropin-releasing hormone. GHRP=growth hormone-releasing peptide. GH=growth hormone. TSH=thyroid-stimulating hormone. GHRH=growth hormone-releasing hormone. PRL=prolactin. T4=thyroxine. GnRH=gonadotropin-releasing hormone. DIPTA=di-iodothyropropionic acid.
Pathogenesis of NTIS
HPT axis feedback regulation and local thyroid hormone metabolism
Severe illness induces large changes in thyroid hormone economy, resulting in a downregulation of the HPT axis both at the hypothalamic and pituitary levels with an associated decrease in circulating thyroid hormone concentrations.3 This finding points to substantial changes to the negative feedback regulation in the HPT axis during NTIS.2 In people, central downregulation of the HPT axis during NTIS was supported by the observation in autopsy samples of decreased TRH gene expression in the hypothalamic paraventricular nucleus. TRH mRNA expression in the paraventricular nucleus showed a positive correlation with antemortem concentrations of plasma TSH and T3.33 Additionally, simultaneous changes in liver metabolism of thyroid hormone contribute to the characteristic changes in thyroid hormone plasma concentrations: low plasma T3 and high plasma rT3, normal or low to normal plasma TSH, and low plasma T4 during severe illness.3
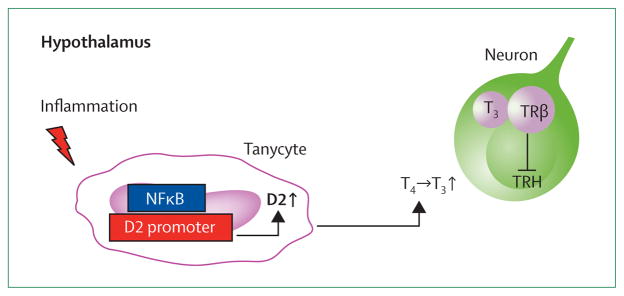
Although the mechanisms associated with these seemingly paradoxical HPT axis changes are not completely understood, findings from animal studies using various NTIS models have elucidated some aspects of NTIS pathogenesis. NTIS induces specific changes in enzymes associated with thyroid hormone metabolism (deiodinases type 1 [D1], 2 [D2], and 3 [D3]), thyroid hormone transporters, and thyroid hormone receptors (TRα and TRβ).3 For example, induction of acute inflammation in rodents by a single peripheral injection of bacterial endotoxin or lipopolysaccharide stimulates D2 mRNA expression in tanycytes lining the third ventricle in the hypothalamus.34,35 This D2 upregulation is followed by an increased local conversion of T4 to T3, which subsequently lowers TRH mRNA expression in the paraventricular nucleus, as noted in people (figure 1).33,36,37 Although an increase in D2 activity has not yet been proven, experiments in an in-vitro co-culture system showed that glial D2 modulates T3 concentrations and gene expression in neighbouring neurons.38 Thus, inflammation inhibits hypophysiotropic TRH neurons probably via increased D2 activity, thereby accounting for hypothalamic downregulation of the HPT axis during NTIS.
Figure 1. Schematic representation of hypothalamic thyroid hormone signalling during inflammation.
Inflammation activates the NFκB pathway in tanycytes, specialised cells lining the third ventricle. Tanycytes express D2, the main T3 producing enzyme in the brain, the promoter of which contains NFκB responsive elements. Binding of NFκB increases D2 expression and activity, and this stimulates the conversion of T4 into T3. T3 will enter adjacent neurons and bind to neuronal TRβ thereby regulating transcriptional activity of TRH. T3=tri-iodothyronine. TRβ=thyroid hormone receptor β. TRH=thyrotropin-releasing hormone. NFκB=nuclear factor kappa-light-chain-enhancer of activated B cells. D2=deiodinase type 2. T4=thyroxine.
The liver is one of the key metabolising organs of thyroid hormone. It expresses the thyroid hormone transporters mono carboxylate transporter 8 (MCT8) and MCT10, both D1 and D3 (although D3 is expressed at very low concentrations in a healthy liver), TRβ1, and TRα1. Figure 2 shows a detailed overview of cellular thyroid hormone metabolism. Although liver-expressed D1 contributes about only 20% of the circulating T3 in man,47 this enzyme’s involvement in NTIS pathogenesis has been extensively studied.48 Investigations showed reduced liver D1 mRNA expression and enzyme activity in people during illness, suggesting a role for liver-expressed D1 in the pathogenesis of illness-induced changes in plasma T3 and rT3.48
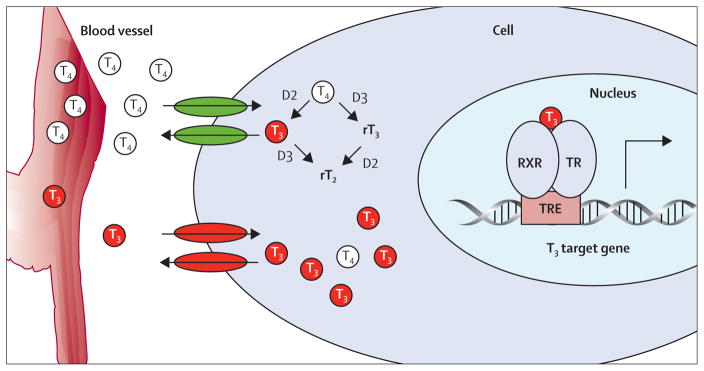
Figure 2. Schematic representation of cellular thyroid hormone metabolism.
Cellular entry of thyroid hormones is necessary for intracellular conversion and for T3 to exerts its actions in the nucleus. Two categories of thyroid hormone transporters have been noted: the organic anion transporters and the aminoacid transporters.1,39–41 Once transported into the cell, thyroid hormones (T4 and T3) can be metabolised by outer or inner ring deiodination through the iodothyronine deiodinases. These enzymes belong to a selenocysteine containing enzyme family and comprise three types; type 1 (D1), 2 (D2), and type 3 (D3).42 D1 is able to deiodinate the inner ring and outer rings of T4 as well as the outer ring of rT3. D1 is expressed in the liver, kidney, thyroid, and pituitary and localised in the plasma membrane.43,44 D2 is localised in the endoplasmic reticulum and deiodinates T4 into the biologically active T3. D2 is the main enzyme involved in the production of tissue T3 and therefore heavily involved in local thyroid hormone metabolism.45,46 D3 is localised in the plasma membrane and can be viewed as the major thyroid hormone inactivating enzyme, as it catalyses inner-ring deiodination of both T4 and T3, exclusively resulting in the production of biologically inactive rT3 and rT2, respectively.47 The balance between D2 and D3 determines the availability of cellular T3, which enters the nucleus and binds to the nuclear receptor complex (RXR and TR). T3 exerts its nuclear actions via the RXR and TR complex that binds to thyroid hormone response elements in target genes. TR complex regulates transcriptional activity of T3-target genes. TR=thyroid hormone receptor. T3=tri-iodothyronine. T4=thyroxine. rT3=reverse T3. rT4=reverse T4. RXR=retinoid-X receptor. TRE=thyroid hormone response elements.
Critical illnesses that include a substantial hypoxia or ischaemia component show a large increase in thyroid hormone catabolism via induction of D3. This effect was shown originally in post-mortem tissues of patients from the ICU49 and later in a series of animal models, including in models for myocardial50 and brain infarction.38 Thus, induction of D3 during NTIS in tissues that do not normally express, or express only very little, D3 is likely to greatly contribute to the abnormalities in thyroid economy reported during ischaemic injury.51
NTIS: part of the acute phase response
Several clinical studies done more than 20 years ago showed a clear association between the changes in thyroid hormone metabolism and the activation of various proinflammatory cytokines.52,53 Cytokines are import ant mediators of the acute phase response affecting fever, leucocytosis, the release of stress hormones and the production of acute phase proteins. Cytokines are also able to affect the expression of many proteins connected with thyroid hormone metabolism and are causally involved in the pathogenesis of NTIS.54 Lipopolysaccharide stimulation of a range of cells results in a strong inflammatory response characterised by the production of various cytokines, including tumour necrosis factor α (TNFα), interleukin 1, and interleukin 6. For the induction of cytokines, the activation of inflammatory signalling pathways, including nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and activator protein 1, is mandatory.55 Activation of NFκB was shown to play an important part in the up regulation of D2 in hypothalamic tanycytes during inflammation.56,57 D1 is also sensitive to cytokines; D1 expression in a liver cell line decreases upon interleukin 1β stimulation and this response can be abolished by simultaneous inhibition of NFκB and activator protein 1.58 In summary, cytokines that are activated as a result of the inflammatory response are causally associated with the pathogenesis of NTIS, making NTIS part of the acute phase response.
Differential effects of illness on thyroid hormone action in metabolic organs
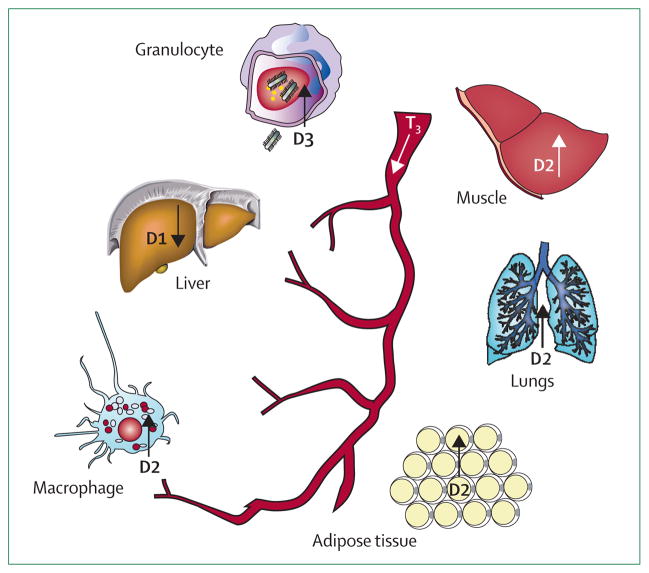
Energy homeostasis changes dramatically during illness, since activation of the immune system induces energy expenditure at the same time as food intake is decreased. Although the common view is that NTIS results in overall downregulation of metabolism in the organism to save energy, investigations have shown great variability between various key metabolic organs and tissues in the expression of genes encoding proteins involved in thyroid hormone metabolism.3 These tissues include cells that are now known to be thyroid hormone responsive, such as granulocytes,59 macrophages,60 and lung epithelial cells (figure 3).61
Figure 3. Simplified overview of various reported differential effects of NTIS on deiodinase activities in various tissues.
Deiodinase effects probably induce interorgan differences in T3 bioavailability in the presence of similarly decreased plasma concentrations of T3. T3=tri-iodothyronine. D1=deiodinase type 1.
D2=deiodinase type 2. D3=deiodinase type 3.
Thyroid hormones are important for skeletal muscle function because a range of genes expressed in the muscles are regulated by T3. Thyroid hormone signalling was shown to be different in skeletal muscle tissue during illness depending on the type (eg, acute inflammation or bacterial sepsis) and stage (ie, acute or long-term) of illness.62–64 Whether the differential changes in thyroid hormone metabolism in muscles during illness are clinically relevant is unknown; however, muscle dysfunction has been associated with changes in muscle metabolism of thyroid hormones during extended critical illness.65–68
Thyroid hormones are now known to target cells and organs that respond to inflammation by increasing D2 or D3 expression, thereby affecting cellular function. For example, stimulation of macrophages with bacterial endotoxin increases D2 expression, which is essential for cytokine production and phagocytosis.60 Moreover, granulocytes show increased D3 expression when infiltrating an infected organ.69 A functional role for this event was suggested by the finding that the absence of D3 in mice severely impaired the bacterial clearance capacity of the host.70 Induction of D3 in activated granulocytes not only inactivates thyroid hormone, but also yields substantial amounts of iodide that can be used by the cell for bactericidal and tissue-toxic systems.71 To summarise, the presence of D3 in activated granulocytes suggests a novel and protective role for the deiodinating enzymes in the defence against acute bacterial infection.
Role of nutrition
Critical illness is associated with loss of appetite and poor oral and enteral nutritional intake.72 Because fasting in healthy individuals induces a similar NTIS response as that reported in patients who are critically ill,12 decreased caloric intake during illness might greatly contribute to the development of NTIS. Fasting induces a decrease in serum thyroid hormones through a multifactorial mechanism and includes a decrease in serum leptin, and downregulation of hypothalamic, hypophysiotropic TRH neurons, contributing to persistently low concentrations of serum TSH (figure 4).74 At the organ level, activity of D1, the enzyme driving the conversion of T4 into the biologically active T3 and clearing the biologically inactive rT3, is decreased. Increased activity of D3, the T3 inactivating enzyme, has also been reported.75 Findings from three clinical studies that compared patients on different nutritional strategies suggested that decreased caloric intake during critical illness is associated with pronounced NTIS.76–78
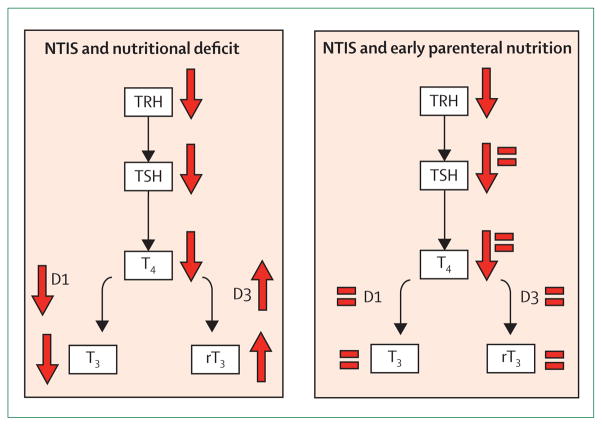
Figure 4. Schematic representation of the effect of parenteral nutrition during NTIS.
Critical illness-induced NTIS is characterised by low circulating T3 and raised concentration of rT3. Low hypothalamic TRH mRNA expression, low circulating TSH, and low T4 are also reported during NTIS. When early, full parenteral support is used to resolve the caloric deficit, the peripheral changes in the thyroid axis partly normalise, but its central suppression does not.73 Solid arrows represent direction of change in concentration or activity. = represents normalisation of concentrations. NTIS=non-thyroidal illness syndrome. TRH=thyrotropin-releasing hormone. TSH=thyroid-stimulating hormone.
T4=thyroxine. T3=tri-iodothyronine. rT3=reverse T3.
In 2011, the large randomised controlled EPaNIC trial79 reported comparisons between two nutritional regimens in 4640 adult patients in the ICU who were at risk of malnutrition: the early parenteral nutrition group received parenteral nutrition within 48 h of admission to the ICU (to supplement insufficient enteral nutrition), whereas the late parenteral nutrition group did not start this feeding regimen to supplement enteral nutrition before day 8 in the ICU. Findings from this study79 showed that toleration of a nutritional deficit during the first week of critical illness resulted in fewer complications and accelerated recovery than with the early administration of supplemental parenteral nutrition. Ability to tolerate a fasting response thus seems to be beneficial for the patient. Of note, a subanalysis of the EPaNIC trial80 (280 participants) showed that late feeding reduced complications and accelerated recovery of patients with NTIS, but also aggravated the changes in circulating concentrations of plasma TSH, total T4, T3, and the ratio of T3 to rT3. By contrast, the opposite was reported with patients given early feeding.80
Thus, the peripheral metabolism of T3 is affected by decreased nutritional intake during acute critical illness. The subanalysis of the EPaNIC trial80 suggested that the inactivation of T3 to rT3, as part of the fasting response, might be a beneficial adaptation during acute illness. Targeting of fasting blood glucose concentrations with intensive insulin treatment in children with critical illness to 2·8–4·4 mmol/L in infants and 3·9–5·6 mmol/L in children, thereby mimicking a fasting response, resulted in improved outcomes,81 but at the same time aggravated peripheral NTIS. Use of a multivariate, Cox proportional hazard analysis showed that the further reduction of T3 and rT3 accounted for part of the therapy improving patient mortality rates.82
Together, these findings suggest that thyroid economy is affected by decreased nutritional intake during acute critical illness and that the inactivation of T4 to rT3 and T3 to T2, as part of the fasting response, might be a beneficial adaptation during acute illness.80 Particularly, the acute peripheral inactivation of thyroid hormones by inner-ring deiodination during critical illness is probably a beneficial adaptation. Indeed, the reduced amount of circulating active T3 could be interpreted as an attempt by the body to decrease the metabolic rate, reduce expenditure of scarce energy, and prevent protein breakdown, thereby, promoting survival. By contrast, the central lowering of T4 could be harmful. Consistent with this interpretation is the finding that especially patients who are severely ill have a decrease in circulating T4 concentrations, whereas virtually all patients who are ill have low T3 and high rT3 concentrations already on admission to the ICU.62
Diagnosis and management of severe primary thyroid disorders in patients in the ICU
The high prevalence of NTIS in patients in the ICU and the extent of HPT axis changes in these patients can make it difficult to distinguish NTIS from untreated primary hypothyroidism. Levothyroxine treatment should be continued during a patient’s stay in the ICU in those who are known to have hypothyroidism. Although this practice seems trivial, prescription and continuation of chronic treatment is not always a main focus of care in the ICU setting. Findings from a retrospective chart review study83 in a tertiary referral ICU, including 133 patients, showed that thyroid replacement therapy was not prescribed for more than 7 days in 23 (17·3%) patients and omitted in three (2·2%). Diagnosis of primary hypothyroidism can be difficult in patients who are severely ill and not known to have hypothyroidism before admission to the ICU because serum thyroid hormones, especially T3, are decreased in most patients in the ICU due to NTIS. In patients clinically suspected to have severe hypothyroidism, the most useful test for diagnosis is measurement of plasma TSH, because a normal plasma TSH excludes primary hypothyroidism. In patients with a combination of primary hypothyroidism and NTIS, serum TSH concentration is still high and responsive to levothyroxine treatment. However, of note is that in patients who have hypothyroidism the high serum TSH concentration might decrease during the acute phase of illness especially if dopamine or high doses of glucocorticoids are given. Thus, high serum TSH in combination with low serum T4 is indicative of hypothyroidism; although, this combination can also be seen in patients recovering from NTIS. A high serum T3 to T4 ratio and a low serum rT3 favour the presence of hypothyroidism, since these ratios are reversed in NTIS, but the diagnostic accuracy of these measurements is poor.4 Especially in patients with long-standing and untreated hypothyroidism, cold exposure, infection, and vascular accidents might trigger the development of myxoedema coma. This disorder is a life-threatening condition with a high mortality of about 50%.84 Key clinical features are hypothermia and altered consciousness, and features of laboratory findings include raised TSH with low or undetectable T4 and T3 concentrations. Of note, the presence of NTIS can reduce the extent of TSH increase. Active management is important for this disorder and depends on the recognition of the clinical features. Treatment of myxoedema coma aims to replace thyroid hormone, treat the underlying condition, and provide supportive care. Additionally, stress dose glucocorticoids should be given (eg, 100 mg hydrocortisone every 8 h) because con comitant autoimmune primary adrenal insufficiency might be present, especially in patients with hypo glycaemia.85
A low serum TSH could suggest thyrotoxicosis, especially if serum free T4 is also high. The extent of TSH suppression is associated with the likelihood of thyrotoxicosis. Combination of suppressed TSH, high concentrations of free T4, and normal T3 concentrations might point to the combination of thyrotoxicosis and NTIS, and has been referred to as T4 thyrotoxicosis.4 Physical examination (eg, for a goitre or proptosis) and the presence of thyroid antibodies (antithyroid peroxidase and thyrotropin binding inhibiting immunoglobulins) might give further information about the probability of thyrotoxicosis. In the ICU setting, some patients can present with decompensated thyrotoxicosis, or thyroid storm, which is a life-threatening condition. Importantly, thyroid hormone concentrations do not distinguish between patients with thyroid storm from those with severe thyrotoxicosis because thyroid storm is a clinical diagnosis. Classic clinical characteristics of thyroid storm include fever, supra ventricular tachycardia, gastrointestinal symptoms, and an altered mental state including confusion, delirium, or even coma.84 Precipitating factors include surgery, parturition, and infection. Treatment generally requires ICU monitoring and aims to restore thyroid gland function and at the same time diminish thyroid hormone effects on peripheral tissues with a combination of β blockers, thyrostatics, intravenous glucocorticoids, and eventually a high-dose of iodide compounds.86 In a retrospective cohort study87 of patients admitted to hospital with acute thyrotoxicosis, the presence of CNS dysfunction was the only significantly different clinical feature between patients with thyroid storm and those with compensated thyrotoxicosis. Thus, patients with thyrotoxicosis, possible thyroid storm, and altered mentation should be treated aggressively with supportive measures and antithyroid drugs.87
Treatment and management of NTIS
Whether interventions aimed at normalising thyroid hormone concentrations in patients with extended critical illness are beneficial has so far not been satisfactorily answered. The table contains clinical studies reporting interventions in patients with NTIS. Only a few, rather small, RTCs have assessed the effects of treatment with thyroid hormones in patients with NTIS. These trials report results obtained in a large range of patient groups—eg, patients with acute renal failure,29 burn injury,24 and cardiac surgery,23,25–27 and those in the ICU with low T4 concentrations.28 Furthermore, the age of the study population varies greatly in these studies,23–29 ranging from premature newborns to children and adults. Disease severity is another variable, ranging from critical illness at the medical ICU to heart surgery in quite healthy participants. Surprisingly little consistency is present in the choice of the active study drug, since both T3 (given orally and intravenously) and levothyroxine have been used. In the context of studies with T4 or T3, of note is that normalising thyroid hormone concentrations in serum does not necessarily result in normal tissue concentrations of thyroid hormone. This finding was clearly shown in a study48 of patients who were critically ill and received thyroid hormone treatment. In these patients, increases in liver T3 concentrations after thyroid hormone treatment was disproportionally high compared with the increase in serum and muscle T3 concentrations.48 In addition to treatment with thyroid hormones, the effect of selenium on NTIS per se and on clinical outcomes has been studied.32,88 A small RCT89 investigated the effect of N-acetylcysteine, an antioxidant that restores intracellular glutathione (a cofactor required for D1 catalytic activity), on NTIS. N-acetylcysteine administration seemed to prevent the derangement in thyroid hormone concentrations that commonly happens in the acute phase of acute myocardial infarction, suggesting that oxidative stress is part of the pathogenesis of NTIS in acute myocardial infarction.
An unresolved and somewhat controversial issue is whether there is a place for thyroid hormone treatment in patients with heart failure. Although a failing heart shows molecular changes partly overlapping with a hypothyroid heart, this does not necessarily imply that treatment of patients with heart failure and low serum T3 in the setting of NTIS will improve their disorder.8 Although the use of thyroid hormone treatment in patients with heart failure has not been adequately studied, some trials have reported encouraging findings. A small RCT30 in patients with dilated cardiomyopathy showed beneficial effects of medium-term (3 months) levothyroxine treatment on cardiac performance, whereas another RCT31 showed that the thyroid hormone analogue 3,5 di-iodothyropropionic acid improved some haemodynamic variables albeit without evidence for symptomatic benefit in heart failure.
Finally, hypothalamic neuropeptides (including combinations of growth hormone releasing hormone, growth hormone releasing peptide 2, gonadotropin releasing hormone, and TRH) have been used in patients with long-term critical illness in an attempt to stimulate the anterior pituitary gland and thereby restore endocrine function, in terms of plasma concentrations and hormone pulsatility.10,19–22 Overall, the RCTs of T3 or T4 in critical illness have been largely negative in terms of clinical benefit (table). However, of note is that hypothalamic neuropeptides, especially TRH in combination with growth hormone releasing peptide 2 given intravenously, can restore circulating concentrations of thyroid hormone and TSH pulsatility to a remarkable extent. Additionally, this strategy improved overall metabolism, including bone markers and anabolic variables. Interpretation of these findings as representative of an insufficient hypothalamic drive of pituitary TSH release in protracted critical illness was supported by a study33 reporting that deceased patients with NTIS seemed to have decreased hypothalamic TRH mRNA expression in the paraventricular nucleus, correlating with decreased antemortem plasma TSH and T3 concentrations. However, the studies undertaken with hypothalamic neuropeptides consisted of only small numbers of patients. At present whether treatment with those neuropeptides offers clinical benefit in terms of morbidity and mortality is unclear. In summary, no definitive conclusion about the effectiveness of thyroid hormone treatment in patients in the ICU with NTIS can yet be made.8
Treatment with hypothalamic neuropeptides could possibly be harmful. The study by Acker and colleagues29 in patients with acute renal failure raised some concern because of a tendency towards increased mortality after treatment with levothyroxine without any beneficial effect on outcome—eg, in terms of dialysis need (table). However, the reported mortality in the control group of this study29 was lower than expected, which does not necessarily justify the interpretation that the intervention was harmful. Clearly, the reported studies were not adequately powered to detect clinically meaningful differences. Finally, most of these trials used rather high doses of either T4 or T3, probably inducing further suppression of pituitary TSH release and altered tissue deiodinase activities. Use of neuropeptides, including TRH, to stimulate the HPT axis could be promising in this respect. Large RCTs in well defined patient groups will be needed to investigate possible positive effects of this approach in terms of outcome. High priority should be given to RCTs comparing the effect of hypothalamic neuropeptides including TRH with placebo, because this approach has already been shown to partly normalise concentrations of serum thyroid hormones and at the same time improve metabolic markers.10 However, these effects have been shown only in small studies that were not powered to study clinically relevant outcome measures, such as mortality or morbidity. Another possibility will be to investigate treatment with recombinant human TSH, because this is a physiological stimulus—similar to TRH—for thyroid hormone release from the thyroid. A pilot study90 showed that once a day low dose (30 μg) TSH treatment in patients with central hypothyroidism was sufficient to increase plasma TSH concentrations to the normal range. Furthermore, this treatment improved patients’ quality of life and sleep behaviour.90 Of interest will be to investigate whether this approach can be used in patients in the ICU with NTIS to normalise TSH and thyroid hormones, and, if possible, whether this approach can improve clinical outcomes. Again, these investigations should be done in adequately powered, randomised, placebo-controlled studies.
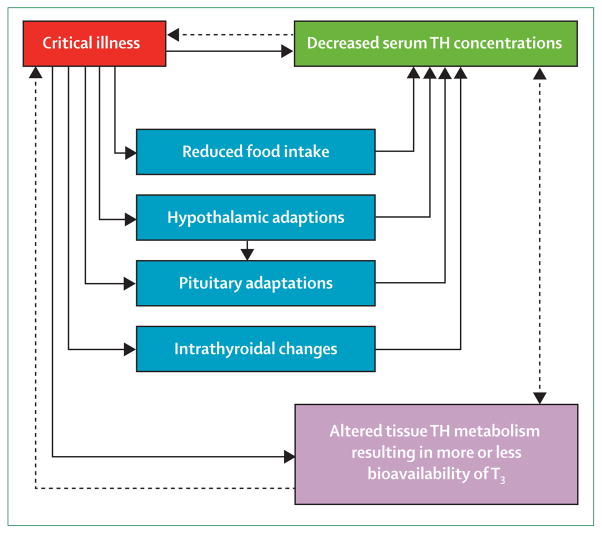
Conclusions
From a classic perspective, NTIS is a syndrome that occurs during various illnesses and is identified by decreased plasma concentrations of thyroid hormones with unclear resulting effects. Recent studies have shown that the changes in thyroid economy during NTIS suggest substantial and complex changes at the level of the HPT axis, in terms of setpoint regulation, and at the organ level, in terms of local metabolism of thyroid hormones (figure 5). Whether the noted changes in critically ill patients are beneficial or harmful in terms of outcome probably depends on disease stage and severity, the need for long-term vital support, and environmental factors (including parenteral nutrition). At present, no evidence-based consensus or guideline advocates thyroid hormone treatment of NTIS in patients who are critically ill. Adequately powered RCTs need to be undertaken to define whether active management of NTIS—eg, with hypothalamic neuropeptides including TRH—will yield clinical benefit in terms of patient outcome.
Figure 5. Schematic representation of the various changes during critical illness.
The scheme is based on both experimental investigations and studies with people. The net result of altered tissue thyroid hormone metabolism could be beneficial or maladaptive, dependent on disease duration and severity. TH=thyroid hormone. T3=tri-iodothyronine. Solid lines represent a causal association. Dashed lines represent a probable effect.
Search strategy and selection criteria.
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials for articles published in English from inception to March 6, 2014, with the search terms “critically ill patients”, “intensive care”, or “sepsis” in combination with “thyroid dysfunction”, “euthyroid sick syndrome”, or “thyroid hormones”. References chosen were selected on the basis of their title.
Footnotes
Contributors
EF, AB, and LL wrote the manuscript. EF, AB, and ACB revised the manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Prof Eric Fliers, Department of Endocrinology and Metabolism, Academic Medical Centre, University of Amsterdam, AZ, Amsterdam, Netherlands.
Prof Antonio C Bianco, Division of Endocrinology and Metabolism, Rush University Medical Center, Chicago, IL, USA.
Lies Langouche, Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, University of Leuven, Leuven, Belgium.
Prof Anita Boelen, Department of Endocrinology and Metabolism, Academic Medical Centre, University of Amsterdam, AZ, Amsterdam, Netherlands.
References
- 1.Alkemade A, Friesema EC, Unmehopa UA, et al. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab. 2005;90:4322–34. doi: 10.1210/jc.2004-2567. [DOI] [PubMed] [Google Scholar]
- 2.Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol. 2007;28:97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32:670–93. doi: 10.1210/er.2011-0007. [DOI] [PubMed] [Google Scholar]
- 4.Wiersinga WM, van den Berghe G. Nonthyroidal illness syndrome. In: Braverman LE, Cooper DS, editors. Werner & Ingbar’s the thyroid: a fundamental and clinical text. 10. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 203–17. [Google Scholar]
- 5.Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocr Rev. 1982;3:164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- 6.Michalaki M, Vagenakis AG, Makri M, Kalfarentzos F, Kyriazopoulou V. Dissociation of the early decline in serum T(3) concentration and serum IL-6 rise and TNFalpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab. 2001;86:4198–205. doi: 10.1210/jcem.86.9.7795. [DOI] [PubMed] [Google Scholar]
- 7.Arem RTJ, Deppe SA. Comparison of thyroid hormone and cortisol measurements with APACHE II and TISS scoring systems as predictors of mortality in the medical intensive care unit. J Intensive Care Med. 1997;12:12–17. [Google Scholar]
- 8.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385–93. doi: 10.1161/CIRCULATIONAHA.109.917922. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid. 2014;24:1456–65. doi: 10.1089/thy.2014.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab. 1999;84:1311–23. doi: 10.1210/jcem.84.4.5636. [DOI] [PubMed] [Google Scholar]
- 11.Kaptein EM, Kaptein JS, Chang EI, Egodage PM, Nicoloff JT, Massry SG. Thyroxine transfer and distribution in critical nonthyroidal illnesses, chronic renal failure, and chronic ethanol abuse. J Clin Endocrinol Metab. 1987;65:606–16. doi: 10.1210/jcem-65-4-606. [DOI] [PubMed] [Google Scholar]
- 12.Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid. 2008;18:123–29. doi: 10.1089/thy.2007.0253. [DOI] [PubMed] [Google Scholar]
- 13.Arem R, Wiener GJ, Kaplan SG, Kim HS, Reichlin S, Kaplan MM. Reduced tissue thyroid hormone levels in fatal illness. Metabolism. 1993;42:1102–08. doi: 10.1016/0026-0495(93)90266-q. [DOI] [PubMed] [Google Scholar]
- 14.Bello G, Pennisi MA, Montini L, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135:1448–54. doi: 10.1378/chest.08-1816. [DOI] [PubMed] [Google Scholar]
- 15.Bettendorf M, Schmidt KG, Grulich-Henn J, Ulmer HE, Heinrich UE. Tri-iodothyronine treatment in children after cardiac surgery: a double-blind, randomised, placebo-controlled study. Lancet. 2000;356:529–34. doi: 10.1016/S0140-6736(00)02576-9. [DOI] [PubMed] [Google Scholar]
- 16.Schönberger W, Grimm W, Emmrich P, Gempp W. Reduction of mortality rate in premature infants by substitution of thyroid hormones. Eur J Pediatr. 1981;135:245–53. doi: 10.1007/BF00442098. [DOI] [PubMed] [Google Scholar]
- 17.Smith LM, Leake RD, Berman N, Villanueva S, Brasel JA. Postnatal thyroxine supplementation in infants less than 32 weeks’ gestation: effects on pulmonary morbidity. J Perinatol. 2000;20:427–31. doi: 10.1038/sj.jp.7200417. [DOI] [PubMed] [Google Scholar]
- 18.Zuppa AF, Nadkarni V, Davis L, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004;32:2318–22. doi: 10.1097/01.ccm.0000146133.52982.17. [DOI] [PubMed] [Google Scholar]
- 19.Van den Berghe G, Wouters P, Bowers CY, de Zegher F, Bouillon R, Veldhuis JD. Growth hormone-releasing peptide-2 infusion synchronizes growth hormone, thyrotrophin and prolactin release in prolonged critical illness. Eur J Endocrinol. 1999;140:17–22. doi: 10.1530/eje.0.1400017. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berghe G, de Zegher F, Bowers CY, et al. Pituitary responsiveness to GH-releasing hormone, GH-releasing peptide-2 and thyrotrophin-releasing hormone in critical illness. Clin Endocrinol (Oxf) 1996;45:341–51. doi: 10.1046/j.1365-2265.1996.00805.x. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe G, de Zegher F, Baxter RC, et al. Neuroendocrinology of prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab. 1998;83:309–19. doi: 10.1210/jcem.83.2.4575. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berghe G, Baxter RC, Weekers F, et al. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf) 2002;56:655–69. doi: 10.1046/j.1365-2265.2002.01255.x. [DOI] [PubMed] [Google Scholar]
- 23.Sirlak M, Yazicioglu L, Inan MB, et al. Oral thyroid hormone pretreatment in left ventricular dysfunction. Eur J Cardiothorac Surg. 2004;26:720–25. doi: 10.1016/j.ejcts.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Becker RA, Vaughan GM, Ziegler MG, et al. Hypermetabolic low triiodothyronine syndrome of burn injury. Crit Care Med. 1982;10:870–75. doi: 10.1097/00003246-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Güden M, Akpinar B, Sagğbaş E, Sanisoğlu I, Cakali E, Bayindir O. Effects of intravenous triiodothyronine during coronary artery bypass surgery. Asian Cardiovasc Thorac Ann. 2002;10:219–22. doi: 10.1177/021849230201000306. [DOI] [PubMed] [Google Scholar]
- 26.Choi YS, Shim JK, Song JW, Song Y, Yang SY, Kwak YL. Efficacy of perioperative oral triiodothyronine replacement therapy in patients undergoing off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2013;27:1218–23. doi: 10.1053/j.jvca.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Choi YS, Kwak YL, Kim JC, Chun DH, Hong SW, Shim JK. Peri-operative oral triiodothyronine replacement therapy to prevent postoperative low triiodothyronine state following valvular heart surgery. Anaesthesia. 2009;64:871–77. doi: 10.1111/j.1365-2044.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 28.Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- 29.Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP. A trial of thyroxine in acute renal failure. Kidney Int. 2000;57:293–98. doi: 10.1046/j.1523-1755.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- 30.Moruzzi P, Doria E, Agostoni PG. Medium-term effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. Am J Med. 1996;101:461–67. doi: 10.1016/s0002-9343(96)00281-1. [DOI] [PubMed] [Google Scholar]
- 31.Goldman S, McCarren M, Morkin E, et al. DITPA (3,5-Diiodothyropropionic Acid), a thyroid hormone analog to treat heart failure: phase II trial veterans affairs cooperative study. Circulation. 2009;119:3093–100. doi: 10.1161/CIRCULATIONAHA.108.834424. [DOI] [PubMed] [Google Scholar]
- 32.Berger MM, Reymond MJ, Shenkin A, et al. Effect of selenium supplements on the low T3 syndrome after trauma: a randomized trial. Intensive Care Med. 1996;22:575–81. doi: 10.1007/BF01708099. [DOI] [PubMed] [Google Scholar]
- 33.Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab. 1997;82:4032–36. doi: 10.1210/jcem.82.12.4404. [DOI] [PubMed] [Google Scholar]
- 34.Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. J Endocrinol. 2004;182:315–23. doi: 10.1677/joe.0.1820315. [DOI] [PubMed] [Google Scholar]
- 35.Fekete C, Gereben B, Doleschall M, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–55. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 36.Lechan RM, Fekete C. Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome. J Endocrinol Invest. 2004;27(suppl):105–19. [PubMed] [Google Scholar]
- 37.Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. Prog Brain Res. 2006;153:189–207. doi: 10.1016/S0079-6123(06)53011-0. [DOI] [PubMed] [Google Scholar]
- 38.Freitas BC, Gereben B, Castillo M, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010;120:2206–17. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugiyama D, Kusuhara H, Taniguchi H, et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem. 2003;278:43489–95. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- 40.Heuer H, Maier MK, Iden S, et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146:1701–06. doi: 10.1210/en.2004-1179. [DOI] [PubMed] [Google Scholar]
- 41.Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Köhrle J. The deiodinase family: selenoenzymes regulating thyroid hormone availability and action. Cell Mol Life Sci. 2000;57:1853–63. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobs TC, Schmutzler C, Meissner J, Köhrle J. The promoter of the human type I 5′-deiodinase gene--mapping of the transcription start site and identification of a DR+4 thyroid-hormone-responsive element. Eur J Biochem. 1997;247:288–97. doi: 10.1111/j.1432-1033.1997.00288.x. [DOI] [PubMed] [Google Scholar]
- 44.Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol Cell Biol. 1995;15:5100–12. doi: 10.1128/mcb.15.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burmeister LA, Pachucki J, St Germain DL. Thyroid hormones inhibit type 2 iodothyronine deiodinase in the rat cerebral cortex by both pre- and posttranslational mechanisms. Endocrinology. 1997;138:5231–37. doi: 10.1210/endo.138.12.5602. [DOI] [PubMed] [Google Scholar]
- 46.Sagar GD, Gereben B, Callebaut I, et al. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol. 2007;27:4774–83. doi: 10.1128/MCB.00283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65:570–90. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peeters RP, van der Geyten S, Wouters PJ, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab. 2005;90:6498–507. doi: 10.1210/jc.2005-1013. [DOI] [PubMed] [Google Scholar]
- 49.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88:3202–11. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 50.Olivares EL, Marassi MP, Fortunato RS, et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology. 2007;148:4786–92. doi: 10.1210/en.2007-0043. [DOI] [PubMed] [Google Scholar]
- 51.Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 2008;4:148–55. doi: 10.1038/ncpendmet0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mooradian AD, Reed RL, Osterweil D, Schiffman R, Scuderi P. Decreased serum triiodothyronine is associated with increased concentrations of tumor necrosis factor. J Clin Endocrinol Metab. 1990;71:1239–42. doi: 10.1210/jcem-71-5-1239. [DOI] [PubMed] [Google Scholar]
- 53.Chopra IJ, Sakane S, Teco GN. A study of the serum concentration of tumor necrosis factor-alpha in thyroidal and nonthyroidal illnesses. J Clin Endocrinol Metab. 1991;72:1113–16. doi: 10.1210/jcem-72-5-1113. [DOI] [PubMed] [Google Scholar]
- 54.Pang XP, Hershman JM, Mirell CJ, Pekary AE. Impairment of hypothalamic-pituitary-thyroid function in rats treated with human recombinant tumor necrosis factor-alpha (cachectin) Endocrinology. 1989;125:76–84. doi: 10.1210/endo-125-1-76. [DOI] [PubMed] [Google Scholar]
- 55.Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–62. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vries EM, Kwakkel J, Eggels L, et al. NFκB signaling is essential for the lipopolysaccharide-induced increase of type 2 deiodinase in tanycytes. Endocrinology. 2014;155:2000–08. doi: 10.1210/en.2013-2018. [DOI] [PubMed] [Google Scholar]
- 57.Zeöld A, Doleschall M, Haffner MC, et al. Characterization of the nuclear factor-kappa B responsiveness of the human dio2 gene. Endocrinology. 2006;147:4419–29. doi: 10.1210/en.2005-1608. [DOI] [PubMed] [Google Scholar]
- 58.Kwakkel J, Wiersinga WM, Boelen A. Differential involvement of nuclear factor-kappaB and activator protein-1 pathways in the interleukin-1beta-mediated decrease of deiodinase type 1 and thyroid hormone receptor beta1 mRNA. J Endocrinol. 2006;189:37–44. doi: 10.1677/joe.1.06354. [DOI] [PubMed] [Google Scholar]
- 59.Boelen A, Kwakkel J, Alkemade A, et al. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology. 2005;146:5128–34. doi: 10.1210/en.2005-0608. [DOI] [PubMed] [Google Scholar]
- 60.Kwakkel J, Surovtseva OV, de Vries EM, Stap J, Fliers E, Boelen A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology. 2014;155:2725–34. doi: 10.1210/en.2013-2066. [DOI] [PubMed] [Google Scholar]
- 61.Barca-Mayo O, Liao XH, DiCosmo C, et al. Role of type 2 deiodinase in response to acute lung injury (ALI) in mice. Proc Natl Acad Sci USA. 2011;108:E1321–29. doi: 10.1073/pnas.1109926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005;90:4559–65. doi: 10.1210/jc.2005-0535. [DOI] [PubMed] [Google Scholar]
- 63.Kwakkel J, van Beeren HC, Ackermans MT, et al. Skeletal muscle deiodinase type 2 regulation during illness in mice. J Endocrinol. 2009;203:263–70. doi: 10.1677/JOE-09-0118. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Perez A, Palos-Paz F, Kaptein E, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) 2008;68:821–27. doi: 10.1111/j.1365-2265.2007.03102.x. [DOI] [PubMed] [Google Scholar]
- 65.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 66.Weitzel JM, Iwen KA. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342:1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Harper ME, Seifert EL. Thyroid hormone effects on mitochondrial energetics. Thyroid. 2008;18:145–56. doi: 10.1089/thy.2007.0250. [DOI] [PubMed] [Google Scholar]
- 68.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–53. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- 69.Boelen A, Boorsma J, Kwakkel J, et al. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid. 2008;18:1095–103. doi: 10.1089/thy.2008.0090. [DOI] [PubMed] [Google Scholar]
- 70.Boelen A, Kwakkel J, Wieland CW, St Germain DL, Fliers E, Hernandez A. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology. 2009;150:1984–90. doi: 10.1210/en.2008-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967;126:1063–78. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schütz P, Bally M, Stanga Z, Keller U. Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target? Swiss Med Wkly. 2014;144:w13957. doi: 10.4414/smw.2014.13957. [DOI] [PubMed] [Google Scholar]
- 73.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370:2450–51. doi: 10.1056/NEJMc1404896. [DOI] [PubMed] [Google Scholar]
- 74.Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–94. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galton VA, Hernandez A, St Germain DL. The 5′-deiodinases are not essential for the fasting-induced decrease in circulating thyroid hormone levels in male mice: possible roles for the type 3 deiodinase and tissue sequestration of hormone. Endocrinology. 2014;155:3172–81. doi: 10.1210/en.2013-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richmand DA, Molitch ME, O’Donnell TF. Altered thyroid hormone levels in bacterial sepsis: the role of nutritional adequacy. Metabolism. 1980;29:936–42. doi: 10.1016/0026-0495(80)90036-0. [DOI] [PubMed] [Google Scholar]
- 77.Chourdakis M, Kraus MM, Tzellos T, et al. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury: an open-labeled randomized trial. JPEN J Parenter Enteral Nutr. 2012;36:108–16. doi: 10.1177/0148607110397878. [DOI] [PubMed] [Google Scholar]
- 78.Ouchi K, Matsubara S, Matsuno S. Effects of supplementary parenteral nutrition on thyroid hormone patterns in surgical patients with liver cirrhosis. Nutrition. 1991;7:189–92. [PubMed] [Google Scholar]
- 79.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 80.Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab. 2013;98:1006–13. doi: 10.1210/jc.2012-2809. [DOI] [PubMed] [Google Scholar]
- 81.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–56. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 82.Gielen M, Mesotten D, Wouters PJ, et al. Effect of tight glucose control with insulin on the thyroid axis of critically ill children and its relation with outcome. J Clin Endocrinol Metab. 2012;97:3569–76. doi: 10.1210/jc.2012-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrett NA, Jones A, Whiteley C, Yassin S, McKenzie CA. Management of long-term hypothyroidism: a potential marker of quality of medicines reconciliation in the intensive care unit. Int J Pharm Pract. 2012;20:303–06. doi: 10.1111/j.2042-7174.2012.00205.x. [DOI] [PubMed] [Google Scholar]
- 84.Ringel MD. Management of hypothyroidism and hyperthyroidism in the intensive care unit. Crit Care Clin. 2001;17:59–74. doi: 10.1016/s0749-0704(05)70152-4. [DOI] [PubMed] [Google Scholar]
- 85.Fliers E, Wiersinga WM. Myxedema coma. Rev Endocr Metab Disord. 2003;4:137–41. doi: 10.1023/a:1022985902253. [DOI] [PubMed] [Google Scholar]
- 86.Papi G, Corsello SM, Pontecorvi A. Clinical concepts on thyroid emergencies. Front Endocrinol (Lausanne) 2014;5:102. doi: 10.3389/fendo.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Angell TE, Lechner MG, Nguyen CT, Salvato VL, Nicoloff JT, LoPresti JS. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab. 2015;100:451–59. doi: 10.1210/jc.2014-2850. [DOI] [PubMed] [Google Scholar]
- 88.Mishra V, Baines M, Perry SE, et al. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr. 2007;26:41–50. doi: 10.1016/j.clnu.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99:4537–45. doi: 10.1210/jc.2014-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixit K, Iwen A, Lehmphul I, Hoefig C, Köhrle J, Brabant G. Treatment of central hypothyroidism with recombinant human TSH—a pilot study. Eur Thyroid J. 2013;2(suppl 1):75–194. P153. [Google Scholar]