Abstract
Background
Dementia is the leading cause of loss of independence in older adults worldwide. In the U.S., approximately 15 million family members provide care to relatives with dementia. This paper presents the rationale and design for a translational study in which an evidence-based, non-pharmacologic intervention for older adults with dementia and family caregivers (CGs) is incorporated into a publicly-funded home care program for older adults at risk for nursing home admission.
Methods
The 4-month Care of Persons with Dementia in their Environments (COPE) intervention is designed to optimize older adults’ functional independence, and to improve CG dementia management skills and health-related outcomes. COPE features 10 in-home occupational therapy visits, and 1 in-home visit and 1 telephone contact by an advanced practice nurse. COPE was deemed efficacious in a published randomized clinical trial. In the present study, older adults with dementia enrolled in the Connecticut Home Care Program for Elders (CHCPE) and their CGs are randomly assigned to receive COPE plus their ongoing CHCPE services, or to continue receiving CHCPE services only.
Outcomes
The primary outcome for older adults with dementia is functional independence; secondary outcomes are activity engagement, quality of life, and prevention or alleviation of neuropsychiatric symptoms. CG outcomes include perceived well-being and confidence in using activities to manage dementia symptoms. Translational outcomes include net financial benefit of COPE, and feasibility and acceptability of COPE implementation into the CHCPE. COPE has the potential to improve health-related outcomes while saving Medicaid waiver and state revenue-funded home care program costs nationwide.
Introduction
Dementia, an umbrella term encompassing multiple causes of brain neurodegeneration and multiple patterns of associated cognitive decline and neuropsychiatric symptoms, affects more than 46 million people worldwide; by 2050, this number will reach more than 130 million people.1 Considered a global public health priority, dementia is the principal cause of morbidity burden; in 2015, the estimated cost of dementia worldwide was $818 billion.1 More than 5 million Americans have dementia and more than 15 million unpaid caregivers, mostly family members, provide care to these individuals. Persons with dementia plus other health problems generate greater Medicare and Medicaid expenditures than those with similar health problems without dementia.2
In the absence of widely effective pharmacotherapy to combat dementia and its health-related consequences, translation and implementation of evidence-based, non-pharmacologic interventions into existing service programs are sorely needed to improve outcomes for persons with dementia and their family and other informal caregivers (CGs), thereby potentially avoiding or delaying costly hospitalizations and nursing home admissions.3 The Connecticut Home Care Program for Elders (CHCPE), a combined Medicaid and state-funded program for older adults at high risk for nursing home admission, provides in-home and community-based services coordinated by care managers.4 However, CHCPE clients with dementia (25-30% of all clients) do not routinely receive evidence-based services directed at their cognitive impairment that could potentially improve their health-related outcomes and sustain living at home. Moreover, no CHCPE services currently engage CGs to help improve their dementia-related symptom management skills and health-related outcomes. Thus, the CHCPE is an ideal setting for translating an evidence-based, non-pharmacologic intervention designed to reduce functional disability in older adults with dementia and improve CG dementia management skills.
In this paper, we describe a translational study in which an evidence-based intervention, Care of Persons with Dementia in their Environments (COPE), is incorporated into the CHCPE. COPE is a 4-month, in-home, non-pharmacologic intervention using occupational therapists and advanced practice nurses to optimize functional independence in older adults with dementia, and to improve CG dementia management skills. In the original COPE randomized trial with community volunteers, persons with dementia receiving COPE experienced less functional decline and more activity engagement designed to keep them independent, compared to an attention control group. CGs receiving COPE, compared to controls, reported improved well-being, increased confidence in using behavioral strategies to address dementia symptoms, and greater ability to keep their family member at home.5
We will randomly assign 290 CHCPE clients with dementia and their CGs to receive the COPE intervention plus customary CHCPE services or customary CHCPE services alone. Primary and secondary client-specific and CG-specific outcomes will be similar to those in the original COPE trial, to determine whether similar outcomes are obtained in a real-world setting. To maximize translational effort, we will: conduct a cost-benefit analysis to determine potential economic benefits of adding COPE to customary CHCPE services; gather interview-based and focus group data from CHCPE care managers and other stakeholders to evaluate feasibility and acceptability of COPE as a new CHCPE service; and convene a Translational Advisory Committee of experts in publicly-funded home care programs for older adults to help guide COPE dissemination and implementation activities.
Translational study and intervention rationale
Several health-related and scientific issues helped frame the rationale for this translational study. First, as already noted, dementia is a rapidly growing national and global public health problem, with dementia prevalence projected to nearly triple worldwide and in the U.S. by mid-century.1,2 The National Alzheimer's Project Act in the U.S. (NAPA), legislated in 2011, includes objectives aimed at supporting families caring for relatives with dementia from pre-diagnosis to end of life stages of the journey, and numerous countries have developed national dementia plans that include similar objectives.6 A recent Institute of Medicine (IOM) report emphasized the need for studies testing models of care linking existing community and healthcare systems to develop and understand payment considerations associated with comprehensive care for persons with dementia.7 Other reports similarly have identified the need for and challenges of translating evidence-based programs in service delivery settings.8-10 This translational study directly addresses this national public health imperative, legislative goal, and IOM directive.
Second, dementia leads to poor health-related outcomes. In addition to behavioral symptoms and cognitive decline, functional disability is a hallmark of dementia but is less studied as an outcome in its own right.11 Also, there are no known pharmacological treatments that address daily functional concerns such that there is an urgent need to advance nonpharmacological approaches to help families manage increasing dependencies among individuals with dementia. Due in part to these poor outcomes, dementia is an independent risk factor for nursing home admission in numerous studies of community-dwelling older adults, even when controlling for numerous comorbidities.12 Among community-dwelling older adults with dementia, functional disability and family caregiver physical and emotional strain, are the most important predictors of nursing home admission, pointing to the need to keep older adults with dementia at the highest levels of functioning as possible to remain living at home.13,14 Physical and emotional burdens of providing help with activities of daily living, as well as the challenges of managing disruptive behaviors such as wandering and resistance to care, place CGs at risk for depression, physical health problems, and admitting their relative to a nursing home.1,15-21
Third, while dementia adds considerably to Medicare and Medicaid costs due primarily to excess hospitalization and nursing home use,2,22 evidence-based interventions that might help reduce costs for the community-dwelling population with dementia are severely lacking. An appropriate test bed for such interventions is Medicaid and state revenue-funded home care programs that are designed to avoid or delay nursing home admission. Most states are aggressively rebalancing their Medicaid long-term care budgets, growing such home care program options while reducing nursing home use.23-27 Yet no known evidence-based interventions designed to slow functional decline in older adults with dementia have been introduced into these publicly-funded home care programs in an effort to achieve cost savings while improving health-related outcomes and those of their CGs.
Fourth, COPE is an evidence-based intervention well-suited for older adults with dementia and their CGs already participating in publicly-funded home care programs. In an efficacy trial, COPE helped sustain greater functional independence in older adults with dementia by providing a non-pharmacologic intervention that included CG education to build their dementia management skills, in-home environmental modifications, and clinical and laboratory tests designed to detect undiagnosed medical conditions in the older adults with dementia.5 The in-home nature of the COPE intervention and its focus on functional independence is highly consistent with the programmatic goals of Medicaid waiver and state revenue-funded home care programs for older adults nationwide.
Finally, publicly-funded home care programs are fertile yet understudied service settings for translational studies: Feasibility, sustainability, and cost-benefit can be simultaneously investigated along with health-related outcomes of interventions being translated. Embedding and testing evidence-based interventions for persons with dementia in existing publicly-funded home care programs and their CGs can help inform state and federal governments concerned with delaying or avoiding Medicaid-covered nursing home admissions and Medicare-covered hospitalizations. Translation of interventions with proven economic benefit would help address the rapidly growing problem of Medicaid and Medicare expenditures for America's costly dually-eligible population, more than half of whom have cognitive impairment.28
Study design
Translational design overview
Many non-pharmacologic interventions for older adults living at home with dementia and their CGs delivered by health and social service professionals have been found to produce positive health-related outcomes.5,29-46 This translational study, which targets the same population, is designed conceptually as an effectiveness-implementation hybrid design.47 The effectiveness-implementation hybrid design blends components of clinical effectiveness and implementation research. Of the three types of such hybrid designs explained by Curran and colleagues, in this study we are dually testing clinical and implementation interventions and strategies.47 This approach allows conclusions to be drawn simultaneously about the effectiveness of an evidence-based non-pharmacologic intervention on meaningful health-related outcomes at the individual level in a real-world service setting, and about the degree of success in incorporating the intervention into that service setting from the viewpoints of feasibility, acceptability, sustainability, and cost-benefit. Hybrid designs with these features have exciting potential to lead to rapid translation of research-based evidence into practice, thereby enhancing public health impact by speeding the traditional 17-year gap from research to application.47,48 For people with dementia and their family caregivers, the effectiveness-implementation hybrid design is especially attractive because in the effectiveness component health-related outcomes could be gathered from both members of the dyad affected by dementia, while implementation could be tested within the real-world settings of the rapidly growing array of community-based health and social services established to serve this challenging target population.
Study Aims
This study employs a randomized design to compare health-related outcomes between CHCPE clients and their CGs who receive the COPE intervention in addition to their CHCPE-funded services, with clients and CGs who receive CHCPE-funded services only. Study aims for CHCPE clients: Aim 1.1: Determine COPE effect on functional independence 4 months after randomization (primary study endpoint). Aim 1.2: Determine COPE effects on engagement in activities, quality of life, and prevention or alleviation of neuropsychiatric symptoms (NPS), 4 months after randomization. Aim 1.3: Determine COPE effects on functional independence, engagement in activities, quality of life, and prevention or alleviation of NPS, 12 months after randomization. We hypothesize that CHCPE clients receiving COPE will show greater functional independence, greater engagement in activities, better quality of life, and fewer NPS, compared to controls, 4 months and 12 months after randomization.
Study aims for CGs: Aim 2.1: Determine COPE effect on perceived CG well-being 4 months after randomization. Aim 2.2: Determine COPE effects on CG confidence in using dementia management strategies 4 months after randomization. Aim 2.3: Determine COPE effects on CG perceived well-being, confidence in using activities, and ability to keep client at home, 12 months after randomization. We hypothesize that COPE CGs will report improvement in all specified outcomes compared to controls, 4 and 12 months after randomization.
Translational study aims: Aim 3.1: Determine the net financial benefit of COPE, accounting for COPE intervention costs, CHCPE usual care costs, nursing home costs, and other service costs, 4 months and 12 months after randomization. The 12 month cost-benefit analysis will test whether financial benefits of COPE accrue over a longer time horizon than the 4-month intervention period. Aim 3.2: Determine the feasibility and acceptability of COPE implementation into the CHCPE from multiple stakeholder viewpoints, including CHCPE care managers and state Medicaid and public policy decision makers.
To address study aims 1.1 through 2.3, client and CG data will be collected by research staff via in-person interviews at 3 time points: pre-randomization; 4 months after randomization (coinciding with the end of the intervention); and 12 months after randomization. Effectiveness will also be tested via cost-benefit analyses (study aim 3.1) to provide important knowledge about the cost of COPE relative to its financial benefits 4 months and 12 months after randomization. To date, few published studies report costs in enough detail to provide evidence of economic costs and benefits of interventions supporting people with dementia and their CGs.49 Data for the cost analysis regarding nursing home, hospital, and emergency department use by study clients will be collected during routine monthly telephone contacts initiated by staff from the participating care management organization. CHCPE care plan service costs for study clients will be provided by the care management organization. Based on current Connecticut Medicaid hourly reimbursement rates for occupational therapists and skilled nurses, the estimated cost of COPE intervention delivery (i.e., 10 in-home occupational therapy visits and 1 in-home visit and 1 telephone follow-up visit by an APN) will be approximately $1,200 per client over the 4 month program. In order for COPE to provide a positive net financial benefit (i.e. save money compared to usual care) it will have to offset other costs. For example, in Connecticut the Medicaid reimbursement rate for nursing home care was $221 per day in 2011;50 therefore, COPE will result in a net financial benefit if it yields an average of 6 fewer nursing home days in the intervention group compared to the control group. Nursing home avoidance is an especially important financial consideration in this study because it is the primary public policy goal of the CHCPE as well as Medicaid waiver and state-funded home and community-based care programs nationwide. For the implementation study component (study aim 3.2), we will gather information on key COPE implementation processes via focus groups and key informant interviews to determine COPE's practical potential for implementation in the CHCPE. Guided by the overarching effectiveness-implementation hybrid design, our study will yield a comprehensive understanding of COPE's translational potential in the CHCPE and in Medicaid waiver and state-funded home and community-based care programs for older adults nationwide.
Theoretical framework for the COPE intervention
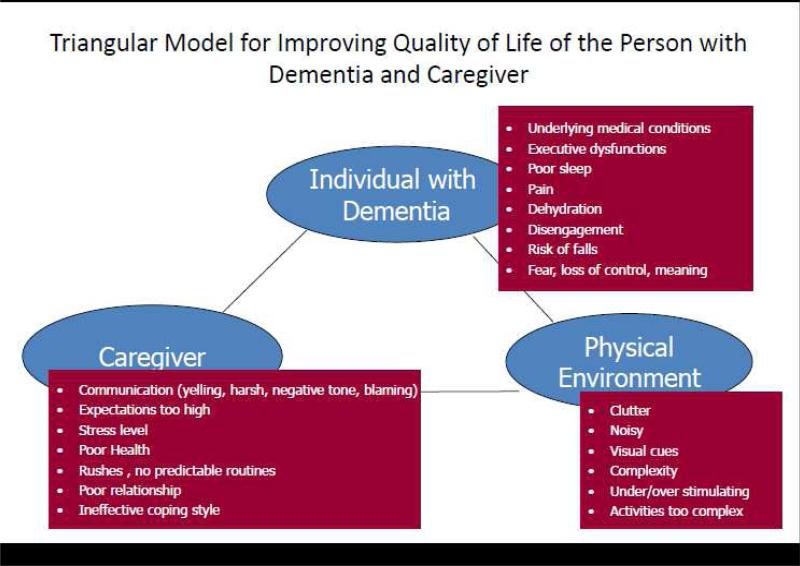
COPE is a 4- month, non-pharmacologic intervention based on the principles of competence-environmental press51 that seeks to maximize physical function and quality of life in people with dementia and build dementia management skills in CGs by realigning capabilities of persons with dementia with environmental demands. Figure 1 illustrates the interplay and interrelationships among the person with dementia, the CG, and the physical environment of the home setting in which the person with dementia lives. This “triangular” model guides the COPE intervention by giving equal emphasis to in-home assessment by clinical interventionists in the domains of the person with dementia, the CG, and the home environment in considerable depth. Figure 1 also shows areas of assessment focus within each of the domains in the triangular model. This figure suggests that various modifiable factors related to the person, caregiver and the living environment often serve as barriers to daily functioning in the home. Modifying these factors (e.g., addressing pain, simplifying caregiver communications, tasks, and the environment) may, therefore, help to reduce excess disability (disability over and above level of cognitive impairment) and optimize functional independence.
Figure 1.
Guiding Conceptual Framework for COPE CT Intervention, Based on Competence-Environmental Press Theory
COPE features 10 in-home visits by an occupational therapist and a single home visit and follow-up telephone contact by an advanced practice nurse. COPE efficacy trial results showed that at the end of the 4-month intervention period, COPE subjects with dementia had greater functional independence in activities of daily living, and were more engaged in daily activities, compared to controls. Also at the 4-month point, COPE CGs, compared to controls, reported improved well-being and increased confidence in using environmental modifications and dementia management activities learned from COPE interventionists (all p<0.05).5 An economic analysis was not conducted in the COPE trial, but COPE CGs were twice as likely as controls (40% vs 20%, p=0.02) to report 9 months after randomization that the intervention helped “a great deal” in keeping their relative at home.5 These results demonstrated the efficacy of COPE. COPE also built on several previous trials, all showing that a combination of training CGs in problem solving, environmental modification, and task simplification can address CG distress and minimize behavioral disturbances and functional difficulties.29,30,52,53 As these were efficacy trials, the next step in the research pipeline is to determine effectiveness of COPE and its translational potential in the “real world” CHCPE service setting.54,55
Study setting and translational target population
The study's target population is older adults (age ≥65 years) with dementia and their CGs who receive services from the CHCPE. The CHCPE features care management professionals coordinating home and community-based services, and is designed to delay or avoid long-term nursing home admission of frail older adults. The CHCPE is administered by 3 care management organizations in Connecticut, each responsible for a distinct geographic area, under contract with the Connecticut Department of Social Services.
For this study, the participating care management organization is Connecticut Community Care, Inc. (CCCI). Established in 1979 as a pioneer care management organization for older adults, today CCCI provides care management services to more than 8,000 CHCPE clients daily.
CCCI shares important features of all care management organizations nationally that are responsible for administering their states’ Medicaid waiver and state-funded home and community-based care service programs for older adults at risk or eligible for nursing home admission, including more than 200 Area Agencies on Aging. The most important shared features are that care managers must: conduct the same uniform clinical assessments of clients for eligibility determination and care plan development; coordinate a range of direct service providers delivering in-home and community-based services; and closely monitor the cost of care plans for each client. Accordingly, lessons learned in this study about effectiveness and implementation success of COPE within existing care plans will be highly generalizable to all care management organizations with identical responsibilities as CCCI.
Eligibility criteria for CHCPE clients and family caregivers (CGs)
Most study entry criteria were adopted from the COPE efficacy trial,5 revised primarily to accommodate client data available from the CCCI electronic database. Client inclusion criteria: (1) Active CHCPE client; (2) diagnosis of dementia or ≥4 errors on the Mental Status Questionnaire (MSQ), which is updated for each client every 6 months; (3) speaks or understands English. The MSQ threshold of ≥4 errors is considered moderate cognitive impairment.56 Client exclusion criteria: (1) Diagnosed schizophrenia or bipolar disorder; (2) bedbound and unresponsive; (3) participation in concurrent experimental drug study designed to treat agitation; (4) home environment deemed unsafe and/or unsanitary. CG inclusion criteria: (1) ≥21 years of age; (2) willing and able to participate in all aspects of the study; (3) plans to live in area for 12 months; (4) speaks English. CG exclusion criteria: (1) Terminal illness with life expectancy of <12 months (2) participation in concurrent non-pharmacologic trial designed to help CGs of people with dementia; (3) planning to admit client to a nursing home within 6 months.
Recruitment, enrollment and randomization
Our approach embeds recruitment within CCCI's daily operations, which is a critical element of the translation of COPE for this service setting. During routine monthly clinical monitoring telephone calls, CCCI care managers will explain key study features to provisionally eligible clients’ CGs and refer interested CGs and clients to the research study coordinator. Research staff will conduct telephone screenings with CGs to determine whether clients and CGs fulfill all remaining inclusion and exclusion criteria. Consenting and pre-randomization data collection will be done in the home setting. Randomization will be stratified according to whether the CHCPE client receives support from Medicaid funds or state general revenues. The study protocol was approved by the University of Connecticut Health Center Institutional Review Board.
Intervention condition
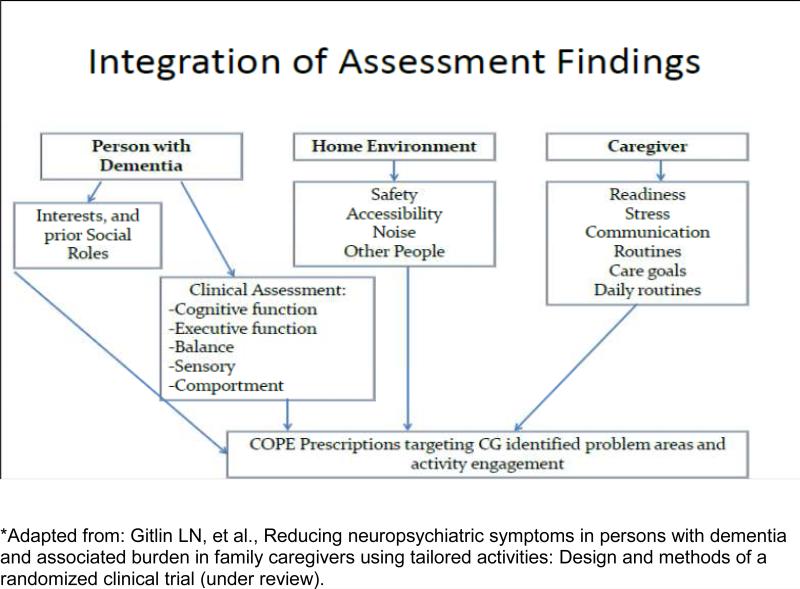
Dyads randomized to intervention will receive COPE plus usual CHCPE services. COPE seeks to support client capabilities by reducing environmental stressors, and enhancing caregiver skills. COPE dyads receive up to 10 sessions over 4 months by occupational therapists (OT); and 1 face-to-face and 1 telephone session by an advanced practice nurse (APN). OTs initially interview CGs to identify client routines, previous and current roles, habits and interests, and CG concerns. OTs also conduct cognitive and functional testing to identify client capacities and deficits in cognitive functioning, including attention, initiation/perseveration, construction, conceptualization, memory, planning and problem solving, as well as in physical functioning, including fall risks and mobility performance. OTs then train CGs how to modify home environments, simplify daily activities, and communicate effectively to support client capabilities, use problem-solving to identify solutions for CG-identified concerns (e.g., behavioral challenges, difficulties managing patient self-care), and use stress reduction techniques to lower their own distress. For each targeted concern jointly identified by CG and OT, a written action plan, referred to as a “COPE prescription”, is devised describing treatment goals, client capacities, and specific strategies for the CG to implement. Figure 2 illustrates how OTs integrate assessment information from the person with dementia, caregiver, and home environment to derive the COPE prescriptions.
Figure 2.
Link between Triangular Model and Assessment Flow Guiding COPE CT intervention*
In a separate home visit, the APN provides CGs with information to help them identify and monitor common health-related concerns (pain detection, hydration, constipation, medication management), and how to be a medical advocate. The APN also obtains blood/urine samples from the client, and examines the client for signs of dehydration. Laboratory evaluations include complete blood count, blood chemistry, and thyroid testing of serum samples, and culture and sensitivity of urine samples. Client medications are also reviewed for polypharmacy and dosing appropriateness. The purpose of these clinical tests is to rule out underlying medical conditions, infections or medication issues that may be negatively contributing to functioning at home. The APN informs CGs by telephone of laboratory results within 48 hours and mails two copies of the results to CGs (one for their records and the other copy to share with clients’ physicians). For positive laboratory results, CGs are asked if they prefer the APN to fax results to the physician and/or care manager, and discuss with them directly. A study geriatrician will be available to consult with the APN regarding any signs, symptoms, and laboratory tests requiring further clinical interpretation before the APN provides her assessments to CGs.
All COPE dyads receive exposure to each treatment component. Specific issues discussed and COPE prescriptions devised are tailored to address each CG's self-identified concerns and client capabilities. Table 1 summarizes the content of all COPE intervention visits, and Table 2 summarizes all treatment principles governing the COPE intervention.
Table 1.
COPE Intervention Content by Visit and Discipline
| Session number | Who | When | Content |
|---|---|---|---|
| 1 | OT | Within 7 days of baseline data collection | Introduce COPE program Introduce Caregiver Guide to Dementia Conduct Clinical Interview with Caregiver Conduct assessments with person with dementia: -Cognitive functioning - Executive function (CG report) Socialability (Comportment) Assess Caregiver Readiness to use new care strategies Assess caregiver Communications Teach caregiver simple stress reduction technique (e.g., breathing) |
| 2 | OT | Within 7 days of previous session | Review stress reduction Continue with Clinical Interview to identify and prioritize care challenges Continue with assessments of person with dementia and home environment |
| 3 | APRN | Between first and second OT sessions | Provide caregiver education about person with dementia: Pain Dehydration Infection Constipation Polypharmacy Talking to doctor Collect and test urine sample from person with dementia for evidence of infection Collect and test blood sample from person with dementia for abnormalities Discuss how caregiver can take care of him/her self Discuss how to talk to doctor and questions to ask |
| 4 | APRN | Telephone contact; within 2 days of Session #3 | Share laboratory results with and mail results to caregiver Encourage caregiver to contact primary care provider with any abnormal findings |
| 5 | OT | 1-2 weeks after previous OT session | Review stress reduction Deliver Assessment Report Problem Area 1 Problem-solving process Brainstorming process Provide 1 or 2 strategies to try |
| 6-10 | OT | Each session 1-2 weeks after previous session | Review stress reduction Problem solving and brainstorming for Problem Areas 2 and 3 (Sessions 6, 7) Introduce COPE Prescription – assess confidence (Sessions 6 through 9) Determine level of effectiveness using activity prescription – using Prescription Record (Sessions 7 through 10) |
| 11 | OT | 1-2 weeks after last session | Follow up on stress reduction Review ways to modify strategies when cognitive abilities change over time Review ways to simplify activities when cognitive abilities of the Person with Dementia change over time Model by identifying ways to simplify the activity Together simplify the activity Caregiver simplifies the activity on his/her own |
| 12 | OT | 1-2 weeks after last session | Review stress reduction Assess caregiver confidence for each Prescription If caregiver reports low confidence (≤5) using any one prescription, then troubleshoot and review specific strategies. If caregiver reports moderate to high confidence, validate how much the caregiver has learned and how well they are doing. Review ways to generalize strategies to new problem areas that may occur in the future Closure |
Table 2.
Immutable COPE Treatment Principles
| 1. Client-centered and client directed |
| – Meeting the caregiver where they are |
| – Working on areas of most concern to families |
| – Tailoring to abilities of individuals with dementia |
| 2. Customized |
| – Tailoring to abilities and interests of person with dementia |
| – Tailoring education and strategies to caregiver readiness and identified problems |
| – Consideration of environmental factors |
| 3. Culturally relevant |
| – Strategies fit previous/current roles, habits and beliefs |
| – Activities are meaningful |
| 4. Problem solving based |
| – Identifying strategies and activities |
| – Determining what works and what does not |
| 5. Actively engaged |
| –Learning through doing |
| 6. Caregiver driven |
| – modify delivery of intervention protocol if needed (e.g., phone vs home visit, or spend more time on one prescription versus another; however, ALWAYS adhere to Treatment Principles |
In order to embed COPE within the routine operation of the Connecticut Home Care Program for Elders (CHCPE), which is essential to the translational effort of this study, the care manager responsible for the total care plan for each CHCPE client will be notified by the study coordinator when a client is assigned to the intervention group, as well as the name of the assigned OT and APN for that client. This will trigger contact between the care manager and assigned COPE interventionists, so that the care manager can help insure that interventionists do not visit client homes at the same time as service providers already providing care to clients per their approved care plans and that care managers can be informed of pertinent clinical information as needed (e.g., positive laboratory results). Care managers will also notify COPE interventionists if they learn, in the course of routine care plan monitoring activities, that a client experiences a change in location due to hospitalization or short-term nursing home admission. In these instances, when the client returns home, care managers routinely re-evaluate the entire care plan and make changes accordingly. Accordingly, the COPE interventionists will re-evaluate such clients upon their return home and adjust COPE prescriptions as appropriate to the client's and CG's situation at that time.
Control condition
Dyads randomly assigned to the control group will continue to receive CHCPE program services only per approved care plans, but will receive no additional services as a result of study participation. The rationale for offering only usual care to the control group is that, as a translational study, our goal is to determine the added value of the COPE intervention to customary CHCPE program services. Control group dyads will, however, receive a free copy of A Caregiver's Guide to Dementia57 after they complete the 12-month follow up research interview.
Fidelity monitoring plan
Our fidelity plan is based on a conceptualization of fidelity as composed of three components: delivery; receipt; and enactment; each of which can be enhanced, measured, and monitored, as used in the COPE efficacy trial and recommended by others.5,58,59 COPE interventionists will complete fidelity checklists for each home visit for purposes of monitoring delivery, receipt and enactment of imparted strategies. Completed fidelity forms and COPE prescriptions will be reviewed for the first three dyads assigned to each OT interventionist. Interventionists also will participate in bi-monthly phone calls with the investigative team to review case studies and discuss any challenges that might disrupt treatment fidelity. The study coordinator will conduct in-home fidelity visits for each interventionist at least once per year and complete a fidelity checklist and write up a report based on this fidelity visit.
As an important translational component of this study, COPE fidelity monitoring responsibilities will be transferred from Drs. Gitlin and Piersol to CCCI care managers after a minimum of two years of intervention implementation. Members of the investigative team will conduct COPE fidelity monitoring training for CCCI care managers. Fidelity monitoring forms from the original COPE trial will be modified with input from care managers and their director, to reflect charting methods routinely used by CCCI care managers to track all CHCPE services. As part of their service oversight responsibilities, care managers will then begin using revised fidelity monitoring forms to track home visits made by COPE interventionists. This approach seeks to assure continued maintenance of fidelity as the program becomes fully integrated into and normalized within routine practice. Evaluation of success of this fidelity transfer process will be an important component of the implementation process in this study.
Data collection and management
Interviews with clients and CGs
In-person pre-randomization and 4-month follow-up interviews will be conducted in the home setting, while 12-month follow-up interviews will be conducted with CGs by telephone. To minimize response bias during in-person interviews, each dyad member will be interviewed separately, out of listening range from the other dyad member. Client and CG retention strategies will include sending thank you notes after each interview and sending birthday cards and/or condolence cards as applicable.
CCCI database
The CCCI electronic database is a repository of all clients’ approved CHCPE care plan services, hospitalizations, ED visits, rehabilitation nursing home stays, and long-term nursing home stays, all updated monthly by CCCI care managers. All data stored in the CCCI database pertaining to use of these services for the 12 months following randomization will be linked to data from other study sources at the client level. Service use data from the CCCI database also will be used by investigators responsible for constructing analytic variables for purposes of conducting economic analyses.
Client and family caregiver measurements
Primary client outcome
For functional independence, the primary client outcome measure, we will use the 15-item Caregiver Assessment of Function and Upset (CAFU) modeled after the Functional Independence Measure, as used in the COPE efficacy trial.5 Items include 7 self-care activities of daily living (ADLs): bathing, dressing upper/lower body, toileting, grooming, eating, and getting into/out of bed; and 8 instrumental ADLs (IADLs); telephone use, shopping, meal preparation, housework, laundry, transportation, taking medicine, and managing finances. CGs report level of assistance required by clients to complete each item, based on a 7-point scale ranging from completely independent (score of 7) to needing complete help (>75% assistance; score of 1). The total mean functional independence score is derived by summing scores across all items and dividing by the number of items. In the COPE efficacy trial, internal consistency reliability was very high (α=0.92).
Secondary client outcomes
Activity engagement of clients, reported by CGs, will be measured using the 5-item scale used in the COPE efficacy trial, including items such as “showed signs of pleasure/enjoyment”, with responses ranging from 1 (never) to 3 (often; α=0.62 in the COPE efficacy trial).5 Quality of life will be measured using the 12-item Quality of Life-Alzheimer's Disease (QOL-AD) scale.60 The QOL-AD is designed for completion by people with dementia and, independently, by CGs. The COPE efficacy trial used CG reports (α=0.78); in this study, we will report results of client and CG derived measures. Neuropsychiatric symptoms will be measured using the psychometrically sound Neuropsychiatric Inventory (NPI) which assesses, via CG report, presence and frequency of 10 symptoms: anxiety, agitation, apathy, irritability, aberrant motor activity, euphoria, dysphoria, disinhibition, delusions, and hallucinations. Total NPI scores range from 0-120, with higher scores representing greater symptoms.61
CG outcomes
Perceived well-being, the primary outcome for CGs as in the COPE efficacy trial, will be measured using the 13-item Perceived Change Index (α=0.86).5 CGs rate their ability to manage dementia-related cognitive and behavioral symptoms in the previous month, with responses ranging from 1 (got much worse) to 5 (improved a lot). Confidence in using activities over the previous month will be measured using a 5-item scale developed in previous work and used in the original COPE trial.5 CGs rate each item (e.g., involve client in activities; use activities to distract client) from 0 (not confident) to 10 (very confident; α=0.87).
Covariates
Covariates were chosen by balancing parsimony with evidence of their associations with proposed study outcomes. Covariates include: living arrangements (CG and client live together or apart); racial and ethnic group membership of clients and CGs (non-Hispanic White; Black or African-American; other group); CG familial relationship to client (spouse, adult child; other relationship); number of hours/week CG provides care; CG educational attainment (<high school; high school grad; >high school) as a measure of socioeconomic status; and severity of client's cognitive impairment at study entry, measured using the St. Louis University Mental Status (SLUMS) examination.62 These variables have been used previously as covariates in dementia and caregiving studies such as the original COPE efficacy trial,5 the NIH-funded REACH II study,29 and others including a recent systematic review of predictors of nursing home admission among individuals with dementia.13
Sample size and power calculations
We estimate sample size needs and associated statistical power based on expected mean changes from baseline to 4 months between intervention and control groups for client and CG primary and secondary outcomes. Based on COPE efficacy trial results,5 a sample size of 128 dyads per treatment group provides >80% power for these outcomes, as shown below. Based on recruitment experience reported in the COPE efficacy trial,5 to achieve 256 dyads completing the intervention period and 4-month data collection, we must randomize 290 dyads (12% attrition from randomization to 4 months), or 145 dyads in the intervention group and 145 dyads in the control group.
| Outcome source | Outcome Level | Variable description | Proposed Δ in original scale | SD | Effect size (=Δ/SD) | Power for N=128/group |
|---|---|---|---|---|---|---|
| Client | Primary | Functional Independence | 0.27 | 0.75 | 0.36 | 0.82 |
| Client | Secondary | Activity Engagement | 0.15 | 0.42 | 0.36 | 0.81 |
| Caregiver | Primary | Perceived Well-Being | 0.25 | 0.58 | 0.43 | 0.93 |
| Caregiver | Secondary | Confidence in Using Activities | 0.75 | 1.90 | 0.40 | 0.88 |
Due to the translational nature of this study, for the client primary outcome (functional independence), we also intend to assess subject benefit in more clinically meaningful terms. We will categorize subjects according to whether they improve, remain stable, or decline in functional independence from baseline to 4 months in either direction, based on a 0.5 SD change in the continuous measure. The table below shows an expected distribution of this categorical variable by study group, based closely on findings shown in the COPE efficacy trial. When the sample size in each of the two groups is 128, a 0.05 level Chi-square test will have 80% power to distinguish between the groups when the proportions in the 3 categories are as shown in this table.63 In summary, we have demonstrated that, with N=128 dyads per treatment group, the study will be sufficiently powered based on a set of assumptions derived from the COPE efficacy trial.
| Functional independence: Changes from baseline to 4 months | |||
|---|---|---|---|
| Group | Improved | Stayed about the same | Declined |
| Intervention | 55% | 40% | 5% |
| Control | 40% | 45% | 15% |
Analytic approach
Data will be analyzed primarily using analysis of covariance (ANCOVA) methods in which baseline values of outcomes are used as covariates; this approach is more efficient than analysis of change scores.64 Distributions of outcome variables will be tested for normality; appropriate transformation or non-parametric alternatives will be employed. The treatment group variable in the ANCOVA model will test the main hypothesis, which is whether there is an intervention vs. control group mean difference in functional independence at 4 months (Aim 1.1). Other client outcomes (Aim 1.2), and CG outcomes (Aims 2.1, 2.2), are continuous variables and will be treated the same as functional independence. ANCOVA models can be fit in GLM or REG procedures in SAS® using OLS regression.65 For covariates included to evaluate effect modification, (covariate × treatment) interaction terms will assess the extent to which they modify the treatment-outcome relationship.66 If interaction terms for specific covariates are statistically significant, we will conduct subgroup analyses using those covariates to identify clients and caregiving situations that benefit most from the intervention.66 Covariates that show no or low association with outcomes and do not contribute any adjustments, as deemed by comparing model coefficients with covariates in and out of models, may be dropped for parsimony.67 Adjusted and unadjusted results will be reported. Treatment group differences in the categorical outcome measure created from functional independence scores at baseline and 4 months (i.e., improved, stayed the same, declined) will be assessed using chi square tests for bivariate analyses or, by adding covariates, in multivariate logistic regression models to produce adjusted odds ratios and 95% confidence limits. To determine long-term COPE effects on client outcomes (Aim 1.3) and CG outcomes (Aim 2.3), analyses based on outcome measures at 12 months post-randomization will use the same ANCOVA methods as described above for 4 month outcomes, using baseline values of outcomes as covariates. We will also conduct analyses to assess the extent to which any COPE effects observed at 4 months are sustained at 12 months, using longitudinal data models with baseline, 4 month, and 12 month values of each outcome measure. For continuous outcomes we will fit linear mixed-effects models for repeated measures, while for categorical outcomes we will use general estimating equations. Models will include main effects of group and time and can test (group × time) interactions. In all these models, we will evaluate the effects of additional covariates as noted above in analysis plans for outcomes 4 months after randomization. Missing data assumptions will be evaluated for each approach.
Cost analyses
The economic analysis will consist of a net financial benefit analysis to inform decision makers in state Medicaid agencies and in care management organizations responsible for Medicaid waiver and state-funded home and community-based service programs for older adults about the economic value of COPE. The net financial benefit of COPE will be calculated based on best practices in cost benefit analysis, as published by Drummond and colleagues.68 Specifically, using a cost benefit analytic framework, we will determine whether the net financial benefit of COPE is ≥ $0; that is, whether the cost of the COPE intervention is equal to or exceeded by its financial benefits. Sensitivity analyses will be conducted in order to determine the robustness of the cost-benefit analysis. Univariate sensitivity analysis will be performed, whereby one variable is changed at a time and impact on net benefit is determined. Sensitivity analyses will include those variables where we anticipate real world uncertainty and will include, at a minimum, modification of key cost variables, e.g., inpatient and outpatient medical costs, based on 25th and 75th percentiles of Medicare reimbursement. This approach, a standard for decision-makers, provides a concrete understanding of financial benefits of COPE. Findings will inform future investments in COPE as well as scalability. More broadly, findings using this approach will contribute to a refined understanding of the economic value of non-pharmacologic treatments to maintain health and independence in older adults with dementia and their CGs.
Intervention and supervisory costs will be calculated using wage rates for COPE intervention personnel multiplied by reported time in preparation, documentation, training and supervision, and fringe benefit costs. Total COPE intervention costs will be defined as the sum of the following direct cost categories: COPE interventionist home and telephone sessions; training time; intervention materials; interventionist travel; care planning; and supervision.69,70 Table 3 provides greater detail on components of intervention and supervisory costs for the COPE CT study.
Table 3.
COPE Intervention Costs
| Cost Type | Description | How Measured |
|---|---|---|
| Staff Training | Training of interventionists, nurses, and screeners | Wage + fringe benefit costs for interventionists, nurses, and screeners multiplied by time spent in training |
| Screening for Eligibility | Screening delivered by a trained staff member | Wage of screeners + fringe benefit cost, multiplied by time spent delivering screening |
| OT Intervention Delivery | Home intervention delivered by OTs | Wage of OTs + fringe benefit costs multiplied by time delivering OT home visits |
| Nurse Initial Assessment | Nurse home assessment of patients | Wage of nurse + fringe benefit cost multiplied by time spent conducting the home assessment |
| Nurse Follow Up Phone Call to Caregiver | Nurse reporting laboratory test results to caregiver | Wage of nurse + fringe benefit cost multiplied by time spent reporting back to caregiver by phone |
| OT Contacts Outside of Intervention Delivery | OT contacts outside of delivering the intervention; includes telephone support, preparation for visits and documentation of visits. Communications are with CCCI, study nurse, other healthcare providers, caregiver/family clients, or other relevant individuals | Wage + fringe benefit costs of OT and personnel being communicated to, multiplied by time spent |
| OT and Nurse Travel Time | OT and nurse travel to and from patient homes and training sites | Wage + fringe benefit costs of OT and nurse personnel multiplied by time spent in travel |
| Communication Between Nurse and Healthcare Providers | Nurse communication (telephone or in person) to geriatrician or other physicians/other care providers (excludes nurse communications with study OT personnel since this time is separately costed) | Wage + fringe benefit costs of nurse and healthcare provider being communicated to, multiplied by time spent |
| Supervision | Supervision of screeners, OTs and nurses by a trained staff member | Wage + fringe benefit costs of supervisor multiplied by time spent supervising |
| Activity Supplies | All activity supplies purchased specifically for this grant, such as cards, folding towels, making flower arrangements etc. | Cost of activity supplies |
| Materials | All office materials (print and otherwise) used for screening, and mailing test results | Cost of materials used for screening, and mailing test results (Note: exclude costs of recruitment materials such as consent forms, since these are research costs not program costs) |
| Mileage | Mileage for OT and nurse travel | Roundtrip mileage travel by OT and nurses multiplied by prevailing mileage reimbursement rate |
| Care Plan | Monthly cost of care plan | Cost of care plan developed and implemented by OTs |
Direct medical costs will be estimated using the Medicare payer perspective using published sources of Medicare reimbursement rates for inpatient and outpatient medical services. Hospitalization, emergency room, post-acute care, and ambulatory medical care costs will be calculated using client-specific data extracted from the CCCI electronic database. Costs for inpatient services will be based on Medicare reimbursements for the DRGs pertaining to medical reasons for seeking inpatient care.71 Costs for outpatient services will be based on Medicare reimbursement rates for the CPT code pertaining to the medical reasons for seeking outpatient care.72 Medication costs: Medications taken by clients will be identified through the CCCI database. We will plan to apply Medicare Part D average sales prices (ASP) to estimate medication costs; if unavailable at the time of analysis, we will estimate costs based on discounted Average Wholesale Prices obtained from the Red Book drug pricing reference guide.73
Approach for Translational Implementation aim (Aim 3.2)
Theoretical framework
An important aspect of our effectiveness-implementation hybrid design is understanding implementation processes47 from which to enable widespread scaling up of COPE within CHCPE as well as within Medicaid waiver and state-funded home care programs elsewhere. To this end, we draw upon Normalization Process Theory (NPT) which posits 4 criteria for judging the implementation potential of an intervention, how practice settings may be affected by the intervention, and how the intervention can be modified to support its implementation.74 This framework provides a systematic way of accounting for both individual participants (e.g., care manager, interventionists) and organizational factors (e.g., daily practice routines, supports and constraints, and supervisory structures) that influence implementation and potential for sustaining COPE within the service context. NPT criteria defining feasibility and acceptability are: coherence, which evaluates whether the intervention makes sense to participants, and if it fits within their practice goals and activities; cognitive participation, which refers to whether participants are willing to invest the time and energy needed to learn and practice the intervention; collective action, or how compatible the intervention is with work practices and daily routines; and reflexive monitoring, or, whether participants perceive intervention benefits and added value. According to NPT, all four criteria must be met or adjustments made for an intervention to have full implementation potential. These criteria will guide the development of focus group and key informant interview questions and probes and provide an analytic framework for evaluating implementation processes.
Focus groups
We will conduct focus groups annually in years 2-4 with all CCCI care managers (CMs) whose clients received COPE. In years 3-4, a focus group will also be held with all COPE interventionists together (up to 8 OTs and an APN). For all focus groups conducted each year, the investigative team and Translational Advisory Committee (TAC) will develop guiding questions and structured probes based on the four NPT criteria. Of specific interest are: feasibility of identifying CHCPE clients who are eligible for COPE; perceived barriers and supports to screening clients for eligibility; acceptability of the intervention in terms of added value and added benefit; whether training time is perceived as worth the effort; and whether the fidelity plan is effective and fits within daily routines and practices of care managers. Focus group and key informant results will be discussed at annual meetings with the TAC to derive a plan for widespread diffusion.
Key informant interviews
Key informants interviewed during year 5 will include the CHCPE program manager in the Department of Social Services, the state Medicaid director, leaders in the state legislature's public health committee, and older adult advocacy organization leaders, all in Connecticut. Based on study evidence and their knowledge of the current and emerging public policy environment, key informants will be asked to specify practical and policy-related steps needed to integrate COPE services into the CHCPE after study completion. Interview results will be presented at the final meeting with the TAC to help inform a diffusion plan for COPE into the CHCPE and further evaluate if adjustments to client identification, referral and intervention components or fidelity monitoring are recommended. Taken as a whole, the focus group, key informant and formal review by the TAC will yield an in-depth understanding of implementation processes, adaptations needed, and a plan for widespread training and implementation.
Qualitative analyses
Focus groups and key informant interviews will be audiotaped, formatted as single transcripts per session or interview, and imported into Atlas.ti software. Specific references to modifications to client identification and referral procedures, interventionist training, conduct of the intervention, and fidelity monitoring procedures will be coded and categorized following emerging cataloguing schemes for adapting interventions to practice settings.75 Dr. Robison will lead the development of coding guides; recurrent themes will be noted and text will be clustered under these themes using the constant comparative method, until saturation is achieved.76,77 Illustrative and supportive quotations will be included in analysis reports.
CG interviews
To determine acceptability and perceived benefit of the COPE to families, we will administer a modified version of the COPE efficacy trial evaluation questionnaire as part of the 12 month post-randomization interview. This questionnaire assesses CG satisfaction and elicits information about perceived COPE benefits and areas needing improvement. Descriptive quantitative results along with supportive quotations from this CG interview component will be shared with TAC members as additional evidence regarding the degree of COPE implementation success.
Discussion
The translational study explained here represents the first known comprehensive effort to test the effectiveness and net financial benefit of an evidence-based in-home dementia care intervention designed to improve health-related outcomes for both the person with dementia and family caregivers when incorporated directly into an ongoing Medicaid waiver and state-funded home care program for older adults. This study is significant and timely given two concurrent and complementary trends: (1) the projected increase in the prevalence of dementia in the U.S. over the next several decades2; and (2) the anticipated growth of Medicaid and other publicly-funded long term home care programs in lieu of nursing home growth in most states, spurred by “rebalancing” initiatives dominating long-term services and supports policies in the U.S.78.79
Our long-term goal is to successfully guide others to integrate COPE (if effective) within Medicaid and other publicly-funded home care programs throughout the country, and to establish a variety of technical assistance approaches to enable other states to implement COPE. To this end, we have planned numerous national dissemination activities, including: COPE program manual for care management organizations detailing procedures and considerations for integrating COPE into Medicaid waiver and state-funded home care programs for older adults with dementia and their CGs; COPE implementation manual for CMs working with these clients and CGs, including how to identify and recruit OTs and APNs in their geographic areas to serve as COPE providers and how to monitor COPE delivery fidelity; and COPE training manual that will be disseminated in partnership with the American Occupational Therapy Association (AOTA) to attract OTs to the field of aging and dementia care. As currently planned, dissemination and technical assistance activities will be offered on-line as well as at scientific and professional conferences. Additionally, beginning in 2016, selected baseline results and reports on progress toward incorporating COPE into the daily practice schedule of care managers will be presented annually at national and international scientific and professional meetings including the Alzheimer's Association International Conference, Gerontological Society of America (GSA), American Public Health Association (APHA); National Association of Area Agencies on Aging, and AOTA. At GSA and APHA annual meetings, where APNs in the fields of geriatrics, geropsychiatry, and public health attend in large numbers, efforts will be made to reach these nursing specialties so they are aware of study presentations during these meetings. Study results related to outcomes and implementation successes and challenges will be prepared for publication review in high-impact, peer-reviewed journals in the fields of aging, public health, OT, nursing, implementation science, and/or health services research.
Acknowledgments
Research reported in this manuscript was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG044504. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trials.gov number: NCT02365051
References
- 1.Alzheimer's Disease International . World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Costs, and Trends. Alzheimer's Disease International; London: 2015. [Google Scholar]
- 2.Alzheimer's Association. 2015 Alzheimer's Disease Facts and Figures. Chicago. 2015 [Google Scholar]
- 3.Gitlin LN, Vause Earland T. In: Improving Quality of Life in Individuals with Dementia: The Role of Nonpharmacologic Approaches in Rehabilitation. Stone B JH, editor. International encyclopedia of rehabilitation; 2009. [Google Scholar]
- 4.Connecticut Home Care Program for Elders . Home Care at a Glance: SFY 2010 Annual Report to the Legislature July 2009-June 2010. Hartford; 2010. [Google Scholar]
- 5.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983–91. doi: 10.1001/jama.2010.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortinsky RH, Downs M. Optimizing person-centered transitions in the dementia journey: A comparison of national dementia strategies. Health Aff. 2014;33(4):566–573. doi: 10.1377/hlthaff.2013.1304. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (IOM) Living well with chronic illness: A call for public health action. The National Academies Press; Washington, D.C.: 2012. [Google Scholar]
- 8.Alzheimer's Association National Plan Care and Support Milestone Workgroup. Borson S, Boustani MA, Buckwalter KC, Burgio LD, Chodosh J, Fortinsky RH, Gifford DR, Gwyther LP, Koren MJ, Lynn J, Roherty R, Phillips C, Ronch J, Stahln C, Rodgers L, Kimp H, Baumgart M, Geiger A. Report on milestones for care and support under the U.S. National Plan to Address Alzheimer's Disease. Alzheimer's Dement. doi: 10.1016/j.jalz.2016.01.005. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Gitlin L, Leff B. Lessons Learned from Implementing Proven Interventions into Real World Contexts. In: Gitlin L, Czaja S, editors. Behavioral Intervention Research: Designing, Evaluating and Implementing. Springer Publications, Inc.; New York: 2016. [Google Scholar]
- 10.Gitlin L, Marx K, Stanley I, Hodgson N. Translating evidence-based dementia caregiving interventions into practice: State-of-the-science and next steps. Gerontologist. 2015;55(2):210–226. doi: 10.1093/geront/gnu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitlin LN, Hodgson N, Choi S, Marx K. Interventions to Address Functional Decline in Persons with Dementia: Closing the Gap between what a Person “Does Do” and what they “Can Do:”. In: Park R, et al., editors. Neuropsychology of Alzheimer's Disease and Other Dementias. 2nd Ed. Oxford University Press; New York: (in press) [Google Scholar]
- 12.Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaugler JE, Yu F, Krichbaum K, Wyman JF. Predictors of nursing home admission for persons with dementia. Med Care. 2009;47:191–8. doi: 10.1097/MLR.0b013e31818457ce. [DOI] [PubMed] [Google Scholar]
- 14.Spillman BC, Long SK. Does high caregiver stress predict nursing home entry? Inquiry. 2009;46:140–61. doi: 10.5034/inquiryjrnl_46.02.140. [DOI] [PubMed] [Google Scholar]
- 15.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimer's Dement. 2011;7:532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilworth-Anderson P, Williams IC, Gibson BE. Issues of race, ethnicity, and culture in caregiving research: a 20-year review (1980-2000). Gerontologist. 2002;42:237–72. doi: 10.1093/geront/42.2.237. [DOI] [PubMed] [Google Scholar]
- 17.Pinquart M, Sorensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: a meta-analysis. Gerontologist. 2005;45:90–106. doi: 10.1093/geront/45.1.90. [DOI] [PubMed] [Google Scholar]
- 18.Alzheimer's Association. 2011 Alzheimer's Disease Facts and Figures. Chicago. 2011 doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta- analysis. Psychol Bull. 2003;129:946–72. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 20.Vitaliano PP, Murphy M, Young HM, Echeverria D, Borson S. Does caring for a spouse with dementia promote cognitive decline? A hypothesis and proposed mechanisms. J Am Geriatr Soc. 2011;59:900–8. doi: 10.1111/j.1532-5415.2011.03368.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–9. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 22.Fortinsky RH, Fenster JR, Judge JO. Medicare and Medicaid home health and Medicaid waiver services for dually eligible older adults: risk factors for use and correlates of expenditures. Gerontologist. 2004;44:739–49. doi: 10.1093/geront/44.6.739. [DOI] [PubMed] [Google Scholar]
- 23.National Health Policy Forum . National Spending for Long-Term Services and Supports (LTSS) The George Washington University; Washington, D.C.: 2012. [Google Scholar]
- 24.Eiken S, Burwell B, Gold L, Sredl K. Medicaid 1915(C) Waiver Expenditures: 2011 Update. Cambridge: 2011. [Google Scholar]
- 25.Mollica R, Simms-Kastelein K, Kassner E. State-Funded Home and Community-Based Services Programs for Older Adults. Washington, DC: 2009. [Google Scholar]
- 26.Kassner E. Home and Community-Based Long-Term Services and Supports for Older People. Washington, DC: 2011. [Google Scholar]
- 27.Kunkel S, Carpio E. The National Aging Network Survey: 2010 Results. Scripps Gerontology Center, Miami University; Oxford, Ohio: 2011. [Google Scholar]
- 28.Kaiser Commission . Dual eligibles: Medicaid's role for low-income Medicare beneficiaries. Washington, DC: 2010. [Google Scholar]
- 29.Belle SH, Burgio L, Burns R, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitlin LN, Belle SH, Burgio LD, et al. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychol Aging. 2003;18:361–74. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–9. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 32.Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. Am J Psychiatr. 2004;161:850–6. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 33.Nichols LO, Chang C, Lummus A, et al. The cost-effectiveness of a behavior intervention with caregivers of patients with Alzheimer's disease. J Am Geriatr Soc. 2008;56:413–20. doi: 10.1111/j.1532-5415.2007.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols LO, Martindale-Adams J, Burns R, Graney MJ, Zuber J. Translation of a dementia caregiver support program in a health care system--REACH VA. Arch Intern Med. 2011;171:353–9. doi: 10.1001/archinternmed.2010.548. [DOI] [PubMed] [Google Scholar]
- 35.Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: a randomized controlled trial. Gerontologist. 2005;45:802–11. doi: 10.1093/geront/45.6.802. [DOI] [PubMed] [Google Scholar]
- 36.Hepburn KW, Tornatore J, Center B, Ostwald SW. Dementia family caregiver training: affecting beliefs about caregiving and caregiver outcomes. J Am Geriatr Soc. 2001;49:450–7. doi: 10.1046/j.1532-5415.2001.49090.x. [DOI] [PubMed] [Google Scholar]
- 37.Ostwald SK, Hepburn KW, Caron W, Burns T, Mantell R. Reducing caregiver burden: a randomized psychoeducational intervention for caregivers of persons with dementia. Gerontologist. 1999;39:299–309. doi: 10.1093/geront/39.3.299. [DOI] [PubMed] [Google Scholar]
- 38.Whitlatch CJ, Judge K, Zarit SH, Femia E. Dyadic intervention for family caregivers and care receivers in early-stage dementia. Gerontologist. 2006;46:688–94. doi: 10.1093/geront/46.5.688. [DOI] [PubMed] [Google Scholar]
- 39.Bass DM, Clark PA, Looman WJ, McCarthy CA, Eckert S. The Cleveland Alzheimer's managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73–85. doi: 10.1093/geront/43.1.73. [DOI] [PubMed] [Google Scholar]
- 40.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148–57. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 41.Fortinsky RH, Kulldorff M, Kleppinger A, Kenyon-Pesce L. Dementia care consultation for family caregivers: collaborative model linking an Alzheimer's association chapter with primary care physicians. Aging Ment Health. 2009;13:162–70. doi: 10.1080/13607860902746160. [DOI] [PubMed] [Google Scholar]
- 42.Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Inter Med. 2006;145:713–26. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 43.Tremont G, Davis JD, Bishop DS, Fortinsky RH. Telephone-Delivered Psychosocial Intervention Reduces Burden in Dementia Caregivers. Dementia. 2008;7:503–20. doi: 10.1177/1471301208096632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortinsky RH, Unson C, Garcia RI. Helping Family Caregivers by Linking Primary Care Physicians with Community-based Dementia Care Services: The Alzheimer's Service Coordination Program. Dementia. 2002;1:227–40. [Google Scholar]
- 45.Burgio LD, Collins IB, Schmid B, Wharton T, McCallum D, Decoster J. Translating the REACH caregiver intervention for use by area agency on aging personnel: the REACH OUT program. Gerontologist. 2009;49:103–16. doi: 10.1093/geront/gnp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thinnes A, Padilla R. Effect of educational and supportive strategies on the ability of caregivers of people with dementia to maintain participation in that role. Am J Occup Ther. 2011;65:541–9. doi: 10.5014/ajot.2011.002634. [DOI] [PubMed] [Google Scholar]
- 47.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–26. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine (IOM) Priority Areas for National Action: Transforming Health Care Quality. National Acadamies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 49.Jones C, Edwards RT, Hounsome B. A systematic review of the cost-effectiveness of interventions for supporting informal caregivers of people with dementia residing in the community. Int Psychogeriatr. 2012;24:6–18. doi: 10.1017/S1041610211001207. [DOI] [PubMed] [Google Scholar]
- 50.AARP Public Policy Institute . Across the States: Profiles of Long Term Services and Supports. AARP; Washington, DC: 2012. [Google Scholar]
- 51.Lawton MP, Nahemow LE. Ecology and the aging process. In: Lawton CP, editor. The psychology of adult development and aging. American Psychology Association; Washington, D.C.: 1973. [Google Scholar]
- 52.Gitlin LN, Winter L, Vause Earland T, et al. The Tailored Activity Program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. Gerontologist. 2009;49:428–39. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc. 2010;58:1465–74. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson KM, Brady TJ, Lesesne C. An organizing framework for translation in public health: the Knowledge to Action Framework. Prev Chronic Dis. 2011;8:A46. [PMC free article] [PubMed] [Google Scholar]
- 55.Gitlin LN, Czaja S J. Behavioral Intervention Research: Designing, Evaluating and Implementing. Springer Publishing Company; New York: 2016. [Google Scholar]
- 56.Kahn RL, Goldfarb AI, Pollack M, Peck A. Brief objective measures for the determination of mental status in the aged. Am J Psychiatry. 1960;117:326–8. doi: 10.1176/ajp.117.4.326. [DOI] [PubMed] [Google Scholar]
- 57.Gitlin LN, Piersol CV. A Caregiver's Guide to Dementia: Using Activities and Other Strategies to Prevent, Reduce and Manage Behavioral Symptoms. Camino Books, Inc.; Philadelphia, PA: 2014. [Google Scholar]
- 58.Burgio L, Lichstein KL, Nichols L, et al. Judging outcomes in psychosocial interventions for dementia caregivers: the problem of treatment implementation. Gerontologist. 2001;41:481–9. doi: 10.1093/geront/41.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichstein JL, Riedel BW, Grieve R. Fair tests of clinical trials: A treatment implementation model. Adv Behav Res Ther. 1994;16:1–29. [Google Scholar]
- 60.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosomat Med. 2002;64:510–9. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 61.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 62.Tariq S, Tumosa N, Chibnall J, Perry III H, Morley J. The Saint Louis University Mental Status (SLUMS) Examination for Detecting Mild Cognitive Impairment and Dementia is more sensitive than the Mini-Mental Status Examination (MMSE) for detecting dementia and mild neurocognitive disorder - A pilot study. Am J Geriatr Psychiatr. 2006;14:900–10. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 63.Elashoff JD. nQuery Advisor Version 5.0 User's Guide. Los Angeles, CA: 2007. [Google Scholar]
- 64.Grady J. Analysis of Change. In: Ambrosius WT, editor. Methods in Molecular Biology: Topics in Biostatistics. Humana Press Inc; 2007. pp. 261–72. [Google Scholar]
- 65.SAS Institute INC. SAS/STAT® 9.1 User's Guide. SAS Institute Inc; Cary, NC: 2004. [Google Scholar]
- 66.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–9. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 67.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epi. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Drummond M. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford University Press; New York: 2005. al e. [Google Scholar]
- 69.Gitlin LN, Harris LF, McCoy M, Chernett NL, Jutkowitz E, Pizzi LT. A community-integrated home based depression intervention for older African Americans: descripton of the Beat the Blues randomized trial and intervention costs. BMC Geriatr. 2012;12:4. doi: 10.1186/1471-2318-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jutkowitz E, Gitlin LN, Pizzi LT. Evaluating willingness-to-pay thresholds for dementia caregiving interventions: application to the tailored activity program. Value Health. 2010;13:720–5. doi: 10.1111/j.1524-4733.2010.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agency for Healthcare Research and Quality HCUPNet 2012 doi: 10.1080/15360280802537332. (Accessed at www.hcupnet.arhq.gov.) [DOI] [PubMed]
- 72.National Fee Analyzer . National Fee Analyzer: Charge data for evaluating fees nationally. Ingenix; 2011. [Google Scholar]
- 73.Social Security Administration . Red Book. Thomson Reuters; Montvale, NJ: 2012. [Google Scholar]
- 74.Murray E, Treweek S, Pope C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med. 2010:8. doi: 10.1186/1741-7015-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stirman SW, Miller CJ, Toder K, Calloway A. Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Imp Science. 2013:8. doi: 10.1186/1748-5908-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCracken GD. The long interview. Sage; Newbury Park, CA: 1988. [Google Scholar]
- 77.Glaser B, Strauss A. The discovery of grounded theory: Strategies for qualitative research. Aldine Publishing Company; Chicago: 1967. [Google Scholar]
- 78.Mollica R, Reinhard S. Rebalancing state long-term care systems. Ethics, Law and Aging Review. 2005;11:23–41. [Google Scholar]
- 79.Kaye HS. Gradual Rebalancing of Medicaid Long-Term Services and Supports Saves Money and Serves More People, Statistical Model Shows. Health Aff. 2012;31(6):1195–1203. doi: 10.1377/hlthaff.2011.1237. [DOI] [PubMed] [Google Scholar]