Abstract
Some drugs that are positive allosteric nAChR modulators in vitro, desformylflustrabromine (dFBr), PNU-120596 and LY 2087101, have not been fully characterized in vivo. These drugs were examined for their capacity to share or modify the hypothermic and discriminative stimulus effects of nicotine (1 mg/kg s.c.) in male C57Bl/6J mice. Nicotine, dFBr, and PNU-120596 produced significant hypothermia, whereas LY 2087101 (up to 100 mg/kg) did not. Nicotine dose-dependently increased nicotine-appropriate responding and decreased response rate; the respective ED50 values were 0.56 mg/kg and 0.91 mg/kg. The modulators produced no more than 38% nicotine-appropriate responding up to doses that disrupted operant responding. Rank order potency was the same for hypothermia and rate-decreasing effects: nicotine>dFBr>PNU-120596=LY 2087101. Mecamylamine and the α4β2 nAChR antagonist dihydro-β-erythroidine, but not the α7 antagonist methyllycaconitine, antagonized the hypothermic effects of nicotine. In contrast, mecamylamine did not antagonize the hypothermic effects of the modulators. The combined discriminative stimulus effects of DFBr and nicotine were synergistic, whereas the combined hypothermic effects of nicotine with either dFBr or PNU-120596 were infra-additive. PNU-120596 did not modify the nicotine discriminative stimulus, and LY 2087101 did not significantly modify either effect of nicotine. Positive modulation of nicotine at nAChRs by PNU-120596 and LY 2087101 in vitro does not appear to confer enhancement of the nAChR-mediated hypothermic or discriminative stimulus effects of nicotine. However, dFBr appears to be a positive allosteric modulator of some behavioral effects of nicotine at doses of dFBr smaller than the doses producing unwanted effects (e.g. hypothermia) through non-nAChR mechanisms.
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels activated by acetylcholine as well as various exogenous compounds, including nicotine. Disruption and dysregulation of nAChR signaling has been implicated in multiple pathologies, including addiction (Henningfield et al., 1985; Stolerman and Jarvis, 1995), Alzheimer’s disease (Court et al., 2001), schizophrenia (Young and Geyer, 2013), and Parkinson’s disease (Quik and Wonnacott, 2011). Currently approved therapeutic strategies that target nAChRs include nAChR agonism and acetylcholinesterase inhibition (Buccafusco, 2004; Newhouse et al., 2004).
There are limitations to using these therapeutic strategies, however. Acetylcholine is also the endogenous ligand for muscarinic acetylcholine receptors (mAChRs), and unintended activation of mAChRs by therapies targeting nAChRs can cause undesired effects, most commonly nausea and vomiting (Inglis, 2002). Although many nAChR agonists have some degree of selectivity for nicotinic versus muscarinic receptors, acetylcholinesterase inhibitors act indiscriminately at both types of receptor. Furthermore, orthosteric activation by acetylcholine and other agonists triggers rapid desensitization of nAChRs, reducing the effectiveness of continuous or repeated dosing regimens (James et al., 1994).
Allosteric modulators differ from orthosteric agonists in that they bind to a receptor site that is distinct from the orthosteric site. Allosteric modulators often do not have effects in the absence of an orthosteric ligand; instead allosteric modulators change the affinity and/or efficacy of the orthosteric ligand (Uteshev, 2014). Positive allosteric nAChR modulation of an orthosteric ligand (e.g. nicotine) decreases the amount of orthosteric ligand required for an effect, thereby resulting in less receptor desensitization produced by the orthosteric ligand (Williams et al., 2011). Thus, allosteric modulation of nAChRs is an alternative therapeutic strategy than can potentially circumvent the limitations inherent in targeting the orthosteric site.
Two nAChR subtypes predominate in the central nervous system: the heteromeric α4β2 subtype and the homomeric α7 subtype. There is evidence to suggest that the α4β2 nAChR subtype is of primary importance in the abuse- and dependence-producing properties of nicotine (Besson et al., 2006; Picciotto et al., 1998; Tapper et al., 2004), while the α7 nAChR subtype is responsible for the cognitive-enhancing effects of nicotine (Pichat et al., 2007; Roncarati et al., 2009; Wallace et al., 2011). Drugs that selectively target a particular nAChR subtype have the advantage of producing only those effects mediated by that nAChR subtype. Although allosteric modulators selective for specific nAChR subtypes in vitro have been developed, there is limited in vivo data to support the effectiveness of these compounds. Therefore, three positive nAChR allosteric modulators reportedly differing in nAChR subtype selectivity in vitro were chosen for examination in vivo: desformylflustrabromine (dFBr), PNU-120596 and LY 2087101.
In cells transfected with one subtype of nAChRs, dFBr, PNU-120596 and LY 2087101 alone do not mimic the effects of an orthosteric nAChR agonist and they vary in selectivity for nAChR subtypes: dFBr is selective for the α4β2 nAChR subtype (Kim et al., 2007; Sala et al., 2005), PNU-120596 is selective for the α7 nAChR subtype (Hurst et al., 2005) and LY 2087101 is non-selective for α4β2 and the α7 nAChRs (Broad et al., 2006). When tested in combination with acetylcholine, dFBr was initially found to increase the maximum current for cells transfected with α4β2 nAChRs, but not for α7 nAChRs (Sala et al., 2005). Additional studies replicated this finding at α4β2 nAChRs, but found that the concentration-response function was biphasic, revealing subsequent inhibition at higher concentrations of dFBr (Kim et al., 2007). In tests with other nAChR agonists, combination with dFBr increased the maximum effect of low efficacy agonists (e.g. cytisine) more than it increased the maximum effect of high efficacy agonists (e.g. nicotine); dFBr had no effect on the potency of the nAChR agonists studied (Kim et al., 2007; Sala et al., 2005; Weltzin and Schulte, 2010). PNU-120596, on the other hand, was shown to increase the maximum effect of acetylcholine-induced current by 7-fold at the α7 nAChR, with no effect at other nAChR subtypes (Gronlien et al., 2007; Hurst et al., 2005). Furthermore, the selectivity of the effects of PNU-120596 in vivo was demonstrated by the ability to increase the antinociceptive effects of nicotine but not the antinociceptive effects of morphine (Freitas et al., 2013a). Unlike both dFBr and PNU-120596, LY 2087101 did not show selectivity for either the α4β2 or the α7 nAChR subtype in vitro; it did, however, produce increases in both the potency and the maximum amount of nicotine-induced currents (Broad et al., 2006).
There is evidence in vitro that dFBr, PNU-120596 and LY 2087101 are positive allosteric modulators with differing selectivity for the α4β2 and α7 nAChR subtypes, because they do not appear to activate these receptors on their own, but do increase the effects of both acetylcholine and nicotine. The aims of the current study were to examine the extent to which positive allosteric nAChR modulators: 1) exert nicotine-like effects in vivo; 2) enhance or otherwise modify the in vivo effects of nicotine; and 3) modulate nicotine differently as a function of the type of effect being modulated. Nicotine was chosen because it is used widely from tobacco products and in the form of nicotine replacement therapy, and the effects of the novel compounds were studied both alone and in combination with nicotine. The discriminative stimulus effects and hypothermic effects of nicotine were both studied because there is evidence that nAChR subtypes differentially mediate these effects (Rodriguez et al., 2014), which was expected to confer drug- and effect-dependent differences in their modulation. Drug discrimination appears to be mediated by the α4β2 nAChR subtype (Gommans et al., 2000; Shoaib et al., 2002). However, published data suggest that the hypothermic effects of nicotine are mediated by not only the α4β2 nAChR subtype (Rodriguez et al., 2014), but also perhaps the α7 nAChR subtype (Freitas et al., 2013a). Therefore, the nicotinic antagonists mecamylamine, dihydro-β-erythroidine (DHβE), and methyllycaconitine (MLA) were used to further characterize the nAChR mechanism underlying the hypothermic effects of nicotine.
2. Materials and Methods
2.1 Subjects
Male C57BL/6J mice were purchased at eight weeks of age from The Jackson Laboratory (Bar Harbor, ME). Forty mice were used for the hypothermia experiments and six mice were used for drug discrimination. Mice were habituated in the colony room for at least seven days before experiments began and housed in groups of four for hypothermia experiments or singly for drug discrimination experiments in cages (28×18×13 cm) with water continuously available. The mice used in the hypothermia experiment had continuous access to food (Harlan, Teklad 7912, Houston, TX) in the home cage. The mice used in the drug discrimination experiment were maintained at 85% free-feeding weight; these mice had access during experimental sessions to 0.6 cm3 of 50% condensed milk (Borden Milk Products, Dallas, TX) and after experimental sessions to 2.5 g of Dustless Precision Pellets (500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ). All experiments were conducted during the light period of a 14/10 hour light-dark cycle (lights on at 0600 h). The maintenance and experimental use of animals was carried out in accordance with the National Institute of Health’s Guide for Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center, San Antonio.
2.2 Apparatus
Temperature was measured by inserting a probe (RET-3) (Physitemp Instruments, Inc., Clifton, NJ) attached to a microcomputer thermometer (7001H) (Physitemp Instruments, Inc., Clifton, NJ) 2 cm into the rectum. For drug discrimination, mice were placed in ventilated, sound-attenuating mouse operant chambers (MedAssociates, St. Albans, VT) with a house light located in the ceiling. On one wall were three holes spaced 5.5 cm apart (2.2 cm diameter each); each hole contained a photo beam and a light, and the center of each hole was 1.6 cm from the floor. In the center of the opposite wall was a fourth hole (also 2.2 cm diameter, center 1.6 cm from the floor) containing a dipper containing 0.01 cm3 condensed milk. An interface (MED-SYST-8, MedAssociates) connected the operant chambers to a computer running Med-PC software (MedAssociates), which controlled and recorded all experimental events.
2.3 Drugs
Doses are expressed as the weight, in mg/kg, of the forms listed below, except in the case of nicotine, which is expressed as the weight of the free base. Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO), mecamylamine (Waterstone Technology, Carmel, IN), dihydro-β-erythroidine (DHβE; Tocris Bioscience, Bristol, UK), methyllycaconitine citrate (MLA; Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD), and desformylflustrabromine hydrochloride (dFBr; Tocris) were dissolved in physiological saline. LY 2087101 (Tocris) and PNU-120596 (Research Technology Branch of the National Institute on Drug Abuse) were dissolved in 1 part propylene glycol (Fisher Scientific, Hampton, NH): 1 part Tween 80 (Sigma-Aldrich): 18 parts saline. Drugs were administered s.c. in a volume of 10 ml/kg except for mecamylamine, LY 2087101, and PNU-120596, which were administered i.p. Due to limitations of solubility, doses of 100 mg/kg LY 2087101 and 100 mg/kg PNU-120596 were administered in a volume of 20 ml/kg and their effects were compared to an equivalent volume of vehicle. Doses for each compound were selected based on the literature, when available. Drugs were studied from ineffective doses up to a dose producing discriminative stimulus, rate-decreasing, or hypothermic effects or up to the limit of drug solubility.
2.4 Hypothermia
For each mouse, a dose or dose combination was administered once every 7 days at the same time of day. Mice were assigned to drug treatments non-systematically and no dose or dose combination was tested more than once in the same mouse. Mice were tested an average of 14 times. Mice were removed from group housing, weighed, and placed in individual containers with water continuously available at least 1 h prior to the first measurement of rectal temperature (baseline). The ambient room temperature was 23°C. Following the baseline temperature reading, saline or a dose of DHβE or MLA was administered followed by vehicle or a dose of nicotine, dFBr or PNU-120596. DFBr or LY 2087101 was also administered prior to nicotine. Mecamylamine was administered 5 min before the baseline temperature, after which vehicle or a dose of nicotine, dFBr or PNU-120596 was administered. PNU-120596 was administered 15 min before the baseline temperature reading followed by a dose of nicotine. Temperature was measured immediately before each s.c. or i.p. injection and at 10, 30, 60, 90, and 120 min following baseline. Dose and dose combinations were administered non-systematically and no more than two mice received the same dose or dose combination on the same day. Six mice were tested for each dose and dose combination, except when a dose or dose combination resulted in lethality, and then it was not tested further.
2.5 Drug discrimination
Mice were trained to discriminate nicotine (1 mg/kg free base weight) from saline daily, seven days per week. Mice received the training dose of nicotine or saline and were placed in an operant chamber. Sessions began with a 10-min timeout during which stimulus lights were not illuminated and responses had no programmed consequence. The timeout was followed by a 15-min period during which milk was available under an FR10 schedule signaled by illumination of both the left and right nose-poke holes; the center hole was inactive and not illuminated throughout the study. Four mice were chosen for which the left hole was designated as correct after the training dose of nicotine and the right hole was designated as correct after saline; for the other four mice this relationship was reversed. Nose pokes in either illuminated hole disrupted a photobeam and were counted. Completion of 10 responses on the correct hole resulted in access to 0.01 cm3 of milk from the dipper on the opposite side of the operant chamber for 10 s. Responses in the incorrect hole had no programmed consequence. During each 10-s period of milk availability the lights in the holes were extinguished, the house light was illuminated, and nose pokes had no programmed consequence. Two days of nicotine training were alternated with two days of saline training. Tests were initiated after mice satisfied the training criteria for five consecutive or six out of seven days. To meet the training criteria, mice were required to make greater than 80% of their responses in the correct hole and fewer than 10 responses in the incorrect hole prior to delivery of the first reinforcer. On test days, 10 nose pokes in either the nicotine- or the saline-associated hole were reinforced. Three consecutive days of passing the training criteria were required between test days, including one nicotine and one saline training day. The same training condition was not repeated for more than two consecutive days. On drug combination test days, a modulator was administered immediately before an injection of nicotine or saline.
2.6 Data analyses
Hypothermia data were expressed as a change from baseline in °C and plotted as a function of dose or time. The effects of vehicle or dose(s) over time were expressed as a change from the pre-injection(s) baseline, whereas dose-response functions were expressed as a change from the time-matched vehicle control. Dose-response functions for hypothermic effects were constructed from data obtained 30 min post-injection because this was the time of peak effect. Non-linear regression with GraphPad Prism version 6.0 for Windows (San Diego, CA) was used to fit the individual dose-response data simultaneously. If the slope of a dose-response function was significantly different from 0, then the effect was considered significantly different from control. If the slopes of the dose-response functions being compared were not significantly different from each other, then a common best-fit slope was used to compare potencies (i.e. intercepts). For nicotine, the function included doses from 0.32 mg/kg to 1 mg/kg. For dFBr, PNU-120596 and LY 2087101, all three doses for each drug were included for analysis. The potency of each drug, defined as the dose required to decrease rectal temperature by 2.5°C, was estimated using the equation obtained from the non-linear regression.
Two-way ANOVAs were used to examine changes in rectal temperature separately for each drug, with one factor consisting of dose versus vehicle a second, repeated-measures factor consisting of time post-injection; significant interactions were followed by Sidak’s multiple comparisons test with GraphPad (P<0.05). The time course was used to calculate an area from 10 to 120 min for vehicle and each dose and dose combination. The boundaries of the area were a horizontal line drawn at 1.5 °C above baseline and the experimentally determined time course. Two-way ANOVAs were used to compare areas of each curve, with one factor consisting of dose of antagonist versus vehicle and a second factor consisting of a dose of nicotine or positive modulator versus vehicle, followed by Tukey’s Multiple Comparison test using GraphPad. If the area of the curve for a dose was significantly different from that after vehicle, then the dose was considered to have significantly decreased rectal temperature. To examine antagonism of hypothermic effects, the area of the vehicle curve was subtracted from the area of the curve for each dose and dose combination. Antagonism was defined as a significant difference between the area of the curve for a dose of drug alone and the area of the curve for the same dose in combination with a dose of antagonist.
The principle of dose equivalence (e.g. Neelakantan et al., 2015) was used to evaluate the combined effects of nicotine and an allosteric modulator on rectal temperature. The change in rectal temperature at 30 min following 32 mg/kg dFBr and 100 mg/kg PNU-120596 was substituted into the non-linear regression equation calculated for the nicotine dose-response function and solved for dose; this yielded the dose of nicotine required to produce the effect obtained at 32 mg/kg dFBr and 100 mg/kg PNU-120596, i.e. nicotine-equivalent dose. The nicotine-equivalent dose of each modulator was added to each dose of nicotine studied. The sum of the dose combination expressed as total nicotine dose was substituted into the equation of the line defining the nicotine dose-response function; this equation was solved for the expected effect for each dose combination. Non-linear regression was used to fit the expected effects and the observed effects of nicotine in combination with either 32 mg/kg dFBr or 100 mg/kg PNU-120596. The lines for the expected and observed effects of the dose combinations were compared with an F-ratio test in GraphPad; if the lines were not significantly different from each other, the combined effects of nicotine and the dose of modulator were considered to be additive.
Discrimination data were expressed and analyzed as a percentage of responding on the nicotine-associated hole, i.e. number of nicotine-appropriate responses divided by the sum of nicotine- and saline-appropriate responses multiplied by 100. Response rate data were expressed and analyzed as a percentage of the saline control, where the saline control was the average rate in responses per s for five saline training sessions immediately preceding the test, excluding training sessions for which the mouse did not pass the training criteria. Non-linear regression with GraphPad was used to fit individual dose-response data for each drug and drug combination separately, and an F-ratio test was used to determine whether or not the lines of multiple functions (i.e. nicotine alone versus nicotine in combination with a positive modulator) were significantly different from each other. The best-fit common slope was used to calculate ED50 values and potency ratios. Paired t-tests were used to compare the discriminative stimulus and rate-decreasing effects of a dose of nicotine alone to the effects of the same dose of nicotine in combination with a dose of PNU-120596.
3. Results
3.1 Hypothermic effects of nicotine, dFBr, PNU-120596 and LY 2087101 alone
Following administration of saline or the vehicle consisting of propylene glycol, Tween-80, and saline, rectal temperature 30 min post-injection (i.e. the time used to construct dose-response functions) was 37 (±0.3) and 37.2 (±0.21) °C, respectively (Fig. 1 Sal and PGT, respectively, expressed as change from pre-injection baseline). Nicotine dose-dependently decreased rectal temperature by 6.8 (±0.47) °C at 1 mg/kg and 7.1 (±0.35) °C at 1.78 mg/kg (Fig. 1, circles). DFBr dose-dependently decreased rectal temperature by 2.7 (±0.62) °C at 32 mg/kg and by 5.0 (±0.31) °C at 100 mg/kg (Fig. 1, triangles). PNU-120596 significantly decreased rectal temperature by 2.8 (±0.27) °C at the largest dose tested (100 mg/kg) (Fig. 1, inverted triangles). LY 2087101 did not significantly alter rectal temperature up to a dose of 100 mg/kg (F1,16=1.06, P=0.32) (Fig. 1, squares). The slopes of the nicotine, dFBr and PNU-120596 dose-effect functions were significantly different from each other (F2,48=16.7, P<0.0001). The dose of each drug estimated to decrease rectal temperature by 2.5 °C was 0.43 mg/kg for nicotine, 29.2 mg/kg for dFBr and 102 mg/kg for PNU-120596.
Fig. 1.
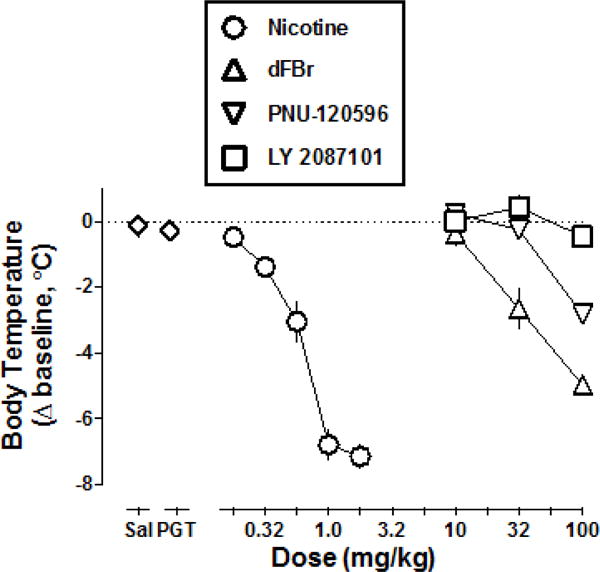
Change in rectal temperature following nicotine, dFBr, PNU-120596, or LY 2087101 as a function of dose. Abscissa: Saline (Sal), vehicle consisting of propylene glycol, Tween 80, and saline (PGT) and dose in milligram per kilogram body weight. Ordinate: change in rectal temperature from pre-injection baseline expressed as °C.
3.2 Hypothermia time course and antagonism
The hypothermic effects of nicotine, dFBr and PNU-120596 varied significantly as a function of time. The maximum effect of 1 mg/kg nicotine occurred 30 min post-injection and rectal temperature was no longer significantly different from control 90 min post-injection (F4,20=53.3, P<0.0001) (Fig. 2 left, open circles). When studied alone, 3.2 mg/kg mecamylamine significantly decreased rectal temperature by 1.7 °C at 10 min (i.e. 15 min post-injection) and by 1.4 °C at 30 min (i.e. 35 min post-injection) (F8,40=12.0, P<0.0001), whereas 1 mg/kg mecamylamine did not significantly alter temperature (Fig. 2 left, squares). Mecamylamine (1 and 3.2 mg/kg) significantly antagonized the hypothermic effects of 1 mg/kg nicotine (F2,30=25.7, P<0.0001) (Fig. 2 left, filled circles top and bottom, respectively).
Fig. 2.
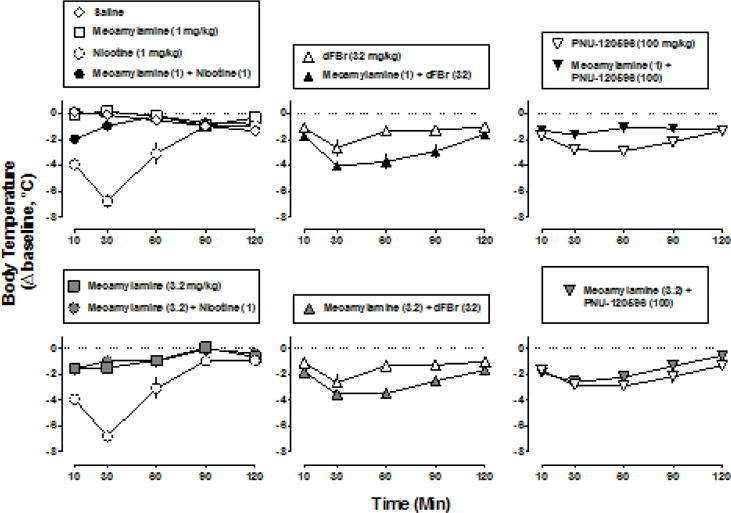
Change in rectal temperature over time following nicotine (1 mg/kg), dFBr (32 mg/kg) or PNU-120596 (100 mg/kg) alone and in combination with either 1 mg/kg mecamylamine (top) or 3.2 mg/kg mecamylamine (bottom). Abscissae: time in min following administration of agonist or vehicle. Ordinates: change in rectal temperature from pre-injection baseline (i.e. immediately before time 0) expressed as °C. Data for nicotine alone (open circles, left), dFBr alone (open triangles, middle) and PNU-120596 alone (open inverted triangles, right) are re-plotted in the top and bottom panels.
DFBr produced significant hypothermia 10 and 30 min post-injection; rectal temperature was no longer significantly different from control 60 min post-injection (F4,20=7.58, P<0.001) (Fig. 2 middle, open triangles). Mecamylamine (1 and 3.2 mg/kg) significantly modified the effects of 32 mg/kg dFBr (F2,30=6.15, P<0.01); however, instead of antagonism, both doses of mecamylamine enhanced the hypothermic effects of dFBr, evidenced primarily by an increase in the duration of action of dFBr (Fig. 2 middle, filled triangles). PNU-120596 (100 mg/kg) significantly decreased rectal temperature at every time point except 120 min (F4,20=17.1, P<0.0001) (Fig. 2 right, open inverted triangles). Neither 1 nor 3.2 mg/kg mecamylamine significantly modified the hypothermic effects of PNU-120596 (F2,30=0.77, P=0.47) (Fig. 2 right, filled inverted triangles).
Administered alone, 3.2 mg/kg DHβE did not significantly alter rectal temperature (Fig. 3 top left, squares), whereas 10 mg/kg DHβE produced significant hypothermia at all time points except 120 min (F8,40=4.20, P<0.01) (10 mg/kg DHβE data not shown). DHβE (3.2 and 10 mg/kg) significantly antagonized the hypothermic effects of nicotine (F2,30=5.80, P<0.01), evidenced by a decrease in latency to onset of maximum effect and a decrease in duration of effect (Fig. 3 top left, 3.2 mg/kg DHβE filled circles). However, the larger dose of DHβE (10 mg/kg) produced less antagonism than 3.2 mg/kg (10 mg/kg DHβE data not shown). DHβE (3.2 mg/kg) did not significantly antagonize the hypothermic effects of 32 mg/kg dFBr (F1,20=1.49, P=0.24) (Fig. 3, top right). The larger dose of DHβE (10 mg/kg) in combination with 32 mg/kg of dFBr resulted in lethality in 2/2 mice tested. MLA (10 mg/kg) did not significantly modify rectal temperature (Fig. 3 bottom left, filled squares) and did not significantly antagonize the hypothermic effects of 1 mg/kg nicotine (F1,20=0.61, P=0.44) (Fig. 3 bottom left) or the hypothermic effects of 100 mg/kg PNU-120596 (F1,20=0.77, P=0.39) (Fig. 3 bottom right). A larger dose of MLA (32 mg/kg) alone was lethal in 3/4 mice tested; that dose of MLA did not antagonize the hypothermic effects of nicotine (1 mg/kg) and did not result in lethality in any of the 4 mice tested.
Fig. 3.
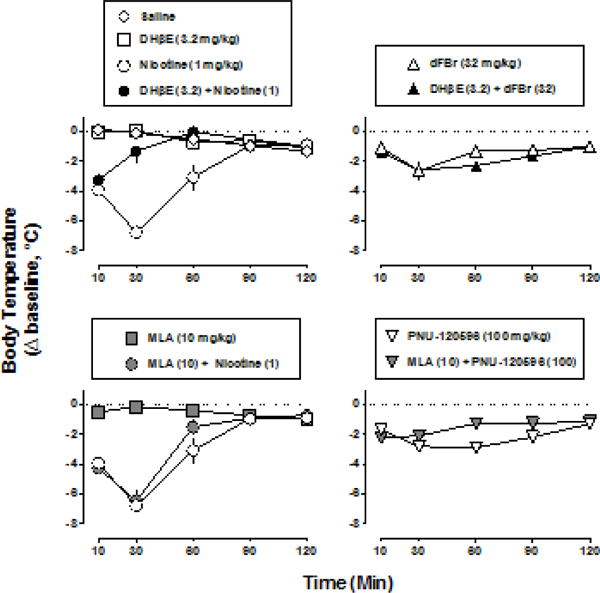
Change in rectal temperature over time following nicotine (1 mg/kg) or dFBr (32 mg/kg) alone and in combination with either 3.2 mg/kg DHβE (top) or 10 mg/kg MLA (bottom). Abscissae: time in min following administration of agonist or vehicle. Ordinates: change in rectal temperature from pre-injection baseline (i.e. immediately before time 0) expressed as °C. Data for nicotine alone (open circles, left) and dFBr alone (open triangles, right) are re-plotted in the top and bottom panels.
3.3 Hypothermic effects of nicotine in combination with dFBr, PNU-120596 and LY 2087101
A dose of dFBr (32 mg/kg) that produced significant hypothermia when administered alone also produced a significant leftward and downward shift of the nicotine dose-response function (F2,32=8.50, P<0.01), whereas a smaller dose of dFBr (10 mg/kg) which by itself lacked hypothermic effects did not significantly modify the effects of nicotine (F1,34=0.08, P=0.79) (Fig. 4 left). In contrast, a dose of PNU-120596 (100 mg/kg) that produced significant hypothermia on its own did not significantly modify the nicotine dose-response function (Fig. 4 right) (F1,34=0.83, P=0.37). A smaller dose of PNU-120596 (32 mg/kg) was similarly ineffective in modifying the effects of nicotine (data not shown) (F1,34=3.66, P=0.06). LY 2087101 (32 mg/kg) alone had no effect on rectal temperature and did not significantly modify the nicotine dose-response function (F1,34=3.19, P=0.08). A larger dose of LY 2087101 (100 mg/kg) did not appear to alter the hypothermic effects of nicotine in a subset of mice (n=2); this dose of LY 2087101 was not tested further due to limitations in drug supply (data not shown).
Fig. 4.
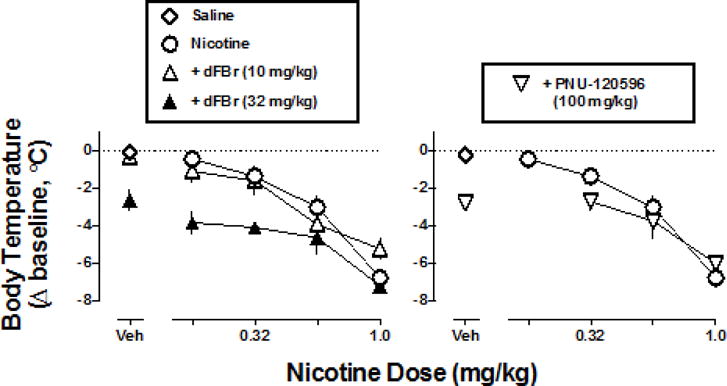
Change in rectal temperature as a function of nicotine dose administered alone and in combination with dFBr (10 and 32 mg/kg; left) or PNU-120596 (100 mg/kg; right). Abscissae: dose of positive allosteric modulator alone or vehicle (Veh) and dose of nicotine in milligram per kilogram bodyweight. Ordinates: change in rectal temperature from pre-injection baseline (i.e. immediately before time 0) expressed as °C. The same control nicotine dose-response function (circles) is plotted in the left and right panels.
The linear regression of the nicotine dose-response function was used to estimate the doses of nicotine required to produce the hypothermic effects that were experimentally determined for 32 mg/kg dFBr and 100 mg/kg PNU-120596. These derived nicotine equivalents were added to the doses of nicotine tested experimentally, and the total nicotine equivalent doses were re-entered into the linear regression to calculate the expected effects and corresponding lines of additivity for the combinations (Fig. 5, open symbols). The observed dose-response function for nicotine in combination with 32 mg/kg dFBr was shifted modestly, but significantly, to the right of the expected line of additivity, indicative of a significant antagonistic interaction (Fig. 5 left) (F1,4=23.5, P<0.01). Similarly, the observed dose-response function for nicotine in combination with 100 mg/kg PNU-120596 was shifted significantly rightward of the expected line of additivity, also indicative of less than additive effects (Fig. 5 right) (F1,4=298.1, P<0.0001).
Fig. 5.
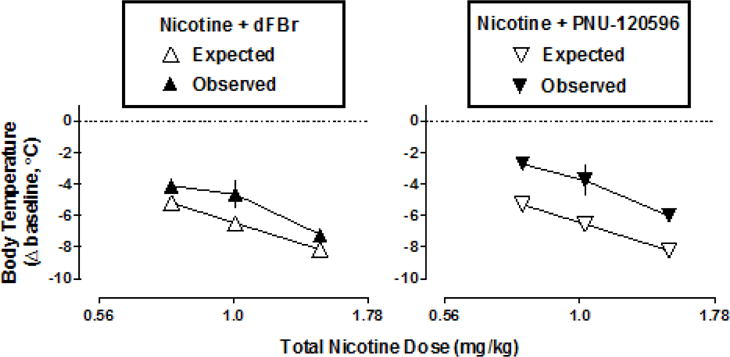
The expected effects (open symbols) and observed effects (filled symbols) of nicotine in combination with either 32 mg/kg dFBr (left) or 100 mg/kg PNU-120596 (right). The expected effects were calculated as described in section 2.6 Data analyses. Abscissae: nicotine dose expressed as the sum of the experimentally administered dose and the dose of nicotine corresponding to the change in rectal temperature produced by 32 mg/kg dFBr (left) or 100 mg/kg PNU-120596 alone (right), as determined from non-linear regression of the experimentally derived nicotine dose-response function. Ordinates: change in rectal temperature from pre-injection baseline (i.e. immediately before time 0) expressed as °C.
3.4 Discriminative stimulus effects of nicotine, dFBr, PNU-120596 and LY 2087101 alone
In mice discriminating nicotine (1 mg/kg free base weight) from saline, absolute rate of responding averaged over 10 saline training sessions was a mean ±S.E.M. of 0.41±0.040 responses per s. Response rate averaged over 10 nicotine training sessions was 0.23±0.039 responses per s. Discrimination performance expressed as the mean ±S.E.M. percentage of nicotine-appropriate responding was 3±0.5% during saline training sessions and 95±1% during nicotine training sessions. Nicotine dose-dependently increased drug-appropriate responding to a mean of 94% at the training dose (Fig. 6, circles); the ED50 value (95% confidence limits) for nicotine to produce discriminative stimulus effects was 0.56 (0.48–0.66) mg/kg. Nicotine also dose-dependently decreased rate of responding to 37% at the training dose; the ED50 value of nicotine to produce rate-decreasing effects was 0.91 (0.73–1.09) mg/kg. Maximum responding after dFBr administered alone reached 38% nicotine-appropriate responding at 10 mg/kg; this dose of dFBr also produced a decrease in response rate to 28% of the saline control (Fig. 6, triangles). PNU-120596 (32 mg/kg) decreased response rate to 22% of control and produced 19% nicotine-appropriate responding (Fig. 6, inverted triangles). LY 2087101 (100 mg/kg) dose-dependently decreased response rate to 23% of control and produced a maximum of 11% nicotine-appropriate responding at 56 mg/kg (Fig. 6, squares). The ED50 values (95% confidence limits) for decreases in response rate were 4.96 (3.45–7.12) mg/kg for dFBr, 21.7 (17.5–27.0) mg/kg for PNU-120596, and 67.9 (50.9–90.5) mg/kg for LY 2087101.
Fig. 6.
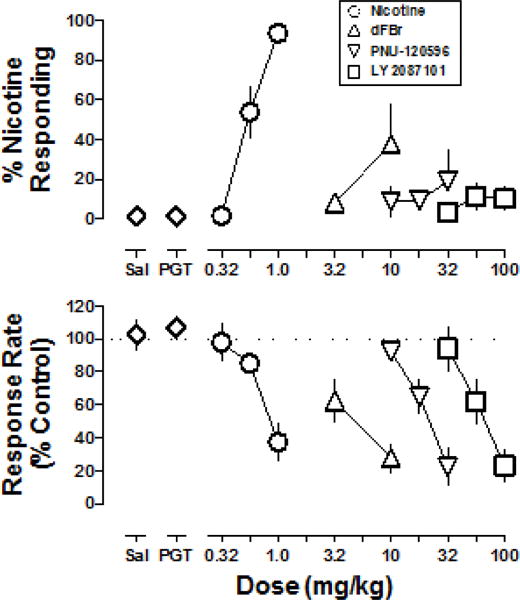
Dose-effect functions for the discriminative stimulus effects (top) and rate effects (bottom) of nicotine, dFBr, PNU-120596 and LY 2087101. Abscissae: Saline (Sal), vehicle consisting of propylene glycol, Tween 80, and saline (PGT) and dose in milligram per kilogram body weight. Ordinates: the percentage of nicotine-appropriate responses (top) and rate of responding in responses per s calculated as a percent of the control response rate (bottom).
3.5 Discriminative stimulus effects of nicotine in combination with dFBr, PNU-120596 and LY 2087101
The largest doses of dFBr, PNU-120596 and LY 2087101 that did not reduce response rate to less than 50% of the saline control were studied in combination with nicotine. DFBr (3.2 mg/kg) alone produced 8% nicotine-appropriate responding; when 3.2 mg/kg dFBr was combined with a dose of nicotine that alone produced 54% nicotine-appropriate responding (0.56 mg/kg), nicotine-appropriate responding increased to 93%. Combination of nicotine with 3.2 mg/kg dFBr significantly decreased the ED50 value of nicotine to 0.29 (0.24–0.34) mg/kg, i.e. shifted the nicotine dose-response function 2-fold to the left (Fig. 7 top left) (F1,38=34.9, P<0.0001). The rate-decreasing effects of 3.2 mg/kg dFBr alone (63% of control) were not significantly altered when combined with nicotine up to 0.56 mg/kg (Fig. 7 bottom left). PNU-120596 (17.8 mg/kg) did not significantly modify the nicotine dose-response function for discriminative stimulus effects (F1,37=0.29, P=0.60) or rate-decreasing effects (F1,37=0.79, P=0.38). Similarly, nicotine-appropriate responding and response rate after 56 mg/kg LY 2087101 in combination with 0.56 mg/kg nicotine did not significantly differ from data obtained with 0.56 mg/kg nicotine alone (P>0.05).
Fig. 7.
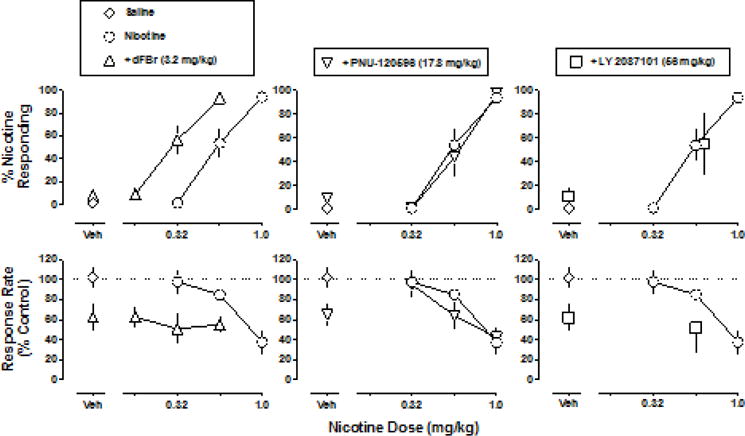
Dose-effect functions for the discriminative stimulus effects (top) and rate effects (bottom) of nicotine alone and in combination with 3.2 mg/kg dFBr (left), 17.8 mg/kg PNU-120596 (middle) or 56 mg/kg LY 2087101 (right). Abscissae: doses of positive allosteric modulator alone or vehicle (Veh) and dose of nicotine in milligram per kilogram bodyweight. Ordinates: the percentage of nicotine-appropriate responses (top) and rate of responding in responses per s calculated as a percent of the control response rate (bottom). The same control nicotine dose-response function (circles) are plotted in the top panels for discriminative stimulus effects and the bottom panels for rate-decreasing effects.
4. Discussion
In mice discriminating nicotine (1 mg/kg), dFBr had effects that were consistent with positive allosteric modulation; i.e. dFBr did not substitute for the nicotine discriminative stimulus but increased the potency of nicotine. Synergistic effects of dFBr and nicotine were selective; the combined effects of dFBr and nicotine to decrease rectal temperature were infra-additive. There was no evidence of positive allosteric modulation of nicotine by either PNU-120596 or LY 2087101 up to doses of each drug that produced either hypothermia (PNU-120596) or decreased rate of operant responding (PNU-120596 and LY 2087101). The effects of dFBr and PNU-120596 alone did not appear to be mediated by nAChR agonism, as evidenced by the failure of mecamylamine to antagonize the hypothermic effects of either drug. Of the three compounds studied, only dFBr produced effects consistent with positive allosteric nAChR modulation, and that activity was observed for discriminative stimulus but not hypothermic effects. Collectively, these results suggest that dFBr can be used to enhance the effects of some but not all behavioral effects of nicotine at doses of dFBr that by themselves are behaviorally inactive.
Changes in body temperature can be measured in response to treatment with a variety of drugs acting at many different types of receptor; however, nicotine-induced hypothermia in the present study was sensitive to antagonism by mecamylamine, indicative of nAChR involvement. Nicotine-induced hypothermia was also antagonized by the β2-selective antagonist DHβE, but not by the α7-selective antagonist MLA, which is consistent with other in vivo effects of nicotine being differentially antagonized by these antagonists in mice (Walters et al., 2006) and, further, with the hypothermic effects of nicotine being mediated by β2-containing, but not α7, nAChR subtypes. The current results appear to differ from previous results showing that PNU-120596 enhanced the hypothermic effects of nicotine and, further, that the enhanced effects of nicotine were attenuated by either MLA or deletion of α7 nAChRs through a transgenic approach (Freitas et al., 2013b). In the current study mice received drugs more than once, though the interval of dosing was a minimum of 7 days, whereas mice in the previous study were used once for drug treatment (Freitas et al., 2013b). Moreover, the strains were different (C57BL/6J versus Institute for Cancer Research mice). As noted in that previous study, the evidence for involvement of α7 nAChRs in the hypothermic effects of nicotine was unexpected.
The hypothermic effects of both dFBr and PNU-120596 were not antagonized by mecamylamine, indicating that positive modulation of ACh is not the underlying mechanism. Positive allosteric modulators increase the binding affinity and/or efficacy of an orthosteric agonist (Pandya and Yakel, 2013). One expected outcome of combining a positive allosteric modulator with an orthosteric agonist is synergy. In combination with nicotine, neither dFBr nor PNU-120596 enhanced the hypothermic effects of nicotine. Instead the opposite occurred, i.e., the combined hypothermic effects were less than additive. Thus, the antagonism was not evident by a rightward shift of the nicotine dose-response function for producing hypothermic effects, but rather by a less-than-expected leftward shift of the nicotine dose-response function based on the individual hypothermic effects of each drug. While the lack of antagonism of the hypothermic effects of dFBr and PNU-120596 by mecamylamine suggests that neither drug is exerting nAChR agonist activity, the infra-additive effects might suggest that dFBr and PNU-120596 are negatively modulating nicotine-induced hypothermia mediated by β2-containing nAChRs. That the antagonism of nicotine occurred at relatively large doses of dFBr is consistent with the biphasic effects of dFBr in vitro, with positive modulation occurring at relatively small concentrations of dFBr and negative modulation occurring at larger concentrations (Kim et al., 2007).
Although none of the compounds clearly functioned as positive allosteric modulators in the hypothermia assay, there was evidence for positive allosteric nAChR modulation by dFBr, but not PNU-120596 or LY 2087101, in the nicotine discrimination assay. The discriminative stimulus effects of nicotine in mice appear to be mediated by β2-containing nAChRs (Shoaib et al., 2002), whereas α7 nAChRs appear to be less involved (Quarta et al., 2009). This would suggest that the discriminative stimulus effects of nicotine in mice would be minimally altered in combination with an allosteric modulator that is selective for the α7 subtype of nAChR (i.e. PNU-120596), but sensitive to modification by an allosteric modulator that is selective for the α4β2 subtype of nAChR (i.e. dFBr). Thus, the effects of dFBr in the nicotine discrimination assay provide evidence for positive allosteric modulation of nAChRs in vivo that is consistent with the nAChR subtype selectivity of dFBr in vitro. Similarly, the effects of PNU-120596 in the discrimination are consistent with the nAChR subtype selectivity of PNU-120596 in vitro, as an allosteric modulator acting selectively at the α7 subtype of nAChR might not be expected to modify the nicotine discriminative stimulus. The failure of dFBr to produce a similar enhancement of nicotine-induced hypothermia could reflect differences in the pharmacological mechanisms underlying the discriminative stimulus and hypothermic effects of nicotine. The nicotine discrimination assay is relatively selective for actions at nAChRs, whereas hypothermic effects can occur through any number of receptor mechanisms. Any non-nAChR mediated effects of dFBr could potentially interfere with the expression of its positive allosteric modulatory effects.
Drug discrimination has been used as a pre-clinical model of subjective effects and to measure aspects of nicotine pharmacology underlying nicotine abuse and dependence. Although both abused and non-abused drugs can be discriminated, drugs sharing discriminative stimulus effects with known drugs of abuse are more likely to be abused than drugs not sharing discriminative stimulus effects with abused drugs. PNU-120596 did not produce nicotine-like effects in the current study. The absence of nicotine-like subjective effects is highly relevant in the context of the current literature examining PNU-120596 in vivo as a potential therapeutic for cognitive dysfunction in humans, as it evaluates a dimension of PNU-120596’s behavioral effects heretofore unknown. In contrast to PNU-120596, which has been used in in vivo studies for several years, little is known about the effects of dFBr in vivo, apart from its ability to attenuate nicotine self-administration in rats (Liu, 2013). In vitro evidence suggests that dFBr can act as both a positive allosteric modulator of some nAChR subtypes as well as an antagonist at nAChRs, and as either of these two mechanisms could be predicted to attenuate nicotine self-administration, this is the first study that provides strong evidence that the in vivo effects of dFBr are consistent with positive allosteric modulation of nAChRs.
Acknowledgments
The authors thank J. Threadgill for technical assistance. The National Institutes of Health National Institute on Drug Abuse [DA25267] supported this research.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- dFBr
desformylflustrabromine
- DHβE
dihydro-β-erythroidine
- FR
fixed ratio
- LY 2087101
[2-[(4-fluorophenyl)amino]-4-methyl-5-thiazolyl]-3-thienylmethanone
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- PNU-120596
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: Beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, Emkey R, Hollinshead SP, Dell CP, Baker SR, Sher E. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. Neuronal nicotinic receptor subtypes: Defining therapeutic targets. Mol Interv. 2004;4:285–295. doi: 10.1124/mi.4.5.8. [DOI] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in alzheimer’s disease. Biol Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI. The antinociceptive effects of nicotinic receptors alpha7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013a;344:264–275. doi: 10.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Negus SS, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013b;169:567–579. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther. 1985;234:1–12. [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: In vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;(127):45–63. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 8th. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 2011. [Google Scholar]
- James JR, Villanueva HF, Johnson JH, Arezo S, Rosecrans JA. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology (Berl) 1994;114:456–462. doi: 10.1007/BF02249336. [DOI] [PubMed] [Google Scholar]
- Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg Med Chem Lett. 2007;17:4855–4860. doi: 10.1016/j.bmcl.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Positive allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors as a new approach to smoking reduction: Evidence from a rat model of nicotine self-administration. Psychopharmacology (Berl) 2013;230:203–213. doi: 10.1007/s00213-013-3145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 2015;26:304–314. doi: 10.1097/FBP.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Pandya AA, Yakel JL. Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies. Biochem Pharmacol. 2013;86:1054–1062. doi: 10.1016/j.bcp.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, Gueudet C, Voltz C, Steinberg R, Stemmelin J, Oury-Donat F, Avenet P, Griebel G, Scatton B. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP. Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl) 2009;2009(203):399–410. doi: 10.1007/s00213-008-1281-x. [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. alpha6beta2* and alpha4beta2* nicotinic acetylcholine receptors as drug targets for parkinson’s disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Cunningham CS, Moura FB, Ondachi P, Carroll FI, McMahon LR. Discriminative stimulus and hypothermic effects of some derivatives of the nAChR agonist epibatidine in mice. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, Jow B, Kowal D, Gianfriddo M, Kelley C, Zanelli U, Ghiron C, Haydar S, Dunlop J, Terstappen GC. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 2009;329:459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- Sala F, Mulet J, Reddy KP, Bernal JA, Wikman P, Valor LM, Peters L, Konig GM, Criado M, Sala S. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a flustra foliacea metabolite. Neurosci Lett. 2005;373:144–149. doi: 10.1016/j.neulet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol. 2014;727:181–185. doi: 10.1016/j.ejphar.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Callahan PM, Tehim A, Bertrand D, Tombaugh G, Wang S, Xie W, Rowe WB, Ong V, Graham E, Terry AV, Jr, Rodefer JS, Herbert B, Murray M, Porter R, Santarelli L, Lowe DA. RG3487, a novel nicotinic alpha7 receptor partial agonist, improves cognition and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2011;336:242–253. doi: 10.1124/jpet.110.171892. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther. 2010;334:917–926. doi: 10.1124/jpet.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


