Abstract
Many distantly-related insect species are specialized feeders of cardenolide-containing host plants such as milkweed (Asclepias spp.). Studies have revealed frequent, parallel substitution of a functionally important amino acid substitution (N122H) in the alpha subunit of Na+,K+-ATPase (N122H) in many of these species. This substitution facilitates the ability of these insects to feed on their toxic hosts. Among milkweed butterflies of the genus Danaus, the previously established phylogeny for this group suggests that N122H arose independently and fixed in two distinct lineages. We re-evaluate this conclusion by examining Danaus phylogenetic relationships using >400 orthologous gene sequences assembled from transcriptome data. Our results indicate that the three Danaus species known to harbor the N122H substitution are more closely related than previously thought, consistent with a single, common origin for N122H. However, we also find evidence of both incomplete lineage sorting and post-speciation genetic exchange among these butterfly species, raising the possibility of collateral evolution of cardenolide-insensitivity in this species group.
Keywords: Danaus; Danaidae; milkweed butterfly; incomplete lineage sorting; genetic introgression; Na+,K+-ATPase
Introduction
Studies of convergent evolution have improved our understanding of the genetic architecture underlying adaptive traits and yielded insights into the extent of constraint on the evolution of novel phenotypes (Stern 2013). One example of this is insects that feed on plants that produce toxic secondary metabolites called cardenolides (Dobler et al. 2011; Agrawal et al. 2012). These toxins provide defense against herbivory, yet many specialist insect species have nonetheless evolved the ability to feed on cardenolide-containing plants. Some of these specialists also actively sequester cardenolides for use in their own defense against predators (Dobler et al. 2011; Agrawal et al. 2012). Among these insects are milkweed butterflies in the genus Danaus, which as larvae feed almost exclusively on Asclepias host plants that often contain cardenolides (Ackery and Vane-Wright 1984; Petschenka and Agrawal, 2015). Three Danaus species (D. plexippus, D. erippus and D. eresimus) have been shown to harbor a specific, functionally important asparagine to histidine amino acid substitution (N122H) in the alpha subunit of Na+K+-ATPase (ATPα, see Fig. 1). This particular substitution has evolved in parallel numerous times in cardenolide-sequestering insects belonging to different orders (Zhen et al. 2012; Dobler et al. 2012).
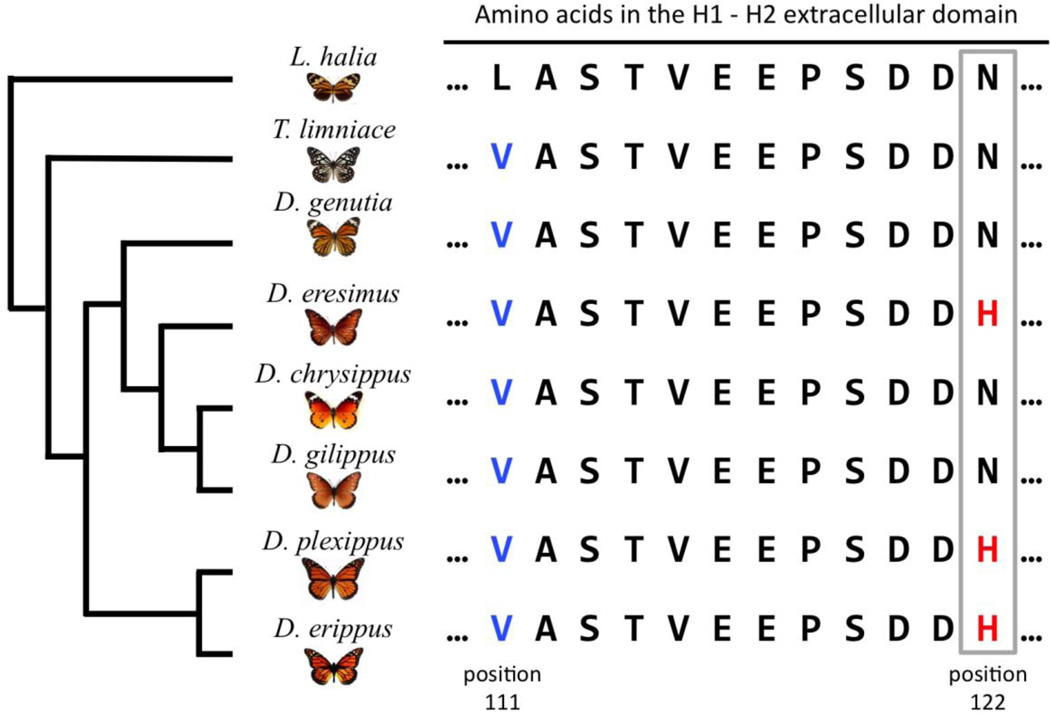
Figure 1.
Previously inferred phylogeny of select Danaus species and additional milkweed butterflies (Danainae subfamily; based on: Ackery and Vane-Wright 1984; Lushai et al. 2003; Smith et al. 2005; Brower et al. 2010). Note: Brower et al. (2010) placed D. eresimus and D. gilippus as sister species, with the D. chrysippus group their sister lineage. Also shown are amino acid sequences of the H1–H2 extracellular domain of the ATPα in these species. The presence of a valine at position 111 (blue letters) likely enhances these butterflies’ ability to feed on milkweed and possibly sequester cardenolides (Petschenka et al. 2013). The N122H mutation (red letters), observed in D. plexippus, D. erippus and D. eresimus, likely further contributes to increased cardenolide feeding and sequestration abilities (Zhen et al. 2012; Petschenka et al. 2013).
Despite sharing the N122H substitution, D. eresimus is considered a distant relative of D. plexippus and D. erippus within the Danaus genus (Fig 1; Ackery and Vane-Wright 1984; Lushai et al. 2003; Smith et al. 2005; Brower et al. 2010). Given these relationships, a straightforward interpretation is that N122H is phylogenetically incongruent and arose in parallel within the D. plexippus and D. eresimus lineages (Zhen et al. 2012). However, several other possibilities exist. For example, it is also possible that N122H fixed early on in the evolution of the Danaus genus and subsequently reverted back to asparagine in the D. gilippus/D. chrysippus lineage. Such a reversion is hypothesized to have occurred in a similar context involving varanid lizards (Ujvari et al. 2015). Another possibility is that of “collateral evolution” (Stern 2013). For example, both asparagine (N) and histidine (H) may have been segregating in a common ancestor of these species and histidine subsequently fixed independently in the D. plexippus and D. eresimus lineages, while being lost in other Danaus species. Another form of collateral evolution is adaptive introgression, which may also explain the sharing of N122H among Danaus species. While previously thought to be rare or unimportant, post-speciation genetic exchange has become increasingly recognized as an important contributor to the evolutionary history of many species (Mallet et al. 2015),
In addition to N122H substitution of ATPα, other phylogenetic incongruences in Danaus have also been noted. These include differences in chromosome number (Brown et al. 2004), allozyme phenotypes (Kitching 1985) and morphological traits (Ackery and Vane-Wright 1984). In this study, we evaluate the most likely evolutionary scenario underlying the evolution of the N122H substitution by examining species relationships among the North American species of Danaus butterfly using ATPα sequences and a large set of protein coding sequences derived from comparative transcriptomic data. We also use this dataset to evaluate the extent to which incomplete lineage sorting (ILS) and genetic introgression may have contributed broadly to phylogenetic incongruences between these species. Both ILS and genetic introgression have previously been proposed to explain observed morphological and genetic incongruences in the Danaus genus (Lushai et al. 2003; Smith et al. 2005), but to date neither hypothesis has been examined explicitly.
Materials and Methods
Species phylogeny based on ATPα
We first examined phylogenic relationships among Danaus species using available genomic and transcriptomic data for the alpha subunit of Na+K+-ATPase (ATPα). Specific information for all Danaus samples used is given in Table S1. With the previously reported ATPα coding sequence for D. plexippus (Zhen et al. 2012, GenBank Accession No: JQ771507), we employed a nucleotide Blast (‘blastn’, Altschul et al. 1990) to locate the exons of this gene on scaffold DPSCF300050 of the D. plexippus genome assembly (Zhan et al. 2011). We next mapped quality trimmed genomic reads for each sample (Phred QV ≥ 20, contiguous length ≥ 30 nucleotides) to this scaffold using Stampy (v. 1.0.17; Lunter and Goodson 2011) with default parameters except substitution rate, which we set to 0.01. We used SAMtools (v. 0.1.4) to filter the mapped reads (MAPQ > 20, Li et al. 2009), and the MarkDuplicates utility in Picard tools (v. 1.77; http://broadinstitute.github.io/picard/) to remove PCR duplicates. We called genetic variants using the HaplotypeCaller utility in GATK (v. 3.3; McKenna et al. 2010). Finally, for each sample we pulled the full exons of this gene and assembled the coding sequence of ATPα.
This data was combined with the previously reported ATPα sequences for D. plexippus, D. gilippus and D. eresimus, plus the outgroup milkweed butterfly Lycorea halia (Table S1, Zhen et al. 2012). Using jModelTest 2 (v. 2.1.7, Guindon and Gascuel 2003; Darriba et al. 2012), we determined the best-fitting mutation model for this dataset (based on AIC score) was the generalized time reversible model (Tavaré 1986) with a gamma distribution of rate heterogeneity (i.e. GTR + Γ model). We used RAxML (v. 8.1.7, Stamatakis 2014) to generate 1000 bootstrap replicate phylogenies to assess support for each branch.
We used PhyloNetHMM (Liu et al. 2014) to evaluate the level of support across the ATPα coding sequence for alternative phylogenetic relationships among Danaus species (specifically [[D. eresimus, D. gilippus], D. plexippus] versus [[D. eresimus, D. plexippus], D. gilippus]). To do this, we used a four-sequence alignment of the coding region of ATPα covering amino acid residues 22 through 996. Included in this alignment were one sequence each from D. plexippus, D. eresimus, D. gilippus and the outgroup species, L. halia (from Zhen et al. 2012). We specified priors on substitution rates using RAxML-based estimates of these parameters. Since PhyloNetHMM explicitly models ILS given a particular species tree, variation in support for a species tree is interpreted as evidence for gene flow (introgression) between species.
De novo transcriptome assembly
To examine hypotheses of shared ancestry or adaptive introgression to explain the origins of the N122H substitution in Danaus butterflies, it was necessary to have high confidence in species relationships within this genus. Therefore, to broadly examine phylogenetic relationships within Danaus, we took advantage of previously produced RNAseq data (Zhen et al. 2012) for the three North American Danaus species (D. plexippus, D. eresimus and D. gilippus) and an outgroup (L. halia). Using three focal species and an outgroup allowed us to compare the major topology to two possible minor topologies in a series of four-taxa trees, and assess whether these minor topologies occur at similar frequencies to one another. Such assessments are a common way to investigate the origins of phylogenetic incongruence (e.g. Green et al. 2010).
Mapping sequence reads to a reference can lead to biases in phylogenetic analyses that become more serious as divergence from the reference increases (Hornett and Wheat 2012). Therefore, we instead produced independent de novo transcriptome assemblies for each species using the programs Velvet (v. 1.2.10) and Oases (v. 0.2.08) with a kmer length of 31 and a minimum read depth of 10 (Zerbino and Birney 2008; Schulz et al. 2012). In cases where multiple isoforms were assembled, we retained the longest one. To locate orthologous nuclear sequences, the four transcriptome assemblies were compared to amino acid sequences of predicted proteins for the D. plexippus reference genome (Dp_geneset_OGS2_pep.fasta, http://monarchbase.umassmed.edu/resource.html), using Blast with a translated nucleotide query (‘blastx’) and a minimum e-value of 1e-50. Only Blast hits that were at least 100 nucleotides long were retained for subsequent ortholog comparison between the four species. This was intended to reduce the number of regions with limited phylogenetic information and improve alignment accuracy (Talavera and Castresana 2007). We discarded gene regions for which any species had more than one overlapping transcript matching the D. plexippus reference protein set (http://monarchbase.umassmed.edu/resource.html) to avoid potential duplicates. Generally, low levels of polymorphism do not affect contig assembly in Velvet, and only a single allele is retained at polymorphic sites (Zerbino and Birney 2008). However, it is possible that highly polymorphic regions will assemble into more than one distinct transcript. In our pipeline, such regions would resemble gene duplicates and subsequently be removed.
We aligned orthologous regions based on amino acid similarity with MUSCLE (Edgar 2004) as implemented in SeaView (v. 4.5.4; Gouy et al. 2010). We then checked each alignment by eye and manually trimmed them to the length of the shortest region observed among the four species. Additional information on these assemblies is given in Table S2.
Species trees
We concatenated all loci into single alignments (i.e. no partitioning between genes) independently for our de novo-assembled nuclear gene datasets, and used jModelTest 2 (v. 2.1.7, Guindon and Gascuel 2003; Darriba et al. 2012) to determine the best-fitting mutation model as above. The best fit was the generalized time reversible model (Tavaré 1986) with a proportion of invariable sites and a gamma distribution of rate heterogeneity (i.e. GTR + I + Γ model). We next produced maximum likelihood phylogenies in RAxML (v. 8.1.7, Stamatakis 2014) with 1000 bootstrap replicates for each of the three possible topologies among the Danaus species. The site log likelihoods from RAxML were used to perform the approximately unbiased (AU) test implemented in CONSEL 0.2 (Shimodaira and Hasegawa 2001). Two trees were considered statistically different from one another if p ≤ 0.05.
Discordance among nuclear gene trees
To determine what proportion of genes concur with the primary species tree, and what proportions support the two alternative phylogenetic relationships, we employed a Bayesian concordance analysis (BCA), as implemented in BUCKy (v. 1.4.3, Larget et al. 2010; Ané et al. 2007). We first produced individual trees for each genetic region in our dataset using MrBayes (v. 3.2.2, Ronquist and Huelsenbeck 2003), with two independent runs of 107 generations and a “burn in” period of 105 generations. Rather than a priori selecting a mutation model for each gene, we used the reversible-jump Markov chain Monte Carlo approach to examine the parameter space for each locus and find the best set of parameters for that particular dataset (Huelsenbeck et al. 2004).
We combined the two independent MrBayes runs for each gene using the mbsum command in BUCKy (v. 1.4.3, Larget et al. 2010). We then examined the extent of discordance as measured by the sample-wide concordance factor (CF) using an α prior of 1. α is an a priori discordance parameter that indicates the expected level of discordance among the different genes being analyzed (ranging from 0 to ∞; Larget et al. 2010). When α=0, this indicates that there is no expected discordant topologies among gene trees, whereas when α=∞, this indicates that all gene trees are expected to have independent topologies. In our analysis, using other values of α (0.1 & 10) did not greatly alter inferred levels of incongruence (Table S3).
Distinguishing incomplete lineage sorting and introgression
While BUCKy can reveal the extent of nuclear discordance among taxa, it does not indicate whether it is more likely to be due to ILS or introgression after species splitting (although these are not mutually exclusive). To examine these two hypotheses we employed the ABBA/BABA test to calculate Patterson’s D statistic for our dataset (Green et al. 2010). This statistic uses comparisons between three focal samples and an outgroup to determine whether phylogenetically-informative sites are in agreement with the primary phylogeny (AABB sites), or support one of the two possible alternative relationships (ABBA or BABA, respectively). If incomplete lineage sorting is the primary contributor to observed phylogenetic incongruence, then ABBA and BABA sites should be present in approximately equal frequencies (D statistic ≈ 0). However, genetic exchange that occurs after splitting may result in an excess of either ABBA or BABA sites (D statistic ≠ 0). It should be noted that population structure in the ancestral population could also produce an asymmetry in ABBA/BABA sites, whereas post-splitting, symmetrical genetic exchange could produce equal frequencies of sites (Durand et al. 2011).
The ABBA/BABA test was performed with all informative sites, 4-fold synonymous sites and 0-fold replacement sites from our 478 gene set. The standard deviation of D was determined by block jackknife sampling (Kunsch 1989) with a block size of 40 genes. 95% confidence intervals based on this jackknifed dataset were used to assess if D differed significantly from 0.
Genetic diversity and divergence times
Changes in the effective population size of a species (Ne) over time can influence divergence estimates and patterns of evolution (Charlesworth 2009). Therefore, we examined relative differences in contemporary Ne for each species by calculating 4-fold synonymous genetic diversity (π4f, Nei and Li 1979) within each of the four individual, wild-caught samples. To do this, we first mapped trimmed reads to the species specific, de novo-assembled transcriptome for each of the four butterflies. Trimming, filtering and variant calling were performed as described above. Likely coding sequences were determined by independently comparing each of the four species’ de novo assembled transcriptomes to the set of D. plexippus genome assembly predicted proteins (http://monarchbase.umassmed.edu/resource.html), using Blast with a translated nucleotide query (‘blastx’; Altschul et al. 1990) and a minimum e value of 1e-50. Table S4 gives summary information for this read mapping.
We quantified species diversity levels as the length-weighted mean number of nucleotide differences per 4-fold degenerate synonymous site (i.e. π4f). 95% confidence intervals on π4f were estimated by bootstrap sampling each gene set 1,000 times with replacement. We employed a Bayesian method to examine approximate species’ splitting times. As there are no known fossils that would allow us to independently calibrate any nodes in our phylogeny, we employed a rate-based estimate of evolution at neutral sites following methods described by Obbard and colleagues (Obbard et al. 2012). For evaluation of neutral evolution, we utilized all 4-fold synonymous sites from the concatenated dataset of our de novo assemblies. While mutations at these sites may not be entirely neutral due to codon usage bias (Hershberg and Petrov 2008) and other types of direct or indirect selection (Lawrie et al. 2013; Sella et al. 2009), they are the best available option in this dataset.
To account for variation in evolution rates among genes, we modeled a lognormal distribution of rate variation around the estimated mean mutation rate per year for H. melpomene (2.9 × 10−9, 95% CIs: 6.5 × 10−9 to 2.8 × 10−8), assuming five generations per year (Malcolm et al. 1987). This allowed us to partially capture potential variation in rates among branches and may have reduced overestimating the length of shorter branches (Schwartz and Mueller 2010).
We ran two MCMC chains of 108 iterations in the program BEAST (v. 1.8.1, Drummond and Rambaut 2007), with a log-normal relaxed molecular clock. We used the Hasegawa, Kishino and Yano (HKY, Hasegawa et al. 1985) substitution model with a proportion of invariant sites and a gamma distribution of rate heterogeneity and a starting tree based on our previous analysis of nuclear genes (Fig. 2C), with a birth-death process (Gernhard 2008). The “burn in” period was the initial 10% of states, and parameters were logged every 1,000 iterations. LogCombiner was used to merge our two separate runs (v. 1.8.1, included with the BEAST package). Log files were checked using Tracer (v. 1.6; Rambaut et al. 2014) to ensure that an effective sampling size (ESS) greater than 200 were achieved for each parameter. Divergence times were estimated based on the 95% highest posterior density (HPD) interval.
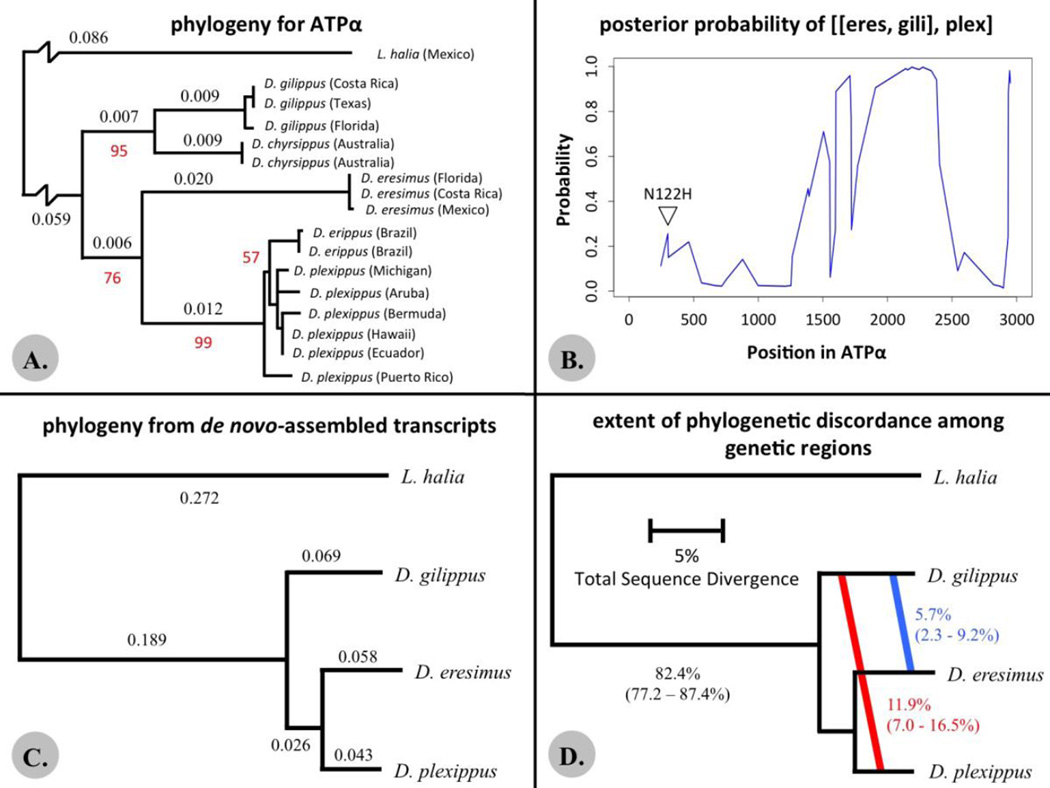
Figure 2.
A) Phylogenetic relationships among Danaus butterflies based on ATPα sequences. Black numbers indicate branch lengths for species-level branches. Red numbers indicate bootstrap support for between species branches when less than 100%. B) The level of support across ATPα for alternative phylogenetic relationships among Danaus species from PhyloNetHMM, using a four-sequence alignment of the coding region of ATPα. C) The best supported phylogenetic relationship as determined using data from 478 de novo-assembled nuclear gene sequences. Numbers indicate branch lengths. This tree topology had 100% bootstrap support at all branches. D) Levels of phylogenetic concordance/discordance between the best-supported species tree and individual gene trees. The red text and diagonal line indicates the level of discordance (with credibility intervals) that supports a closer relationship between D. plexippus and D. gilippus (with α = 1.0). The blue text and diagonal line indicated the level of discordance (with credibility intervals) that supports a closer relationship between D. gilippus and D. eresimus (with α = 1.0). NOTE: Branch lengths in these trees (A, C & D) are not drawn to the same scale between trees.
Results
Phylogenic analysis based on ATPα
The maximum likelihood phylogeny for Danaus species based on ATPα sequences is shown in Figure 2A. Contrary to previous findings, this phylogeny groups together D. eresimus and D. plexippus as sister lineages relative to D. gilippus, albeit with limited bootstrap support (75% on the branch leading to D. eresimus/D. plexippus). Removing the single non-synonymous substitution encoding N122H results in a phylogeny with the same topology, though bootstrap support for the branch is even lower at 65%. To better establish the cause of this lower level of branch support, we carried out a spatial evaluation of the phylogenetic signature across the ATPα gene using the program PhyloNetHMM (Liu et al. 2014). This analysis reveals that ATPα exhibits conflicting phylogenetic signature across its length with only the middle third of the protein exhibiting strong support for sister-species status for D. eresimus and D. gilippus (Fig 2B). These results imply either that D. eresimus is more closely related to D. plexippus than previously thought, or that there has been introgression of at least part of the ATPα gene between the D. eresimus and D. plexippus lineages.
Phylogenic analysis based on transcriptome data
To distinguish among possible causes for the apparent discordant pattern found in ATPα, we carried out a phylogenetic analysis using transcriptome data. Our de novo assembled nuclear gene dataset contained 478 sequences, totaling 220,188 nucleotides, with an average sequence length of 461 nucleotides. This dataset was limited in the total number of genes analyzed as overlapping regions in each gene had to be assembled in all four species for inclusion. The best-supported topology placed D. plexippus and D. eresimus as sister taxa, with D. gilippus a more distant relative (Fig. 1C). The two alternative topologies were significantly worse fits than the primary tree, but they were not significantly different from each other (Table 1). There was 100% bootstrap support for the best tree topology. This relationship is also supported by a dataset of genes mapped to predicted D. plexippus coding sequences and also the complete sequences of the mitochondrial genomes for numerous Danaus species (see Figs. S1 and S2 for details).
Table 1.
Comparison of the three possible phylogenetic relationships among the butterflies in this study for our de novo-assembled nuclear gene dataset. Significance between trees was determined using the AU test. See Materials and Methods for more details.
| Tree Comparison (see Fig. 3) |
|Δ lnL| | P-value of AU test |
|---|---|---|
| Tree A – Tree B | 282.9 | <0.001 |
| Tree A – Tree C | 297.6 | <0.001 |
| Tree B – Tree C | 22.6 | 0.057 |
Phylogenetic discordance
Our BUCKy analysis reveals that the primary concordance tree is the same as that determined in the maximum-likelihood analyses (Fig. 2D). However, it also reveals significant discordance (>5%) among individual gene trees and the inferred species tree. A higher proportion of genes supported a closer relationship between D. gilippus and D. plexippus, than between D. gilippus and D. eresimus.
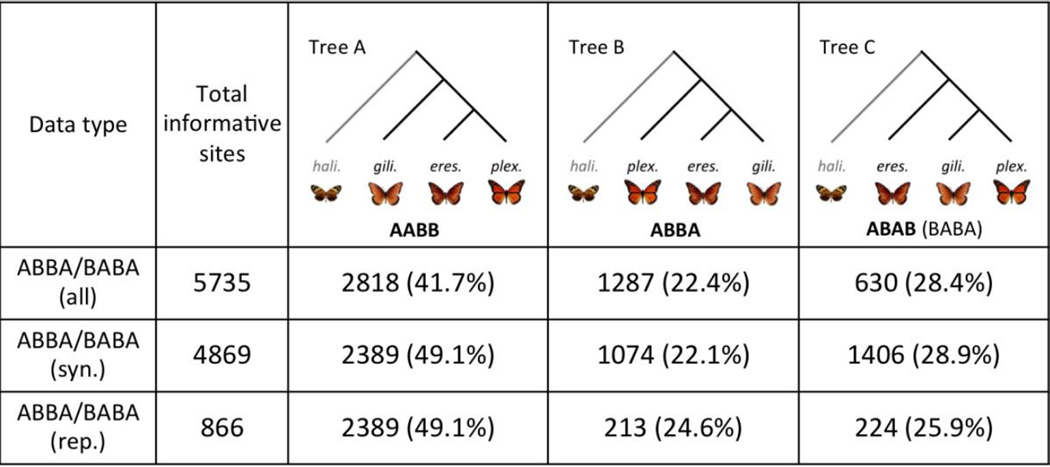
The full dataset contained 5,735 informative sites for the ABBA/BABA test. The percentages of sites supporting each of the three possible relationships are given in Figure 3, along with the number of 4-fold synonymous and 0-fold replacement sites. The D statistic for the full dataset was −0.118 (95% CIs: −0.121 to −0.114), for 4-fold synonymous sites it was− −0.134 (95% CIs: −0.138 to −0.130), and for 0-fold, non-synonymous sites it was −0.025 (95% CIs: −0.031 to −0.019). For all classes of sites, the D statistic was significantly less than 0, indicating that ABBA and BABA sites were not equally represented. This was always due to a larger number of BABA sites than ABBA sites, which suggests that a greater amount of gene flow has occurred between D. plexippus and D. gilippus after splitting than between D. eresimus and D. gilippus. It is also possible that this result is due to structure in the ancestral populations of these species (Green et al. 2010).
Figure 3.
The percentages of ABBA/BABA informative sites that match the best-supported species tree (Tree A) and the two alternative trees for the three North American Danaus species in this study for either all 4-fold/0-fold sites (all), just 4-fold synonymous sites (syn.) or just 0-fold replacement sites (rep.). (‘gili.’ = D. gilippus, ‘eres.’ = D. eresimus, ‘plex’ = D. plexippus, ‘hali’ = L. halia).
Genetic diversity and divergence time estimates
To assess the relative contemporary effective population sizes (Ne) of these species, we compared levels of 4-fold synonymous site diversity (π4f, Table S5). Of the four species, D. eresimus had the lowest synonymous diversity (π4f = 0.010, 95% CIs: 0.009 – 0.011). The other three species all had similar levels of diversity (L. halia: π4f = 0.022, 95% CIs: 0.021 – 0.023; D. gilippus: π4f = 0.021, 95% CIs: 0.018 – 0.023; D. plexippus: π4f = 0.021, 95% CIs: 0.021 – 0.022). The similarities in genetic diversity levels exhibited in D. plexippus and D. gilippus suggest that they have comparable contemporary Ne, while D. eresimus likely has a somewhat smaller Ne.
Differences in Ne between these species could affect estimates of divergence times. Specifically, D. eresimus may have had a higher rate of evolution over time than the other three species. However, Tajima’s relative rate test (Tajima 1993), using all sites from the concatenated de novo dataset suggests that D. eresimus has had a similar rate of evolution to D. gilippus (χ2 = 2.46, p=0.074), whereas the rate of evolution in D. plexippus was higher than both D. gilippus (χ2 = 31.11, p<0.001) and D. eresimus (χ2 = 20.25, p<0.001). Thus, we do not infer any evolutionary patterns in relation to contemporary Ne in these species.
Bayesian estimates of divergence times suggest that D. plexippus and D. eresimus diverged 7.2 MYA (95% highest posterior density [HPD]: 6.9 – 7.5), whereas D. gilippus diverged from the common ancestor of D. plexippus and D. eresimus 11.0 MYA (95% HPD: 10.7 – 11.4). These estimates of splitting time are similar to simple estimates based on synonymous divergence and a fixed mutation rate (Table S6), and suggest that the three species diverged relatively rapidly from one another (within 3 – 4 million years). The close timing of speciation events in this clade may have increased the likelihood that ILS occurred.
We explicitly examined the extent of ILS given our estimated divergence times by simulating the coalescent of 10,000 genes in ms (Hudson 2002). We used 0.02 for our estimate of θ (4Neμ) and converted our estimated divergence times to units of 4Ne generations (command line: ms 3 10000 -t 0.02 -I 3111 -ej 2.412-ej 3.623 –T). From these simulations, we infer that ~8% of genetic regions may exhibit an alternative topology to that of the true species relationship given the divergence times calculated here. This is less than the ~18% phylogenetic discordance observed, and is consistent with the inference that genetic exchange after these species diverged contributed to some of the observed phylogenetic incongruence observed between these species.
Discussion
The close phylogenetic relationship of D. eresimus and D. plexippus/D. erippus suggests either that the N122H substitution of ATPα is shared due to common descent or that it introgressed from one lineage into the other. The best-supported phylogeny from whole nuclear gene datasets of the three North American species of Danaus supports the former hypothesis (Fig. 2C.). Therefore, the presence of the N122H mutation in both the D. plexippus/D. erippus lineage and D. eresimus is most parsimoniously explained by a scenario in which it arose (and possibly fixed) in their shared common ancestor (Fig. S3A). However, it is also possible that the N122H mutation arose in a more ancestral Danaus species, and was subsequently lost in other species (Fig. S3B). We cannot confidently differentiate between this and the previous hypothesis with the data currently available, although this second explanation is less parsimonious.
One curious finding of this study is that there is a phylogenetically discordant signal across the length of ATPα and evidence for gene flow between the D. plexippus and D. gilippus lineages as well as the D. eresimus and D. gilippus lineages. Thus, while we currently lack the ability to directly test for on-going gene flow between D. plexippus and D. eresimus, it seems plausible given the evidence for gene flow in the comparisons we can make. The implication is that introgression of ATPα, and thus the N122H mutation, is not substantially less parsimonious than shared ancestry, particularly given the adaptive significance of this substitution. A crude way to evaluate evidence for introgression between D. plexippus and D. eresimus is to examine levels of sequence divergence. The estimated synonymous divergence (dS) between D. plexippus and D. eresimus for ATPα (0.15 per site) is somewhat lower than the genome-wide average (0.233). However, the large number of fixed differences between D. plexippus and D. eresimus implies that if introgression of the ATPα gene occurred, it did not happen recently.
It is becoming increasingly recognized that species divergence is often accompanied by post-splitting genetic exchange and other factors that obscure our ability to make evolutionary inferences. This may be especially true when divergence occurs rapidly. Among North American Danaus butterflies, we observe significant phylogenetic discordance between species, which may be attributed to several factors including incomplete lineage sorting (ILS) and post-speciation gene flow. Using coalescent simulations, we infer that ILS is likely to explain some of the observed phylogenetic incongruence. However, we also detect significant evidence for (asymmetrical) gene flow between species, implicating post-speciation gene flow as a contributor to phylogenetic discordance. Similar explanations have been proposed to explain phylogenetic discordance in many other species groups (e.g. Moody and Rieseberg 2012; Cui et al. 2013; Liu et al 2015), including butterflies (Kozak et al. 2015).
This study raises questions about the origins of the extensive phenotypic divergence observed in D. plexippus such as changes in wing size and mating strategy (Ackery and Vane-Wright 1984). Interestingly, recent work suggests all contemporary D. plexippus populations (including non-migratory ones) originated from a single North American migratory population (Brower et al. 2007; Zhan et al. 2014). The uniquely derived traits of D. plexippus may have obscured phylogenetic relationships between Danaus species in previous studies.
Supplementary Material
Acknowledgments
V. Miró Pina, M. Schumer and two anonymous reviewers provided helpful comments on earlier versions of this work. This work was supported by a National Institute of Health grant R01-GM115523 to PA.
Footnotes
Data Accessibility
The raw RNAseq data used in this study can be found at: http://genomics-pubs.princeton.edu/insect_genomics/data.shtml. All other data is available on GenBank as indicated in the text and supplementary information.
References
- Aardema ML, Zhen Y, Andolfatto P. The evolution of cardenolide-resistant forms of Na+,K+-ATPase in Danainae butterflies. Mol. Ecol. 2012;21:340–349. doi: 10.1111/j.1365-294X.2011.05379.x. [DOI] [PubMed] [Google Scholar]
- Ackery PR, Vane-Wright RI. Milkweed butterflies: their cladistics and biology. Ithaca, NY: Cornell Univ. Press; 1984. [Google Scholar]
- Agrawal AA, Petschnka G, Bingham RA, Weber MG, Rasmann S. Toxic cardenolides: chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol. 2012;194:28–45. doi: 10.1111/j.1469-8137.2011.04049.x. [DOI] [PubMed] [Google Scholar]
- Ané C, Larget B, Baum DA, Smith SD, Rokas A. Bayesian estimation of concordance among gene trees. Mol. Biol. Evol. 2007;24:412–426. doi: 10.1093/molbev/msl170. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baack EJ, Rieseberg LH. A genomic view of introgression and hybrid speciation. Curr. Opin. Genet. Dev. 2007;17:513–518. doi: 10.1016/j.gde.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D, Thornton K, Clark A, Andolfatto P. Extensive introgression of mitochondrial DNA relative to nuclear genes in the Drosophila yakuba species group. Evolution. 2006;60:292–302. [PubMed] [Google Scholar]
- Brower AVZ, Boyce TM. Mitochondrial DNA variation in monarch butterflies. Evolution. 1991;45:1281–1286. doi: 10.1111/j.1558-5646.1991.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Brower LP, Moffit CM. Palatability dynamics of cardenolides in the monarch butterfly. Nature. 1974;249:280–283. doi: 10.1038/249280b0. [DOI] [PubMed] [Google Scholar]
- Brower LP, Oberhauser KS, Boppré M, Brower AVZ, Vane-Wright RI. Monarch sex: ancient rites, or recent wrongs? Antenna. 2007;31:12–18. [Google Scholar]
- Brower AVZ, Wahlberg N, Ogawa JR, Boppré M, Vane-Wright RI. Phylogenetic relationships among genera of danaine butterflies (Lepidoptera: Nymphalidae) as implied by morphology and DNA sequences. Syst. Biodivers. 2010;8:75–89. [Google Scholar]
- Brown KS, Jr, von Schoultz B, Suomalainen E. Chromosome evolution in Neotropical Danainae and Ithomiinae (Lepidoptera) Hereditas. 2004;141:216–236. doi: 10.1111/j.1601-5223.2004.01868.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 2009;10:195–205. doi: 10.1038/nrg2526. [DOI] [PubMed] [Google Scholar]
- Cui R, Schumer M, Kruesi K, Walter R, Andolfatto P, Rosenthal GG. Phylogenomics reveals extensive reticulate evolution in Xiphophorus fishes. Evolution. 2013;67:2166–2179. doi: 10.1111/evo.12099. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol. Evol. 2009;24:332–340. doi: 10.1016/j.tree.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Dobler S, Petschenka G, Pankoke HC. Coping with toxic plant compounds - the insect's perspective on iridoid glycosides and cardenolides. Phytochemistry. 2011;72:1593–1604. doi: 10.1016/j.phytochem.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Dobler S, Dalla S, Wagschal V, Agrawal AA. Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na, K-ATPase. Proc. Natl. Acad. Sci. USA. 2012;109:13040–13045. doi: 10.1073/pnas.1202111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler S, Petschnka G, Wagschal V, Flacht L. Convergent adaptive evolution – how insects master the challenge of cardiac glycoside-containing host plants. Entomol. Exp. Appl. 2015;157:30–39. [Google Scholar]
- Dockx C. Directional and stabilizing selection on wing size and shape in migrant and resident monarch butterflies, Danaus plexippus (L.), in Cuba. Biol. J. Linn. Soc. 2007;92:605–616. [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eanes WF, Koehn RK. Analysis of genetic structure in the monarch butterfly, Danaus plexippus L. Evolution. 1978;32:784–797. doi: 10.1111/j.1558-5646.1978.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7197. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernhard T. The conditioned reconstructed process. J. Theor. Biol. 2008;253:769–778. doi: 10.1016/j.jtbi.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, et al. A draft sequence of the Neanderthal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Hershberg R, Petrov DA. Selection on codon bias. Annu. Rev. Genet. 2008;42:287–299. doi: 10.1146/annurev.genet.42.110807.091442. [DOI] [PubMed] [Google Scholar]
- Holzinger F, Wink M. Mediation of cardiac glycoside insensitivity in the monarch butterfly (Danaus plexippus): role of an amino acid substitution in the ouabain binding site of Na+,K+-ATPase. J. Chem. Ecol. 1996;22:1921–1937. doi: 10.1007/BF02028512. [DOI] [PubMed] [Google Scholar]
- Hornett EA, Wheat CW. Quantitative RNA-Seq analysis in non-model species: assessing transcriptome assemblies as a scaffold and the utility of evolutionary divergent genomic reference species. BMC Genomics. 2012;13:361. doi: 10.1186/1471-2164-13-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RR. Generating samples under a Wright-Fisher neutral model of genetic variation. Bioinformatics. 2002;18:337–338. doi: 10.1093/bioinformatics/18.2.337. [DOI] [PubMed] [Google Scholar]
- Hudson RR, Kreitman M, Aguadé M. A test of neutral molecular evolution based on nucleotide data. Genetics. 1987;116:153–159. doi: 10.1093/genetics/116.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Larget B, Alfaro ME. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol. Biol. Evol. 2004;21:1123–1133. doi: 10.1093/molbev/msh123. [DOI] [PubMed] [Google Scholar]
- Keightley PD, Pinharanda A, Ness RW, Simpson F, Dasmahapatra KK, Mallet J, Davey JW, Jiggins CD. Estimation of the spontaneous mutation rate in Heliconius melpomene. Mol. Biol. Evol. 2015;32:239–243. doi: 10.1093/molbev/msu302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KM, Wahlberg N, Neild A, Dasmahapatra KK, Mallet J, Jiggins CD. Multilocus species trees show the recent adaptive radiation of the mimetic Heliconius butterflies. Syst. Bio. 2015;64:505–524. doi: 10.1093/sysbio/syv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsch H. The jackknife and the bootstrap for general stationary observations. Ann. Stat. 1989;17:1217–1241. [Google Scholar]
- Larget BR, Kotha SK, Dewey CN, Ané C. BUCKy: Gene tree/species tree reconciliation with Bayesian concordance analysis. Bioinformatics. 2010;26:2910–2911. doi: 10.1093/bioinformatics/btq539. [DOI] [PubMed] [Google Scholar]
- Lawrie DS, Messer PW, Hershberg R, Petrov DA. Strong purifying selection at synonymous sites in D. melanogaster. PLoS Genet. 2013;9:e1003527. doi: 10.1371/journal.pgen.1003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R 1000 Genome Project Data Processing Subgroup. The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KJ, Steinberg E, Yozzo A, Song Y, Kohn MH, Nakhleh L. Interspecific introgressive origin of genomic diversity in the house mouse. Proc. Natl. Acad. Sci. USA. 2015;112:196–201. doi: 10.1073/pnas.1406298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KJ, Dai J, Truong K, Song Y, Kohn MH, Nakhleh L. An HMM-based comparative genomic framework for detecting introgression in eukaryotes. PLoS Comput. Biol. 2014;10:e1003649. doi: 10.1371/journal.pcbi.1003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunter G, Goodson M. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2011;21:936–939. doi: 10.1101/gr.111120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushai G, Smith DAS, Goulson D, Allen JA, Maclean N. Mitochondrial DNA clocks and the phylogeny of Danaus butterflies. Insect Sci. Appl. 2003;23:309–315. [Google Scholar]
- Lyons JI, Pierce AA, Barribeau SM, Sternberg ED, Mongue AJ, de Roode JC. Lack of genetic differentiation between monarch butterflies with divergent migration destinations. Mol. Ecol. 2012;21:3433–3444. doi: 10.1111/j.1365-294X.2012.05613.x. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Knowles LL. Inferring phylogeny despite incomplete lineage sorting. Syst. Biol. 2006;55:21–30. doi: 10.1080/10635150500354928. [DOI] [PubMed] [Google Scholar]
- Malcolm SB, Cockrell BJ, Brower LP. Monarch butterfly voltinism: effects of temperature constraints at different latitudes. Oikos. 1987;49:77–82. [Google Scholar]
- Mallet J, Besansky N, Hahn MW. How reticulated are species? Bioessays. 2015;37 doi: 10.1002/bies.201500149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Haigh J. The hitchhiking effect of a favourable gene. Genet. Res. 1974;23:23–35. [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebs D, Reuss E, Schneider M. Studies on the cardenolide sequestration in African milkweed butterflies (Danaidae) Toxicon. 2005;45:581–584. doi: 10.1016/j.toxicon.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Moody ML, Rieseberg LH. Sorting through the chaff, nDNA gene trees for phylogenetic inference and hybrid identification of annual sunflowers (Helianthus sect. Helianthus) Mol. PhyloGenet. Evol. 2012;64:145–155. doi: 10.1016/j.ympev.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Nei M. Molecular evolutionary genetics. New York: Columbia Univ. Press; 1987. [Google Scholar]
- Nei M, Li W-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Obbard DJ, Maclennan J, Kim K-W, Rambaut A, O’Grady PM, Jiggins FM. Estimating divergence dates and substitution rates in the Drosophila phylogeny. Mol. Biol. Evol. 2012;29:3459–3473. doi: 10.1093/molbev/mss150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Amino acid substitution at the Adh locus of Drosophila is facilitated by small population size. Proc. Natl. Acad. Sci. USA. 1993;90:4548–4551. doi: 10.1073/pnas.90.10.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschenka GS, Agrawal AA. Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc. R. Soc. B. 2015;282:20151865. doi: 10.1098/rspb.2015.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschenka G, Fandrich S, Sander N, Wagschal V, Boppré M, Dobler S. Stepwise evolution of resistance to toxic cardenolides via genetic substitutions in the Na+/K+-ATPase of milkweed butterflies (Lepidoptera: Danaini) Evolution. 2013;67:2753–2761. doi: 10.1111/evo.12152. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. Tracer v1.6. 2014 Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Ritland DB, Brower LP. The viceroy butterfly is not a batesian mimic. Nature. 1991;350:497–498. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: Robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RS, Mueller RL. Branch length estimation and divergence dating: estimates of error in Bayesian and maximum likelihood frameworks. BMC Evol. Biol. 2010;10:5. doi: 10.1186/1471-2148-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sella G, Petrov DA, Przeworski M, Andolfatto P. Pervasive natural selection in the Drosophila genome? PLoS Genet. 2009;5:e1000495. doi: 10.1371/journal.pgen.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001;17:1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Smith DAS, Lushai G, Allen JA. A classification of Danaus butterflies (Lepidoptera: Nymphalidae) based upon data from morphology and DNA. Zool. J. Linn. Soc. 2005;144:191–212. [Google Scholar]
- Stamatakis A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL. The genetic causes of convergent evolution. Nat. Rev. Genet. 2013;14:751–764. doi: 10.1038/nrg3483. [DOI] [PubMed] [Google Scholar]
- Tajima F. Simple methods for testing the molecular evolutionary clock hypothesis. Genetics. 1993;135:599–607. doi: 10.1093/genetics/135.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré S. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences. Lectures Math. Life Sci. 1986;17:57–86. [Google Scholar]
- Ujvaria B, Casewell NR, Sunagar K, Arbuckle K, Wüster W, Lo N, O’Meally D, Beckmann C, King GF, Deplazes E, Madsen T. Widespread convergence in toxin resistance by predictable molecular evolution. Proc. Natl. Acad. Sci. USA. 2015;112:11911–11916. doi: 10.1073/pnas.1511706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells H, Wells PH, Rogers SH. Is multiple mating an adaptive feature of monarch butterfly winter aggregation? In: Malcolm SB, Zalucki MP, editors. Biology and Conservation of the Monarch Butterfly. No. 38 Science Series. Los Angeles, CA, USA: Nat. Hist. Mus. of Los Angeles Co.; 1993. pp. 61–68. [Google Scholar]
- Woofit M. Effective population size and the rate and pattern of nucleotide substitutions. Biol. Lett. 2009;5:417–420. doi: 10.1098/rsbl.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: a program package for phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan S, Zhang W, Niitepõld K, Hsu J, Haeger JF, Zalucki MP, Altizer S, de Roode JC, Reppert SM, Kronforst MR. The genetics of monarch butterfly migration and warning coloration. Nature. 2014;514:317–321. doi: 10.1038/nature13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Aardema ML, Medina EM, Schumer M, Andolfatto P. Parallel molecular evolution in an herbivore community. Science. 2012;337:1634–1637. doi: 10.1126/science.1226630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.