Abstract
Aims
Postural Tachycardia Syndrome (POTS) represents an autonomic disorder predominantly affecting females between 15 and 50 years of age. POTS is a chronic disorder (>6 months) characterized by an excessive heart rate increment on standing (>30 beats/min) in the presence of characteristic symptoms of cerebral hypoperfusion or sympathetic activation. Patients have clinically been noted to describe lower urinary tract symptoms (LUTS), although urologic symptoms have not been methodically assessed in the POTS population. Herein we present data from a pilot study designed to identify and quantitate overactive bladder (OAB) in patients diagnosed with POTS.
Methods
Patients admitted to the Vanderbilt Autonomic Dysfunction Center between June 2009 and October 2010 for evaluation for the potential diagnosis of POTS completed a validated, standardized questionnaire for OAB (OAB-q) at presentation. Symptom score and subscale analyses were conducted. Subscale health related quality of life (HRQL) scores were transformed into a 0–100 scale, with higher scores reflecting superior HRQL. Data are presented as mean ± SD.
Results
Thirty-two females presented for evaluation of symptoms consistent with POTS. Twenty-nine women were subsequently diagnosed with POTS with 19 of these patients completing the OAB-q questionnaire (65.5% response rate). Average age was 33.5 ± 8.3 years. Symptom severity transformed score was 26.0 ± 16.4, with 13 of 19 patients (68.4%) meeting clinical criteria for diagnosis of probable clinically significant OAB. Nocturia was the most bothersome symptom, followed by frequency and urgency.
Conclusions
This pilot study describes bothersome lower urinary tract dysfunction in patients presenting with POTS as assessed by patient-reported questionnaire data. Nocturia demonstrated the greatest negative impact on health-related quality of life (HRQL), while social interaction was the least affected HRQL domain. In patients with dysautonomia, this data provides a critical baseline for mechanistic insight into both disease-specific and global pathophysiology of nocturia and OAB.
Keywords: Overactive bladder, autonomic dysfunction, Postural Tachycardia Syndrome, OAB-q
INTRODUCTION
The relationship between autonomic dysfunction and lower urinary tract symptoms is intricate and likely underappreciated in the current literature. The sophisticated signalling pathways required for normal bladder function are infinitely subject to perturbations of autonomic innervations. [1] However, the defining factors linking distinct autonomic disorders and resultant lower urinary tract manifestations remains at the early stages of investigation. [2] Indeed, initial studies have demonstrated a potential relationship between autonomic dysfunction and idiopathic OAB. [3] The female predominant dysautonomia, Postural Tachycardia Syndrome (POTS), has previously been linked to sleep disturbance, fibromyalgia and chronic fatigue syndrome and remains one of the most common manifestations of orthostatic intolerance. [4] [5]
POTS is classically defined by an excessive increase in heart rate (>30 beats per minute) with upright posture, associated with orthostatic symptoms including palpitations, chest pain, dyspnea on standing, mental clouding, and difficulties with concentration in the absence of orthostatic hypotension. [6]–[7] POTS is diagnosed markedly more frequently in women with a 4.5:1 female to male ratio. Although there are select instances of known prodromal viral syndromes or heredity contributions, the etiology and pathophysiology of POTS remains elusive.
Perturbations in the delicate regulation of the continence-micturition cycle by the autonomic nervous system may contribute dramatically to both sensory and functional detrusor abnormalities which frequently manifest with symptoms of OAB. OAB is defined by the International Continence Society as “urgency, with or without urgency urinary incontinence, usually with frequency and nocturia in the absence of pathological or metabolic conditions that might be able to explain these symptoms.” [8] Voiding symptoms of urgency and frequency, hallmarks of OAB, may manifest from a vast array of primary pathologies. Many etiologies for urinary urgency/frequency have been theorized which include broad categories of neurogenic, myogenic, and urothelial signaling pathways. [9] Current concepts embrace an integrative hypothesis for urgency/frequency which encompasses complex signaling interactions between urothelium, detrusor, as well as accommodating the role of interstitial cells and neural complexes. [10] However, these sophisticated theories do not address the contribution of comorbid systemic disease processes which may be compounded by an infinite array of pharmacologic compounds and environmental insults. In addition to the multiple potential pathways which may result in similar symptomatology, the penetrance of each of these processes is unlikely to be equal leading to a spectrum of disease which is highly dependent on a patient’s independent physiology.
Most clinicians utilize the patient’s subjective report to define OAB and initiate therapy. Clinical assessment of the disease is often performed with standardized patient assessment tools and for patients refractory to first line therapy, with functional lower urinary tract analysis with urodynamics. [11, 12] Critically, OAB and the urodynamic finding of detrusor overactivity (DO) which is defined as involuntary detrusor contractions during the cystometric filling phase, are not interchangeable terms as it has been recognized that approximately half of patients with OAB symptoms will not display hallmark DO, particularly patients without associated incontinence. [13, 14]. This weak correlation, along with the invasive nature of the procedure, precludes widespread use of urodynamics as a means of providing front line analysis to diagnose OAB, therefore clinical symptoms and standardized questionnaires remain a mainstay for diagnosis. [15]
The Overactive Bladder Questionnaire (OAB-q) was designed to ascertain patient-reported perception of symptom bother and impact on Health Related Quality of Life (HRQL) and has previously demonstrated high responsiveness to treatment-related change. [12] OAB-q consists of 33 questions - 8 urinary symptom questions and 25 HRQL questions. Further, the 8 urinary symptom questions of the OAB-q have been used to develop an OAB screening awareness tool which allows primary care providers to diagnose patients with probable OAB. [16] [17]
Secondary to prior studies implicating autonomic dysfunction in patients with idiopathic OAB, as well as the potential contribution of nocturia to previously published data from our center regarding sleep disturbances significantly contributing to diminished quality of life, we hypothesized that POTS patients would demonstrate patient-reported symptoms consistent with OAB. [3, 18] Herein we present the first evidence utilizing an OAB-specific standardized questionnaire to demonstrate the presence of moderate OAB symptoms in the POTS population.
MATERIALS AND METHODS
Following protocol approval by the Vanderbilt Institutional Review Board, consecutive female patients presenting to the Vanderbilt University Clinical Research Center between June 2009 and October 2010 for evaluation of the autonomic dysfunction POTS were administered an OAB-q standardized questionnaire at admission. The OAB-q is composed of the 8-item urinary symptom bother scale (0–5 scale) combined with the 25 HRQL items that form 4 subscales: 8 questions regarding coping, 7 referring to concern, 5 for assessment of sleep, and finally 5 for evaluation of social interaction. [12] Patients rated each item on a 6 point Likert scale ranging from “none of the time” to “all of the time” for the HRQL items and “not at all” to “a very great deal” for the symptom bother items. Subscales were summed and transformed into scores ranging from 0–100 with the following formula: (highest possible score − actual raw score)/possible raw score range × 100). Higher symptom bother scores indicate worsening bother, with higher HRQL scores reflecting superior HRQL. Demographic data was additionally collected. Data are presented as mean ± SD. Symptom scores were employed to subdivide patients into a probable OAB group based on prior validation studies with probable OAB correlating to symptom scores ≥16. [17]
RESULTS
During the time course of the study, thirty-two females presented for evaluation of symptoms consistent with POTS. Twenty-nine women were subsequently diagnosed with POTS with 19 of these patients completing the OAB-q questionnaire (65.5% response rate). Average age was 33.5 ± 8.3 years. Symptom severity transformed score for the POTS patients was 26.0 ± 16.4 (Fig. 1). 13 of 19 POTS patients (68.4%) met criteria for diagnosis of probable OAB by reporting a symptom score ≥16. With regards to the HRQL domains, nocturia was determined the most bothersome symptom, followed by frequency and urgency. On the scale of 0–100 with 100 representing the highest HRQL score, transformed scores for the coping, concern, sleep and social interaction domains were 86±21, 81±21, 72± 28 and 98±6, respectively (Figs. 2–5). For this population of POTS patients, the sleep domain demonstrated the lowest scores correlating to the most prominent impact with regards to HRQL.
Fig. 1.
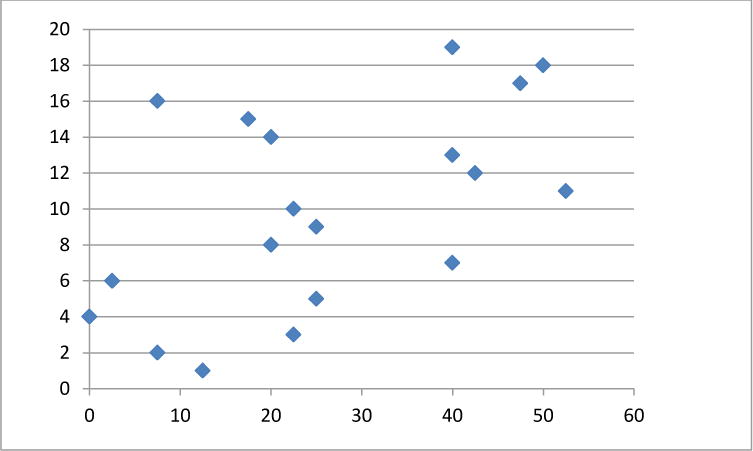
Symptom severity scores per patient number (y axis) utilizing the OABq 0–5 scale (mean 26.0 ± 16.4). Higher values correlate to worsening symptom bother. Probable OAB was assigned to patients with scores ≥ 16.
Fig. 2.
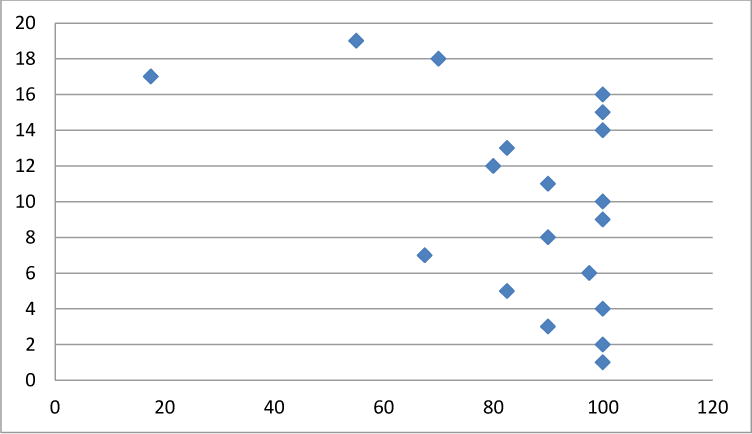
Coping domain scores per patient (y axis) transformed on a 1–100 scale (x axis) (mean 86±21). Higher scores correlate to less impact on HRQL domain of coping.
Figure 5.
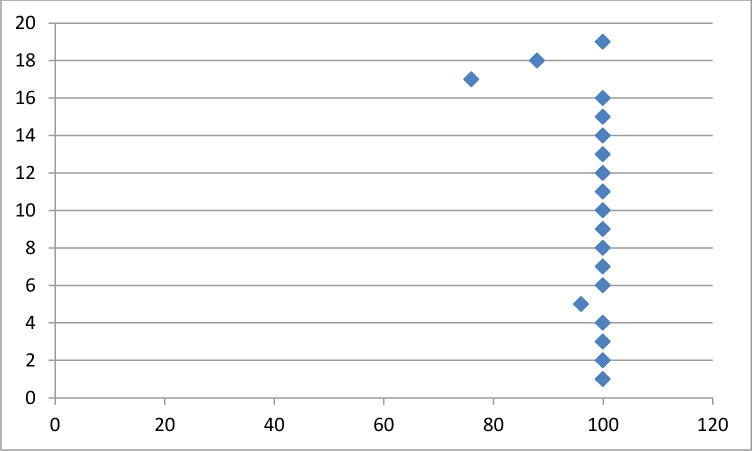
Social domain scores per patient (y axis) transformed on a 1–100 scale (x axis) (mean 98±6). Higher scores correlate to less impact on HRQL in the social domain.
DISCUSSION
Our tertiary care referral intuition encompasses an independent large volume center for the evaluation and management of patients with a wide range of autonomic disorders, including POTS. In addition to typical orthostatic symptoms, a multitude of systemic manifestations have been reported by these patients including chronic headache, fatigue, sleep disturbance, fibromyalgia, and gastrointestinal distress. [5] [19] The delicate relationship between dysautonomia and OAB symptoms has been postulated due to known autonomic control of lower urinary tract function; however the pathophysiologic mechanisms remain elusive. [20–22] Fascinating data is emerging revealing potential correlations of sympathetic autonomic dysfunction as assessed by cardiovascular testing with the presence of detrusor overactivity on urodynamic analysis. [3] Likewise, prior investigations regarding arterial response reported OAB symptoms in women demonstrating sympathetic predominant autonomic dysfunction. [23] Additional studies have described regional autonomic dysfunction in OAB patients via altered sympathetic skin response. [24]
Prior data from our center has demonstrated POTS patients manifest nocturnal sleep disturbances which contribute significantly to diminished quality of life, however the role of nocturia was not assessed in this study. [18] Herein, utilizing the OAB-q, we report nocturia demonstrates the most substantial disruption impacting HRQL for the POTS population, providing insight into the mechanisms of the previously noted sleep disturbance and correlative fatigue symptoms.
Patient-reported outcomes are particularly critical in defining the negative impact of, and HRQL for, conditions such as OAB that are predominantly driven by patient symptoms. In the current analysis, symptom bother sub scores placed the majority of POTS patients into the probable OAB category. However, one limitation to this study involves just such utilization of a patient-reported outcomes based system for analysis without direct clinical correlation. Indeed, despite efforts to create a benchmark for scoring with both a clinical sample and a community population, no true standard normalized value exists for OAB-q scoring. [25] Therefore, determining the true symptom bother from a discrete measurement in relation to a normative value becomes a challenging issue. Additional limitations include the limited sample size, although by design, such a pilot study demonstrating a strong correlation is suggestive that future analysis of the correlation between POTS and OAB is indicated. Indeed, well known disease processes, such as diabetes, which impact voiding function are likely to share similar autonomic dysfunctions and may represent a spectrum of processes which the POTS patients herein are included.[26]
CONCLUSIONS
This pilot data suggests substantial lower urinary tract dysfunction in patients presenting with POTS as assessed by the patient-reported questionnaire OAB-q. Nocturia demonstrated the greatest negative impact on HRQL, while social interaction was the least affected HRQL domain. In addition to providing information regarding disease-specific urinary symptoms in patients with dysautonomia, this data provides a critical baseline for mechanistic insight into global pathophysiology of nocturia and OAB.
Figure 3.
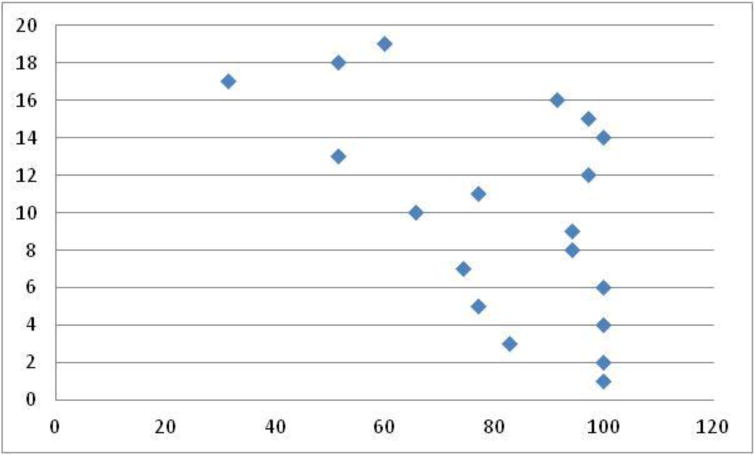
Concern domain scores per patient (y axis) transformed on a 1–100 scale (x axis) (mean 81±21). Higher scores correlate to less impact on HRQL domain of concern.
Figure 4.
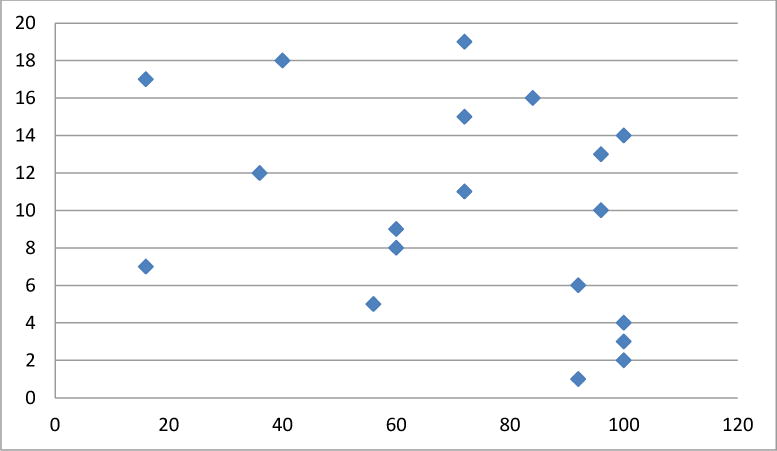
Sleep domain scores per patient (y axis) transformed on a 1–100 scale (x axis) (mean 72± 28). Higher scores correlate to less impact on HRQL domain of sleep.
References
- 1.Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra - Chapter 60. In: K LR, Wein AJ, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th. Elselvier; Philadelphia: 2011. [Google Scholar]
- 2.Ulas UH, Chelimsky TC, Chelimsky G, et al. Comorbid health conditions in women with syncope. Clin Auton Res. 2010;20(4):223–7. doi: 10.1007/s10286-010-0070-x. [DOI] [PubMed] [Google Scholar]
- 3.Hubeaux K, Deffieux X, Raibaut P, et al. Evidence for autonomic nervous system dysfunction in females with idiopathic overactive bladder syndrome. Neurourol Urodyn. 2011;30(8):1467–72. doi: 10.1002/nau.21154. [DOI] [PubMed] [Google Scholar]
- 4.Raj SR. Postural tachycardia syndrome (POTS) Circulation. 2013;127(23):2336–42. doi: 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–25. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 7.Garland EM, Raj SR, Black BK, et al. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69(8):790–8. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 8.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 9.Drake MaPA. Overactive Bladder. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th. Elselvier; Philadelphia, PA: 2011. [Google Scholar]
- 10.Drake MJ, I, Mills W, Gillespie JI. Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet. 2001;358(9279):401–3. doi: 10.1016/s0140-6736(01)05549-0. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res. 1994;3(5):291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 12.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–74. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 13.Digesu GA, Khullar V, Cardozo L, Salvatore S. Overactive bladder symptoms: do we need urodynamics? Neurourology and Urodynamics. 2003;22(2):105–8. doi: 10.1002/nau.10099. [DOI] [PubMed] [Google Scholar]
- 14.Hashim H, Abrams P. Overactive bladder: an update. Curr Opin Urol. 2007;17(4):231–6. doi: 10.1097/MOU.0b013e32819ed7f9. [DOI] [PubMed] [Google Scholar]
- 15.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2455–63. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 16.Coyne KS, Matza LS, Thompson CL. The responsiveness of the Overactive Bladder Questionnaire (OAB-q) Qual Life Res. 2005;14(3):849–55. doi: 10.1007/s11136-004-0706-1. [DOI] [PubMed] [Google Scholar]
- 17.Coyne KS, Zyczynski T, Margolis MK, et al. Validation of an overactive bladder awareness tool for use in primary care settings. Adv Ther. 2005;22(4):381–94. doi: 10.1007/BF02850085. [DOI] [PubMed] [Google Scholar]
- 18.Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7(2):204–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Loavenbruck A, Iturrino J, Singer W, et al. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol Motil. 2015;27(1):92–8. doi: 10.1111/nmo.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanc F, Pichot V, Roche F, et al. Activity of the autonomous nervous system measured based on the variability of heart rate in female urinary incontinence. Prog Urol. 2001;11(3):492–7. [PubMed] [Google Scholar]
- 21.Wein AJaRRD. Neuromuscular dysfunction of the lower urinary tract - Chapter 65. In: K LR, Wein AJ, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th. Elsevier; Philadelphia: 2013. [Google Scholar]
- 22.Ochodnicky P, Uvelius B, Andersson KE, Michel MC. Autonomic nervous control of the urinary bladder. Acta Physiol (Oxf) 2013;207(1):16–33. doi: 10.1111/apha.12010. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao SM, Su TC, Chen CH, et al. Autonomic dysfunction and arterial stiffness in female overactive bladder patients and antimuscarinics related effects. Maturitas. 2014;79(1):65–9. doi: 10.1016/j.maturitas.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Ates E, Ipekci T, Akin Y, et al. Impact of sympathetic dysfunction in the etiology of overactive bladder in women: A preliminary study. Neurourol Urodyn. 2014 doi: 10.1002/nau.22652. [DOI] [PubMed] [Google Scholar]
- 25.Cardozo L, Staskin D, Currie B, et al. Validation of a bladder symptom screening tool in women with incontinence due to overactive bladder. Int Urogynecol J. 2014;25(12):1655–63. doi: 10.1007/s00192-014-2417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Daneshgari F. Diabetic bladder dysfunction. Chinese medical journal. 2014;127(7):1357–64. [PMC free article] [PubMed] [Google Scholar]


