Abstract
Background
More than one-quarter of the US population qualify as excessive alcohol consumers. Alcohol use impacts several lung diseases, and heavy consumption has been associated with poor clinical outcomes. The fractional excretion of exhaled nitric oxide (Feno) has clinical implications in multiple airways diseases. We hypothesized that excessive alcohol intake is associated with lower Feno levels.
Methods
To test this hypothesis, we examined a sample consisting of 12,059 participants, aged 21 to 79 years, interviewed between 2007 and 2012 from the National Health and Examination Survey. Two valid Feno measurements that were reproducible were recorded. Alcohol questionnaire data were used to define the following alcohol groups: never drinkers, nonexcessive drinkers, excessive drinkers, and former excessive drinkers. The natural logarithm of Feno values [ln(Feno)] as well as blood eosinophil count and C-reactive protein were used as dependent variables to test the association with alcohol groups including multivariable linear regression models with adjustment for predictors of Feno.
Results
Excessive alcohol consumption comprised 3,693 (26.9%) of the US sample population. Controlling for all other factors, excessive alcohol consumption had a negative association and was an independent predictor for ln(Feno) levels in comparison with the never-drinker group (−0.11; 95% CI, −0.17 to −0.06; P < .001). ln(Feno) levels decreased across categories of increasing alcohol use (P < .001).
Conclusions
Accounting for alcohol use in the interpretation of Feno levels should be an additional consideration, and further investigations are warranted to explore the complex interaction between alcohol and nitric oxide in the airways.
Key Words: airway inflammation, alcohol, C-reactive protein, epidemiology (pulmonary), exhaled nitric oxide
Abbreviations: CRP, C-reactive protein; Feno, fractional excretion of exhaled nitric oxide; NHANES, National Health and Nutrition Examination Survey; NOS, nitric oxide synthase; ppb, parts per billion
Fractional excretion of exhaled nitric oxide (Feno) is a simple, noninvasive measurement of nitric oxide in an exhaled breath that has been commonly used as a biomarker of eosinophilic airway inflammation in patients with asthma.1, 2 In addition, the American Thoracic Society has recommended that Feno levels in patients with asthma be used to determine corticosteroid responsiveness and compliance.3 Use of Feno has expanded to several other applications in pulmonary diseases including corticosteroid responsiveness in COPD, diagnosing primary ciliary dyskinesia, and treatment responses in pulmonary artery hypertension and cystic fibrosis.4, 5, 6, 7 Nitric oxide is an important gaseous mediator, synthesized by the nitric oxide synthase (NOS) family of enzymes, that is involved in regulating several lung physiologic and pathologic processes. Whereas nitric oxide can arise from multiple anatomic locations in the lungs, Feno levels are currently thought to represent predominantly production from airway epithelial cells via nitric oxide synthase 2 (NOS2; inducible NOS).4, 8, 9 The effect of alcohol use on Feno levels has not been investigated, but this information might be important because alcohol use impacts several lung diseases and could thereby influence the interpretation and application of Feno measurements and the usefulness of the assay.
Heavy alcohol consumption impairs lung defenses, as reflected in the well-recognized association of alcoholism and pneumonia.10 However, alcohol can have positive effects and was used historically to treat asthma and a variety of lung diseases.11 Pure ethanol can act as a bronchodilator in humans11 and has been shown to reduce allergic asthma in mice.12 On the other hand, nonalcohol components within alcoholic beverages such as sulfites, phenolic compounds/resveratrol (inhibitors of cyclooxygenase-1), wheat/gluten, and hop content can act as potential triggers of asthma.13, 14, 15 The Third National Health and Nutrition Examination Survey (NHANES III; 1994) data set demonstrated an increased risk for airflow obstruction in former excessive alcohol consumers, independent of potential confounders.16 Interestingly, there was a reduction in the risk for lung restriction in this group.16
We hypothesize that alcohol is an important predictor of Feno, with reduced levels being observed in excessive alcohol consumers. This hypothesis is based on the observation that intravenous alcohol reduces exhaled nitric oxide in anesthetized rabbits17 and, moreover, modest alcohol consumption reduces exhaled nitric oxide in subjects with asthma, but not normal subjects.18 Information regarding the effect of alcohol on Feno levels might be important in the interpretation and therapeutic considerations in subjects with risky alcohol consumption and respiratory disease and/or provide insight into potential mechanisms of alcohol effects in the lung.
Methods
Study Population and Inclusion Criteria
NHANES is a nationally representative cross-sectional survey designed to assess the health and nutritional status of the civilian, noninstitutionalized US population. The survey is conducted annually using stratified multistage probability sampling to provide estimates of the US population. Household interviews were performed in person and standardized health measurements were performed in a mobile examination center. Samples are weighted on the basis of age, sex, race, and ethnicity to represent the distribution of the US population. Beginning in 2007, NHANES expanded to include Feno measurements. We analyzed data beginning in 2007 through the last available public use data files released in 2012.
Participants 21 years of age or older with demographic data, alcohol questionnaire data, and completed Feno testing were included in the analysis. The age cutoff was chosen on the basis of the legal US drinking age and to reduce bias in survey response from those below the legal drinking age. Pregnant participants were excluded from analysis. Additional data collected for analysis included C-reactive protein (CRP) level and peripheral blood eosinophil count to represent systemic inflammation. All participants provided written consent, and the National Center for Health Statistics Research Ethics Review Board approved all protocols.
Exhaled Nitric Oxide
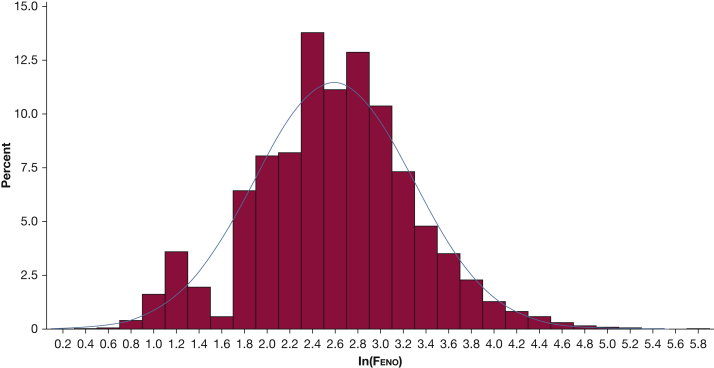
A US Food and Drug Administration-approved, portable, hand-held nitric oxide analyzer (Aerocrine NIOX MINO) was used with automated prompts and coaching by health technicians. Subjects were allowed 10 attempts to meet the NHANES protocol for two valid Feno measurements that were reproducible, in accordance with the testing procedures recommended by the manufacturer and similar to those published by the American Thoracic Society and European Respiratory Society.3 The device uses an online chemiluminescence analyzer to provide measurements between 5 and 300 parts per billion (ppb). Participants with Feno results below the level of detection (5 ppb) comprised 922 (6.27%) of all tested participants. Multiple imputations were performed for values below the instrument’s limit of detection to provide a normal distribution in the natural log transformation of Feno (Fig 1). Subjects with active chest pain, difficulty performing forceful expiration, or using supplemental oxygen were excluded from participating.
Figure 1.
Natural log-transformed distribution of fractional excretion of exhaled nitric oxide (Feno) with percentage of subjects in comparison with Feno levels for adult subjects ≥ 21 years with adequate testing from NHANES between 2007 and 2012. NHANES = National Health and Nutrition Examination Survey.
To address potential confounders in Feno measurements, data regarding consumption of nitrate-rich foods was captured by the following questions: “Within the last three hours have you eaten beets, broccoli, cabbage, celery, lettuce, spinach, or radishes?” and “Within the last three hours have you eaten bacon, ham, hot dogs, or smoked fish?” Upper respiratory infection was defined as an affirmative answer to the following question: “In the past seven days, have you had a cough, cold, phlegm, runny nose, or other respiratory illness? Do not count allergies or hay fever.”
Alcohol Exposure
Alcohol questionnaire data were used to identify participants who met the following definition for excessive alcohol consumption: binge alcohol consumption (four or more drinks per occasion for a woman and five or more drinks per occasion for a man); heavy alcohol consumption (more than one drink per day on average for a woman, and more than two drinks per day on average for a man). This definition is consistent with the Centers for Disease Control and Prevention standards used to identify harmful patterns of alcohol consumption.19 In NHANES, the following questions were used to identify consumers meeting the given definition of excessive alcohol consumption: “Had at least 12 drinks alcohol in life?” and “Had at least 12 drinks in last year?” If drinkers answered yes to both, then any of the following could be used: “Average number of drinks per day?”; “Number of days had 5 or more drinks/past 12 months” (response had to equal or exceed 12 to average one binge per month); and “Ever have 5 or more drinks every day?” Former excessive drinkers met the criteria for excessive drinking but answered no to the question, “Had at least 12 drinks in last year?” Lifetime never drinkers answered no to the question, “Had at least 12 drinks alcohol in life?” The remainder were classified as nonexcessive alcohol consumers.
Alcohol-related liver disease was presumed in participants who reported excessive alcohol use and had elevated liver enzyme levels at the time of medical examination in the absence of other chronic liver diseases including chronic hepatitis C and chronic hepatitis B. Elevated liver enzyme levels were defined as serum alanine aminotransferase (ALT) level > 40 units/L or aspartate aminotransferase (AST) level > 37 units/L in men and ALT or AST > 31 units/L in women.20
Other Variables
Age was categorized to represent the inflection points that occur in Feno levels across the age ranges previously shown in NHANES.21 In women, the categories for age range are 21 to 45 years and 46 to 79 years. In men, the categories for age range are 21 to 59 years and 60 to 79 years (NHANES limited Feno testing to a maximum of 79 years of age).
Never smokers were participants who smoked < 100 cigarettes in a lifetime and do not currently smoke. Current smokers reported smoking ≥ 100 cigarettes and currently smoke. Former smokers previously smoked ≥ 100 cigarettes but do not currently smoke cigarettes. In addition, smokers were evaluated in regression analysis using the survey question: “During the past 30 days, on the days that you smoked, about how many cigarettes did you smoke per day?”
Inhaled steroid and oral corticosteroid use was determined by asking survey participants to report use of prescription medications during a 1-month period prior to the survey date. The medications included the following: fluticasone/salmeterol, mometasone, beclomethasone, budesonide, budesonide/formoterol, flunisolide, fluticasone, triamcinolone, prednisone, prednisolone, and methylprednisolone.
Asthma was self-reported by indicating yes to the questions “Ever been told you have asthma?” and “Still have asthma?” Self-report for hay fever status in the past 12 months was used to reflect atopy; although poorly sensitive, it has been shown to have good specificity (up to 97%).22
Race and ethnicity were categorized into four groups: non-Hispanic whites, non-Hispanic blacks, Mexican American/Hispanics, and other (Alaska Native, American Indian, Asian, or Pacific Islander).
Body mass index (BMI) was calculated by dividing the weight (kilograms) by the square of the height (meters). BMI was then divided into six classifications based on the equivalent World Health Organization criteria for conventional BMI: < 18.5 (underweight); 18.5 to 24.9 (normal weight); 25.0 to 29.9 (overweight); 30.0 to 34.9 (class I, obese); 35.0 to 39.9 (class II, severely obese); ≥ 40.0 (class III, morbidly obese).23 Height was also recorded separately.
Analytic Approach
Feno values were positively skewed and were natural log (ln) transformed to approximate normality (Fig 1). The primary outcome was ln(Feno). In secondary analyses, CRP level and peripheral blood eosinophil count were evaluated as continuous variables. All estimates were calculated using the sampling weight to represent people 21 years of age or older in the United States. Baseline characteristics were presented as means with standard errors and compared by t test for continuous variables and by χ2 test for comparison of two or more proportions. Linear regression was used to test associations of ln(Feno) levels with covariables. Variable selection for the final model was made a priori and based on a review of previous literature and biologic plausibility. For average cigarettes per day, the distribution was largely skewed to zero cigarettes per day for never smokers, and therefore this variable was categorized into the following, based on its distribution: zero cigarettes per day, one to 10 cigarettes per day, 11 to 20 cigarettes per day, and > 20 cigarettes per day. The final adjusted model included age, sex, BMI, height, ethnicity, education level, poverty index, asthma status, average number of cigarettes smoked per day, inhaled steroid use, hay fever status, nitric oxide-rich vegetable consumption, alcohol-related liver disease, and alcohol consumption. Percent change and back transformation of the ln(Feno) was performed for the alcohol groups in the multivariable model. Main effects were tested in univariate analysis, and then two-way interactions were tested including smoking and alcohol, asthma and alcohol, and age and alcohol. Test for interactions with multiple levels was performed according to a previously published method using Bonferroni-adjusted t statistics for β coefficients from multivariable linear regression analysis using survey methods.24 Test for linear and quadratic trend was performed using contrasts in the coefficients corresponding to variables representing the alcohol categories. The Pearson product-moment correlation was used to test the correlation between ln(Feno) and CRP level/blood eosinophil count. The analyses were performed with SAS (SAS Institute) and Survey Data Analysis (SUDAAN; Research Triangle Institute).
Results
Between 2007 and 2012, 30,442 individuals were screened and selected. From those screened, 50.9% met inclusion criteria and were enrolled, representing 15,501 people 21 years of age or older. Of the 15,501 NHANES participants, 1,788 (11.5%) did not have completed questionnaire or demographics data. Another 1,654 (10.7%) did not undergo two valid Feno measurements for one of the following reasons: measurements were nonreproducible; Feno examination was attempted but no measurements were produced; or the participant was excluded for medical reasons, including breathing problems or chest pain. The final study population for evaluation of Feno by alcohol group consisted of 12,059 people. A comparison of participants with missing data with the final study population is shown in e-Table 1. Participants with missing data included a lower proportion of non-Hispanic whites, current smokers, and those with alcohol-related liver disease. In addition, those with missing data had lower socioeconomic status and comprised a greater proportion of women.
Excessive alcohol consumption was recorded in 3,693 participants (26.9%) who underwent the Feno examination. Monthly binge alcohol consumption was the most common form of excessive alcohol consumption, described by 2,682 participants (72.6%). Heavy daily alcohol consumption accounted for 197 excessive alcohol consumers (5.3%), and 814 of the excessive alcohol group (22.1%) described both binge alcohol consumption and heavy daily alcohol consumption. Excessive alcohol consumers were younger than members of the other alcohol groups and included a greater proportion of males, non-Hispanic whites, overweight participants, and never smokers. Among the alcohol groups, excessive alcohol consumers had the lowest median and ln(Feno) levels at 11.32 ppb (interquartile range, 7.11-17.82 ppb) and 2.45 ppb (SE 0.02; P < .0001), respectively. Demographics and characteristics by alcohol group are shown in Table 1.
Table 1.
Descriptive Statistics of Study Population From the NHANES, 2007-2012: Adults Aged ≥ 21 Years
| Variable | Total (13,713) | Alcohol Group |
P Value | |||
|---|---|---|---|---|---|---|
| Never Drinker (1,812) | Excessive (3,693) | Nonexcessive (8,068) | Former Excessive (140) | |||
| Baseline Characteristic | ||||||
| Age group 1,a No. (%) | 7,791 (62.68) | 770 (47.97) | 2,552 (74.66) | 4,379 (59.41) | 90 (78.06) | < .001 |
| Age group 2,a No. (%) | 5,922 (37.32) | 1,042 (52.03) | 1,141 (25.34) | 3,689 (40.59) | 50 (21.94) | |
| Age, mean (SEM), y | 46.48 (0.32) | 49.64 (0.60) | 43.23 (0.41) | 47.43 (0.34) | 47.81 (1.25) | < .001 |
| Height, mean (SEM), cm | 169.26 (0.14) | 163.95 (0.29) | 172.84 (0.18) | 168.48 (0.18) | 170.50 (0.80) | < .001 |
| BMI, No. (%) | ||||||
| Normal weight | 3,659 (28.46) | 454 (26.30) | 1,035 (29.69) | 2,134 (28.31) | 36 (24.83) | |
| Underweight | 199 (1.39) | 23 (1.40) | 61 (1.36) | 115 (1.43) | 0 (0.0) | |
| Overweight | 4,572 (33.88) | 571 (32.47) | 1,291 (34.95) | 2,666 (33.65) | 44 (31.70) | |
| Obese | ||||||
| Class I | 2,936 (20.59) | 399 (20.88) | 780 (21.11) | 1,728 (20.33) | 29 (19.30) | < .001 |
| Class II | 1,362 (9.13) | 204 (9.94) | 317 (7.85) | 829 (9.57) | 12 (9.31) | |
| Class III | 985 (6.55) | 161 (9.01) | 209 (5.05) | 596 (6.72) | 19 (14.86) | |
| Female, No. (%) | 6,804 (50.10) | 1,338 (71.38) | 987 (29.83) | 4,428 (56.11) | 51 (32.90) | < .001 |
| Race/ethnicity, No. (%) | ||||||
| Non-Hispanic white | 5,961 (68.72) | 547 (51.59) | 1,770 (71.67) | 3,585 (70.26) | 59 (65.33) | |
| Non-Hispanic black | 2997 (11.19) | 426 (15.96) | 745 (9.76) | 1801 (11.07) | 25 (10.06) | < .001 |
| Hispanic | 3,654 (13.63) | 586 (18.89) | 984 (14.58) | 2,039 (12.27) | 45 (18.18) | |
| Other race/ethnicity | 1,101 (6.45) | 253 (13.57) | 194 (4.00) | 643 (6.40) | 11 (6.44) | |
| Asthma, No. (%) | 1,127 (7.98) | 134 (7.27) | 319 (8.38) | 659 (7.88) | 15 (9.83) | .708 |
| Smoker status, No. (%) | ||||||
| Never | 3,154 (21.98) | 108 (6.07) | 1,538 (38.94) | 1,465 (16.75) | 43 (29.67) | |
| Current | 3,264 (24.49) | 164 (8.11) | 1,025 (29.09) | 2,023 (24.93) | 52 (35.20) | < .001 |
| Former | 7,295 (53.53) | 1,540 (85.82) | 1,130 (31.97) | 4,580 (58.32) | 45 (35.13) | |
| Cigarettes per day (current smokers), mean (SEM) | 13.30 (0.50) | 12.76 (1.45) | 13.80 (0.53) | 12.61 (0.60) | 20.92 (2.60) | < .001 |
| Education, No. (%) | ||||||
| 12 y | 3,685 (17.64) | 651 (26.59) | 1,155 (21.10) | 1,823 (14.36) | 56 (32.29) | |
| < 12 y | 3,137 (22.63) | 438 (27.30) | 931 (25.07) | 1,729 (20.61) | 39 (31.59) | < .001 |
| ≥ 12 y | 6,891 (59.74) | 723 (46.12) | 1,607 (53.84) | 4,516 (65.03) | 45 (36.12) | |
| Poverty income ratio < 1, No. (%) | 3,887 (19.74) | 656 (28.82) | 1,138 (21.99) | 2,040 (17.05) | 53 (30.68) | < .001 |
| Inhaled steroid use, No. (%) | 369 (2.59) | 36 (1.86) | 98 (2.35) | 224 (2.75) | 11 (6.60) | .055 |
| Oral corticosteroid use, No. (%) | 154 (0.97) | 27 (0.89) | 36 (0.86) | 86 (0.98) | 5 (4.41) | .492 |
| Hay fever, No. (%) | 2,141 (18.15) | 232 (14.58) | 558 (17.09) | 1,329 (19.22) | 22 (18.29) | .002 |
| URI in past 7 d, No. (%) | 2,485 (17.89) | 288 (18.00) | 777 (20.19) | 1,393 (16.78) | 27 (19.95) | .062 |
| Ate NO-rich vegetables in past 3 h, No. (%) | 506 (4.60) | 68 (5.44) | 116 (3.65) | 317 (4.94) | 5 (3.00) | .002 |
| Ate NO-rich meat in past 3 h, No. (%) | 415 (3.61) | 39 (3.19) | 138 (4.15) | 229 (3.42) | 9 (4.24) | .453 |
| Alcohol-related liver disease, No. (%) | 627 (4.54) | 0 (0.00) | 627 (16.27) | 0 (0.00) | 0 (0.00) | < .001 |
| Outcomes | ||||||
| CRP, mean (SEM), ng/mL; n = 9,122 | 0.38 (0.01) | 0.44 (0.04) | 0.38 (0.02) | 0.37 (0.01) | 0.42 (0.07) | .079 |
| Blood eosinophil level, mean (SEM), cells/mm3; n = 13,173 | 0.20 (0.00) | 0.19 (0.00) | 0.21 (0.00) | 0.20 (0.00) | 0.19 (0.01) | < .001 |
| Fractional excretion of exhaled NO (n = 12,059) | ||||||
| Feno, mean (SEM), ppb | 16.71 (0.27) | 17.72 (0.50) | 15.22 (0.40) | 17.29 (0.30) | 14.40 (1.00) | < .001 |
| ln(Feno), mean (SE), ppb | 2.58 (0.02) | 2.69 (0.03) | 2.45 (0.02) | 2.63 (0.02) | 2.46 (0.08) | < .001 |
| Geometric mean (SE), ppb | 13.20 (12.81-13.60) | 14.73 (13.87-15.49) | 11.59 (11.13-12.06) | 13.87 (13.33-14.30) | 11.70 (9.97-13.74) | < .001 |
| Feno, median (SE), ppb | 12.90 (0.33) | 14.60 (0.58) | 11.32 (0.38) | 13.49 (0.36) | 11.61 (1.57) | < .001 |
| Feno, interquartile range, ppb | 8.42-19.70 | 9.47-21.20 | 7.11-17.82 | 8.88-20.11 | 7.27-20.20 | |
CRP = C-reactive protein; Feno = fractional excretion of exhaled nitric oxide; NHANES = National Health and Nutrition Examination Survey; NO = nitric oxide; ppb = parts per billion; URI = upper respiratory illness.
Age group 1: women, 21 to 45 years and men, 21 to 59 years; age group 2: women, 46 to 79 years and men, 60 to 79 years.
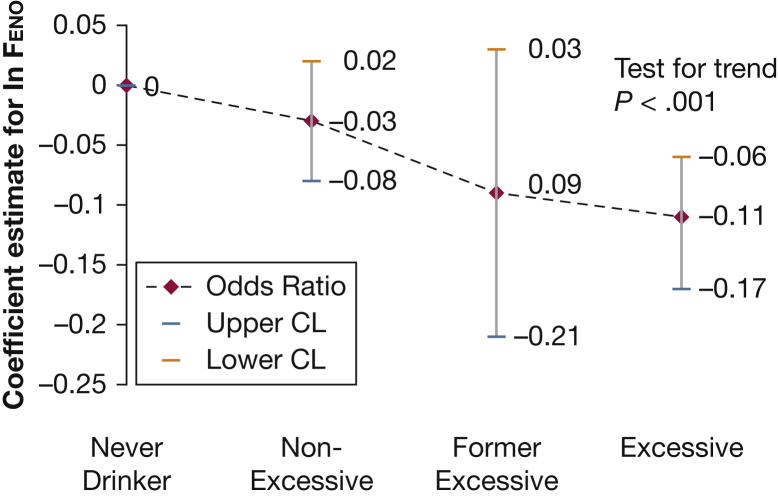
In univariate linear regression, the coefficients for all the alcohol groups were negatively associated, in a dose-dependent manner, with ln(Feno) levels in comparison with the never-drinker group (Table 2). In multivariable analysis adjusting for asthma, inhaled steroid use, cigarettes per day, diet, hay fever, alcohol-related liver disease, and baseline demographics, the coefficient for excessive alcohol consumption continued to have a negative association and remained an independent predictor for ln(Feno) levels (Table 3). ln(Feno) levels also decreased with increasing numbers of cigarettes smoked per day, female sex, and education > 12 years. On the other hand, Feno levels increased with higher age group, increasing height, Hispanic race, asthma, hay fever, and recent consumption of nitrogen-rich vegetables. The association of ln(Feno) with the alcohol groups had a decreasing linear trend (P < .001), with the highest levels for nonexcessive alcohol consumers and the lowest levels for excessive alcohol consumers (Fig 2). Back transformation of the natural log for the alcohol groups from multiple linear regression demonstrates changes of 0.42, 1.18, and 1.47 ppb in comparison with the never-drinker group for nonexcessive, former excessive, and excessive drinker groups, respectively. These represent 3.00%, 8.48%, and 10.59% changes, respectively. In the multivariable regression model, no interaction was demonstrated between alcohol categories and age (P = 0.100), alcohol use and self-reported asthma (P = 0.284), and alcohol categories and average cigarettes smoked per day (P = 0.549). No significant associations were likewise found between the excessive alcohol group and peripheral blood eosinophil count or CRP level in multivariable linear regression. Furthermore, neither blood eosinophil count (P = 0.692) nor CRP level (P = 0.330) had a linear trend by alcohol group. A significant but weak correlation was found between ln(Feno) levels and peripheral blood eosinophil counts (Pearson’s coefficient, 0.21; P < .001) but not with CRP level (Pearson’s coefficient, −0.02; P = 0.16). Stratified analysis based on participants with asthma did not show any association between excessive alcohol consumption and Feno levels (e-Table 2), with no linear trend identified (P = 0.461).
Table 2.
Coefficients From Univariate Linear Regression for Primary Outcome of ln(Feno) Levels and Secondary Outcomes of Blood Eosinophil and C-Reactive Protein Levels
| Variable | ln(Feno) |
Blood Eosinophil Level |
CRP Levela |
|||
|---|---|---|---|---|---|---|
| Regression Coefficient (95% CI) (n = 12,059) |
P Value | Regression Coefficient (95% CI) (n = 11,795) |
P Value | Regression Coefficient (95% CI) (n = 8,115) | P Value | |
| Age group 1b | Referent | Referent | Referent | |||
| Age group 2b | 0.12 (0.09-0.14) | < .001 | 0.00 (−0.01 to 0.01) | .758 | 0.09 (0.05-0.13) | < .001 |
| Height (per 10 cm of height) | 0.05 (0.03-0.07) | < .001 | 0.00 (0.00-0.01) | .169 | −0.05 (−0.07 to −0.02) | < .001 |
| BMI | ||||||
| Normal | Referent | Referent | Referent | |||
| Underweight | −0.15 (−0.29 to −0.02) | .027 | 0.01 (−0.03 to 0.05) | .488 | −0.07 (−0.16 to 0.01) | .089 |
| Overweight | 0.09 (0.05-0.13) | < .001 | 0.02 (0.01-0.03) | < .001 | 0.06 (0.02-0.10) | .009 |
| Obese | ||||||
| Class I | 0.10 (0.05-0.14) | < .001 | 0.02 (0.01-0.03) | < .001 | 0.19 (0.14-0.24) | < .001 |
| Class II | 0.07 (0.00-0.13) | 0.041 | 0.04 (0.03-0.05) | < .001 | 0.42 (0.34-0.50) | < .001 |
| Class III | 0.08 (0.00-0.16) | 0.052 | 0.05 (0.04-0.07) | < .001 | 0.71 (0.60-0.82) | < .001 |
| Sex | ||||||
| Male | Referent | Referent | Referent | |||
| Female | −0.13 (−0.16 to −0.10) | < .001 | −0.03 (−0.03 to −0.02) | < .001 | 0.11 (0.07-0.14) | < .001 |
| Race | ||||||
| Non-Hispanic white | Referent | Referent | Referent | |||
| Non-Hispanic black | −0.03 (−0.08 to 0.02) | .229 | −0.02 (−0.03 to −0.01) | < .001 | 0.14 (0.09-0.20) | < .001 |
| Hispanic | 0.09 (0.04-0.13) | .001 | 0.01 (0.00-0.02) | .047 | 0.03 (−0.03 to 0.08) | .331 |
| Other race/ethnicity | 0.14 (0.07-0.20) | < .001 | 0.01 (0.00-0.03) | .055 | −0.13 (−0.20 to −0.06) | < .001 |
| Asthma | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.16 (0.10-0.22) | < .001 | 0.05 (0.04-0.06) | < .001 | 0.14 (0.09-0.20) | < .001 |
| Average cigarettes/d | ||||||
| 0 | Referent | Referent | Referent | |||
| 1-10 | −0.27 (−0.41 to −0.12) | < .001 | 0.02 (−.03 to 0.06) | < .001 | 0.05 (−0.08, 0.18) | .411 |
| 11-20 | −0.47 (−0.62 to −0.32) | < .001 | 0.01 (0.00-0.02) | .012 | 0.06 (−0.08, 0.20) | .389 |
| > 20 | −0.57 (−0.73 to −0.40) | < .001 | 0.00 (−0.02 to 0.02) | .786 | 0.13 (−0.06, 0.32) | .183 |
| Education | ||||||
| 12 y | Referent | Referent | Referent | |||
| ≤ 12 y | −0.06 (−0.10 to −0.01) | .009 | 0.01 (0.01-0.02) | .002 | 0.01 (−0.05 to 0.08) | .647 |
| > 12 y | 0.13 (0.09-0.18) | < .001 | −0.01 (−0.02 to 0.00) | .009 | −0.08 (−0.04 to −0.03) | .002 |
| Poverty income ratio < 1 | ||||||
| No | Referent | Referent | Referent | |||
| Yes | −0.13 (−0.18 to −0.09) | < .001 | 0.01 (0.01-0.02) | .002 | 0.01 (0.01-0.02) | .002 |
| Inhaled steroid use | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.21 (0.11-0.30) | < .001 | 0.09 (0.05-0.12) | < .001 | 0.19 (0.08-0.30) | .001 |
| Ate NO-rich vegetables in past 3 h | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.17 (0.09-0.24) | < .001 | −0.10 (−0.03 to 0.00) | .059 | −0.10 (−0.17 to −0.03) | .005 |
| Hay fever | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.18 (0.14-0.23) | < .001 | 0.03 (0.02-0.04) | < .001 | 0.03 (−0.03 to 0.08) | .340 |
| Alcohol-related liver disease | ||||||
| No | Referent | Referent | Referent | |||
| Yes | −0.08 (−0.16 to −0.01) | .036 | 0.02 (0.01-0.04) | .003 | 0.10 (−0.06 to 0.26) | .201 |
| Alcohol category | ||||||
| Never drinker | Referent | Referent | Referent | |||
| Nonexcessive | −0.06 (−0.12 to −0.01) | .028 | 0.01 (0.00-0.02) | .012 | −0.07 (−0.14 to 0.01) | .079 |
| Excessive | −0.24 (−0.29 to −0.19) | < .001 | 0.02 (0.01-0.03) | < .001 | −0.06 (−0.14 to 0.03) | .171 |
| Former excessive | −0.22 (−0.39 to −0.06) | .008 | 0.00 (−0.02 to 0.02) | .786 | −0.02 (−0.15 to 0.11) | .760 |
Coefficients are expressed as change in ln(Feno) per 1-unit change in the independent variable. Blood eosinophil level is expressed as cells/mm3; CRP level is expressed as ng/mL. See Table 1 legend for expansion of abbreviations.
CRP data available from 2007 to 2010.
Age group 1: women, 21 to 45 years and men, 21 to 59 years; age group 2: women, 46 to 79 years and men, 60 to 79 years.
Table 3.
Coefficients From Multivariable Linear Regression for Primary Outcome of Feno Levels and Secondary Outcomes of Blood Eosinophil and C-Reactive Protein Levels
| Variable | ln(Feno) |
Blood Eosinophil Level |
CRP Levela |
|||
|---|---|---|---|---|---|---|
| Regression Coefficient (95% CI) (n = 12,059) |
P Value | Regression Coefficient (95% CI) (n = 11,795) |
P Value | Regression Coefficient (95% CI) (n = 8,115) |
P Value | |
| Age group 1b | Referent | Referent | Referent | |||
| Age group 2b | 0.15 (0.11-0.18) | < .001 | 0.01 (0.00-0.02) | .032 | 0.04 (0.01-0.08) | .013 |
| Height (per 10 cm of height) | 0.05 (0.03-0.07) | < .001 | −0.01 (−0.01 to 0.00) | .002 | 0.00 (−0.03 to 0.03) | .801 |
| BMI | ||||||
| Normal | Referent | Referent | Referent | |||
| Underweight | −0.01 (−0.15 to 0.13) | .917 | 0.01 (−0.03 to 0.05) | .645 | −0.14 (−0.20 to −0.08) | < .001 |
| Overweight | 0.02 (−0.02 to 0.05) | .264 | 0.02 (0.01-0.03) | < .001 | 0.07 (0.02-0.12) | .006 |
| Obese | ||||||
| Class I | 0.02 (−0.03 to 0.06) | .409 | 0.02 (0.01-0.03) | < .001 | 0.19 (0.14-0.24) | < .001 |
| Class II | 0.00 (−0.06 to 0.05) | .847 | 0.03 (0.02-0.05) | < .001 | 0.40 (0.31-0.50) | < .001 |
| Class III | 0.00 (−0.08 to 0.09) | .902 | 0.06 (0.04-0.07) | < .001 | 0.68 (0.55-0.80) | < .001 |
| Sex | ||||||
| Male | Referent | Referent | Referent | |||
| Female | −0.17 (−0.21 to −0.13) | < .001 | −0.04 (−0.05 to −0.03) | < .001 | 0.08 (0.04-0.12) | < .001 |
| Race | ||||||
| Non-Hispanic white | Referent | Referent | Referent | |||
| Non-Hispanic black | 0.01 (−0.03 to 0.06) | .573 | −0.02 (−0.03 to −0.01) | < .001 | 0.05 (0.01-0.10) | .018 |
| Hispanic | 0.14 (0.10-0.18) | < .001 | 0.01 (−0.01 to 0.02) | .372 | 0.03 (−0.02 to 0.08) | .224 |
| Other race/ethnicity | 0.14 (0.08-0.19) | < .001 | 0.02 (0.01-0.04) | .006 | −0.05 (−0.12 to 0.01) | .083 |
| Asthma | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.19 (0.13-0.26) | < .001 | 0.03 (0.02-0.04) | < .001 | 0.00 (−0.07 to 0.07) | .933 |
| Average cigarettes/d | ||||||
| 0 | Referent | Referent | Referent | |||
| 1-10 | −0.46 (−0.51 to −0.41) | < .001 | 0.03 (0.02-0.05) | < .001 | 0.09 (0.01-0.18) | .034 |
| 11-20 | −0.66 (−0.74 to −0.57) | < .001 | 0.04 (0.02-0.05) | .003 | 0.12 (0.04-0.21) | .006 |
| > 20 | −0.78 (−0.87 to −0.68) | < .001 | 0.04 (0.01-0.06) | .002 | 0.24 (0.06-0.42) | .008 |
| Education | ||||||
| 12 y | Referent | Referent | Referent | |||
| ≤ 12 y | −0.02 (−0.06 to 0.01) | .238 | 0.01 (0.00-0.03) | .013 | −0.01 (−0.07 to 0.04) | .665 |
| > 12 y | 0.04 (0.01-0.08) | .018 | 0.00 (−0.01 to 0.01) | .901 | −0.03 (−0.08 to 0.03) | .313 |
| Poverty income ratio < 1 | ||||||
| No | Referent | Referent | Referent | |||
| Yes | −0.03 (−0.07 to 0.01) | .114 | 0.01 (0.00-0.02) | .099 | 0.02 (−0.02 to 0.06) | .290 |
| Inhaled steroid use | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.02 (−0.09 to 0.13) | .673 | 0.05 (0.02-0.09) | .006 | 0.10 (−0.05 to 0.25) | .178 |
| Ate NO-rich vegetables in past 3 h | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.10 (0.03-0.18) | < .001 | −0.01 (−0.03 to 0.00) | .155 | −0.06 (−0.13 to 0.00) | .063 |
| Hay fever | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.15 (0.11-0.18) | < .001 | 0.02 (0.01-0.03) | < .001 | 0.02 (−0.02 to 0.07) | .298 |
| Alcohol-related liver disease | ||||||
| No | Referent | Referent | Referent | |||
| Yes | −0.02 (−0.10 to 0.06) | .625 | 0.00 (−0.01 to 0.01) | .929 | 0.05 (−0.13 to 0.22) | .610 |
| Alcohol category | ||||||
| Never drinker | Referent | Referent | Referent | |||
| Nonexcessive | −0.03 (−0.08 to 0.02) | .264 | 0.01 (0.00-0.02) | .019 | −0.06 (−0.13 to 0.02) | .136 |
| Excessive | −0.11 (−0.17 to −0.06) | < .001 | 0.01 (0.00-0.02) | .281 | −0.05 (−0.13 to 0.04) | .267 |
| Former excessive | −0.09 (−0.21 to 0.03) | .141 | −0.01 (−0.04 to 0.01) | .260 | −0.02 (−0.12 to 0.07) | .605 |
Coefficients are expressed as change in ln(Feno) per 1-unit change in the independent variable. Blood eosinophil level is expressed as cells/mm3; CRP level is expressed as ng/mL. See Table 1 legend for expansion of abbreviations.
Data available from 2007 to 2010.
Age group 1: women, 21 to 45 years and men, 21 to 59 years; age group 2: women, 46 to 79 years and men, 60 to 79 years.
Figure 2.
Trend for ln(Feno) by alcohol group. Points represent adjusted multivariable analysis and error bars represent 95% CIs. Adjusted for age, height, body mass index, sex, race, asthma, hay fever, smoking, education, poverty income ratio, inhaled steroid use, alcohol-related liver disease, and diet. Test for trend P < .001 for each alcohol group in comparison with the never-drinker reference group (OR, 1.0). CL = confidence limit. See Figure 1 legend for expansion of other abbreviation.
Discussion
In a representative sample of the US population, Feno levels are linearly associated with level of alcohol consumption, with excessive alcohol consumers having the lowest levels in reference to never drinkers. More than one-quarter of the US population can be described as excessive alcohol consumers, and they have an inverse relationship with Feno levels when compared with never drinkers even after adjusting for asthma, atopy, demographics, steroid use, diet, cigarette use, socioeconomic status, education, and other confounders. These changes are not demonstrated in the blood eosinophil or CRP level, indicating a role for excessive alcohol consumption in airway inflammation and biology.
Excessive alcohol consumption accounts for one in 10 deaths among working-age adults and is the third leading preventable cause of death in the United States.25, 26 We show that more than one-quarter of the US population qualifies as excessive alcohol consumers, which is similar to another report from a nationally representative sample of US residents.27 Binge drinking is the most common form of excessive alcohol consumption and is responsible for more than one-half of alcohol-associated deaths and three-quarters of an estimated $223.5 billion dollars in economic costs.19 We found that nearly three-quarters of US residents with excessive alcohol consumption are binge alcohol consumers. Much of the biological effects of unhealthy alcohol consumption have been investigated in chronic alcohol consumers (ie, alcohol use disorder), but < 5% of excessive alcohol consumers are characterized as having an alcohol use disorder.27
Alcoholic lung disease and alcohol impairment of airway host defenses occur in individuals with an alcohol use disorder. Airway ciliary dysfunction, glutathione depletion, epithelial barrier dysfunction, cilia desensitization, and a proinflammatory state represent well-described pathologic consequences resulting from chronic alcohol use, which increase the risk for pneumonia and acute respiratory distress syndrome.10, 28, 29, 30, 31 However, despite the fact that excessive alcohol consumers constitute a large proportion of the US population, the clinical significance of the depressed Feno levels found in this population is unknown and requires future investigations. Studies from NHANES to identify reference standards and thresholds for Feno in healthy adults have been reported.32, 33 The results from these NHANES studies and the American Thoracic Society clinical practice guideline identify cutoff points between 39 and 50 ppb as clinically relevant,3 but no data are available regarding low Feno levels in healthy subjects. In a small cohort study in which patients with normal lung function and upper respiratory symptoms were monitored for 2 years, nearly one-half were subsequently diagnosed with asthma,34 and Feno levels above 7 ppb demonstrated an area under the receiver-operating characteristic curve of 0.896 with 82.5% sensitivity and 88.9% specificity, respectively, for an asthma diagnosis. Measurement of Feno levels have been examined mainly in the diagnosis and management of patients with asthma; however, we have shown that no meaningful differences of excessive alcohol consumers were found in Feno levels when examining asthma participants only. Our finding of a 21.3% reduction in the geometric mean between never drinkers and excessive alcohol consumers and a 10.59% reduction from the multivariable model among all participants may represent clinical relevance. Although the back transformation from the natural log shows a small change in parts per billion from the multivariable model, this interpretation is limited because the calculation is negatively biased.35 More importantly, the decreasing dose-dependent linear trend shown in our results should be investigated further in individuals with recent excessive alcohol consumption or detectable blood alcohol concentrations, in whom the effect on Feno levels may be stronger.
We did not find a role for alcohol in individuals with asthma: no interaction between asthma and alcohol consumption, or between Feno level and alcohol consumption, was demonstrated. This supports the notion that alcohol consumption does not contribute significantly to asthma activity as measured by Feno. However, our data are limited to self-reported asthma and likely constitute a majority of healthy individuals without symptoms at the time of testing. Historically, alcoholic beverages have been used to treat asthma symptoms and have been demonstrated in experiments to have beneficial effects via bronchodilation and an increase in vital capacity.11, 36, 37 However, adverse asthma reactions have been described in groups with abnormal acetaldehyde accumulation or sensitivities to nonalcohol components of alcoholic beverages (such as brewer’s yeast or sulfites), subsequent to alcohol consumption.13, 14, 15 More recently, survey studies have identified wine as the most common alcohol trigger for asthma symptoms; however, the underlying mechanism is not well understood.38, 39 Additional studies are needed to evaluate Feno levels in individuals with symptomatic or more severe asthma to fully understand the magnitude and clinical relevance of alcohol consumption in asthma control and symptoms.
Nitric oxide is produced by the human lung and is a biological mediator of airway inflammation with abnormal levels in multiple lung diseases, most notably asthma.40 In the airway, nitric oxide also functions as a vasodilator and bronchodilator41 and as a ciliary beat frequency stimulator.42 It is also a free radical that provides cytotoxic effects important in the antimicrobial function of the airway.43 Research on the association of short-term alcohol consumption with Feno levels in humans is limited to a small study in which only participants with asthma had a decrease in Feno levels and healthy participants had no appreciable change.18 On the other hand, results from our US population sample show that excessive alcohol consumption is an independent predictor of lower Feno levels, with a dose-dependent association. In comparison, CRP and blood eosinophil levels (markers of systemic and allergic inflammation, respectively) did not demonstrate a significant association. Whereas peripheral blood eosinophils have been shown to be a predictor for Feno levels in people with asthma,3, 44 our findings show a weak correlation with Feno in the general US population and no association with excessive alcohol consumers in adjusted analysis. Collectively, we interpret these observations to suggest a previously unrecognized role for excessive alcohol consumption in modulating nitric oxide pathways specific to the airway.
The mechanism by which alcohol might reduce Feno levels is not known, although there are intriguing possibilities based on laboratory and small-animal studies. Airway epithelial cell culture studies have shown that brief alcohol exposure increases nitric oxide-dependent airway ciliary beat frequency by activating the endothelial isoform of nitric oxide synthase (eNOS or NOS3).31 In contrast, prolonged alcohol ingestion by mice resulted in reduced lung nitric oxide levels as determined in bronchoalveolar lavage fluid and was associated with low l-arginine blood levels, suggesting lung nitric oxide depletion.45 Last, the role of eNOS in the production of exhaled nitric oxide in airways is probably minor compared with that produced by the inducible form of NOS (iNOS or NOS2), which is thought to be the primary source of Feno in airways diseases.46
The major strength of our study is the availability of data from NHANES, which is a nationally representative and comprehensive survey drawing from a large and diverse sample of participants. The survey design avoids volunteer bias and enhances the generalizability of our results. However, several limitations apply to this cross-sectional survey. Our missing data analysis indicated that missing data did not occur at random and that bias may have occurred in Feno testing and questionnaire data capture. Changes in Feno over time could not be captured to better assess the relationship to alcohol consumption patterns. Alcohol consumption was self-reported, and a misclassification bias of the alcohol groups may have occurred. In addition, environmental exposures, second-hand smoke exposure, and diurnal variability may have confounded Feno results. Similar to prior studies, we show that Feno levels are decreased in smokers and increased in subjects with atopy and asthma, supporting the robustness of our findings.32, 47 The application of our results to populations outside the United States is less reliable because of differences in legal drinking age and definition of excessive alcohol consumption. Many European nations have lower drinking ages and, therefore, include a younger population and also classify higher drinking thresholds for unhealthy alcohol consumption.48
In conclusion, we demonstrate a significant negative association of excessive alcohol consumption with Feno levels that was independent of other well-described factors that influence Feno. We propose that interpretation of Feno levels should account for alcohol use. Our observation underscores the role of excessive alcohol use in impacting biological responses in the lung, and moreover, highlights the importance of alcohol in mediating the response of nitric oxide pathways in the lung. Further investigations are warranted to explore the complex interaction between alcohol and nitric oxide in the airways in order to identify populations at risk for airways disease and as an aid in the search for therapeutic interventions.
Acknowledgments
Author contributions: M. A. is the guarantor of the manuscript. Concept and design: M. A., J. A. P., R. C. C., E. J. K., and J. H. S.; analysis and interpretation of data: M. A., J. A. P., G. C., R. D., R. C. C., J. H. S.; drafting of the article: M. A., J. A. P., R. D., R. C. C., E. J. K., and J. H. S.; final approval of the article: M. A., J. A. P., G. C., R. D., R. C. C., E. J. K., and J. H. S.; administrative, technical, or logistic support: M. A., J. A. P., G. C., R. D., R. C. C., and J. H. S.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This work was performed at the Loyola University Public Health Sciences Department.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Dr Kovacs is currently at the Department of Surgery, University of Colorado (Aurora, CO).
FUNDING/SUPPORT: This research was supported in part by National Institutes of Health [grants R01 AA008769-23 (J. H. S.) and R01 GM115257-14 (E. J. K.)], and by the Loyola Research Funding Committee (M. A.).
Supplementary Data
References
- 1.Dweik R.A., Sorkness R.L., Wenzel S. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A.D., Cowan J.O., Brassett K.P., Herbison G.P., Taylor D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. NEJM. 2005;352(21):2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 3.Dweik R.A., Boggs P.B., Erzurum S.C. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (Feno) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes P.J., Dweik R.A., Gelb A.F. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138(3):682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 5.Boon M., Meyts I., Proesmans M., Vermeulen F.L., Jorissen M., De Boeck K. Diagnostic accuracy of nitric oxide measurements to detect primary ciliary dyskinesia. Eur J Clin Invest. 2014;44(5):477–485. doi: 10.1111/eci.12254. [DOI] [PubMed] [Google Scholar]
- 6.Michl R.K., Hentschel J., Fischer C., Beck J.F., Mainz J.G. Reduced nasal nitric oxide production in cystic fibrosis patients with elevated systemic inflammation markers. PLoS One. 2013;8(11):e79141. doi: 10.1371/journal.pone.0079141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker W.T., Liew A., Harris A., Cole J., Lucas J.S. Upper and lower airway nitric oxide levels in primary ciliary dyskinesia, cystic fibrosis and asthma. Respir Med. 2013;107(3):380–386. doi: 10.1016/j.rmed.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Guo F.H., De Raeve H.R., Rice T.W., Stuehr D.J., Thunnissen F.B., Erzurum S.C. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92(17):7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George S.C. How accurately should we estimate the anatomical source of exhaled nitric oxide? J Appl Physiol (1985) 2008;104(4):909–911. doi: 10.1152/japplphysiol.00111.2008. [DOI] [PubMed] [Google Scholar]
- 10.Sisson J.H. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayres J.G., Clark T.J. Intravenous ethanol can provide bronchodilatation in asthma. Clin Sci. 1983;64(5):555–557. doi: 10.1042/cs0640555. [DOI] [PubMed] [Google Scholar]
- 12.Oldenburg P.J., Poole J.A., Sisson J.H. Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302(3):L308–L315. doi: 10.1152/ajplung.00077.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vally H., Thompson P.J. Allergic and asthmatic reactions to alcoholic drinks. Addict Biol. 2003;8(1):3–11. doi: 10.1080/1355621031000069828. [DOI] [PubMed] [Google Scholar]
- 14.Payne S.C. Re: alcohol-induced respiratory symptoms are common in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2014;2(5):644. doi: 10.1016/j.jaip.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Mittag D., Niggemann B., Sander I. Immunoglobulin E-reactivity of wheat-allergic subjects (baker’s asthma, food allergy, wheat-dependent, exercise-induced anaphylaxis) to wheat protein fractions with different solubility and digestibility. Mol Nutr Food Res. 2004;48(5):380–389. doi: 10.1002/mnfr.200400016. [DOI] [PubMed] [Google Scholar]
- 16.Sisson J.H., Stoner J.A., Romberger D.J. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36(1):19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Persson M.G., Gustafsson L.E. Ethanol can inhibit nitric oxide production. Eur J Pharmacol. 1992;224(1):99–100. doi: 10.1016/0014-2999(92)94826-h. [DOI] [PubMed] [Google Scholar]
- 18.Yates D.H., Kharitonov S.A., Robbins R.A., Thomas P.S., Barnes P.J. The effect of alcohol ingestion on exhaled nitric oxide. Eur Respir J. 1996;9(6):1130–1133. doi: 10.1183/09031936.96.09061130. [DOI] [PubMed] [Google Scholar]
- 19.Bouchery E.E., Harwood H.J., Sacks J.J., Simon C.J., Brewer R.D. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 20.Trimble G., Zheng L., Mishra A., Kalwaney S., Mir H.M., Younossi Z.M. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther. 2013;38(6):596–602. doi: 10.1111/apt.12432. [DOI] [PubMed] [Google Scholar]
- 21.Jacinto T., Malinovschi A., Janson C., Fonseca J., Alving K. Evolution of exhaled nitric oxide levels throughout development and aging of healthy humans. J Breath Res. 2015;9(3):036005. doi: 10.1088/1752-7155/9/3/036005. [DOI] [PubMed] [Google Scholar]
- 22.Hoppin J.A., Jaramillo R., Salo P., Sandler D.P., London S.J., Zeldin D.C. Questionnaire predictors of atopy in a US population sample: findings from the National Health and Nutrition Examination Survey, 2005-2006. Am J Epidemiol. 2011;173(5):544–552. doi: 10.1093/aje/kwq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finucane M.M., Stevens G.A., Cowan M.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korn E.L., Graubard B.I. Simultaneous testing of regression coefficients with complex survey data: use of Bonferroni t statistics. Am Stat. 1990;44(4):270–276. [Google Scholar]
- 25.Stahre M., Roeber J., Kanny D., Brewer R.D., Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.L. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 27.Esser M.B., Hedden S.L., Kanny D., Brewer R.D., Gfroerer J.C., Naimi T.S. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:E206. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss M., Guidot D.M., Wong-Lambertina M., Ten Hoor T., Perez R.L., Brown L.A. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161(2 Pt 1):414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 29.Guidot D.M. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest. 2002;122(90060):309S–314S. doi: 10.1378/chest.122.6_suppl.309s. [DOI] [PubMed] [Google Scholar]
- 30.Guidot D.M., Hart C.M. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investig Med. 2005;53(5):235–245. doi: 10.2310/6650.2005.53506. [DOI] [PubMed] [Google Scholar]
- 31.Sisson J.H., Pavlik J.A., Wyatt T.A. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res. 2009;33(4):610–616. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brody D.J., Zhang X., Kit B.K., Dillon C.F. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir Med. 2013;107(11):1682–1691. doi: 10.1016/j.rmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 33.See K.C., Christiani D.C. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: results from the National Health and Nutrition Examination Survey 2007-2010. Chest. 2013;143(1):107–116. doi: 10.1378/chest.12-0416. [DOI] [PubMed] [Google Scholar]
- 34.Berkman N., Avital A., Breuer R., Bardach E., Springer C., Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60(5):383–388. doi: 10.1136/thx.2004.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning W.G. The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ. 1998;17(3):283–295. doi: 10.1016/s0167-6296(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 36.Vally H., Thompson P.J. Alcohol drinks and asthma. Clin Exp Allergy. 2002;32(2):186–191. doi: 10.1046/j.1365-2222.2002.01303.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown E. The use of intravenous ethyl aclohol in the treatment of status asthmaticus. Ann Allergy. 1947;5(3):193–195. [PubMed] [Google Scholar]
- 38.Vally H., de Klerk N., Thompson P. Ashma induced by alcoholic drinks: a new food allergy questionnaire (FAQ) Aust NZ J Public Health. 1999;23(6):26–30. doi: 10.1111/j.1467-842x.1999.tb01542.x. [DOI] [PubMed] [Google Scholar]
- 39.Vally H., de Klerk N., Thompson P. Alcoholic drinks: important triggers for asthma. J Allergy Clin Immunol. 2000;105(3):462–467. doi: 10.1067/mai.2000.104548. [DOI] [PubMed] [Google Scholar]
- 40.Dweik R.A., Comhair S.A., Gaston B. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98(5):2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 42.Jain B., Rubinstein I., Robbins R.A., Leise K.L., Sisson J.H. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191(1):83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- 43.Nathan C.F., Hibbs J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 44.Batra J., Pratap S.T., Mabalirajan U., Sinha A., Prasad R., Ghosh B. Association of inducible nitric oxide synthase with asthma severity, total serum immunoglobulin E and blood eosinophil levels. Thorax. 2007;62(1):16–22. doi: 10.1136/thx.2006.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simet S.M., Pavlik J.A., Sisson J.H. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol. 2013;47(8):629–635. doi: 10.1016/j.alcohol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brindicci C., Ito K., Barnes P.J., Kharitonov S.A. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007;132(2):581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- 47.Jouaville L.F., Annesi-Maesano I., Nguyen L.T., Bocage A.S., Bedu M., Caillaud D. Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy. 2003;33(11):1506–1511. doi: 10.1046/j.1365-2222.2003.01800.x. [DOI] [PubMed] [Google Scholar]
- 48.Smyth A., Teo K.K., Rangarajan S. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386(10007):1945–1954. doi: 10.1016/S0140-6736(15)00235-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.