ABSTRACT
WKYMVm hexapeptide has been identified as a strong FPR2 agonist through a library screening of synthetic peptides. The FPR2 has been reported to play a crucial role in inflammation and angiogenic responses via stimulation of chemotaxis, migration, cell proliferation, wound healing and vessel growth. Recently, the therapeutic effects of WKYMVm have been reported in various disease models. In cutaneous wound model in diabetic mice, WKYMVm facilitated wound healing processes by stimulating the formation of capillary and arteriole and re-epithelialization. In coronary artery stenosis model, WKYMVm coating on stent promoted re-endothelialization and lowered restenosis rate. In hindlimb ischemia mouse model, intramuscular injection of WKYMVm promoted homing of exogenously transplanted endothelial colony-forming cells and neovascularization, resulting in salvaging hindlimb. Furthermore, a single injection of WKYMVm encapsulated in poly (lactide-co-glycolide) microspheres was demonstrated to be as efficient as multiple injections of WKYMVm in restoring blood flow in hindlimb ischemia model. These observations may open up promising biomedical applications of WKYMVm for tissue repairs and regenerations.
KEYWORDS: anti-restenosis, angiogenesis, formyl peptide receptor-2, neovascularization, re-endothelialization, WKYMVm, wound healing
INTRODUCTION
Formyl peptide receptors (FPRs) belong to the transmembrane domain G-protein-coupled receptor (GPCR) family. Formyl peptide receptor 2 (FPR2) has been mainly identified to activate immune cells like phagocytes, lympho-cytes and play a crucial role in host defense and inflammation.1 The FPR2 has been known to stimulates chemotaxis, cell proliferation, wound healing, migration and vessel growth.2 Various FPR2 agonists stimulating different cell lines have been identified. Excepting lipoxin A4, resolvins and D-series resolvins, most of the FPR2 agonists are peptides, which include microbe-derived peptides, endogenous peptides such as mitochondrial peptides, amyloidogenic peptides, and inflammatory response related peptides.2 Through a screening of synthetic peptide library, a hexapeptide WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met) was identified as a strong agonist of FPR2 with weak affinity to FPR1 and FPR3 (Fig. 1).1 WKYMVm has been reported to activate immune cells, including neutrophils, monocytes, and NK cells.3-5 Recently, along with high affinity of WKYMVm to FPR2 receptor and its ability on modulating inflammatory cell activity, several studies identified that WKYMVm can induce angiogenesis of endothelial cells via FPR2 dependent stimulation of proliferation, migration, tube formation, and sprouting activity.6,7 In addition, several studies showed that WKYMVm improved tissue repair with enhanced neovascularization through promoting homing, proliferation, and tube formation of endothelial colony-forming cells (ECFCs).8-11 Furthermore, synthesis of WKYMVm peptides composed of few amino-acids can be easily and quickly performed and cost-effective. With these properties of WKYMVm, recent studies are using these small peptides in various biomedical application fields. The commentary highlights the biomedical application of WKYMVm in the repair of various tissues with different delivery methods.
Stimulation of cutaneous wound healing
Cutaneous wound healing is mediated by complex processes: inflammation, re-epithelialization, granulation tissue formation, neovascularization, wound contraction, and ECM reor-ganization.12 Various cells such as platelets, macrophages, leukocytes, fibroblasts, endothelial cells, and keratinocytes are involved during the inflammatory and tissue regeneration processes. Wound healing processes are also regulated by various molecules such as interferon, integrin, matrix metalloproteinase, cytokines and growth factors.13
Many drugs have been developed and investigated to facilitate repair of acute or chronic skin wounds and stimulate tissue regeneration. For instance, erythropoietin (EPO) has been identified that reduced tissue damage by modulating inflammatory cell activity, and induced neovascularization through induction of stem cells and endothelial progenitor cells recruitment.14 Furthermore, EPO has been identified to stimulate the re-epithelialization and ECM formation.14 Among FPR2 agonists, LL-37 was shown to expedite wound healing by accelerating re-epithelialization and granulation tissue formation in excisional wounds of ob/ob mice.15
Recently, Kwon et al. identified that the WKYMVm effectively stimulated the healing of a cutaneous wound in streptozotocin-induced diabetic mice.9 By topical treatment with a small amount of WKYMVm (20 μL of 1 μM in HBSS) on wound sites for 12 days, they found that WKYMVm accelerated re-epithelialization and angiogenesis in dermal tissues. Histological analysis showed WKYMV-m-treated group accelerated re-epithelialization at the early time points compared with the HBSS-treated control group. Furthermore, regeneration of hair follicles was promoted on day 12. Insufficient angiogenesis is known to cause chronic and non-healing wounds in diabetic models.16 WKYMVm treated group showed a higher number of endothelial cells and mature blood vessels in wound site when the wound dermis immuno-stained with vWF, an endothelial marker, and α-SMA, a mature blood vessels marker. Furthermore, they identified that the WKYMVm promoted immune cells infiltration into dermal tissues in an early stage of wound healing and ECM organization. Previous studies reported that immune cells were recruited via activation of FPR2, to which WKYMVm is a strong agonist.3,17 Cytokines from numerous inflammatory cells play a crucial role to facilitate epidermal regeneration, wound vascularization, fibroblast-mediated synthesis of extracellular matrix, and granulation tissue formation.12 Inflammatory cells have additional functions, including host defense and removal of apoptotic cells during wound repair process.18 Though exact target cell and downstream molecular mechanism of WKYMVm-mediated FPR2 in accelerating wound repair process remain unclear, treatment of WKYMVm peptides has shown the remarkable efficacy in several stages of wound healing process (Fig. 2).
Induction of re-endothelialization and anti-restenosis in stent implantation
Coronary artery stenosis, atherosclerosis, is characterized by the accumulation of cells, lipids, and connective-tissue elements within artery wall.19 Myocardial infarction occurs when the growth of atheroma disturbs blood flow through the coronary artery.20 In order to treat coronary artery stenosis, intracoronary stents have been developed to prevent occlusion and restenosis.21 However, stent implantation revealed limitations by early acute vessel closure due to stent thrombosis and late stent failure due to in-stent restenosis.22 These limitations led to the development of drug-eluting stents, which release anti-proliferative agents targeting smooth-muscle cells, such as sirolimus or paclitaxel, and have proven effective in lowering stent failure rate by reducing the risk of restenosis and repeat revascularization.23 The long-term safety of drug-eluting stents, however, has been questioned due to delayed arterial healing, incomplete endothelialization and vessel remodeling, which increases the risk of stent thrombosis beyond 1 y after stent implantation.23
Recently, a dual drug-coated stent was developed to simultaneously promote re-endothelialization and anti-restenosis.11 The stent was coated with WKYMVm to recruit circulating ECFCs and promote re-endothelialization. In addition, the stent was coated with sirolimus to inhibit restenosis by preventing the proliferation of smooth muscle cells. Hyaluronic acid and WKYMVm mixtures were coated onto the bare metal stent and sirolimus was consecutively applied to the peptide-coated stent. To identify treatment efficacy, WKYMVm- and sirolimus-coated stent, bare-metal stent and a commercial everolimus-eluting stent was implanted into rabbit iliac artery. At 6 week post implantation, rabbit iliac arteries were isolated and histological analysis was conducted. Histological analysis showed WKYMVm- and sirolimus-coated stent provided a consecutive linear staining of CD31-positve cells with low restenosis rate whereas bare-metal stent or everolimus-eluting stent showed incomplete CD31 staining. Through the in vivo study, they identified that the dual-drug coating stent prevented the restenosis and promotedd endothelial healing.
In addition, a bi-directional stent coatings with WKYMVm and sirolimus were developed and the therapeutic efficacy was investigated.24 The efficacy of drug-eluting stents was investigated by altering the drug position and comparing the single and simultaneous drug delivery. Bi-directional drug-coated stent showed en-hancement of human vein endothelial cells proliferation and inhibition of smooth muscle cell proliferation and movement when analyzed in the instrument mimicking the body’s circulation system. Therefore, the simultaneous and sustained release of WKYMVm and sirolimus had therapeutic efficacy in decreasing restenosis and improving the healing of endothelial cells covering stent for vascular wall healing. These results suggest a biomedical application of WKYMVm as a strong candidate in reducing long-term risks of stent implantation by promoting re-endothelialization.
Stimulation of ischemic neovascularization
Since the discovery of endothelial progenitor cells (EPCs) in 1997, EPCs have drawn attention as a good source to treat various ischemic diseases including critical limb ischemia.25,26 EPCs have been shown to contribute to neovascularization through direct incorporation into new vessels or indirect paracrine secretion to boost angiogenesis by preexisting endothelial cells.27,28 Identifying the effect of WKYMVm on enhancing the therapeutic efficacy of EPCs was pioneered by Heo et al in the study of exogenously transplanted ECFCs in mouse hindlimb ischemia model.8 ECFCs were derived from 7 day culture of human cord blood mononuclear cells and transplanted through tail vein in mouse hindlimb ischemia model. Intramuscular injection of WKYMVm at ischemic sites (20 µL of 10 µM in HBSS, three sites, three times per week for 28 days) promoted homing of transplanted ECFCs and significantly incre-ased restoration of blood flow and degree of limb salvage. Therapeutic augmentation of ECFCs by WKYMVm as well as up-regulation of migration and tube formation of ECFCs in vitro by WKYMVm were dependent on FPR2 expression in ECFCs. These findings opened up the biomedical application of WKYMVm in promoting tissue repair and regeneration.
Therapeutic activities of peptides are known to diminish with their short circulation time and rapid degradation when delivered by direct injection. Because of this limitation, repeated injection of peptides is necessary to achieve therapeutic efficacy. However, due to the poor patient compliance, repeated injection of peptides is not favored in clinical practice.29 Therefore, in our recent study, we have fabricated the injectable biodegradable microspheres encapsulating WKYMVm and evaluated the effect on neovascularization in vitro and therapeutic efficacy with a single injection in vivo.10 The aim of our study was to achieve the sustainable and controllable delivery of WKYMVm with a single injection of microspheres, which provides a comparable efficacy to that of multiple injections. Poly lactic-co-glycolic acid (PLGA) is a biocompatible and biodegradable polymer that exhibits a broad range of erosion times and tunable mechanical properties and extensively studied for protein and peptide carriers.30,31 We fabricated the PLGA microspheres to encapsulate WKYMVm peptides by W1/O/W2 double emulsion solvent evaporation method. To enhance the stability of peptides, peptides were solubilized in enzyme-free distilled water (W1 phase), and this drug solubilized W1 phase was encapsulated by PLGA polymer (O phase). Finally this emulsion solution was stabilized and hardened by a stabilizing agent PVA (W2 phase). We identified that the size distribution, surface areas, and stability of microspheres were suitable for injection from the syringe for drug release.
In mouse aorta ring ex vivo experiment to measure angiogenic sprout formation, PLGA microspheres encapsulating WKYMVm showed the sustained and efficient release of drugs and increased sprout formation comparable to free WKYMVm treatment in 3 day culture. When PLGA microspheres encapsulating WKYMVm were injected into hindlimb mouse ischemia model, a single injection of high dose microspheres was as efficient as multiple injections of free WKYMVm in restoring blood flow and salvaging limbs. Injection of free WKYMVm was carried out three times per week. 513.6 ng (60 μL × 10 µM) of WKYMVm was injected per injection, and a total of 6163.2 ng (12 × 60 μL × 10 µM) was injected over 4 weeks. Injection of PLGA microspheres encapsulating WKYMVm (6163.2 ng, 60 μL × 12 × 10 µM) was prosecuted at one time when the hind limb ischemia was induced. Both group significantly increased blood perfusion and tissue regeneration compared with the HBSS-injected control group or control PLGA microsphere-injected group. These results demonstrated biomedical efficacy and efficiency of PLGA microspheres encapsulating WKYMVm in treating ischemic disease by facilitating neovascularization at the injury site.
CONCLUSION
WKYMVm, a strong FPR2 agonist, have recently been proven to be effective in promoting recoveries from tissues damages in various disease models (Fig. 3). In cutaneous wound healing of diabetic mice, topical application of WKYMVm promoted re-epithelialization, angiogenesis, and infiltration of immune cells at the injury site. In a stent application, a dual coating of WKYMVm with sirolimus on stent surface decreased in-stent restenosis and increased re-endothelialization, which may reduce the long-term risk of a drug-eluting stent. In hindlimb ischemia model, WKYMVm injection at injury site enhanced homing of transplanted ECFCs and increased the blood flow. With PLGA microspheres encapsulating WKYMVm, a single injection of microspheres in hindlimb ischemia model was shown to be as efficient as multiple injections of free WKYMVm to restore blood flow and salvage limbs. These studies have demonstrated the therapeutic potential of WKYMVm in treating ischemic diseases, and safe delivery methods may expedite the clinical applications of WKYMVm hexapeptide.
FIGURE 1.
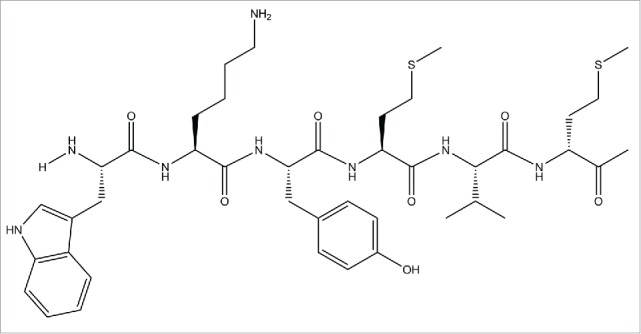
Chemical structure of synthetic WKYMVm hexapeptide. Peptide consists of six amino acid residues: tryptophan, lysine, tryrosine, methionine, valine, and D-form methionine. WKYMVm was selected from random hexapeptide sequence library as suitable agonist of FPR2.
FIGURE 2.
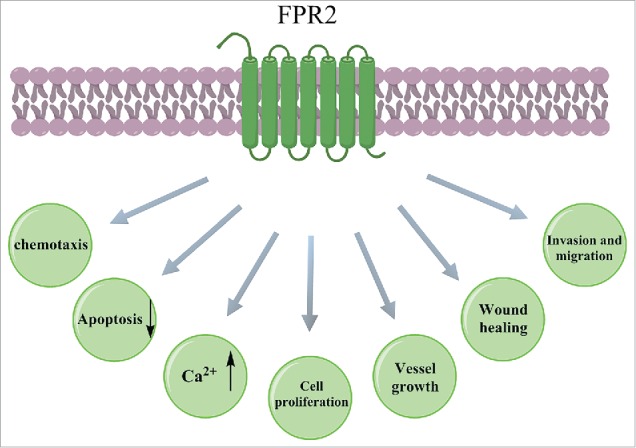
Cellular responses after activation of FRP2. FPR2 activation induces chemotaxis and Ca2+ mobilization and suppresses apoptosis of neutrophils, monocytes and T-lymphocytes. Endothelial progenitor cell proliferation and vessel growth are effectively facilitated by FPR2 activation. Invasion and migration of epithelial cells and wound healing are stimulated by FPR2 activation.
FIGURE 3.
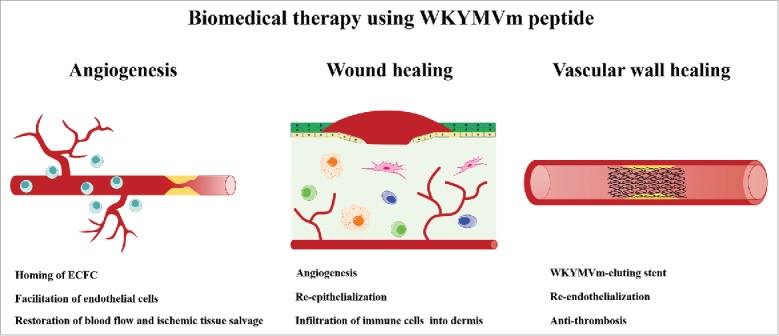
Biomedical therapy using synthetic WKYMVm hexapeptide. WKYMVm-induced FPR2 activation promotes homing of ECFCs (equivalent to endothelial progenitor cells) to the ischemic injury sites and angiogenesis to restore blood flow. Topical application of WKYMVm accelerates skin wound healing by promoting angiogenesis and re-epithelialization. WKYMVm-coated stent reduces restenosis and restores vascular function.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This research was supported by the programs of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012M3A9C7-050184 and NRF-2012M3A9C6050102). This research was also supported in part by the Ministry of Health and Welfare of Korea (HI13C1789).
REFERENCES
- [1].Baek SH, Seo JK, Chae CB, Suh PG, Ryu SH. Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries. J Biol Chem 1996; 271:8170-5; PMID:8626507; http://dx.doi.org/ 10.1074/jbc.271.14.8170 [DOI] [PubMed] [Google Scholar]
- [2].Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl Peptide receptor 2 (FPR2) agonists. Int J Mol Sci 2013; 14:7193-230; PMID:23549262; http://dx.doi.org/ 10.3390/ijms14047193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J biol Chem 2001; 276:21585-93; PMID:11285256; http://dx.doi.org/ 10.1074/jbc.M007769200 [DOI] [PubMed] [Google Scholar]
- [4].Kang HK, Lee HY, Kim MK, Park KS, Park YM, Kwak JY, Bae YS. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. J Immunol 2005; 175:685-92; http://dx.doi.org/ 10.4049/jimmunol.175.2.685 [DOI] [PubMed] [Google Scholar]
- [5].Kim SD, Kim JM, Jo SH, Lee HY, Lee SY, Shim JW, Seo SK, Yun J, Bae YS. Functional expression of formyl peptide receptor family in human NK cells. J Immunol 2009; 183:5511-7; http://dx.doi.org/ 10.4049/jimmunol.0802986 [DOI] [PubMed] [Google Scholar]
- [6].Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M, Lebherz C, et al.. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 2003; 111:1665-72; PMID:12782669; http://dx.doi.org/ 10.1172/JCI17545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee MS, Yoo SA, Cho CS, Suh PG, Kim WU, Ryu SH. Serum amyloid A binding to formyl peptide receptor-like 1 induces synovial hyperplasia and angiogenesis. J Immunol 2006; 177:5585-94; http://dx.doi.org/ 10.4049/jimmunol.177.8.5585 [DOI] [PubMed] [Google Scholar]
- [8].Heo SC, Kwon YW, Jang IH, Jeong GO, Yoon JW, Kim CD, Kwon SM, Bae YS, Kim JH. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells 2014; 32:779-90; PMID:24155208; http://dx.doi.org/ 10.1002/stem.1578 [DOI] [PubMed] [Google Scholar]
- [9].Kwon YW, Heo SC, Jang IH, Jeong GO, Yoon JW, Mun JH, Kim JH. Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen 2015; 23:575-82; PMID:25973651; http://dx.doi.org/ 10.1111/wrr.12-315 [DOI] [PubMed] [Google Scholar]
- [10].Choi YH, Heo SC, Kwon YW, Kim HD, Kim SH, Jang IH, Kim JH, Hwang NS. Injectable PLGA microspheres encapsulating WKYMVM peptide for neovascularization. Acta Bio Materialia 2015; 25:76-85; PMID:26216508; http://dx.doi.org/ 10.1016/j.actbio.2015.07.033 [DOI] [PubMed] [Google Scholar]
- [11].Jang EJ, Bae IH, Park DS, Lee SY, Lim KS, Park JK, Shim JW, Sim DS, Jeong MH. Effect of a novel peptide, WKYMVm- and sirolimus-coated stent on re-endothelialization and anti-restenosis. J Materials Sci Materials Med 2015; 26:251; PMID:26438653; http://dx.doi.org/ 10.1007/s10856-015-5585-1 [DOI] [PubMed] [Google Scholar]
- [12].Singer AJ, Clark RA. Cutaneous wound healing. N Eng J Med 1999; 341:738-46; PMID:10471461; http://dx.doi.org/ 10.1056/NEJM199909023411006 [DOI] [PubMed] [Google Scholar]
- [13].Baum CL, Arpey CJ. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatologic Surg 2005; 31:674-86; PMID:15996419; http://dx.doi.org/ 10.1097/000427-28-200506000-00011 [DOI] [PubMed] [Google Scholar]
- [14].Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmouliere A. Erythropoietin, a novel repurposed drug: An innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen 2014; 22:23-33; PMID:24471742; http://dx.doi.org/ 10.1111/wrr.12135 [DOI] [PubMed] [Google Scholar]
- [15].Carretero M, Escamez MJ, Garcia M, Duarte B, Holguin A, Retamosa L, Jorcano JL, Río MD, Larcher F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol 2008; 128:223-36; PMID:17805349; http://dx.doi.org/ 10.1038/sj.jid.5701043 [DOI] [PubMed] [Google Scholar]
- [16].Demidova-Rice TN, Durham JT, Herman IM. Wound Healing Angiogenesis: Innovations and Challenges in Acute and Chronic Wound Healing. Adv Wound Care 2012; 1:17-22; PMID:24527273; http://dx.doi.org/ 10.1089/wound.2011.0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seo JK, Choi SY, Kim Y, Baek SH, Kim KT, Chae CB, Lambeth JD, Suh PG, Ryu SH. A peptide with unique receptor specificity: stimulation of phosphoinositide hydrolysis and induction of superoxide generation in human neutrophils. J Immunol 1997; 158:1895-901 [PubMed] [Google Scholar]
- [18].Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005; 15:599-607; PMID:16202600; http://dx.doi.org/ 10.1016/j.tcb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- [19].Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995; 92:1355-74; PMID:7648691; http://dx.doi.org/ 10.1161/01.CIR.92.5.1355 [DOI] [PubMed] [Google Scholar]
- [20].Hansson GK. Mechanisms of disease - Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685-95; PMID:15843671; http://dx.doi.org/ 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- [21].Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular Stents to Prevent Occlusion and Restenosis after Trans-Luminal Angioplasty. N Engl J Med 1987; 316:701-6; PMID:2950322; http://dx.doi.org/ 10.1056/NEJM198703193161201 [DOI] [PubMed] [Google Scholar]
- [22].Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? Andreas Gruntzig Lecture ESC 2014; 36(47):3320-31. European heart journal 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stefanini GG, Holmes DR. Drug-Eluting Coronary-Artery Stents. N Engl J Med 2013; 368:254-65; PMID:23323902; http://dx.doi.org/ 10.1056/NEJMra-1210816 [DOI] [PubMed] [Google Scholar]
- [24].Park JK, Lee JH, Nah JW, Kim HK, Lim KS, Bae IH, et al.. Development of a novel drug-eluting stent consisting of an abluminal and luminal coating layer dual therapy system. Rsc Adv 2015; 5:40700-7 [Google Scholar]
- [25].Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964-7; PMID:9020076; http://dx.doi.org/ 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- [26].Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011; 29:1650-5; PMID:21948649; http://dx.doi.org/ 10.1002/stem.745 [DOI] [PubMed] [Google Scholar]
- [27].Alev C, Ii M, Asahara T. Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxidants Redox Signal 2011; 15:949-65; PMID: 21254837; http://dx.doi.org/ 10.1089/ars.2010.3872 [DOI] [PubMed] [Google Scholar]
- [28].Jang IH, Heo SC, Kwon YW, Choi EJ, Kim JH. Role of formyl peptide receptor 2 in homing of endothelial progenitor cells and therapeutic angiogenesis. Adv Biol Regulation 2015; 57:162-72; PMID:25304660; http://dx.doi.org/ 10.1016/j.jbior.2014.09.011 [DOI] [PubMed] [Google Scholar]
- [29].Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003; 2:214-21; PMID:12612647; http://dx.doi.org/ 10.1038/nrd1033 [DOI] [PubMed] [Google Scholar]
- [30].Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011; 3:1377-97; PMID:22577513; http://dx.doi.org/ 10.3390/polym3-031377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000; 21:2475-90; PMID:11055295; http://dx.doi.org/ 10.1016/S0142-9612(00)00115-0 [DOI] [PubMed] [Google Scholar]


