Summary
Emerging evidence has shown that noncoding RNAs, particularly microRNAs (miRNAs), contribute to the pathogenesis of mood and anxiety disorders, although the molecular mechanisms are poorly understood. Here we show altered levels of miR-17-92 in adult hippocampal neural progenitors have a significant impact on neurogenesis and anxiety- and depression-related behaviors in mice. miR-17-92 deletion in adult neural progenitors decreases neurogenesis in the dentate gyrus, while its overexpression increases neurogenesis. miR-17-92 affects neurogenesis by regulating genes in the glucocorticoid pathway, especially serum- and glucocorticoid-inducible protein kinase-1 (Sgk1). miR-17-92 knockout mice show anxiety- and depression-like behaviors, whereas miR-17-92 overexpressing mice exhibit anxiolytic and antidepression-like behaviors. Furthermore, we show that miR-17-92 expression in the adult mouse hippocampus responds to chronic stress, and miR-17-92 rescues proliferation defects induced by corticosterone in hippocampal neural progenitors. Our study uncovers a crucial role for miR-17-92 in adult neural progenitors through regulation of neurogenesis and anxiety- and depression-like behaviors.
Keywords: miR-17-92, adult hippocampal neurogenesis, anxiety, depression, Sgk1
eTOC blurb
The molecular pathogenesis of anxiety and depression disorders is poorly understood. Jin et al. show that microRNA miR-17-92 plays a critical role in regulating adult hippocampal neurogenesis and anxiety- and depression-like behaviors by modifying expression of genes in the glucocorticoid pathway.
Introduction
Mood and anxiety disorders are known to be associated with many risk factors, such as environmental, genetic and epigenetic factors, making the investigation of pathophysiology of these disorders extremely challenging (Krishnan and Nestler, 2008). Antidepressants are used for the treatment of both depression and anxiety disorders, but only 30%–50% of patients show amelioration (Gaynes et al., 2008). Thus, it is essential to better understand the etiology of the disorders and to develop new targets for improved therapies.
The dentate gyrus (DG) in the hippocampus is one of the neurogenic zones in the adult brain. The DG is populated by neural stem cells and neural progenitors, and is continuously generating new neurons (Ming and Song, 2011; Zhao et al., 2008). The impact of adult neurogenesis in the function of hippocampus has drawn significant attentions (Deng et al., 2010; Jacobs et al., 2000; Kheirbek et al., 2012; Sahay and Hen, 2007). While some studies demonstrate the association of adult neurogenesis with depression and anxiety (Kheirbek et al., 2012; Sahay and Hen, 2007), some other reports show inconsistent outcomes (Petrik et al., 2012). Even though the neurogenic theory of depression and anxiety appears controversial, it is still plausible and holds possibility for the adult hippocampal neurogenesis as a therapeutic means for mood and anxiety disorders (Eisch and Petrik, 2012; Hill et al., 2015; Miller and Hen, 2014). Thus, there is an unmet need for research to identify molecules that regulate both hippocampal neurogenesis and depression- and anxiety-like behaviors.
MicroRNAs (miRNAs) normally silence protein-coding target genes and play crucial roles in many aspects of neural development and neurological disorders (Bian and Sun, 2011; Fineberg et al., 2009; Kosik, 2006; Qureshi and Mehler, 2012). Recent studies begin to reveal the contribution of miRNAs in the pathogenesis of mood and anxiety disorders (Issler et al., 2014; Lopez et al., 2014; Malan-Muller et al., 2013; Xu et al., 2012). However, little is known about specific miRNAs being involved in the regulation of adult hippocampal neurogenesis, and/or mood and anxiety behaviors.
The miR-17-92 cluster has been shown to be involved in embryonic brain development and tumorigenesis (Bian et al., 2013; Olive et al., 2010). In this study, we identified the miR-17-92 cluster highly expressed in the adult mouse hippocampus. We found that miR-17-92 regulates adult hippocampal neurogenesis and mood and anxiety-related behaviors in mice. We further established the regulatory role of miR-17-92 in modifying genes in the glucocorticoid pathway including Sgk1, and rescuing reduced proliferation, caused by corticosterone, in hippocampal progenitors. Our studies indicate that the miR-17-92 cluster is a pivotal regulator of adult hippocampal neurogenesis, and anxiety- and depression-like behaviors.
Results
Altering adult hippocampal expression of miR-17-92 in miR-17-92 KO and OE mice
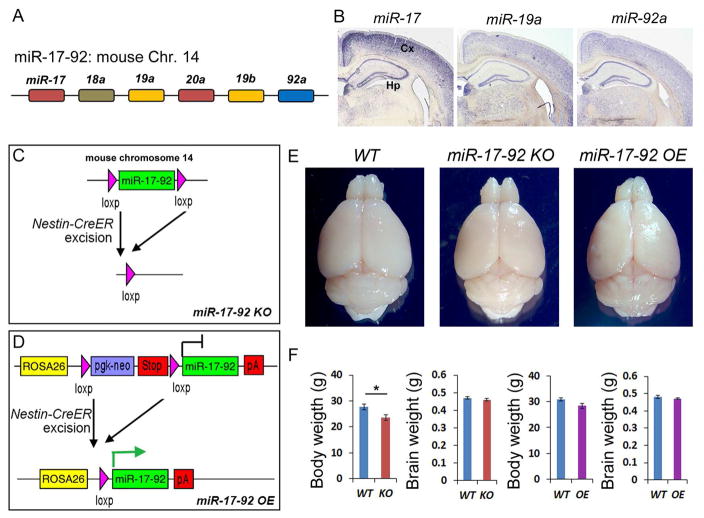
Previous studies suggested a role of the miR-17-92 cluster in embryonic neural stem cell development and proliferation of cancer cells (Bian et al., 2013; Olive et al., 2010), its involvement in adult brain neurogenesis and function is unknown. Transcription of the miR-17-92 cluster generates 6 miRNAs that can be grouped into 4 subfamilies (Mendell, 2008), miR-17 (and -20a), miR-18a, miR-19a (and -19b) and miR-92a, based on their conserved sequences (Figure 1A). To examine expression patterns of miR-17-92, we performed in situ hybridization in 12 weeks old adult mouse brains using locked nucleic acid (LNA) probes for miR-17 (which also recognizes 20a), -19a (which also recognizes 19b) and -92a. All three subfamilies of the miRNA were expressed in the adult hippocampus and in the cortex (Figure 1B).
Figure 1. Altering adult hippocampal expression of miR-17-92 in miR-17-92 KO and OE mice.
(A) Schematic genomic organization of miRNAs in the miR-17-92 cluster on mouse chromosome 14. The color code represents miRNAs with the conserved seed sequence.
(B) miR-17 (miR-20a), miR-19a (miR-19b) and miR-92a were expressed in the 12 weeks old adult mouse hippocampus (Hp) and cortex (Cx).
(C and D) Generation of miR-17-92 knockout (KO) and miR-17-92 overexpressing (OE) mice using the Nestin-CreER line.
(E) The whole brain images of wild type (WT), miR-17-92 KO, and miR-17-92 OE mice at the age of 13 weeks old.
(F) The body weight of miR-17-92 KO was slightly reduced, and the body weight of miR-17-92 OE mice was not changed, compared to WT controls. Brain weights of miR-17-92 KO and miR-17-92 OE mice were indistinguishable from WT controls.
Values plotted were means ± s.e.m. n=6 mice per group. *: p < 0.05. A two-tailed, unpaired Student’s t-test was used for comparisons. See also Figures S1 and S2.
Because miR-17-92 has been shown to regulate proliferation in embryonic neural stem cells and cancer cells (Bian et al., 2013; Olive et al., 2010), we speculated that they might be involved in adult hippocampal neurogenesis and function. To examine the possible role for miR-17-92 in maintaining progenitor cells in adult hippocampus, we generated conditional miR-17-92 knockout (miR-17-92 KO) mice and miR-17-92 overexpressing (miR-17-92 OE) mice. We utilized a Nestin-CreER line (Kuo et al., 2006), in which the Cre recombinase is expressed in neural progenitors in the adult brain upon tamoxifen injection. The Nestin-CreER mice were bred with floxed miR-17-92 knockout mice (Ventura et al., 2008) or floxed miR-17-92 overexpressing mice (Xiao et al., 2008), and tamoxifen was injected at the age of 5-weeks (Figures 1C, 1D, and S1A). To test altered miR-17-92 expression, real-time quantitative Reverse Transcription PCR (qRT-PCR) was performed. Expression levels of miR-17, -18, -19a and -92 were decreased in hippocampus of miR-17-92 KO mice, while they were increased in hippocampus of miR-17-92 OE mice, suggesting expected alteration of miR-17-92 in the adult hippocampus (Figures S2A and S2B).
miR-17-92 KO and OE mice showed normal brain morphology (Figure 1E). In addition, miR-17-92 KO mice displayed slightly reduced body weight but normal brain weight compared to wild type control littermates (Figure 1F). miR-17-92 OE mice were indistinguishable from controls in brain and body weights (Figure 1F). Furthermore, we measured the thickness of the cortical wall. It did not show difference among control, miR-17-92 KO and OE mice (Figures S2C-E). These results suggest that altered miR-17-92 expression in neural progenitors in the adult brain has minimal effects on brain and body growth.
Adult hippocampal neurogenesis is altered in miR-17-92 KO and OE mice
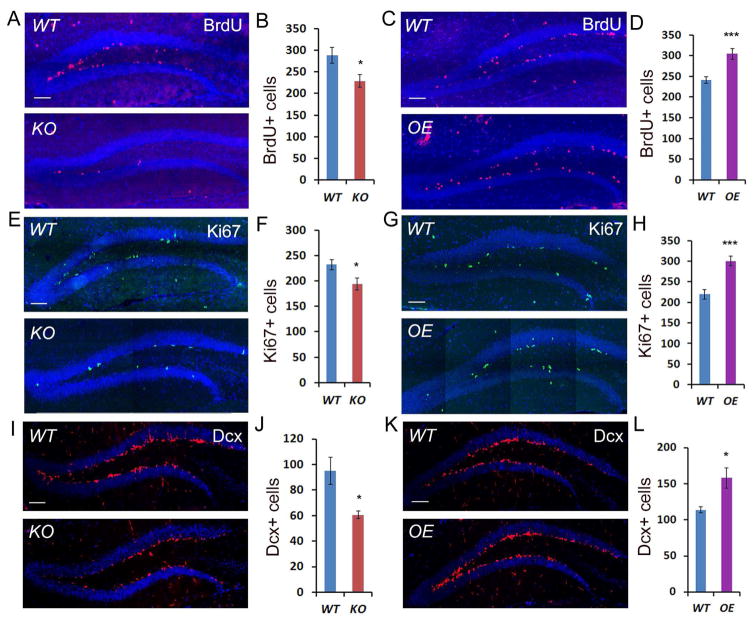
Even though brain weights were not changed in miR-17-92 KO and OE mice, altered miR-17-92 expression might affect neurogenesis in the adult hippocampus. We thus investigated generation of adult hippocampal neural progenitors and newborn neurons in miR-17-92 KO and OE mice. To detect proliferative neural progenitors, bromodeoxyuridine (BrdU) was administered in the 12 weeks old mice, 6 days before brain tissue collection (Figure S1A). The number of BrdU+ cells was reduced in the dentate gyrus of the miR-17-92 KO hippocampus (Figures 2A and 2B), but increased in OE mice (Figures 2C and 2D). Moreover, the number of cells expressing Ki67, which indicates progenitors in the G1-, S-, G2-, and M-phases of the cell cycle, also was decreased in the miR-17-92 KO (Figures 2E and 2F) but increased in the OE hippocampus (Figures 2G and 2H). These results indicate that miR-17-92 function is required for maintaining proliferative neural progenitors in the adult hippocampus.
Figure 2. Deletion or overexpression of miR-17-92 in adult neural progenitors causes altered hippocampal neurogenesis.
(A and B) The number of BrdU+ (red) proliferating cells was decreased in the dentate gyrus (DG) of 13 weeks old miR-17-92 knockout (KO) mice, compared to wild type (WT) mice.
(C and D) The number of BrdU+ cells was significantly increased in miR-17-92 overexpressing (OE) mice.
(E and F) The number of Ki67+ (green) progenitors in the DG was significantly reduced in 13 weeks old miR-17-92 KO mice.
(G and H) The number of Ki67+ cells was significantly increased in OE mice compared to WT.
(I and J) Deletion of miR-17-92 led to significant reduction in the number of Dcx+ neurons (red) in the DG.
(K and L) The number of Dcx+ cells was increased in OE mice.
Scale bars, 100μm in A, C, E and G; 20μm in I and K. DAPI (blue) was used to label nucleus. All data were presented as means ± s.e.m. n=6 mice per group. *: p < 0.05 and ***: p < 0.001. A two-tailed, unpaired Student’s t-test was used for comparisons. See also Figures S1 and S3.
We also examined the number of Doublecortin (Dcx) expressing newborn neurons. Consistent with changes in the number of neural progenitors, the number of Dcx+ cells was reduced in the DG of the miR-17-92 KO hippocampus (Figures 2I and 2J), but increased in OE mice (Figures 2K and 2L), suggesting that generation of newborn neurons is impaired. Moreover, the number of BrdU+ or NeuN+/BrdU+ cells at 13 weeks old, which were labelled by BrdU injection at 7 weeks old to detect newborn neurons (Figure S1B), also was decreased in the miR-17-92 KO hippocampus (Figures S3A and S3B). Taken together, our results indicate that miR-17-92 is required to maintain proliferative neural progenitors and to generate new neurons in the adult hippocampus.
miR-17-92 KO and OE mice exhibit elevated anxiety- or anti-anxiety-like behaviors
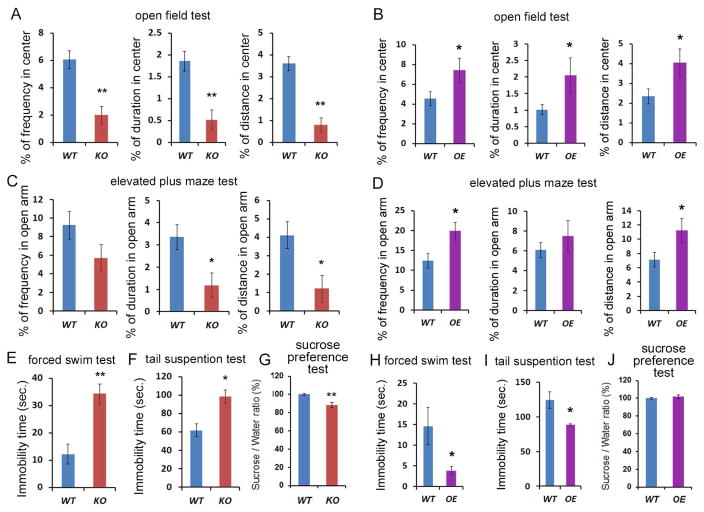
Previous studies have shown that adult hippocampal neurogenesis is associated with mood and anxiety disorders (Eisch and Petrik, 2012; Miller and Hen, 2014). Because altered expression of miR-17-92 caused changes in hippocampal neurogenesis, we examined whether anxiety-related behaviors are affected in miR-17-92 KO and OE mice (Figure S1C). In the open field test, miR-17-92 KO mice and OE mice showed comparable locomotor activity as measured by total distance compared to their wild type controls (Figures S3C). However, miR-17-92 KO mice displayed markedly less frequency of stay, less time spent, and less distance moved in the center of the open field compared to wild type controls (Figure 3A). On the other hand, miR-17-92 OE mice exhibited opposing behaviors (Figure 3B). Moreover, in the elevated plus maze test, miR-17-92 KO mice showed significantly less duration and less distance travelled in open arms (Figure 3C), while miR-17-92 OE mice showed a significantly greater frequency of stay and longer total distance moved in open arms (Figure 3D). These results indicate that miR-17-92 deletion or overexpression in mice results in elevated anxiety-like or antianxiety-like behaviors, respectively.
Figure 3. Deletion or overexpression of the miR-17-92 cluster in adult neural progenitors results in altered anxiety- and depression-like behaviors.
(A) miR-17-92 knockout (KO) mice displayed less frequency, duration, and distance moved in the center in the open field, compared to wild type (WT) mice.
(B) miR-17-92 overexpressing (OE) mice showed significantly anxiolytic behavior in the open field test.
(C) miR-17-92 KO mice showed less duration and distance traveled in open arms in the elevated plus maze test.
(D) miR-17-92 OE mice showed significantly increased frequency and total distance moved in open arms in the elevated plus maze test.
(E) miR-17-92 KO mice showed significantly elevated immobility time in the forced swim test, compared to WT mice. Results are shown as the mean of immobility duration (seconds).
(F) miR-17-92 KO mice showed increased immobility in the tail suspension test. Results are shown as the mean of immobility duration (seconds).
(G) miR-17-92 KO mice showed significantly reduced sucrose consumption in the sucrose preference test, compared to WT mice. Results are shown as the percentage of sucrose consumption ratio (% of WT).
(H) miR-17-92 OE mice had significantly reduced immobility time compared to WT mice in the forced swim test.
(I) miR-17-92 OE mice showed reduced immobility in the tail suspension test.
(J) miR-17-92 OE mice showed no difference in the sucrose preference test.
Values plotted are means ± s.e.m, n=13-18 mice per group. *: p< 0.05 and **: p< 0.01. A two-tailed, unpaired Student’s t-test was used for comparisons. See also Figure S3.
Altered expression of miR-17-92 in the hippocampus regulates depression-like behaviors
Adult hippocampal neurogenesis has been shown to be related to depression (Sahay and Hen, 2007), we also examined depression-like behaviors in miR-17-92 KO and OE mice. In the forced swim test, miR-17-92 KO mice displayed significantly increased immobility time (Figure 3E). In the tail-suspension test, the immobility time was also increased in miR-17-92 KO mice, suggesting that miR-17-92 KO mice exhibit elevated depression-like behaviors (Figure 3F). Moreover, in the sucrose preference test, miR-17-92 KO mice showed reduced intake of sucrose, suggesting anhedonia-like behavior (Figure 3G). Conversely, in both the forced swim and tail-suspension tests, miR-17-92 OE mice had greatly reduced immobility time, compared to their controls, suggesting an antidepression-like effect of miR-17-92 (Figures 3H and 3I). In sucrose preference test, miR-17-92 OE mice did not show detectable difference compared to controls (Figure 3J). These results altogether indicate that miR-17-92 deletion or overexpression in the adult neural progenitors results in increased or decreased anxiety- and depression-like behaviors.
The glucocorticoid pathway is affected in miR-17-92 KO hippocampus
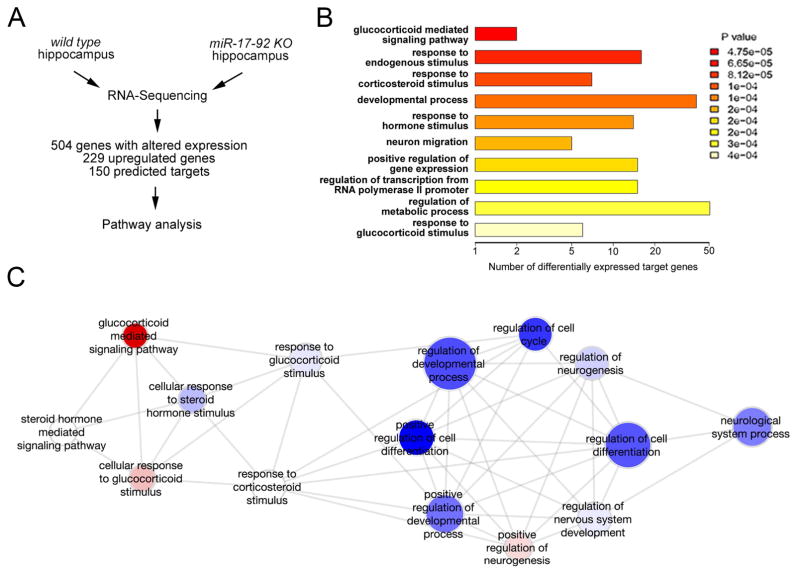
miRNAs exert their functions by silencing target genes. To investigate miR-17-92 regulation mechanisms, we next quantified gene expression levels in the hippocampus of miR-17-92 KO and wild type mice using Illumina RNA-sequencing (RNA-Seq) to identify genes with altered expression when miR-17-92 is knocked out (Figures 4A and S1D). We identified 504 genes significantly altered in the hippocampus of miR-17-92 KO mice (false discovery rate <0.1). Among them, 229 genes were upregulated, suggesting that they are usually directly or indirectly silenced by miR-17-92, and 78 out of 229 genes were predicted targets of miR-17-92 (Table S1). Gene ontology (GO) analyses of the 504 genes with altered expression in miR-17-92 KO hippocampus revealed that many of them regulate cell proliferation and differentiation (Figure S4A). This finding is consistent with the function associated with the 229 upregulated genes, including predicted targets of the miR-17-92 cluster (Figure S4B).
Figure 4. Deletion of miR-17-92 in adult hippocampal neural progenitors alters expression levels of genes in the glucocorticoid pathway.
(A) Schematic flow of gene expression analysis. The adult hippocampal samples from 12 weeks old wild type (WT) and miR-17-92 knockout (KO) mice were analyzed by RNA-Sequencing to detect genes with altered expression.
(B) Gene ontology (GO) analysis of 150 predicted target genes indicated 10 most significantly overrepresented functions. The glucocorticoid-mediated signaling pathway was the top among altered functions.
(C) Gene ontology analysis of signaling pathways that are associated with the Sgk1 function. See details in Experimental Procedures. See also Figure S4.
Moreover, using four target prediction tools (Targetscan, miRDB, DIANA-microT and TarBase), we identified 150 genes, which are either up- or down-regulated, as predicted targets of the miR-17-92 cluster (Table S1). Interestingly, a detailed GO analysis of the 150 predicted target genes revealed their involvement in glucocorticoid-related functions in the absence of miR-17-92 (Figure 4B). Among them, the kinase Sgk1 (Lang et al., 2010) was found to have a significant upregulation in the miR-17-92 KO hippocampus (about 1.3 fold increase), and to be involved in 6 out of the top 10 predictable major biological functions that are associated with the 150 target genes (Figure 4B). Sgk1 is known as a downstream effector of the glucocorticoid receptor (GR) (Anacker et al., 2013). Functional interaction analysis of Sgk1 with 504 genes that showed altered expression in the miR-17-92 KO hippocampus further indicated that Sgk1, as a crucial modulator in the glucocorticoid pathway, interacts with genes directly involved in cell cycle control, developmental process and neurogenesis (Figure 4C). Our results indicate the regulatory effect of the miR-17-92 cluster on the glucocorticoid pathway.
The expression level of Sgk1 is specifically increased in miR-17-92 KO hippocampus
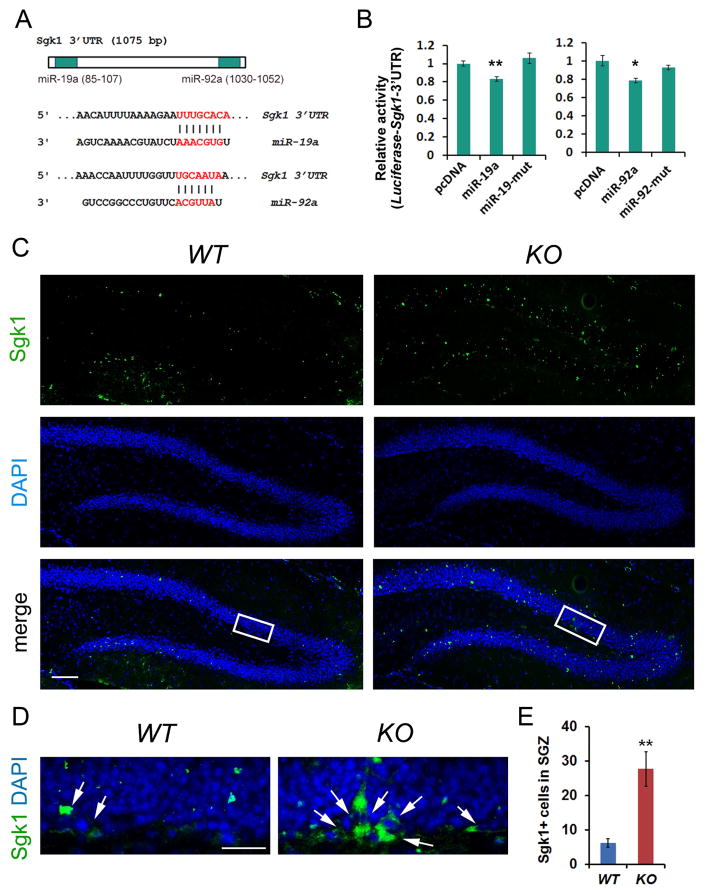
To further test whether Sgk1 is a target of the miR-17-92 cluster, we searched the Sgk1 3’untranslated region (3’UTR), where miRNAs typically bind to silence target gene expression. We found that Sgk1 3’UTR contains binding sites for miR-19a and miR-92a (Figure 5A). We cloned the Sgk1 3’UTR into a luciferase vector. When the Sgk1 3’UTR-luciferase was co-expressed with miR-19a or miR-92a, wild type but not their mutants significantly reduced the relative luciferase activity, suggesting that miR-19a and miR-92a have specific targeting effects on the Sgk1 3’UTR (Figure 5B).
Figure 5. Sgk1 is a target of miR-17-92 in the adult hippocampus.
(A) The 3’untranslated region (3’UTR) of Sgk1 contained targeting sites for miR-19a and miR-92a.
(B) Luciferase fused to Sgk1 3’UTR was used to examine targeting effects of miR-19a and miR-92a on the Sgk1 3’UTR. Both miR-19a and miR-92a, but not their mutants (miR-19-mut and miR-92-mut), recognized the 3’UTR of Sgk1 and reduced the luciferase activity. n=3.
(C) Sgk1 (green) expression in the dentate gyrus in the hippocampus of the 13 weeks old wild type (WT) and miR-17-92 knockout (KO) mice.
(D) High power view of highlighted areas in C. Arrows indicate Sgk1+ cells in the subgranular zone (SGZ).
(E) The number of Sgk1+ cells was significantly increased in the SGZ in miR-17-92 KO mice, compared to WT controls. n=6.
Scale bars, 100μm in C and 20μm in D. DAPI (blue) was used to label nucleus. All data were presented as means ± s.e.m. **p < 0.01. A two-tailed, unpaired Student’s t-test was used for comparisons. See also Figure S5.
We next examined the expression of Sgk1 in the DG of 13 weeks old wild type and miR-17-92 KO mice. Scattered Sgk1+ cells were observed in the subgranular zone (SGZ) and the granule cell layer (GCL) in the DG of wild type and miR-17-92 KO hippocampus (Figure 5C). Interestingly, the number of Sgk1+ cells was significantly increased in the SGZ in miR-17-92 KO mice (Figures 5D and 5E). Moreover, Sgk1+ cells were also detected in the polymorphic layer (PML) (Scharfman and Myers, 2012), the area between the upper and lower GCLs (Figure S5A). The number of Sgk1+ cells also was increased in the PML in miR-17-92 KO mice (Figures S5B and S5C). However, the number of cells expressing GR, an upstream gene of Sgk1 (Anacker et al., 2013), didn’t show significant difference in the DG of control and miR-17-92 KO mice (Figures S5D and S5E). These results, consistent with RNA-seq data, indicate that miR-17-92 knockout in neural progenitors results in elevated level of Sgk1 expression in the DG.
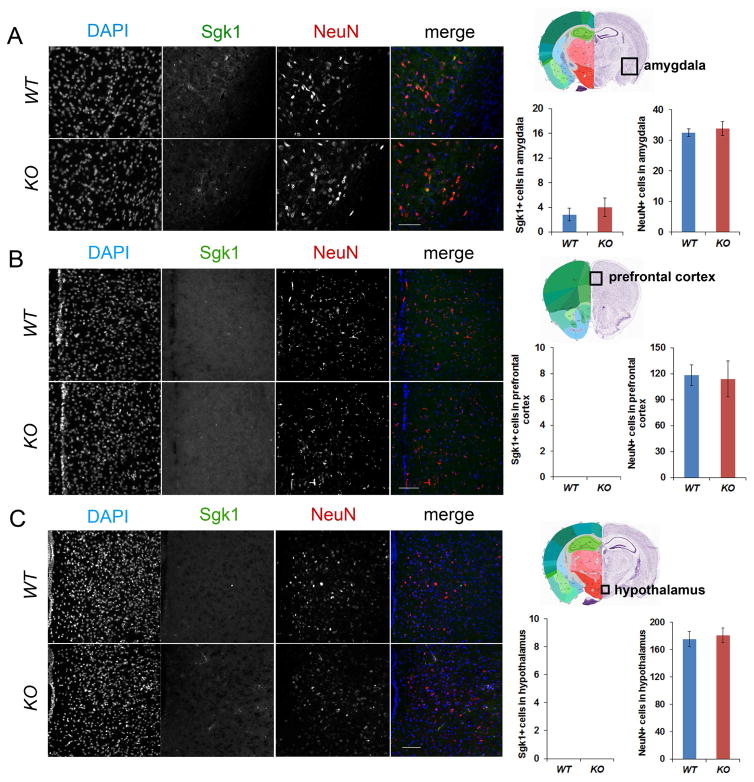
To test whether the number of Sgk1+ cells also is increased in other brain regions in miR-17-92 KO mice, we examined Sgk1 expression in the amygdala, prefrontal cortex and hypothalamus. Sgk1 expression was detected in the amygdala, but the number of Sgk1+ cells did not show detectable change between WT control and miR-17-92 KO mice (Figure 6A). In addition, Sgk1 expression was not detected in the prefrontal cortex and hypothalamus (Figures 6B and 6C). Moreover, the overall neurogenesis, labeled by NeuN+ cells, also did not show significant changes in the amygdala, prefrontal cortex and hypothalamus (Figure 6). Our results suggest that elevated expression of Sgk1 in the hippocampus in miR-17-92 KO mice is brain region specific.
Figure 6. Sgk1 expressions are not changed in other brain regions in miR-17-92 KO mice.
(A) Sgk1 expressing cells were detected in the amygdala. The numbers of Sgk1+ and NeuN+ cells did not show significant difference between 13 weeks old wild type (WT) and miR-17-92 knockout (KO) mice.
(B and C) No Sgk1 expressing cells were detected in the prefrontal cortex and hypothalamus. The number of NeuN+ cells did not show significant difference between WT and miR-17-92 KO mice.
Scale bars, 100μm. DAPI (blue), Sgk1 (green), and NeuN (red) were labelled. All data were presented as means ± s.e.m. A two-tailed, unpaired Student’s t-test was used for comparisons.
miR-17-92 rescues stress induced proliferation defect in hippocampal neural progenitors
A large body of studies has shown that chronic stress alters the release of adrenal glucocorticoid hormones and in turn affects hippocampal neurogenesis, and has a detrimental influence on learning and memory and mood behaviors (Joels, 2008; McEwen, 2001; Saaltink and Vreugdenhil, 2014; Schoenfeld and Gould, 2012). Because we found that miR-17-92 regulates the glucocorticoid pathway, we next examined whether chronic stress also has a direct effect on miR-17-92 expression in the hippocampus. 8 weeks old mice were subjected to restraint stress for 2 weeks, while being administered an antidepressant (antidep) fluoxetine, or vehicle as a control (Seo et al., 2012). Consistent with previous reports (Anacker et al., 2013; Saaltink and Vreugdenhil, 2014), expression levels of GR and Sgk1 were increased due to stress and rescued by antidepressant treatment (Figure S6A). On the contrary, chronic stress significantly reduced expression levels of miR-17, -18, -19a and -92a in the hippocampus, compared to their controls, and the reduction was prevented by antidepressant treatment (Figure S6B). These results suggest that hippocampal expression of miR-17-92 is responsive to chronic stress and antidepressant.
To further test the effect of miR-17-92 on proliferation of hippocampal neural progenitors under stress treatment, we used HT-22 cells, which are immortalized mouse hippocampal neurons (Davis and Maher, 1994). HT-22 cells were treated with corticosterone (CORT) and the vehicle control (Malviya et al., 2013), and were then transfected with the pCAGIG vector containing a green fluorescent protein (GFP) reporter gene, and pulsed with BrdU for 2 hours. CORT-treatment significantly reduced BrdU+/GFP+ cells, compared to the vehicle control (Figures S6C and S6D). Furthermore, miR-19a and miR-92a precursors were cloned into the pCAGIG vector and transfected into CORT-treated HT-22 cells (Bian et al., 2013). Compared to those transfected with the empty pCAGIG vector, BrdU+/GFP+ cells were significantly increased upon miR-19a and miR-92a transfection (Figures S6C and S6D). These results suggest that miR-19a and miR-92a in the miR-17-92 cluster rescues proliferation defect in hippocampal neural progenitors caused by corticosterone.
miR-17-92 mediates antidepressant- and stress-regulated adult hippocampal neurogenesis in vivo
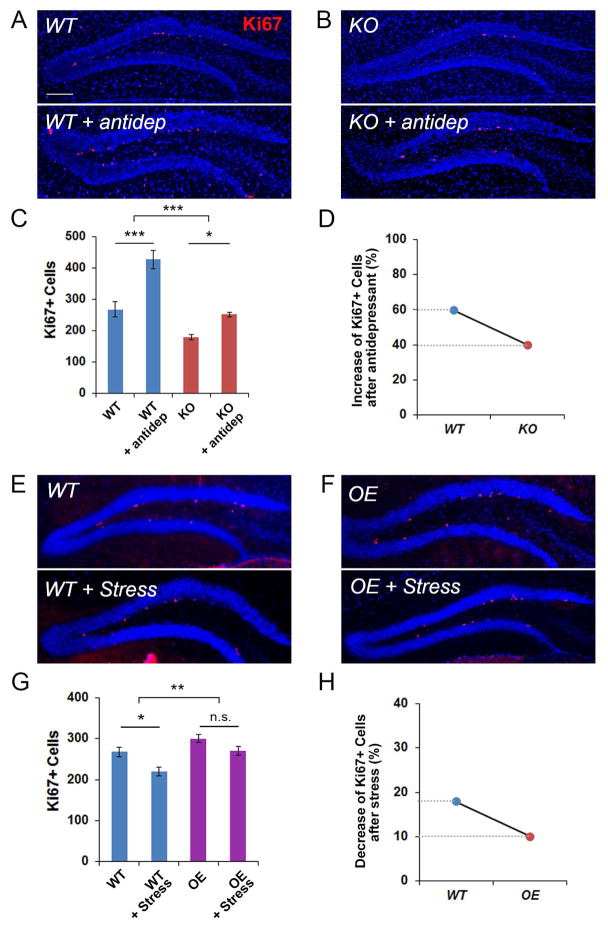
We found that miR-17-92 KO and OE mice show altered hippocampal neurogenesis. We wanted to examine whether there is a direct causal relationship between miR-17-92 expression and hippocampal neurogenesis defects. Previous studies have shown that antidepressant treatment has a positive impact on adult hippocampal neurogenesis (Boldrini et al., 2009; Malberg et al., 2000). We then tested whether antidepressant-regulated neurogenesis is mediated by miR-17-92. Wild type control and miR-17-92 KO mice were administered the antidepressant fluoxetine for 2 weeks. Consistent with previous reports, elevated neurogenesis was observed in control and miR-17-92 KO hippocampus (Figures 7A–C). However, the degree of increased neurogenesis was significantly restrained by miR-17-92 knockout (Figure 7D).
Figure 7. miR-17-92 mediates antidepressant- and stress-regulated adult hippocampal neurogenesis in vivo.
(A–C) The number of Ki67+ (red) progenitors in the dentate gyrus (DG) was significantly increased in antidepressant-treated 13 weeks old wild type (WT) mice, compared to vehicle-treated WT mice. Moreover, the number of Ki67+ progenitors in the DG was significantly increased in antidepressant-treated 13 weeks old miR-17-92 knockout (KO) mice, compared to vehicle-treated miR-17-92 KO mice.
(D) miR-17-92 KO mice showed less degree of increase in the number of Ki67+ progenitors in the DG than WT mice after antidepressant treatment.
(E–G) The number of Ki67+ progenitors in the DG was significantly reduced in stress- administered 13 weeks old WT mice, compared to WT mice without stress treatment. However, stress-administered miR-17-92 overexpressing (OE) mice showed significant rescue of reduced number of Ki67+ progenitors.
(H) miR-17-92 OE mice showed less degree of decrease in the number of Ki67+ progenitors in the DG than WT mice after stress treatment.
Scale bars, 100μm. DAPI (blue) was used to label nucleus. All data were presented as means ± s.e.m. n=6 per group. *: p < 0.05, **: p < 0.01, ***: p < 0.001, and n.s.: not significant. Statistic analyses were assessed using two-way ANOVA with Bonferroni’s posthoc test. See also Figures S6 and S7.
Furthermore, studies have shown that chronic stress has a negative effect on hippocampal neurogenesis (Joels, 2008; McEwen, 2001; Saaltink and Vreugdenhil, 2014; Schoenfeld and Gould, 2012). We next examined whether overexpression of miR-17-92 protects stress-induced decrease of adult hippocampal neurogenesis. Wild type control and miR-17-92 OE mice at 8 weeks old were subjected to restraint stress for 2 weeks (Seo et al., 2012). While stress caused a reduction in neurogenesis in the control hippocampus, its effect on miR-17-92 OE hippocampus was subtle, suggesting that overexpression of miR-17-92 rescues stress induced neurogenesis defect (Figures 7E–H).
Sgk1 expression displays opposite change compared to hippocampal neurogenesis upon antidepressant and stress treatment
If miR-17-92-mediated adult hippocampal neurogenesis is partly regulated through their target gene Sgk1, changes in Sgk1 expression and neurogenesis should be opposite. We examined Sgk1 expression in the hippocampus of wild type control and miR-17-92 KO mice that are administered the antidepressant fluoxetine. In the control hippocampus treated with antidepressant, the number of Sgk1+ cells was not altered compared to untreated (Figure S7A). Moreover, while antidepressant treatment resulted in an increased neurogenesis (Figures 7A–D), it caused decreased number of Sgk1+ cells in miR-17-92 KO hippocampus, compared to untreated miR-17-92 KO (Figure S7A).
Furthermore, while chronic stress caused a decreased neurogenesis in the control hippocampus (Figures 7E–H), it caused an increase in the number of Sgk1+ cells (Figure S7B). Interestingly, in miR-17-92 OE mice treated with stress, miR-17-92 overexpression prevented a reduction in neurogenesis (Figures 7E–H), it caused a decrease in the number of Sgk1+ cells, which further suggests a silencing effect of miR-17-92 on Sgk1 expression (Figure S7B). Moreover, miR-17-92 knockout caused a dramatic increase in the number of Sgk1+ cells in the hippocampus of miR-17-92 KO mice (Figure S7B). These results indicate miR-17-92 targeting effect on Sgk1 in vivo.
Because increased neurogenesis and decreased Sgk1 expression were detected in the hippocampus of miR-17-92 KO mice treated with antidepressant, we further examined the behavioral outcome. The forced swim test and tail suspension test were performed in miR-17-92 KO mice treated with vehicle and antidepressant. miR-17-92 KO mice treated with antidepressant displayed reduced immobility time in both tests, compared to those treated with vehicle, suggesting that antidepressant treatment has a rescue effect on depression-like behaviors in miR-17-92 KO mice (Figures S7C and S7D).
Discussion
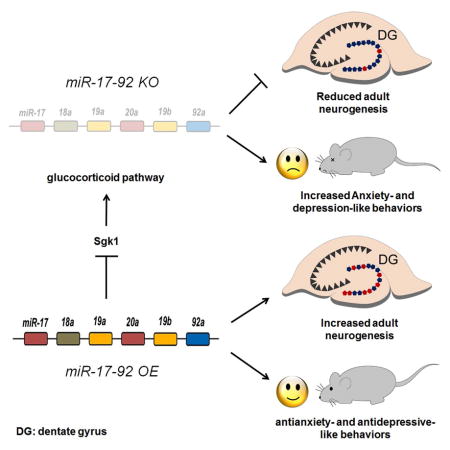
Identification of specific molecules that regulate anxiety- and depression-like behaviors would contribute to our better understanding of pathophysiology of mood and anxiety disorders. In this study, we have shown that miRNAs in the miR-17-92 cluster are highly expressed in the adult mouse hippocampus. Importantly, deletion or overexpression of miR-17-92 in adult neural progenitors causes decreased or increased hippocampal neurogenesis, resulting in elevated anxiety- and depression-like behaviors, or anxiolytic and antidepression-like behaviors, respectively. Our results suggest a molecular mechanism by which miRNAs are associated with adult hippocampal neurogenesis and mood and depression-like behaviors.
Adult hippocampal neurogenesis requires multiple steps including proliferation of neural progenitors, differentiation to neurons and survival of newborn neurons (Ming and Song, 2011; Zhao et al., 2008). Our results have shown that miR-17-92 plays a role in the maintenance of proliferative neural progenitors and the generation of newborn neurons. The role for miR-17-92 in the proliferation of neural progenitors in adult hippocampus is consistent with its demonstrated function in the maintenance of embryonic neural stem cells and the proliferation of various cancer cells (Bian et al., 2013; Olive et al., 2010). We have found that miR-17-92 knockout causes a reduction of antidepressant-induced increase in neurogenesis, while miR-17-92 overexpression is resistant to stress-induced reductions in neurogenesis, indicating a direct role of miR-17-92 in regulating hippocampal neurogenesis. Moreover, here we have identified that the expression levels of multiple genes, known to be involved in cell proliferation and differentiation, are altered in the hippocampus of miR-17-92 KO mice. Particularly, the alterations in the level of multiple genes that are implicated in glucocorticoid-mediated pathways are also observed in the hippocampus of miR-17-92 KO mice. Since glucocorticoid and its signaling are known to affect adult hippocampal neurogenesis (Joels, 2007), altered expression of target genes for miR-17-92 in glucocorticoid pathways may be responsible for impaired adult neurogenesis in miR-17-92 KO mice. Future studies should address which target genes for miR-17-92 contribute to which specific steps of the adult neurogenesis.
Recent studies have suggested an involvement of miRNAs in the regulation of anxiety- and depression-like behaviors. miR-16 was found to play a role in the actions of SSRI (selective serotonin reuptake inhibitor) by targeting serotonin transporter in serotonergic and noradrenergic neurons (Baudry et al., 2010). miR-135 was shown to target serotonin transporter and serotonin receptor-1a in the serotonergic neurons, and regulate anxiety- and depression-like behaviors and behavioral response to antidepressant treatment (Issler et al., 2014). miR-18 might negatively regulate the glucocorticoid pathway (Shimizu et al., 2015; Vreugdenhil et al., 2009). Glucocorticoid is associated with stress-induced anxiety and mood behaviors (McEwen, 2001; Pace and Miller, 2009). Previously, the increased level of Sgk1 was observed in peripheral blood of depressed patients (Anacker et al., 2013). In this study, we have identified Sgk1 as a direct target of miR-19a and miR-92a in the miR-17-92 cluster in vitro and in vivo. The increased Sgk1 expression, as a result of miR-17-92 deletion, was observed in the SGZ of the DG but not in other brain regions. It is possible that the effects of miR-17-92 deletion in hippocampal neurogenesis and mouse behaviors are, at least in part, mediated by increased Sgk1.
Furthermore, although we observed a correlation between the altered hippocampal neurogenesis and behavioral phenotypes in miR-17-92 KO or OE mice, the connection of adult hippocampal neurogenesis to anxiety- and depression-like behaviors remains controversial (Petrik et al., 2012; Samuels and Hen, 2011; Snyder et al., 2011). Nevertheless, because miRNAs are useful tools for the treatment of mood and anxiety disorders (O'Connor et al., 2012), the causal relationship observed in this study between altered levels of miR-17-92 and anxiety- and depression-like behaviors suggests that the miR-17-92 cluster has potential as a therapeutic target for these disorders.
Experimental procedures
Transgenic mouse lines
To delete the miR-17-92 cluster in the adult mouse brain, floxed miR-17-92 transgenic mice (Ventura et al., 2008) (requested from the lab of Dr. Tyler Jacks, MIT, USA) were bred with the tamoxifen-inducible Nestin-Cre line, called Nestin-CreERtm mice (Kuo et al., 2006) (requested from the lab of Dr. Yuh-Nung Jan, UCSF, USA), to generate miR-17-92flox/flox:Nestin-CreER. miR-17-92 knockout (KO) mice were generated after the injection of tamoxifen. To overexpress the miR-17-92 cluster in the adult brain, we employed transgenic mice with a floxed stop codon in front of the miR-17-92 transgene (Xiao et al., 2008). Once bred with the Nestin-CreER line, the overexpression of the miR-17-92 cluster was induced by tamoxifen, called here miR-17-92 overexpressing (OE) mice. Wild type mice used in this project were mice that carry homozygote floxed alleles but are Cre-negative. Tamoxifen was administered all genotyping mice to avoid tamoxifen drug effect.
Transgenic animals were maintained at the facility of Weill Cornell Medical College. Animal use was overseen by the Animal Facility and approved by the IACUC at the Weill Cornell Medical College. Experiments with chronic restraint stress were performed at The Rockefeller University, and the experimental procedure was approved by The Rockefeller University Institutional Animal Care and Use Committee, and was in accordance with guidelines of the National Institutes of Health.
RNA sequencing (RNA-Seq)
The adult hippocampi of the 13 weeks old mice were dissected from three wild-type and three miR-17-92 KO mice, each of them from a different litter. Total RNA was extracted and purified using the RNeasy mini kit with optional on-column DNAse digestion (Qiagen). Whole RNA sequencing was performed at the Weill Cornell Genomics Core Facility using an IlluminaHiseq 1000.
Statistics
All data were shown as means ± standard error of the mean (s.e.m.). Two group comparisons were done by two-tailed, unpaired Student’s t-test. The influence of drug and stress treatment were assessed using two-way ANOVA with Bonferroni’s posthoc tests.
Full Experimental procedures and associated references are available in the online version of the paper.
Supplementary Material
Highlights.
miR-17-92 knockout and overexpression alter adult hippocampal neurogenesis.
Expression levels of miR-17-92 affect mood and anxiety-like behaviors in mice.
miR-17-92 regulates genes in the glucocorticoid pathway and targets Sgk1.
miR-17-92 rescues reduced hippocampal proliferation caused by corticosterone.
Acknowledgments
We thank Dr. Paul Greengard at the Rockefeller University for advice and support, Dr. Tyler Jacks at Massachusetts Institute of Technology and Dr. Yuh-Nung Jan at University of California San Francisco for providing floxed miR-17-92 and Nestin-CreER mice, respectively, Dr. Xingshun Xu at Soochow University for providing HT-22 cells. We thank Drs. Bingfang Liu and Miklos Toth for assistance in behavioral tests. This work was supported by the DOD/USAMRMC Grant W81XWH-09-1-0392 (Y.K.), the National Science Foundation of China (81471152) (X.L.), the Hirschl/Weill-Caulier Trust (T.S.), and an R01-MH083680-08 grant from the NIH/NIMH (T.S.).
Footnotes
Author Contributions
JJ, YK, and TS designed experiments. JJ did in situ hybridizations, analyzed mouse model phenotypes, performed tissue collection, immunostainings and cell counting for neurogenesis assays, and behavioral tests. SK did immunostainings and cell counting for all Sgk1 expression, treated mice with stress and antidepressant. XL performed HT-22 cell culture experiments. HZ performed RT-PCR analysis and luciferase experiments. JS performed the restraint stress test. CZ performed RNA-Seq analyses and GO analyses. The manuscript was written by JJ, YK, and TS. TS supervised the project.
RNA sequencing (RNA-Seq) data
All RNA-Seq data from the adult hippocampus of wild type and miR-17-92 knockout mice were deposited at the GEO website. The GEO accession number is GSE83636.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2013;110:8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Bian S, Hong J, Li Q, Schebelle L, Pollock A, Knauss JL, Garg V, Sun T. MicroRNA Cluster miR-17-92 Regulates Neural Stem Cell Expansion and Transition to Intermediate Progenitors in the Developing Mouse Neocortex. Cell Rep. 2013;3:1398–1406. doi: 10.1016/j.celrep.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Sun T. Functions of Noncoding RNAs in Neural Development and Neurological Diseases. Mol Neurobiol. 2011 doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Maher P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain research. 1994;652:169–173. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75:57–66. doi: 10.3949/ccjm.75.1.57. [DOI] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, et al. MicroRNA 135 Is Essential for Chronic Stress Resiliency, Antidepressant Efficacy, and Intact Serotonergic Activity. Neuron. 2014;83:344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Joels M. Role of corticosteroid hormones in the dentate gyrus. Prog Brain Res. 2007;163:355–370. doi: 10.1016/S0079-6123(07)63021-0. [DOI] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosteroids in the hippocampus. European journal of pharmacology. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588:3349–3354. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang JP, Yerko V, Vigneault E, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med. 2014;20:764–768. doi: 10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan-Muller S, Hemmings SM, Seedat S. Big effects of small RNAs: a review of microRNAs in anxiety. Mol Neurobiol. 2013;47:726–739. doi: 10.1007/s12035-012-8374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya SA, Kelly SD, Greenlee MM, Eaton DC, Duke BJ, Bourke CH, Neigh GN. Estradiol stimulates an anti-translocation expression pattern of glucocorticoid co-regulators in a hippocampal cell model. Physiology & behavior. 2013;122:187–192. doi: 10.1016/j.physbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2014;30C:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry. 2012;17:359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- Olive V, Jiang I, He L. mir-17-92, a cluster of miRNAs in the midst of the cancer network. Int J Biochem Cell Biol. 2010;42:1348–1354. doi: 10.1016/j.biocel.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaltink DJ, Vreugdenhil E. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cellular and molecular life sciences : CMLS. 2014;71:2499–2515. doi: 10.1007/s00018-014-1568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9690–9699. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Tanaka T, Takeda T, Tohyama M, Miyata S. The Kampo Medicine Yokukansan Decreases MicroRNA-18 Expression and Recovers Glucocorticoid Receptors Protein Expression in the Hypothalamus of Stressed Mice. BioMed research international. 2015;2015:797280. doi: 10.1155/2015/797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T, Meijer OC, de Kloet ER, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Hsu PK, Karayiorgou M, Gogos JA. MicroRNA dysregulation in neuropsychiatric disorders and cognitive dysfunction. Neurobiol Dis. 2012;46:291–301. doi: 10.1016/j.nbd.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.