Abstract
The mechanisms underlying neurodegenerative disorders are complex and multifactorial; however, accumulating evidences suggest few common shared pathways. These common pathways include mitochondrial dysfunction, intracellular Ca2+ overload, oxidative stress and inflammation. Often multiple pathways co-exist, and therefore limit the benefits of therapeutic interventions. Nutraceuticals have recently gained importance owing to their multifaceted effects. These food-based approaches are believed to target multiple pathways in a slow but more physiological manner without causing severe adverse effects. Available information strongly supports the notion that apart from preventing the onset of neuronal damage, nutraceuticals can potentially attenuate the continued progression of neuronal destruction. In this article, we i) review the common pathways involved in the pathogenesis of the toxicants-induced neurotoxicity and neurodegenerative disorders with special emphasis on Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), Multiple sclerosis (MS) and Amyotrophic lateral sclerosis (ALS), and ii) summarize current research advancements on the effects of nutraceuticals against these detrimental pathways.
Keywords: Neurodegeneration, mitochondria, calcium, oxidative stress, inflammation, nutraceuticals
1. INTRODUCTION
Neurodegenerative diseases are one of the most debilitating conditions and usually associated with mutated genes, accumulation of abnormal proteins, increased reactive oxygen species (ROS) or destruction of the neurons in a specific part of the brain [1-5]. Improved experimental models, modern research techniques and research in the field of neuroscience have advanced our understanding about the unique mechanisms of the onset and expansion of the neurodegeneration and toxicants-induced neurotoxicity. Among several recognized mechanisms, the damage to neuronal mitochondria, intracellular Ca2+overload, uncontrolled generation of ROS, sustained inflammatory condition or any combination of these are the common busy high-roads for neurotoxicity [6-14]. Increased oxidative stress and mild chronic inflammation are the host of multiple disorders, including neuronal disorders. On the other hand, Ca2+ homeostasis across the membrane (cellular, endoplasmic reticulum (ER) and mitochondrial) is critical for a variety of cellular physiological functions. The multiple order of calcium concentration [Ca2+] gradient across the membrane is precisely regulated through several specialized mechanisms. Any event that perturbs these mechanisms leads to the persistent increase in the intracellular and intra-organelle (ER and mitochondria) [Ca2+], which has been linked to the pathogenesis of several neurodegenerative disorders [15-17]. Among these mechanisms, mitochondrial damage has gained particular attention, as perturbation of its function triggers activation of neurotoxic pathways such as deregulation of Ca2+ homeostasis, generation of ROS from the electron transport chain (ETC) and impaired cellular energy production [18]. Although, the causative factor for the onset of these signaling may differ, the progression of toxicants-induced neuronal damage and neurodegenerative disorders largely involves the same shared mechanisms, reinforcing the importance of these pathways as common targets for the intervention strategies. Advancement in our understanding of the mechanisms underlying the neuronal damage has made it possible to design interventions that can potentially delay the onset and/or prevent further expansion of the unabated neurodegeneration [19, 20].
Utilizing the knowledge of the etiology of the neuro- degenerative diseases, target-based therapies such as neuro- transmitter modulators, direct receptor agonists/antagonists, second messenger modulators, stem cells-based therapies, hormone replacement therapy, neurotrophic factors as well as regulators of the mRNA synthesis and its translation into disease-causing mutant proteins have been developed [21-28]. Although, these strategies are great tools to mitigate the neurodegenerative processes, such treatments are often associated with adverse effects and long-term unknown consequences [22, 25, 29, 30]. Neural transplantation and stem cells-based therapies are emerging with a great hope to fight against the range of neurodegenerative diseases [31-33]. Despite unprecedented advancement in these fields of research, the therapeutic applications of such novel tools are still far from reaching the clinics. So, there is a need to identify a safer alternative treatment option that can be employed over long duration to fight against the debilitating neuronal conditions. Nutraceuticals, which can simultaneously act on multiple targets to improve the overall neuronal health, appear to be an attractive option [34-39]. In the present article, we have reviewed mitochondrial dysfunction, intracellular Ca2+ overload, oxidative stress and inflammation as common pathways involved in the neuronal damage and evaluated current research advancements on the effects of some selected nutraceuticals on these pathways.
2. MECHANISMS OF NEURONAL DAMAGE
2.1. Mitotoxicity-mediated Neuronal Damage
Mitochondrial damage underlies the pathogenesis of several types of toxicants-induced acute neurotoxicity and neurodegenerative diseases [40-48]. Unlike other organelles, the regulation of mitochondrial functions is under the control of two genomes: nuclear DNA (nDNA) and mitochondrial DNA (mtDNA). Mutations in either of these genomes can result in the mitotoxicity-mediated neurodegeneration [49]. Mitochondrial dysfunction could affect distinct compartments of a single neuron or a specific region of the brain. Widely studied glutamate neurotoxicity by Ca2+ influx is also primarily attributed to the mitochondrial permeability transition pore (MPT) [40, 45]. The neuro-toxicant, 1-methyl-4-phenylpyridinium (MPP+), as generated from the mono amine oxidase (MAO)-catalyzed oxidation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) within the brain, is concentrated inside the mitochondria of the dopaminergic cells and inhibits Complex I of the respiratory electron transport chain (ETC), which contributes to the development of Parkinson’s disease (PD) [50, 51]. Poly ADP ribose polymerase (PARP) activation-associated neuronal cell death (both apoptotic and necrotic forms) after cerebral ischemia is mediated by cyclophilin D (CypD)-dependent MPT [50, 51]. Neurotoxicity that occurs with various chemotherapeutic agents such as taxanes [52], bortezomib [53] and platinum compounds [54] is also associated with the mitochondrial dysfunction.
Pathophysiology of several neurodegenerative diseases such as Alzheimer’s disease (AD), PD, Huntington’s disease (HD), multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) largely involves damaged mitochondria [55-59]. Several mitochondrial-dependent molecular mechanisms such as inhibition of mitochondrial ETC's complexes, generation of ROS, perturbations of mitochondrial clearance mechanisms, altered functioning of the enzymes involved in tricarboxylic acid cycle, impairment of mitochondrial dynamics, and interference with the timely supply of the functional mitochondria to the specific sites within a neuron could contribute to the pathogenesis of neuronal injury and neurodegenerative diseases [40, 41, 43-46]. Defective ETC (Complex I) in the substantia-nigra has been considered as a central cause of the sporadic form of PD [50, 51, 57, 60, 61]. Mitochondrial abnormalities also underlie the neuronal damage in AD [55, 62] and ALS [59, 63, 64]. Neuronal degeneration in MS and in experimental autoimmune encephalomyelitis (EAE) is also attributed to the abnormal activities of Complexes I and IV of the ETC as well as the loss of mitochondrial membrane potential (ΔΨm) [65, 66]. Evidence from pre-clinical and clinical studies suggested the role of mitotoxicity in the pathogenesis of neuronal degeneration in HD [56, 67-70]. Mutant huntingtin, the gene responsible for the development of HD, is reported to directly impair mitochondrial functions [71]. Defective mitochondrial Complex II, III and IV have been found in the postmortem tissue from HD patients [72, 73]. Several studies have concluded a positive relationship between mtDNA fragmentation in striatal and cortical neurons and the development of HD [74].
Collectively, these findings suggest that mitochondrial damage is the primary event observed in toxicants-induced neurotoxicity as well as various neurodegenerative diseases. Owing to complex interplay between mitotoxicity, oxidative stress and sustained inflammatory status, it has always remained unclear if mitochondrial damage is the cause or consequence of neuronal damage. Several lines of evidence suggest that mitotoxicity-associated intracellular Ca2+ overload, oxidative stress and inflammation are the leading causes for the development of neurodegeneration [43]. The role of Ca2+overload, oxidative stress and inflammation in the development of neurotoxicity and neurodegeneration has been discussed below.
2.2. Ca2+ Overload-mediated Neuronal Damage
Research about the role of Ca2+ ions in neurotoxicity and neurodegenerative diseases started in 1970s [75, 76]. In general, the [Ca2+] in extracellular space remains in millimolar range, while inside cells, it remains in micromolar range [75, 77-79]. This multiple order of [Ca2+] difference across plasma membrane under normal physiological conditions is attributed to the selective permeability of plasma membrane, the dynamic functions of the several adenosine triphosphate (ATP)-dependent Ca2+-ATPase pumps and sodium/calcium exchange system that actively pumps Ca2+ions outside the cells [17, 80, 81]. Moreover intracellular low level of [Ca2+] is maintained by the buffering functions of ER and mitochondria by virtue of their ability to store the Ca2+ions [17, 47, 82]. Storage of Ca2+ inside the ER occurs after active transport of cytoplasmic Ca2+ through ATP-dependent pumps in the ER membrane [17]. However, uptake of cytoplasmic Ca2+ into mitochondria occurs through a membrane protein known as electrophoretic uniporter present in the mitochondrial outer membrane [47]. Cellular events that compromise integrity of the plasma membranes or the functions of ATP-dependent Ca2+-ATPase pumps/uniporter system can potentially be responsible for rise in intracellular [Ca2+]. In addition to these mechanisms, Ca2+ accumulation in neuronal cells can also occur through several other routes such as activation of voltage-sensitive Ca2+channels, receptor-operated Ca2+ channels (N-methyl-D-aspartate (NMDA)), ATP-dependent Ca2+ channel (P2j receptor), cyclic nucleotide-gated Ca2+ channels, Ca2+ channels coupled to G protein receptors and are sensitive to inositol 1,3,4,5 tetrakisphosphate [83]. Notably, the plasma membrane, ER and mitochondria can only handle rise in intracellular Ca2+ up to certain extent. Persistent rise in intracellular Ca2+ leads to disturbed ER and mitochondrial Ca2+ homeostasis, which has been linked to the pathogenesis of various neurodegenerative diseases [16, 17]. Excitotoxicity is also related to the influx of Ca2+ ions into the neurons [84]. Further, some pathological conditions such as cerebral ischemia and traumatic brain injury are also associated with an abrupt increase in intracellular [Ca2+] [85, 86]. Sudden rise in intracellular [Ca2+] is an immediate threat to the cell, as it triggers a cascade of neurotoxic events, including mitotoxicity and cessation of ATP synthesis, over-activation of several Ca2+-dependent hydrolytic enzymes such as proteases, phospholipases, nucleases, nitric oxide synthase and phosphatases [87, 88]. These events lead to the onset of neurotoxicity, impaired neuronal functions and eventually death of the neuronal cells. This explains why the maintenance of the intracellular Ca2+ ions homeostasis is of crucial importance for the survival of the neuronal cells. Exposure to several neurotoxic chemicals result in the perturbed intracellular Ca2+ homeostasis, which eventually leads to the death of neuronal cells [89]. Increased intracellular [Ca2+] is not only implicated in the traumatic and toxicants-induced neurotoxicity, but several neurodegenerative diseases also have the same underlying mechanisms [90-93]. In AD, amyloid-beta (Aβ)-induced neuronal cell death is associated with the dysregulation of Ca2+-dependent pathways [93-95]. Moreover, deregulation of the intracellular Ca2+ could explain the selective destruction of dopaminergic neurons in the PD [96]. Neuronal destabilization and injury in MS is due to the overactivation of the Ca2+-dependent proteases, calpain and protein phosphatases [97]. Opening of the MPT due to mitochondrial Ca2+ overload and release of the Ca2+ ions from the ER by mutant huntingtin is considered as the mechanism of the neuronal death in HD [71, 98]. Collectively, this information indicates that the death of the neuronal cells in toxicants-induced neurotoxicity and neurodegenerative disease follow the common [Ca2+]-dependent mechanisms. However, further studies are needed to understand the downstream pathway that precisely orchestrate the expansion of high intracellular [Ca2+]-mediated death of neuronal cells in neurodegenerative diseases.
2.3. Oxidative Stress-mediated Neuronal Damage
Excessive production of ROS as a result of the imbalance among cellular biochemical processes gives rise to a condition known as oxidative stress [99]. ROS and reactive nitrogen species (RNS)-mediated damage to cellular macromolecules is involved in the pathogenesis of several neurodegenerative diseases [14, 100, 101]. Nervous system is particularly vulnerable to the oxidative stress due to several reasons such as: i) high oxygen consumption due to higher demand of energy (ATP), ii) relatively lower level of the endogenous antioxidants reserve, iii) abundance of polyunsaturated lipids, which are particularly susceptible to free radical attack, in the plasma membrane of large neuronal cells, iv) high levels of redox-active transition metals, v) presence of excitatory amino acids and neurotransmitters, whose metabolism can produce ROS, and vi) almost complete dependency of the neuronal cells on the membrane-dependent dynamic synaptic transmission for the effective communication [102-107]. These characteristics make neuronal cells an easy target for the ROS- and RNS-mediated damage. Although, initial events after MPP+ exposure is the inhibition of Complex I in ETC, subsequent oxidative stress is the underlying cause of the PD like condition [108]. The loss of dopaminergic and serotonergic nerve terminals in the methamphetamine (METH) and 3,4-methlyenedioxymethamphetamine (MDMA) drug abusers has been attributed to the oxidative stress [109, 110]. Neuronal degeneration and Aβ neurotoxicity in AD patients is associated with the oxidative damage to DNA, RNA, proteins and lipids [111-116]. Oxidative damage to DNA [117-119] and protein [120, 121] has been reported in the nigro-striatal region of the brain in PD. Oxidation of dopamine to a reactive 6-hydroxydopamine (6-OHDA) provides an important endogenous mechanism for the development of PD like condition [122-124]. Iron, a transition metal, overload has been recognized to play a key role in the Lewy body formation in PD [125]. Perturbed homeostasis of redox-active and inactive metals has been associated with the affected area in the brain of AD [113, 114] and PD [126-129]. Oxidative stress-induced mutations in the gene encoding for the ubiquitous Cu/Zn-superoxide dismutase (SOD-1) enzyme [130] and damage to the proteins, lipids and DNA [131-133] have been associated with the familial and sporadic forms of the ALS. Pathology of MS is no different when the detrimental effect of the oxidative stress is considered. ROS and RNS produced by the activated microglia and mononuclear cells play a major role in the pathogenesis of MS. Oxidative damage to nDNA, mtDNA and decreased level of endogenous antioxidants [134-136] have been linked to the demyelination [137] and axonal injury in MS and EAE [138]. Increased incidence of the oxidative DNA strand breaks and exacerbated lipofuscin, a pigment produced by the reaction of cellular amino compounds with the aldehydic products of the oxidative damage to tissue macromolecules, is associated with HD [139, 140]. Significant increase in the levels of 8-OH-dG in nDNA has been found in the postmortem tissue of HD cases [141]. All these evidences clearly indicate a causal relationship between the oxidative stress and neuronal cell death in neurodegenerative diseases and toxicants-induced neurotoxicity. This implies that interventional agents such as antioxidants, metal chelators and those able to boost the cellular endogenous antioxidants may offer a therapeutic strategy to prevent the continued progression and sometimes even initiation of the diseases.
2.4. Inflammation-mediated Neuronal Damage
Emerging evidence underscores an active role of neuro- inflammation in the pathophysiology of neurodegenerative diseases [142]. The initiation and subsequent propagation of the neuroinflammation appear to rely on the interaction between glia, immune cells and neurons. Immunologic privileges of brain owing to absence of physical connection between central nervous system and classical lymphatic system has recently been challenged with identification of functional and classical lymphatic system in the brain [143] suggesting an important role in the pathogenesis of neuronal diseases. Macrophages are present in the brain near glia and microglia and play a fundamental role in the inflammation-mediated neurodegenerative disorders. In the disease state, activated microglia mediate neuronal injury through the production of pro-inflammatory factors such as cytokines and chemokines [144]. Production of pro-inflammatory cytokines and chemokines leads to the trans-endothelial migration of immune cells across the blood-brain-barrier [145]. Several mechanisms have been identified for the microglia-mediated phagocytic and cytotoxic actions that are responsible for neuronal damage. The first and foremost mechanism is phagocytic oxidase (PHOX)-mediated oxidative stress-induced neurotoxicity [145, 146]. Unlike healthy brain, when PHOX (normally expressed at high levels in microglia) is acutely stimulated by inflammatory condition, it causes oxidative stress by rapidly producing high levels of superoxides (O2-).
Inflammatory activation of PHOX also causes activation of microglia [146] resulting in the production of TNFα, IL-1β and inducible NO Synthase (iNOS) [147, 148]. Thus, PHOX activation provides an important link between inflammation and oxidative stress [149]. Thus, inhibition of PHOX has been reported as a potential therapeutic strategy for several neuroinflammatory conditions. iNOS is expressed in microglia, astrocytes and is upregulated during inflammation [150, 151], resulting in increased NO production and thereby RNS leading to neuronal death through the inhibition of the mitochondrial cytochrome oxidase in the neurons [145, 152, 153]. Increased iNOS expression during inflammation may potentiate the neuronal death by hypoxic condition, as it can compete with cytochrome oxidase for the molecular oxygen [153, 154]. However, it should be noted that, activation of either NADPH oxidase (NOX/PHOX) or production of NO through iNOS expression alone is not sufficient to induce neurotoxicity, but their combined activation underlies the mechanism of inflammatory neurodegeneration [145, 155]. Apart from this, chemokines secreted by astrocytes play multiple roles in the pathology of a chronic inflammatory neurodegenerative disease such as AD [154, 156, 157]. Given the self-perpetuating cyclic nature of inflammatory processes in the progression of neurodegeneration [158], intervention with the natural compounds having strong anti-inflammatory activities may offer a safer way to prevent or at least reduce the progression of such devastating diseases.
2.5. Interplay between Intracellular Mitotoxicity, Ca2+ Accumulation, Oxidative Stress and Inflammation
Although, each mechanism discussed above can independently mediate neuronal damage, they are highly interlinked with each other and often co-exist. Every single mechanism can serve as an initiation point for the vicious cycle. But perturbation in. Ca2+ homeostasis has immediate cytotoxic consequences Ca2+ overload, as observed during excitotoxicity, is sensed by the ER, mitochondria and membrane bound Ca2+-transporter pumps. ER and mitochondria operate to re-establish intracellular Ca2+ homeostasis. Continued uptake of Ca2+ by mitochondria through Ca2+ uniporter results in the dissipation of ΔΨm, hindering ATP synthase activity. Inhibition of ATP synthesis impairs the regulatory functions of ATP-dependent Ca2+-ATPase pumps located in the membrane of ER and cell. This causes further accumulation of Ca2+ in the cytoplasm of the cells. Continuous overload of Ca2+ increases ROS production through interference with the ETC [159]. Ca2+overload can activate constitutively expressed NOS in the neuronal and endothelial cells of the brain to produce excess amount of NO [160, 161]. Coproduction of NO and O2- leads to the formation of highly reactive NOO2-. Furthermore, NOO2- can itself augment its formation through the inactivation of Mn-SOD. ROS and RNS irreversibly bind with the respiratory complexes and inhibit ATP synthesis.
Excessive production of ROS and RNS can induce DNA stand break, which leads to the activation of DNA repair enzymes such as PARP. Activated PARP causes depletion of cellular NAD+, which severely compromises ATP synthesis. Thus, oxidative and nitrosative stress can indirectly lead to the accumulation of cytoplasmic Ca2+ by depriving the functions of ATP-dependent Ca2+ pumps. Furthermore, ROS and RNS can directly inactivate ATP-dependent Ca2+ pumps by reacting with the soft nucleophile, such as thiol group, and contribute towards the cytoplasmic overload of the Ca2+ ions. Moreover, mitochondrial dysfunction in the neuro- degenerative diseases leads to the deregulation of the Ca2+ ion homeostasis [162, 163]. This provides a direct link between mitochondrial damage and intracellular Ca2+ overload. Mitochondria serve as primary site for the generation of the oxidative and nitrosative stress. The proximity of mtDNA to the sites of ROS and RNS generation and lack of protective histones makes it highly vulnerable for ROS-induced damage [164]. Literature evidences suggest that ROS- and RNS-mediated cellular damage activates glial cells to produce NO and pro-inflammatory cytokines with subsequent formation of RNS [147, 155]. Activated glial cells also release H2O2, which is converted to hypochlorous acid (HOCl), a highly toxic pro-oxidant [165]. Thus, a vicious cycle of Ca2+ overload, oxidative stress and inflammation mediates the progression of neurodegenerative diseases. This makes it challenging to distinguish the sequence of the events.
3. NUTRACEUTICALS: BRIEF OVERVIEW
Although, our understanding about the mechanisms involved in the neuronal damage has increased in recent years, we are yet to identify natural compound-based therapeutic agents that positively improve the overall neuronal health. One of the major disadvantages of the currently available therapeutic agents against the neuronal damage is that chronic use of these agents results in the multiple adverse effects. Therefore, protective strategies including nutraceuticals-based and other cost-effective therapies are warranted. The term “Nutraceutical” was coined by Dr. Stephen DeFelice, the then Chairman of the Foundation for Innovation Medicine, in 1989 [166]. Nutraceuticals are food-based products that not only supplement the diet but also help in the prevention and/or treatment of a wide variety of diseases and disorders [167]. About 2000 years ago, Hippocrates correctly stated that “Let food be your medicine and medicine be your food” [168]. According to the epidemiological studies carried out in the 1980s by Professors Doll and Peto for the World Health Organization, it has been postulated that appropriate nutrition could prevent approximately 35% of cancer deaths, and as many as 90% of certain cancers could be avoided by dietary supplementation [169]. As stated above, several mechanistic studies found that mitotoxicity, intracellular Ca2+ overload, oxidative stress and inflammation are the primary reasons for the number of neurodegenerative disorders including AD, PD, HD, MS and ALS [55, 96, 111, 142]. Nutraceuticals by virtue of their better tolerance and ability to act on multiple cellular pathways appear to be favorable candidates for the long-term consumption as required for the management of chronic ailments such as neurodegenerative disorders. Currently nutraceuticals have received considerable global interest because of their presumed safety and potential nutritional and therapeutic effects.
Some nutraceuticals such as resveratrol, α-lipoic acid, coenzyme Q10 (ubiquinone), β-carotene, lycopene, astaxanthin and curcumin, to name a few, are potent antioxidants and have showed therapeutic effects against several diseases by neutralizing oxidative stress, boosting the endogenous antioxidant levels and stabilizing mitochondrial functions [37-39, 170-173]. Several studies have suggested beneficial effects of a wide variety of nutraceuticals against various neurodegenerative disorders; however, the present review focuses on nutraceuticals for which mechanistic evidences for neuroprotection are available (Table 1).
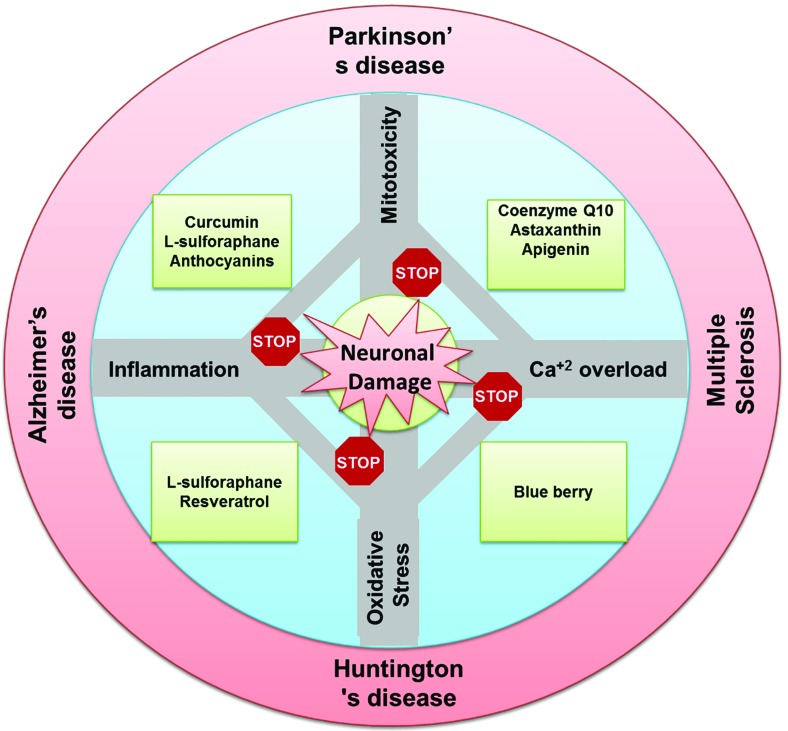
In general, the neuroprotective effects of nutraceuticals can be attributed, but not limited, to their ability to scavenge free radicals, chelate transition metals, improve antioxidant reserve and anti-inflammatory effects. At the molecular level, nutraceuticals regulate several signaling pathways which are known to play role in the cell survival and stress response such as Nrf2/ARE, mitogen-activated protein kinase (MAPK) [174], protein kinase C (PKC) [175], Janus kinase-Signal Transducer and Activator of Transcription (JAK-STAT) [176, 177], MEK/ERK/CREB, PI3K/AKT [178, 179] and insulin-signaling pathways [180, 181]. As nutraceuticals act on multiple molecular pathways, they are anticipated to improve overall neuronal health. Nonetheless, we have categorized the available evidences for some nutraceuticals-mediated neuroprotection (Table 1) based on the primary effect as concluded from the respective study. Major pathways involved in neuronal damage and nutraceuticals-mediated neuroprotection are depicted in Fig. 1.
Table 1.
Summary of the molecular neuronal effects of emerging nutraceuticals.
| S. N. | Nutraceuticals | Targeted Mechanism(s) | Implication | Refs. |
|---|---|---|---|---|
| 1. | Curcumin | • Restored the mitochondrial membrane potential, • Suppressed increase in intracellular ROS • Inhibited pro-inflammatory signaling through NF-κB or toll-like receptors • Inhibited Aβ-induced MAP kinase activation and the phosphorylation of ERK-1/2 • Inhibited phosphorylation of JNK1/2 and c-Jun • Decreased malondialdehyde levels, cytochrome c and cleaved caspase-3 expression and increasing mitochondrial Bcl-2 expression |
• Ameliorated 6-hydroxydopamine-induced neurotoxicity in MES23.5 cells • Inhibited pro-apoptotic signals in mouse models of encephalitis • Prevented Aβ-induced cell death in a human neuroblastoma cell line • Stabilized the blood brain barrier in MS • Protected C57BL/6 N mice and SH-SY5Y cells against dopaminergic neurotoxicity induced by MPTP or 1- methyl-4-phenylpyridnium ion- (MPP (+) • Protected rats against stroke |
[17, 71, 199-204] |
| 2. | α-lipoic acid | • Improved mitochondrial functions and physiology | Protected neurons in vitro and in vivo against the chemotherapy, hypoxia, Aβ, diabetic neuropathy and peripheral neuropathy associated with the cancer chemotherapy | [184-191] |
| 3. | Astaxanthin | Boosted energy production by protecting mitochondria | Protected cultured nerve cells | [192, 193] |
| 4. | Coenzyme Q10 (ubiquinone) | Preserved mitochondrial functions | Protection against stroke, epilepsy, striatal excitotoxic lesions produced by the mitochondrial toxin (Complex II inhibitor), malonate and several other neurodegenerative disorders | [56, 131, 194-196, 198] |
| 5. | L-sulforaphane (isothiocyanate compound) | Decreased ROS and inhibited pro-inflammatory signaling through NF-κB or toll-like receptors | • Inhibited dopamine quinone-induced neuronal death • Stabilized the blood brain barrier in MS |
[202, 205] |
| 6. | Tert-butyl hydroquinone | Stimulated Nrf2-ARE transcriptional pathway | Protected neurons and astrocytes against H2O2-induced oxidative stress | [205] |
| 7. | Blue berry | • Altered ROS signaling through CREB and MAP-kinase signaling pathways • Modulated inflammatory genes expression • Antagonized the increase in intracellular Ca2+ activity |
[206-209] | |
| 8. | Resveratrol | • Antioxidant properties-mediated modulations of Aβ processing and up- regulation of the longevity-linked gene, sirtuin1 • Increased 5-HT activity |
Antidepressant properties | [210, 211] |
| 9. | Carnosic acid | Scavenged ROS | Neuroprotective action both in in vitro models of neuronal death and in in vivo models of neurodegeneration Protected neuroblastoma cells from H2O2-induced oxidative stress |
[201, 212, 213] |
| 10. | Rosmarinic acid | |||
| 11. | Aged garlic extract | Suppressed ROS generation and attenuated caspase-3 activation, DNA fragmentation and PARP cleavage | Protected PC12 cells against Aβ peptide-induced apoptosis | [214] |
| 12. | Eugenol | Decreased 6-hydroxydopamine-induced lipid peroxidation and increased GSH level | Prevented 6-hydroxydopamine-induced reduction in the dopamine level in the mouse striatum | [215] |
| 13. | Anthocyanins | Negatively regulated pro-oxidants and pro-inflammatory cytokines signaling pathways | Neuroprotection | [209, 216, 217] |
| 14. | The green tea flavonoid, epigallocatechin-3-gallate | Inhibited pro-inflammatory signaling through NF-κB or toll-like receptors | Stabilized the blood brain barrier in MS | [202] |
| 15. | Mustard oil glycoside | |||
| 16. | Retinoic acid | Decreased inflammation through balancing T-lymphocyte populations in peripheral blood |
Improve plasticity, regeneration, cognition and behaviour in MS patients | [218] |
| 17. | Vitamin D | Anti- inflammatory activity | Protective in patients with MS, PD and AD | [219] |
| 18. | Vitamin E | Decreased pro-inflammatory cytokines such as IL-1β and TNF-α | Decreased neuroinflammation and neuronal degeneration in the brain of rat with kainic acid-induced status epilepticus | [220] |
| 19. | Omega-3 polyunsaturated fatty acid | Reduced inflammation | Improved neurologic recovery and attenuated white matter injury after experimental traumatic brain injury | [221] |
| 20. | Apigenin | • Stabilized mitochondrial membrane potential • Modulated GABAergic and glutamatergic transmission |
Protected neurons against copper-induced Aβ-mediated toxicity | [222, 223] |
| 21. | Soy isoflavones | Modulated brain cholinergic system | Ameliorated age-related neuronal loss and cognition decline in male rats | [224] |
| 22. | Isoflavones | Mimicked the effects of estrogen through estrogen receptor β in the brain | Improved cognitive function | [225] |
| 23. | Emodin (3-methyl-1,6,8-trihydroxyanthraquinone), an anthraquinone derivative | Attenuated EGF receptor signaling | Protected rats against psychosis and ameliorating behavioral deficits | [226] |
Fig. (1).
A diagrammatic representation of the major pathways involved in neuronal damage and nutraceuticals-mediated neuroprotection.
3.1. Nutraceuticals Targeting Mitochondrial Dysfunction
Neurons are vulnerable to mitochondrial damage [182, 183]. Several nutritional supplements have been tested for their ability to preserve mitochondrial functions and thereby offer protection against the neurodegenerative diseases. Curcumin, derived from the turmeric, ameliorated 6-hydroxydopamine-induced neurotoxicity in MES23.5 cells by partially restoring the ΔΨm, increasing the level of Cu-Zn SOD and suppressing an increase in intracellular ROS and translocation of NF-κB, a transcription-factor involved in inflammation [55]. α-lipoic acid, another potent mitochondrial stabilizer, protected neurons in vitro and in vivo against the chemotherapy, hypoxia, Aβ and other toxicants-induced neurotoxicity by improving the mitochondrial functions and physiology [184-189]. Several clinical trials have been initiated or completed for α- lipoic acid in patients with diabetic neuropathy [190] and peripheral neuropathy associated with the cancer chemotherapy [191]. Astaxanthin protected mitochondria of the cultured nerve cells and boosted energy production, without increasing the production of ROS [192, 193]. Coenzyme Q10 is another powerful mitochondrial antioxidant that protects neuronal cells by preserving the functions of mitochondria in stroke, epilepsy, striatal excitotoxic lesions produced by the mitochondrial toxin (Complex II inhibitor) and several other neurodegenerative disorders [67, 194-196]. Several clinical trials with Coenzyme Q10 suggested its beneficial effects in neurodegenerative disorders [197, 198]. These evidences strongly substantiate the ability of nutraceuticals to fight against the onset and progression of neurodegenerative disorders by primarily preserving the mitochondrial functions.
3.2. Nutraceuticals Targeting Calcium Overload
The neuroprotective effect of blue berry-extract against Aβ was mediated by its ability to antagonize increase in intracellular Ca2+ and aggregated Aβ-induced ATP leakage [207, 208]. Despite being a potentially effective therapeutic strategy [227], only few studies are available that examine the ability of nutraceuticals to antagonize intracellular Ca2+ overload. With the help of advanced in-silico tools, further mechanistic studies are required to identify some potential nutraceuticals that can prevent the pathological rise in intracellular [Ca2+].
3.3. Nutraceuticals Targeting Oxidative Stress
A large number of nutraceuticals exert both direct and indirect antioxidant effects by scavenging ROS and inducing the expression of cytoprotective proteins in a Nrf2-dependent manner, respectively [37-39]. Nrf2-antioxidant response element (ARE) signaling pathway has been identified as a promising therapeutic target for neurodegenerative diseases [228].
Pre-treatment with L-sulforaphane, an isothiocyanate compound found in broccoli and other cruciferous vegetables, markedly inhibited dopamine quinone-induced neuronal death by decreasing accumulation of ROS, attenuating membrane damage and preventing DNA fragmentation in dopaminergic cell lines (Cath. and SK-N-BE (2)C) and in mesencephalic dopaminergic neurons. Also, L-sulforaphane and tert-butylhydroquinone protected neurons and astrocytes against H2O2-induced oxidative stress in a mixed neuron-astrocyte culture system through stimulation of the Nrf2-antioxidant response element (ARE) transcriptional pathway. Activation of Nrf2 pathway induces transcription of various antioxidant genes such as γ-glutamylcysteine ligase (GCL), the rate limiting enzyme in the synthesis of glutathione (GSH), MnSOD, hemeoxygenase and NAD(P) H:quininereductase [205, 229]. Blue berry diet is also known to exert neuroprotection through alteration in ROS signaling through CREB and MAP-kinase signaling pathways [206]. Possible mechanisms for the neuroprotective effect of resveratrol are related to its antioxidant properties and its
capacity to modulate Aβ processing and up-regulation of the longevity-linked gene, sirtuin1 [211]. Curcumin decreased ROS and inhibited pro-apoptotic signals in mouse models of encephalitis [199, 201]. Carnosic acid and rosmarinic acid showed a neuroprotective action both in vitro and in vivo by scavenging ROS [201, 213]. Aged garlic extract protected PC12 cells against Aβ peptide-induced apoptosis by suppressing the generation of ROS and attenuating caspase-3 activation, DNA fragmentation and PARP cleavage [214]. Eugenol, a nutraceutical obtained from cloves, prevented 6-hydroxydopamine-induced reduction in the dopamine level in the mouse striatum by decreasing 6-hydroxydopamine-induced lipid peroxidation and increasing the GSH level [215]. Epidemiological studies have demonstrated that consuming foods rich in antioxidant vitamin supplements such as vitamin C and E are associated with a lower risk of developing PD [230]. However, at present, we are not aware of any conclusive clinical trials for these antioxidants against neurodegenerative disorders.
3.4. Nutraceuticals Targeting Inflammation
The neuroprotective effect of anthocyanins is known to be mediated through phospholipase A2 inhibition [216], which is adversely involved in a complex network of signaling pathways linking pro-oxidants and pro-inflammatory cytokines to the release of arachidonic acid and eicosanoid synthesis [217]. Blue berry diet showed neuroprotection through the modulation of genes expression involved in inflammation [209]. Curcumin inhibited the activation of NF-κB and prevented Aβ-induced cell death in a human neuroblastoma cell line, suggesting its possible role in the treatment for AD [11]. The green tea flavonoid, epigallocatechin-3-gallate, curcumin and the mustard oil glycoside are known to inhibit the pro-inflammatory signaling through NF-κB or toll-like receptors and stabilize the blood brain barrier in MS [202]. Retinoic acid, a metabolite of vitamin A, is known to increase tolerance and decrease inflammation, through balancing T-lymphocyte populations in peripheral blood, and thereby may improve plasticity, regeneration, cognition and behavior in patients with MS [218]. Vitamin D has been shown to alleviate various inflammatory markers in patients with MS, PD and AD [219]. Vitamin E supplementation reduced neuroinflammation and neuronal degeneration in the brain of rat with kainic acid-induced status epilepticus by decreasing the levels of astrocytic and microglial antigens (GFAP and MHC II, respectively) and pro-inflammatory cytokines such as IL-1β and TNF-α [220]. Omega-3 polyunsaturated fatty acid supplementation improved neurologic recovery and attenuated white matter injury after experimental traumatic brain injury by eliciting multifaceted protection against behavioral dysfunction, hippocampal neuronal loss, inflammation, and loss of myelination and impulse conductivity [221].
3.5. Nutraceuticals as Neurotransmitter Modulators
Apigenin, a flavonoid, protected neurons against copper-induced Aβ-mediated toxicity by relieving mitochondrial ΔΨm dissipation [223] and modulating GABAergic and glutamatergic transmission in the cultured cortical neurons [222]. Moreover, soy isoflavones are known to influence the brain cholinergic system and ameliorate age-related neuronal loss and cognition decline in male rats [224]. Resveratrol, a non-flavonoid polyphenol, possesses antidepressant properties, which could be attributed to its ability to increase 5-HT level [210]. Thus, a few compounds have been identified to possess neuromodulatory activities that can be leveraged for the treatment of neurodegenerative disorders.
4. CONCLUSIONS
Mitotoxicity, calcium overload, oxidative stress and inflammation are the common pathways that are involved, often together, in the neuronal damage. Activation of these pathways, viz. mitochondrial dysfunction, intracellular Ca2+ overload, oxidative stress and inflammation, in neuronal damage is widely acknowledged, and it must be coupled with a change in the expression and function of hundreds to thousands of genes. We believe that the experimental studies utilizing advanced microarray-based investigations to identify the ability of nutraceuticals to antagonize such changes are the need of hour. At present limited information is available in this direction and further research is needed.
ACKNOWLEDGEMENTs
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fu X.Y., Yang M.F., Cao M.Z., Li D.W., Yang X.Y., Sun J.Y., Zhang Z.Y., Mao L.L., Zhang S., Wang F.Z., Zhang F., Fan C.D., Sun B.L. Strategy to Suppress Oxidative Damage-Induced Neurotoxicity in PC12 Cells by Curcumin: the Role of ROS-Mediated DNA Damage and the MAPK and AKT Pathways. Mol. Neurobiol. 2014 doi: 10.1007/s12035-014-9021-1. [PMID: 25432891]. [DOI] [PubMed] [Google Scholar]
- 2.Keber U., Klietz M., Carlsson T., Oertel W.H., Weihe E., Schäfer M.K., Höglinger G.U., Depboylu C. Striatal tyrosine hydroxylase-positive neurons are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Neuroscience. 2015;298:302–317. doi: 10.1016/j.neuroscience.2015.04.021. [PMID: 25892702]. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A., Leinisch F., Kadiiska M.B., Corbett J., Mason R.P. Formation and Implications of Alpha-Synuclein Radical in Maneb- and Paraquat-Induced Models of Parkinson’s Disease. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9179-1. [PMID: 25952542]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson M.P., Magnus T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006;7(4):278–294. doi: 10.1038/nrn1886. [PMID: 16552414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zindo F.T., Joubert J., Malan S.F. Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond. Future Med. Chem. 2015;7(5):609–629. doi: 10.4155/fmc.15.12. [PMID: 25921401]. [DOI] [PubMed] [Google Scholar]
- 6.Amor S., Puentes F., Baker D., van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [PMID: 20561356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61(1):71–90. doi: 10.1002/glia.22350. [PMID: 22674585]. [DOI] [PubMed] [Google Scholar]
- 8.Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G.N., Radi E. Mitochondria, oxidative stress and neuro- degeneration. J. Neurol. Sci. 2012;322(1-2):254–262. doi: 10.1016/j.jns.2012.05.030. [PMID: 22669122]. [DOI] [PubMed] [Google Scholar]
- 9.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [PMID: 20303880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [PMID: 16805774]. [DOI] [PubMed] [Google Scholar]
- 11.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [PMID: 17051205]. [DOI] [PubMed] [Google Scholar]
- 12.Moreira P.I., Zhu X., Wang X., Lee H.G., Nunomura A., Petersen R.B., Perry G., Smith M.A. Mitochondria: a therapeutic target in neurodegeneration. Biochim. Biophys. Acta. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [PMID: 19853657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen H.T., Sawmiller D.R., Markov O., Chen M. Elevated [Ca2+]i levels occur with decreased calpain activity in aged fibroblasts and their reversal by energy-rich compounds: new paradigm for Alzheimer’s disease prevention. J. Alzheimers Dis. 2013;37(4):835–848. doi: 10.3233/JAD-131001. [PMID: 24122005]. [DOI] [PubMed] [Google Scholar]
- 14.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calì T., Ottolini D., Brini M. Mitochondrial Ca(2+) and neuro- degeneration. Cell Calcium. 2012;52(1):73–85. doi: 10.1016/j.ceca.2012.04.015. [PMID: 22608276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celsi F., Pizzo P., Brini M., Leo S., Fotino C., Pinton P., Rizzuto R. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim. Biophys. Acta. 2009;1787(5):335–344. doi: 10.1016/j.bbabio.2009.02.021. [PMID: 19268425]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verkhratsky A., Toescu E.C. Endoplasmic reticulum Ca(2+) homeostasis and neuronal death. J. Cell. Mol. Med. 2003;7(4):351–361. doi: 10.1111/j.1582-4934.2003.tb00238.x. [PMID: 14754504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80(5):780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [PMID: 11948241]. [DOI] [PubMed] [Google Scholar]
- 19.Gilgun-Sherki Y., Melamed E., Offen D. Anti-inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr. Pharm. Des. 2006;12(27):3509–3519. doi: 10.2174/138161206778343091. [PMID: 17017944]. [DOI] [PubMed] [Google Scholar]
- 20.Jung Y.W., Hysolli E., Kim K.Y., Tanaka Y., Park I.H. Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. Curr. Opin. Neurol. 2012;25(2):125–130. doi: 10.1097/WCO.0b013e3283518226. [PMID: 22357218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young A.B. Four decades of neurodegenerative disease research: how far we have come! J. Neurosci. 2009;29(41):12722–12728. doi: 10.1523/JNEUROSCI.3767-09.2009. [PMID: 19828782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [PMID: 24756517]. [DOI] [PubMed] [Google Scholar]
- 23.Dantuma E., Merchant S., Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res. Ther. 2010;1(5):37. doi: 10.1186/scrt37. [PMID: 21144012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkel P., Chai C.L., Sperlágh B., Huleatt P.B., Mátyus P. Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin. Investig. Drugs. 2012;21(9):1267–1308. doi: 10.1517/13543784.2012.703178. [PMID: 22741814]. [DOI] [PubMed] [Google Scholar]
- 25.Dye R.V., Miller K.J., Singer E.J., Levine A.J. Hormone replacement therapy and risk for neurodegenerative diseases. 2012. [DOI] [PMC free article] [PubMed]
- 26.Moraes W.A. Current Pharmacological and Non-Pharmacological Therapies for Neurodegenerative Diseases. 2015.
- 27.Moreno J.A., Halliday M., Molloy C., Radford H., Verity N., Axten J.M., Ortori C.A., Willis A.E., Fischer P.M., Barrett D.A., Mallucci G.R. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013;5(206):206ra138. doi: 10.1126/scitranslmed.3006767. [PMID: 24107777]. [DOI] [PubMed] [Google Scholar]
- 28.Weissmiller A.M., Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012;1(1):14. doi: 10.1186/2047-9158-1-14. [PMID: 23210531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrish P. Prescribing in Parkinson’s disease: a story of hope and adverse events. Pract. Neurol. 2012;12(5):335–340. doi: 10.1136/practneurol-2012-000210. [PMID: 22976066]. [DOI] [PubMed] [Google Scholar]
- 30.Roch-Torreilles I., Camu W., Hillaire-Buys D. Therapie. 2000;55(2):303–312. [Adverse efects of riluzole (Rilutek) in the treatment of amyotrophic lateral sclerosis]. [Adverse efects of riluzole (Rilutek) in the treatment of amyotrophic lateral sclerosis]. [PMID: 10967703]. [PubMed] [Google Scholar]
- 31.Abeliovich A., Doege C.A. Reprogramming therapeutics: iPS cell prospects for neurodegenerative disease. Neuron. 2009;61(3):337–339. doi: 10.1016/j.neuron.2009.01.024. [PMID: 19217371]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunn J.S., Sakowski S.A., Hur J., Feldman E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011;70(3):353–361. doi: 10.1002/ana.22487. [PMID: 21905078]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whalley K. Neurodegenerative disease: Discouraging transplant results. Nat. Rev. Neurosci. 2009;10(9):624–624. [Google Scholar]
- 34.Essa M.M., Subash S., Akbar M., Al-Adawi S., Guillemin G.J. Long-term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS One. 2015;10(3):e0120964. doi: 10.1371/journal.pone.0120964. [PMID: 25807081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh N.D., Edwall D., Lindmark L., Kousoulas K.G., Iyer A.V., Haron M.H., Pasco D.S. Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A (H1N1) virus infection. Phytomedicine. 2015;22(2):271–276. doi: 10.1016/j.phymed.2014.12.006. [PMID: 25765832]. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S.S., Kumar A., Arora M., Kaundal R.K. Neuroprotective potential of combination of resveratrol and 4-amino 1,8 naphthalimide in experimental diabetic neuropathy: focus on functional, sensorimotor and biochemical changes. Free Radic. Res. 2009;43(4):400–408. doi: 10.1080/10715760902801509. [PMID: 19291593]. [DOI] [PubMed] [Google Scholar]
- 37.Tripathi D.N., Jena G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. 2010;696(1):69–80. doi: 10.1016/j.mrgentox.2009.12.014. [PMID: 20038455]. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi P.P., Jena G.B. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem. Toxicol. 2013;59:339–355. doi: 10.1016/j.fct.2013.06.019. [PMID: 23793040]. [DOI] [PubMed] [Google Scholar]
- 39.Trivedi P.P., Jena G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2014 doi: 10.1007/s00394-014-0745-5. [PMID: 25074825]. [DOI] [PubMed] [Google Scholar]
- 40.Atlante A., Calissano P., Bobba A., Giannattasio S., Marra E., Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497(1):1–5. doi: 10.1016/s0014-5793(01)02437-1. [PMID: 11376653]. [DOI] [PubMed] [Google Scholar]
- 41.Chen H., Chan D.C. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 2009;18(R2):R169–R176. doi: 10.1093/hmg/ddp326. [PMID: 19808793]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galluzzi L., Blomgren K., Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat. Rev. Neurosci. 2009;10(7):481–494. doi: 10.1038/nrn2665. [PMID: 19543220]. [DOI] [PubMed] [Google Scholar]
- 43.Mancuso M., Coppede F., Migliore L., Siciliano G., Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J. Alzheimers Dis. 2006;10(1):59–73. doi: 10.3233/jad-2006-10110. [PMID: 16988483]. [DOI] [PubMed] [Google Scholar]
- 44.Rintoul G.L., Reynolds I.J. Mitochondrial trafficking and morphology in neuronal injury. Biochim. Biophys. Acta. 2010;1802(1):143–150. doi: 10.1016/j.bbadis.2009.09.005. [PMID: 19747973]. [DOI] [PubMed] [Google Scholar]
- 45.Schinder A.F., Olson E.C., Spitzer N.C., Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 1996;16(19):6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [PMID: 8815895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su B., Wang X., Zheng L., Perry G., Smith M.A., Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim. Biophys. Acta. 2010;1802(1):135–142. doi: 10.1016/j.bbadis.2009.09.013. [PMID: 19799998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White R.J., Reynolds I.J. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J. Neurosci. 1995;15(2):1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [PMID: 7869100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieloch T. Mitochondrial involvement in acute neuro- degeneration. IUBMB Life. 2001;52(3-5):247–254. doi: 10.1080/15216540152846064. [PMID: 11798039]. [DOI] [PubMed] [Google Scholar]
- 49.Beal M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [PMID: 16178023]. [DOI] [PubMed] [Google Scholar]
- 50.Nicklas W.J., Vyas I., Heikkila R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetra- hydropyridine. Life Sci. 1985;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [PMID: 2861548]. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay R.R., Salach J.I., Dadgar J., Singer T.P. Inhibition of mitochondrial NADH dehydrogenase by pyridine derivatives and its possible relation to experimental and idiopathic parkinsonism. Biochem. Biophys. Res. Commun. 1986;135(1):269–275. doi: 10.1016/0006-291x(86)90972-1. [PMID: 3485428]. [DOI] [PubMed] [Google Scholar]
- 52.Zheng H., Xiao W.H., Bennett G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011;232(2):154–161. doi: 10.1016/j.expneurol.2011.08.016. [PMID: 21907196]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng H., Xiao W.H., Bennett G.J. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp. Neurol. 2012;238(2):225–234. doi: 10.1016/j.expneurol.2012.08.023. [PMID: 22947198]. [DOI] [PubMed] [Google Scholar]
- 54.Podratz J.L., Knight A.M., Ta L.E., Staff N.P., Gass J.M., Genelin K., Schlattau A., Lathroum L., Windebank A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011;41(3):661–668. doi: 10.1016/j.nbd.2010.11.017. [PMID: 21145397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aliev G., Seyidova D., Neal M.L., Shi J., Lamb B.T., Siedlak S.L., Vinters H.V., Head E., Perry G., Lamanna J.C., Friedland R.P., Cotman C.W. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels as a central target for the development of human AD and AD-like pathology in aged transgenic mice. Ann. N. Y. Acad. Sci. 2002;977:45–64. doi: 10.1111/j.1749-6632.2002.tb04798.x. [PMID: 12480733]. [DOI] [PubMed] [Google Scholar]
- 56.Beal M.F. Neurochemistry and toxin models in Huntington’s disease. Curr. Opin. Neurol. 1994;7(6):542–547. doi: 10.1097/00019052-199412000-00012. [PMID: 7866587]. [DOI] [PubMed] [Google Scholar]
- 57.Beal M.F. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004;36(4):381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [PMID: 15377876]. [DOI] [PubMed] [Google Scholar]
- 58.Su K., Bourdette D., Forte M. Mitochondrial dysfunction and neurodegeneration in multiple sclerosis. Front. Physiol. 2013;4:169. doi: 10.3389/fphys.2013.00169. [PMID: 23898299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong P.C., Pardo C.A., Borchelt D.R., Lee M.K., Copeland N.G., Jenkins N.A., Sisodia S.S., Cleveland D.W., Price D.L. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14(6):1105–1116. doi: 10.1016/0896-6273(95)90259-7. [PMID: 7605627]. [DOI] [PubMed] [Google Scholar]
- 60.Dawson T.M., Dawson V.L. Molecular pathways of neuro- degeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [PMID: 14593166]. [DOI] [PubMed] [Google Scholar]
- 61.Schapira A.H. Causes of neuronal death in Parkinson’s disease. Adv. Neurol. 2001;86:155–162. [PMID: 11553973]. [PubMed] [Google Scholar]
- 62.Sullivan P.G., Brown M.R. Mitochondrial aging and dysfunction in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(3):407–410. doi: 10.1016/j.pnpbp.2004.12.007. [PMID: 15795049]. [DOI] [PubMed] [Google Scholar]
- 63.Brown R.H. Jr Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995;80(5):687–692. doi: 10.1016/0092-8674(95)90346-1. [PMID: 7889564]. [DOI] [PubMed] [Google Scholar]
- 64.Hensley K., Mhatre M., Mou S., Pye Q.N., Stewart C., West M., Williamson K.S. On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid. Redox Signal. 2006;8(11-12):2075–2087. doi: 10.1089/ars.2006.8.2075. [PMID: 17034351]. [DOI] [PubMed] [Google Scholar]
- 65.Di Filippo M., Tozzi A., Tantucci M., Arcangeli S., Chiasserini D., Ghiglieri V., de Iure A., Calabresi P. Interferon-β1a protects neurons against mitochondrial toxicity via modulation of STAT1 signaling: electrophysiological evidence. Neurobiol. Dis. 2014;62:387–393. doi: 10.1016/j.nbd.2013.09.022. [PMID: 24135008]. [DOI] [PubMed] [Google Scholar]
- 66.Qi X., Lewin A.S., Sun L., Hauswirth W.W., Guy J. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J. Biol. Chem. 2006;281(42):31950–31962. doi: 10.1074/jbc.M603717200. [PMID: 16920708]. [DOI] [PubMed] [Google Scholar]
- 67.Beal M.F., Henshaw D.R., Jenkins B.G., Rosen B.R., Schulz J.B. Coenzyme Q10 and nicotinamide block striatal lesions produced by the mitochondrial toxin malonate. Ann. Neurol. 1994;36(6):882–888. doi: 10.1002/ana.410360613. [PMID: 7998775]. [DOI] [PubMed] [Google Scholar]
- 68.Beal M.F., Swartz K.J., Hyman B.T., Storey E., Finn S.F., Koroshetz W. Aminooxyacetic acid results in excitotoxin lesions by a novel indirect mechanism. J. Neurochem. 1991;57(3):1068–1073. doi: 10.1111/j.1471-4159.1991.tb08258.x. [PMID: 1830613]. [DOI] [PubMed] [Google Scholar]
- 69.Greene J.G., Porter R.H., Eller R.V., Greenamyre J.T. Inhibition of succinate dehydrogenase by malonic acid produces an “excitotoxic” lesion in rat striatum. J. Neurochem. 1993;61(3):1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [PMID: 8360680]. [DOI] [PubMed] [Google Scholar]
- 70.Ludolph A.C., He F., Spencer P.S., Hammerstad J., Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991;18(4):492–498. doi: 10.1017/s0317167100032212. [PMID: 1782616]. [DOI] [PubMed] [Google Scholar]
- 71.Tang T.S., Slow E., Lupu V., Stavrovskaya I.G., Sugimori M., Llinás R., Kristal B.S., Hayden M.R., Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2005;102(7):2602–2607. doi: 10.1073/pnas.0409402102. [PMID: 15695335]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu M., Gash M.T., Mann V.M., Javoy-Agid F., Cooper J.M., Schapira A.H. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996;39(3):385–389. doi: 10.1002/ana.410390317. [PMID: 8602759]. [DOI] [PubMed] [Google Scholar]
- 73.Reddy P.H., Shirendeb U.P. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim. Biophys. Acta. 2012;1822(2):101–110. doi: 10.1016/j.bbadis.2011.10.016. [PMID: 22080977]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trushina E., McMurray C.T. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145(4):1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [PMID: 17303344]. [DOI] [PubMed] [Google Scholar]
- 75.Schanne F.A., Kane A.B., Young E.E., Farber J.L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206(4419):700–702. doi: 10.1126/science.386513. [PMID: 386513]. [DOI] [PubMed] [Google Scholar]
- 76.Schlaepfer W.W., Bunge R.P. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 1973;59(2 Pt 1):456–470. doi: 10.1083/jcb.59.2.456. [PMID: 4805010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borle A.B. Membrane transfer of calcium. Clin. Orthop. Relat. Res. 1967;52(52):267–291. doi: 10.1097/00003086-196700520-00022. [PMID: 6048916]. [DOI] [PubMed] [Google Scholar]
- 78.Hodgkin A.L., Keynes R.D. Movements of labelled calcium in squid giant axons. J. Physiol. 1957;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [PMID: 13526124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970;170(3956):404–412. doi: 10.1126/science.170.3956.404. [PMID: 4318972]. [DOI] [PubMed] [Google Scholar]
- 80.Blaustein M.P., Lederer W.J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 1999;79(3):763–854. doi: 10.1152/physrev.1999.79.3.763. [PMID: 10390518]. [DOI] [PubMed] [Google Scholar]
- 81.Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J. Physiol. 1993;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [PMID: 8229797]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehninger A.L. Mitochondria and calcium ion transport. Biochem. J. 1970;119(2):129–138. doi: 10.1042/bj1190129. [PMID: 4922961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Racay P., Kaplán P., Lehotský J. Control of Ca2+ homeostasis in neuronal cells. Gen. Physiol. Biophys. 1996;15(3):193–210. [PMID: 9076503]. [PubMed] [Google Scholar]
- 84.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4(7):552–565. doi: 10.1038/nrm1150. [PMID: 12838338]. [DOI] [PubMed] [Google Scholar]
- 85.Chao D., Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog. Neurobiol. 2010;90(4):439–470. doi: 10.1016/j.pneurobio.2009.12.007. [PMID: 20036308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon R.P., Griffiths T., Evans M.C., Swan J.H., Meldrum B.S. Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J. Cereb. Blood Flow Metab. 1984;4(3):350–361. doi: 10.1038/jcbfm.1984.52. [PMID: 6470053]. [DOI] [PubMed] [Google Scholar]
- 87.Murphy A.N., Fiskum G., Beal M.F. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J. Cereb. Blood Flow Metab. 1999;19(3):231–245. doi: 10.1097/00004647-199903000-00001. [PMID: 10078875]. [DOI] [PubMed] [Google Scholar]
- 88.Penn R.D., Loewenstein W.R. Uncoupling of a nerve cell membrane junction by calcium-ion removal. Science. 1966;151(3706):88–89. doi: 10.1126/science.151.3706.88. [PMID: 4956065]. [DOI] [PubMed] [Google Scholar]
- 89.Tedesco E., Rigoni M., Caccin P., Grishin E., Rossetto O., Montecucco C. Calcium overload in nerve terminals of cultured neurons intoxicated by alpha-latrotoxin and snake PLA2 neurotoxins. Toxicon. 2009;54(2):138–144. doi: 10.1016/j.toxicon.2009.03.025. [PMID: 19341756]. [DOI] [PubMed] [Google Scholar]
- 90.Manev H., Favaron M., Guidotti A., Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 1989;36(1):106–112. [PMID: 2568579]. [PubMed] [Google Scholar]
- 91.Marcoux F.W., Probert A.W., Jr, Weber M.L. Hypoxic neuronal injury in tissue culture is associated with delayed calcium accumulation. Stroke. 1990;21(11) Suppl.:III71–III74. [PMID: 2146782]. [PubMed] [Google Scholar]
- 92.Mattson M.P. Calcium and neurodegeneration. Aging Cell. 2007;6(3):337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [PMID: 17328689]. [DOI] [PubMed] [Google Scholar]
- 93.Zündorf G., Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011;14(7):1275–1288. doi: 10.1089/ars.2010.3359. [PMID: 20615073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berridge M.J. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. 2010;459(3):441–449. doi: 10.1007/s00424-009-0736-1. [PMID: 19795132]. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto S., Wajima T., Hara Y., Nishida M., Mori Y. Transient receptor potential channels in Alzheimer’s disease. Biochim. Biophys. Acta. 2007;1772(8):958–967. doi: 10.1016/j.bbadis.2007.03.006. [PMID: 17490865]. [DOI] [PubMed] [Google Scholar]
- 96.Chan C.S., Gertler T.S., Surmeier D.J. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32(5):249–256. doi: 10.1016/j.tins.2009.01.006. [PMID: 19307031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurnellas M.P., Donahue K.C., Elkabes S. Mechanisms of neuronal damage in multiple sclerosis and its animal models: role of calcium pumps and exchangers. Biochem. Soc. Trans. 2007;35(Pt 5):923–926. doi: 10.1042/BST0350923. [PMID: 17956247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283(9):5780–5789. doi: 10.1074/jbc.M704704200. [PMID: 18156184]. [DOI] [PubMed] [Google Scholar]
- 99.Betteridge D.J. What is oxidative stress? Metabolism. 2000;49(2) Suppl. 1:3–8. doi: 10.1016/s0026-0495(00)80077-3. [PMID: 10693912]. [DOI] [PubMed] [Google Scholar]
- 100.Harman D. The aging process. Proc. Natl. Acad. Sci. USA. 1981;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [PMID: 6947277]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sayre L.M., Perry G., Smith M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;21(1):172–188. doi: 10.1021/tx700210j. [PMID: 18052107]. [DOI] [PubMed] [Google Scholar]
- 102.Bush A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000;4(2):184–191. doi: 10.1016/s1367-5931(99)00073-3. [PMID: 10742195]. [DOI] [PubMed] [Google Scholar]
- 103.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [PMID: 37532]. [DOI] [PubMed] [Google Scholar]
- 104.Floyd R.A., Carney J.M. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992;32(Suppl.):S22–S27. doi: 10.1002/ana.410320706. [PMID: 1510377]. [DOI] [PubMed] [Google Scholar]
- 105.Gilgun-Sherki Y., Melamed E., Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–975. doi: 10.1016/s0028-3908(01)00019-3. [PMID: 11406187]. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi S., Takahashi I., Sato H., Kubota Y., Yoshida S., Muramatsu Y. Age-related changes in the concentrations of major and trace elements in the brain of rats and mice. Biol. Trace Elem. Res. 2001;80(2):145–158. doi: 10.1385/BTER:80:2:145. [PMID: 11437180]. [DOI] [PubMed] [Google Scholar]
- 107.Zaleska M.M., Floyd R.A. Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem. Res. 1985;10(3):397–410. doi: 10.1007/BF00964608. [PMID: 4000395]. [DOI] [PubMed] [Google Scholar]
- 108.Keeney P.M., Xie J., Capaldi R.A., Bennett J.P. Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006;26(19):5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [PMID: 16687518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quinton M.S., Yamamoto B.K. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8(2):E337–E347. doi: 10.1007/BF02854904. [PMID: 16796384]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamamoto B.K., Bankson M.G. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit. Rev. Neurobiol. 2005;17(2):87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [PMID: 16808729]. [DOI] [PubMed] [Google Scholar]
- 111.Hensley K., Butterfield D.A., Hall N., Cole P., Subramaniam R., Mark R., Mattson M.P., Markesbery W.R., Harris M.E., Aksenov M. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann. N. Y. Acad. Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [PMID: 8687014]. [DOI] [PubMed] [Google Scholar]
- 112.Liu Q., Smith M.A., Avilá J., DeBernardis J., Kansal M., Takeda A., Zhu X., Nunomura A., Honda K., Moreira P.I., Oliveira C.R., Santos M.S., Shimohama S., Aliev G., de la Torre J., Ghanbari H.A., Siedlak S.L., Harris P.L., Sayre L.M., Perry G. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 2005;38(6):746–754. doi: 10.1016/j.freeradbiomed.2004.11.005. [PMID: 15721985]. [DOI] [PubMed] [Google Scholar]
- 113.Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S., Chiba S., Atwood C.S., Petersen R.B., Smith M.A. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [PMID: 11487050]. [DOI] [PubMed] [Google Scholar]
- 114.Nunomura A., Perry G., Pappolla M.A., Wade R., Hirai K., Chiba S., Smith M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 1999;19(6):1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [PMID: 10066249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perry G., Raina A.K., Nunomura A., Wataya T., Sayre L.M., Smith M.A. How important is oxidative damage? Lessons from Alzheimer’s disease. Free Radic. Biol. Med. 2000;28(5):831–834. doi: 10.1016/s0891-5849(00)00158-1. [PMID: 10754280]. [DOI] [PubMed] [Google Scholar]
- 116.Sayre L.M., Zelasko D.A., Harris P.L., Perry G., Salomon R.G., Smith M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997;68(5):2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [PMID: 9109537]. [DOI] [PubMed] [Google Scholar]
- 117.Alam Z.I., Jenner A., Daniel S.E., Lees A.J., Cairns N., Marsden C.D., Jenner P., Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69(3):1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [PMID: 9282943]. [DOI] [PubMed] [Google Scholar]
- 118.Kikuchi A., Takeda A., Onodera H., Kimpara T., Hisanaga K., Sato N., Nunomura A., Castellani R.J., Perry G., Smith M.A., Itoyama Y. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol. Dis. 2002;9(2):244–248. doi: 10.1006/nbdi.2002.0466. [PMID: 11895375]. [DOI] [PubMed] [Google Scholar]
- 119.Zhang J., Perry G., Smith M.A., Robertson D., Olson S.J., Graham D.G., Montine T.J. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999;154(5):1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [PMID: 10329595]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alam Z.I., Daniel S.E., Lees A.J., Marsden D.C., Jenner P., Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997;69(3):1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [PMID: 9282961]. [DOI] [PubMed] [Google Scholar]
- 121.Floor E., Wetzel M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [PMID: 9422371]. [DOI] [PubMed] [Google Scholar]
- 122.Napolitano A., Crescenzi O., Pezzella A., Prota G. Generation of the neurotoxin 6-hydroxydopamine by peroxidase/H2O2 oxidation of dopamine. J. Med. Chem. 1995;38(6):917–922. doi: 10.1021/jm00006a010. [PMID: 7699708]. [DOI] [PubMed] [Google Scholar]
- 123.Orth M., Tabrizi S.J. Models of Parkinson’s disease. Mov. Disord. 2003;18(7):729–737. doi: 10.1002/mds.10447. [PMID: 12815651]. [DOI] [PubMed] [Google Scholar]
- 124.Pezzella A., d’Ischia M., Napolitano A., Misuraca G., Prota G. Iron-mediated generation of the neurotoxin 6-hydroxydopamine quinone by reaction of fatty acid hydroperoxides with dopamine: a possible contributory mechanism for neuronal degeneration in Parkinson’s disease. J. Med. Chem. 1997;40(14):2211–2216. doi: 10.1021/jm970099t. [PMID: 9216840]. [DOI] [PubMed] [Google Scholar]
- 125.Kaur D., Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Res. Rev. 2004;3(3):327–343. doi: 10.1016/j.arr.2004.01.003. [PMID: 15231240]. [DOI] [PubMed] [Google Scholar]
- 126.Nunomura A., Chiba S., Kosaka K., Takeda A., Castellani R.J., Smith M.A., Perry G. Neuronal RNA oxidation is a prominent feature of dementia with Lewy bodies. Neuroreport. 2002;13(16):2035–2039. doi: 10.1097/00001756-200211150-00009. [PMID: 12438921]. [DOI] [PubMed] [Google Scholar]
- 127.Salazar J., Mena N., Núñez M.T. Iron dyshomeostasis in Parkinson’s disease. J. Neural Transm. Suppl. 2006;(71):205–213. doi: 10.1007/978-3-211-33328-0_22. [PMID: 17447431]. [DOI] [PubMed] [Google Scholar]
- 128.Sian-Hülsmann J., Mandel S., Youdim M.B., Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011;118(6):939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [PMID: 21138437]. [DOI] [PubMed] [Google Scholar]
- 129.Youdim M.B., Stephenson G., Ben Shachar D. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann. N. Y. Acad. Sci. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [PMID: 15105275]. [DOI] [PubMed] [Google Scholar]
- 130.Taylor D.M., Gibbs B.F., Kabashi E., Minotti S., Durham H.D., Agar J.N. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 2007;282(22):16329–16335. doi: 10.1074/jbc.M610119200. [PMID: 17389599]. [DOI] [PubMed] [Google Scholar]
- 131.Ferrante R.J., Browne S.E., Shinobu L.A., Bowling A.C., Baik M.J., MacGarvey U., Kowall N.W., Brown R.H., Jr, Beal M.F. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997;69(5):2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [PMID: 9349552]. [DOI] [PubMed] [Google Scholar]
- 132.Kikuchi S., Shinpo K., Ogata A., Tsuji S., Takeuchi M., Makita Z., Tashiro K. Detection of N epsilon-(carboxymethyl)lysine (CML) and non-CML advanced glycation end-products in the anterior horn of amyotrophic lateral sclerosis spinal cord. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2002;3(2):63–68. doi: 10.1080/146608202760196020. [PMID: 12215227]. [DOI] [PubMed] [Google Scholar]
- 133.Pedersen W.A., Fu W., Keller J.N., Markesbery W.R., Appel S., Smith R.G., Kasarskis E., Mattson M.P. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 1998;44(5):819–824. doi: 10.1002/ana.410440518. [PMID: 9818940]. [DOI] [PubMed] [Google Scholar]
- 134.Calabrese V., Raffaele R., Cosentino E., Rizza V. Changes in cerebrospinal fluid levels of malondialdehyde and glutathione reductase activity in multiple sclerosis. Int. J. Clin. Pharmacol. Res. 1994;14(4):119–123. [PMID: 7607784]. [PubMed] [Google Scholar]
- 135.Ferretti G., Bacchetti T., Principi F., Di Ludovico F., Viti B., Angeleri V.A., Danni M., Provinciali L. Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: a relationship with paraoxonase activity. Mult. Scler. 2005;11(6):677–682. doi: 10.1191/1352458505ms1240oa. [PMID: 16320727]. [DOI] [PubMed] [Google Scholar]
- 136.Karg E., Klivényi P., Németh I., Bencsik K., Pintér S., Vécsei L. Nonenzymatic antioxidants of blood in multiple sclerosis. J. Neurol. 1999;246(7):533–539. doi: 10.1007/s004150050399. [PMID: 10463352]. [DOI] [PubMed] [Google Scholar]
- 137.Smith K.J., Kapoor R., Felts P.A. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9(1):69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [PMID: 9989453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gilgun-Sherki Y., Melamed E., Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J. Neurol. 2004;251(3):261–268. doi: 10.1007/s00415-004-0348-9. [PMID: 15015004]. [DOI] [PubMed] [Google Scholar]
- 139.Goebel H.H., Heipertz R., Scholz W., Iqbal K., Tellez-Nagel I. Juvenile Huntington chorea: clinical, ultrastructural, and biochemical studies. Neurology. 1978;28(1):23–31. doi: 10.1212/wnl.28.1.23. [PMID: 145549]. [DOI] [PubMed] [Google Scholar]
- 140.Tellez-Nagel I., Johnson A.B., Terry R.D. Studies on brain biopsies of patients with Huntington’s chorea. J. Neuropathol. Exp. Neurol. 1974;33(2):308–332. doi: 10.1097/00005072-197404000-00008. [PMID: 4150800]. [DOI] [PubMed] [Google Scholar]
- 141.Browne S.E., Bowling A.C., MacGarvey U., Baik M.J., Berger S.C., Muqit M.M., Bird E.D., Beal M.F. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41(5):646–653. doi: 10.1002/ana.410410514. [PMID: 9153527]. [DOI] [PubMed] [Google Scholar]
- 142.Dong H., Zhang X., Qian Y. Mast cells and neuroinflammation. Med. Sci. Monit. Basic Res. 2014;20:200–206. doi: 10.12659/MSMBR.893093. [PMID: 25529562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., Harris T.H., Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [PMID: 26030524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gendelman H.E. Neural immunity: Friend or foe? J. Neurovirol. 2002;8(6):474–479. doi: 10.1080/13550280290168631. [PMID: 12476342]. [DOI] [PubMed] [Google Scholar]
- 145.Bal-Price A., Matthias A., Brown G.C. Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J. Neurochem. 2002;80(1):73–80. doi: 10.1046/j.0022-3042.2001.00675.x. [PMID: 11796745]. [DOI] [PubMed] [Google Scholar]
- 146.Mander P.K., Jekabsone A., Brown G.C. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006;176(2):1046–1052. doi: 10.4049/jimmunol.176.2.1046. [PMID: 16393992]. [DOI] [PubMed] [Google Scholar]
- 147.Jekabsone A., Mander P.K., Tickler A., Sharpe M., Brown G.C. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J. Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [PMID: 16959029]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pawate S., Shen Q., Fan F., Bhat N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004;77(4):540–551. doi: 10.1002/jnr.20180. [PMID: 15264224]. [DOI] [PubMed] [Google Scholar]
- 149.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [PMID: 17180163]. [DOI] [PubMed] [Google Scholar]
- 150.Heneka M.T., Feinstein D.L. Expression and function of inducible nitric oxide synthase in neurons. J. Neuroimmunol. 2001;114(1-2):8–18. doi: 10.1016/s0165-5728(01)00246-6. [PMID: 11240010]. [DOI] [PubMed] [Google Scholar]
- 151.Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29(1):1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [PMID: 10594918]. [DOI] [PubMed] [Google Scholar]
- 152.Bal-Price A., Brown G.C. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [PMID: 11517237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown G.C., Cooper C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356(2-3):295–298. doi: 10.1016/0014-5793(94)01290-3. [PMID: 7805858]. [DOI] [PubMed] [Google Scholar]
- 154.Mander P., Borutaite V., Moncada S., Brown G.C. Nitric oxide from inflammatory-activated glia synergizes with hypoxia to induce neuronal death. J. Neurosci. Res. 2005;79(1-2):208–215. doi: 10.1002/jnr.20285. [PMID: 15558752]. [DOI] [PubMed] [Google Scholar]
- 155.Mander P., Brown G.C. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflammation. 2005;2:20. doi: 10.1186/1742-2094-2-20. [PMID: 16156895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu X., Miller M.J., Joshi M.S., Thomas D.D., Lancaster J.R. Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA. 1998;95(5):2175–2179. doi: 10.1073/pnas.95.5.2175. [PMID: 9482858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Santolini J., Meade A.L., Stuehr D.J. Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases I, II, and III. J. Biol. Chem. 2001;276(52):48887–48898. doi: 10.1074/jbc.M108666200. [PMID: 11684690]. [DOI] [PubMed] [Google Scholar]
- 158.Schwab C., McGeer P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. 2008;13(4):359–369. doi: 10.3233/jad-2008-13402. [PMID: 18487845]. [DOI] [PubMed] [Google Scholar]
- 159.Jacobson J., Duchen M.R. Mitochondrial oxidative stress and cell death in astrocytes--requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002;115(Pt 6):1175–1188. doi: 10.1242/jcs.115.6.1175. [PMID: 11884517]. [DOI] [PubMed] [Google Scholar]
- 160.Murphy M.P. Nitric oxide and cell death. Biochim. Biophys. Acta. 1999;1411(2-3):401–414. doi: 10.1016/s0005-2728(99)00029-8. [PMID: 10320672]. [DOI] [PubMed] [Google Scholar]
- 161.Szabó C., Zingarelli B., O’Connor M., Salzman A.L. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc. Natl. Acad. Sci. USA. 1996;93(5):1753–1758. doi: 10.1073/pnas.93.5.1753. [PMID: 8700830]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frieden M., James D., Castelbou C., Danckaert A., Martinou J.C., Demaurex N. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J. Biol. Chem. 2004;279(21):22704–22714. doi: 10.1074/jbc.M312366200. [PMID: 15024001]. [DOI] [PubMed] [Google Scholar]
- 163.Szabadkai G., Simoni A.M., Chami M., Wieckowski M.R., Youle R.J., Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell. 2004;16(1):59–68. doi: 10.1016/j.molcel.2004.09.026. [PMID: 15469822]. [DOI] [PubMed] [Google Scholar]
- 164.Kim H.R., Won S.J., Fabian C., Kang M.G., Szardenings M., Shin M.G. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann. Lab. Med. 2015;35(1):1–14. doi: 10.3343/alm.2015.35.1.1. [PMID: 25553274]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Andersen J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 2004;10(Suppl.):S18–S25. doi: 10.1038/nrn1434. [PMID: 15298006]. [DOI] [PubMed] [Google Scholar]
- 166.Kalra E.K. Nutraceutical--definition and introduction. AAPS PharmSci. 2003;5(3):E25. doi: 10.1208/ps050325. [PMID: 14621960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brower V. Nutraceuticals: poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998;16(8):728–731. doi: 10.1038/nbt0898-728. [PMID: 9702769]. [DOI] [PubMed] [Google Scholar]
- 168.Prasad C. Improving mental health through nutrition: the future. Nutr. Neurosci. 2001;4(4):251–272. doi: 10.1080/1028415x.2001.11747367. [PMID: 11842893]. [DOI] [PubMed] [Google Scholar]
- 169.Hardy G., Hardy I., Ball P.A. Nutraceuticals--a pharmaceutical viewpoint: part II. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6(6):661–671. doi: 10.1097/00075197-200311000-00010. [PMID: 14557798]. [DOI] [PubMed] [Google Scholar]
- 170.Dadhania V.P., Tripathi D.N., Vikram A., Ramarao P., Jena G.B. Intervention of alpha-lipoic acid ameliorates methotrexate-induced oxidative stress and genotoxicity: A study in rat intestine. Chem. Biol. Interact. 2010;183(1):85–97. doi: 10.1016/j.cbi.2009.10.020. [PMID: 19900424]. [DOI] [PubMed] [Google Scholar]
- 171.Kumar A., Sharma S.S. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem. Biophys. Res. Commun. 2010;394(2):360–365. doi: 10.1016/j.bbrc.2010.03.014. [PMID: 20211601]. [DOI] [PubMed] [Google Scholar]
- 172.Prakash A., Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer׳s disease. Eur. J. Pharmacol. 2014;741:104–111. doi: 10.1016/j.ejphar.2014.07.036. [PMID: 25066110]. [DOI] [PubMed] [Google Scholar]
- 173.Soto-Urquieta M.G., López-Briones S., Pérez-Vázquez V., Saavedra-Molina A., González-Hernández G.A., Ramírez-Emiliano J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014;47(1):74. doi: 10.1186/0717-6287-47-74. [PMID: 25723052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroeter H., Boyd C., Spencer J.P., Williams R.J., Cadenas E., Rice-Evans C. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol. Aging. 2002;23(5):861–880. doi: 10.1016/s0197-4580(02)00075-1. [PMID: 12392791]. [DOI] [PubMed] [Google Scholar]
- 175.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [PMID: 15019969]. [DOI] [PubMed] [Google Scholar]
- 176.Nicolas C.S., Amici M., Bortolotto Z.A., Doherty A., Csaba Z., Fafouri A., Dournaud P., Gressens P., Collingridge G.L., Peineau S. The role of JAK-STAT signaling within the CNS. JAK-STAT. 2013;2(1):e22925. doi: 10.4161/jkst.22925. [PMID: 24058789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim H.Y., Park E.J., Joe E.H., Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 2003;171(11):6072–6079. doi: 10.4049/jimmunol.171.11.6072. [PMID: 14634121]. [DOI] [PubMed] [Google Scholar]
- 178.Spencer J.P. Flavonoids: modulators of brain function? Br. J.Nutr. 2008;99 doi: 10.1017/S0007114508965776. ES60-77. [DOI] [PubMed] [Google Scholar]
- 179.Peng P.H., Chiou L.F., Chao H.M., Lin S., Chen C.F., Liu J.H., Ko M.L. Effects of epigallocatechin-3-gallate on rat retinal ganglion cells after optic nerve axotomy. Exp. Eye Res. 2010;90(4):528–534. doi: 10.1016/j.exer.2010.01.007. [PMID: 20114044]. [DOI] [PubMed] [Google Scholar]
- 180.Craft S., Watson G.S. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–178. doi: 10.1016/S1474-4422(04)00681-7. [PMID: 14980532]. [DOI] [PubMed] [Google Scholar]
- 181.Bahadoran Z., Mirmiran P., Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J. Diabetes Metab. Disord. 2013;12(1):43. doi: 10.1186/2251-6581-12-43. [PMID: 23938049]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aliev G., Palacios H.H., Walrafen B., Lipsitt A.E., Obrenovich M.E., Morales L. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int. J. Biochem. Cell Biol. 2009;41(10):1989–2004. doi: 10.1016/j.biocel.2009.03.015. [PMID: 19703659]. [DOI] [PubMed] [Google Scholar]
- 183.Boveris A., Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60(5):308–314. doi: 10.1002/iub.46. [PMID: 18421773]. [DOI] [PubMed] [Google Scholar]
- 184.Jalali-Nadoushan M., Roghani M. Alpha-lipoic acid protects against 6-hydroxydopamine-induced neurotoxicity in a rat model of hemi-parkinsonism. Brain Res. 2013;1505:68–74. doi: 10.1016/j.brainres.2013.01.054. [PMID: 23415934]. [DOI] [PubMed] [Google Scholar]
- 185.Melli G., Taiana M., Camozzi F., Triolo D., Podini P., Quattrini A., Taroni F., Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008;214(2):276–284. doi: 10.1016/j.expneurol.2008.08.013. [PMID: 18809400]. [DOI] [PubMed] [Google Scholar]
- 186.Müller U., Krieglstein J. Prolonged pretreatment with alpha-lipoic acid protects cultured neurons against hypoxic, glutamate-, or iron-induced injury. J. Cereb. Blood Flow Metab. 1995;15(4):624–630. doi: 10.1038/jcbfm.1995.77. [PMID: 7790411]. [DOI] [PubMed] [Google Scholar]
- 187.Prehn J.H., Karkoutly C., Nuglisch J., Peruche B., Krieglstein J. Dihydrolipoate reduces neuronal injury after cerebral ischemia. J. Cereb. Blood Flow Metab. 1992;12(1):78–87. doi: 10.1038/jcbfm.1992.10. [PMID: 1345759]. [DOI] [PubMed] [Google Scholar]
- 188.Salinthone S., Yadav V., Bourdette D.N., Carr D.W. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr. Metab. Immune Disord. Drug Targets. 2008;8(2):132–142. doi: 10.2174/187153008784534303. [PMID: 18537699]. [DOI] [PubMed] [Google Scholar]
- 189.Zhang L., Xing G.Q., Barker J.L., Chang Y., Maric D., Ma W., Li B.S., Rubinow D.R. Alpha-lipoic acid protects rat cortical neurons against cell death induced by amyloid and hydrogen peroxide through the Akt signalling pathway. Neurosci. Lett. 2001;312(3):125–128. doi: 10.1016/s0304-3940(01)02205-4. [PMID: 11602326]. [DOI] [PubMed] [Google Scholar]
- 190.Mijnhout G.S., Kollen B.J., Alkhalaf A., Kleefstra N., Bilo H.J. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012 doi: 10.1155/2012/456279. 2012, 456279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guo Y., Jones D., Palmer J.L., Forman A., Dakhil S.R., Velasco M.R., Weiss M., Gilman P., Mills G.M., Noga S.J., Eng C., Overman M.J., Fisch M.J. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support. Care Cancer. 2014;22(5):1223–1231. doi: 10.1007/s00520-013-2075-1. [PMID: 24362907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim J.H., Choi W., Lee J.H., Jeon S.J., Choi Y.H., Kim B.W., Chang H.I., Nam S.W. Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J. Microbiol. Biotechnol. 2009;19(11):1355–1363. doi: 10.4014/jmb.0906.06003. [PMID: 19996687]. [DOI] [PubMed] [Google Scholar]
- 193.Lu Y.P., Liu S.Y., Sun H., Wu X.M., Li J.J., Zhu L. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010;1360:40–48. doi: 10.1016/j.brainres.2010.09.016. [PMID: 20846510]. [DOI] [PubMed] [Google Scholar]
- 194.Cleren C., Yang L., Lorenzo B., Calingasan N.Y., Schomer A., Sireci A., Wille E.J., Beal M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2008;104(6):1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [PMID: 17973981]. [DOI] [PubMed] [Google Scholar]
- 195.Shults C.W. Coenzyme Q10 in neurodegenerative diseases. Curr. Med. Chem. 2003;10(19):1917–1921. doi: 10.2174/0929867033456882. [PMID: 12871093]. [DOI] [PubMed] [Google Scholar]
- 196.Spindler M., Beal M.F., Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr. Dis. Treat. 2009;5:597–610. doi: 10.2147/ndt.s5212. [PMID: 19966907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ferrante K.L., Shefner J., Zhang H., Betensky R., O’Brien M., Yu H., Fantasia M., Taft J., Beal M.F., Traynor B., Newhall K., Donofrio P., Caress J., Ashburn C., Freiberg B., O’Neill C., Paladenech C., Walker T., Pestronk A., Abrams B., Florence J., Renna R., Schierbecker J., Malkus B., Cudkowicz M. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65(11):1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [PMID: 16344537]. [DOI] [PubMed] [Google Scholar]
- 198.Storch A., Jost W.H., Vieregge P., Spiegel J., Greulich W., Durner J., Müller T., Kupsch A., Henningsen H., Oertel W.H., Fuchs G., Kuhn W., Niklowitz P., Koch R., Herting B., Reichmann H. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch. Neurol. 2007;64(7):938–944. doi: 10.1001/archneur.64.7.nct60005. [PMID: 17502459]. [DOI] [PubMed] [Google Scholar]
- 199.Dutta K., Ghosh D., Basu A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J. Neuroimmune Pharmacol. 2009;4(3):328–337. doi: 10.1007/s11481-009-9158-2. [PMID: 19434500]. [DOI] [PubMed] [Google Scholar]
- 200.Giri R.K., Rajagopal V., Kalra V.K. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cyto- chemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 trans- cription factor. J. Neurochem. 2004;91(5):1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [PMID: 15569263]. [DOI] [PubMed] [Google Scholar]
- 201.Kelsey N.A., Wilkins H.M., Linseman D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15(11):7792–7814. doi: 10.3390/molecules15117792. [PMID: 21060289]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schmitz K., Barthelmes J., Stolz L., Beyer S., Diehl O., Tegeder I. “Disease modifying nutricals” for multiple sclerosis. Pharmacol. Ther. 2015;148:85–113. doi: 10.1016/j.pharmthera.2014.11.015. [PMID: 25435020]. [DOI] [PubMed] [Google Scholar]
- 203.Yu S., Zheng W., Xin N., Chi Z.H., Wang N.Q., Nie Y.X., Feng W.Y., Wang Z.Y. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 2010;13(1):55–64. doi: 10.1089/rej.2009.0908. [PMID: 20230279]. [DOI] [PubMed] [Google Scholar]
- 204.Zhao J., Yu S., Zheng W., Feng G., Luo G., Wang L., Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem. Res. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [PMID: 19774461]. [DOI] [PubMed] [Google Scholar]
- 205.Kraft A.D., Johnson D.A., Johnson J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004;24(5):1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [PMID: 14762128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brewer G.J., Torricelli J.R., Lindsey A.L., Kunz E.Z., Neuman A., Fisher D.R., Joseph J.A. Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J. Nutr. Biochem. 2010;21(10):991–998. doi: 10.1016/j.jnutbio.2009.08.005. [PMID: 19954954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fuentealba J., Dibarrart A.J., Fuentes-Fuentes M.C., Saez-Orellana F., Quiñones K., Guzmán L., Perez C., Becerra J., Aguayo L.G. Synaptic failure and adenosine triphosphate imbalance induced by amyloid-β aggregates are prevented by blueberry-enriched polyphenols extract. J. Neurosci. Res. 2011;89(9):1499–1508. doi: 10.1002/jnr.22679. [PMID: 21647937]. [DOI] [PubMed] [Google Scholar]
- 208.Petrozzi L., Ricci G., Giglioli N.J., Siciliano G., Mancuso M. Mitochondria and neurodegeneration. Biosci. Rep. 2007;27(1-3):87–104. doi: 10.1007/s10540-007-9038-z. [PMID: 17486441]. [DOI] [PubMed] [Google Scholar]
- 209.Shukitt-Hale B., Lau F.C., Carey A.N., Galli R.L., Spangler E.L., Ingram D.K., Joseph J.A. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 2008;11(4):172–182. doi: 10.1179/147683008X301487. [PMID: 18681986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ogle W.O., Speisman R.B., Ormerod B.K. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology. 2013;59(1):23–31. doi: 10.1159/000342208. [PMID: 22947921]. [DOI] [PubMed] [Google Scholar]
- 211.Pocernich C.B., Lange M.L., Sultana R., Butterfield D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8(5):452–469. doi: 10.2174/156720511796391908. [PMID: 21605052]. [DOI] [PubMed] [Google Scholar]
- 212.Lee H.J., Cho H.S., Park E., Kim S., Lee S.Y., Kim C.S., Kim D.K., Kim S.J., Chun H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology. 2008;250(2-3):109–115. doi: 10.1016/j.tox.2008.06.010. [PMID: 18644421]. [DOI] [PubMed] [Google Scholar]
- 213.Qiao S., Li W., Tsubouchi R., Haneda M., Murakami K., Takeuchi F., Nisimoto Y., Yoshino M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005;39(9):995–1003. doi: 10.1080/10715760500231836. [PMID: 16087481]. [DOI] [PubMed] [Google Scholar]
- 214.Peng Q., Buz’Zard A.R., Lau B.H. Neuroprotective effect of garlic compounds in amyloid-beta peptide-induced apoptosis in vitro. Med. Sci. Monit. 2002;8(8):BR328–BR337. [PMID: 12165737]. [PubMed] [Google Scholar]
- 215.Kabuto H., Tada M., Kohno M. Eugenol [2-methoxy-4-(2-propenyl)phenol] prevents 6-hydroxydopamine-induced dopamine depression and lipid peroxidation inductivity in mouse striatum. Biol. Pharm. Bull. 2007;30(3):423–427. doi: 10.1248/bpb.30.423. [PMID: 17329831]. [DOI] [PubMed] [Google Scholar]
- 216.Frisardi V., Panza F., Solfrizzi V., Seripa D., Pilotto A. Plasma lipid disturbances and cognitive decline. J. Am. Geriatr. Soc. 2010;58(12):2429–2430. doi: 10.1111/j.1532-5415.2010.03164.x. [PMID: 21143446]. [DOI] [PubMed] [Google Scholar]
- 217.Sun G.Y., Xu J., Jensen M.D., Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 2004;45(2):205–213. doi: 10.1194/jlr.R300016-JLR200. [PMID: 14657205]. [DOI] [PubMed] [Google Scholar]
- 218.Fragoso Y.D., Stoney P.N., McCaffery P.J. The evidence for a beneficial role of vitamin A in multiple sclerosis. CNS Drugs. 2014;28(4):291–299. doi: 10.1007/s40263-014-0148-4. [PMID: 24557746]. [DOI] [PubMed] [Google Scholar]
- 219.Gianforcaro A., Hamadeh M.J. Vitamin D as a potential therapy in amyotrophic lateral sclerosis. CNS Neurosci. Ther. 2014;20(2):101–111. doi: 10.1111/cns.12204. [PMID: 24428861]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Betti M., Minelli A., Ambrogini P., Ciuffoli S., Viola V., Galli F., Canonico B., Lattanzi D., Colombo E., Sestili P., Cuppini R. Dietary supplementation with α-tocopherol reduces neuro- inflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 2011;45(10):1136–1142. doi: 10.3109/10715762.2011.597750. [PMID: 21749318]. [DOI] [PubMed] [Google Scholar]
- 221.Pu H., Guo Y., Zhang W., Huang L., Wang G., Liou A.K., Zhang J., Zhang P., Leak R.K., Wang Y., Chen J., Gao Y. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2013;33(9):1474–1484. doi: 10.1038/jcbfm.2013.108. [PMID: 23801244]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Losi G., Puia G., Garzon G., de Vuono M.C., Baraldi M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur. J. Pharmacol. 2004;502(1-2):41–46. doi: 10.1016/j.ejphar.2004.08.043. [PMID: 15464088]. [DOI] [PubMed] [Google Scholar]
- 223.Zhao L., Wang J.L., Wang Y.R., Fa X.Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013;1492:33–45. doi: 10.1016/j.brainres.2012.11.019. [PMID: 23178511]. [DOI] [PubMed] [Google Scholar]
- 224.Lee Y.B., Lee H.J., Won M.H., Hwang I.K., Kang T.C., Lee J.Y., Nam S.Y., Kim K.S., Kim E., Cheon S.H., Sohn H.S. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J. Nutr. 2004;134(7):1827–1831. doi: 10.1093/jn/134.7.1827. [PMID: 15226476]. [DOI] [PubMed] [Google Scholar]
- 225.Lee H., Kim H.J., Kim J.M., Chang N. Effects of dietary folic acid supplementation on cerebrovascular endothelial dysfunction in rats with induced hyperhomocysteinemia. Brain Res. 2004;996(2):139–147. doi: 10.1016/j.brainres.2003.10.027. [PMID: 14697491]. [DOI] [PubMed] [Google Scholar]
- 226.Mizuno M., Kawamura H., Takei N., Nawa H. The anthraquinone derivative Emodin ameliorates neurobehavioral deficits of a rodent model for schizophrenia. J Neural Transm (Vienna) 2008;115(3):521–530. doi: 10.1007/s00702-007-0867-5. [PMID: 18301953]. [DOI] [PubMed] [Google Scholar]
- 227.Duncan R.S., Goad D.L., Grillo M.A., Kaja S., Payne A.J., Koulen P. Control of intracellular calcium signaling as a neuro- protective strategy. Molecules. 2010;15(3):1168–1195. doi: 10.3390/molecules15031168. [PMID: 20335972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calkins M.J., Johnson D.A., Townsend J.A., Vargas M.R., Dowell J.A., Williamson T.P., Kraft A.D., Lee J.M., Li J., Johnson J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009;11(3):497–508. doi: 10.1089/ars.2008.2242. [PMID: 18717629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Han J.M., Lee Y.J., Lee S.Y., Kim E.M., Moon Y., Kim H.W., Hwang O. Protective effect of sulforaphane against dopaminergic cell death. J. Pharmacol. Exp. Ther. 2007;321(1):249–256. doi: 10.1124/jpet.106.110866. [PMID: 17259450]. [DOI] [PubMed] [Google Scholar]
- 230.Seidl S.E., Santiago J.A., Bilyk H., Potashkin J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014;6:36. doi: 10.3389/fnagi.2014.00036. [PMID: 24639650]. [DOI] [PMC free article] [PubMed] [Google Scholar]