Abstract
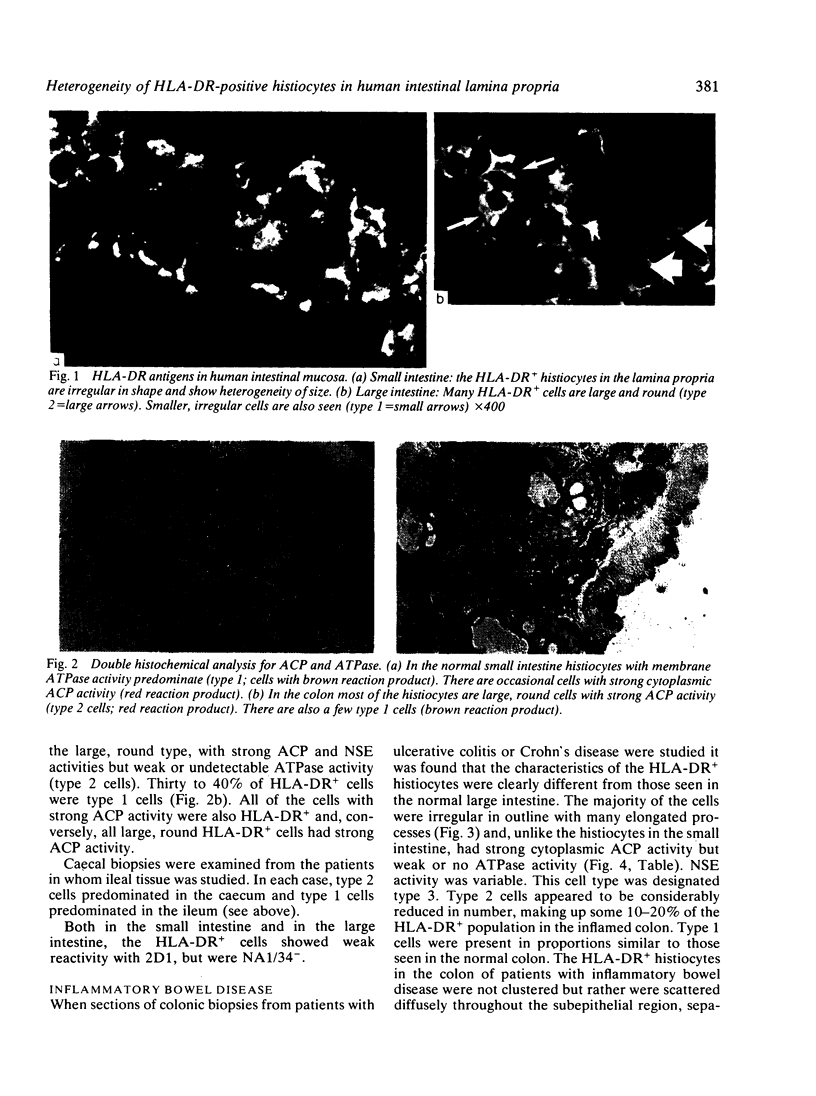
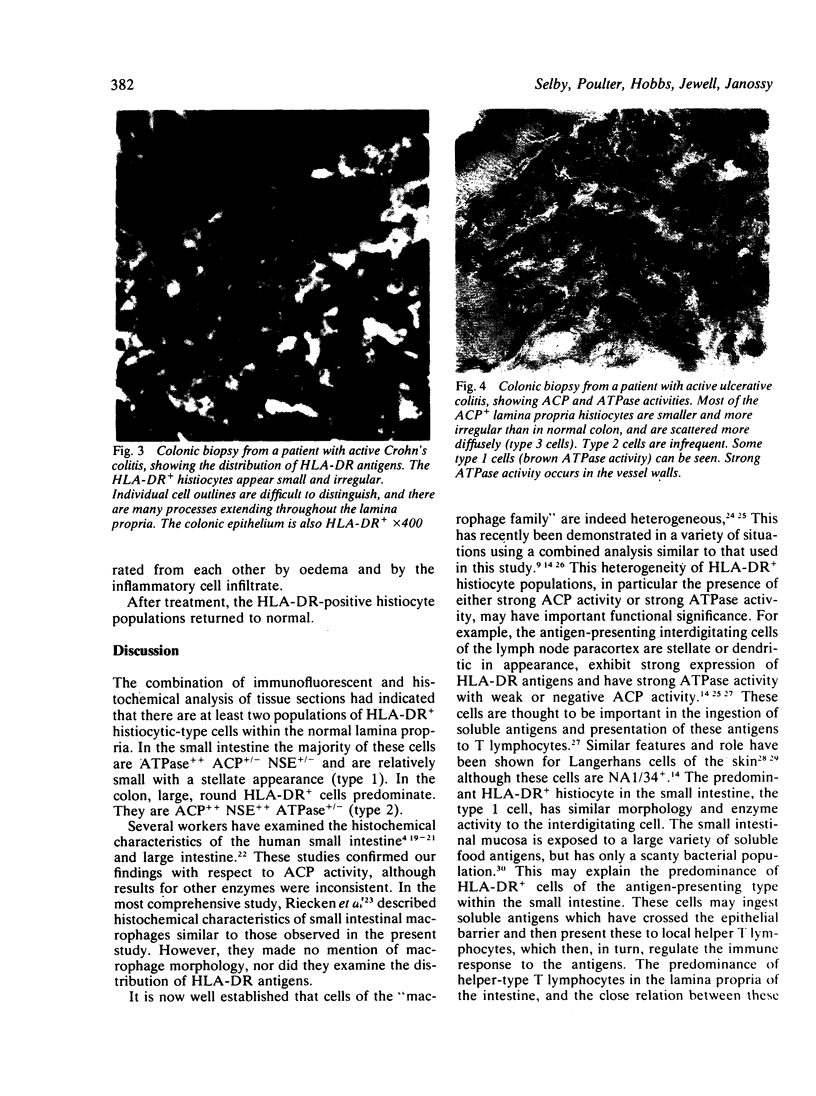
HLA-DR-positive histiocytes in the lamina propria of the human intestine have been characterised using combined histochemical and immunohistological techniques. In the small intestine, 80-90% of the HLA-DR+ histiocytes had irregular surfaces with stellate processes, and exhibited strong membrane adenosine triphosphatase (ATPase) activity, but weak acid phosphatase (ACP) and non-specific esterase (NSE) activities (HLA-DR+ ACP+/- NSA+/- ATP++; type 1 cell). In contrast, in the lamina propria of the colon the majority (60-70%) of HLA-DR+ cells were large, round cells with strong ACP and NSE activities but no detectable ATPase activity (HLA-DR+ ACP++ NSE++ ATP+/-; type 2 cell). The colon also contained a population of type 1 cells (30-40%). In active inflammatory bowel disease affecting the colon a third population of HLA-DR+ histiocytes was seen. These cells were irregular in outline, with many processes, and were ACP++ NSE+ ATP+/- (type 3 cell). The type 3 cells appeared to replace type 2 cells. After treatment, the appearances returned to normal. These findings suggest that the different populations of HLA-DR+ histiocytes in the human intestine may have several functions, reflecting the different forms of antigen present in the intestine. The alterations in inflammatory bowel disease may represent activation in response to an invading antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balfour B. M., Drexhage H. A., Kamperdijk E. W., Hoefsmit E. C. Antigen-presenting cells, including Langerhans cells, veiled cells and interdigitating cells. Ciba Found Symp. 1981;84:281–301. doi: 10.1002/9780470720660.ch15. [DOI] [PubMed] [Google Scholar]
- Braathen L. R., Thorsby E. Studies on human epidermal Langerhans cells. I. Allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11(4):401–408. doi: 10.1111/j.1365-3083.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman M. D., Chappelka A. R., Schaedler R. W. The Microbial ecology of the upper small bowel. Am J Gastroenterol. 1976 Jan;65(1):57–62. [PubMed] [Google Scholar]
- Donnellan W. L. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology. 1965 Nov;49(5):496–514. [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Lerner P. I., Weinstein L. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967 Dec;53(6):845–855. [PubMed] [Google Scholar]
- Humphrey J. H. Differentiation of function among antigen-presenting cells. Ciba Found Symp. 1981;84:302–321. doi: 10.1002/9780470720660.ch16. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Tjernlund U., Forsum U., Peterson P. A. Epidermal Langerhans cells express Ia antigens. Nature. 1977 Jul 21;268(5617):248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- Lampert I. A., Pizzolo G., Thomas A., Janossy G. Immuno-histochemical characterisation of cells involved in dermatopathic lymphadenopathy. J Pathol. 1980 Jun;131(2):145–156. doi: 10.1002/path.1711310207. [DOI] [PubMed] [Google Scholar]
- MONIS B., MENDELOFF A. I. STUDIES IN ULCERATIVE COLITIS: TPN-LINKED DEHYDROGENASES AND NONSPECIFIC ESTERASE IN RECTAL BIOPSY SPECIMENS. Gastroenterology. 1965 Feb;48:173–184. [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Meuret G., Bitzi A., Hammer B. Macrophage turnover in Crohn's disease and ulcerative colitis. Gastroenterology. 1978 Mar;74(3):501–503. [PubMed] [Google Scholar]
- PADYKULA H. A., STRAUSS E. W., LADMAN A. J., GARDNER F. H. A morphologic and histochemical analysis of the human jejunal epithelium in nontropical sprue. Gastroenterology. 1961 Jun;40:735–765. [PubMed] [Google Scholar]
- PADYKULA H. A., STRAUSS E. W., LADMAN A. J., GARDNER F. H. A morphologic and histochemical analysis of the human jejunal epithelium in nontropical sprue. Gastroenterology. 1961 Jun;40:735–765. [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Turk J. L. Studies on the effect of soluble lymphocyte products (lymphokines) on macrophage physiology. II. Cytochemical changes associated with activation. Cell Immunol. 1975 Nov;20(1):25–32. doi: 10.1016/0008-8749(75)90080-5. [DOI] [PubMed] [Google Scholar]
- Riecken E. O., Stewart J. S., Booth C. C., Pearse A. G. A histochemical study on the role of lysosomal enzymes in idiopathic steatorrhoea before and during a gluten-free diet. Gut. 1966 Aug;7(4):317–332. doi: 10.1136/gut.7.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowden G., Lewis M. G., Sullivan A. K. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977 Jul 21;268(5617):247–248. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- SAMLOFF I. M., DAVIS J. S., SCHENK E. A. A CLINICAL AND HISTOCHEMICAL STUDY OF CELIAC DISEASE BEFORE AND DURING A GLUTEN-FREE DIET. Gastroenterology. 1965 Feb;48:155–172. [PubMed] [Google Scholar]
- SPIRO H. M., FILIPE M. I., STEWART J. S., BOOTH C. C., PEARSE A. G. FUNCTIONAL HISTOCHEMISTRY OF THE SMALL BOWEL MUCOSA IN MALABSORPTIVE SYNDROMES. Gut. 1964 Apr;5:145–154. doi: 10.1136/gut.5.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki W., Kucharczyk K., Szymanska K., Kujawa M. Lamina propria macrophages of intestine of the guinea pig. Possible role in phagocytosis of migrating cells. Gastroenterology. 1977 Dec;73(6):1340–1344. [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer H. W. Study of acute localised inflammation of the gastrointestinal tract: the effluent lymph. Gut. 1981 Oct;22(10):827–835. doi: 10.1136/gut.22.10.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Lustig D. S., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974 Jun 1;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Clement L., Green I., Shevach E. M. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978 Nov;121(5):2005–2013. [PubMed] [Google Scholar]
- Thyberg J., Graf W., Klingenström P. Intestinal fine structure in Crohn's disease. Lysosomal inclusions in epithelial cells and macrophages. Virchows Arch A Pathol Anat Histol. 1981;391(2):141–152. doi: 10.1007/BF00437592. [DOI] [PubMed] [Google Scholar]






