Abstract
Objective
To test if intraarticular corticosteroid injection mitigates injury-induced synovitis and collagen degradation after anterior cruciate ligament (ACL) transection and characterize the synovial response using a functional genomics approach in a preclinical model of post- traumatic osteoarthritis.
Methods
Yorkshire pigs received untreated unilateral ACL transection (ACLT, n=6) or transection with immediate injection of 20mg triamcinolone acetonide (STEROID, n=6). Total synovial membrane cellularity and synovial fluid concentration of COL-2 3/4C short neoepitope bearing collagen fragments at 14 days post-injury were primary endpoints and compared between ACLT, STEROID and INTACT (n=6 uninjured knees). Cells were differentiated by histological phenotype and counted, while RNA-seq was used to quantify transcriptome-wide gene expression, monocyte, macrophage and lymphocyte markers.
Results
Total cellularity of 13% (95% confidence interval of 9–16) and COL-2 3/4C short levels of 0.24 Kg/ml (0.08–0.39) were determined in INTACT. Significant increases in total cellularity to 21% (16–27) and COL-2 3/4C short to 0.49 Kg/ml (0.39–0.59) were observed in ACLT. Compared to ACLT, total cellularity was non-significantly and COL-2 3/4C short was significantly decreased in STEROID to 17% (15–18, p=0.26) and 0.29 Kg/ml (0.23–0.35). Between ACLT and INTACT, 255 genes were differentially expressed and enriched pathways related to cellular immune response and proteolysis. Mononuclear leukocytes were the dominant cell type in cell dense areas. MARCO, SOCS3, CCR1, IL4R and MMP2 expression was significantly associated with COL-2 3/4C short levels.
Conclusions
Early intraarticular immunosuppression mitigated the injury-induced increase of collagen fragments, an outcome better predicted by specific marker expression than histological measures of synovitis.
Keywords: Synovitis, Proteolysis, Osteoarthritis, Corticosteroids, Anterior Cruciate Ligament
INTRODUCTION
Injury to the anterior cruciate ligament (ACL) is clearly linked to the development of post- traumatic osteoarthritis, with radiographic signs of the disease present in 50% or more of the injured knees only 15 years after the injury,[1–4] and a substantially earlier need for joint replacement surgery for patients with a previous ACL tear.[5] It is increasingly recognized that the acute injury to the ACL signals the onset of a molecular stage of the disease process where an early therapeutic intervention could be useful,[6,7] particularly if the molecular processes that are elicited in the joint tissues at the time of injury are known.[8]
The role of the synovial membrane in the development of osteoarthritis has been characterized at later stages of the disease process.[9–11] Synovitis, histologically characterized by increased cellularity (intimal thickness and inflammatory cellular infiltrates) and increased angiogenesis, is associated with clinical symptoms, as well as with the progression of cartilage damage.[9,11] This may be due to the secretion of proinflammatory mediators and catabolic enzymes into the synovial fluid which bathes the intraarticular tissues.[12] The synovial fluid and membrane can be targeted by intraarticular injection of small molecules or biologics. Hence, an improved understanding of the molecular and cellular response of this tissue to knee injury might translate into novel therapeutic strategies of acute knee injuries.
Analyses of human synovial fluid after ACL injury indicate early increases of proinflammatory mediators and catabolic enzymes, changes that have been associated with the extent of cartilage damage.[13–17] Those descriptions triggered clinical studies of intraarticular administration of Interleukin-1 receptor antagonist (Anakinra, NCT00332254),[18,19] and the synthetic glucocorticoid triamcinolone acetonide (Kenalog, AAA-Trial, NCT01692756) on pain, function, and synovial fluid markers of inflammation and cartilage degradation following ACL injury. To date, it has been difficult to obtain synovial tissue for gene expression analyses in clinical trials and subsequently difficult to measure the effect of these agents on the synovial membrane.
The porcine ACL transection model has been previously shown to result in post-traumatic osteoarthritis in a pattern identical to that seen in human patients after an ACL injury.[20] Synovial fluid and tissue can be obtained from the porcine model following surgical ACL injury, while controlling for parameters such as time from injury, previous joint and systemic diseases, sex and age. Targeted analyses demonstrated a pronounced increase in synovial gene expression of pro-inflammatory cytokines at the first day post-injury followed by increasing levels of protease gene expression, synovial fluid markers of collagen fragments and CD163 expressing synovial macrophages that remained markedly elevated at 14 days post-injury.[21,22] Using this model, we tested the hypothesis that the immediate intraarticular application of triamcinolone acetonide would result in the mitigation of the injury-induced synovitis and subsequent collagen degradation at 14 days after ACL transection. Further, we have utilized the tissue samples generated in this preclinical efficacy trial in combination with a functional genomics approach to characterize the synovial response to injury on a cellular and molecular level with the aim to identify those changes that are strongly associated with the extent of collagen degradation. This strategy appears promising to delineate those processes within the early synovial response to injury that are contributing to the potentially permanent destruction of the collagenous architecture of articular cartilage.
METHODS
Boston Children’s Hospital Institutional Animal Care and Use Committee approved this study. 18 adolescent female Yorkshire pigs (E.M. Parsons & Sons Inc., Hadley, MA), aged 83–96 days and weighing 31–37 kg, were randomly assigned to one of three groups: ACL transection (ACLT), ACL transection followed by injection of 20 mg triamcinolone acetonide injection (STEROID), and no surgery (INTACT control; n=6 for all groups). In the ACLT and STEROID groups, the ACL was transected at the junction of the proximal and middle thirds as previously described.[20] No post-operative immobilization was used. Pigs were housed in single cages with ad libitum access to water and were fed three times daily. No adverse event was observed throughout the experiment. After 14 days, the animals were euthanized and synovial fluid was drawn from the joints. Adjacent biopsies of the lateral synovial membrane (distant from the site of the original surgery) were collected, placed into sterile tubes (for RNA extraction) or embedded in optimal cutting temperature medium (OCT; Sakura Finetek, Torrance, CA) (for histology), frozen in liquid nitrogen and stored at −80°C until processed for further analysis.
Histology and cellularity measurements
OCT-embedded synovium was cut at 6 Km and stained with hematoxylin & eosin (MassHistology, Worcester, MA). Photomicrographs of the entire biopsy (intimal and subintimal tissue) were obtained in a scanning pattern and merged using the Photomerge tool of Adobe Photoshop CS5 Extended Version 12.0.4 (Adobe Systems, San Jose CA, USA). The area ratio occupied by nuclei within the total tissue area of the biopsy was used as a measure of total cellularity throughout the whole biopsy (ImageJ 64-bit version 1.48, National Institutes of Health, Bethesda, MD). Based on the automated measurements the synovial lining and stromal areas representing the highest cellularity of the respective biopsy were selected to manually distinguish cell types. According to their histological morphology, cell types were differentiated into mononuclear leukocytes (MNL), fibroblast-like synoviocytes (FLS) and polymorphonuclear leukocytes (PMNL) by an experienced reader blinded to the group allocation of the samples (MHW). Cell count was normalized on the tissue area and 0.307 mm2, subsequently referred to as high power field (HPF). A semiquantitative synovitis scoring system was used to assess the degree of intimal hyperplasia, stromal cellularity and inflammatory infiltration (each parameter from 0-absent to 3-strong) throughout the biopsy.[23]
Synovial fluid COL-2 3/4C short concentration
Synovial fluid samples were available for all surgical knees and 5 out of 6 of the INTACT group, due to the limited fluid volume in healthy joints. Samples were centrifuged at 3,000 g for 10 min to pellet cells and the supernatant was stored at −80°C. After thawing, fluids were diluted 1:3 in the assay buffer of the COL-2 3/4C short ELISA kit (60-1002-001, IBEX, Montreal, Canada) and then processed according to the manufacturers protocol.
RNA extraction and preparation for sequencing
Total RNA was extracted from the synovial membranes through homogenization, phenol-chloroform separation and on-column purification using the PureLink™ RNA Mini Kit (Life technologies, Carlsbad, CA). RNA samples were treated with DNAse I (PureLink™ DNase Set, Life technologies, Carlsbad, CA) and assessed with the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The samples were then enriched for polyA+ messenger RNA, reverse transcribed with random hexamers, ligated with indexed adapters and amplified with 15 cycles of PCR using the TruSeq RNA Sample Preparation Kit v2 (Illumina, San Diego, CA). Following the removal of primer-dimers with magnetic bead-based purification, samples were pooled and sequenced with 8 libraries per lane on an Illumina HiSeq 2000 machine with 50 basepair paired-end reads (Partners Healthcare, Cambridge, MA).
RNA-seq data analysis
Raw reads were mapped to the pig genome (Susscr3, released Aug. 2011) with RUM.[24] Reads uniquely aligned to the exons of each gene were counted with a custom R script,[25] that utilizes Rsamtools,[26] and GenomicFeatures packages,[27] and used for differential expression analysis with edgeR.[28] Additionally, reads per kilobase of exon model per million mapped reads (RPKM) were calculated. The p-values calculated in the differential expression analysis were corrected for transcriptome-wide testing using a Benjamini and Hochberg approach.[29] As additional measure, p-values had to remain < 0.05 during a leave-one-out cross validation and expression levels had to be > 5 RPKM in at least one of the compared groups to be considered significant.[25] For each of comparison, the differentially expressed genes were used to analyze the enrichment of specific pathways using the process network ontology of the MetaCore bioinformatics suite (Thomson & Reuters, New York City, NY, USA). Pathways were considered as significantly enriched when the adjusted p-values (false discovery rate) were < 0.05.
Monocyte, macrophage and lymphocyte markers
A specific set of genes including markers of monocytes, macrophages, lymphocytes and their function was analyzed in detail (Table 1). Monocyte surface markers and molecules involved in their trafficking were used as summarized by Shi and Pamer.[30] Commonly used macrophage surface markers as summarized by Murray and Wynn and their proposed combinatorial marker systems for the phenotyping of activated macrophages were included.[31] Lymphocyte markers included commonly used T-, B- and natural killer cell surface markers. Further, additional marker genes related to macrophage function in inflammation and wound healing as described by Murray and Wynn,[31] including the matrix metalloproteinase encoding MMP1–MMP15 were assessed (Table 2).
Table 1.
Synovial expression of monocyte, macrophage and lymphocyte marker genes
| Marker type | Gene symbol | Protein | INTACT | ACLT | STEROID |
|---|---|---|---|---|---|
| mean RPKM | |||||
| Monocyte surface | ITGAM | CD11b or Integrin alpha-M | 41.6 | 78.6 | 56.9 |
| CSF1R | CD115 or Macrophage colony-stimulating factor 1 receptor | 5.6 | 10.7 | 13.9 | |
| CD14 | CD14, Monocyte differentiation antigen CD14 | 4.0 | 9.5 | 19.4 * | |
| Monocyte chemokine receptors | CCR1 | CCR-1, C-C chemokine receptor type 1 | 14.8 | 49.9 * | 31.1 |
| CCR5 | CCR-5, C-C chemokine receptor type 5 | 25.8 | 39.3 | 24.3 | |
| CX3CR1 | CX3CR1, CX3C chemokine receptor 1 | 4.6 | 23.7 * | 5.7 # | |
| CCR6 | CCR-6, C-C chemokine receptor type 6 | 0.6 | 1.1 | 1.5 | |
| CCR2 | CCR-2, C-C chemokine receptor type 2 | 0.5 | 1.1 | 1.3 | |
| CCR8 | CCR-8. C-C chemokine receptor type 8 | 0.2 | 0.1 | 0.1 | |
| CCR7 | CCR-7, C-C chemokine receptor type 7 | 0.1 | 0.1 | 0.1 | |
| - | Pig CXCR2 orthologue (ENSSSCG00000016183) | 0.1 | 0.0 | 1.0 | |
| Monocyte adhesion molecules | PECAM1 | Platelet endothelial cell adhesion molecule | 77.2 | 74.9 | 68.1 |
| SELPLG | PSGL-1, P-selectin glycoprotein ligand 1 | 17.0 | 48.3 | 44.1 | |
| SELL | L-Selectin | 27.0 | 14.5 | 18.5 | |
| ITGA4 | Integrin alpha-4 or VLA-4 subunit alpha | 6.4 | 9.5 | 6.8 | |
| ITGAL | LFA1-1A or Integrin alpha-L | 4.6 | 5.2 | 3.9 | |
| Macrophage surface | LGALS3 | MAC2 or Galectin-3 | 183.4 | 203.3 | 247.6 |
| EMR1 | F4/80 or Adhesion G protein-coupled receptor E1 | 116.2 | 147.1 | 119.8 | |
| ITGAX | CD11c or Integrin alpha-X | 57.0 | 71.7 | 41.2 | |
| CD68 | Macrosialin | 42.0 | 66.7 | 113.7 | |
| IL4R | IL4-RA, Interleukin-4 receptor subunit alpha | 5.7 | 26.0 * | 25.4 * | |
| CD163 | CD163 or Scavenger receptor cysteine-rich type 1 protein M130 | 0.0 | 0.0 | 0.0 | |
| Macrophage M1 activation | MARCO | Macrophage receptor MARCO | 8.9 | 63.4 * | 29.0 |
| IDO1 | IDO-1, Indoleamine 2,3-dioxygenase 1 | 36.1 | 25.2 | 11.5 | |
| SOCS3 | SOCS-3, Suppressor of cytokine signaling 3 | 3.5 | 24.3 * | 19.5 * | |
| NOS2 | iNOS or Nitric oxide synthase, inducible | 0.6 | 0.6 | 0.4 | |
| PTGS2 | COX-2 or Prostaglandin G/H synthase 2 | 0.6 | 0.5 | 0.5 | |
| IL12B | IL-12B, Interleukin-12 subunit beta | 0.0 | 0.0 | 0.0 | |
| Macrophage M2 activation | CHI3L1 | Chitinase-3-like protein 1 | 34.7 | 127.9 * | 279.9 * |
| KLF4 | Krueppel-like factor 4 | 52.7 | 41.4 | 45.0 | |
| CCL24 | C-C motif chemokine 24 | 7.0 | 16.7 | 31.4 * | |
| SOCS2 | SOCS-2, Suppressor of cytokine signaling 2 | 23.9 | 13.1 | 11.4 | |
| CXCL13 | C-X-C motif chemokine 13 | 3.6 | 6.0 | 1.6 | |
| CHI3L2 | Chitinase-3-like protein 2 | 1.6 | 1.4 | 2.5 | |
| CHIA | AMCase, Acidic mammalian chitinase | 1.5 | 1.3 | 1.1 | |
| IRF4 | Interferon regulatory factor 4 | 0.3 | 0.6 | 0.7 | |
| RETNLB | Resistin-like beta, pig Retnla orthologue | 0.1 | 0.0 | 0.0 | |
| Macrophage context- dependent | MRC1 | Macrophage mannose receptor 1 | 103.3 | 78.8 | 119.2 |
| IL10 | IL-10, Interleukin-10 | 8.7 | 15.0 | 16.8 | |
| ARG1 | Arginase-1 | 0.0 | 0.3 | 1.6 | |
| Lymphocyte surface | CD4 | CD4, T-cell surface glycoprotein CD4 | 5.4 | 6.8 | 3.1 |
| CD22 | CD22, B-cell receptor CD22 | 1.5 | 1.8 | 1.2 | |
| NCAM1 | CD56 or Neural cell adhesion molecule 1 | 2.8 | 1.6 | 0.9 | |
| CD3E | CD3e, T-cell surface glycoprotein CD3 epsilon chain | 1.0 | 0.8 | 0.4 | |
| CD3G | CD3g, T-cell surface glycoprotein CD3 gamma chain | 1.2 | 0.7 | 0.4 | |
| CD3D | CD3d, T-cell surface glycoprotein CD3 delta chain | 0.7 | 0.7 | 0.3 | |
| CD8A | CD8a, T-cell surface glycoprotein CD8 alpha chain | 0.5 | 0.5 | 0.2 | |
| CD8B | CD8b, T-cell surface glycoprotein CD8 beta chain | 0.1 | 0.1 | 0.1 | |
| CD19 | CD19, B-lymphocyte antigen CD19 | 0.2 | 0.1 | 0.1 | |
| MS4A1 | CD20, B-lymphocyte antigen CD20 | 0.1 | 0.1 | 0.0 | |
Significantly differentially expressed to INTACT
Significantly differentially expressed between ACLT and STEROID
Table 2.
Synovial expression of marker genes related to macrophage function in inflammation and wound healing
| Marker type | Gene symbol | Protein | INTACT | ACLT | STEROID |
|---|---|---|---|---|---|
| mean RPKM | |||||
| Inflammatory response | MMP1 | MMP-1 or Interstitial collagenase | 149.5 | 1452.9 * | 1086.7 * |
| MMP2 | MMP-2 or 72 kDa type IV collagenase | 127.3 | 488.8 * | 501.4 * | |
| MMP3 | MMP-3 or Stromelysin-1 | 264.8 | 457.3 | 1068.3 * | |
| MMP7 | MMP-7 or Matrilysin | 0.6 | 0.4 | 0.3 | |
| MMP8 | MMP-8 or Neutrophil collagenase | 8.4 | 6.6 | 37.2 *, § | |
| MMP9 | MMP-9, Matrix metalloproteinase-9 | 0.0 | 0.4 | 0.3 | |
| MMP11 | MMP-11 or Stromelysin-3 | 5.1 | 5.7 | 5.5 | |
| MMP13 | MMP-13 or Collagenase 3 | 0.1 | 0.6 | 0.2 | |
| MMP14 | MT1-MMP or Matrix metalloproteinase-14 | 24.5 | 60.1 | 62.2 | |
| MMP15 | MT2-MMP or Matrix metalloproteinase-15 | 0.5 | 1.0 | 0.5 | |
| TNFA | TNF-alpha, Tumor necrosis factor | 0.7 | 1.4 | 1.3 | |
| IL1A | IL-1 alpha, Interleukin-1 alpha | 1.0 | 0.8 | 0.5 | |
| IL1B | IL-1 beta, Interleukin-1 beta | 0.7 | 0.5 | 0.4 | |
| TIMP1 | TIMP-1, Metalloproteinase inhibitor 1 | 28.1 | 88.2 * | 95.1 * | |
| Wound healing and fibrosis | TGFB1 | TGF-beta-1, Transforming growth factor beta-1 | 18.7 | 26.7 | 17.4 |
| PDGFB | PDGF subunit B, Platelet-derived growth factor subunit B | 1.2 | 3.1 | 2.8 | |
| PDGFA | PDGF subunit A, Platelet-derived growth factor subunit A | 0.6 | 1.0 | 1.2 | |
| PDCD1LG2 | PD-L2, Programmed cell death 1 ligand 2 | 0.4 | 0.2 | 0.2 | |
| MMP12 | MMP-12, Macrophage metalloelastase | 0.0 | 0.0 | 0.2 | |
Significantly differentially expressed to INTACT
Significantly differentially expressed between ACLT and STEROID
Statistical analysis
Total synovial membrane cellularity and synovial fluid concentration of COL-2 3/4C short neoepitope bearing collagen fragments at 14 days post-injury were the primary endpoints and compared between ACLT, STEROID and INTACT. The significance of differences was determined using a one-way analysis of variance with subsequent Tukey post-hoc test to correct for the comparison of multiple groups. Similarly, the Kruskal-Wallis rank sum test was used for ordinal variables, specifically the synovitis score and its subscales. Adjusted p-values < 0.05 were considered as statistically significant.
The association of histological parameters and marker gene expression levels with the synovial fluid COL-2 3/4C concentration level was determined using linear regression analysis. Only the parameters and markers that were significantly different in at least one comparison of the three groups were included. Associations with p < 0.05 were considered as statistically significant. All analyses were performed in R version 3.1.0.
RESULTS
Synovial membrane total cellularity and COL-2 3/4C short fragments
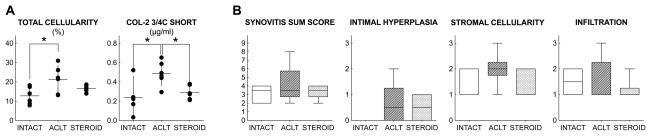
The area occupied by nuclei throughout the synovial membrane biopsy (intima and stroma) increased after ACL transection from 13% (95% confidence interval of 9–16) to 21% (16–27) in INTACT and ACLT, respectively (p = 0.02). The mean total cellularity in STEROID and corresponding confidence intervals, were in between ACLT and INTACT mean values at 17% (15–18). However, these differences did not reach statistical significance after correcting for multiple comparisons (p=0.26 and 0.40, respectively; Figure 1A).
Figure 1.
(A) Effects of ACL transection and intraarticular triamcinolone administration on synovial membrane total cellularity and synovial fluid COL-2 3/4C short concentration. Dots represent individual samples, horizontal lines group means, and vertical bars 95% confidence intervals. Total cellularity and COL-2 3/4C short levels were significantly increased after untreated ACL transection. Corticosteroid injection mitigated the injury-induced increase in COL-2 3/4C short fragment levels. (B) Synovitis sum score and subscores. Subscores range from 0-absent to 3-strong. Horizontal lines depict medians, boxes 25th–75th percentiles and whiskers ranges. Significant group differences (adjusted p<0.05) are indicated by *.
The synovial fluid concentration of COL-2 3/4C short collagen fragments doubled after ACL transection from 0.24 Kg/ml (0.08–0.39) to 0.49 Kg/ml (0.39–0.59) in INTACT and ACLT, respectively (p=0.02). Compared to ACLT, the COL-2 3/4C short collagen fragment concentrations were significantly reduced to 0.29 Kg/ml (0.23–0.35, p=0.04). STEROID was considered similar to the INTACT group (p=0.78) (Figure 1A).
The changes in total cellularity only explained a small portion of the variance in COL-2 3/4C short levels (R2=0.09, p=0.24). We thus utilized a functional genomics approach to characterize the early synovial response to injury and the association of cellular and molecular processes with the extent of intraarticular collagen degradation.
Transcriptome-wide differential expression and pathway analysis
255 protein-coding transcripts were differentially expressed in the comparison of ACLT with INTACT (Supplementary Table 1), and 33 protein-coding transcripts were differentially expressed in the comparison of STEROID with ACLT (Supplementary Table 2). These genes mainly enriched pathways related to cellular immune response in both comparisons, while proteolysis and angiogenesis were only enriched in the comparison of ACLT with INTACT (Supplementary Table 3).
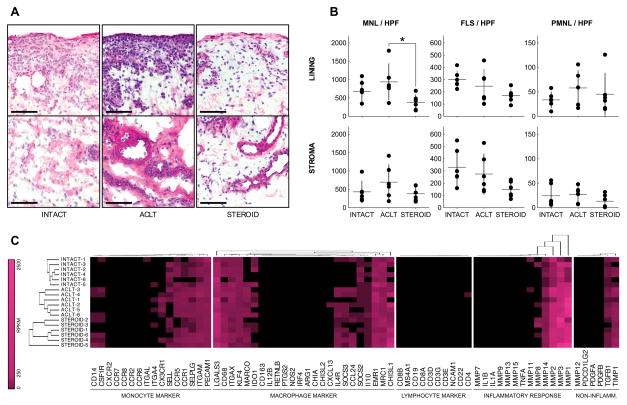
Cell type specific changes in synovial membrane cellularity
From each synovial membrane sample, we selected lining and stromal areas that demonstrated the highest measured total cellularity, and counted individual cell types based on their (nuclear) morphology. MNL were more abundant than FLS, and the latter more abundant than PMNL (Figure 2A and B). The number of MNL per HPF was increased from 680 (439–920) in INTACT to 939 (560–1317) in ACLT lining and from 431 (203–660) to 697 (332–1062) in the stroma. However, a considerable variance in MNL count after ACL transection was observed and the increase was thus not considered statistically significant in both regions (p= 0.41 and p=0.37, respectively). Compared to ACLT, the number of MNL per HPF decreased significantly to 379 (217–540, p=0.03) in STEROID lining and decreased non-significantly to 380 (224–537) in the stroma (p=0.25); values slightly, but not significantly, below INTACT (p=0.31, p=0.96, respectively). No significant differences were observed in the number of FLS and PMNL per HPF in synoval lining and stroma (Figure 2B; Supplementary Table 4).
Figure 2.
Cell type specific changes in synovial membrane biopsies. (A) Using a hematoxylin-eosin stained section of each biopsy, synovial lining and stromal areas with the highest total cellularity were identified and used to manually count the number of mononuclear leukocytes [MNL], fibroblast-like synoviocytes [FLS] and polymorphonuclear leukocytes [PMNL]. Photomicrographs correspond to the median MNL count per high power field [MNL / HPF] for each group. Bars represent 100 Km. MNL were more abundant than FLS and the latter were more abundant than PMNL in synovial areas of high cellularity. (B) Number of MNL, FLS or PMNL per HPF. Dots represent individual samples, horizontal lines group means, and vertical bars 95% confidence intervals. Significant group differences (adjusted p<0.05) are indicated by *. (C) Gene expression profiles of synovial membrane biopsies. Abundance of transcripts encoding markers of monocytes, macrophages, lymphocytes and their function is encoded by color using a logarithmic scale. Black indicates absence of detectable expression, pink corresponds to 2500 reads per kilobase of exon model per million mapped reads [RPKM]. Of note, lymphocyte marker gene expression is almost absent, indicating that monocytes and macrophages are the predominant MNLs in homeostatic and post-injury synovial membrane biopsies.
Synovial expression of monocyte, macrophage and lymphocyte marker genes
While 11 of 16 monocyte markers (69%) and 15 of 24 macrophage markers (63%) were expressed at a substantial level, defined by > 5 RPKM in one or more of the groups, only 1 of 10 lymphocyte markers (10%) was expressed above this threshold (Figure 2C). The transcripts with the highest mean abundances were encoding ITGAM (78.6 RPKM) amongst the monocyte markers, LGALS3 (203.3 RPKM) amongst the macrophage markers and CD4 (6.8 RPKM) amongst the lymphocyte markers in the ACLT group. In STEROID, PECAM1 (68.1 RPKM), CHI3L1 (279.9 RPKM) and CD4 (3.1 RPKM) were the most abundant transcripts of the assessed subsets, respectively (Table 1). Amongst the monocyte markers, all of 3 monocyte surface markers (100%), 3 of 8 monocyte chemokine receptors (38%), and all of 5 monocyte adhesion molecules (100%) were substantially expressed. Amongst the macrophage markers, 5 of 6 macrophage surface markers (83%), 3 of 6 markers of M1 activation (50%), 5 of 9 markers of M2 activation (56%) and 2 of 3 context-dependent markers of macrophage activation (67%) were substantially expressed (Table 1).
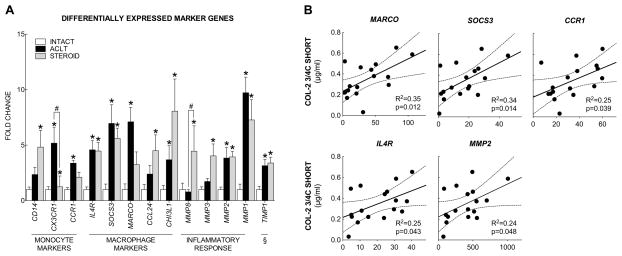
Further, the differential expression of these markers between groups was investigated. Out of the 50 assessed monocyte, macrophage and lymphocyte marker genes (Table 1), eight were significantly differentially expressed in at least one of the comparisons (all included in Figure 3A). In the comparison of ACLT with INTACT, six of these genes were significantly upregulated in the ACLT group, and none were significantly downregulated. Specifically, the monocyte chemokine receptors, CX3CR1 and CCR1, were upregulated by 5-fold (p<0.001) and 3-fold (p=0.004) and the macrophage marker IL4R was upregulated 4-fold (p<0.001).
Figure 3.
Synovial marker gene expression and association with COL-2 3/4C short levels. (A) Amongst the assessed set of 69 markers of monocytes, macrophages, lymphocytes and their function 13 were differentially expressed in at least one comparison of the three groups. ACL transection induced an upregulation of monocyte (CX3CR1, CCR1) and macrophage surface receptors (IL4R), M1 (SOCS3 and MARCO) and M2 (CHI3L1) macrophage markers, as well as proteases (MMP1, MMP2) and a protease inhibitor (TIMP1) when compared to uninjured controls, indicated by *. Corticosteroid administration resulted in significant downregulation of CX3CR1 and significant upregulation of MMP8 when compared to untreated ACL transection, indicated by #. Bars represent mean fold-changes relative to intact controls, error bars indicate standard errors of the means. (B) Amongst these genes, MARCO, SOCS3, CCR1, IL4R and MMP2 were significantly associated with the synovial fluid concentration of COL-2 3/4C short fragment levels. X-axis depicts the reads per kilobase of exon model per million mapped reads [RPKM], dotted lines depict 95% confidence intervals of regressed lines.
Further, MARCO and SOCS3, both markers of an M1 activation state (both 7-fold, p<0.001), as well as the M2 activation marker CHI3L1 (4-fold, p=0.001) were upregulated in the ACLT group. In the comparison of STEROID with INTACT, five of these genes were significantly upregulated in the STEROID group and none were downregulated. Specifically, an upregulation of the monocyte surface marker CD14 was observed (5-fold, p<0.001), while the macrophage marker IL4R was upregulated 5-fold (p<0.001) to a similar level as in ACLT. Further, CHI3L1 and CCL24, two markers of an M2 activation state (8-fold and 5-fold, both p<0.001), and the M1 activation marker SOCS3 (6-fold, p<0.001) were upregulated in the STEROID group. However, in the direct comparison of STEROID with ACLT, only CX3CR1 was significantly differentially expressed out of the assessed subset of marker genes (Downregulated to 22% of the post-injury expression levels in STEROID vs. ACLT, p<0.001).
Synovial expression of marker genes related to macrophage function in inflammation and wound healing
Out of the 19 assessed genes that are typically associated with macrophage function in inflammation and wound healing (Table 2), 5 were significantly differentially expressed in at least one of the comparisons (all included in Figure 3A). In the comparison of ACLT with INTACT, 3 of these genes were significantly upregulated and none were downregulated. Specifically, the metalloproteinase encoding MMP1 (9-fold, p<0.001) and MMP2 (4-fold, p<0.001), as well as the protease inhibitor TIMP1 were upregulated (3-fold, p<0.001). In the comparison of STEROID with INTACT, 5 genes of this subset were significantly upregulated, encompassing MMP1 (7-fold), MMP2 (4-fold), MMP3 (4-fold), MMP8 (5-fold, all p<0.001) and TIMP1 (3-fold, p=0.001), while none were downregulated. Surprisingly, in the direct comparison of STEROID with ACLT, the metalloproteinase encoding MMP8 was significantly upregulated by 6-fold in STEROID (p<0.001).
Association of marker gene expression and histological measures with synovial fluid COL-2 3/4C short
Amongst the two histological outcome measures that detected significant differences between the groups, none was significantly associated with the synovial fluid COL-2 3/4C short fragment concentration. Specifically, associations of total cellularity (R2=0.09, p=0.244) and number of MNL per HPF of synovial lining (R2=0.10, p=0.210) only explained small parts of the variance observed in the fluid fragment levels.
In contrast, amongst the 13 differentially expressed marker genes of monocytes, macrophages and their function, 5 were significantly associated with the synovial fluid COL- 2 3/4C short fragment levels (all positively correlated). The strongest associations were observed for MARCO (R2=0.35, p=0.012) and SOCS3 (R2=0.34, p=0.014), both previously described as markers of an M1 macrophage activation state.[31] Further, the monocyte chemokine receptor CCR1 (R2=0.25, p=0.039), the cytokine receptor IL4R (R2=0.25, p=0.043) and the metalloproteinase encoding MMP2 (R2=0.24, p=0.048) were also significantly associated with the synovial fluid COL-2 3/4C short levels (Figure 3B).
DISCUSSION
This study demonstrates that early intraarticular therapy with triamcinolone acetonide after joint injury entirely mitigates the injury-induced increase in synovial fluid collagen fragments in a preclinical model, resulting in values similar to those observed in healthy control animals, although the concomitant mitigating effect on the injury-induced increase in synovial total cellularity was smaller and did not reach the predefined level of statistical significance. As this study was based on the assumption that an early post-injury synovitis contributes to the intraarticular collagen degradation, we investigated the relationship between the total cellularity in the synovial membrane and the collagen fragment levels in the synovial fluid. We found that it only explained a small portion of the variance in COL-2 3/4C short levels (R2=0.09), underlining that an improved understanding of the response to injury is needed.
The synovial response to ACL transection
To better characterize the cellular and molecular processes in the early response of the synovial membrane to injury, we utilized a functional genomics approach. Using RNA-seq, we obtained transcriptome-wide expression data and detected 255 differentially expressed protein-coding transcripts in synovium obtained from untreated ACL transected and healthy joints, and 33 transcripts in synovium obtained from corticosteroid treated and non-treated ACL transected joints. Due to their predominance amongst all transcriptome-wide changes, we further investigated pathways related to the cellular immune response and proteolysis, pathways that appear upstream of our measured primary outcomes. Using standard histology, we found MNL as the predominant cell type in synovial areas of high cellularity and further analyzed specific sets of genes (Table 1 and 2) to distinguish between cells that share this nuclear morphology. We detected that 69% and 63% of our monocyte and macrophage markers were expressed at a substantial level, defined by > 5 RPKM in one or more of the groups, while only 10% of lymphocyte markers were expressed above this threshold (corresponding to one marker, CD4). Combined with the differential expression observed after untreated ACL transection in these gene sets, we propose that the early synovial response to injury induces an M1 macrophage-driven synovitis. This process includes an increased trafficking and migration of monocytes into the synovial membrane (upregulated chemokine receptors CX3CR1 and CCR1, both previously described to be involved in monocyte trafficking),[30] resulting in an increased presence of macrophages (upregulated cytokine receptor IL4R, previously described to be expressed by macrophages) predominantly in the M1 activation state (upregulated M1 activation markers MARCO and SOCS3).[31] Further, the protease expression (upregulated MMP1 and MMP2) indicate a pro-inflammatory function of macrophages. Simultaneously, the increased expression of one M2 activation marker (upregulated CHI3L1) and metalloproteinase inhibitor (upregulated TIMP1) suggests an initiation of a regulatory function in some of the present macrophages. Previous reports using a dog ACL transection model of post-traumatic osteoarthritis demonstrated definite MNL infiltration that remained constant throughout 3, 5 and 54 months post-injury, while no infiltration by PMNL was observed.[32,33] In combination with our findings, this suggests that mononuclear infiltration of the synovium is a process that is initiated very early after injury and remains present throughout the entire development of post-traumatic osteoarthritis. Although the role of macrophages in osteoarthritis is only sparsely described, some reports suggest a causal role of macrophages in various features of osteoarthritis, such as osteophyte development and extracellular matrix catabolism.[34, 35, 36] Thus, the migration and activation of macrophages in the synovial membrane might contribute to the pathogenesis of post-traumatic osteoarthritis. As it is an early feature of the porcine ACL transection model, it appears as a suitable model to further study this phenomenon and the effect of immunosuppressive therapies.
The effect of triamcinolone on synovitis
In response to triamcinolone, the number of MNL per HPF of synovial lining and the expression of the monocyte chemokine receptor CX3CR1 was significantly reduced compared to the untreated ACL transection. However, the observed effect on the number of MNL in the synovial stroma and the total cellularity (measured throughout the whole biopsy) was smaller and did not reach the level of statistical significance. Further the general macrophage marker IL4R was unaffected, suggesting that there were no substantial changes in the abundance of macrophages. However, markers for the activation state of macrophages were modulated to some degree. In later stage OA, synovial macrophages express markers indicative of both M1 (CD86, Tumor necrosis factor-alpha, inducible nitric oxide synthase) and M2 (CD206, Interleukin-10 and Transforming growth factor-beta) activation.[37,38] In general, it is thought that M1 macrophages perpetuate inflammation and proteolysis and that M2 macrophages have anti-inflammatory function and regulate wound healing.[31] Thus, a conversion from M1 to M2 macrophages may move the synovium towards an anti-inflammatory function. Here we observed that, in comparison to the healthy samples, two M1 macrophage markers were upregulated in the untreated ACL transection group (SOCS3 and MARCO), while only SOCS3 was significantly upregulated after corticosteroid treatment. Further, only one M2 macrophage marker (CHI3L1) was significantly upregulated in the untreated ACL transection groups, while two (CHI3L1 and CCL24) were significantly upregulated after corticosteroid treatment. However, the observed differences were not statistically significant in the direct comparison of corticosteroid treated with untreated ACL transection, and thus require further study.
The effect of triamcinolone on collagen degradation and structural progression
In response to triamcinolone, the level of synovial fluid COL-2 3/4C short fragments was significantly reduced at 14 days post-injury. While this experiment was designed to evaluate the early effects of an immediate post-injury corticosteroid injection, previous studies analyzed the effect of a long-term systemic administration of corticosteroid on the structural progression of post-traumatic osteoarthritis. While high doses of oral prednisone (0.3 mg/kg/day) protected against the development of osteophytes and cartilage lesions after ACL transection in dogs, lower doses (0.1 mg/kg/day) administered troughout 12 weeks post-injury did not modulate synovial inflammation or protect against structural progression.[39,40] Similar to high dose oral prednisone, the intraarticular injection of 5 mg triamcinolone hexacetonide immediately and 4 weeks post-injury protected against structural post-traumatic ostearthritis in the Pond-Nuki modell at 8 weeks.[39] In summary of these preclinical experiments, corticosteroids appear to prevent degradation of cartilage extracellular matrix (measured directly or by associated synovial fluid biomarkers) after surgical injury to the joint capsule and anterior cruciate ligament, if a sufficient local dose is achieved. We expected that this effect is mediated by a reduced synovial expression of proteases and assessed MMP1-MMP15 and other markers of macrophage function in inflammation and wound healing (Table 2). Unexpectedly, the corticosteroid treatment did not reduce the injury induced expression of MMP1 and MMP2, but further increased protease expression including a significant upregulation of MMP8 in comparison to the untreated ACL transection group. Thus, the observed changes in synovial membrane protease expression do not intuitively explain the changes in synovial fluid collagen fragment levels.
The association of cellular and molecular outcomes with collagen degradation
To identify cellular and molecular changes that are strongly associated with the extent of collagen degradation in this early post-injury stage, we performed linear regression analysis to detect the association between the assessed histological outcomes and marker gene expression levels with the synovial fluid COL-2 3/4C short concentration, and found that the expression of MARCO, SOCS3, CCR1, IL4R and MMP2 were significantly positively associated with the fragment level. Further, the expression of these marker genes (R2=0.35–0.24) better predicted the synovial fluid fragment concentration than histological measures of cellularity (R2=0.10–0.09). This strategy appears promising to delineate those processes within the early synovial response to injury that are associated with and could possibly contribute to the permanent destruction of the collagenous architecture of articular cartilage. It would be expected that some of these genes have previously been described to be implicated in osteoarthritis, and indeed those reports exist for MMP2,[42, 43] CCR1,[44] and IL4R.[45] MMP2 expression levels in human synovial membranes were associated with the clinical stage of osteoarthritis,[41] and upregulated after destabilization of the medial meniscus in mice.[42] CCR1 is abundantly expressed in synovial membranes of patients with osteoarthritis,[43] and was previously described to be involved in the recruitment of monocytes into inflamed tissues.[30] IL4R encodes Interleukin-4 receptor subunit alpha [IL-4RA], which is further cleaved into soluble IL-4RA. Soluble IL-4RA is present in elevated concentrations in the serum of knee osteoarthritis patients.[44] Interestingly, the 5 genes significantly associated with the synovial fluid level of cartilage fragments included one chemokine receptor involved in monocyte recruitment (CCR1),[30] one cytokine receptor of general macrophages (IL4R) and two markers of an M1 macrophage activation state (MARCO, SOCS3).[31] We further report that the respective genes were increasingly expressed in the synovial membrane in response to joint injury. However, it is yet unclear whether the identified genes have a causal role in osteoarthritis.
In conclusion, the immediate intraarticular corticosteroid administration mitigated the injury-induced increase of synovial fluid COL2 3/4C short levels in a preclinical model. Amongst cellular and molecular measures related to synovitis, markers of monocyte recruitment and M1 macrophage activation were significantly associated with the collagen fragment levels and were more predictive than histological measures of synovial cellularity.
Supplementary Material
Acknowledgments
The authors thank the ARCH staff, Arthur Nedder, Kathryn Donovan, Dana Bolgen and Courtney White, for their assistance and care in handling the minipigs. Further we want to thank Elise Magarian, Ryu Yoshida, Patrick Vavken and Brian Kelly for their engagement in surgery and tissue collection. We thank Matthew Warman for his contributions to the experimental design, Carla Haslauer for her contributions to the experimental design, tissue harvesting and processing and Andre Steinert for his contributions to the histological assessment of synovitis. Bioanalyzer analysis was performed in the BCH IDDRC Molecular Genetic Core that is supported by National Institutes of Health award NIH-P30-HD 18655. This investigation was supported by the National Institutes of Health under NIAMS AR054099, AR056834 and the Boston Children’s Hospital Translational Research Program.
Footnotes
COMPETING INTEREST AND DATA SHARING
The authors have no competing interests. Transcriptome-wide gene expression data is available at ArrayExpress (E-MTAB-4294).
References
- 1.Øiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, et al. Knee Function and Prevalence of Knee Osteoarthritis After Anterior Cruciate Ligament Reconstruction A Prospective Study With 10 to 15 Years of Follow-up. Am J Sports Med. 2010;38:2201–2210. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander LS, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis & Rheumatism. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 3.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries Osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Brophy RH, Gray BL, Nunley RM, Barrack RL, Clohisy JC. Total knee arthroplasty after previous knee surgery: expected interval and the effect on patient age. J Bone Joint Surg Am. 2014;96:801–805. doi: 10.2106/JBJS.M.00105. [DOI] [PubMed] [Google Scholar]
- 6.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis and Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen C, Lohmander LS. Osteoarthritis: Does post-injury ACL reconstruction prevent future OA? Nat Rev Rheumatol. 2014;10:577–578. doi: 10.1038/nrrheum.2014.120. [DOI] [PubMed] [Google Scholar]
- 8.Conaghan PG, Kloppenburg M, Schett G, Bijlsma JWJ. Osteoarthritis research priorities: a report from a EULAR ad hoc expert committee. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204660. annrheumdis–2013–204660. [DOI] [PubMed] [Google Scholar]
- 9.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 10.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis and Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 13.Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. Journal of Orthopaedic Research. 2013;31:315–321. doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 14.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. The Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Cuellar VG, Cuellar JM, Golish SR, Yeomans DC, Scuderi GJ. Cytokine Profiling in Acute Anterior Cruciate Ligament Injury. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26:1296–1301. doi: 10.1016/j.arthro.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Marks PH, Donaldson MLC. Inflammatory Cytokine Profiles Associated With Chondral Damage in the Anterior Cruciate Ligament–Deficient Knee. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2005;21:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Research & Therapy. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus VB, Birmingham J, Stabler TV, Feng S, Taylor DC, Moorman CT, 3rd, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial ( NCT00332254) Osteoarthr Cartil. 2012;20:271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Murray MM, Fleming BC. Use of a Bioactive Scaffold to Stimulate Anterior Cruciate Ligament Healing Also Minimizes Posttraumatic Osteoarthritis After Surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haslauer CM, Proffen BL, Johnson VM, Hill A, Murray MM. Gene expression of catabolic inflammatory cytokines peak before anabolic inflammatory cytokines after ACL injury in a preclinical model. J Inflamm. 2014;11:34. doi: 10.1186/s12950-014-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslauer CM, Elsaid KA, Fleming BC, Proffen BL, Johnson VM, Murray MM. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthr and Cartil. 2013;21:1950–1957. doi: 10.1016/j.joca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 24.Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, et al. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM) Bioinformatics. 2011;27:2518–2528. doi: 10.1093/bioinformatics/btr427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayturk UM, Jacobsen CM, Christodoulou DC, Gorham J, Seidman JG, Seidman CE, et al. An RNA-seq protocol to identify mRNA expression changes in mouse diaphyseal bone: Applications in mice with bone property altering Lrp5 mutations. Journal of Bone and Mineral Research. 2013;28:2081–2093. doi: 10.1002/jbmr.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson M, Aboyoup P, Falcon S, Morgan M, Sarkar D, Lawrence M. R package version 1.8.3. GenomicFeatures: Tools for making and manipulating transcript centric annotations. [Google Scholar]
- 28.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 30.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers SL, Brandt KD, O’Connor BL, Visco DM, Albrecht ME. Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum. 1990;33:1406–1415. doi: 10.1002/art.1780330913. [DOI] [PubMed] [Google Scholar]
- 33.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 34.Blom AB, van Lent PLEM, Holthuysen AEM, van der Kraan PM, Roth J, van Rooijen N, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2004;12:627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 35.van Lent PLEM, Blom AB, van der Kraan P, Holthuysen AEM, Vitters E, van Rooijen N, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 36.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuneyoshi Y, Tanaka M, Nagai T, Sunahara N, Matsuda T, Sonoda T, et al. Functional folate receptor beta-expressing macrophages in osteoarthritis synovium and their M1/M2 expression profiles. Scand J Rheumatol. 2012;41:132–140. doi: 10.3109/03009742.2011.605391. [DOI] [PubMed] [Google Scholar]
- 38.Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis and Cartilage. 2014;22:1167–1175. doi: 10.1016/j.joca.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier JP, Martel-Pelletier J. Protective effects of corticosteroids on cartilage lesions and osteophyte formation in the pond-nuki dog model of osteoarthritis. Arthritis & Rheumatism. 1989;32:181–193. doi: 10.1002/anr.1780320211. [DOI] [PubMed] [Google Scholar]
- 40.Myers SL, Brandt KD, O’Connor BL. Low dose prednisone treatment does not reduce the severity of osteoarthritis in dogs after anterior cruciate ligament transection. J Rheumatol. 1991;18:1856–1862. [PubMed] [Google Scholar]
- 41.Yang C-C, Lin C-Y, Wang H-S, Lyu S-R. Matrix Metalloproteases and Tissue Inhibitors of Metalloproteinases in Medial Plica and Pannus-like Tissue Contribute to Knee Osteoarthritis Progression. PLoS ONE. 2013;8:e79662. doi: 10.1371/journal.pone.0079662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis & Rheumatism. 2012;64:705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haringman JJ, Smeets TJM, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvestri T, Pulsatelli L, Dolzani P, Facchini A, Meliconi R. Elevated serum levels of soluble interleukin-4 receptor in osteoarthritis. Osteoarthritis and Cartilage. 2006;14:717–719. doi: 10.1016/j.joca.2006.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.