Case presentations
Case 1
After induction using 7+3 cytarabine-idarubicin, a 64-year-old man with cytogenetically normal acute myeloid leukemia (AML) without FLT3-ITD, NPM1, and CEBPA mutations achieved a morphologic complete remission (CR). The patient was not interested in allogeneic hematopoietic cell transplantation (allo-HCT) at that time and underwent 3 cycles of consolidation with high-dose cytarabine 3 g/m2 for 6 doses per cycle. Would maintenance therapy after completion of high-dose cytarabine be of any value in prolonging survival?
Case 2
A 41-year-old otherwise healthy woman was diagnosed with normal karyotype AML and an FLT3-ITD mutation. After 7+3 induction, she achieved a morphologic CR. Treatment was consolidated with a myeloablative T-replete allo-HCT from her HLA-matched brother. Her early posttransplant course was uneventful, and full donor chimerism was documented on her day +30 bone marrow examination with no evidence of morphologic or cytogenetic relapse and no graft-versus-host disease. Should maintenance therapy be recommended to reduce the risk of relapse and improve posttransplant outcomes?
Introduction
Achievement of complete remission (CR) is an important milestone for patients with acute myeloid leukemia (AML) undergoing curative-intent therapy.1 Still, even after consolidation and/or hematopoietic cell transplantation (HCT)—modalities with well-documented efficacy in reducing the risk of relapse2-5—many patients will experience disease recurrence. Thus, there has been long-standing interest in the use of lower-intensity (maintenance) therapies after the intensive treatment phase has been concluded to prolong remission durations and improve survival and likelihood of cure. Although the concept of maintenance therapy in AML dates back to the 1960s,6 it continues to be a subject of controversy. We conducted this systematic review to comprehensively examine the evidence supporting maintenance therapy in AML patients after completion of consolidation chemotherapy or HCT.
Methods
Literature search strategy and study selection criteria
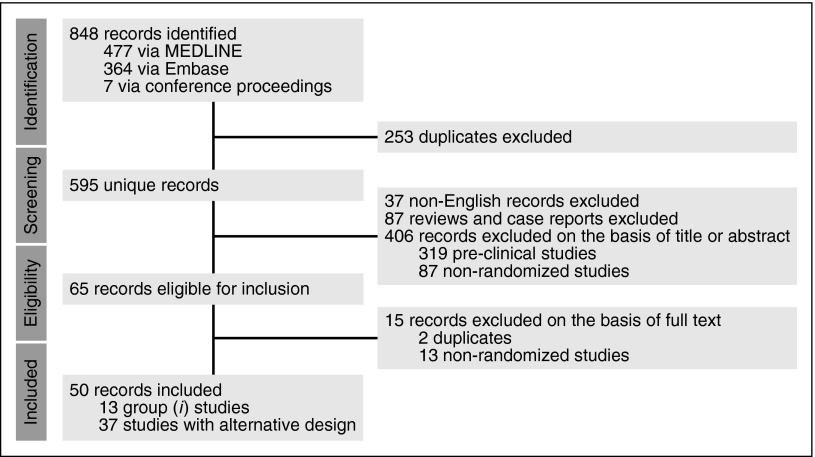
A systematic literature review was conducted using MEDLINE and Embase (April 15, 2016; Figure 1). Proceedings of 2 major annual conferences (56th annual meeting of the American Society of Hematology in 2014 and 51st annual meeting of the American Society of Oncology in 2015) were also searched for abstracts whose corresponding manuscripts have not yet been published in peer-reviewed journals; the Clinical Trial Registry (www.clinicaltrials.gov; April 15, 2016) was also searched for ongoing trials. Two authors (A.R. and R.B.W.) independently reviewed all abstracts for eligibility assessment, with a third author (J.F.D.) mediating discordant results. The full article was reviewed if eligibility was clearly met or if there was uncertainty regarding a priori defined eligibility criteria based on the title and abstract. Studies were included if they were randomized trials evaluating maintenance therapy in AML in one of these two scenarios: (1) after intensive induction (containing at least an anthracycline and cytarabine) and at least 1 cycle of consolidation, and (2) after autologous HCT (auto-HCT) or allogeneic HCT (allo-HCT). Although the value and necessity of preclinical and early-phase clinical trials is unquestioned, we focused on randomized clinical trials (RCTs) because they provide the highest level of evidence for comparative effectiveness, and such a synthesis provides an evidence-based summary to help physicians with clinical decision-making when a trial is not readily available. Studies specifically focused on acute promyelocytic leukemia were not included. Although there is some arbitrariness in the definition of maintenance therapy, we considered maintenance therapy as a treatment with a lower intensity than and administered after induction and consolidation. Consolidation was defined as one or more courses of cytotoxic chemotherapy administered to patients in remission at doses close to those used for induction.
Figure 1.
PRISMA article flow diagram. Group 1 studies are those in which patients randomly assigned between maintenance and observation were similar with regard to previous treatments (preplanned, stratified randomization, or differences accounted for in multivariate analysis). “Alternative design” refers to studies in which either patients randomly assigned between maintenance and observation were dissimilar with regard to previous treatments (induction and/or consolidation) or none of the strategies mentioned in group 1 were used to account for potential differences between the groups. Also included in this group are studies in which patients received intensification courses (in addition to standard consolidation) that are not standard in today’s practice, as well as studies without an observation-only arm or those evaluating maintenance therapies after auto-HCT.
Considering the great heterogeneity of the reported trials in terms of design, setting, induction, and consolidation regimens and/or schedules, and era (over 4 decades), studies included in this review were classified into 2 general categories. The first category (group 1) included studies in which patients randomly assigned between maintenance and observation were similar with regard to previous treatments (preplanned, stratified randomization, or differences accounted for in multivariate analysis). The second category (group 2) included studies in which either patients randomly assigned between maintenance and observation were dissimilar with regard to previous treatments (induction and/or consolidation) or none of the strategies mentioned in group 1 were used to account for potential differences between the groups. Also included in group 2 were studies in which patients received intensification courses (in addition to standard consolidation) that are not standard in today’s practice. Studies in group 2 along with those without an observation-only arm or those evaluating maintenance therapies after auto-HCT were collectively named “trials with alternative design.” Studies in group 1 were evaluated in more detail because they represent the most rigorous design to address the question of whether maintenance therapy in AML is beneficial.
Outcome measures and quality assessment
Outcomes of interest were overall survival (OS), relapse-free survival (RFS), disease-free survival (DFS), and event-free survival (EFS), cumulative incidence of relapse, remission duration, and quality of life (QoL). A trial was considered positive if it showed a statistically significant improvement in at least 1 of the studied outcomes in the maintenance arm. Studies in group 1 were scored by detailed evaluation of their methodology by using a tool from Cochrane Collaboration for assessing risk of bias7 and of their report quality by using the CONSORT recommendations for abstracts and articles.8 Although the actual publication (journal article or abstract/conference proceeding) was the basis for evaluating the quality of reports, we researched related previous and subsequent publications to find unreported protocol details when evaluating the quality of the study (rather than the published report). We also considered the specifics of induction and consolidation in each trial and assessed their relevance to current standards. Specifically, we evaluated the following questions: (1) Was induction adequate, that is, according to the 7+3 protocol (cytarabine in addition to daunorubicin, idarubicin, or mitoxantrone)? (2) Did induction include a single course, with a second course only if needed, or was double induction with 2 courses administered regardless of response to the first course? and (3) Was consolidation adequate, that is, at least 3 cycles of cytotoxic chemotherapy? Final recommendations were developed on the basis of the Grading of Recommendations Assessment Development, and Evaluation system (supplemental Data, available on the Blood Web site).9
Search results and discussion
Our systematic review resulted in 595 unique records (Figure 1). A total of 13 studies in group 1 (Table 1)10-22 and 37 studies in group 2 with alternative design (Table 2)23-60 met our inclusion criteria. All of these studies were in the non-HCT setting. Table 3 provides quality assessment for group 1 studies, Tables 4 and 5 provide quality assessment for the reports resulting from the studies, and Table 6 provides additional details about these studies.
Table 1.
Randomized trials of maintenance vs observation (group 1 studies)
| Reference | No. of patients* | Age (y) | Regimen |
|---|---|---|---|
| Immunotherapy | |||
| 22 | 41 | NA | BCG + irradiated allogeneic myeloblasts vs observation |
| 16 | 45 | 15-59 | IFN-α vs chemotherapy (3 y) vs observation |
| 10 | 163 | ≥60 | IL-2 (3 mo) vs observation |
| 21† | 528 | ≤60 | IL-2 (1 y) vs observation |
| 17 | 161 | 50-70 | IL-2 (1 y) vs observation |
| 19 | 154 | 0-18 | IL-2 (1 y) vs observation |
| Conventional chemotherapy | |||
| 20 | 74 | 7-65 | Chemotherapy (2 y) vs observation |
| 11 | 145 | ≥15 | Chemotherapy (3 y) vs observation |
| 13 | 48 | 18-75 | Chemotherapy (6 mo) vs observation |
| 15 | 147 | ≥60 | Chemotherapy (1 y) vs observation |
| 18 | 70 | <20 | Chemotherapy (1.5 y) vs observation |
| Other approaches | |||
| 14 | 102 | >60 | Cytarabine + all-trans retinoic acid (1 y) vs observation |
| 12 | 226 | >60 | Azacitidine (1 y) vs observation |
Positive trials are shown in bold.
NA, not available.
Randomly assigned between maintenance and observation arms.
Maintenance randomization occurred after 1 cycle of consolidation and auto-HCT.
Table 2.
Randomized trials with alternative design
| Reference | No. of patients* | Age (y) | Reason for classification as group 2 | QoL† |
|---|---|---|---|---|
| Conventional chemotherapy | ||||
| 39 | 965 | 16-60 | No observation-only arm | No |
| 43 | 598 | ≤64 | More consolidation vs less + maintenance | No |
| 26 | 325 | Any | No strategy used to balance groups for previous therapies‡ | No |
| 32 | 150 | Any | No observation-only arm | No |
| 58 | 208 | ≤50 | No strategy used to balance groups for previous therapies‡ | No |
| 33 | 41 | ≤65 | No strategy used to balance groups for previous therapies‡ | No |
| 59 | NA | ≤21 | No strategy used to balance groups for previous therapies‡ | No |
| 47 | 131 | ≥16 | No observation-only arm | No |
| 28 | 170 | ≤65 | Maintenance arm received no consolidation | No |
| 27 | NA | >16 | Maintenance arm received no consolidation | No |
| 54 | 82 | ≥51 | No strategy used to balance groups for previous therapies‡ | No |
| 44 | 47 | 50-70 | No strategy used to balance groups for previous therapies‡ | No |
| 35 | 248 | NA | No observation-only arm | No |
| 34 | 48 | NA | No observation-only arm | No |
| 51 | 275 | Any | More consolidation vs less + maintenance | No |
| Hypomethylating agents | ||||
| 46 | 214 | ≥61 | No strategy used to balance groups for previous therapies‡ | No |
| 29 | 727 | ≥60 | No strategy used to balance groups for previous therapies‡ | No |
| 60 | 176 | NA | No observation-only arm | No |
| 24 | 45 | ≥18 | No observation-only arm | No |
| Immunotherapy (alone or combined with chemotherapy) | ||||
| 37,38 | 214 | <60 | No strategy used to balance groups for previous therapies‡ | No |
| 50§ | 169 | ≤60 | No strategy used to balance groups for previous therapies‡ | No |
| 41 | 289 | ≤21 | No strategy used to balance groups for previous therapies‡ | No |
| 25 | 261 | ≥18 | No strategy used to balance groups for previous therapies‡ | No |
| 31 | 362 | >55 | No strategy used to balance groups for previous therapies‡ | No |
| 23 | 78 | <50 | Following auto-SCT | No |
| 45 | 91 | ≥15 | No strategy used to balance groups for previous therapies‡ | No |
| 48 | 66 | ≥15 | No observation-only arm | No |
| 57 | 215 | ≥15 | No strategy used to balance groups for previous therapies‡ | No |
| 40 | 35 | ≤84 | No observation-only arm | No |
| 49 | 97 | >15 | No strategy used to balance groups for previous therapies‡ | No |
| 30 | 47 | Any | No observation-only arm | No |
| 56 | 15 | 18-65 | No strategy used to balance groups for previous therapies‡ | No |
| Small molecules | ||||
| 42 | 144 | Any | No strategy used to balance groups for previous therapies‡ | No |
| 55 | 717 | ≤60 | No strategy used to balance groups for previous therapies‡ | No |
| 52 | 267 | ≤60 | No strategy used to balance groups for previous therapies‡ | No |
| 53 | 197 | >60 | No strategy used to balance groups for previous therapies‡ | No |
| 36 | 111 | ≥15 | No strategy used to balance groups for previous therapies‡ | No |
Randomly assigned between maintenance arms.
QoL was formally assessed.
See text for details.
This study was terminated early.
Table 3.
Quality assessment of randomized trials of maintenance treatment in AML
| Reference | No. of patients* | Quality domains (risk of bias) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of postremission courses | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias | |||
| 1 | 22 | 41 | 1 | Unclear | Unclear | High | Unclear | Unclear | High |
| 2 | 20 | 74 | 2-3 | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| 3 | 11 | 145 | 1 | Unclear | Unclear | Unclear | High | High | Unclear |
| 4 | 13 | 32 | 1 | Unclear | Low | Unclear | High | Unclear | High |
| 5 | 16 | 45 | 6 | Unclear | Unclear | Unclear | Low | Low | Low |
| 6 | 15 | 76 | 1 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| 7 | 18 | 70 | 2 | Unclear | Low | Unclear | Unclear | Unclear | High |
| 8 | 10 | 163 | 1 | Unclear | Unclear | Unclear | High | Unclear | High |
| 9 | 14 | 102 | 1 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| 10 | 21 | 528 | 1 | Unclear | Low | Unclear | Unclear | Low | Unclear |
| 11 | 17 | 161 | 2 | Unclear | Unclear | Unclear | Unclear | Low | Low |
| 12 | 19 | 154 | 3 | Unclear | Unclear | Unclear | Unclear | High | Low |
| 13 | 12 | 226 | 1 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
Positive trials are shown in bold. Details of scoring are provided in Higgins et al.7 Low corresponds to low risk of bias; high corresponds to high risk of bias. The following score/domain correspondence was used: sequence generation (method to generate the allocation sequence [eg, random number table, computer random number generator, or minimization], allocation concealment (method used to conceal the allocation sequence [eg, central allocation, sequentially numbered drug containers of identical appearance]), binding (measures used to blind study participants and personnel from knowledge of which intervention a participant received [eg, no blinding, single blinded, or double blinded]), incomplete outcome data (completeness of outcome data for each main outcome, including attritions and exclusions and reasons for each [eg, no missing outcome data, imputation, reasons for missing outcome data unlikely to be related to true outcome, missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups]), selective outcome reporting (the possibility of selective outcome reporting [eg, all prespecified outcomes have been reported in the prespecified way]), other sources of bias (any concerns for bias from other sources [eg, early termination as a result of some data-dependent process]).
Randomly assigned between maintenance and observation arms.
Table 4.
CONSORT quality assessment of RCT articles on maintenance treatment in AML
| Reference | Report domains | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title/abstract | Introduction | Methods | Results | Discussion | Other information | ||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |||||||||||||
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | ||||||||||||||
| 22 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 11 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 13 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 15 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 14 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
Items not applicable to each study as well as those assessed adequate are coded 1. Details of scoring are provided in Moher et al.8 The designation of 0 corresponds to the absence and 1 corresponds to the presence of a potential deficiency. The following score/domain correspondence was used: 1a, identification as RCT in the title; 1b, structured summary of trial design, methods, results, and conclusions; 2a, background and rationale; 2b, objectives and/or hypotheses; 3a, design including allocation ratio; 3b, important changes to methods after trial commencement; 4a, eligibility criteria; 4b, settings and locations; 5, interventions; 6a, prespecified outcomes; 6b, changes to outcomes after trial commencement; 7a, sample size determination; 7b, interim analyses, if applicable; 8a, random allocation sequence generation; 8b, randomization type; 9, allocation concealment; 10, who generated the random allocation sequence, who enrolled participants, and who assigned them to interventions; 11a, blinding; 11b, similarity of interventions, if applicable; 12a, statistical methods for primary and secondary end points; 12b, methods for additional analyses; 13a, flow diagram or equivalent; 13b, losses and/or exclusions after randomization, with reasons; 14a, recruitment and/or follow-up dates; 14b, why the trial ended or was stopped, if applicable; 15, baseline demographics and clinical characteristics of each group; 16, number analyzed in each group and whether analysis was intention to treat; 17a, results in each group, effect size and its precision for each outcome; 17b, absolute and relative effect size recommended for binary outcomes; recommended; 18, results of other analyses; 19, harms and unintended effects in each group; 20, limitations, including biases and imprecisions; 21, generalizability; 22, consistency of interpretation and results, balancing benefits and harms, other relevant evidence; 23, registration number and name of registry; 24, where the full protocol can be accessed, if available; 25, funding and its role.
Table 5.
CONSORT quality assessment of abstracts from RCTs on maintenance treatment in AML
| Reference | Authors |
Design |
Methods | Results | Conclusions |
Registration |
Funding |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 3c | 3d | 3e | 3f | 4a | 4b | 4c | 4d | 4e | 5 | 6 | 7 | |
| 18 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 21 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 19 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| 12 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
Positive trials are shown in bold. Details of scoring are provided in Moher et al.8 0 corresponds to the absence and 1 corresponds to the presence of a potential deficiency. The following score/domain correspondence was used: 1, authors; 2, design; 3a, participants; 3b, interventions; 3c, objective; 3d, outcome; 3e, randomization; 3f, blinding (masking); 4a, numbers randomized; 4b, recruitment; 4c, numbers analyzed; 4d, outcomes; 4e, harms; 5, conclusions; 6, registration; 7, funding.
Table 6.
Additional details of randomized trials of maintenance treatment in AML
| Reference | Induction | Consolidation | Outcome and follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| Randomized | Regimen | Randomized | Regimen | Primary | Secondary | QoL* | Median follow-up | |
| 22 | No | Cytarabine-daunorubicin (BART III) × 2 | No | Cyclophosphamide + 6-thioguanine | NA | NA | No | NA |
| 20 | No | Cytarabine-daunorubicin (7+3)-vincristine; cytarabine-daunorubicin (5+2)-vincristine | No | Cytarabine-daunorubicin (7+3)-vincristine | NA | NA | No | NA |
| 11 | No | 6-Thioguanine-cytarabine-daunorubicin | No | 6-Thioguanine-cytarabine-daunorubicin | NA | NA | No | NA |
| 13 | No | Cytarabine-daunorubicin (5+2) × 2 | No | C1: 5+2; C2: intermediate-dose cytarabine |
NA | NA | No | NA |
| 16 | No | 6-Thioguanine-cytarabine-daunorubicin | No | C1 and C4: 6-thioguanine-cytarabine-daunorubicin; C2 and C5: azacitidine- cyclophosphamide-etoposide-vincristine; C3 and C6: methotrexate |
NA | NA | No | 82 mo |
| 15 | Yes | Cytarabine with or without daunorubicin vs mitoxantrone | No | Same as induction | DFS | OS | No | NA |
| 18 | No | Cytarabine-mitoxantrone | No | Daunorubicin–intermediate-dose cytarabine; daunorubicin–high-dose cytarabine-Ams-Asp | NA | NA | No | NA |
| 10 | Yes | Cytarabine-daunorubicin-etoposide with or without PSC-833 | No | Same as induction | NA | NA | No | NA |
| 14 | Yes | Cytarabine + daunorubicin vs liposomal daunorubicin | No | Same as induction | DFS, CR (induction) | DFS (maintenance) | No | NA |
| 21 | Yes | Cytarabine-daunorubicin-etoposide vs high-dose cytarabine-daunorubicin-etoposide | No | Daunorubicin–intermediate-dose cytarabine | OS | DFS/EFS/ Toxicity | No | 3.6 y |
| 17 | Yes | Cytarabine + daunorubicin vs idarubicin3 vs idarubicin4 | No | Idarubicin-daunorubicin + intermediate-dose cytarabine | EFS | NA | No | 49 mo |
| 19 | No | Cytarabine-mitoxantrone | No | C1 and C3: high-dose cytarabine | Relapse/EFS | Toxicity | No | 5 y |
| 12 | Yes | Cytarabine + daunorubicin vs clofarabine with or without gemtuzumab ozogamicin × 2 | Yes | 5+2 vs none | OS | TTR, Toxicity | No | 50 mo |
Positive trials are shown in bold.
Ams, amsacrine; Asp, asparaginase; BART III, BCG + allogeneic cells vs no maintenance randomized trial; idarubicin3, 3 days of idarubicin; idarubicin4, 4 days of idarubicin; NA, not available; PSC-833, Valspodar; TTR, time to relapse.
QoL formally assessed.
Case 1: Maintenance therapy after consolidation
Immunotherapy trials.
The three most widely explored immunotherapeutic strategies for maintenance therapy are Bacille Calmette-Guérin (BCG) vaccination, interferon alpha (IFN-α), and interleukin-2 (IL-2), largely with negative results. The idea of using BCG as maintenance immunotherapy in acute leukemia dates back to the late 1960s,61 before the combination of cytarabine and daunorubicin was recognized as a potent induction regimen in AML.62 Of the four RCTs conducted in the 1970s and 1980s to assess the value of BCG vaccination as maintenance therapy over observation after consolidation,22,49,56,63 only 1 was a group 1 study.22 In that small study, 41 patients were randomly assigned to observation vs BCG vaccination together with irradiated allogeneic AML cells, and remission duration (median, 35 vs 20 weeks) and OS (median, 90 vs 45 weeks) were significantly longer in the maintenance arm.22 Induction and consolidation in this study would be considered suboptimal today, which, along with other limitations of the study (Tables 3 and 6), precludes any definitive conclusion. The other 3 BCG studies, all with alternative design, found no outcome differences between the maintenance and observation arms.49,56,63
Because of its direct effects on AML cells as well as its stimulatory effects on natural killer, dendritic, and T cells, IFN-α has been studied in clinical trials of AML treatment in various settings since the late 1970s.64 The only RCT in group 1 that explored the value of IFN-α as maintenance therapy in AML found no differences between the groups in remission duration, OS, or RFS.16 That small study randomly assigned 45 patients after induction (6-thioguanine, cytarabine, and daunorubicin [TAD]) and 6 cycles of consolidation to receive no intervention, monthly IFN-α, or monthly chemotherapy for 3 years. Two other maintenance IFN-α studies, both with alternative design, were equally negative.31,45
A meta-analysis of individual patient data in 6 major RCTs (3 classified as group 110,17,21) on IL-2 maintenance therapy in AML patients in first CR (CR1) conducted in the 2000s10,17,21,23,38,41 found no evidence of improved survival and indicated that neither small sample sizes nor study heterogeneity was the reason for the negative results.65 An updated meta-analysis supported the earlier findings.66 Similarly, the recently reported French study in pediatric patients using 1 year of IL-2 maintenance vs observation showed no difference in outcomes between the 2 groups.19 In that multicenter study, presented only in abstract form so far, 154 children (younger than age 18 years) in CR1 after induction (cytarabine and mitoxantrone) and 3 courses of consolidation (cycles 1 and 3 with high-dose cytarabine) were randomly assigned between monthly IL-2 (days 1-5) and observation for 1 year. With a median follow-up of 5 years, there was no difference in DFS or relapse between the 2 groups. Only 51% of patients in the maintenance arm completed all 12 cycles, and 60% of discontinuations were for reasons other than relapse. Difficulties in prolonged IL-2 maintenance were reported in other series as well. Approximately one quarter of patients randomly assigned to IL-2 in 2 studies did not initiate treatment and another quarter discontinued treatment prematurely.10,37 Fewer than 40% of patients were able to complete all planned cycles of therapy without dose reduction in 2 studies.10,17 In contrast to these negative results, another RCT (n = 320) found improved 3-year DFS (but not OS) for patients randomly assigned to low-dose IL-2 plus histamine dihydrochloride vs observation (40% vs 26%, respectively).25 Approximately 20% of patients in that study were in second (or later) CR at the time of random assignment, more than 10% had a previous auto-HCT, and about one third were not treated with high-dose cytarabine–containing regimens during consolidation.67 Although the 2 groups were balanced for the mentioned features, it is difficult to use the results of that study for addressing our specific question. Most importantly, induction regimens (and their intensity) were not reported in that study, and any induction was apparently allowed. Nonetheless, the regimen used for maintenance was remarkably well tolerated, with 92% of the patients completing all ten 3-week cycles of therapy. Subsequent to this trial, histamine dihydrochloride in combination with IL-2 was approved in 2008 by the European Medicines Agency for remission maintenance in adult patients with AML in CR1. An ongoing single-arm German study (NCT01770158) is evaluating the role of IL-2 together with histamine dihydrochloride given for 10 cycles in adults (older than age 18 years) who achieved a morphologic CR but have minimal residual disease (MRD) based on molecular testing for NPM1, CBFβ-MYH11, or MLL-AF9. Finally, a recently completed study (not reported yet) evaluated MRD status in CR1 patients receiving maintenance IL-2 and histamine dihydrochloride (NCT01347996).
Maintenance immunotherapy studies with alternative design are summarized in Table 2, and Table 7 lists ongoing trials. There are currently 3 ongoing group 1 randomized phase 2 studies investigating the unconjugated recombinant anti-killer cell immunoglobulin-like receptor antibody lirilumab (NCT01687387), the PD-1 inhibitor nivolumab (NCT02275533), and a WT1-targeted dendritic cell vaccine (NCT01686334).
Table 7.
Ongoing trials of maintenance therapy in AML
| ClinicalTrials.gov number | Phase | Agent | Maintenance duration | Age (y) | Prior therapy and other requirements | Primary end point | Comparator | Status |
|---|---|---|---|---|---|---|---|---|
| Non-HCT immunotherapy, phase 1/2, 2, or 3 | ||||||||
| NCT02275533 | r2 | Nivolumab | 92 wk | ≥18 | Any except low risk and age <60 y after consolidation | PFS | Observation | Enrolling |
| NCT01687387 | r2 | Lirilumab | NA | 60-80 | 1-2 consolidation cycles | LFS | Placebo | Ongoing* |
| NCT02229266 | r2 | Natural killer cells + IL-2 | NA | ≥60 | High risk, after 0-1 consolidation cycles | OS | Chemotherapy† | Enrolling |
| NCT01686334 | r2 | Dendritic cell vaccine (WT1) | NA | ≥65 | 1 consolidation cycle | Relapse | Observation | Enrolling |
| NCT02405338 | 1/2 | Dendritic cell vaccine (WT1, PRAME) | NA | 18-75 | — | Toxicity, feasibility | None | Enrolling |
| Non-HCT chemotherapy/small molecules, phase 1/2, 2, or 3 | ||||||||
| NCT02668653 | 3 | Quizartinib | 1 y | 18-75 | FLT3-ITD positive, ≤4 consolidation cycles | EFS | Placebo | Not open |
| NCT01371981 | 3 | Sorafenib | 1 y | <29 | — | EFS | Observation | Enrolling |
| NCT01041703 | 3 | Decitabine | 1 y | ≥60 | — | OS | Observation | Enrolling |
| NCT01757535 | 3 | Oral azacitidine | Indefinite | ≥55 | — | OS | Placebo | Enrolling |
| NCT02013648 | 3 | Dasatinib | 1 y | ≥18 | CBF AML, 4 consolidation cycles | EFS | Observation | Enrolling |
| NCT01420926 | r2 | Bortezomib + decitabine | Indefinite | ≥60 | No intensive induction or consolidation | OS | Decitabine | Ongoing1 |
| NCT02126553 | 2 | Lenalidomide | NA | ≥18 | High risk | RFS | None | Enrolling |
| NCT01873495 | 2 | Omacetaxine | 6 mo | ≥55 | — | Relapse | Observation | Enrolling |
| NCT00509093 | 2 | Imatinib | 1 y | ≥18 | c-KIT positive, ≥1 consolidation cycle | PFS | None | Ongoing1 |
| NCT01806571 | 2 | Nilotinib | 96 wk | 18-69 | KIT positive, 4 consolidation cycles | CR | None | Enrolling |
| NCT02302846 | 2 | Ixazomib | 1 y | ≥18 | 2 induction or ≥1 consolidation cycles | RFS | None | Enrolling |
| NCT01477606 | 2 | Midostaurin | 1-1.5 y | 18-70 | FLT3-ITD positive | EFS | None | Enrolling |
| NCT01830361 | 2 | Midostaurin | 1 y | 18-65 | c-KIT or FLT3-ITD mutated t(8;21), 3 consolidation cycles | EFS | None | Enrolling |
| NCT02560025 | 2 | Alisertib | 36 wk | ≥18 | High risk | CR | None | Enrolling |
| NCT01253070 | 2 | Sorafenib | 1 y | ≥60 | Any except low risk | OS | None | Ongoing1 |
| NCT02472626 | 1/2 | CPI-613 | 1 y | ≥60 | — | MTD, CR | None | Enrolling |
| Post-HCT, phase 1, 2, or 3 | ||||||||
| NCT01773395 | r2 | GVAX | NA | ≥18 | Not in CR before transplant | PFS | Placebo | Enrolling |
| NCT02400255 | 2 | Crenolanib | 2 y | ≥18 | FLT3 mutated | PFS | None | Enrolling |
| NCT01578109 | 2 | Sorafenib | 2 y | >18 | FLT3-ITD positive | DLT | None | Enrolling |
| NCT02723435 | 2 | Midostaurin | 1 y | ≥60 | FLT3 mutated | EFS/OS | None | Not open |
| NCT02124174 | 2 | Azacitidine + valproic acid | 4 mo | 2-89 | Any except low risk | OS | None | Enrolling |
| NCT01995578 | 2 | Azacitidine | 1 y | 1-75 | High-risk T-depleted allo-HCT | Relapse | None | Enrolling |
| NCT02204020 | 2 | Azacitidine | 1 y | ≥18 | High risk | Relapse | None | Enrolling |
| NCT01700673 | 2 | Azacitidine + granulocyte-macrophage colony-stimulting factor | 1 y | Any | Allo-HCT/cytarabine consolidation | RFS | None | Enrolling |
| NCT02038153 | 1/2 | Lenalidomide | Indefinite | 60-75 | ≥1 consolidation cycle and auto-HCT | RFS | None | Enrolling |
| NCT01835587 | 1/2 | Oral azacitidine | 1 y | ≥18 | ≤10% bone marrow blasts, ≤5% circulating blasts | MTD | None | Ongoing1 |
| NCT01451268 | 1/2 | Panobinostat | 1 y | ≥18 | High risk | MTD/DLT | None | Enrolling |
| NCT01398501 | 1 | Sorafenib | NA | 18-75 | FLT3 mutated | MTD | None | Ongoing1 |
| NCT02326584 | 1 | SGN-CD33A | 48 wk | ≥18 | — | DLT | None | Enrolling |
Group 1 studies are shown in bold. Trials that have been completed, with unknown status, on relapsed/refractory AML, or on patients not in CR (non-HCT setting) are not included. Studies in each group are sorted according to the phase of the trial in descending order and randomization.
CBF, core binding factor; DLT, dose-limiting toxicity; LFS, leukemia-free survival; MTD, maximum-tolerated dose; NA, not available; r2, randomized phase 2; WT1, Wilm's tumor antigen-1.
Active but not enrolling.
One consolidation cycle.
Chemotherapy and small molecule trials.
Among RCTs that investigated the role of chemotherapy or small molecules as maintenance therapy in AML, 7 are classified as group 1 studies11-15,18,20 (Tables 1 and 2). A German Cooperative Group trial randomly assigned 145 patients after TAD induction and 1 cycle of consolidation using TAD to monthly maintenance (alternating courses of cytarabine-daunorubicin, cytarabine–6-thioguanine, cytarabine-cyclophosphamide) for 3 years vs observation and found significantly improved RFS at 2.5 years in the maintenance arm (30% vs 17%).11 OS was not reported. One cycle of consolidation is not considered adequate at the present time, and maintenance chemotherapy may have simply replaced at least part of the missing consolidation in that study, resulting in improved RFS. This notion was also supported by several studies with alternative design, including 2 large pediatric RCTs (CCG251 and CCG213).59 Similarly, in another study, 74 evaluable patients age 60 years or older were randomly assigned between low-dose cytarabine 10 mg/m2 every 12 hours for 12 days each cycle maintenance therapy and observation for eight 6-week cycles. All patients had undergone a first random assignment between cytarabine-daunorubicin and cytarabine-mitoxantrone for induction (1 cycle) and received 1 cycle of consolidation using the same regimen as that used for induction.15 Higher 3- and 5-year DFS rates (20% and 13% vs 7% and 7%, respectively), but no OS difference, were observed with maintenance. Finally, after random assignment between double induction arms (cytarabine-daunorubicin vs cytarabine-clofarabine, each with or without gemtuzumab ozogamicin) and a second random assignment with either 0 or 1 cycles of consolidation (5+2 regimen), 453 patients in CR were randomly assigned between azacitidine 75 mg/m2 per day on days 1 to 5 for nine 6-week courses and observation in the United Kingdom National Cancer Research Institute AML16 trial.12 Randomizations were stratified for induction regimens and demographics. There was no significant OS difference between maintenance and observation arms, irrespective of the number of consolidation cycles. However, in an unplanned subset analysis, maintenance azacitidine improved 5-year OS of MRD-negative patients (40% for patients with maintenance vs 13% for patients without maintenance; P = .003). The other group 1 studies were negative, with no improvement in any of the studied outcomes in the maintenance arm (Table 1). In fact, 2 studies (1 in group 118 and the other with alternative design54) showed lower CR rates after relapse in the maintenance chemotherapy arm, an observation attributed (correctly or incorrectly) to acquired resistance resulting from prolonged exposure to chemotherapy. A detailed critique of both positive and negative studies is provided in Tables 3-6, and trials with alternative design are summarized in Table 2.
Small molecules have been the focus of a number of recent maintenance studies. In the largest maintenance trial with alternative design, 717 patients with FLT3-mutated AML (age 18 to 60 years) received 7+3 induction followed by 4 cycles of consolidation.55 The experimental group also received midostaurin with induction and consolidation, followed by 1 year of maintenance midostaurin 50 mg twice per day. Both OS (median, 74.7 vs 26.0 months) and EFS (median, 8.0 vs 3.0 months) were significantly improved in the midostaurin group, even after censoring at the time of transplantation. In the SORAML trial, patients (age 18 to 60 years) received 2 courses of induction (7+3) and 3 courses of high-dose cytarabine consolidation.52 In the experimental arm, sorafenib was added to induction and consolidation and continued as maintenance (400 mg twice per day) for 1 year. The overall frequency of FLT3-ITD in this trial was 17%. Among the 267 evaluable patients, those in the sorafenib arm had significantly longer median EFS of 21 vs 9.5 months in the control group, but no OS difference was observed. Among the 46 randomly assigned FLT3-ITD patients, EFS, DFS, and OS were not significantly different between the treatment groups, suggesting that the beneficial effect of sorafenib was not specific to inhibition of FLT3-ITD. The same strategy was used in a study of older patients (older than age 60 years) who received 7+3 induction and up to 2 cycles of consolidation (intermediate-dose cytarabine).53 In that study, however, EFS and OS rates were similar between the groups (197 evaluable patients). Importantly, 60-day mortality (mostly as a result of infections) was significantly higher in the sorafenib arm. The specific design of the midostaurin and SORAML studies poses an inherent limitation to specifically evaluating the role of maintenance because midostaurin and sorafenib were used during all 3 phases of treatment. Furthermore, most, if not all, of the separation between the survival curves for maintenance and placebo groups occurred during the first 6 to 12 months, which brings into question the value of maintenance alone in prolonging survival.
Ongoing trials of maintenance therapy in the non-HCT setting have moved away from traditional cytotoxic chemotherapy. Instead, a relatively large number of ongoing studies are investigating hypomethylating agents (HMAs), small molecules, and other targeted agents used as maintenance therapy for AML (Table 7). One trial (NCT01757535) is considered a group 1 study. With the idea of delivering lower doses of systemic azacitidine over a longer period of time as maintenance, the investigators of that large multicenter phase 3 placebo-controlled RCT are using the oral formulation of azacitidine (CC-486) in older adults (age 55 years or older) in CR1 after induction with or without consolidation. Treatment is given on days 1 to 14 every 28 days. Randomization is stratified for consolidation. QoL and use of health care resources have been explicitly included as secondary outcomes. This study could shed light on the role of maintenance therapy using an HMA and whether (and to what extent) prior consolidation can mask the effect of maintenance therapy. A number of other trials were terminated or completed with no reports yet published. These include studies of lenalidomide (NCT00957385 and NCT00957385), sequential azacitidine and lenalidomide (NCT01301820), and the integrin inhibitor Cilengitide (NCT00089388; terminated). Health-related QoL and the use of health care resources have been explicitly incorporated as secondary outcomes in some of the ongoing studies (NCT01757535, NCT01180322, NCT01371981, NCT02085408, and NCT01477606).
Recommendation.
Outside a well-designed clinical trial, and potentially with the exception (pending approval by the US Food and Drug Administration) of using midostaurin during induction, consolidation, and maintenance in FLT3-mutated patients, we do not recommend maintenance therapy following adequate induction and consolidation (grade 2B). Although the majority of previous trials on maintenance therapy showed little improvement in outcomes with maintenance therapy, induction and (more commonly) consolidation in many of these trials are not considered standard today, limiting their applicability to the question of interest in this review. In addition, important details are missing from several older studies. Furthermore, the alternative design used in some of the previous studies precludes a definitive conclusion regarding the value of maintenance therapy per se.
Case 2: post-HCT maintenance therapy in AML
The post–allo-HCT setting may represent a unique situation in which maintenance can, at least theoretically, contribute to cure by maintaining the disease burden in a minimal state until the immunologic effect of graft-versus-leukemia becomes dominant, potentially resulting in eradication of residual disease. Although no maintenance RCT has yet been performed in the post-HCT setting, a number of early-phase studies have been performed to assess safety and feasibility of maintenance therapy after allo-HCT using HMAs,68-72 FLT3 inhibitors,73-76 histone deacetylase (HDAC) inhibitors,77 and lenalidomaide.78 In the largest of these trials, 42 patients with high-risk myelodysplastic syndrome (n = 5) or AML (n = 37) in CR after reduced-intensity allo-HCT were treated with the HDAC inhibitor panobinostat using 2 different schedules; 67% of patients received transplantation in active disease. Treatment was started between days 60 and 150 posttransplant and continued for up to 1 year. Although grade 3 to 4 toxicity (mostly hematologic) developed in 57% of patients, it was rapidly reversible upon discontinuation of the drug with no evidence of impaired immune reconstitution. The 2-year DFS was 74%.77
A number of phase 1 and 2 studies are evaluating FLT3 inhibitors (NCT02400255, NCT01398501, NCT01578109, and NCT02723435), HMAs (NCT01835587, NCT01835587, NCT02124174, NCT01995578, NCT02204020, and NCT01700673), lenalidomide (NCT02038153), antibody-drug conjugates such as SGN-CD33A (NCT02326584), and HDACs (NCT01451268) as maintenance after allo-HCT. In addition, a randomized phase 2 study (NCT01773395), currently enrolling participants, is comparing posttransplant outcomes in patients with AML (not in CR before allo-HCT) randomly assigned to receive placebo vs GVAX. GVAX is composed of irradiated adenovirus vector–transferred granulocyte-macrophage colony-stimulating factor–secreting autologous AML cells. Treatment begins 30 to 45 days posttransplant with the idea of potentiating an early graft-versus-leukemia effect in high-risk patients. That study is a larger follow-up study of previous trials in post–auto-HCT79 and post–allo-HCT80 settings. An unanswered question, particularly important in the post-HCT setting, is related to accurate identification of patients who are at high risk for relapse and would more likely benefit from maintenance. MRD testing before and/or after transplant may add to the discriminatory power of patient-, disease-, and transplant-related characteristics as predictors of relapse81,82 and perhaps have an impact on maintenance therapy.
Recommendation.
Patient 2 underwent an allo-HCT for high-risk AML in CR1. Although her FLT3-positive disease is both high-risk and potentially targetable, there is not yet any convincing evidence that maintenance therapy after allo-HCT improves outcomes. In the absence of randomized studies showing efficacy of maintenance therapy after allo-HCT, we do not recommend this strategy outside the setting of a well-designed clinical trial (grade 2D). However, we would encourage the patient to consider participation in one of the several ongoing trials of maintenance therapy post-HCT. Post-HCT maintenance is an active and promising area of current research, and the results of ongoing studies may change our recommendation regarding maintenance therapy after allo-HCT.
In conclusion, despite 40 years of research and a large number of RCTs exploring the role of maintenance therapy in AML, there is no consensus on the efficacy of this strategy. In this comprehensive and critical review, we attempted to find the roots of the controversies and found several. First, many of the available reports are relatively old, and they used induction and consolidation regimens and/or schedules that today would be considered inadequate. Although a number of studies showed improvements in 1 or more outcomes in the maintenance arm, their generalizability to current practice is at best unclear. Most of these studies were conducted before the current diagnostic and therapeutic standards were developed. Second, the design and quality of several older studies as well as their report quality are suboptimal or unclear according to current well-established and standardized criteria. Third, although well-designed and well-conducted, some of the studies were not primarily designed to evaluate the role of maintenance therapy, and many were not sufficiently powered to address this question. Given these limitations, the quality of evidence for or against maintenance therapy in AML is poor.
Although the synthesis provided here does not eliminate the possibility of benefit with maintenance therapy, the benefit of maintenance seems more apparent after suboptimal induction and consolidation. This may be relevant to patients who cannot tolerate consolidation (eg, some elderly patients or those who develop serious complications during induction). In these patients, maintenance therapy after induction could conceivably be beneficial. Unfortunately, elderly patients were, and continue to be, excluded from most clinical trials of AML, and the quality of evidence for or against maintenance therapy in this population is poor. The importance of designing mechanistic, biologically driven trials cannot be overemphasized. A weakness of most previous RCTs in this field is that therapies were tested in unselected patients. Targeting a selected group of patients on the basis of the specifics of their disease and the intervention should improve the chances of success. An obvious example is the use of midostaurin in FLT3-mutated patients.
Maintenance therapy using small molecules targeting leukemia-specific antigens, dendritic cell–based vaccination, checkpoint inhibitors, and natural killer cell–based therapies are some of the strategies that are being explored in high-risk patients who are not eligible for a transplant. Alternatively, these strategies can be investigated posttransplant in patients who, on the basis of their pretransplant characteristics, are deemed high-risk for relapse. Using midostaurin as a component of induction, consolidation, and maintenance in FLT3-mutated patients will possibly be considered by the US Food and Drug Administration as a registration study for accelerated approval by the US and European regulatory agencies. A variety of agents ranging from conventional chemotherapy to novel agents such as small molecules and targeted immunotherapeutic drugs are being tested in trials of various phases. In addition, a great deal of interest has been developed in the use of small molecules as maintenance after allo-HCT, a unique setting in which no RCTs have yet been performed. The results of these studies would shed light on the value of maintenance therapy in AML.
Historically, the outcome of greatest interest in AML trials has been survival. However, survival is not the only important outcome in RCTs, and more emphasis should be placed on QoL as an end point of future trials. The importance of QoL measures and patient-reported outcomes is apparent from the fact that a number of previous maintenance studies had to be closed early because of excessive toxicity. By using QoL measures, trials are becoming more patient centered, and the traditional focus on what physicians interpret as outcome is being shifted to the status of patients’ health from their own perspective. A number of recent attempts have been made to incorporate QoL in AML trials.83-85 Questionnaires such as the European Organization for Research and Treatment of Cancer 30-item questionnaire (EORTC QLQ-C30)86 and the Functional Assessment of Cancer Therapy Leukemia-specific (FACT-Leu) Trial Outcome Index (TOI) score87 that include items related to global health, various QoL domains (physical, role, emotional, social, and cognitive function), and symptoms should be used more frequently in future studies.
Acknowledgments
The authors thank Graham A. Colditz for his help and guidance on the methods for critical evaluation of previous trials.
This study was supported by grants from the National Institutes of Health Center for Advancing Translational Sciences/Washington University Institute of Clinical and Translational Sciences (UL1 TR000448) (A.R.) and from the Specialized Program of Research Excellence in Leukemia (P50 CA171963) (J.F.D.). R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: A.R. and R.B.W. were responsible for the concept of this review, contributed to the literature search and data collection/quality assessment, analyzed and interpreted data, and wrote the manuscript; A.R. designed the literature search and performed the data extraction; and M.S.T., F.R.A., and J.F.D. contributed to the concept of the review, assessed the literature, and critically revised the manuscript.
Conflict of interest: The authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Campus Box 8007, Washington University School of Medicine, 660 South Euclid Ave, St. Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.
References
- 1.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 2010;28(10):1766–1771. doi: 10.1200/JCO.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H, Estey EH, Amadori S, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 6.Ellison RR, Holland JF, Weil M, et al. Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood. 1968;32(4):507–523. doi: 10.1182/blood-2016-06-725101. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26(30):4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Büchner T, Urbanitz D, Hiddemann W, et al. Intensified induction and consolidation with or without maintenance chemotherapy for acute myeloid leukemia (AML): two multicenter studies of the German AML Cooperative Group. J Clin Oncol. 1985;3(12):1583–1589. doi: 10.1200/JCO.1985.3.12.1583. [DOI] [PubMed] [Google Scholar]
- 12.Burnett A, Russell N, Freeman S, et al. A comparison of limited consolidation chemotherapy therapy or not, and demethylation maintenance or not in older patients with AML and high risk MDS: Long term results of the UK NCRI AML16 trial [abstract]. Haematologica. 2015 100(S1). Abstract 513. [Google Scholar]
- 13.Johnson SA, Prentice AG, Phillips MJ. Treatment of acute myeloid leukaemia with early intensive induction therapy. Acta Oncol. 1988;27(5):527–529. doi: 10.3109/02841868809093582. [DOI] [PubMed] [Google Scholar]
- 14.Latagliata R, Breccia M, Fazi P, et al. Liposomal daunorubicin versus standard daunorubicin: long term follow-up of the GIMEMA GSI 103 AMLE randomized trial in patients older than 60 years with acute myelogenous leukaemia. Br J Haematol. 2008;143(5):681–689. doi: 10.1111/j.1365-2141.2008.07400.x. [DOI] [PubMed] [Google Scholar]
- 15.Löwenberg B, Suciu S, Archimbaud E, et al. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy--the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. J Clin Oncol. 1998;16(3):872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 16.Palva IP, Almqvist A, Elonen E, et al. Value of maintenance therapy with chemotherapy or interferon during remission of acute myeloid leukaemia. Eur J Haematol. 1991;47(3):229–233. doi: 10.1111/j.1600-0609.1991.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 17.Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 18.Perel Y, Auvrignon A, Leblanc T, et al. Group LAME; French Society of Pediatric Hematology and Immunology. Maintenance therapy in childhood acute myeloid leukemia. Ann Hematol. 2004;83(Suppl 1):S116–S119. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 19.Petit A, Ducassou S, Leblanc T, et al. Relevance of a one-year maintenance therapy with interleukin-2 in the treatment of childhood acute myeloid leukemia: Results from the French multicenter, phase III, randomized controlled SFCE trial, ELAM02 [abstract]. Blood. 2014 124(21). Abstract 378. [Google Scholar]
- 20.Sauter C, Berchtold W, Fopp M, et al. Acute myelogenous leukaemia: maintenance chemotherapy after early consolidation treatment does not prolong survival. Lancet. 1984;1(8373):379–382. doi: 10.1016/s0140-6736(84)90424-0. [DOI] [PubMed] [Google Scholar]
- 21.Willemze R, Suciu S, Mandelli F, et al. Value of low dose IL-2 as maintenance following consolidation treatment or autologous transplantation in acute myelogenous leukemia (AML) patients aged 15-60 years who reached CR after high dose (HD-AraC) vs standard dose (SD-AraC) Cytosine Arabinoside during induction: Results of the AML-12 trial of EORTC and GIMEMA Leukemia Groups [abstract]. Blood. 2009 114(22). Abstract 791. [Google Scholar]
- 22.Zuhrie SR, Harris R, Freeman CB, et al. Immunotherapy alone vs no maintenance treatment in acute myelogenous leukaemia. Br J Cancer. 1980;41(3):372–377. doi: 10.1038/bjc.1980.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaise D, Attal M, Reiffers J, et al. Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw. 2000;11(1):91–98. [PubMed] [Google Scholar]
- 24.Boumber Y, Kantarjian H, Jorgensen J, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012;26(11):2428–2431. doi: 10.1038/leu.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 26.Büchner T, Hiddemann W, Berdel WE, et al. German AML Cooperative Group. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): a randomized trial of the German AML Cooperative Group. J Clin Oncol. 2003;21(24):4496–4504. doi: 10.1200/JCO.2003.02.133. [DOI] [PubMed] [Google Scholar]
- 27.Büchner T, Hiddemann W, Wörmann B, et al. Longterm effects of prolonged maintenance and of very early intensification chemotherapy in AML: data from AMLCG. Leukemia. 1992;6(Suppl 2):68–71. [PubMed] [Google Scholar]
- 28.Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79(8):1924–1930. [PubMed] [Google Scholar]
- 29.Foran JM, Sun Z, Claxton DF, et al. North American Leukemia, Intergroup phase III randomized trial of single agent clofarabine as induction and post-remission therapy, and decitabine as maintenance therapy in newly-diagnosed acute myeloid leukemia in older adults (age ≥60 years): A trial of the ECOG-ACRIN Cancer Research Group (E2906) [abstract]. Blood. 2015 126(23). Abstract 217. [Google Scholar]
- 30.Gale RP, Foon KA, Cline MJ, Zighelboim J. Intensive chemotherapy for acute myelogenous leukemia. Ann Intern Med. 1981;94(6):753–757. doi: 10.7326/0003-4819-94-6-753. [DOI] [PubMed] [Google Scholar]
- 31.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE Medical Research Council Adult Leukemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 32.Hewlett J, Kopecky KJ, Head D, et al. A prospective evaluation of the roles of allogeneic marrow transplantation and low-dose monthly maintenance chemotherapy in the treatment of adult acute myelogenous leukemia (AML): a Southwest Oncology Group study. Leukemia. 1995;9(4):562–569. [PubMed] [Google Scholar]
- 33.Jehn U. Long-term outcome of postremission chemotherapy for adults with acute myeloid leukemia using different dose-intensities. Leuk Lymphoma. 1994;15(1-2):99–112. doi: 10.3109/10428199409051684. [DOI] [PubMed] [Google Scholar]
- 34.Jehn U, Knüppel W, Wilmanns W. Intensive maintenance treatment in acute myelogenous leukemia (AML): single institution experience of a multicenter randomized trial. Onkologie. 1988;11(1):13–17. doi: 10.1159/000216473. [DOI] [PubMed] [Google Scholar]
- 35.Jehn U, Zittoun R, Suciu S, et al. A randomized comparison of intensive maintenance treatment for adult acute myelogenous leukemia using either cyclic alternating drugs or repeated courses of the induction-type chemotherapy: AML-6 trial of the EORTC Leukemia Cooperative Group. Haematol Blood Transfus. 1990;33:277–284. doi: 10.1007/978-3-642-74643-7_50. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi T, Miyawaki S, Tanimoto M, et al. The Japan Leukemia Study Group. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/intensification therapy in adult acute myeloid leukemia. J Clin Oncol. 1996;14(1):204–213. doi: 10.1200/JCO.1996.14.1.204. [DOI] [PubMed] [Google Scholar]
- 37.Kolitz JE, George SL, Benson DM, Jr, et al. Alliance for Clinical Trials in Oncology. Recombinant interleukin-2 in patients aged younger than 60 years with acute myeloid leukemia in first complete remission: results from Cancer and Leukemia Group B 19808. Cancer. 2014;120(7):1010–1017. doi: 10.1002/cncr.28516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolitz JE, Hars V, DeAngelo DJ, et al. Phase III trial of immunotherapy with recombinant interleukin-2 (rIL-2) versus observation in patients <60 years with acute myeloid leukemia (AML) in first remission (CR1): preliminary results from Cancer and Leukemia Group B (CALGB) 19808 [abstract]. Blood. 2007 110(11). Abstract 157. [Google Scholar]
- 39.Krug U, Berdel WE, Gale RP, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia. 2016;30(6):1230–1236. doi: 10.1038/leu.2016.25. [DOI] [PubMed] [Google Scholar]
- 40.la Cour Petersen E, Hokland P, Ellegaard J. Adjuvant immune stimulation with Corynebacterium parvum during maintenance chemotherapy of acute myeloid leukemia. A prospective randomized study. Cancer Immunol Immunother. 1983;16(2):88–92. doi: 10.1007/BF00199237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111(3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luger S, Wang V, Paietta E, et al. Tipifarnib as maintenance therapy in acute myeloid leukemia (AML) improves survival in a subgroup of patients with high risk disease. Results of the phase III intergroup trial E2902 [abstract]. Blood. 2015 126(23). Abstract 1308. [Google Scholar]
- 43.Miyawaki S, Sakamaki H, Ohtake S, et al. Japan Adult Leukemia Study Group AML 97 Study. A randomized, postremission comparison of four courses of standard-dose consolidation therapy without maintenance therapy versus three courses of standard-dose consolidation with maintenance therapy in adults with acute myeloid leukemia: the Japan Adult Leukemia Study Group AML 97 Study. Cancer. 2005;104(12):2726–2734. doi: 10.1002/cncr.21493. [DOI] [PubMed] [Google Scholar]
- 44.Montastruc M, Reiffers J, Stoppa AM, et al. Treatment of acute myeloid leukemia in elderly patients: the influence of maintenance therapy (BGM 84 protocol). Nouv Rev Fr Hematol. 1990;32(2):147–152. [PubMed] [Google Scholar]
- 45.Morrison FS, Kopecky KJ, Head DR, et al. Late intensification with POMP chemotherapy prolongs survival in acute myelogenous leukemia--results of a Southwest Oncology Group study of rubidazone versus adriamycin for remission induction, prophylactic intrathecal therapy, late intensification, and levamisole maintenance. Leukemia. 1992;6(7):708–714. [PubMed] [Google Scholar]
- 46.Müller-Tidow C, Tschanter P, Röllig C, et al. Azacitidine in combination with intensive induction chemotherapy in older patients with acute myeloid leukemia: The AML-AZA trial of the study alliance leukemia. Leukemia. 2016;30(3):555–561. doi: 10.1038/leu.2015.306. [DOI] [PubMed] [Google Scholar]
- 47.Ohno R, Kobayashi T, Tanimoto M, et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance-intensification therapy between 4 or 12 courses in adult acute myeloid leukemia. AML-87 Study of the Japan Adult Leukemia Study Group. Cancer. 1993;71(12):3888–3895. doi: 10.1002/1097-0142(19930615)71:12<3888::aid-cncr2820711216>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 48.Ohno R, Nakamura H, Kodera Y, et al. Randomized controlled study of chemoimmunotherapy of acute myelogenous leukemia (AML) in adults with Nocardia rubra cell-wall skeleton and irradiated allogeneic AML cells. Cancer. 1986;57(8):1483–1488. doi: 10.1002/1097-0142(19860415)57:8<1483::aid-cncr2820570808>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Omura GA, Vogler WR, Lefante J, et al. Treatment of acute myelogenous leukemia: influence of three induction regimens and maintenance with chemotherapy or BCG immunotherapy. Cancer. 1982;49(8):1530–1536. doi: 10.1002/1097-0142(19820415)49:8<1530::aid-cncr2820490804>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 50.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council’s 8th acute myeloid leukaemia trial. Lancet. 1986;2(8518):1236–1241. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 52.Röllig C, Serve H, Hüttmann A, et al. Study Alliance Leukaemia. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699. doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 53.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–3118. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 54.Stein RS, Vogler WR, Winton EF, Cohen HJ, Raney MR, Bartolucci A. Therapy of acute myelogenous leukemia in patients over the age of 50: a randomized Southeastern Cancer Study Group trial. Leuk Res. 1990;14(10):895–903. doi: 10.1016/0145-2126(90)90179-d. [DOI] [PubMed] [Google Scholar]
- 55.Stone RM, Mandrekar S, Sanford BL, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): An international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]) [abstract]. Blood. 2015 126(23). Abstract 6. [Google Scholar]
- 56.Summerfield GP, Gibbs TJ, Bellingham AJ. Immunotherapy using BCG during remission induction and as the sole form of maintenance in acute myeloid leukaemia. Br J Cancer. 1979;40(5):736–742. doi: 10.1038/bjc.1979.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogler WR, Winton EF, Gordon DS, Raney MR, Go B, Meyer L. A randomized comparison of postremission therapy in acute myelogenous leukemia: a Southeastern Cancer Study Group trial. Blood. 1984;63(5):1039–1045. [PubMed] [Google Scholar]
- 58.Volger WR, Weiner RS, Moore JO, Omura GA, Bartolucci AA, Stagg M. Long-term follow-up of a randomized post-induction therapy trial in acute myelogenous leukemia (a Southeastern Cancer Study Group trial). Leukemia. 1995;9(9):1456–1460. [PubMed] [Google Scholar]
- 59.Wells RJ, Woods WG, Lampkin BC, et al. Impact of high-dose cytarabine and asparaginase intensification on childhood acute myeloid leukemia: a report from the Childrens Cancer Group. J Clin Oncol. 1993;11(3):538–545. doi: 10.1200/JCO.1993.11.3.538. [DOI] [PubMed] [Google Scholar]
- 60.Zhai X, Xuan L, Sun J, et al. Autologous HSCT followed by immunotherapy and maintenance chemotherapy compared with allogeneic HSCT for intermediate-risk molecules/cytogenetics acute myeloblastic leukemia in first complete remission [abstract]. Blood. 2014 124(21). Abstract 3949. [Google Scholar]
- 61.Mathé G, Amiel JL, Schwarzenberg L, et al. Active immunotherapy for acute lymphoblastic leukaemia. Lancet. 1969;1(7597):697–699. doi: 10.1016/s0140-6736(69)92648-8. [DOI] [PubMed] [Google Scholar]
- 62.Crowther D, Bateman CJ, Vartan CP, et al. Combination chemotherapy using L-asparaginase, daunorubicin, and cytosine arabinoside in adults with acute myelogenous leukaemia. BMJ. 1970;4(5734):513–517. doi: 10.1136/bmj.4.5734.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris R, Zuhrie SR, Freeman CB, et al. Active immunotherapy in acute myelogenous leukaemia and the induction of second and subsequent remissions. Br J Cancer. 1978;37(2):282–288. doi: 10.1038/bjc.1978.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anguille S, Lion E, Willemen Y, Van Tendeloo VF, Berneman ZN, Smits EL. Interferon-α in acute myeloid leukemia: an old drug revisited. Leukemia. 2011;25(5):739–748. doi: 10.1038/leu.2010.324. [DOI] [PubMed] [Google Scholar]
- 65.Buyse M, Squifflet P, Lange BJ, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117(26):7007–7013. doi: 10.1182/blood-2011-02-337725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao C, Fu XH, Yuan JQ, et al. Interleukin-2 as maintenance therapy for children and adults with acute myeloid leukaemia in first complete remission. Cochrane Database Syst Rev. 2015;11:CD010248. doi: 10.1002/14651858.CD010248.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buyse M, Squifflet P, Lucchesi KJ, Brune ML, Castaigne S, Rowe JM. Assessment of the consistency and robustness of results from a multicenter trial of remission maintenance therapy for acute myeloid leukemia. Trials. 2011;12:86. doi: 10.1186/1745-6215-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antar A, Kharfan-Dabaja MA, Ghaddara HA, Mahfouz R, Bazarbachi A. Azacitidine maintenance after allogeneic stem cell transplantation is feasible in patients with acute myeloid leukemia and myelodysplasia [abstract]. Blood. 2014 124(21). Abstract 5884. [Google Scholar]
- 69.Oshikawa G, Kakihana K, Saito M, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol. 2015;169(5):756–759. doi: 10.1111/bjh.13248. [DOI] [PubMed] [Google Scholar]
- 70.Pusic I, Choi J, Fiala MA, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761–1769. doi: 10.1016/j.bbmt.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.William BM, de Lima M, Oran B, et al. CC-486 (oral azacitidine) following allogeneic hematopoietic stem cell transplantation (AlloHSCT) in patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) [abstract]. Blood. 2014 124(21). Abstract 990. [Google Scholar]
- 72.Gill H, Lie AKW, Kwong YL, Leung AYH. Prospective evaluation of azacitidine maintenance following allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia and myelodysplastic syndrome [abstract]. Blood. 2015 126(23). Abstract 5476. [Google Scholar]
- 73.Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic HSCT for patients with FLT3-ITD AML. Biol Blood Marrow Transplant. 2014;20(2):S152. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandmaier BM, Khaled SK, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib (AC220) as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic cell transplantation [abstract]. Blood. 2014;124(21) doi: 10.1002/ajh.24959. Abstract 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins R, Kantarjian HM, Ravandi F, et al. Full doses of crenolanib, a type I FLT3 inhibitor, can be safely administered in AML patients post allogeneic stem cell transplant [abstract]. Blood. 2015 126(23). Abstract 4359. [Google Scholar]
- 76.Pratz KW, Gojo I, Karp JE, et al. Prospective study of peri-transplant use of sorafenib as remission maintenance for FLT3-ITD patients undergoing allogeneic transplantation [abstract]. Blood. 2015 126(23). Abstract 3164. [Google Scholar]
- 77.Bug G, Burchert A, Wagner E-M, et al. Phase I/II study of the deacetylase inhibitor panobinostat as maintenance therapy after an allogeneic stem cell transplantation in patients with high-risk MDS or AML: The Panobest-Trial [abstract]. Blood. 2015 doi: 10.1038/leu.2017.242. 126(23). Abstract 4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. German MDS and Cooperative Transplant Study Group (GCTSG) Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97(9):e34–e35. doi: 10.3324/haematol.2012.067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML). Blood. 2009;114(9):1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho VT, Vanneman M, Kim H, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci USA. 2009;106(37):15825–15830. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kayser S, Schlenk RF, Grimwade D, Yosuico VE, Walter RB. Minimal residual disease-directed therapy in acute myeloid leukemia. Blood. 2015;125(15):2331–2335. doi: 10.1182/blood-2014-11-578815. [DOI] [PubMed] [Google Scholar]
- 82.Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34(4):329–336. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Efficace F, Lo-Coco F. Using patient-reported health status to improve prognostic assessment in patients with acute myeloid leukemia: current challenges and future applications. Haematologica. 2013;98(1):7–9. doi: 10.3324/haematol.2012.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohamedali H, Breunis H, Timilshina N, et al. Older age is associated with similar quality of life and physical function compared to younger age during intensive chemotherapy for acute myeloid leukemia. Leuk Res. 2012;36(10):1241–1248. doi: 10.1016/j.leukres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 85.Alibhai SM, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015;6(4):262–271. doi: 10.1016/j.jgo.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 87.Cella D, Jensen SE, Webster K, et al. Measuring health-related quality of life in leukemia: the Functional Assessment of Cancer Therapy--Leukemia (FACT-Leu) questionnaire. Value Health. 2012;15(8):1051–1058. doi: 10.1016/j.jval.2012.08.2210. [DOI] [PubMed] [Google Scholar]