Abstract
Glial restricted precursors (GRP) are a promising cellular source for transplantation therapy of spinal cord injury (SCI), capable of creating a permissive environment for axonal growth and regeneration. However, there are several issues regarding the nature of their permissive properties that remain unexplored. For example, cellular transplantation strategies for spinal cord repair require the preparation of a large number of cells, but it is unknown whether the permissive properties of GRP are maintained following the process of in vitro expansion. We used rat GRP isolated from the embryonic day 13.5 spinal cord to compare the properties of early (10-20 days) and late (120-140 days) passage GRP. We found that late passage GRP showed comparable effects on neurite outgrowth of adult rat DRG to early passage GRP in both in vitro co-culture and conditioned medium experiments. In addition, to further examine the effects of the inflammatory cascade activated in the aftermath of SCI on the microenvironment, we studied the direct effects of strong inflammatory mediators, Lipopolysaccharide and interferon gamma (LPS and IFNɤ, respectively), on the properties of GRP. We showed that exposure to these pro-inflammatory mediators altered GRP phenotype and attenuated their growth-promoting effects on neurite outgrowth in a dose dependent manner. Taken together, our data suggest that GRP maintain their growth-promoting properties following extensive in vitro passaging and underscore the importance of modulating the inflammatory environment at the injured spinal cord.
Keywords: Spinal cord injury, cell transplantation, DRG neurons, proinflammatory mediators, LPS, Interferon gamma
1. Introduction
Spinal cord injury (SCI) is a devastating disorder characterized by the disruption of ascending and descending axonal pathways and death of a variety of cells in the central nervous system (CNS) (Simpson et al., 2012; Verma et al., 2008). One of the most significant obstacles for repair following SCI is the inability of injured neurons to regenerate and restore connectivity (Fitch and Silver, 2008). Transplantation of neural progenitors is a promising therapeutic strategy that has the potential to replace lost cells, modulate the inhibitory microenvironment of the CNS, and promote axonal growth (Eftekharpour et al., 2008; Tetzlaff et al., 2011).
Extensive studies using multiple types of cells such as Schwann cells, olfactory ensheathing glial cells, neural stem cells, fate-restricted neural/glial precursor cells, and bone marrow stromal cells have been examined for their therapeutic potential for SCI repair (Falnikar et al., 2014; Lopez-Vales et al., 2006; Medalha et al., 2014; Mendonca et al., 2014). Once appropriate candidates for cell transplantation have been identified, the transition to clinical applications requires culturing and expansion to prepare adequate cell stocks and to determine whether the cells retain their original properties. These are particularly important considerations for primary cells derived from fetal or adult tissue, which unlike embryonic stem (ES)-derived cells, are a limited resource. Several studies have shown that some cells retain their growth-supportive properties following expansion (Akesson et al., 2007; Radtke et al., 2010; Tang et al., 2000; Zhu et al., 2014), but in other cases the transplantation protocols have been restricted to a low cell passage (Huang et al., 2006; Saberi et al., 2008). It is therefore important to define the expansion parameters for specific cell types and use these data to develop appropriate transplantation protocols.
Another issue concerning transplantation following CNS trauma relates to the presence of pro-inflammatory molecules, which are released at the injury site and elicit an immune response leading to astrogliosis and glial scar formation (Donnelly and Popovich, 2008; Fitch and Silver, 2008; Sofroniew, 2009). The inflammatory environment encountered by transplanted cells may produce changes that will affect the phenotypic and functional properties of transplanted cells. These changes can be studied in vitro by modeling the direct effects of pro-inflammatory molecules on particular cell types. Lipopolysaccharide (LPS) and interferon gamma (IFNɤ) represent powerful, canonical innate inflammatory mediators and have been used extensively to study cellular responses to inflammation (Dafny and Yang, 2005; Fok-Seang et al., 1998; Fu et al., 2014; Lehnardt et al., 2003). Although the responses of a variety of cell subtypes, such as astrocytes, microglia/ macrophages, and oligodendrocyte precursor cells (OPCs) to inflammation have been previously documented (Tanner et al., 2011; Walter et al., 2011; Zamanian et al., 2012), the changes to the properties of multipotent glial restricted precursors (GRP) following exposure to inflammatory factors has not been examined.
The present report continues our previous studies, which have shown that a combination of neuronal and glial-restricted precursors (NRP/GRP) promoted host axon regeneration of sensory neurons into the injury site to form synaptic connections with transplant-derived neurons and created a function relay across the injury (Bonner et al., 2011; Lepore and Fischer, 2005). The presence of GRP was shown to be a necessary and sufficient component for regeneration, creating a permissive environment even in the presence of inhibitory chondroitin sulfate proteoglycans (CSPGs), a major inhibitory component of the glial scar (See et al., 2010). Conditioned medium harvested from rat GRP also promoted neurite outgrowth of DRG neurons in vitro, suggesting that the secretion of soluble factors was responsible the growth (Ketschek et al., 2012).
In the present study we wanted to examine the properties of GRP in the context of their potential application for SCI repair, with specific aims designed to test their ability to support axonal growth following in vitro expansion and exposure to inflammatory factors. Here, we demonstrate the consistently permissive nature of late passage GRP (P19-21; grown over 120 days) on neurite outgrowth from adult rat DRG utilizing two experimental protocols, direct DRG-GRP co-culture and the use of conditioned medium, indicating the role of secreted factors. In addition, we examined the direct effects of pro-inflammatory mediators, LPS and IFNɤ, on the phenotypic and functional properties of GRP with respect to neurite outgrowth using co-culture experiments. This treatment resulted in a reduced capacity to support neurite outgrowth, which correlated with phenotypic changes of GRP. Taken together, these results underscore the therapeutic potential of GRP to serve as permissive cellular transplants in SCI, even after prolonged expansion in vitro, but emphasize the importance of the inflammatory microenvironment of the injured spinal cord and its potential influence on the properties of GRP.
2. RESULTS
2.1. Characterization of late passage GRP
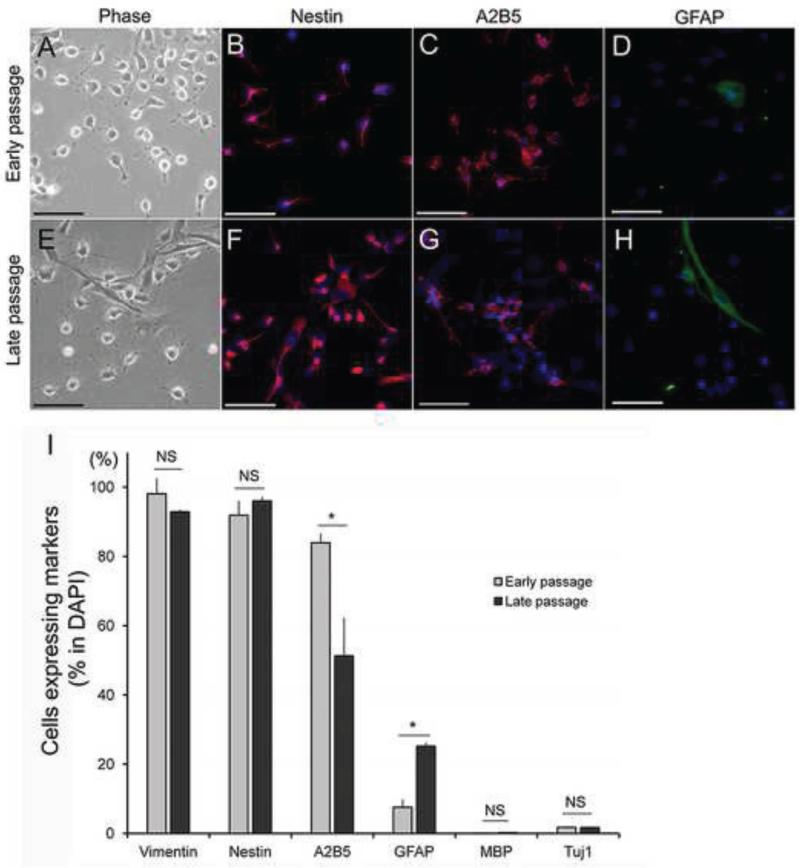
We have previously shown that serum-free defined basal medium supplemented with basic fibroblast growth factor (bFGF) maintains rat GRP in an undifferentiated state for at least 14 days as compared to media supplemented with FBS, BMP-4 or CNTF, which induce differentiation into astrocytes (Haas et al., 2012). Here, we followed a similar culturing method using basal medium supplemented with bFGF, but continued to grow GRP for over 19 passages (ranging from 120 – 140 days in vitro) to compare early and late passage GRP with respect to their proliferative potential, morphology, and lineage specific markers. Early passage GRP showed robust proliferation with a population doubling time (PDT: 22.3 ± 1.1 hr), while late passage GRP showed reduced proliferation (PDT: 27.5 ± 1.5 hr), as shown in Figure S1A (see supplemental information). Immunostaining with Ki67, a marker of cell proliferation, supported these results, showing a large population of cells positive for Ki67 (Figure S1B), with 60.0 ± 1.3 % early passage GRP and 47.2 ± 2.4 % of late passage GRP staining for Ki67 (P=0.03). Early passage GRP had a typical morphology of a non-differentiated progenitor characterized by small refractive cell bodies and a short stellate or bipolar appearance (Figure 1A). The vast majority of late passage GRP showed a comparable morphology with only a small number of cells displaying larger cell bodies and multi-branching shapes or cells with a flattened morphology, typical of mature astrocytes (Figure 1E). We have previously described the developmental continuum of GRP (Haas et al., 2012), ranging from immature GRP, characterized by the A2B5 marker, to mature astrocytes characterized by the GFAP marker. Accordingly, early passage GRP showed that 83.9 ± 1.5 % were A2B5+, while 7.5 ± 1.3 % were GFAP+ (Figures 1C-D and I). Of the GFAP+ GRP, 76.3 ± 4.2 % were also A2B5+ (data not shown), indicating that the majority of early passage GRP were in an undifferentiated state. Late passage GRP demonstrated significant changes in A2B5 and GFAP markers, but still retained high levels of A2B5+ of 51.3 ± 6.2 %, P=0.04 relative to early passage GRP (Figure 1D and I) and low levels of GFAP+ (25.2 ± 1.1 %, P=0.03 relative to early passage GRP) (Figures 1H and I). The A2B5+ cells within the GFAP+ population also remained high and showed a comparable ratio to that of early passage GRP (53.9 ± 3.2 %, P=0.07, data not shown). Nestin and Vimentin, stem cell and progenitor markers that are down-regulated in differentiated astrocytes, were expressed in both early and late passage GRP at high levels (Nestin: 91.9 ± 2.4% and 96.0 ± 0.6%, (P=0.30), Vimentin: 98.1 ± 0.3% and 92.9 ± 2.5%, (P=0.43), respectively) (Figure 1B, F and I). There were no mature oligodendrocytes or neuronal lineages in either early or late passage cultures as evidenced by the absence of staining for myelin basic protein (MBP) and βIII tubulin (Tuj1), respectively. MBP staining was 0.1 ± 0.0% and 0.2 ± 0.0%, (P=0.47), while Tuj1 staining was 1.8 ± 0.1% and 1.7 ± 0.1%, (P=0.70), in early and late passage GRP cultures, respectively (Figure 1I). These results indicated that although late passage GRP exhibited a reduced proliferation rate and some phenotypic changes, overall, they maintained their immature/stem cell properties following long-term in vitro passaging.
Figure 1.
Phenotypic analysis of early and late passage GRP. Early passage GRP showed small refractive cell bodies and a short stellate or bipolar appearance (A), while a majority of late passage GRP showed comparable morphology with only a small number of cells displaying larger cell bodies and multi branching shapes or cell with flattened morphology, typical of mature astrocytes (F). Quantitative analysis using immunostaining demonstrated that late passage GRP showed astrocyte lineage commitment (increase of GFAP+ and decrease of A2B5+) but still kept immature phenotype (high in Vimentin+ and Nestin+) (B-D, F-G and I). Data are presented in graph as mean ± standard error of the mean (SEM). Asterisk represents significant difference relative in comparison between early and late passage GRP (Student T-test, *P<0.05, **P<0.005). Scale bar = 50μm
2.2. Late Passage GRP still promote neurite outgrowth in vitro
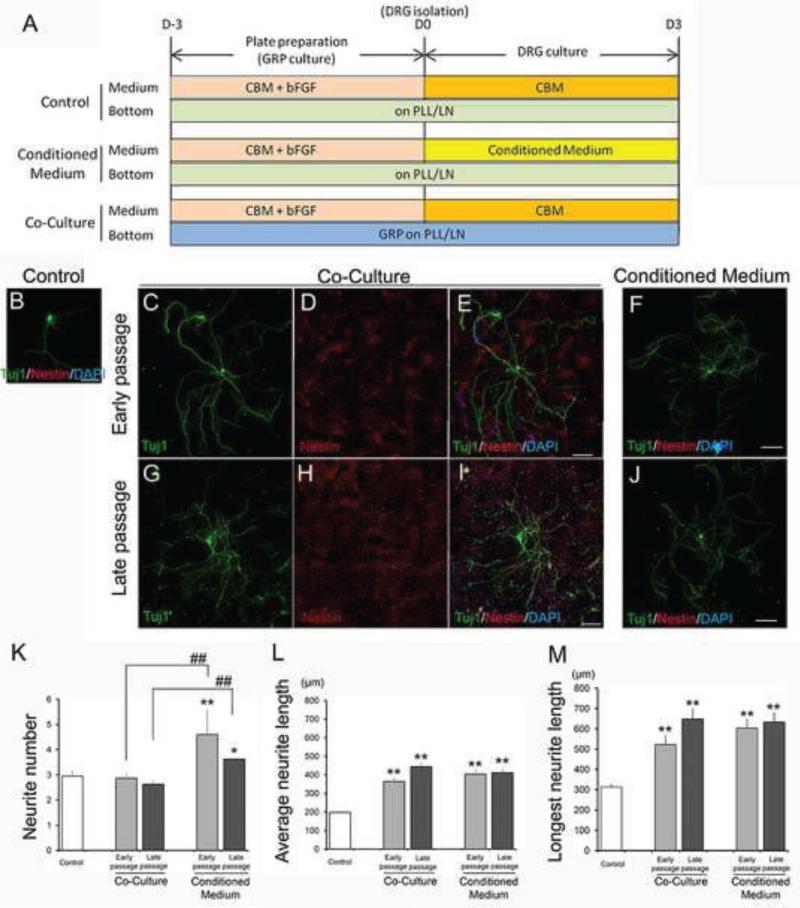
We assessed whether long-term passage of GRP affects their functional properties in an assay designed to examine their ability to support neurite outgrowth of adult DRG neurons. We first used DRG-GRP co-culture experiments and immunostained with specific cell markers to distinguish Tuj1+ DRG neurons from Nestin+/AP+ GRP (Figures 2A-E, G-F and S2A-C) and to quantify the effects of GRP on neurite outgrowth. Early and late passage GRP did not show significant differences in neurite number relative to controls of DRG neurons alone (Control: 2.945 ± 0.2122, early passage: 2.866 ± 0.1950, late passage: 2.625 ± 0.1481, P=1.0), (Figure 2K). In contrast, cultures of early and late passage GRP showed significant differences in the average neurite length and the size of the longest neurite relative to controls; Average neurite length: Control: 197.4 ± 11.20 μm, early passage: 363.5 ± 21.27 μm (P<0.001), late passage: 444.1 ± 21.21 μm (P<0.001) (Figure 2L); Longest neurite length: Control: 313.1 ± 14.70 μm, early passage: 522.7 ± 46.75 μm (P<0.001), late passage: 648.2 ± 55.07 μm (P=0.005) (Figure 2M). Importantly, there were no significant differences between early and late passage GRP with respect to their ability to increase average neurite length and the length of the longest neurite (Figure 2L-M).
Figure 2.
Analysis of the growth-promoting effects on neurite outgrowth from DRG in early and late passage GRP. (A) Experimental design of co-culture and culture with conditioned medium. Immunostaining of DRG in control of DRG alone (B), co-culture with early (C-E) and late passage GRP (G-I), and in conditioned media from early (F) and late passage GRP (J). Scale bars = 150μm. Quantitative analysis of growth-promoting effects on neurite outgrowth from DRG in early and late passage GRP; (K) Primary neurite number, (L) Average neurite length, (M) Longest neurite length are shown in each graph. Asterisk represents significant differences relative to control (*P<0.05, **P<0.005). Pound sign represents significant differences relative to correspondents between co-culture and conditioned medium group (#P<0.05, ##P<0.005). Experiments were repeated three times and representative data are shown as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA with Bonferroni test (N>70).
We then performed conditioned medium experiments and compared the results of neurite growth between the various conditions, as well as to the DRG-GRP co-culture experiments (Figure 2A, F and J). DRG cultured in conditioned media from early and late passage GRP showed a significant increase in average neurite length and the size of the longest neurite relative to control cultures of DRG neurons alone; Average neurite length: early passage: 404.1 ± 25.30 μm (P<0.001), late passage: 411.4 ± 23.86 μm (P<0.001) (Figure 2L); Longest neurite length: early passage: 603.8 ± 46.31 μm (P<0.001), late passage: 635.5 ± 46.21 μm (P<0.001) (Figure 2M). In contrast to the results of direct DRG-GRP co-culture experiments, the number of primary neurites from DRG neurons were increased in conditioned medium experiments from both early and late passage GRP relative to controls; early passage: 4.59 ± 0.97 (P=0.009), late passage: 3.62 ± 0.06 (P<0.001), respectively (Figure 2K). As in co-culture studies, conditioned media from early and late passage GRP showed no significant differences in DRG neurite number, average neurite length, or longest neurite length (P=1.0 in all comparisons) (Figure 2K-M).
Next, we compared the results of direct DRG-GRP co-culture experiments (effects of cell-surface interaction) with results obtained from conditioned medium experiments (effects of secreted factors). Both average neurite length and the length of the longest neurite from early passage GRP conditioned media showed comparable responses to co-culture experiments (P=1.0, P=1.0, respectively) (Figure 2L-M). Similarly, conditioned media harvested from late passage GRP showed comparable effects on average neurite length and length of the longest neurite length compared to direct co-culture studies (P=1.0, P=1.0, respectively) (Figure 2L-M). In contrast, conditioned medium harvested from early or late passage GRP showed a significant increase in the number of primary neurites relative to those in co-culture experiments (early passage: P=0.001, late passage: P<0.001), (Figure 2K). These results indicated that secreted factors from both early and late passage GRP were sufficient to promote neurite outgrowth from DRG with respect to the neurite number, average and longest neurite length. Importantly, the results demonstrated that late passage GRP maintained their permissive properties on neurite outgrowth in both assays.
2.3. LPS and IFNɤ treatment altered the phenotypic properties of GRP distinct from a reactive astrocyte state
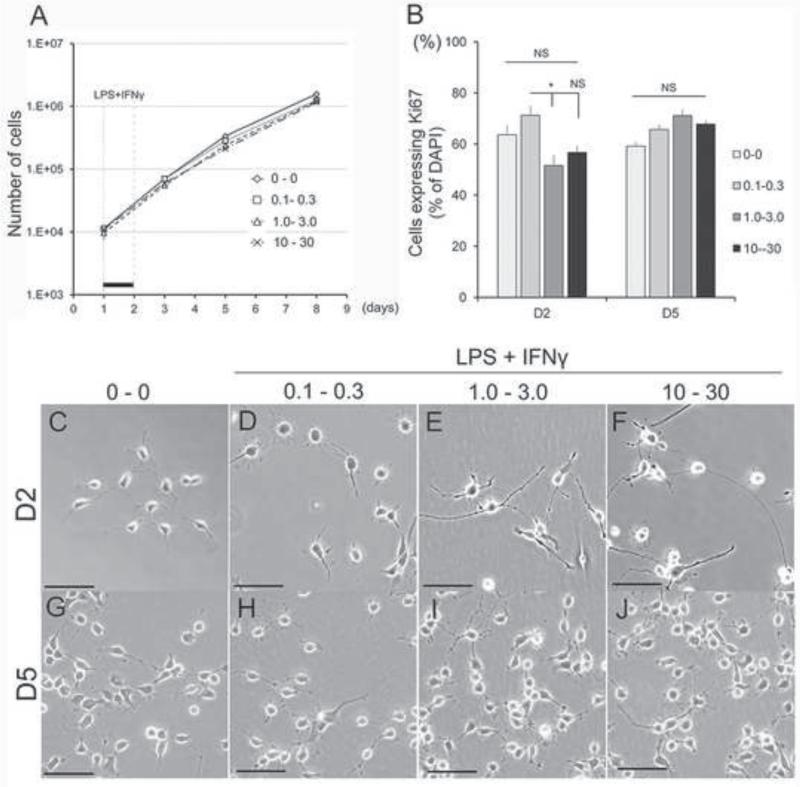
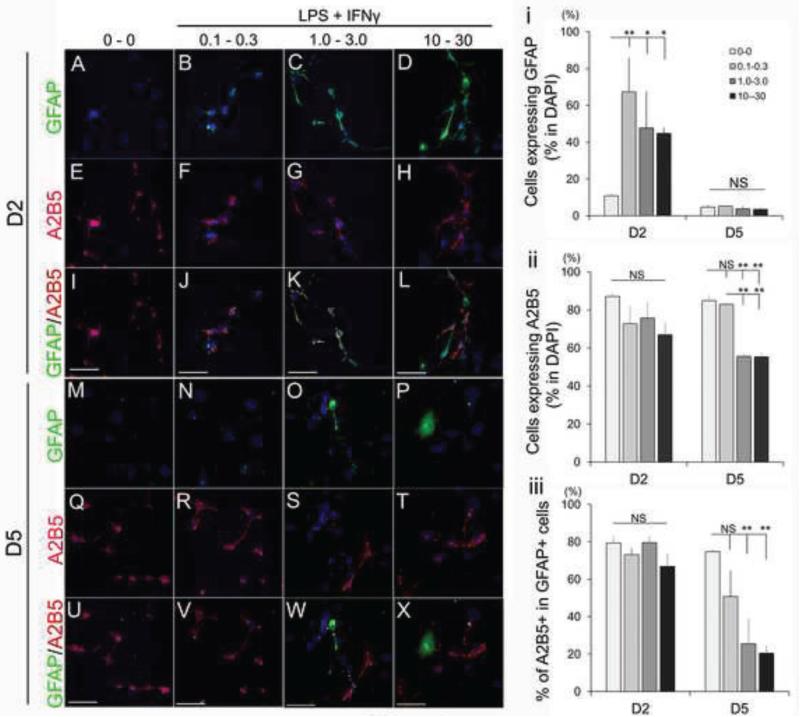
To mimic the inflammatory environment encountered by GRP following transplantation into the injured spinal cord, we next examined the direct effects of LPS and IFNɤ as pro-inflammatory mediators on the morphological, phenotypic, and functional properties of GRP. First, we confirmed receptor expression for the cognate LPS and IFNɤ ligands, Toll-like receptor 4 (TLR4) (Lehnardt et al., 2003) and Interferon gamma receptor alpha (IFNɤRα) (Tsuda et al., 2009) respectively, by immunocytochemistry (Figure S3A and B, respectively). To address whether LPS in combination with IFNɤ, which have been shown to have synergistic effects (Schroder et al., 2006; Yoo et al., 2008), can alter the properties of GRP, we exposed GRP to selected concentrations of these reagents for 24 hr and observed their proliferation and morphology for 8 days. Treatment of GRP with increasing concentrations of LPS and IFNɤ (0.1–10 μg/ml of LPS and 0.3–30 ng/ml of IFNɤ) for 24 hr did not induce apparent growth inhibition by 7 days after treatment (Figure 3A). Similarly, analysis of Ki67 staining demonstrated that there was no inhibition of cell division by LPS and IFNɤ at day 2 and day 5 relative to untreated control; Day2: LPS 0 μg/ml - IFNɤ 0 ng/ml, 63.6 ± 3.6%; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 71.2 ± 3.5% (P=0.89); LPS 1.0 μg/ml - IFNɤ 3.0 μg/ml, 51.5 ± 4.0% (P=0.22); LPS 10 μg/ml - IFNɤ 30 μg/ml, 56.7 ± 2.5% (P=1.0), Figure 3B). Interestingly, although GRP exposed to lower concentrations of LPS and IFNɤ (LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, Figure 3D) did not show morphological changes relative to untreated GRP (Figure 3C), when GRP were exposed to higher concentrations of LPS and IFNɤ (LPS 1.0 μg/ml - IFNɤ 3.0 ng/mL and LPS 10 μg/ml - IFNɤ 3 0 ng/ml, Figure 3E-F), they developed longer processes one day after treatment. These changes, however, were transient and at day five of the experiment all GRP cultures had an undifferentiated morphology and were indistinguishable from each other (Figure 3G-J). We then examined the phenotypic profiles of GRP following LPS-IFNɤ treatment and found that GFAP expression was induced in all LPS-IFNɤ treatment groups at day 2 but returned to control levels at day 5 (Figure 4A-D and M-P) as follows: Day2: Control 10.8 ± 0.6 %; LPS 0.1μg/ml - IFNɤ 0.3 μg/ml, 67.3 ± 1.8 % (P<0.001); LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 47.6 ± 6.2 % (P=0.009); LPS 10 μg/ml - IFNɤ 30 ng/ml, 44.7 ± 5.3 % (P=0.015); Day5: Control 4.6 ± 0.6 %; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 5.2 ± 1.8 %; LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 3.8 ± 0.5 %; LPS 10 μg/ml - IFNɤ 30 ng/ml, 34.2 ± 0.4 % (P=1.0 in all comparisons) (Figure 4i). Nestin and Vimentin, which were originally expressed in a large proportion of GRP on day 0 did not change during LPS-IFNɤ treatment (Figure S4). To assess the possibility that high concentrations of LPS-INFɤ affected lineage commitment, we performed immunostaining with lineage-specific antibodies. At day 2, A2B5 expression in each group were comparable to untreated GRP, but were down-regulated at higher concentration groups at day 5 (Figure 4E-H, Q-T). A2B5+ expression in GFAP+ cells was also decreased in higher concentration groups at day 5 (Figure 4I-L, U-X). Quantitative analysis confirmed a significant decrease of A2B5+ at higher concentrations LPS-IFNɤ treated groups at day 5 as follows: Control, 84.9 ± 1.6 %; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 82.9 ± 1.2 % (P=1.0); LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 55.6 ± 0.7 % (P<0.001); LPS 10 μg/ml - IFNɤ 30 ng/ml, 55.3 ± 2.6 % (P<0.001) (Figure 4ii); A2B5+/GFAP+: Control 74.7 ± 4.6 %; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 50.7 ± 3.9 % (P=0.54); LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 25.4 ± 2.6 % (P=0.026); LPS 10 μg/ml - IFNɤ 30 ng/ml, 20.4 ± 3.5 % (P=0.015) (Figure 4iii). These population changes, however, were transient and recovered to the level equivalent to untreated GRP by day 8 (data not shown). We also measured cell populations of other lineages such as MBP (oligodendrocytes), Tuj1 (neurons), NG2 (oligodendrocyte precursors), and Iba1 (microglia) but did not find significant changes (Figure S5 and data not shown).
Figure 3.
Effect of LPS/IFNɤ on GRP morphology and proliferation. (A) Growth curve of GRPs after LPS/IFNɤ exposure. Black bar represents time window of LPS/IFNɤ treatment. (B) Quantitative analysis of Ki67+ cell population after LPS/IFNɤ treatment. Asterisk represents significant difference relative (*P<0.05). (C-J) Morphology of GRP at each time point after exposure to LPS/IFNɤ with different concentrations. Experiments were repeated for three times and representative data are shown in Figures as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA with Bonferroni test. Scale bar = 50 μm.
Figure 4.
Phenotypic analysis of GRP after LPS/IFN treatment. Immunostaining with antibodies for GFAP and A2B5. GFAP expression was transiently induced at day 2 by LPS/IFN treatment in all concentrations (A-D and M-P), while A2B5 expression was diminished at day 5 only in higher concentration of LPS/IFN (E-L and Q-X). Scale bar = 50μm. (i-iii) Quantitative analysis of GFAP and A2B5+ populations in LPS/IFN treated GRP. Cells with each marker expression were represented as relative to DAPI+ cells (i and ii) and A2B5+ cells in GFAP+ cells (iii) at day 2 and 5. Asterisk indicates significant difference relative to untreated GRP (*P<0.05, **P<0.005). Experiments were repeated for three times and representative data are shown in Figure as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA with Bonferroni test.
2.4. GRP stimulated with pro-inflammatory mediators attenuated their growth promoting properties
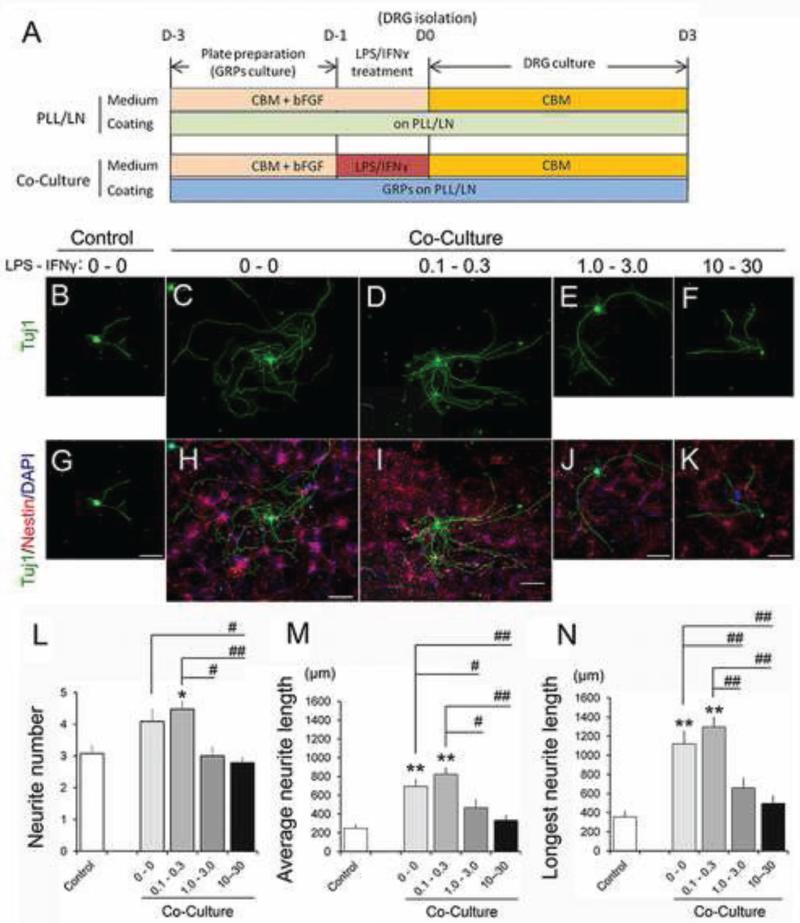
We assessed the effects of LPS-IFNɤ-treated GRP on neurite outgrowth using our standard DRG-GRP co-culture assays (Fu et al., 2014; Hamby et al., 2012). GRP were exposed to selected concentrations of LPS-IFNɤ for 24 hr (LPS-IFNɤ: 0.1-0.3, 1.0-3.0, and 10-30 μg/ml-ng/ml, respectively) and were utilized in phenotypic analyses. Following incubation cells were washed and cultured with dissociated DRG in CBM for three additional days (Figure 5A). We did not observe DRG cell death in any of the treated groups (Figure 5B-K). There were also no significant differences in primary neurite number, average length, or longest neurite length in DRG co-cultured with GRP treated with low concentrations of LPS-IFNɤ relative to untreated GRP. Primary neurite number: Control 4.09 ± 0.08; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 4.48 ± 0.05 (P=1.0); Average length: control, 693.7 ± 15.9 μm; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml, 823.1 ± 15.4 μm (P=0.093); Longest length: Control 1118.9 ± 27.9 μm; LPS 0.1 μg/ml - IFNɤ 0.3 ng/ml 1295.5 ± 23.1 μm (P=1.0). However, we found significant attenuation of growth in all three parameters at higher concentrations. Primary neurite number: LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 3.00 ± 0.06 (P=0.093); LPS 10.0 μg/ml - IFNɤ 30.0 ng/ml, 2.79 ± 0.05 (P=0.015); Average length: LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 467.1 ± 16.1 μm (P=0.025), LPS 10 μg/ml - IFNɤ 30 ng/ml, 332.8 ± 9.8 μm (P<0.001); Longest length: LPS 1.0 μg/ml - IFNɤ 3.0 ng/ml, 659.0 ± 19.6 μm (P=0.004), LPS 10 μg/ml - IFNɤ 30 ng/ml, 496.3 ± 14.8 μm (P<0.001), as shown in Figure 5L-N. We also performed the same experiments using conditioned medium from GRP stimulated with LPS-IFNɤ and obtained a similar trend (data not shown). These data indicate that GRP treatment with the pro-inflammatory combination of LPS-IFNɤ reduces the permissive properties of GRP with respect to promoting neurite outgrowth in a dose dependent manner.
Figure 5.
LPS/IFNɤ treatment altered GRP's promoting effect on neurite outgrowth from DRG in vitro. (A) Experimental design of co-culture with GRP treated with LPS/IFNɤ. (B-K) Immunostaining of DRG and DRG co-cultured with GRP treated with each concentration of LPS/IFNɤ (μg/ml-ng/ml). Upper panels: Tuj1 (DRG) (B-F), Lower panels: marge of Tuj1 and Nestin (GRP) (G-K). Scale bars = 150μm. Quantitative analysis of neurite outgrowth from DRG co-cultured with GRP treated with LPS-IFNɤ demonstrated that higher concentration of LPS-IFNɤ treatment (1.0-3.0 and 10-30 (μg/ml- ng/ml) attenuated GRPs’ promoting effect on neurite outgrowth in all parameters. (L) Primary neurite number, (M) average neurite length, and (N) longest neurite length. Asterisk represents significant difference relative to control (*P<0.05, **P<0.005). Sharp represents significant difference relative to correspondents among LPS-IFNɤ treated groups (#P<0.05, ##P<0.005). Experiments were repeated for three times and representative data are shown in Figure as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA with Bonferroni test. (N>30)
3. DISCUSSION
Astrocytes are abundant cells in the CNS (Wang and Bordey, 2008), produced during development by glial progenitors, such as GRP, and provide critical supportive functions for axonal and synaptic connectivity. Such progenitors can be isolated from embryonic tissue and expanded for therapeutic applications to reduce inhibition and provide a permissive environment for neuroprotection and axonal growth (Mothe and Tator, 2013; Okano et al., 2007; Tetzlaff et al., 2011). It is therefore critical to establish efficient culture protocols for large-scale cell preparation, and characterize these cell populations in terms of their phenotypic and functional properties under different environments (Haas et al., 2012; Haas and Fischer, 2013). The present study addresses these issues by analyzing the properties of glial progenitors after long term culturing and following exposure to inflammatory mediators.
3.1. Phenotypic and functional properties of late passage cells
Many of the early studies of cell transplantation for SCI repair have used acutely isolated cells or cells that have been expanded with minimal in vitro expansion because of concern about phenotypic and functional changes during long-term culturing and cell passaging (Tetzlaff et al., 2011). For example, a study describing the co-culturing of primary astrocytes isolated from newborn rat cerebral cortices showed increased in neurite outgrowth from E18 rat cerebral cortical and E7 chick retinal neurons (Smith et al., 1990). However, when astrocytes were maintained for a month in the presence of fetal bovine serum, forming “mature cultured astrocytes,” the cells showed an attenuated ability to support neurite outgrowth (Smith et al., 1990). Similarly, olfactory ensheathing cells, specialized glial cells with properties of both Schwann cells and astrocytes, have the potential to proliferate, but showed an impaired ability to remyelinate the injured spinal cord after 6 weeks culture (Radtke et al., 2010). Alternatively, neural cells can be prepared from stem/progenitor cells, instead of acute primary cultures, with sources ranging from ES cells to multipotent neural stem cells and lineage specific progenitors. For example, OPCs isolated from neonatal rat optic nerve can be cultured continuously for many months without losing their functional properties, but they gradually acquire the characteristics of adult OPCs, suggesting that they have an intrinsic maturation program (Tang et al., 2000). Other studies have shown that OPCs from rat neonatal brain maintained as oligospheres for several months could differentiate into functional oligodendrocytes capable of myelinating axons of primary cortical neurons in a co-culture (Zhu et al., 2014). As an alternative, multipotent neural stem cells derived from embryonic human spinal cord and grown as neurospheres can be successfully expanded for up to 25 passages (> 350 days) in vitro. They continue to proliferate and maintain their multipotent differentiation property in vivo when grafted into an SCI model (Akesson et al., 2007). These examples emphasize the importance of identifying an appropriate source for derivation and expansion of cells used in transplantation therapy with respect to their developmental continuum. For primary cells, long-term culture of differentiated glial cells induces a maturation process that results in the loss of permissive properties. In contrast, long-term culturing of cells prepared from progenitors such as OPC (Tang et al., 2000) and GRP (current study) appear to retain their original properties despite some phenotypic changes, likely reflecting the self-renewal potential of these cells when grown with mitogens and without differentiation factors for a period of several months. In this sense, using ES or iPS cells can provide an unlimited potential for the initial expansion process, which is proceeded by specific differentiation protocols (Krencik and Zhang, 2011; Sareen et al., 2014). For example, OPC derived from ES cells have been used as cells for transplantation in a clinical trial of SCI initiated by Geron (Alper, 2009).
In this study, we cultured GRP in serum-free, defined media supplemented with bFGF mitogen for over 120 days, and compared the cells from early and late passages with respect to proliferative property, morphology, and differentiation state by lineage marker expression. Late passage GRP were still proliferative, although they showed reduced cell growth compared to early passage GRP, and mostly retained the typical morphology of immature GRP with small refractive cell bodies and a short or bipolar appearance. The phenotypic classification of early and late passage GRP along an immature GRP to differentiated astrocyte spectrum showed that the majority of both early and late passage GRP remained immature, expressing the A2B5 marker, but a small population of late passage GRP began to differentiate into GFAP+ astrocytes. As an indication of a transitional state, the majority of these GFAP+ cells co-expressed the A2B5 marker and nearly 90% also expressed progenitor markers such as Nestin and Vimentin, without evidence of other differentiation markers for oligodendrocytes or neuronal lineage such as MBP or Tuj1, respectively. Taken together, our results indicated that although late passage GRP began a maturation process, they maintained most of the immature characteristics of early passage GRP for 120 days of culturing.
For functional analysis we compared neurite outgrowth from adult rat DRG co-cultured with early or late passage GRP. Both early and late passage cultures significantly promoted neurite outgrowth as measured by average neurite length and the length of the longest neurite, without affecting neurite number. These comparable effects on growth in average and longest neurite length were also observed in conditioned medium experiments indicating the presence of growth promoting factors secreted by GRP. We, however, observed increased neurite number only from DRG cultured in conditioned media from early and late passage GRP, but not in co-culture. These phenomena may imply the presence of different effects of cell-cell interaction and secreted factors on neurite growth, although possible effect of cells from dissociated DRG (e.g. Schwann cells) has not been completely excluded (Jeon et al., 2011; Park et al., 2012). Taken together, these results indicated that late passage GRP remained in an immature state and maintained their permissive effects on neurite growth, suggesting that these cells can be expanded in the presence of the bFGF mitogen and potentially used for transplantation therapy in the repair of SCI.
3.2. Effects of pro-inflammatory mediators
In response to trauma the CNS initiates a cascade of events mediated by inflammatory signaling cascades initially involving resident microglia and astrocytes and later other immune cells. The reactive astrogliosis associated with this process triggers astrocytes to undergo a complex and heterogeneous set of morphological, gene expression, and functional changes that depend on location, severity, and timing following the insult (Fitch and Silver, 2008; Hamby et al., 2012; Yuan and He, 2013). Reactive astrocytes, however, can exert both beneficial and detrimental effects in a context-dependent manner, as determined by the activation of specific molecular signaling cascades (Sofroniew, 2009). DNA microarray analysis revealed temporal changes in the reactive astrocyte transcriptome initiated by either ischemic stroke injury or LPS-induced neuro-inflammation and demonstrated the presence of distinct populations of reactive astrocytes that possess beneficial or detrimental effects (Zamanian et al., 2012).
If GRP are going to be used in treatment of SCI, it is important to understand how such an inflammatory microenvironment affects the grafted cells and whether it will induce changes typical of reactive astrocytes. We therefore examined the direct effects of inflammation on GRP using LPS and IFNɤ as powerful pro-inflammatory mediators and studied the phenotypic and functional response of the GRP cells. We applied these two agents to maximize the pro-inflammatory effects through independent pathways and synergistic signal integration (Hamby et al., 2012; Schroder et al., 2006; Yoo et al., 2008). This combination treatment could be used because GRP express both TRL4 and IFNɤRα, signal transducers of LPS and IFNɤ, respectively (Lehnardt et al., 2003; Tsuda et al., 2009). However, it is important to note that other molecules associated with the compromised blood-brain barrier, such as albumin and thrombin, can also affect astrocytes and change their morphological and functional properties as part of the pro-inflammatory cascade (Chodobski et al., 2011). In our study, LPS and IFNɤ treatment of GRP did not induce apparent cell growth arrest, but when treated with high concentrations, the cells transiently changed their morphology to cells with long processes. In addition, GFAP expression was induced immediately after treatment even at low concentration of LPS and IFNɤ, but the expression was transient and disappeared at day 5. Interestingly, the GFAP expression induced by LPS and IFNɤ was accompanied by co-expression of the immature A2B5 marker, which was diminished only when GRP were treated with high concentrations of the pro-inflammatory agents. These results suggest that the effects of LPS and IFNɤ on GRP (e.g. induced GFAP, reduced A2B5 expression) were the result of direct pro-inflammatory signaling, acting in a dose dependent manner.
Previous studies have shown that treatment of OPC with IFNɤ arrested their cell cycle, inhibited them from generating oligodendrocytes and increased their tendency to generate GFAP+ astrocytes. These results suggest that OPC (which are similar to GRP) withstand inflammation and respond by differentiating into astrocytes (Dafny and Yang, 2005). Application of IFNɤ to mouse NSC induced developmentally atypical GFAP expression along with neural stem cell markers and prevented normal differentiation (Walter et al., 2011). It appears therefore that the GFAP expression observed in this study may be induced by IFNɤ and represent either a direct effect on gene expression or the early stages of astrocyte differentiation. The effects of LPS are mediated through TLR and represent the first step of innate immune cell activation. TLR signaling in astrocyte activation is not well understood but they appear to mediate the pro-inflammatory response by the interplay between NFκB, MAPK, and Jak1/Stat1 signaling pathways (Gorina et al., 2011). Modulation of this signaling may affect the various functional responses of astrocytes in CNS injury, some of which are beneficial (Sofroniew, 2009). In contrast, LPS appear to be detrimental to oligodendrocytes, inducing cell death and demyelination (Felts et al., 2005).
To examine whether LPS/IFNɤ treatment altered the ability of GRP to promote neurite outgrowth, we used our standardized DRG-GRP co-culture assay. Importantly, high concentrations of LPS/IFNɤ that induce phenotypic alteration in GRP dramatically attenuated their supportive effects on neurite outgrowth with respect to neurite number, average neurite length, and the length of the longest neurite. To eliminate the possibility of contaminating microglia, which could mediate inflammation and induce neurotoxicity, we stained with the ED1/Iba1 marker and found no positive cells (data not shown). Thus, we confirmed that LPS/IFNɤ can phenotypically alter GRP and at high concentrations abolish their permissive properties with respect to neurite growth. This result may reflect the conditions observed during the peak of the inflammatory process and astrogliosis (Yang et al., 2005).
4. Conclusions
As a promising candidate for therapeutic intervention in CNS injury, we examined the ability of GRP ability to support axon growth following in vitro expansion (designed to achieve enough cells for transplantation) and exposure to inflammatory factors (designed to test their properties at the injury site). We have shown that even after 120 days in culture GRP maintained their permissive characteristics in an assay of neurite outgrowth from adult DRG underscoring the advantages of using progenitors for derivation of immature astrocytes. Exposure of GRP to the pro-inflammatory combination of LPS and IFNɤ at high concentrations attenuated their growth-promoting effects underscoring the importance of modulating the inflammatory environment of the injured CNS. This study demonstrates the value of the in vitro co-culture system for testing and screening the properties of cells and then applying the lessons gained from in vitro evaluation for effective transplantation protocols. These lessons highlight the ability of GRP to support axon growth following in vitro expansion and the consequences of their exposure to inflammatory factors.
5. Experimental Procedure
5.1. GRP cultures
Rat GRP were harvested from embryonic day 13.5 spinal cords of transgenic Fischer-344 rats expressing the human placental alkaline phosphatase (AP) reporter (Akiyama et al., 2004), and prepared as previously described.(Haas et al., 2012) GRP were cultured on Poly-L-Lysine (PLL; 15 mg/ml; Sigma-Aldrich) and Laminin (LN; 15 mg/ml; Invitrogen) coated dishes in GRP culture basal medium (CBM; DMEM-F12 (Gibco), B27 supplement (20 μl/ml; Gibco), N2 supplement (10 μl/ml; Gibco), Pen-Strep (1000 IU/ml; Gibco), BSA (1 mg/ml; Sigma-Aldrich)) supplemented with 30 ng/ml of bFGF (Peprotech) at 37°C in a 5% CO2 incubator. GRP were cultured for 2-3 passages (grown for 10-20 days in vitro; Early passage) or 19-21 passages (grown for 120-140 days in vitro; Late passage) prior to harvest and use in subsequent experiments.
5.2. Phenotypic characterization of cultured GRP
To determine the cellular phenotypes of early and late passage GRP, we performed immunocytochemical staining with following cell type specific antibodies for A2B5: glial precursors, Glial fibrillary acid protein (GFAP): astrocytes, Nestin and Vimentin: immature progenitors, respectively, Myelin basic protein (MBP): oligodendrocytes, βIII tubulin (Tuj1): neuronal cells, and Ki67: proliferating cells, followed by the appropriately conjugated second antibodies (see Table 1 for detailed information). Cells were fixed with 4% Paraformaldehyde (Electron Microscopy Services). Live staining with primary and secondary antibodies was performed for A2B5 prior to fixation and staining with other antibodies as previously described (Haas et al., 2012). Immunostaining was visualized using a Leica DM5500B fluorescent microscope (Leica Microsystems) with a Retiga-SRV camera (QImaging) or a Leica SP2 AOBS VIS/405 confocal microscope (Leica Microsystems). For quantification of in vitro staining, cells from at least three randomly selected fields per coverslip were counted. A minimal of 300 cells per coverslips were counted for each condition with a total of three different coverslips counted per condition. Counting was performed using Slidebook software, ver.4.2 (Olympus) and cells stained with each marker were expressed as percentage of the total number of DAPI+ cells. Each experiment was performed in triplicate.
Table 1.
Primary and secondary antibodies used in this study
| Name | Type | Dillusion | Source |
|---|---|---|---|
| A2B5 | Mouse IgM | 1:2 | Hybrydoma |
| GFAP | Rabbit | 1:2000 | Chemicon |
| Vimentin | Mouse | 1:1000 | DAKO |
| Nestin | Mouse IgG1 | 1:1000 | BD Pharmigen |
| MBP | Mouse IgG2b | 1:1000 | Invitrogen |
| Tuj1 | Rabbit IgG | 1:1000 | Covance |
| Ki67 | Rabbit IgG | 1:200 | ThermoFisher |
| goat α mouse-IgM RITC | 1:400 | Jackson | |
| goat α mouse-IgG RITC | 1:400 | Jackson | |
| goat α rabbit RITC | 1:400 | Jackson | |
| goat α mouse-IgG FITC | 1:400 | Jackson | |
| goat α rabbit FITC | 1:400 | Jackson |
5.3. Treatment of GRP with pro-inflammatory mediators
To address how the inflammatory micro-environment alters the phenotypic characteristics of GRP, we exposed GRP to Lipopolysaccharide (LPS: serotype O127; Sigma) and Interferon gamma (IFNɤ; R&D) in selected concentrations in CBM, (LPS - IFNɤ : 0.1 - 0.3, 1.0 - 3.0, and 10 - 30 μg/ml - ng/ml, respectively), or CBM only as experimental control (LPS - IFNɤ; 0 - 0 μg/ml - ng/ml) for 24 hrs. Early passage GRP were plated and maintained for appropriate culture periods, and then LPS and IFNɤ were added to CBM in the presence of bFGF. After exposure, cells were washed two times for three minutes in CBM and utilized in further experiments.
For analyses with respect to morphology, proliferative capacity, and phenotypic changes of GRP after LPS and IFNɤ treatment, cells were plated at a density of 1000 cells/cm2 and maintained in CBM supplemented with bFGF without reaching confluency during the assays. On the day following plating, cells were exposed to LPS and IFNɤ for 24 hrs. Media was changed every two days after LPS and IFN exposure and cell numbers were counted at days 1, 3, 5, and 8, and immunostaining was performed on fixed cells at days 2, 5, and 8. Staining and counting was performed as described above (section 5.2.). Each experiment was performed in triplicate on three separate occasions.
5.4. Dissociated DRG cultures
Primary dorsal root ganglia were prepared from adult Sprague-Dawley rats as described previously (Jang et al., 2008). Dissociated DRG were utilized in the following experiments: i) DRG-GRP co-culture, ii) GRP conditioned media, and iii) DRG-GRP co-culture using GRP pre-treated with LPS and IFNɤ.
5.5. DRG-GRP co-cultures
-
i)
DRG-GRP co-culture experiments were prepared by plating 200 dissociated DRG/well onto 24 well plates coated with PLL/LN or onto monolayer cultures of early passage or late passage GRP prepared three days earlier. GRP were plated at a density of 25,000 cells/cm2. DRG were suspended in CBM without any growth factors and plated into wells.
-
ii)
Conditioned medium was collected from cultures of both early and late passage GRP. Briefly, early or late passage GRP were plated at 25,000 cells/cm2 and cultured for two days in CBM supplemented with 30 ng/ml bFGF. Media was then removed and cells were washed once with warmed HBSS (Gibco). CBM without additional growth factors was added and incubated for an additional 24hrs. On the following day, medium was harvested and utilized as conditioned medium for further experiments. Dissociated DRG were suspended in conditioned medium from early or late passage GRP culture or in CBM without any growth factors as an experimental control.
-
iii)
In DRG-GRP co-culture experiments using GRP pre-treated with LPS and IFNɤ, early passage GRP were exposed to LPS and IFNɤ two days after plating. Following 24hrs exposure, cells were washed and DRG, suspended in CBM, were added. DRG-GRP co-cultures and DRG cultures with conditioned medium were maintained for three days without any media changes and subsequently prepared for immunocytochemistry. Each experiment was performed in triplicate on three separate occasions.
5.6. Analysis of neurite growth
For measurement of neurite growth, cells were fixed and stained with cell type specific antibodies for Tuj1 and Nestin to distinguish neurons from GRP and other satellite cells. A minimum of 30 of Tuj1+ soma with neurite longer than 20μm were chosen in randomly selected fields and utilized for further analysis. Counting was performed manually with Slidebook software by a blinded observer. Average neurite length, longest neurite length, and primary neurite number were measured using NeuronJ software, ver. 1.4.2.
5.7. Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using SPSS statistical software using student T-test and one-way ANOVA followed by Bonferroni test for multiple comparisons. Significance was set at P<0.05.
Supplementary Material
HIGHLIGHTS.
GRP promoted neurite outgrowth of DRG using co-cultures and conditioned media
Late passage GRP (grown for 120 days) maintained growth-promoting effects
Treatment of GRP with inflammatory factors (LPS/IFNɤ) attenuated growth-promoting effects
Transplants of GRP could potentially be used even after prolonged in vitro expansion
Modulating the inflammatory environment in the aftermath of injury could improve the efficacy of GRP transplants
ACKNOWLEDGMENTS
We thank B. T. Himes, T. Connors, M. P. cote, X. Yuan, V. Zhukarev, L. Zholudeva, S. Pandey, for their invaluable comments and discussion, M. Obrocka for experimental management and technical assistance. KH is thankful to Ayano Hayakawa for constant encouragement and support during this study. This work was supported by the Craig H. Neilsen Foundation and the NIH grant NS055976.
ABRREVIATIONS
- GRP
Glial restricted precursors
- SCI
Spinal cord injury
- LPS
Lipopolysaccharide
- IFNɤ
Interferon gamma
- OPC
oligodendrocyte precursor cell
- NRP
Neuronal restricted precursors
- ES cells
embryonic stem cells
- TRL4
Toll-like receptor 4
- IFNɤRα
Interferon gamma receptor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DISCLOSURE
No conflict of interest.
REFERENCES
- Akesson E, Piao JH, Samuelsson EB, Holmberg L, Kjaeldgaard A, Falci S, Sundstrom E, Seiger A. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 2007;92:60–6. doi: 10.1016/j.physbeh.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Lankford K, Radtke C, Greer CA, Kocsis JD. Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene. Neuron Glia Biol. 2004;1:47–55. doi: 10.1017/S1740925X04000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–4. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–86. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafny N, Yang PB. Interferon and the central nervous system. Eur J Pharmacol. 2005;523:1–15. doi: 10.1016/j.ejphar.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–88. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008;24:E19. doi: 10.3171/FOC/2008/24/3-4/E18. [DOI] [PubMed] [Google Scholar]
- Falnikar A, Li K, Lepore AC. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Woolston AM, Fernando HB, Asquith S, Gregson NA, Mizzi OJ, Smith KJ. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain. 2005;128:1649–66. doi: 10.1093/brain/awh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok-Seang J, DiProspero NA, Meiners S, Muir E, Fawcett JW. Cytokine-induced changes in the ability of astrocytes to support migration of oligodendrocyte precursors and axon growth. Eur J Neurosci. 1998;10:2400–15. doi: 10.1046/j.1460-9568.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- Fu HQ, Yang T, Xiao W, Fan L, Wu Y, Terrando N, Wang TL. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One. 2014;9:e106331. doi: 10.1371/journal.pone.0106331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–55. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–32. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–52. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32:14489–510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Chen L, Wang H, Xi H, Gou C, Zhang J, Zhang F, Liu Y. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up with MRI. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:439–43. [PubMed] [Google Scholar]
- Jang I, La JH, Kim GT, Lee JS, Kim EJ, Lee ES, Kim SJ, Seo JM, Ahn SH, Park JY, Hong SG, Kang D, Han J. Single-Channel Recording of TASK-3-like K Channel and Up-Regulation of TASK-3 mRNA Expression after Spinal Cord Injury in Rat Dorsal Root Ganglion Neurons. Korean J Physiol Pharmacol. 2008;12:245–51. doi: 10.4196/kjpp.2008.12.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon EJ, Xu N, Xu L, Hansen MR. Influence of central glia on spiral ganglion neuron neurite growth. Neuroscience. 2011;177:321–34. doi: 10.1016/j.neuroscience.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–37. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–7. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–9. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–42. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Verdu E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. 2006;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Medalha CC, Jin Y, Yamagami T, Haas C, Fischer I. Transplanting neural progenitors into a complete transection model of spinal cord injury. J Neurosci Res. 2014;92:607–18. doi: 10.1002/jnr.23340. [DOI] [PubMed] [Google Scholar]
- Mendonca MV, Larocca TF, Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, Dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701–13. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Okano H, Kaneko S, Okada S, Iwanami A, Nakamura M, Toyama Y. Regeneration-based therapies for spinal cord injuries. Neurochem Int. 2007;51:68–73. doi: 10.1016/j.neuint.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. Multi-compartment neuron-glia co-culture platform for localized CNS axon-glia interaction study. Lab Chip. 2012;12:3296–304. doi: 10.1039/c2lc40303j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke C, Lankford KL, Wewetzer K, Imaizumi T, Fodor WL, Kocsis JD. Impaired spinal cord remyelination by long-term cultured adult porcine olfactory ensheathing cells correlates with altered in vitro phenotypic properties. Xenotransplantation. 2010;17:71–80. doi: 10.1111/j.1399-3089.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Sareen D, Gowing G, Sahabian A, Staggenborg K, Paradis R, Avalos P, Latter J, Ornelas L, Garcia L, Svendsen CN. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J Comp Neurol. 2014;522:2707–28. doi: 10.1002/cne.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- See J, Bonner J, Neuhuber B, Fischer I. Neurite Outgrowth of Neural Progenitors in Presence of Inhibitory Proteoglyccans. JURNAL OF NEUROTRAUMA. 2010;27:951–957. doi: 10.1089/neu.2009.1158. [DOI] [PubMed] [Google Scholar]
- Simpson LA, Eng JJ, Hsieh JT, Wolfe DL, Spinal Cord Injury Rehabilitation Evidence Scire Research, T. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548–55. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–90. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–84. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner DC, Cherry JD, Mayer-Proschel M. Oligodendrocyte progenitors reversibly exit the cell cycle and give rise to astrocytes in response to interferon-gamma. J Neurosci. 2011;31:6235–46. doi: 10.1523/JNEUROSCI.5905-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J of Neurotrauma. 2011;28:1611–1628. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:8032–7. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Garcia-Alias G, Fawcett JW. Spinal cord repair: bridging the divide. Neurorehabil Neural Repair. 2008;22:429–37. doi: 10.1177/1545968307313500. [DOI] [PubMed] [Google Scholar]
- Walter J, Honsek SD, Illes S, Wellen JM, Hartung HP, Rose CR, Dihne M. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol Neurodegener. 2011;6:18. doi: 10.1186/1750-1326-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–67. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12:276–84. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Choi JW, Shin CY, Jeon SJ, Park SJ, Cheong JH, Han SY, Ryu JR, Song MR, Ko KH. Activation of p38 MAPK induced peroxynitrite generation in LPS plus IFN-gamma-stimulated rat primary astrocytes via activation of iNOS and NADPH oxidase. Neurochem Int. 2008;52:1188–97. doi: 10.1016/j.neuint.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–35. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Zhao C, Young FI, Franklin RJ, Song B. Isolation and long-term expansion of functional, myelinating oligodendrocyte progenitor cells from neonatal rat brain. Curr Protoc Stem Cell Biol. 2014;31:2D 17 1–2D 17 15. doi: 10.1002/9780470151808.sc02d17s31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.