Abstract
Fluorine is a highly attractive element for both medicinal chemistry and imaging technologies. To facilitate protein tyrosine phosphatases (PTPs)-targeted drug discovery and imaging-guided PTP research with fluorine, several highly potent and 19F MR sensitive PTP inhibitors were discovered through a structure-based focused library strategy.
Graphical abstract
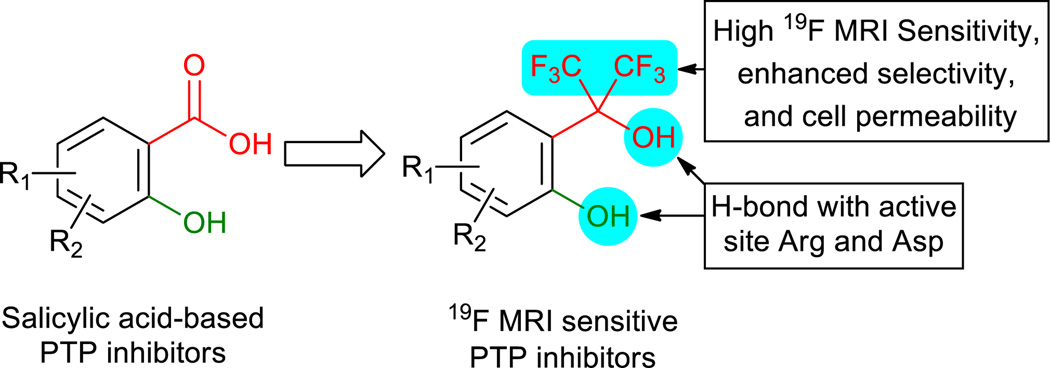
The first 19F MRI sensitive PTP inhibitors were discovered through a structure-based focus library strategy. Ortho-bis(trifluoromethyl)carbinol phenol not only successfully mimics the interactions between salicylic acid and PTPs, but also sheds new light on PTPs.
Introduction
PTPs play crucial roles in such fundamental cellular processes as proliferation, differentiation, survival, apoptosis, motility and adhesion.1 Abnormal PTP activity is well known to be associated with a broad spectrum of human diseases.2 As a superfamily of more than 100 signalling enzymes, many PTPs have emerged as attractive drug targets, such as mPTPB for tuberculosis, SHP2 for many types of cancers, LYP for autoimmune diseases, PTP1B for type 2 diabetes, obesity and breast cancer.3 To this end, the discovery of highly potent and specific small-molecule PTP inhibitors and their application in probing the biological and pathological mechanisms of PTPs, especially with the aid of modern imaging and spectroscopy technologies, are the cornerstone for PTPs-targeted drug discovery.
As a versatile element in biomedical research, fluorine has promising utility in PTPs-targeted drug discovery. On one hand, the introduction of fluorine(s) into bioactive molecules is usually accompanied by improved pharmacokinetic properties and protein-ligand binding interactions.4 Thus, fluorination has become a routine strategy in drug discovery and fluorinated compounds have made up over 20% of all pharmaceuticals. On the other hand, fluorinated molecules can be monitored in vivo without ionizing radiation and background signals by 19F magnetic resonance (19F MR) which provides high-contrast and non-invasive spectroscopy (19F NMR) and images (19F MRI). In recent years, 19F MRI/NMR has been widely used in tracking targets of interest5 and monitoring biological reactions.6 Therefore, the discovery of fluorinated small-molecule PTP inhibitors with high 19F MR sensitivity may provide easy access to PTPs-targeted drugs and detailed understanding of PTPs’ biological and pathological mechanisms.
A recent discovery of a 19F MRI sensitive salinomycin derivative with specific toxicity towards cancer cells7 by this group promoted us to develop novel fluorinated PTP inhibitors. Herein, ortho-bis(trifluoromethyl)carbinol phenol was designed as a novel chemical scaffold for 19F MRI sensitive PTP inhibitors (Scheme 1). Due to the strong electron-withdraw ability of 2 trifluoromethyl groups, the bis(trifluoromethyl)-carbinol is a weak acid and therefore a suitable substitute for the carboxylic group in salicylic acid from which a number of highly potent and selective PTP inhibitors have recently been discovered.8 Consequently, the ortho-bis(trifluoromethyl)-carbinol phenols may mimic the well-established binding mode of salicylic acid-based inhibitors at the highly positively charged active site of PTPs.8 It is noteworthy that the 6 symmetric fluorines in the bis(trifluoromethyl)carbinol, which were recently employed in the construction of highly 19F MRI sensitive dendritic drug delivery vehicles,9 aggregately provide a strong 19F MR signal for conveniently probing the mode of interaction and related biological reactions with 19F NMR and 19F MRI. Moreover, cell permeability is a challenge for PTP inhibitors. The bis(trifluoromethyl)carbinol-based PTP inhibitors without negative charge may exhibit favorable cell permeability, bioavailability and pharmacokinetic properties by the introduction hydrophobic trifluoromethyl groups.4
Scheme 1.
Design of 19F MR sensitive PTP inhibitors.
Materials and methods
Chemistry general information
1H, 19F and 13C NMR spectra were recorded at 400 MHz. Chemical shifts (δ) are in ppm and coupling constants (J) are in Hertz (Hz). 1H NMR spectra were referenced to tetramethylsilane (d, 0.00 ppm) using CDCl3, Acetone-d6 or DMSO-d6 as solvents. 13C NMR spectra were referenced to solvent carbons (77.16 ppm for CDCl3, 29.84, 206.26 ppm for Acetone-d6 and 39.52 ppm for DMSO-d6). 19F NMR spectra were referenced to 2% perfluorobenzene (s, −164.90 ppm). The splitting patterns for 1H NMR spectra are denoted as follows: s (singlet), d (doublet), q (quartet), m (multiplet), dd (doublet of doublets), td (triplet of doublets). High resolution mass spectra were recorded using Electron Spray Ionization (ESI).
Unless otherwise indicated, all reagents were obtained from commercial supplier and used without prior purification. DCM and DMF were dried and freshly distilled prior to use. Flash chromatography was performed on silica gel (200–300 mesh) with either petroleum ether/EtOAc as eluents.
Synthesis of Compounds
Phenol 1c
Hexafluoroacetone trihydrate (9.71 g, 6.1 mL, 44.1 mmol) was dried over concentrated sulfuric acid and the resulting anhydrous hexafluoroacetone was bubbled into to a solution of 4-phenylphenol (5.00 g, 29.4 mmol) and aluminium chloride (0.39 g, 2.94 mmol) in 1,2-dichloroethane (250 mL) slowly. After the addition, the mixture was heated to reflux at 80°C until 4-phenylphenol was consumed as indicated by TLC. The reaction mixture was then cooled to rt, washed with 2 N HCl (100 mL) and extracted with DCM (50 mL × 2). The combined organic layers were dried over anhydrous Na2SO4, concentrated under vacuum and purified by flash chromatography on silica gel (5% EtOAc/petroleum ether) to give 1c as white wax (3.6 g, 85% yield). 1H NMR (CDCl3, 400 MHz) δ 7.00 (d, J = 8.5 Hz, 1H), 7.35 (t, J = 7.2 Hz, 1H), 7.44 (t, J = 8.0 Hz, 2H), 7.48–7.54 (m, 2H), 7.57 (dd, J = 8.5, 2.1 Hz, 1H), 7.66 (s, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.53; 13C NMR (Acetone-d6, 100 MHz) δ 80.0–81.2 (m), 116.0, 119.5, 124.2 (q, J = 286 Hz), 127.4, 127.7, 128.2, 129.9, 131.0, 134.7, 140.7, 156.6; HRMS (ESI) calcd for C15H11F6O2+ ([M+H]+) 337.0658, found 337.0671.
Phenol 1a
was prepared from benzene by following the general procedure as clear oil (2.5 g, 30%). 1H NMR (CDCl3, 400 MHz) δ 7.39–7.53 (m, 3H), 7.73 (dd, J = 7.4, 0.9 Hz, 2H); 19F NMR (CDCl3, 376 MHz) δ −78.69.
Phenol 1b
was prepared from p-cresol (3.0 g, 27.7 mmol) by following the general procedure as white wax (6.1 g, 80%). 1H NMR (CDCl3, 400 MHz) δ 2.31 (s, 3H), 6.16 (s, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.88 (s, 1H), 7.15 (dd, J = 8.3, 1.6 Hz, 1H), 7.23 (s, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.64.
Phenol 1d
Prepared from [1,1'-biphenyl]-3-ol (5.00 g, 29.4 mmol) in the same manner as described for 1c (8.6 g, 87% yield). 1H NMR (CDCl3, 400 MHz) δ 7.13 (d, J = 1.8 Hz, 1H), 7.19–7.26 (m, 1H), 7.35–7.47 (m, 3H), 7.47–7.59 (m, 3H); 19F NMR (CDCl3, 376 MHz) δ −78.72; 13C NMR (Acetone-d6, 100 MHz) δ 79.9–81.1 (m), 114.5, 117.0, 120.2, 124.2 (q, J = 286 Hz), 128.8, 129.1, 129.9, 140.0, 145.3, 157.5; HRMS (ESI) calcd for C15H11F6O2+ ([M+H]+) 337.0658, found 337.0651.
Phenol 1e
Prepared from [1,1'-biphenyl]-2-ol (5.00 g, 29.4 mmol) in the same manner as described for 1c (3.6 g, 74% yield). 1H NMR (CDCl3, 400 MHz) δ 7.11 (t, J = 7.9 Hz, 1H), 7.35 (dd, J = 7.5, 1.5 Hz, 1H), 7.43–7.50 (m, 3H), 7.50–7.59 (m, 3H); 19F NMR (CDCl3, 376 MHz) δ −78.41; 13C NMR (Acetone-d6, 100 MHz) δ 80.6–81.8 (m), 115.5, 121.4, 124.1 (q, J = 286 Hz), 128.2, 128.3, 129.2, 130.5, 133.1, 133.8, 138.4, 154.9; HRMS (ESI) calcd for C15H11F6O2+ ([M+H]+) 337.0658, found 337.0654.
Phenol 1f
Prepared from 2-naphthalenol (5.00 g, 34.7 mmol) in the same manner as described for 1c (6.2 g, 57% yield). 1H NMR (CDCl3, 400 MHz) δ 7.29 (s, 1H), 7.42 (dd, J = 11.1, 3.9 Hz, 1H), 7.52 (dd, J = 11.1, 4.0 Hz, 1H), 7.68 (d, J = 8.3 Hz, 1H), 7.82 (d, J = 8.2 Hz, 1H), 8.04 (s, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.40; 13C NMR (Acetone-d6, 100 MHz) δ 80.2–81.4 (m), 113.3, 117.7, 124.2 (q, J = 286 Hz), 125.5, 126.6, 128.9, 129.0, 129.5, 130.6, 135.7, 154.0; HRMS (ESI) calcd for C13H9F6O2+ ([M+H]+) 311.0501, found 311.0489.
Phenol 1g
Prepared from 1-naphthalenol (5.00 g, 34.7 mmol) in the same manner as described for 1c (6.5 g, 60% yield). 1H NMR (CDCl3, 400 MHz) δ 7.39 (s, 2H), 7.46–7.63 (m, 2H), 7.71–7.84 (m, 1H), 8.24–8.38 (m, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.55; 13C NMR (Acetone-d6, 100 MHz) δ 81.2–82.4 (m), 107.0, 120.6, 123.5, 124.18 (q, J = 287 Hz), 124.19, 126.8, 126.9, 128.2, 129.0, 135.9, 155.5; HRMS (ESI) calcd for ([M+H]+) C13H9F6O2+ 311.0501, found 311.0498.
Naphthol 3
Prepared from 2,7-naphthalenediol (30.0 g, 187.2 mmol) in the same manner as described for 1c (10.2 g, 17% yield). 1H NMR (Acetone-d6, 400 MHz) δ 7.01–7.13 (m, 2H), 7.25 (s, 1H), 7.85 (d, J = 8.0 Hz, 1H), 8.04 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.10; 13C NMR (Acetone-d6, 100 MHz) δ 80.1–81.3 (m), 107.9, 111.7, 114.2, 118.4, 124.1, 124.3 (q, J = 286 Hz), 130.3, 131.5, 137.6, 154.5, 158.2; HRMS (ESI) calcd for C13H9F6O3+ ([M+H]+) 327.0450, found 327.0444.
Naphthol 4
To an ice-cold suspension of diol 3 (2.40 g, 7.36 mmol) in trifluoroacetic acid was added acetone (2.2 mL, 29.5 mmol), then TFA (10.8 mL, 145.87 mmol) was added to the mixture dropwise. The reaction mixture was warmed slowly to rt and then stirred for 48 h. After evaporation of the solvent, the residue was purified by flash chromatography on silica gel (2% EtOAc/petroleum ether) to give 4 as white wax (0.85 g, 34% yield). 1H NMR (Acetone-d6, 400 MHz) δ 1.61 (s, 6H), 7.11–7.24 (m, 2H), 7.34 (s, 1H), 7.95 (d, J = 8.8 Hz, 1H), 8.09 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −78.30; 13C NMR (Acetone-d6, 100 MHz) δ 27.0, 76.6–77.8 (m), 102.8, 108.5, 110.0, 113.7, 119.2, 123.2 (q, J = 287 Hz), 125.2, 128.4, 131.6, 138.0, 150.0, 158.5; HRMS (ESI) calcd for C16H13F6O3+ ([M+H]+) 367.0763, found 367.0773.
Ester 8
To a solution of 4 (470.0 mg, 1.28 mmol) and methyl bromoacetate (588.7 mg, 3.85 mmol) in acetone was added K2CO3 (381.5 mg, 3.85 mmol), then the reaction mixture was heated at reflux until 4 was consumed as indicated by TLC. After removal of the solvent under reduced pressure, the residue was dissolved in EtOAc (20 mL), and washed with water (50 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated under vacuum and purified by flash chromatography on silica gel (2% EtOAc/petroleum ether) to give ester 8 as light yellow oil (480.0 mg, 86% yield). 1H NMR (CDCl3, 400 MHz) δ 1.60 (s, 6H), 3.83 (s, 3H), 4.76 (s, 2H), 6.96 (d, J = 2.4 Hz, 1H), 7.19 (dd, J = 9.0, 2.5 Hz, 1H), 7.29 (s, 1H), 7.78 (d, J = 9.0 Hz, 1H), 7.99 (s, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.24; 13C NMR (CDCl3, 100 MHz) δ 26.8, 52.4, 65.2, 76.0–76.6 (m), 101.8, 105.5, 113.6, 118.3, 122.1 (q, J = 287 Hz), 125.1, 127.6, 130.7, 136.2, 149.5, 157.5, 169.0; HRMS (ESI) calcd for C19H17F6O5+ ([M+H]+) 439.0975, found 439.0981.
Acid 9
Ester 8 (400.0 mg, 0.91 mmol) was dissolved in THF/H2O (5 mL/5 mL) and the solution was stirred at 0°C. Then NaOH (43.8 mg, 1.10 mmol, 10 N aqueous solution) was added at 0°C. The reaction mixture was stirred at rt until 8 was consumed as indicated by TLC. The solution was acidified to pH 6.0, then extracted with EtOAc (20 mL × 2) and washed with water (10 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under vacuum to give acid 9 as white wax (370 mg, 96% yield). 1H NMR (CDCl3, 400 MHz) δ 1.60 (s, 6H), 4.82 (s, 2H), 6.99 (d, J = 2.4 Hz, 1H), 7.19 (dd, J = 9.0, 2.5 Hz, 1H), 7.29 (s, 1H), 7.79 (d, J = 9.1 Hz, 1H), 7.99 (s, 1H); 19F NMR (CDCl3, 376 MHz) δ −78.33; 13C NMR (Acetone-d6, 100 MHz) δ 27.0, 65.4, 76.6–77.8 (m), 102.9, 106.6, 111.0, 114.6, 119.5, 123.2 (q, J = 286 Hz), 126.0, 128.4, 131.5, 137.6, 150.2, 159.0, 170.0; HRMS (ESI) calcd for C18H15F6O5+ ([M+H]+) 425.0818, found 425.0798.
Amide 7a
Potassium carbonate (170.0 mg, 1.23 mmol) was added to a solution of 4 (150.0 mg, 0.41 mmol) and 6a (111.6 mg, 0.62 mmol) in acetone (5 mL), then the result suspension was heated at reflux until 4 was consumed as indicated by TLC. After removal of the solvent under reduced pressure, the residue was dissolved in EtOAc (25 mL), washed with 2N HCl (30 mL) and brine (30 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated under vacuum and used without purification. The residue was dissolved in TFA/H2O (9/1, 11.3 mL), then anisole (45 µL) was added and the resulting mixture was stirred overnight. The reaction mixture was concentrated under vacuum and then diluted with EtOAc (25 mL), washed with brine (30 mL × 2), the organic layer was dried over anhydrous Na2SO4, concentrated under vacuum and purified by flash chromatography on silica gel (20–80% EtOAc/petroleum ether) to give 7a as clear oil (131 mg, 77% yield). 1H NMR (Acetone-d6, 400 MHz) δ 0.88 (t, J = 7.4 Hz, 3H), 1.41–1.66 (m, 2H), 3.24–3.31 (m, 2H), 4.64 (s, 2H), 7.09–7.25 (m, 2H), 7.42 (s, 1H), 7.91 (d, J = 9.0 Hz, 1H), 8.09 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.08; 13C NMR (Acetone-d6, 100 MHz) δ 11.1, 23.0, 41.0, 67.4, 79.7–80.3 (m), 105.4, 111.9, 114.8, 117.8, 123.6 (q, J = 286 Hz), 124.1, 129.5, 130.8, 136.6, 154.4, 157.8, 168.6; HRMS (ESI) calcd for C18H18F6NO4+ ([M+H]+) 426.1135, found 426.1118.
Amide 7b
Prepared from 4 (110.0 mg, 0.30 mmol) in the same manner as described for 7a (100 mg, 78% yield). 1H NMR (Acetone-d6, 400 MHz) δ 0.48–0.64 (m, 2H), 0.66–0.80 (m, 2H), 2.06 (dt, J = 4.4, 2.2 Hz, 1H), 4.61 (s, 2H), 7.08–7.23 (m, 2H), 7.42 (s, 1H), 7.90 (d, J = 9.0 Hz, 1H), 8.08 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.06; 13C NMR (Acetone-d6, 100 MHz) δ 5.9, 22.8, 67.6, 79.9–80.5 (m), 105.5, 112.1, 114.9, 117.9, 123.8 (q, J = 286 Hz), 124.2, 129.7, 131.0, 136.7, 154.5, 158.0; HRMS (ESI) calcd for C18H16F6NO4+ ([M+H]+) 424.0978, found 424.0976.
Amide 7c
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (130 mg, 72% yield). 1H NMR (Acetone-d6, 400 MHz) δ 1.12 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 8.0 Hz, 3H), 3.42 (q, J = 7.0 Hz, 2H), 3.51 (q, J = 7.1 Hz, 2H), 4.95 (s, 2H), 7.02–7.18 (m, 2H), 7.30 (s, 1H), 7.85 (d, J = 8.8 Hz, 1H), 8.02 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.04; 13C NMR (Acetone-d6, 100 MHz) δ 12.8, 14.1, 40.6, 66.0, 78.0–79.2 (m), 104.7, 110.0, 116.5, 122.4, 123.1 (q, J = 288 Hz), 129.6, 130.2, 136.0, 153.9, 157.6, 166.0; HRMS (ESI) calcd for C19H20F6NO4+ ([M+H]+) 440.1291, found 440.1298.
Amide 7d
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (90 mg, 42% yield). 1H NMR (DMSO-d6, 400 MHz) δ 1.62 (s, 6H), 2.00 (d, J = 10.7 Hz, 10H), 4.53 (s, 2H), 7.06 (d, J = 8.4 Hz, 2H), 7.18 (s, 1H), 7.44 (s, 1H), 7.85 (d, J = 8.7 Hz, 1H); 19F NMR (DMSO-d6, 376 MHz) δ −76.01; 13C NMR (DMSO-d6, 100 MHz) δ 28.7, 35.9, 40.9, 51.0, 67.0, 78.0–79.1 (m), 104.6, 110.1, 116.6, 122.5, 123.1 (q, J = 288 Hz), 129.6, 130.2, 136.0, 153.9, 157.3, 166.4; HRMS (ESI) calcd for C25H26F6NO4+ ([M+H]+) 518.1761, found 518.1766.
Amide 7e
Prepared from 4 (100.0 mg, 0.27 mmol) in the same manner as described for 7a (91 mg, 71% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.51 (d, J = 8.0 Hz, 2H), 4.71 (s, 2H), 7.10–7.16 (m, 1H), 7.17–7.33 (m, 6H), 7.40 (s, 1H), 7.89 (d, J = 8.0 Hz, 1H), 8.08 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.03; 13C NMR (DMSO-d6, 100 MHz) δ 41.8, 67.0, 78.0–78.9 (m), 104.8, 110.1, 116.7, 122.6, 123.1 (q, J = 287 Hz), 126.7, 127.1, 128.2, 129.7, 130.2, 136.0, 139.3, 153.9, 157.1, 167.6; HRMS (ESI) calcd for C22H18F6NO4+ ([M+H]+) 474.1135, found 474.1138.
Amide 7f
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (200 mg, 99% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.50 (d, J = 5.9 Hz, 2H), 4.72 (d, J = 2.0 Hz, 2H), 6.98–7.07 (m, 2H), 7.13 (dd, J = 9.0, 2.5 Hz, 1H), 7.20 (d, J = 2.4 Hz, 1H), 7.30–7.38 (m, 2H), 7.42 (s, 1H), 7.90 (d, J = 9.0 Hz, 1H), 8.09 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −117.73, −76.14; 13C NMR (DMSO-d6, 100 MHz) δ 41.1, 67.0, 78.0–79.2 (m), 104.8, 110.2, 114.8, 115.0, 116.7, 122.6, 123.1 (q, J = 287 Hz), 129.0, 129.1, 129.7, 130.2, 135.5, 136.0, 153.9, 157.1, 159.9, 162.3, 167.6; HRMS (ESI) calcd for C22H17F7NO4+ ([M+H]+) 492.1040, found 492.1041.
Amide 7g
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (170 mg, 78% yield). 1H NMR (Acetone-d6, 400 MHz) δ 3.65 (s, 3H), 3.74 (s, 3H), 4.43 (d, J = 6.2 Hz, 2H), 4.70 (s, 2H), 6.81 (d, J = 1.0 Hz, 2H), 6.89 (s, 1H), 7.08–7.26 (m, 2H), 7.39 (s, 1H), 7.90 (d, J = 9.0 Hz, 1H), 8.09 (s, 2H); 19F NMR (Acetone-d6, 376 MHz) δ −76.03; 13C NMR (Acetone-d6, 100 MHz) δ 42.7, 55.4, 55.6, 67.5, 79.8–80.4 (m), 105.6, 112.0, 112.1, 115.0, 118.0, 120.2, 123.8 (q, J = 286 Hz), 124.2, 129.7, 131.0, 131.8, 136.7, 149.1, 149.8, 154.4, 158.0, 168.8; HRMS (ESI) calcd for C24H22F6NO6+ ([M+H]+) 534.1346, found 534.1369.
Amide 7h
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (210 mg, 95% yield). 1H NMR (Acetone-d6, 400 MHz) δ 2.98 (t, J = 7.0 Hz, 2H), 3.59 (dd, J = 13.2, 6.8 Hz, 2H), 4.65 (s, 2H), 7.03–7.21 (m, 3H), 7.26 (d, J = 8.2 Hz, 1H), 7.35–7.47 (m, 2H), 7.91 (d, J = 9.0 Hz, 2H); 19F NMR (Acetone-d6, 376 MHz) δ −75.99; 13C NMR (Acetone-d6, 100 MHz) δ 33.2, 39.0, 67.7, 80.0–80.6 (m), 105.7, 112.2, 115.1, 118.2, 123.9 (q, J = 287 Hz), 124.5, 127.7, 129.4, 129.9, 131.2, 132.9, 133.0, 135.2, 136.4, 137.9, 154.5, 158.1, 168.9; HRMS (ESI) calcd for C23H18Cl2F6NO4+ ([M+H]+) 556.0512, found 556.0510.
Amide 7i
Prepared from 4 (115.1 mg, 0.31 mmol) in the same manner as described for 7a (28 mg, 16% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.84 (s, 2H), 6.26 (s, 1H), 7.05 (dd, J = 9.0, 2.5 Hz, 1H), 7.21–7.32 (m, 3H), 7.36–7.43 (m, 3H), 7.51 (d, J = 7.5 Hz, 2H), 7.81 (t, J = 8.4 Hz, 3H), 8.04 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.08; 13C NMR (DMSO-d6, 100 MHz) δ 54.3, 67.3, 78.8–79.4 (m), 105.1, 110.6, 117.1, 120.6, 123.0, 123.7 (q, J = 288 Hz), 125.2, 128.0, 128.9, 130.1, 130.7, 136.5, 140.6, 144.9, 154.9, 157.7, 169.0; HRMS (ESI) calcd for C28H20F6NO4+ ([M+H]+) 548.1291, found 548.1284.
Amide 7j
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (88 mg, 95% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.81 (s, 2H), 7.11 (t, J = 7.4 Hz, 1H), 7.20–7.39 (m, 4H), 7.42 (s, 1H), 7.72–7.83 (m, 2H), 7.95 (d, J = 9.0 Hz, 1H), 8.12 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.04; 13C NMR (Acetone-d6, 100 MHz) δ 67.7, 79.7–80.3 (m), 105.6, 111.9, 114.8, 117.9, 120.5, 123.6 (q, J = 286 Hz), 124.2, 124.5, 129.0, 129.6, 130.9, 136.6, 138.6, 154.2, 157.9, 166.8; HRMS (ESI) calcd for C21H16F6NO4+ ([M+H]+) 460.0978, found 460.0982.
Amide 7k
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (130 mg, 63% yield). 1H NMR (Acetone-d6, 400 MHz) δ 1.20 (d, J = 6.9 Hz, 6H), 2.71–2.98 (m, 1H), 4.81 (s, 2H), 7.12–7.33 (m, 4H), 7.42 (s, 1H), 7.60–7.73 (m, 2H), 7.93 (d, J = 9.0 Hz, 1H), 8.12 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.11; 13C NMR (Acetone-d6, 100 MHz) δ 23.9, 33.8, 67.9, 79.8–80.4 (m), 105.7, 112.1, 114.9, 118.0, 120.8, 123.7 (q, J = 287 Hz), 124.3, 127.0, 129.7, 131.0, 136.3, 136.7, 145.2, 154.3, 158.0, 166.7; HRMS (ESI) calcd for C24H22F6NO4+ ([M+H]+) 502.1448, found 502.1424.
Amide 7l
Prepared from 4 (120.0 mg, 0.33 mmol) in the same manner as described for 7a (47 mg, 27% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.82 (s, 2H), 7.18–7.29 (m, 2H), 7.34 (d, J = 7.4 Hz, 1H), 7.44 (dd, J = 16.7, 8.8 Hz, 3H), 7.60–7.70 (m, 4H), 7.83–7.98 (m, 3H), 8.09 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.04; 13C NMR (Acetone-d6, 100 MHz) δ 68.0, 80.0–80.5 (m), 105.9, 112.2, 115.0, 118.2, 120.9, 121.0, 123.9 (q, J = 286 Hz), 124.4, 127.1, 127.6, 127.7, 127.8, 129.4, 130.0, 131.1, 136.8, 137.2, 138.3, 140.9, 154.4, 158.1, 167.0; HRMS (ESI) calcd for C27H20F6NO4+ ([M+H]+) 536.1291, found 536.1272.
Amide 7m
1,3-diisopropylcarbodiimide (42.8 mg, 0.34 mmol) was added slowly to a solution of acid 9 (120.0 mg, 0.28 mmol) and 1-hydroxytriazole (45.8 mg, 0.34 mmol) in dry DMF (3 mL) at 0°C. After 15 minutes, a solution of 4-morpholinoaniline (60.5 mg, 0.34 mmol) in dry DMF (2 mL) was added and the resulting mixture was stirred at rt overnight. The reaction mixture was diluted with brine (40 mL) and extracted with EtOAc (20 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated under vacuum and used without purification. The residue was dissolved in TFA/H2O (v/v, 9/1, 7.6 mL), then anisole (30 µl) was added and the resulting mixture was stirred overnight. The reaction mixture was concentrated under vacuum and then diluted with EtOAc (20 mL), washed with brine (30 mL × 2), the organic layer was dried over anhydrous Na2SO4, concentrated under vacuum and purified by flash chromatography on silica gel (20–80% EtOAc/petroleum ether) to give 7m as white wax (115 mg, 75% yield). 1H NMR (Acetone-d6, 400 MHz) δ 3.03–3.17 (m, 4H), 3.71–3.83 (m, 4H), 4.77 (s, 2H), 6.93 (d, J = 9.0 Hz, 2H), 7.17–7.31 (m, 2H), 7.42 (s, 1H), 7.57–7.69 (m, 2H), 7.94 (d, J = 8.9 Hz, 1H), 8.10 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.06; 13C NMR (DMSO-d6, 100 MHz) δ 48.8, 66.0, 67.2, 78.3–78.9 (m), 104.7, 110.2, 115.3, 116.6, 121.0, 122.6, 123.1 (q, J = 286 Hz), 129.7, 130.3, 136.0, 147.6, 153.9, 157.3, 165.7; HRMS (ESI) calcd for C25H23F6N2O5+ ([M+H]+) 545.1506, found 545.1490.
Amide 7n
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (120 mg, 57% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.98 (s, 2H), 7.29–7.41 (m, 2H), 7.41–7.60 (m, 4H), 7.76–8.06 (m, 5H), 8.14 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.07; 13C NMR (Acetone-d6, 100 MHz) δ 68.1, 80.0–80.6 (m), 105.8, 112.1, 115.0, 118.3, 122.6, 122.8, 124.5, 123.9 (q, J = 287 Hz), 126.0, 126.6, 126.7, 126.8, 128.87, 128.90, 129.8, 131.2, 133.1, 134.8, 136.8, 154.4, 158.1, 167.8; HRMS (ESI) calcd for C25H18F6NO4+ ([M+H]+) 510.1135, found 510.1110.
Amide 7o
Prepared from 4 (110.0 mg, 0.30 mmol) in the same manner as described for 7a (110 mg, 68% yield). 1H NMR (Acetone-d6, 400 MHz) δ 4.79 (s, 2H), 6.98–7.11 (m, 2H), 7.39 (d, J = 45.1 Hz, 11H), 7.81–7.95 (m, 1H), 8.06 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.07; 13C NMR (DMSO-d6, 100 MHz) δ 66.03, 78.4–80.0 (m), 104.5, 110.2, 116.2, 122.5, 123.2 (q, J = 288 Hz), 129.6, 130.3, 136.0, 154.3, 157.3, 166.7; HRMS (ESI) calcd for C27H20F6NO4+ ([M+H]+) 536.1291, found 536.1296.
Amide 7p
Prepared from 4 (150.0 mg, 0.41 mmol) in the same manner as described for 7a (53 mg, 35% yield). 1H NMR (Acetone-d6, 400 MHz) δ 5.06 (s, 2H), 6.86–7.02 (m, 2H), 7.26–7.49 (m, 4H), 7.52–7.65 (m, 2H), 7.65–7.89 (m, 4H), 8.03 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.12; 13C NMR (DMSO-d6, 100 MHz) δ 65.5, 78.0–79.2 (m), 104.0, 104.1, 110.0, 116.4, 122.5, 123.1 (q, J = 286 Hz), 126.9, 127.5, 128.1, 129.6, 130.3, 132.0, 135.76, 135.83, 136.8, 137.2, 137.5, 153.88, 153.93, 156.0, 157.0, 166.0; HRMS (ESI) calcd for C27H20F6NO4+ ([M+H]+) 536.1291, found 536.1296.
Amide 7q
Prepared from 9 (200.0 mg, 0.47 mmol) in the same manner as described for 7m (126 mg, 76% yield). 1H NMR (Acetone-d6, 400 MHz) δ 5.09 (s, 2H), 7.24–7.54 (m, 5H), 7.77 (d, J = 8.0 Hz, 1H), 7.96 (dd, J = 12.5, 5.3 Hz, 2H), 8.11 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.12; 13C NMR (Acetone-d6, 100 MHz) δ 67.5, 80.0–81.1 (m), 106.0, 112.4, 115.3, 118.3, 121.8, 122.2, 124.1 (q, J = 286 Hz), 124.70, 124.74, 127.0, 130.1, 131.4, 133.0, 137.0, 149.6, 154.8, 158.1, 158.3, 168.2; HRMS (ESI) calcd for C22H15F6N2O4S+ ([M+H]+) 517.0651, found 517.0646.
Amide 7r
Prepared from 9 (120.0 mg, 0.28 mmol) in the same manner as described for 7m (95 mg, 61% yield). 1H NMR (Acetone-d6, 400 MHz) δ 2.34 (s, 3H), 4.65 (s, 2H), 7.03–7.16 (m, 2H), 7.21 (d, J = 8.1 Hz, 2H), 7.37 (s, 1H), 7.67 (d, J = 8.3 Hz, 2H), 7.89 (dd, J = 22.1, 8.7 Hz, 1H), 8.12 (s, 1H); 19F NMR (Acetone-d6, 376 MHz) δ −76.03; 13C NMR (Acetone-d6, 100 MHz) δ 21.1, 66.7, 79.9–80.5 (m), 105.7, 112.1, 118.2, 123.8 (q, J = 286 Hz), 124.4, 128.8, 130.0, 131.1, 136.1, 136.8, 144.5, 154.4, 158.0, 167.1; HRMS (ESI) calcd for C22H19F6N2O6S+ ([M+H]+) 553.0863, found 553.0861.
19F MRI experiments
19F MRI experiments were performed on a 9.4 T microimaging system with a 10 mm inner diameter 19F coil (376.4 MHz) for both radiofrequency transmission and reception. The MSME (Multi Slice Multi Echo) pulse sequence was employed for all MRI acquisitions with single average. FOV = 8 × 8 mm2, SI = 40.0 mm TR = 2500 ms and TE = 7.6 ms were used. The data collection time was 160 ms.
Computational analysis
For computational analysis, PDB code 3O5X was used as model structure. Molecular docking was done using AutoDock Vina. Small molecule binding mode was modelled manually using Moloc(Gerber Mulecular Design:Switzerland). The image was produced by PyMOL.
PTPs activity assay
PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 mM EDTA, 150 mM NaCl) at 25 °C. The library compounds was screened in a 96-well format. The amount of product p-nitrophenol was determined from the absorbance at 405 nm detected by a Spectra MAX340 microplate spectrophotometer (Molecular Devices). The nonenzymatic hydrolysis of pNPP was corrected by measuring the control without the addition of enzyme. All PTPs used in the study were recombinant proteins prepared in-house.
Results and discussion
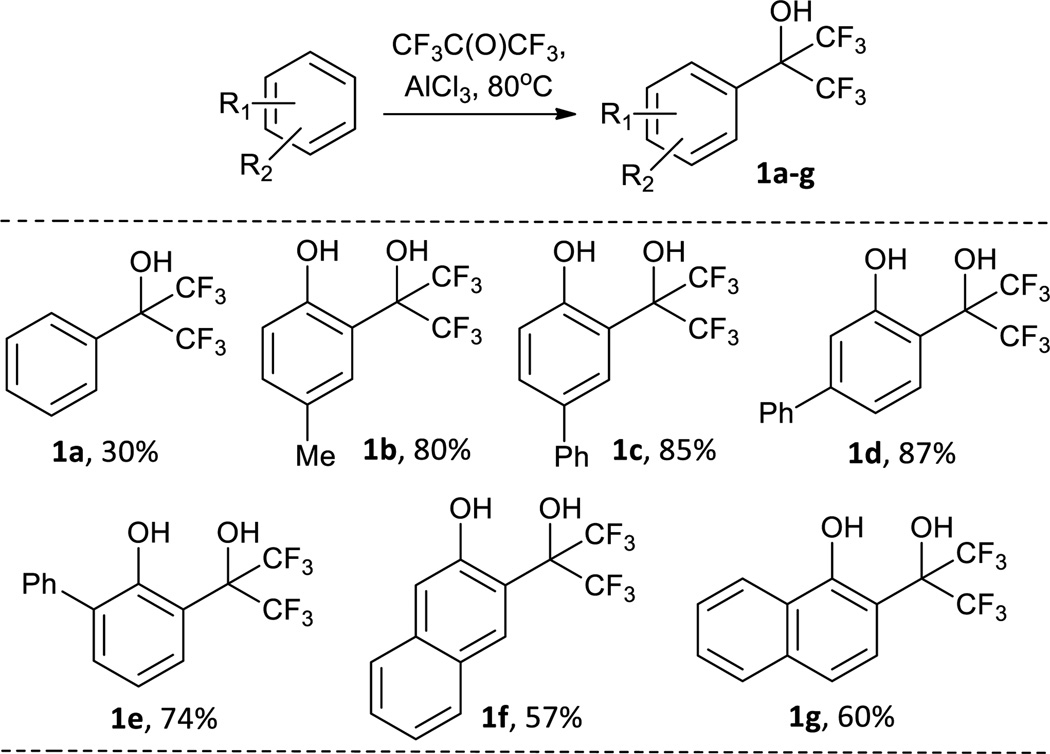
To probe the structure-activity relationship of the ortho-bis(trifluoromethyl)carbinol phenol-based inhibitors, a structure-based focused library strategy was employed. Our initial effort involved the construction of a focused library of 7 bis(trifluoromethyl)carbinol-substituted benzene to identify the optimal relative positions for these substituents (Scheme 2). Through the Lewis acid-catalysed Friedel-Crafts reaction, the bis(trifluoromethyl)-carbinol moiety was conveniently anchored to benzene, phenols, and naphthols with good yields. Due to the strong directing effect of phenolic hydroxyl group, the desired ortho-bis(trifluoromethyl)carbinol phenols were isolated as the major products (1b–g).
Scheme 2.
Synthesis of bis(trifluoromethyl)carbinol library.
The ability of library compounds 1a–g to inhibit a selected panel of PTPs of therapeutic interest, including mPTPB, SHP2, PTP1B, CD45, LYP, and FAP-1, was assessed at pH 7 and 25°C (Table 1). The results indicate that the phenolic hydroxyl group plays a crucial role in PTP binding through which the inhibitors may mimic the binding mode of salicylic acid-based inhibitors. No appreciable activity was found for 1a, which lacks phenolic hydroxyl group in the scaffold. The PTP inhibitory activity is also very sensitive to the size and position of substituent. Neither 1c with a para-phenyl group nor 1d with a meta-phenyl group has appreciable activity, while 1b with a small-sized para-methyl group has moderate activity. Among the library compounds 1a–g, 2-naphthol derived 1f is the most potent one for the selected panel of PTPs which was then selected for further optimization.
Table 1.
IC50 (µM) of 1a–g for a selected panel of PTPs.
| 1a | 1b | 1c | 1d | 1e | 1f | 1g | |
|---|---|---|---|---|---|---|---|
| mPTPB | - | 179.5 ± 19 |
351.0 ± 154 |
- | 148.4 ± 6 |
105.6 ± 10 |
- |
| SHP2 | - | 201.8 ± 37 |
392.2 ± 71 |
- | 136.0 ± 28 |
114.8 ± 17 |
- |
| PTP1B | - | 360.2 ± 207 |
- | - | 422.4 ± 311 |
260.4 ± 47 |
- |
| CD45 | - | 207.1 ± 17 |
- | - | 157.2 ± 14 |
112.4 ± 9 |
- |
| LYP | - | 302.9 ± 165 |
- | - | 268.4 ± 149 |
133.9 ± 34 |
- |
| FAP-1 | - | 454.3 ± 754 |
- | - | 448.8 ± 1506 |
127.9 ± 85 |
- |
A “-“ indicates IC50 >> 500 µM.
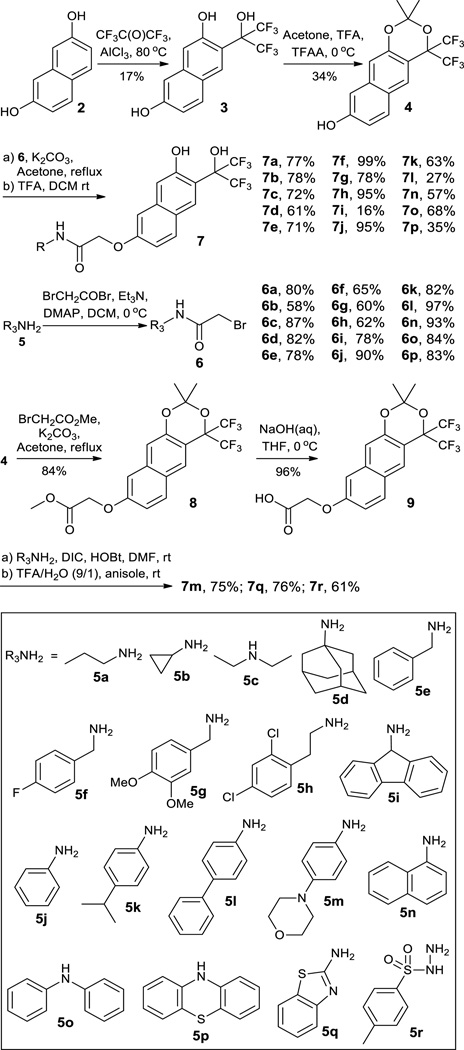
To further improve the potency and selectivity, 1f was modified into a focused library to target both the active site and a peripheral secondary binding site of PTPs (Scheme 3).8,10 Starting from 2,7-naphthalene-diol 2, a core compound 3, with an extra 7-hydroxyl group compared to 1f, was constructed through Friedel-Crafts reaction with good yield. Then, a panel of amines 5a–r with structural diversity were selected for the side chains 6a–r construction by reacting with bromoacetyl bromide, respectively. After protecting the 2 neighbouring hydroxyl groups in 3 with acetones, side chains 6a–r were anchored to the 7-hydroxyl group in 4 in the presence of K2CO3 to give ester intermediates after which the acetonide protecting group was removed with TFA to give amides 7a–p with good yields over 2 steps. However, the preparation of 7m, 7q and 7r was unsuccessful. So, an alternative method was developed by first anchoring an acetic acid side chain to 4 and then coupling amines 5m, 5q, and 5r, respectively, to give the corresponding amides 7m, 7q, and 7r. In these ways, the focused library of 18 ortho-bis(trifluoromethyl)carbinol phenols 7a–r with an amide side chain was conveniently prepared.
Scheme 3.
Synthesis of focused library of PTP inhibitors.
To illustrate the structures of ortho-bis(trifluoro methyl)carbinol phenols 7a–r, a single-crystal X-ray structure of 7c was obtained (Fig. 1). However, many attempts to prepare a single-crystal of 7p were unsuccessful.
As expected, the activities of library compounds 7a–r are much higher than these of 1f (Table 2). Compound 7r with sulfonohyrazide side chain was identified as a highly potent and selective mPTPB inhibitor with an IC50 value of 2.3 µM and more than 7-fold selectivity compared to SHP2, PTP1B, CD45, LYP, and FAP-1. It is interesting to point out that most of aliphatic amine derived compounds 7a–c and 7e–g show no appreciable PTP inhibitory activity, while bulky aliphatic amine derived compounds 7d, 7h, and 7i exhibit moderate activities. In contrast, most of aromatic amine derived compounds 7j–r have good activities and selectivity except for the positively charged 7m. Among them, compound 7p with a bulky aromatic group on the side chain exhibits very high activity toward the selected panel of PTPs with IC50 ranging from 2.2 µM for FAP-1 to 6.6 µM for PTP1B. Based on these observation, it is obvious that the potency of ortho-bis(trifluoromethyl) carbinol phenols-based inhibitors can be considerably optimized up to 58-fold by tethering an amide side chain. A bulky aromatic group containing side chain, i.e. 7l and 7p, can efficiently promote the binding affinity between PTPs and inhibitors by interacting with a peripheral pocket in the vicinity of the PTPs active sites, probably through steric effects and π-π stacking.
Table 2.
IC50 (µM) of 7a–r for a selected panel of PTPs.
| 7a–c | 7d | 7e–f | 7g | 7h | 7i | 7j | 7k | 7l | 7m | 7n | 7o | 7p | 7q | 7r | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mPTPB | - | - | - | 18.1 ± 9.5 |
5.1 ± 0.4 |
9.4 ± 0.2 |
14.4 ± 1.9 |
7.3 ± 0.5 |
4.8 ± 0.1 |
- | 4.7 ± 0.2 |
- | 2.9 ± 0.1 |
2.6 ± 0.1 |
2.3 ± 0.1 |
| SHP2 | - | 9.0 ± 2.2 |
- | - | 6.7 ± 1.2 |
6.9 ± 1.4 |
19.0 ± 25.6 |
8.5 ± 1.4 |
3.5 ± 0.5 |
- | - | 19.5 ± 47.8 |
3.2 ± 0.3 |
12.8 ± 3.6 |
20.3 ± 18.6 |
| PTP1B | - | 14.7 ± 3.0 |
- | - | 12.6 ± 4.8 |
13.8 ± 2.0 |
17.2 ± 8.9 |
- | - | - | - | 6.6 ± 1.3 |
- | - | |
| CD45 | - | 7.6 ± 0.8 |
- | - | 5.2 ± 0.6 |
6.6 ± 0.6 |
- | 7.8 ± 0.5 |
3.4 ± 0.2 |
- | 29.1 ± 38.2 |
14.9 ± 7.2 |
2.8 ± 0.2 |
10.9 ± 1.4 |
16.1 ± 5.0 |
| LYP | - | 10.1 ± 0.8 |
- | - | 6.9 ± 1.1 |
7.5 ± 0.8 |
- | 8.8 ± 1.1 |
- | - | - | 20.6 ± 35.9 |
3.4 ± 0.3 |
14.3 ± 3.1 |
15.4 ± 3.1 |
| FAP-1 | - | 7.4 ± 0.8 |
- | - | 4.3 ± 1.8 |
5.6 ± 0.7 |
- | 6.5 ± 0.7 |
2.8 ± 0.3 |
- | - | 12.4 ± 8.3 |
2.2 ± 0.3 |
9.9 ± 1.5 |
14.9 ± 5.2 |
A “-“ indicates IC50 >> 20µM.
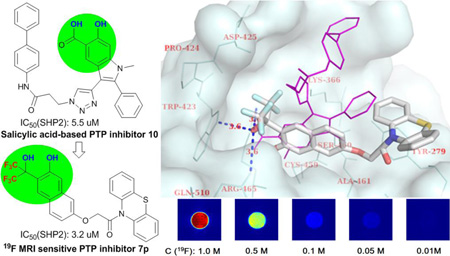
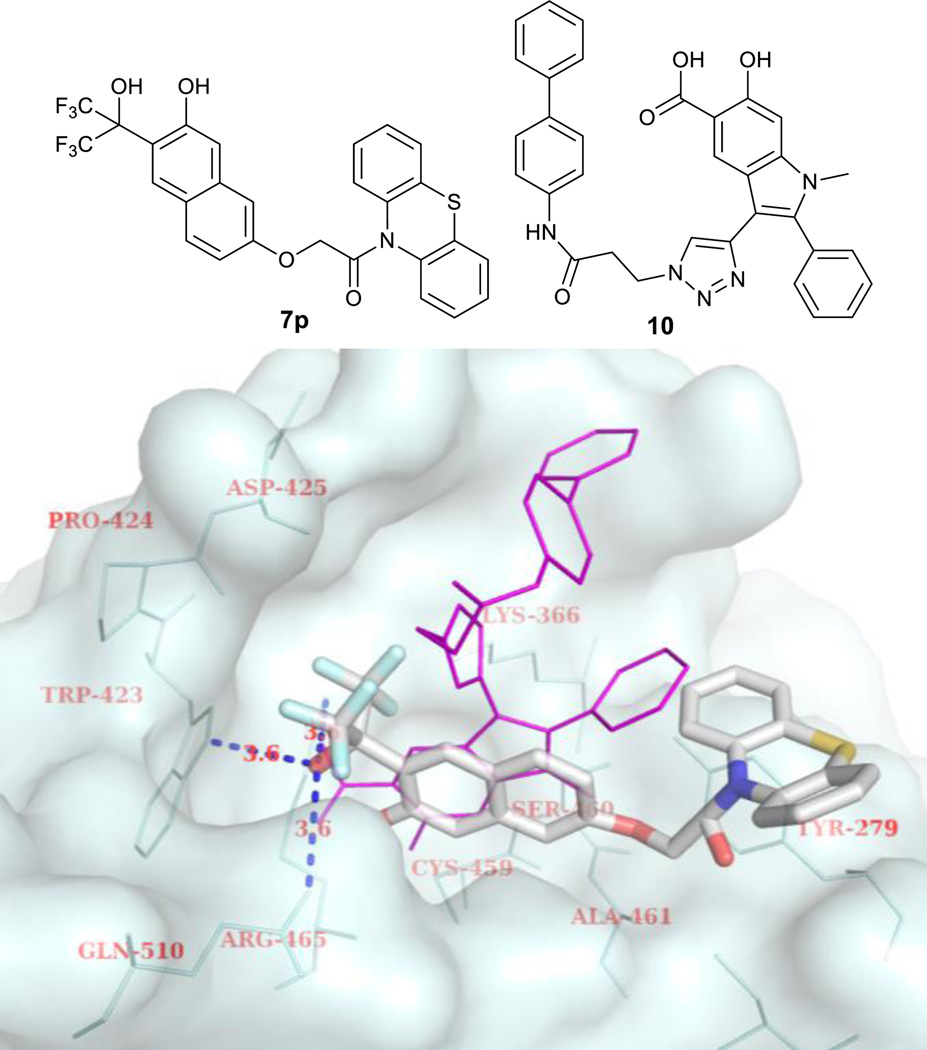
Computational analysis of the binding activity of 7p in the highly conservative active site of PTPs provided some insight into the structure-activity relationship between these novel inhibitors and PTPs. Oncogenic SHP2 with known complex structure (PDB ID: 3O5X) was selected as a model. Fig. 2 shows the binding mode of 7p with SHP2 compared to that of a known salicylic acid-based SHP2 inhibitor 10 which has a IC50 of 5.5 µM toward SHP2.8a As expected, ortho-bis(trifluoromethyl) carbinol phenol moiety can mimic the binding mode of salicylic acid by interacting with the corresponding amino acid residues Trp423, Arg465 and Gln510 (the distances between O of ortho-bis(trifluoromethyl)carbinol and the three hydrogen bonding heavy atoms of the residues are 3.6 Å). However, due to the difference in molecular geometry, the side chains of 7p and 10 interacted with SHP2 in different ways. Instead of interacting with Arg362 and Lys364 of SHP2, the aromatic side chain in 7p has a strong π-π interaction with Tyr-279.
Fig. 2.
Calculated structure of 7p bound to SHP2 with salicylic acid-based inhibitor 10 as a comparison.
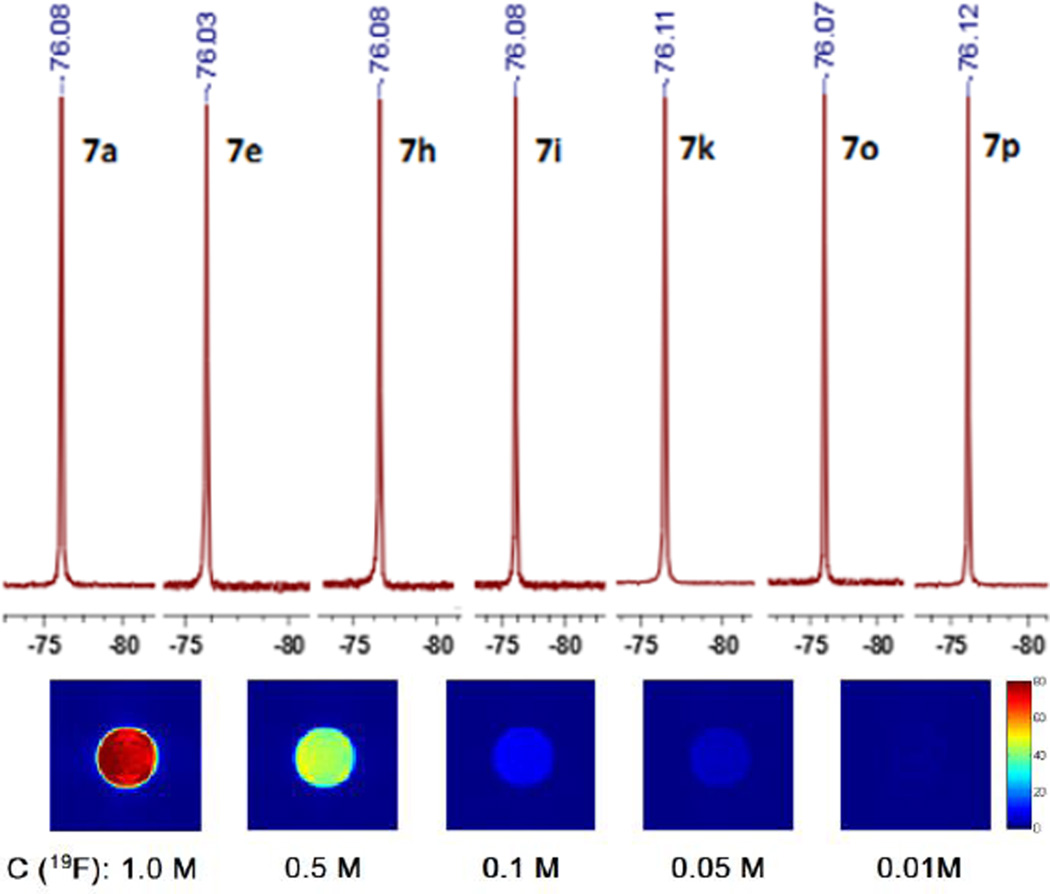
Finally, 19F magnetic resonance properties of PTP inhibitors 7a–r were investigated. As designed, all 6 symmetrical fluorines in 7a–r generated a strong singlet 19F NMR signal, respectively (Fig. 3). Uniformed 19F signal dramatically improved the 19F NMR sensitivity of these fluorinated inhibitors for downstream applications. Then, 7p with high potencies toward a panel of PTPs was selected for 19F MRI study. It was found that 7p has a very short longitudinal relaxation time T1 of 299 ms which could further improve its 19F MRI sensitivity by allowing the collection of more transient signals without prolonging the data acquisition time. 19F MRI phantom experiment on an array of 7p solution indicated that 7p could be clearly imaged by 19F MRI with a scan time of 120 seconds at a concentration of as low as 8.3 mM (or 50 mM in 19F concentration, Fig. 3). Therefore, 7p is a novel PTP inhibitor as well as a highly valuable tool molecule whose local information, such as distribution and concentration, and interactions with PTPs, such as binding mode and affinity, can be conveniently monitored by 19F MR spectroscopy and imaging without extra modification in the absence of background signals.
Fig. 3.
19F NMR of selected inhibitors (upper) and 19F MRI of 7p (lower).
Conclusions and outlook
In summary, we have successfully demonstrated a strategy of developing novel 19F magnetic resonance sensitive small molecule PTP inhibitors for drug discovery and biomedical research through rational molecular design and symmetrical fluorination. Ortho-bis(trifluoromethyl)carbinol phenol is a valuable substitute for salicylic acid in PTP inhibitor discovery, which successfully integrates the PTP binding ability and high 19F NMR signal generating ability. As fluorinated drugs are booming in pharmaceutical industry, it is of great importance to utilize their inherent 19F magnetic resonance properties in target identification, pharmacology study, in vivo drug tracking, image/spectroscopy-guided drug therapy and beyond.
Finally, we want to point out that both 19F NMR and 19F MRI are valuable modalities for biomedical research. 19F MRI provides high-contrast images at 19F concentrations of mM and above, while 19F NMR provides sensitive spectroscopies even at sub-µM 19F concentrations. Improving the PTP inhibition potency and selectivity, 19F MRI sensitivity of these inhibitors and their application in 19F magnetic resonance-guided PTP mechanism study are currently in progress and will be published in due course.
Supplementary Material
Acknowledgments
We are thankful for financial support from the National Natural Science Foundation of China (21372181, 21402144, 21572168, and 21575157), Key Laboratory of Synthetic Chemistry of Natural Substances (Shanghai Institute of Organic Chemistry) and State Key Laboratory for Modification of Chemical Fibers and Polymer Materials (Donghua University). LW and ZYZ are supported by NIH RO1 CA69202 and P30 CA023168.
Footnotes
The authors declare no competing interests.
Electronic Supplementary Information (ESI) available: Copies of 1H NMR, 13C NMR, 19F NMR and HRMS of compounds, and single-crystal X-ray diffractograms of 7c (CCDC1470244). See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Tonks NK. Nat. Rev. Mol. Cell Biol. 2006;7:833. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 2.(a) Zhang Z-Y. Curr. Opin. Chem. Biol. 2001;5:416. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]; (b) Bialy L, Waldmann H. Angew. Chem., Int. Ed. 2005;44:3814. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]
- 3.(a) Krause DS, van Etten RA. New Engl. J. Med. 2005;353:172. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]; (b) Jiang Z-X, Zhang Z-Y. Cancer metastasis Rev. 2008;27:263. doi: 10.1007/s10555-008-9113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ahrens ET, Flores R, Xu H, Morel PA. Nat. Biotechnol. 2005;23:983. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]; (b) Vivian D, Cheng K, Khuranan S, Xu S, Kriel EH, Dawson PA, Raufman JP, Polli JE. Mol. Pharmaceutics. 2014;11:1575. doi: 10.1021/mp400740c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Mizukami S, Takikawa R, Sugihara F, Hori Y, Tochio H, Walchli M, Shirakawa M, Kikuchi K. J. Am. Chem. Soc. 2008;130:794. doi: 10.1021/ja077058z. [DOI] [PubMed] [Google Scholar]; (b) Bruemmer KJ, Merrikhihaghi S, Lollar CT, Morris SN, Bauer JH, Lippert AR. Chem. Commun. 2014;50:12311. doi: 10.1039/c4cc04292a. [DOI] [PubMed] [Google Scholar]
- 7.Shi Q, Li Y, Bo S, Li X, Zhao P, Liu Q, Yang Z, Cong H, Deng H, Chen M, Chen S, Zhou X, Ding H, Jiang Z-X. Chem. Commun. 2016;52:5136–5139. doi: 10.1039/c6cc01508e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Zhang X, He Y, Liu S, Yu Z, Jiang Z-X, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM, Wang L, Chan RJ, Zhang Z-Y. J. Med. Chem. 2010;53:2482. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He Y, Xu J, Yu Z-H, Gunawan AM, Wu L, Wang L, Zhang Z-Y. J. Med. Chem. 2013;56:832. doi: 10.1021/jm301781p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He Y, Liu S, Menon A, Stanford S, Oppong E, Gunawan AM, Wu L, Wu DJ, Barrios AM, Bottini N, Cato AC, Zhang Z-Y. J. Med. Chem. 2013;56:4990. doi: 10.1021/jm400248c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zeng LF, Zhang R-Y, Yu Z-H, Liu S, Wu L, Gunawan AM, Lane BS, Mali RS, Li X, Chan RJ, Kapur R, Wells CD, Zhang Z-Y. J. Med. Chem. 2014;57:6594. doi: 10.1021/jm5006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Yu W, Yang Y, Bo S, Li Y, Chen S, Yang Z, Zheng X, Jiang Z-X, Zhou X. J. Org. Chem. 2015;80:4443. doi: 10.1021/acs.joc.5b00294. [DOI] [PubMed] [Google Scholar]; (b) Bo S, Song C, Li Y, Yu W, Chen S, Zhou X, Yang Z, Zheng X, Jiang Z-X. J. Org. Chem. 2015;80:6360. doi: 10.1021/acs.joc.5b00810. [DOI] [PubMed] [Google Scholar]
- 10.(a) Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang Z-Y. Proc. Natl. Acad. Sci., USA. 1997;94:13420. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu X, Sun J-P, He Y, Guo X-L, Liu S, Zhou B, Hudmon A, Zhang Z-Y. Proc. Natl. Acad. Sci., USA. 2007;104:19767. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.