Abstract
Objectives
To evaluate if optimal the dose of either oral or injectable regimens of MTX of 25mg per week was used in the comparator arms of studies comparing biologic drugs with methotrexate in rheumatoid arthritis (RA).
Methods
A systematic literature search was carried out in MEDLINE, EMBASE and the Cochrane Library searching for randomized controlled trials comparing biologics with methotrexate in RA. A systematic review was performed among studies that met predefined criteria focusing on assessment of dose of methotrexate used in the comparator arm. Study authors were contacted when necessary. Study quality was assessed.
Results
A total of 3276 references were identified and 13 trials were included. We obtained maximal dose and regimen for all. The maximal dose of methotrexate used in the comparator arm of the trials was no more than 20mg per week in any trial and for all but one trial, MTX was given orally and not by injection. The trial that used an injectable form reached a maximum of 15mg/week.
Conclusion
A suboptimal dose of MTX was used in biological clinical trials performed in RA, particularly regarding route of administration. This may have biased findings in favor of biologic agents.
Keywords: rheumatoid arthritis, methotrexate, biologics, dosage
Numerous studies have demonstrated that studies sponsored by the manufacturing company are often more favorable to the sponsor's product compared with studies with other sources of sponsorship. [1] One of the limitations in pharmaceutical sponsored clinical trials is the use of a suboptimal dose of the comparator drug in the control arm, to provide an artefactual superiority for the investigational drug. [2,3]
Methotrexate is the anchor drug in rheumatoid arthritis (RA). In trials conducted among DMARD naïve RA patients, trials have often compared methotrexate (MTX) to biologic agents and have reported superiority of biologic treatment over MTX monotherapy. No study, to our knowledge has examined whether the dose of MTX used in such trials was optimal.
There exists enough evidence to support that the absorption of oral MTX is variable, when used at doses greater then 15 mg/week and that injectable MTX at the higher doses reaches higher therapeutic levels [4] and efficacy [5] than oral MTX. The European League against Rheumatism (EULAR) and expert opinion recommends up-titration of MTX up to 25mg/week for achieving disease control. [6, 7]
Trials comparing biologic agents to MTX serve as the basis for practice and for recommendations as to which treatments are efficacious. We carried out a systematic review of RA clinical trials to assess if optimal doses of either oral or injectable regimens of MTX were used in the comparator arms of foundational trials comparing biologic agents with MTX.
Methods
Research Question
We aimed to determine if methotrexate was used at its recommended dose in clinical trials evaluating the efficacy of biologic drugs in RA. We reformulated the research question using the PICOS method. Patients were subjects with rheumatoid arthritis; the intervention was a biologic drug; the comparison was methotrexate; the outcome was clinical measurement of disease activity and the study design was randomized controlled trial.
Systematic literature search
A systematic literature search for articles published up to November 2014 was carried out in MEDLINE, EMBASE and the Cochrane Library, using the following search terms: rheumatoid arthritis, anti tnf, anti tnf alpha, tumor necrosis factor alpha inhibitor, infliximab, cA2, remicade, adalimumab, D2E7, humira, etanercept, TNFR-Fc fusion protein, p75TNFR-FC, enbrel, golimumab, CNTO-148, simponi, certolizumab, certolizumab pegol, cdp870, cimzia, rituximab, anti-CD20, rituxan, mabthera, abatacept, ctla4 Ig, orencia, tocilizumab, atlizumab, actemra, roactemra, tofacitinib, xeljanz. No language restriction was used. Review articles were retrieved to identify additional references by hand search. For the purposes of simplicity of labeling, we shall designate all the primary drugs compared to MTX as biologics even though we realize that tofacitinib, developed to have biologic effects, is in fact, a small molecule.
The following inclusion criteria were applied: RCT, RA, 18 years old or greater, biologic therapy in one treatment group, methotrexate in one of the treatment groups, clinical outcome measures and study duration of at least 6 months and less or equal to 24 months. Articles that did not fulfill all the inclusion criteria, included MTX partial responders, used combined biologic drugs or presented only radiological outcomes were excluded.
Studies were assessed by two independent investigators (JD and DD or MB). Any disagreements regarding study eligibility were resolved by discussion. Relevant articles were selected in a three-step procedure. First, titles were screened. When a title seemed relevant the abstract was reviewed. Articles that addressed the topic of interest in the abstract were selected and reviewed in full paper.
Data extraction
Data extraction was performed by two independent investigators (JD and DD or MB) using a piloted form. Disagreements were resolved by discussion. From each study we collected the source (main author, journal, publication year), disease duration, former treatment, biologic used (route, combination with MTX), MTX dose and regimen, use of injectable placebo, time-point of outcome, number of subjects, outcome measure (DAS28, ACR20/50/70), superiority of biologic reported (y/n) according to the conclusion presented in the paper, industry sponsorship and intention to treat analysis. If necessary, authors were contacted to provide additional information regarding maximum dosage of MTX. To evaluate the methodological quality of studies the Cochrane risk of bias tool was used. [8]
Results
A total of 3276 references were identified with the systematic search strategy after eliminating duplicates. Title screening left 414 abstracts for revision. After selecting abstracts, 75 articles/conference abstracts were retrieved for full paper review. For our current study 13 fulfilled the inclusion criteria. (Figure 1) Of these, three studied adalimumab, two infliximab, two etanercept, one golimumab, one abatacept, one rituximab, two tocilizumab and one tofacitinib. (Table 1) [9,10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20]
Figure 1. Flowchart of study selection process.
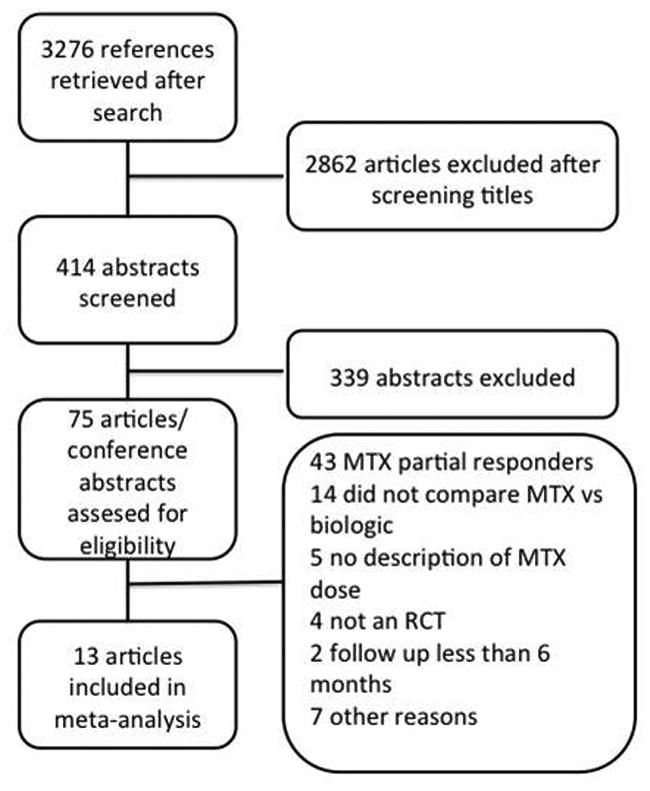
Table 1. Summary of all included studies in the meta-analysis and the characteristics of patients.
| Author (year) | Biologic | Biologic branch-MTX combination | RA Duration (months) | Total subjects | MTX Subjects | Biologic Subjects* | Biologic reported superior |
|---|---|---|---|---|---|---|---|
| Bathon (2000) | ETN | monotherapy | 12 | 632 | 217 | 208/207 | N/Ya |
| Bong Lee (2014) | TOFA | monotherapy | 36 | 956 | 186 | 373/397 | Y |
| Breedveld (2006) | ADA | monotherapy | 8 | 799 | 257 | 274 | N |
| Combined | 268 | Y | |||||
| Burmester (2015) | TCZ | monotherapy | 6 | 1157 | 287 | 292 | Y |
| Combined | 291 | Y | |||||
| Detert (2013)b | ADA | Combined | 2 | 172 | 85 | 87 | Y |
| Durez (2007) | INF | Combined | 4 | 44 | 14 | 15 | Y |
| Emery (2008) | ETN | Combined | 10 | 528 | 263 | 268 | Y |
| Emery (2009) | GLM | monotherapycombined | 48 | 637 | 160 | 159 | N |
| 159/159 | Y | ||||||
| Jones (2009)c | TCZ | monotherapy | 72 | 673 | 284 | 288 | Y |
| Smolen (2014) | ADA | Combined | 3 | 1032 | 517 | 515 | Y |
| St Clair (2004) | INF | Combined | 11 | 1049 | 298 | 373 | Y |
| Tak (2010) | RTX | Combined | 11 | 748 | 249 | 251 | Y |
| Westhovens (2009) | ABA | Combined | 6 | 509 | 253 | 256 | Y |
ABA, abatacept; ADA, adalimumab; CZP, certolizumab; ETA, etanercept; GLM, golimumab; INF, infliximab; RTX, rituximab; TCZ, tocilizumab; TOFA, tofacitinib; Mono, monotherapy; Comb, combined therapy; inj, injectable.
activity scores were not superior at follow up but the study conclusion states the biologic is beneficial due more rapid effect and less radiologic progression
trial not sponsored by industry
there was also a branch that received placebo 12wks and then tcz 12 wks. It was not included due to the short follow up using tcz
two numbers in a same trial correspond to different dose branches
Only 8 of the 13 trials reported the maximum dose of methotrexate. We were able to obtain additional information regarding methotrexate doses from the first author for the remaining 5 studies. Overall, none of the studies used doses of more than 20 mg. Further, only 1 study allowed the use of injectable methotrexate and in this study the maximum dose was 15 mg/week. (table2)
Table 2. Methotrexate Dosage regimen in Biologic Trials in RA.
| Author (year) | Biologic | MTX maximum dose mg/week | MTX mean dose mg/week | MTX route of administration |
|---|---|---|---|---|
| Bathon (2000) | ETN | 20 | 19 | Oral |
| Bong Lee (2014) | TOFA | 20 | 19 | Oral |
| Breedveld (2006) | ADA | 20 | 17 | Oral |
| Burmester (2015) | TCZ | 20 | 19 | Oral |
| Detert (2013) | ADA | 15 | 15 | Subcutaneous |
| Durez (2008) | INF | 20 | 20 | Oral |
| Emery (2008) | ETN | 20 | NR* * | Oral |
| Emery (2009) | GLM | 20 | 19 | Oral |
| Jones (2009) | TCZ | 20 | 16 | Oral |
| Smolen (2014) | ADA | 20 | NR** | Oral |
| St Clair (2004) | INF | 20 | 15 | Oral |
| Tak (2010) | RTX | 20 | >18 | Oral |
| Westhovens (2009) | ABA | 20 | 19 | Oral* |
ABA, abatacept; ADA, adalimumab; CZP, certolizumab; ETA, etanercept; GLM, golimumab; INF, infliximab; RTX, rituximab; TCZ, tocilizumab; TOFA, tofacitinib; NR, not reported; SC, subcutaneous.
In the Westhovens et al. trial 1.5% of subjects received injectable MTX, but it was not part of the protocol
In all but two studies subjects received injected placebo.(9,13) All but one study (12) was sponsored by industry and in all industry sponsored studies, the product of the industrial sponsor was the biologic agent being evaluated. Regarding clinical outcomes, reports from biologic monotherapy trials, with the exception of those studying tocilizumab and tofacitinib, reported no difference in efficacy. However, Bathon et al [8], in their conclusion emphasized that etanercept monotherapy had a quicker onset of action and was superior in radiological outcomes. All studies concluded that combination of MTX with biologic drugs was superior to MTX monotherapy.
Methodological quality of studies
All studies were randomized trials. Detail regarding internal validity of these trials is presented in Supplement Table 1. None of them was considered at high risk of bias, but not all items were clearly reported in 8 studies.
Discussion
To our knowledge, this is the first systematic review that evaluates the dosage of the comparator MTX in biologic drug trials in RA. We found that in all trials in which there was a direct comparison with biologic drugs, MTX was not used at the maximum recommended dose. Moreover, injectable forms of methotrexate were only used in one of these trials, and in this trial it was not used at full dose.
A dose-effect relationship exists for MTX in RA treatment. Therefore, for it to be an appropriate comparator, the maximum dose should be used in subjects who require and tolerate it. Multiple studies have shown oral methotrexate is a drug with variable bioavailability between individuals and with a decreasing relative bioavailability with increasing doses. Response to therapy is achieved only with maximum dose (25-30mg/week) in a proportion of subjects and there are no identified predictors that identify these patients [3]. On the other hand, subcutaneous MTX has been shown to have a better bioavailability at high doses and clinical studies support that it is more effective at an equal dose than oral MTX. As Schiff et al, concluded recently in a cross-over study evaluating routes of administration in the same subject, drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration.(3)
Industry sponsorship bias has been documented by studies in different fields of medicine. [1] A common theme in these studies has been the under-dosing of the standard treatment comparator when a new drug is being tested by their sponsor. We suggest that the same phenomenon has occurred with the development of biologics in RA. Further, this suggests that the presumed superiority of some biologics either in combination with MTX or as monotherapy over MTX may be overstated given the suboptimal MTX regimens used. Bias generated due to the use of suboptimal dose of MTX as a comparator that would favor biologics may lead to exposing patients to unnecessary risk or expense.
It may be argued that using injectable methotrexate in case of oral methotrexate failure would compromise blinding, but internal validity would probably not be harmed to an extent to justify not using the maximum effective dose. An alternative would have been the use of a study design like HIT HARD trial in which only subcutaneous methotrexate was used in order to make sure everybody reached maximum levels of the drug without affecting blinding. [12]
Regarding the quality of studies there was lack of reporting of important aspects in several trials, such as allocation concealment and blinding. Patients and physician reported outcomes used in activity scores may be affected by knowledge of the intervention.
Strengths of this study are that a comprehensive of the literature search was performed and no study was excluded due to language restrictions. In addition, authors were contacted when MTX dose/route was not clear in the article and they all provided information about the MTX regimen used. A potential limitation is we did not perform a ‘grey literature’ search but thirty meta-analyses were hand searched and it is unlikely that studies were missed.
Conclusion
A suboptimal dose of MTX was used in biological drugs clinical trials performed in RA, particularly in relation to route of administration. This may have biased findings in favor of biologic agents in RA trials.
Supplementary Material
Acknowledgments
We would like to thank the researchers who kindly provided additional data from their studies: Josef S. Smolen, Gerd R. Burmester, Rene Westhovens, Eun Bong Lee, Patrick Durez.
References
- 1.Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews. 2012;(12) doi: 10.1002/14651858.MR000033.pub2. Art. No.: MR000033. [DOI] [PubMed] [Google Scholar]
- 2.Rochon P, Gurwitz JH, Simms RW, Fortin PR, Felson DT, Minaker KL, Chalmers TC. A Study of Manufacturer-Supported Trials of Nonsteroidal Anti-inflammatory Drugs in the Treatment of Arthritis. Arch Intern Med. 1994;154:157–163. [PubMed] [Google Scholar]
- 3.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann RheumDis. 2014;73:1549–1551. doi: 10.1136/annrheumdis-2014-205228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun J, Kaestner P, Flaxenberg P, Waehrisch J, Hanke P, Demary W, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis. Arthritis Rheum. 2008;58:73–81. doi: 10.1002/art.23144. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086–1093. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JPT, Altman DG, Gøtzsche PC, J(x00075)ni P, Moher D, Oxman AD, Savovi(x00107) J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 9.Bong Lee E, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus Methotrexate in Rheumatoid Arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 10.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis and rheumatism. 54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 11.Burmester GR, Rigby WF, van Vollenhoven RF, et al. Ann Rheum Dis 2015. 2015 Oct 28; doi: 10.1136/annrheumdis-2015-207628. Published Online First: [DOI] [Google Scholar]
- 12.Detert J, Bastian H, Listing J, Weiβ A, Wassenberg S, Liebhaber A, Rockwitz K, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naïve patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. 2013;72:844–850. doi: 10.1136/annrheumdis-2012-201612. [DOI] [PubMed] [Google Scholar]
- 13.Durez P, Malghem J, Nzeusseu Toukap A, Depresseux G, Lauwerys BR, Westhovens R, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56:3919–27. doi: 10.1002/art.23055. [DOI] [PubMed] [Google Scholar]
- 14.Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti–tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four–week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 15.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 16.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolen JS, Emery P, Fleischmann R, van Vollenhoven RF, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383:321–32. doi: 10.1016/S0140-6736(13)61751-1. [DOI] [PubMed] [Google Scholar]
- 18.Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 19.Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, et al. for the IMAGE Investigators. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis. 2011;7:39–46. doi: 10.1136/ard.2010.137703. [DOI] [PubMed] [Google Scholar]
- 20.Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


