Abstract
Congenital hyperinsulinism (HI) is the most common cause of persistent hypoglycemia in infants and children. In cases of diazoxide unresponsive HI, alternative medical and surgical approaches may be required to reduce risk of hypoglycemia. Octreotide, a somatostatin analog, often has a role in the management of these children, but a dose dependent reduction in splanchnic blood flow is a recognized complication. Necrotizing enterocolitis (NEC) has been reported within the first few weeks of initiating predominantly high doses of octreotide. We describe the case of an infant with Beckwith Wiedemann Syndrome (BWS) and diazoxide unresponsive HI, who who had persistent hypoglycemia after two pancreatectomy surgeries. She developed NEC two months after beginning octreotide therapy at a relatively low dose of 8 μg/kg/day. This complication has occurred later, and at a lower dose, than has previously been described. We review the case and identifiy the known and suspected multifactorial risk factors for NEC that may contribute to the development of this complication in patients with HI.
Keywords: octreotide, hyperinsulinism, necrotizing enterocolitis, infant, Beckwith Wiedemann
INTRODUCTION
Congenital hyperinsulinism (HI) is the most common cause of persistent hypoglycemia in infants and children. The consequences of delayed recognition and treatment of hypoglycemia include seizures and developmental and cognitive delay [1]. Mutations in nine genes are currently known to cause congenital HI [1, 2], but in approximately 20-50 % of cases a mutation in these genes is not identified [3]. HI can also be associated with syndromes such as Beckwith Wiedemann Syndrome (BWS) and these cases, particularly those in which there is paternal UPD of 11p, can be severe [4]. The most common and severe form of congenital HI is caused by inactivating mutations in the genes encoding the ATP sensitive potassium channel (KATP). In pancreatic ß-cells, KATP channels play a critical role in regulating insulin secretion by coupling metabolism to membrane potential. Glucose entry into the ß-cell results in a change in energy potential of the ß-cell followed by closure of the KATP channel leading to depolarization of the ß-cell membrane, calcium influx, stimulating exocytosis of insulin-containing granules [5]. Most mutations associated with KATP HI completely disrupt KATP channel formation [6] inducing persistent membrane depolarization and resultant unregulated insulin secretion,
Diazoxide, which has been the first line therapeutic approach to HI since its use was first described in 1968[7], acts by maintaining the KATP channel in an open state, thus preventing beta cell depolarization and insulin secretion. For patients with KATP HI in whom diazoxide is not effective in, a near-total pancreatectomy is often required in order to control their hypoglycemia [2, 3]. The most commonly used medical alternatives or adjuncts to surgery are limited but include somatostatin analogs and continuous enteral dextrose [1]. Use of sirolimus [8], calcium channel blockers [9] and glucagon pumps [10] have all been described, but are not currently regarded as standard care.
Octreotide is a somatostatin analog that reduces insulin secretion by inhibiting the activation of voltage-gated calcium channels on the membrane of pancreatic β-cells [11]. A side effect of octreotide therapy is a dose dependent reduction in splanchnic blood flow [12, 13], which is thought to increase the risk of necrotizing enterocolitis (NEC) in susceptible infants. NEC is characterized by coagulation necrosis of the intestine, bacterial overgrowth and inflammation [14]. There have been six reported cases of NEC associated with octreotide in infants with HI [15, 16], all of which occurred within the first month of life and within 15 days of starting treatment.
We describe the first case of delayed presentation of NEC in an infant with HI treated with octreotide, and we explore the possible multifactorial etiology of this complication. Consequently, we advise caution and close monitoring of children treated with this medication even beyond the early phase of treatment.
CASE
Our patient is a baby girl born at 38 weeks gestation weighing 4.56kg via an uncomplicated spontaneous vaginal delivery. The pregnancy was complicated only by maternal pruritic urticarial papules and plaques of pregnancy, for which her mother was treated with prednisolone briefly. Hypoglycemia was noted with a blood glucose less than 20 mg/dL within the first hour of age, and she commenced intravenous dextrose at a glucose infusion rate (GIR) of 14 mg/kg/min. HI was confirmed by an inappropriately detectable insulin level (71 uIU/ml) and suppressed beta-hydroxybutyrate (<0.3 mmol/L) in the setting of hypoglycemia. Her examination was significant for macroglossia and hemi hypertrophy, but was otherwise normal.
Her GIR was increased to 18.7 mg/kg/min via umbilical venous catheter and diazoxide was commenced (with chlorthiazide to reduce the risk of fluid retention). Diazoxide was discontinued after 5 days, as it was not possible to reduce her GIR despite maximal dosing. She was found to have a paternally inherited heterozygous autosomal recessive mutation in ABCC8 (c.692g>a/p.W231X) which was predicted to be disease causing, and also had paternal uniparental disomy of 11p15.5, consistent with BWS.
Our patient underwent surgery following a preoperative fluorine-18-L-dihydroxyphenylalanine positron emission tomography (18F-DOPA PET) scan, which showed slightly more 18FDOPA activity at the end of the pancreatic tail, which was suggestive of a focal lesion. An intraoperative ultrasound did not identify a potential focal lesion and frozen section biopsies demonstrated diffusely increased endocrine tissue with islet cell nucleomegaly. A near-total (98%) pancreatectomy with gastrostomy tube placement was performed. Following surgery, she continued to have an increased glucose requirement and a completion pancreatectomy was performed two weeks later, on day 32 of life. She developed a central line infection one week after this procedure, and was transitioned from intravenous to enteral dextrose 20% after this had been treated. Feeding intolerance and reflux were treated with ranitidine.
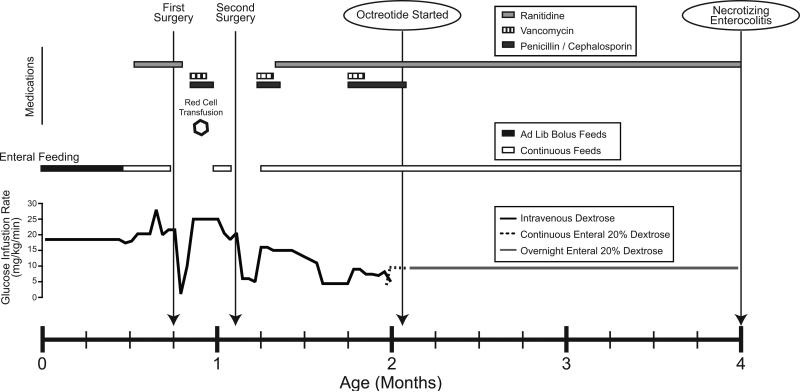
Continuous enteral 20% dextrose provided a GIR of 9.4 mg/kg/min, in addition to continuous feedings of 20 kcal/oz feeds (breastmilk with Enfamil) at 30 ml/hr 24 hrs/day. At two months of age, she started twice daily subcutaneous octreotide at 3 μg/kg at 8am and 5 μg/kg at 3pm. This daytime octreotide allowed her continuous enteral dextrose 20% to be discontinued during the day. This timeline is shown graphically in Figure 1.
Figure 1.
Timeline of clinical course including Glucose Infusion Rates (GIR), enteral feeds, surgeries, antibiotics and ranitidine.
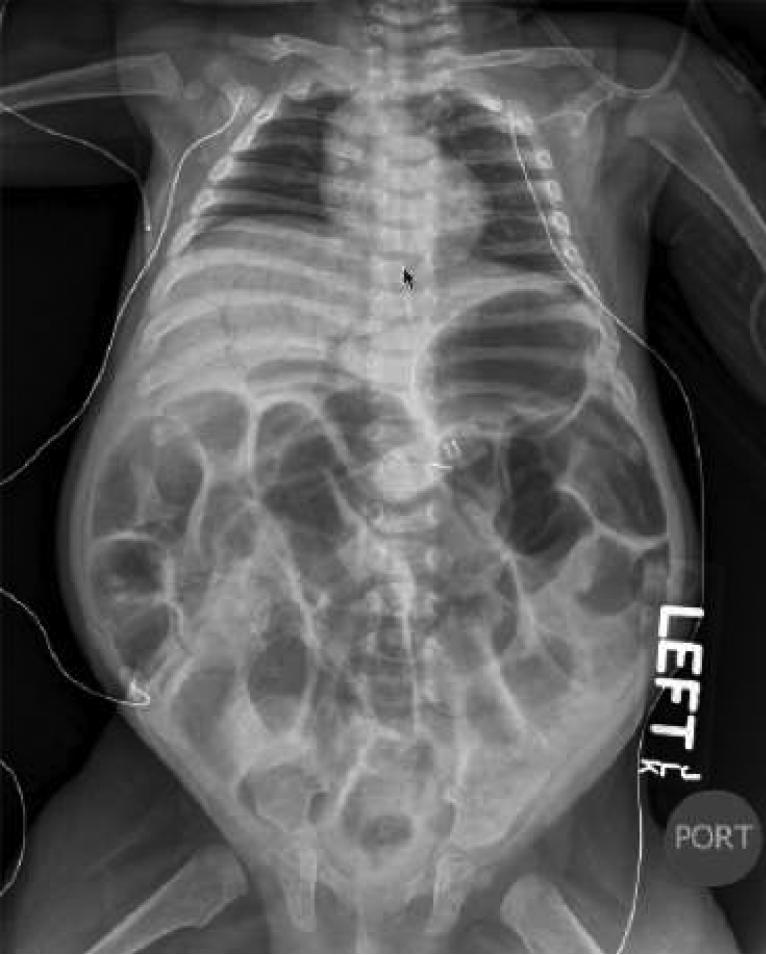
She was discharged at three months of age on this regimen as well as oral ranitidine and pancreatic enzymes. At four months of age she presented with a two day history of reduced activity and abdominal distension. She was noted to have metabolic acidosis and hypernatremia, with tachycardia and hypotension. Clinical examination and abdominal radiography (Figure 2) were consistent with fulminant necrotizing enterocolitis. Care was redirected to palliation and she died within 12 hours of presentation. Post-mortem examination showed necrosis of the entire small and large intestines and congestion of the mucosa with regenerative epithelial changes of residual glands, and focal pneumatosis in the submucosa. There was evidence of bacterial overgrowth in the lumen. These findings were compatible with the clinical diagnosis of NEC.
Figure 2.
Abdonimal radiograph on presentation with necrotizing enterocolitis. Notable features include generalized bowel loop distention, intestinal intramural pneumatosis, portal venous air, and pneumoperitoneum.
DISCUSSION
We describe a delayed presentation of NEC associated with octreotide use in an infant with HI on a lower octreotide doses than previously described in cases of NEC associated with octreotide. This case describes a four month old infant who developed NEC after 2 months of therapy at a relatively low dose (8 μg/kg/day) of octreotide. All other reported cases of NEC associated with octreotide use in infants with HI occurred in neonates younger than 1 month of age and within 15 days of commencing octreotide therapy at doses of 15 - 27 mcg/kg/day [15, 16].
Octreotide increases splanchnic vascular resistance and reduces splanchnic and portal venous blood flow[17]. These effects have been used therapeutically in other settings: reduced splanchnic blood flow may reduce chyle flow in children with refractory chylothoraces[18, 19]; and reduced portal venous flow may be beneficial in the treatment of esophageal variceal hemorrhage in hepatic cirrhosis[20]. Although the vasoconstrictive effects of octreotide alone may be insufficient to cause significant splanchnic ischemia, it may represent an additional insult in the infant at risk. This has been seen in neonates treated with octreotide for chylothoraces, where necrotizing ileus has been reported with coexisting total anomalous pulmonary venous connection[21], and NEC was reported with coexisting coarctation of the aorta[22].
The additive effects of multiple risk factors for NEC is particularly relevant in infants with severe, diazoxide unresponsive HI and may have played a role in this case. These may be broadly be categorized as causes of bowel ischemia[23] or alterations in the intestinal flora (Table 1).
Table 1.
Potential multifactorial etiologies of necrotizing enterocolitis in children with hyperinsulinism treated with octreotide
Reduced bowel perfusion can compromise mucosal integrity both as a result of the original insult and secondary reperfusion injury and the small bowel is at greater risk than the colon[24]. There are numerous factors that can put the infant with HI at risk of this. Firstly, these infants require the central venous provision of concentrated dextrose solutions, and umbilical venous access can be critical in the early management. Critical perinatal illness may necessitate umbilical arterial catheter placement for blood sampling and cardiovascular monitoring. The infant described in this case had umbilical venous and arterial access in place for the first week of life, and this vascular access is associated with an increased risk of NEC[25, 26]. These procedures may confer additional risk in children with HI due to disordered coagulation. Hyperinsulinemia has been associated with impaired fibrinolysis[28], and infants with HI are at high risk of thrombosis[29]. which may be due to a combination of this effect and their requirement for central venous access.
Other factors that may affect bowel perfusion include red blood cell transfusions[30], although some studies refute the association of red blood cell transfusions and NEC[31]. The mechanism for this link is unclear and recent studies suggest that this may be related to bowel inflammation[32] rather than ischemia[33]. Intraabdominal surgery can affect bowel motility via neural mechanisms and through triggering an inflammatory cascade, both of which could possibly affect bowel perfusion[27]. Postoperative opiate medications can also increase splanchnic blood flow[34] and alter bowel motility, although whether or not there is a direct link between this and the development of NEC is not known.
Bowel mucosa can also be compromised by direct local effects by hyperosmolar feeds, or alteration of the gut flora. In this case, 20% enteral dextrose was required to maintain normoglycemia [1]. This solution has an osmolarity of 1010 mOsm/L, over twice that of most commonly used infant formulas [35]. While the evidence linking hyperosmolar feeds with increased necrotizing enterocolitis is based on a small randomized controlled trial including 16 infants [36, 37], it is common practice in neonatology to avoid hyperosmolar feeds and medications in high risk infants [38]. In addition to being hyperosmolar, it is also possible that the continuous delivery of enteral 20% dextrose will unfavorably alter the local gut flora and further increase risk.
Increased gastric pH may increase bacterial colonization and risk of necrotizing enterocolitis [39], and an association of ranitidine with increased risk has been demonstrated [40, 41]. Reflux and feeding issues are common in this population, and ranitidine is commonly prescribed for these infants. The infant described here had significant reflux and feeding difficulties, and was treated with ranitidine for almost three months.
The gut flora is a therapeutic target for infants at high risk of NEC. Prophylactic proobiotics have shown a reduction in the development of NEC[42]. Postulated mechanisms include blocking the migration of pathogenic bacteria across the intestinal mucosa, the competitive exclusion of these bacteria, or the alteration in local immune response in the inflammatory cascade. Enteral antibiotics reduce the intestinal bacterial load, and are associated with a reduction in bowel ischemic lesions in the animal model[43]. However, short term antibiotic use have longstanding effects on the infant's bowel microbiota[44] and it is now known if this disruption may represent a risk factor in infants predisposed to the development of NEC. The infant in this case had three short courses of antibiotic treatment.
In this patient, octreotide use is likely to have contributed to the multifactorial risk factors for NEC of bowel ischemia, inflammation and altered gut flora. We advise that consideration is given to stewardship of each of these factors in these patients, but acknowledge that the vast majority are unavoidable risks. Breast milk is a known protective factor for the development of NEC[45], and should be promoted. Probiotics may have a role in the prevention of NEC, but have not been studied in this population. We advise close monitoring of these high risk infants for the the development of NEC and, in addition to the previously reported early risk of NEC, continuation of this close monitoring beyond the previously reported high risk first few weeks of octreotide use.
Established Facts.
Medical management of diazoxide unresponsive congenital hyperinsulinism includes somatostatin analogues, but many children require pancreatectomy to alleviate risk of severe hypoglycemia.
Necrotizing enterocolitis has been described as a complication seen within two weeks of starting octreotide treatment in these infants.
Novel Insights In addition to the recognized early risk of necrotizing enterocolitis in infants treated with octreotide, delayed presentation can occur. We advise ongoing observation for the development of this complication.
Infants with diazoxide unresponsive hyperinsulinism have multiple risk factors for necrotizing enterocolitis, and these should be considered when contemplating and using octreotide..
Acknowledgments
Funding Sources: Dr. Hawkes is supported by a PhD grant by the National Children's Research Centre, Dublin, Ireland. Dr. De Leon is supported by grant 5R01DK098517 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- 18F-DOPA PET
Fluorine-18-L-dihydroxyphenylalanine positron emission tomography
- BWS
Beckwith Wiedemann Syndrome
- HI
Hyperinsulinism
- NEC
Necrotizing Enterocolitis
Footnotes
Society Memberships
Drs. Colin P. Hawkes, Andrew A. Palladino and Diva D. de León are members of the Pediatric Endocrine Society
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Disclosure Summary: CPH, NSA, AAP and DDDL have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Palladino AA, Stanley CA. A specialized team approach to diagnosis and medical versus surgical treatment of infants with congenital hyperinsulinism. Seminars in Pediatric Surgery. 2011;20:32–37. doi: 10.1053/j.sempedsurg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Lord K, De Leon DD. Monogenic hyperinsulinemic hypoglycemia: Current insights into the pathogenesis and management. International journal of pediatric endocrinology. 2013;2013:3. doi: 10.1186/1687-9856-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord K, Dzata E, Snider KE, Gallagher PR, De Leon DD. Clinical presentation and management of children with diffuse and focal hyperinsulinism: A review of 223 cases. J Clin Endocrinol Metab. 2013;98:E1786–1789. doi: 10.1210/jc.2013-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalish JM, Boodhansingh KE, Bhatti TR, Ganguly A, Conlin LK, Becker SA, Givler S, Mighion L, Palladino AA, Adzick NS, De Leon DD, Stanley CA, Deardorff MA. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and beckwith-wiedemann syndrome. J Med Genet. 2015 doi: 10.1136/jmedgenet-2015-103394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seino S, Miki T. Physiological and pathophysiological roles of atp-sensitive k+ channels. Progress in biophysics and molecular biology. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 6.De Leon DD, Stanley CA. Pathophysiology of diffuse atp-sensitive potassium channel hyperinsulinism. In: De Leon DD, Stanley CA, editors. Monogenic hyperinsulinemic hypoglycemia disorders. Vol. 21. Karger; Basel: 2012. pp. 18–29. [Google Scholar]
- 7.Drash A, Kenny F, Field J, Blizzard R, Langs H, Wolff F. The therapeutic application of diazoxide in pediatric hypoglycemic states. Annals of the New York Academy of Sciences. 1968;150:337–355. doi: 10.1111/j.1749-6632.1968.tb19059.x. [DOI] [PubMed] [Google Scholar]
- 8.Senniappan S, Alexandrescu S, Tatevian N, Shah P, Arya V, Flanagan S, Ellard S, Rampling D, Ashworth M, Brown RE, Hussain K. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med. 2014;370:1131–1137. doi: 10.1056/NEJMoa1310967. [DOI] [PubMed] [Google Scholar]
- 9.Bas F, Darendeliler F, Demirkol D, Bundak R, Saka N, Gunoz H. Successful therapy with calcium channel blocker (nifedipine) in persistent neonatal hyperinsulinemic hypoglycemia of infancy. Journal of pediatric endocrinology & metabolism : JPEM. 1999;12:873–878. doi: 10.1515/jpem.1999.12.6.873. [DOI] [PubMed] [Google Scholar]
- 10.Neylon OM, Moran MM, Pellicano A, Nightingale M, O'Connell MA. Successful subcutaneous glucagon use for persistent hypoglycaemia in congenital hyperinsulinism. Journal of pediatric endocrinology & metabolism : JPEM. 2013;26:1157–1161. doi: 10.1515/jpem-2013-0115. [DOI] [PubMed] [Google Scholar]
- 11.D'Alessio DA, Sieber C, Beglinger C, Ensinck JW. A physiologic role for somatostatin 28 as a regulator of insulin secretion. The Journal of clinical investigation. 1989;84:857–862. doi: 10.1172/JCI114246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper AM, Braatvedt GD, Qamar MI, Brown H, Thomas DM, Halliwell M, Read AE, Corrall RJ. Fasting and post-prandial splanchnic blood flow is reduced by a somatostatin analogue (octreotide) in man. Clinical science. 1991;81:169–175. doi: 10.1042/cs0810169. [DOI] [PubMed] [Google Scholar]
- 13.Schiedermaier P, Goke B, Sauerbruch T. Effects of different octreotide dosages on splanchnic hemodynamics and glucagon in patients with tips. The American journal of gastroenterology. 2001;96:2218–2224. doi: 10.1111/j.1572-0241.2001.03960.x. [DOI] [PubMed] [Google Scholar]
- 14.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: A ten-year experience. J Pediatr. 1990;117:S6–13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 15.Laje P, Halaby L, Adzick NS, Stanley CA. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes. 2010;11:142–147. doi: 10.1111/j.1399-5448.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 16.Reck-Burneo CA, Parekh A, Velcek FT. Is octreotide a risk factor in necrotizing enterocolitis? J Pediatr Surg. 2008;43:1209–1210. doi: 10.1016/j.jpedsurg.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 17.Schiedermaier P, Brensing KA, Goke B, Schatzle T, Sauerbruch T. Effects of different octreotide dosages on splanchnic hemodynamics and glucagon in healthy volunteers. Digestion. 1999;60:132–140. doi: 10.1159/000007638. [DOI] [PubMed] [Google Scholar]
- 18.Das A, Shah PS. Octreotide for the treatment of chylothorax in neonates. Cochrane Database Syst Rev. 2010:CD006388. doi: 10.1002/14651858.CD006388.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Roehr CC, Jung A, Proquitte H, Blankenstein O, Hammer H, Lakhoo K, Wauer RR. Somatostatin or octreotide as treatment options for chylothorax in young children: A systematic review. Intensive Care Med. 2006;32:650–657. doi: 10.1007/s00134-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD, Practice Guidelines Committee of American Association for Study of Liver D, Practice Parameters Committee of American College of G Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. The American journal of gastroenterology. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo T, Matsumoto M, Sugita T, Nishizawa J, Matsuyama K, Tokuda Y, Yoshida K. Treatment of persistent chylothorax with somatostatin. Ann Thorac Surg. 2003;76:340–341. doi: 10.1016/s0003-4975(02)04397-7. author reply 341-342. [DOI] [PubMed] [Google Scholar]
- 22.Mohseni-Bod H, Macrae D, Slavik Z. Somatostatin analog (octreotide) in management of neonatal postoperative chylothorax: Is it safe? Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2004;5:356–357. doi: 10.1097/01.pcc.0000123552.36127.22. [DOI] [PubMed] [Google Scholar]
- 23.Bolisetty S, Lui K, Oei J, Wojtulewicz J. A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr. 2000;89:1226–1230. doi: 10.1080/080352500750027619. [DOI] [PubMed] [Google Scholar]
- 24.Hundscheid IHMD, Grootjans JMDP, Lenaerts KP, Schellekens DHMD, Derikx JPMDP, Boonen BTB, von Meyenfeldt MFMDP, Beets GLMDP, Buurman WAP, Dejong CHMDP. The human colon is more resistant to ischemia-reperfusion-induced tissue damage than the small intestine: An observational study. Annals of surgery. 2015;262:304–311. doi: 10.1097/SLA.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 25.Barrington KJ. Umbilical artery catheters in the newborn: Effects of position of the catheter tip. Cochrane Database Syst Rev. 2000:CD000505. doi: 10.1002/14651858.CD000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oelberg DG, Baker A, Quast D, Worley L. Impact of umbilical catheterization on morbidity and mortality in extremely premature newborns. J Neonatal Perinatal Med. 2014;7:13–19. doi: 10.3233/NPM-1475313. [DOI] [PubMed] [Google Scholar]
- 27.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 28.Stegenga ME, van der Crabben SN, Levi M, de Vos AF, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55:1807–1812. doi: 10.2337/db05-1543. [DOI] [PubMed] [Google Scholar]
- 29.Al-Agha AE, Ahmad IA. Characterization of the abcc8 gene mutation and phenotype in patients with congenital hyperinsulinism in western saudi arabia. Saudi medical journal. 2013;34:1002–1006. [PubMed] [Google Scholar]
- 30.AlFaleh K, Al-Jebreen A, Baqays A, Al-Hallali A, Bedaiwi K, Al-Balahi N, AlGhamdi A, AlKharfi T, Alzahem A. Association of packed red blood cell transfusion and necrotizing enterocolitis in very low birth weight infants. J Neonatal Perinatal Med. 2014;7:193–198. doi: 10.3233/NPM-14814048. [DOI] [PubMed] [Google Scholar]
- 31.Wallenstein MB, Arain YH, Birnie KL, Andrews J, Palma JP, Benitz WE, Chock VY. Red blood cell transfusion is not associated with necrotizing enterocolitis: A review of consecutive transfusions in a tertiary neonatal intensive care unit. J Pediatr. 2014;165:678–682. doi: 10.1016/j.jpeds.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho TT, Groer MW, Luciano AA, Schwartz A, Ji M, Miladinovic BS, Maheshwari A, Ashmeade TL. Red blood cell transfusions increase fecal calprotectin levels in premature infants. Journal of perinatology : official journal of the California Perinatal Association. 2015;35:837–841. doi: 10.1038/jp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White L, Said M, Rais-Bahrami K. Monitoring mesenteric tissue oxygenation with near-infrared spectroscopy during packed red blood cell transfusion in preterm infants. J Neonatal Perinatal Med. 2015;8:157–163. doi: 10.3233/NPM-15814090. [DOI] [PubMed] [Google Scholar]
- 34.Leaman DM, Levenson L, Zelis R, Shiroff R. Effect of morphine on splanchnic blood flow. Br Heart J. 1978;40:569–571. doi: 10.1136/hrt.40.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson F, Johnson MJ, Leaf AA. Milk osmolality: Does it matter? Archives of disease in childhood Fetal and neonatal edition. 2013;98:F166–169. doi: 10.1136/adc.2011.300492. [DOI] [PubMed] [Google Scholar]
- 36.Book LS, Herbst JJ, Atherton SO, Jung AL. Necrotizing enterocolitis in low-birth-weight infants fed an elemental formula. J Pediatr. 1975;87:602–605. doi: 10.1016/s0022-3476(75)80835-3. [DOI] [PubMed] [Google Scholar]
- 37.Willis DM, Chabot J, Radde IC, Chance GW. Unsuspected hyperosmolality of oral solutions contributing to necrotizing enterocolitis in very-low-birth-weight infants. Pediatrics. 1977;60:535–538. [PubMed] [Google Scholar]
- 38.Srinivasan L, Bokiniec R, King C, Weaver G, Edwards AD. Increased osmolality of breast milk with therapeutic additives. Archives of disease in childhood Fetal and neonatal edition. 2004;89:F514–517. doi: 10.1136/adc.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrion V, Egan EA. Prevention of neonatal necrotizing enterocolitis. Journal of pediatric gastroenterology and nutrition. 1990;11:317–323. doi: 10.1097/00005176-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, Phelps DL, National Institute of Child H, Human Development Neonatal Research N Association of h2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–142. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 41.Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, Messina F, Paludetto R, Canani RB. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129:e40–45. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]
- 42.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011:CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Jensen ML, Thymann T, Cilieborg MS, Lykke M, Molbak L, Jensen BB, Schmidt M, Kelly D, Mulder I, Burrin DG, Sangild PT. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. American journal of physiology Gastrointestinal and liver physiology. 2014;306:G59–71. doi: 10.1152/ajpgi.00213.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fouhy F, Guinane CM, Hussey S, Wall R, Ryan CA, Dempsey EM, Murphy B, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56:5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]