Abstract
Background and aims
To determine whether epicardial (EAT) and paracardial adipose tissue (PAT) volume and attenuation are associated with high-risk coronary plaque features.
Methods
In subjects with suspected acute coronary syndrome (ACS) enrolled in the ROMICAT II trial, EAT and PAT volumes indexed to body surface area (BSA) and attenuation were measured on noncontrast coronary artery calcium score (CACS) CT. High-risk plaque features (napkin-ring sign, positive remodeling, low density plaque, spotty calcium) and stenosis were assessed on coronary CT angiography (CTA). The association of EAT and PAT volume and attenuation with high-risk plaque and whether this was independent of clinical risk assessment, CACS and significant coronary artery disease (CAD) was determined.
Results
Of 467 (mean 54±8 yrs, 53% male) with CACS and CTA, 167 (36%) had high-risk plaque features. Those with high-risk plaque had significantly higher indexed EAT (median 59 (Q1–Q3:45–75) cc/m2 vs. 49 (35–65) cc/m2, p <0.001) and PAT volume (median:51 (36–73) cc/m2 vs. 33 (22–52) cc/m2, p <0.001). Higher indexed EAT volume was associated with high-risk plaque [univariate OR 1.02 (95%-CI:1.01 – 1.03) per cc/m2 of EAT, p <0.001], which remained significant [univariate OR 1.04 (95%-CI:1.00–1.08) per cc/m2 of EAT, p=0.040] after adjustment for risk factors, CACS, and stenosis ≥50%. Higher indexed PAT volume was associated with high-risk plaque in univariate analysis [OR 1.02 (1.01 – 1.03) per cc/m2 of PAT, p <0.001], though this was not significant in multivariate analysis. At a threshold of >62.3 cc/m2, EAT volume was associated with high-risk plaque [univariate OR 2.50 (95%-CI:1.69–3.72), p <0.001)], which remained significant [OR 1.83 (95%-CI:1.10–3.05), p=0.020] after adjustment. Subjects with high-risk plaque had lower mean attenuation EAT (−88.1 vs. −86.9 HU, p=0.008) and PAT (−106 vs. −103 HU, p <0.001), though this was not significant in multivariable analysis.
Conclusions
Greater volumes of EAT are associated with high-risk plaque independent of risk factors, CACS and obstructive CAD. This observation supports possible local influence of EAT on development of high-risk coronary plaque.
Keywords: Coronary CT Angiography, high-risk coronary plaque, coronary atherosclerosis, epicardial adipose tissue, paracardial adipose tissue
Introduction
Epicardial adipose tissue (EAT), defined as fat within the pericardial sac, is a metabolically and immunologically active fat depot implicated in atherogenesis. EAT encases the coronary arteries without an intervening fascial barrier1. EAT also shares common innervation and blood supply with the coronary arterial wall. Due to this unique anatomic relationship, several authors have suggested that inflamed EAT may release cytokines with a local paracrine proatherogenic effect on the underlying coronary arteries2, 3. By this mechanism EAT may be the link between dysregulated metabolism, inflammation of the arterial wall and subsequent atherogenesis. Paracardial adipose tissue (PAT) lies outside the external surface of the pericardium, with separate blood supply from the pericardiophrenic artery4. PAT may also potentiate atherogenesis through endocrine mediators, though this has not yet been clarified.
Cardiac computed tomography allows for both accurate quantification of EAT/PAT5–7 and identification of high-risk coronary plaque features. High-risk plaque features include positive remodeling, low-density plaque <30 Hounsfield Units (HU), spotty calcification, and napkin ring sign and are associated with future adverse cardiovascular events8–13. The volume of EAT has been linked to coronary risk factors, coronary artery calcification, plaques causing significant (>50%) stenosis, and adverse events6, 14–19. Furthermore, the “quality” of fat can also be evaluated via CT attenuation (density) measurements. While lower CT density of visceral and subcutaneous abdominal fat have been linked to greater extent of coronary artery calcium20 and cardiovascular events21 in the Framingham Heart Study, the relationship between epicardial and paracardial fat attenuation and high-risk plaque features is not well understood.
Similarly, the evidence linking the quantity of epicardial and paracardial fat depots with high-risk coronary plaque features is limited. The aim of this study was to investigate the association between the quantity and density of EAT and PAT and high-risk coronary plaque seen on coronary CT angiography (CTA). Furthermore, we examined whether this association is independent of traditional cardiovascular risk factors, coronary artery calcification, and coronary artery stenosis.
Patients and methods
Study population
The study cohort consisted of subjects randomized to CTA in the ROMICAT II trial. The trial was designed to compare CTA versus standard evaluation for suspected acute coronary syndrome (ACS) in the emergency department. A detailed description of the patient population and CT protocol was recently reported22. Briefly, 1,000 patients presenting to the emergency department of nine hospitals in the United States with chest pain, clinical suspicion of acute coronary syndrome, and no known history of CAD were enrolled between April 2010 and January 2012. Of these, 501 were randomized to CT and had both prospectively ECG-triggered non-contrast coronary artery calcium scoring (CACS) CT to evaluate for coronary artery calcium and contrast-enhanced retrospectively ECG-gated or prospectively ECG-triggered CTA to evaluate for coronary artery stenosis (Supplemental Figure 1). CT images were acquired using a 64 multi-detector row or more recent scanner23. Baseline clinical characteristics, medical history and cardiovascular risk factors (arterial hypertension, hyperlipidemia, diabetes mellitus, and former or current smoking) were collected22. Body mass index (BMI) was calculated as body weight divided by the square of height. Body surface area (BSA) was calculated using the Mosteller formula.
Previous investigations of the trial’s CTA cohort found that those with high-risk coronary plaque on CT were more likely to have ACS13, and that non-alcoholic fatty liver disease is associated with high-risk plaque24. There have been no previous investigations of epicardial or paracardial fat in this cohort. All study participants provided written consent, and local institutional review boards approved the study.
Noncontrast CT assessment of epicardial and paracardial adipose tissue
Epicardial adipose tissue (EAT) and paracardial adipose tissue (PAT) were measured on the non-contrast CACS images. For the purposes of this study, epicardial adipose tissue (EAT) was defined as fat within the pericardial sac. Paracardial adipose tissue (PAT) was defined as fat along the external surface of the pericardium1. We defined total adipose tissue (TAT) as the sum of EAT and PAT. It is worth acknowledging another commonly used term for fat around the heart – pericardial adipose tissue. Some authors use pericardial adipose tissue synonymously with EAT25; others define pericardial adipose tissue as the sum of EAT and PAT4. In light of this inconsistency, we elected to use the above definitions of EAT and PAT.
Measurement of EAT and PAT volume and mean attenuation was performed using the non-contrast CACS CTs on a dedicated offline workstation (Volume Viewer, Siemens Medical Solutions, Forchheim, Germany) using a semi-automated technique divided between a team of two readers (at least 1 year of experience in cardiac CT) blinded to the results of the CTA. For EAT, regions of interest (ROI) were manually drawn tracing the contour of the pericardium at 1 cm intervals, starting at the mid-level of the right pulmonary artery and extending inferiorly to the diaphragm. Interpolated contours were manually adjusted as necessary16. For PAT, ROIs were drawn around the adipose tissue outside the pericardial sac. As suggested in previous literature, attenuation thresholds of −195 to −45 HU were applied to identify voxels containing adipose tissue14. Volume and mean attenuation of adipose tissue was recorded in cubic centimeters (cc) and Hounsfield units (HU), respectively. Twenty-five randomly chosen subjects’ EAT and PAT measurements were analyzed by both readers to assess inter-observer variability. (Figure 1)
Figure 1. Non-Contrast CT measurements of epicardial and paracardial adipose tissue (EAT and PAT).
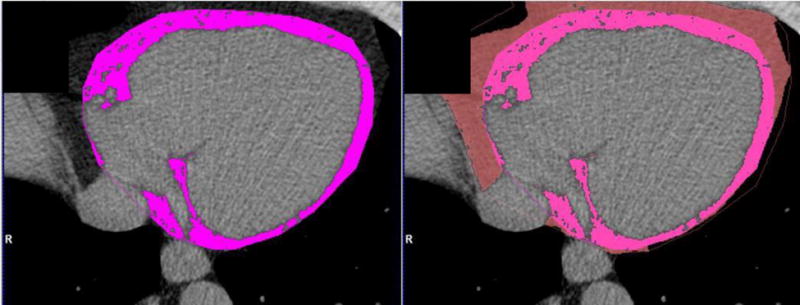
Epicardial adipose tissue within the pericardium (left) and paracardial adipose tissue outside the pericardium (right) are traced manually. Voxels with attenuation between −195 and −45 HU (fat tissue) are marked in pink for EAT and salmon for PAT.
CTA assessment of high-risk coronary plaque (primary outcome)
The contrast-enhanced CTA images were divided between a second team of three core laboratory CTA readers (at least 5 years of experience in cardiac CTA and level III training), who performed the image analysis of coronary plaque and high-risk plaque features on a dedicated cardiac workstation (TeraRecon, San Mateo, CA). The CTA analysis was performed on a per coronary segment basis using the model of the Society of Cardiovascular Computed Tomography26. For each coronary segment, the reader determined whether the image quality was sufficient to evaluate for the presence of stenosis and plaque with confidence. Coronary segments that were assessed as non-diagnostic in image quality were treated as non-informative for the purpose of the analysis.
The CTA readers assessed each evaluable coronary segment for noncalcified plaque, calcified plaque, and stenosis ≥50% as previously described8. The primary outcome was the presence of one or more high-risk plaque features which were defined as positive remodeling, low Hounsfield unit (HU) plaque, napkin-ring sign and spotty calcium (Supplemental Figure 2). Positive remodeling was analyzed on long and short axis multiplanar reformatted images, and defined by a ratio of at least 1.1 of the maximal outer diameter of the vessel at the plaque divided by the average of the diameter of proximal and distal normal reference vessels27. If a low density non-calcified plaque was noted, readers placed three region-of-interest measurements (approximately 0.5 – 1.0 mm2) in the non-calcified low density, with low HU plaque defined as a mean HU number <30 HU28. The napkin-ring sign was defined as ring of peripheral higher attenuation surrounding a central core of low attenuation in a non-calcified coronary plaque29, 30. Spotty calcium was defined by calcified plaque with measuring <3 mm, extending <1.5 times the vessel diameter along the long axis of the vessel, and occupying two thirds of the circumference of the vessel31.
Statistical analysis
All statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas). Continuous data are presented as mean ± standard deviation or median with interquartile ranges. Categorical and ordinal variables are presented as absolute and relative frequencies. Comparisons between groups were performed with the use of an unpaired Student’s t-test or Wilcoxon rank-sum test for continuous variables, Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables. Inter-observer variability for volume and mean attenuation was assessed using the intra-class correlation coefficient (ICC).
To determine the association between high-risk plaque features and (continous) EAT volume, we performed univariable (i.e. unadjusted) and multivariable (i.e. adjusted) logistic regression analyses. The models included the presence of high-risk plaque as dependent variable, absolute and BSA-indexed fat depot volume, fat depot attenuation, age, sex, number of cardiovascular risk factors, (log) calcium score, and obstructive CAD (≥50% stenosis) as independent variables. To address any possible non-linear relationship, i.e. convex or concave, we also added a quadratic variable (Squared Indexed Epicardial Fat Volume/Squared Indexed Paracardial Fat Volume) to each regression model.
In order to provide a potentially clinically useful threshold for “high” EAT volume, an optimal cutoff value for EAT regarding high-risk plaque features was then calculated using the maximum sum of sensitivity and specificity in ROC analysis. The association between high-risk plaque features and the (binary) EAT measurement based on the cutoff value was then performed in univariate and stepwise multivariate logistic regression analyses, excluding variables with p >0.10. The above analyses were then repeated for PAT. For all analyses, a 2-tailed p value <0.05 was required to reject the null hypothesis.
Results
Study population and high-risk plaque features
Of the 501 subjects randomized to the CTA arm of the ROMICAT II trial, 28 were excluded who did not undergo CT, 1 due to insufficient image quality of the CTA, and 5 who did not undergo non-contrast CT (Supplemental Figure 1). 467 subjects (mean age 54 ± 8 yrs, 47 % female) who underwent both contrast CTA and non-contrast CACS imaging with diagnostic quality were included in the analysis. Baseline characteristics are described in Table 1.
Table 1.
Clinical Characteristics of Study Patients
| Total (n = 467) |
High-risk Plaque (n = 167) |
No High-risk Plaque (n = 300) |
p value | |
|---|---|---|---|---|
| Age (mean ± SD), years | 53.9 ± 8.0 | 56.8 ± 8.4 | 52.3 ± 7.3 | <0.001 |
| Female sex, n (%) | 221 (47.3) | 49 (29.3) | 172 (57.3) | <0.001 |
| Body Mass Index (mean ± SD), kg/m2 | 29.4 ± 5.1 | 30.0 ± 4.7 | 29.1 ± 5.3 | 0.046 |
| Cardiovascular risk factors, n (%) | ||||
| Hypertension | 249 (53.3) | 105 (62.9) | 144 (48.0) | 0.003 |
| Diabetes mellitus | 78 (16.7) | 36 (21.6) | 42 (14.0) | 0.039 |
| Dyslipidemia | 212 (45.4) | 98 (58.7) | 114 (38.0) | <0.001 |
| Former or current smoker | 233 (49.9) | 98 (58.7) | 135 (45.0) | 0.005 |
| Family history of premature CAD | 130 (27.8) | 38 (22.8) | 92 (30.7) | 0.085 |
| Number of cardiovascular risk factors, % | <0.001 | |||
| 0 or 1 | 170 (36.4) | 43 (25.8) | 128 (42.3) | |
| 2 or 3 | 253 (54.2) | 98 (58.7) | 155 (51.7) | |
| ≥ 4 | 44 (9.4) | 26 (15.6) | 18 (6.0) |
On CTA, 36% (167/467) had high-risk plaque features which were more prevalent in male (p <0.001), older (p <0.001) and obese (p=0.046) subjects. The frequencies of the individual high-risk plaque features among the subjects with high-risk plaque were as following: Napkin-ring sign 15.0%, positive remodeling 32.3%, low HU plaque 23.4% and spotty calcium 91.0%. The overall number of risk factors was greater among subjects with high-risk plaque features (p <0.001), with a higher rate of hypertension (p=0.003), diabetes (p=0.039), dyslipidemia (p <0.001) and smoking (p=0.005).
CAD was present in 260 (56%), with non-obstructive CAD (<50% stenosis) in 216 (46%) and obstructive CAD (≥50% stenosis) in 44 (9%) subjects (Table 2). Coronary artery calcium was present in 217 (46%) subjects. Stenosis and calcium were more prevalent in subjects with high-risk plaque (p <0.001).
Table 2.
Coronary Computed Tomography Angiography Characteristics of Patients Stratified According to Presence of Any High-risk Plaque
| Total (n = 467) |
High-risk Plaque (n = 167) |
No High-risk Plaque (n = 300) |
p value | |
|---|---|---|---|---|
| Coronary Artery Disease (CAD) category, n (%) | ||||
| No CAD (0% Stenosis) | 207 (44.3) | 0 (0) | 207 (69.0) | — |
| Mild CAD (1–49% Stenosis) | 216 (46.3) | 127 (76.1) | 89 (29.7) | <0.001 |
| Significant CAD (≥ 50% Stenosis) | 44 (9.4) | 40 (24.0) | 4 (1.3) | <0.001 |
| Coronary Artery Calcium Score Category, n (%) | <0.001 | |||
| CACS 0 | 250 (53.5) | 14 (8.4) | 236 (78.7) | |
| CACS 1 – 100 | 137 (29.3) | 93 (55.7) | 44 (14.7) | |
| CACS 101 – 300 | 42 (9.0) | 30 (18.0) | 12 (4.0) | |
| CACS > 300 | 38 (8.1) | 30 (18.0) | 8 (2.7) | |
| Epicardial Fat Measurements, median (iqr) | ||||
| Fat Volume [cc] | 108.5 (76.4 – 140.6) |
123.0 (93.4 – 155.8) |
97.9 (68.2 – 126.5) |
<0.001 |
| Indexed Fat Volume [cc/m2] | 53.7 (37.8 – 69.4) |
59.2 (45.2 – 75.2) |
49.2 (35.2 – 64.9) |
<0.001 |
| Mean Attenuation [HU] | −87.3 (−90.2 – −85.0) |
−88.1 (−90.9 – −85.7) |
−86.9 (−89.9 – −84.7) |
0.008 |
| Paracardial Fat Measurements, median (iqr) | ||||
| Fat Volume [cc] | 79.0 (46.2 – 128.3) |
106.8 (68.9 – 157.1) |
67.8 (42.1 – 103.1) |
<0.001 |
| Indexed Fat Volume [cc/m2] | 40.4 (24.4 – 60.6) |
50.5 (36.1 – 73.3) |
33.4 (22.1 – 52.2) |
<0.001 |
| Mean Attenuation [HU] | −104.8 (−108.9 – −100.5) |
−106.4 (−110.6 – −102.7) |
−103.4 (−107.5 – −99.6) |
<0.001 |
| Total Fat Measurementsa, median (iqr) | ||||
| Fat Volume [cc] | 189.3 (129.1 – 261.2) |
232.8 (169.8 – 324.2) |
166.8 (113.8 – 242.1) |
<0.001 |
| Indexed Fat Volume [cc/m2] | 96.6 (68.1 – 127.0) |
113.4 (86.5 – 149.6) |
84.9 (60.6 – 118.9) |
<0.001 |
| Mean Attenuation [HU] | −94.9 (−98.6 – −91.9) |
−96.9 (−99.7 – −93.8) |
−93.8 (−97.5 – −91.0) |
<0.001 |
CAD = Coronary artery disease; CACS = Coronary artery calcium score;
Total fat = epicardial + paracardial fat
Inter-rater agreement
EAT and PAT measurements were highly reproducible between the two EAT/PAT readers, with excellent inter-rater agreement for EAT volume (ICC = 0.994), PAT volume (ICC = 0.991), EAT mean attenuation (ICC = 0.849) and PAT mean attenuation (ICC = 0.988) (Supplemental Figure 3).
Fat volume and density
Subjects with high-risk plaque showed a higher median EAT volume than those without high-risk plaque [123 (IQR 93 – 156) cc vs. 98 (IQR 68–127) cc, p <0.001]. PAT volume was significantly higher in subjects with high-risk plaque than in those without [107 (IQR 69 – 157) cc vs. 68 (IQR 42 – 103) cc, p <0.001] (Table 2).
Figure 4-2 illustrates EAT volume stratified by BMI categories and the presence of high-risk plaque features. Subjects with high-risk plaque had significantly higher EAT volume for all four standard BMI categories including subjects with a BMI <25 (all with p ≤0.05). Similar significant differences across BMI categories were observed for PAT (Supplemental Figure 4). We then indexed EAT and PAT volumes to body surface area to adjust for metabolic mass independent of body habitus. Indexed EAT and PAT volumes remained significantly higher in subjects with high-risk plaque (both p <0.001).
In subjects with high-risk plaque, the median EAT density was lower by one Hounsfield unit compared to subjects without high-risk plaque [−88 HU vs. −87 HU, p=0.008]. For PAT, the median density was lower by 3 HU [−106 vs. −103 HU, p <0.001] for subjects with high-risk plaque (Table 2).
Association of EAT and PAT volume with high-risk plaque – Continuous approach
With EAT treated as a continuous variable, higher indexed EAT volume was associated with high-risk plaque [univariate OR 1.02 (95%-CI:1.01 – 1.03) per cc/m2 of EAT, p <0.001], which remained significant (multivariate OR 1.04 (95%-CI:1.00–1.08) per cc/m2 of EAT, p=0.040) after adjustment for the number of risk factors, CACS, and stenosis ≥50% (Table 3). Higher indexed PAT volume was associated with high-risk plaque in univariate analysis [OR 1.02 (1.01 – 1.03) per cc/m2 of PAT, p <0.001], though this was not significant in multivariate regression analysis [OR 1.01 (0.99 – 1.044), p=0.22).
Table 3.
Unadjusted and Adjusted Associations Between Continuous Epicardial and Paracardial Fat Volume, Demographic/Clinical Risk Factors, and High-risk Plaque Features
| Dependent Variable: Any High-risk Plaque Feature Independent Variables |
Univariable Analysis | Multivariable Analysis Model: EAT (indexed volume) | Multivariable Analysis Model: PAT (indexed volume) | |||
|---|---|---|---|---|---|---|
| Unadjusted OR | p value | Adjusted OR | p value | Adjusted OR | p value | |
| Indexed Epicardial Fat Volume (continuous, cc/m2) | 1.02 (1.01–1.03) | <0.001 | 1.04 (1.00–1.08) | 0.040 | NA | NA |
| Squared Indexed Epicardial Fat Volume | 1.00 (1.00–1.00) | 0.001 | 1.00 (1.00–1.00) | 0.031 | NA | NA |
| Indexed Paracardial Fat Volume (continuous, cc/m2) | 1.02 (1.01–1.03) | <0.001 | NA | NA | 1.01 (0.99–1.04) | 0.215 |
| Squared Indexed Paracardial Fat Volume | 1.00 (1.00–1.00) | <0.001 | NA | NA | 1.00 (1.00–1.00) | 0.065 |
| Age (years) | 1.08 (1.05–1.10) | <0.001 | 1.03 (1.00–1.07) | 0.074 | 1.04 (1.00–1.08) | 0.030 |
| Female | 0.31 (0.21–0.46) | <0.001 | 0.36 (0.21–0.63) | 0.001 | 0.34 (0.19–0.63) | <0.001 |
| No. of cardiovascular risk factorsa | 1.48 (1.24–1.76) | <0.001 | 1.05 (0.84–1.32) | 0.655 | 1.09 (0.86–1.37) | 0.478 |
| Coronary artery calcium score (log) | 1.91 (1.71–2.13) | <0.001 | 1.69 (1.49–1.93)) | <0.001 | 1.71 (1.50–1.94) | <0.001 |
| Significant CAD (≥50% Stenosis) | 23.31 (8.17–66.51) | <0.001 | 5.66 (1.73–18.51) | 0.004 | 4.97 (1.53–16.20) | 0.008 |
The summary is based on all 467 observations; 95% Confidence Intervals in brackets
Risk factors include arterial hypertension, diabetes mellitus, dyslipidemia, former or current smoker, family history of CAD CAD = Coronary artery disease; OR = Odds Ratio, NA = not applicable
Association of EAT and PAT volume with high-risk plaque – Threshold approach
The optimal threshold which maximized the sensitivity and specificity of indexed EAT volume for prediction of high-risk plaque was 62.3 cc/m2, with a sensitivity of 48.5% (95% CI 40.7–56.3%) and a specificity of 72.7% (95% CI 67.2–77.6%). For indexed PAT the optimal cut-point was 33.6 cc/m2. An indexed EAT volume greater than 62.3 cc/m2 was significantly associated with high-risk plaque [univariate odds ratio (OR): 2.50 (CI 1.69 – 3.72), p <0.001]. In the stepwise logistic regression analysis, the association remained significant [OR 1.83 (CI 1.11 – 3.05), p=0.020] after adjustment for female sex [OR 0.42 (CI 0.25 – 0.70), p=0.001], CACS [OR 1.75 (1.56 – 1.97), p <0.001], and stenosis ≥ 50% [OR 5.19 (CI 1.64 – 16.41), p=0.005]. Age and the number of cardiovascular risk factors were excluded from the model since they did not meet the criteria of p <0.1 (Table 4).
Table 4.
Unadjusted and Adjusted Associations Between Epicardial and Paracardial Fat Volume Thresholds, Demographic/Clinical Risk Factors, and High-risk Plaque Features
| Dependent Variable: Any High-risk Plaque Feature Independent Variables |
Univariable Analysis | Multivariable Analysisb Model: EAT (indexed volume) | Multivariable Analysisb Model: PAT (indexed volume) | |||
|---|---|---|---|---|---|---|
| Unadjusted OR | p value | Adjusted OR | p value | Adjusted OR | p value | |
| Indexed Epicardial Fat Volume (threshold ≥62.3 cc/m2) | 2.50 (1.69–3.72) | <0.001 | 1.83 (1.10–3.05) | 0.020 | NA | NA |
| Indexed Paracardial Fat Volume (threshold ≥33.6 cc/m2) | 4.12 (2.64–6.41) | <0.001 | NA | NA | 2.03 (1.15–3.59) | 0.015 |
| Age (years) | 1.08 (1.05–1.10) | <0.001 | 1.03 (1.00–1.07) | 0.087 | ||
| Female | 0.31 (0.21–0.46) | <0.001 | 0.42 (0.25–0.70) | 0.001 | 0.45 (0.25–0.80) | 0.007 |
| No. of cardiovascular risk factorsa | 1.48 (1.24–1.76) | <0.001 | ||||
| Coronary artery calcium score (log) | 1.91 (1.71–2.13) | <0.001 | 1.75 (1.56–1.97) | <0.001 | 1.69 (1.49–1.91) | <0.001 |
| Significant CAD (≥50% Stenosis) | 23.31 (8.17–66.51) | <0.001 | 5.19 (1.64–16.41) | 0.005 | 5.03 (1.54–16.47) | 0.008 |
The summary is based on all 467 observations; 95% Confidence Intervals in brackets
Risk factors include arterial hypertension, diabetes mellitus, dyslipidemia, former or current smoker, family history of CAD
Stepwise regression approach including number of cardiovascular risk factors, age, sex and CTA findings, excluding variables p>0.1
CAD = Coronary artery disease; OR = Odds Ratio; NA = not applicable
Similarly, indexed PAT volumes greater than 33.6 cc/m2 were significantly associated with high-risk plaque in uni- and multivariable analysis. For indexed PAT, the number of cardiovascular risk factors was excluded from the model as it did not meet the prespecified criteria of p <0.1 (Table 4).
The cut-offs and odds ratios for absolute (non-indexed) EAT and PAT volumes can be found in Supplemental Table 2b. Both absolute EAT and PAT volumes were significant predictors of high-risk plaque in univariable analysis. However in multivariable analysis only absolute EAT volume remained a significant predictor of high-risk plaque [adjusted OR 1.88 (CI 1.14 – 3.10), p=0.013].
Cutoffs and odds ratios for absolute and BSA-indexed total adipose tissue (EAT + PAT) are described in Supplemental Table 1b. Both absolute and indexed total adipose tissue volume had a significant association with high-risk plaque; however in multivariable analysis only the indexed total fat volume was significant [adjusted OR 1.74 (CI 1.05 – 2.88), p=0.031].
Discussion
In 467 subjects with acute chest pain who had both non-contrast CT and CTA, we found a significant association between excess epicardial adipose tissue volume and high-risk plaque features, which persisted after adjusting for potential confounders such as traditional cardiovascular risk factors, coronary artery calcium score, and coronary artery stenosis. In addition, lower attenuation EAT and PAT were associated with high-risk plaque features, but only in univariate analyses. These findings support the possible local influence of epicardial adipose tissue depots on high-risk coronary plaque features.
EAT volume and high-risk plaque
In multivariate analyses we found that each additional cc/m2 of EAT was associated with an 1.04 fold higher odds of having high risk coronary plaque (OR 1.04 (1.00–1.08), p=0.040). These results add to the body of evidence linking EAT and coronary atherosclerosis. There is no fascial barrier between EAT and the coronary arteries it encases. Due to this unique anatomic relationship, EAT and the coronaries share a common blood supply and innervation32. In animal models selective surgical excision of EAT slows atherosclerosis in the underlying coronary artery33. Analysis of excised human specimens2 suggests that EAT secretes cytokines with a paracrine proatherogenic effect on the underlying coronary arteries14, 15, 34. Furthermore on a local level, increased EAT around a coronary artery segment detected on CT is associated with increased CAD in that segment, including high-risk plaque features35–40.
Several studies have demonstrated an association between EAT volume and risk factors, progression of coronary artery calcification, plaques causing significant (>50%) stenosis, and adverse events6, 14–19, 41. Two studies have found an association between the volume of EAT and high-risk plaque features16, 42. Our findings extend these studies by assessing for independence from coronary calcium score and significant stenosis to adjust for established predictors of cardiovascular risk43 in the setting of a multicenter randomized controlled trial. We also provide results indexed to body surface area to adjust for changes related to habitus that are known to alter EAT volume44. These findings suggest that EAT is associated with specific features representing high-risk plaque independent of general features of CAD such as CAC or degree of stenosis, and support the hypothesis that fat depot inflammation plays a role in the development of high-risk coronary plaque. While information about EAT is of limited additional value when patients already undergo evaluation with contrast ECG-gated coronary CTA, which allows direct visualization of high risk plaque features, excess EAT may be useful to identify persons at high risk who have only non-contrast CT or a non-ECG gated CTA.
EAT, PAT and VAT
Epicardial and visceral adipose tissue (VAT) share the same embryological origin and show similar morphological and physiological characteristics4. Although VAT is not measureable on standard cardiac CT, we accounted for other anthropometric variables using body surface area and BMI. Our findings are supported by sub-analyses of the Framingham Heart and the Jackson Heart Study, in which EAT was associated with coronary calcification and an adverse cardiovascular risk profile after correction for VAT, respectively6, 14, 45. Doesch et al. report that indexed and absolute EAT volumes quantified by cardiac magnetic resonance imaging were lower in subjects with a clinical diagnosis of ischemic heart failure (NYHA ≥ II) compared to a healthy control group. These findings suggest a possible dynamic character of EAT volume that evolves with the severity of ischemic cardiomyopathy46. However as the ROMICAT II population consisted of persons at low to intermediate risk for CAD and no known history of CAD, we expect that most were in the early phase of disease. EAT was more strongly associated with high risk plaque than PAT. A significant association between EAT and high risk plaque was found in all analyses, whereas PAT fell out of the analysis when assessed as a continuous variable or when not indexed to BSA. These observations are concordant with those of Chen et al. which found excess PAT volume to be associated with the presence of metabolic syndrome but not with CAD47. High amounts of PAT were shown to be closely connected to VAT, metabolic risk factors and further hypothesized to be a surrogate for overall obesity48. EAT is the true visceral fat of the heart and shares the vascular supply as the coronary arteries without an intervening fascial barrier, and thus could have a local paracrine effect on the coronary arteries. In contrast PAT lies outside the pericardial sac, and thus may have a less direct effect.
Volume thresholds in comparison to published values
We analyzed first EAT and PAT as continuous variables, then as binary variables using a threshold approach. Thresholds can be useful to define a “high” volume in clinical practice. Our volume thresholds for EAT were similar to those previously reported to be “high” or associated with adverse events. Our BSA-indexed EAT threshold of 62.3 cc/m2 was slightly below the 68 cc/m2 reported to be at the 95th percentile in a healthy population49. We calculated an absolute EAT threshold of 117 cc for optimal prediction of high-risk plaque features (Supplemental Table 2a); this was also slightly below the cutoff of 125 cc described as a predictor of major adverse cardiac events in an asymptomatic population50.
CT attenuation of EAT/PAT and high-risk plaque
Subjects with high-risk plaque had slightly lower mean attenuation of both EAT [−88 HU vs. −87 HU, p=0.008] and PAT [−106 vs. −103 HU, p <0.001]. While the magnitude of the attenuation difference was small (between one and three HU) and there was not an independent association in multivariate analysis, these hypothesis-generating data raise the possibility that the quality of epicardial and paracardial fat play a role in the development of high-risk plaque features. Furthermore the overall lower mean density of PAT relative to EAT suggests an underlying difference in the makeup of these adjacent but embryologically distinct fat depots. Our findings extend similar observations in the Framingham Heart Study in which lower visceral and subcutaneous fat mean attenuation was associated with an adverse cardiometabolic risk profile21, coronary calcification20, and death51. It is hypothesized that lower CT density of fat is the result of poor vascularization and lipid dense fat tissue20. Conversely, higher fat density might be a CT-morphological correlate for inflammation52 or fibrosis53. The interplay between high-risk coronary plaque and adipose density and how these changes are reflected in CT morphology requires further investigations.
Strengths and limitations
Strengths include a relatively large, well-defined subject cohort from a multi-center randomized clinical trial. A potential limitation lies in the cross-sectional nature of the study which only allows us to infer associations, not causality. Furthermore, our subject cohort consisted of patients 40–74 years old referred for acute chest pain, and thus may not be generalizable to the broader population without acute chest pain for whom high-risk plaque is presumably less common. PAT often extends above and below the heart, and thus the full extent of PAT may not have been captured due to limited z-axis coverage on these coronary calcium score CTs. Lastly, the pathophysiological mechanisms which connect the morphological characteristics of these fat depots to the formation of high-risk plaque features have yet to be fully uncovered.
Conclusion
Greater volumes of EAT are associated with high-risk plaque in patients referred for acute chest pain independent of traditional risk factors, CACS and stenosis. These observations suggest that local ectopic adipose tissue may play a role in the development of high-risk coronary plaque.
Supplementary Material
Figure 2.
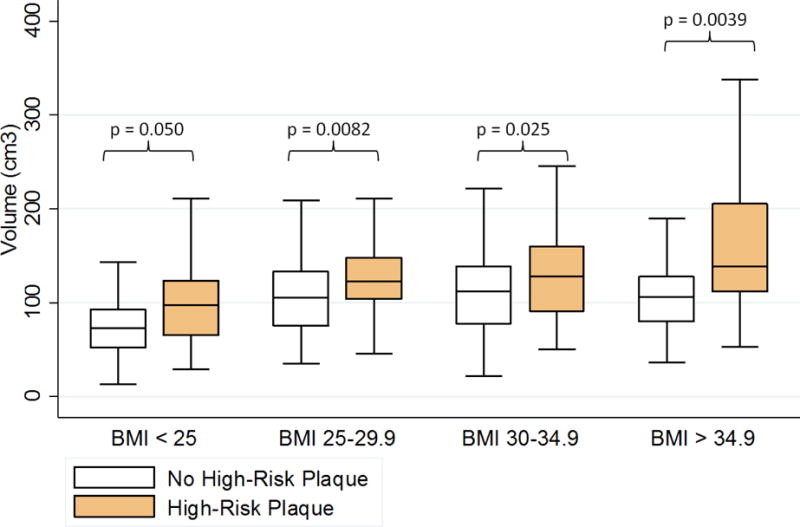
Absolute epicardial adipose tissue volume is increased in individuals with high-risk coronary plaque features within across BMI categories.
Acknowledgments
The authors acknowledge NIH 5T32HL076136, the MGH Executive Committee on Research and the American Roentgen Ray Society Scholarship (MTL), the German Cardiac Society and German National Academic Foundation (JP), the American Heart Association 13FTF16450001 (MF), Siemens Healthcare, NIH/NHLBI 5K24HL113128 and 5U01HL092040-04 (UH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
MTL and JP contributed equally to the manuscript.
References
- 1.Fitzgibbons TP, Czech MP. Epicardial and Perivascular Adipose Tissues and Their Influence on Cardiovascular Disease: Basic Mechanisms and Clinical Associations. Journal of the American Heart Association. 2014;3:e000582–e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 3.Verhagen SN, Vink A, Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225:99–104. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. American heart journal. 2007;153:907–17. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Nichols JH, Samy B, Nasir K, Fox CS, Schulze PC, Bamberg F, Hoffmann U. Volumetric measurement of pericardial adipose tissue from contrast-enhanced coronary computed tomography angiography: a reproducibility study. Journal of cardiovascular computed tomography. 2008;2:288–95. doi: 10.1016/j.jcct.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. European Heart Journal. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat - Noninvasive measurement and clinical implications. Cardiovasc Diagn Ther. 2012;2:85–93. doi: 10.3978/j.issn.2223-3652.2012.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, Pohle K, Baum U, Anders K, Jang IK, Daniel WG, Brady TJ. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, Dohi Y, Kunita E, Utsunomiya H, Kohno N, Kihara Y. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovascular imaging. 2009;2:153–60. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Motoyama S, Sarai M, Narula J, Ozaki Y. Coronary CT angiography and high-risk plaque morphology. Cardiovascular Intervention and Therapeutics. 2013;28:1–8. doi: 10.1007/s12928-012-0140-1. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki Y, Okumura M, Ismail TF, Motoyama S, Naruse H, Hattori K, Kawai H, Sarai M, Takagi Y, Ishii J, Anno H, Virmani R, Serruys PW, Narula J. Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy. European Heart Journal. 2011;32:2814–2823. doi: 10.1093/eurheartj/ehr189. [DOI] [PubMed] [Google Scholar]
- 13.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-Risk Plaque Detected on Coronary CT Angiography Predicts Acute Coronary Syndromes Independent of Significant Stenosis in Acute Chest Pain: Results From the ROMICAT-II Trial. J Am Coll Cardiol. 2014;64:684–92. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Hsu F, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) The American journal of clinical nutrition. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlett CL, Ferencik M, Kriegel MF, Bamberg F, Ghoshhajra BB, Joshi SB, Nagurney JT, Fox CS, Truong QA, Hoffmann U. Association of pericardial fat and coronary high-risk lesions as determined by cardiac CT. Atherosclerosis. 2012;222:129–34. doi: 10.1016/j.atherosclerosis.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forouzandeh F, Chang SM, Muhyieddeen K, Zaid RR, Trevino AR, Xu J, Nabi F, Mahmarian JJ. Does quantifying epicardial and intrathoracic fat with noncontrast computed tomography improve risk stratification beyond calcium scoring alone? Circulation Cardiovascular imaging. 2013;6:58–66. doi: 10.1161/CIRCIMAGING.112.976316. [DOI] [PubMed] [Google Scholar]
- 18.Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Oka T, Utsunomiya H, Urabe Y, Tsushima H, Awai K, Budoff MJ, Kihara Y. Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis. 2014;233:447–53. doi: 10.1016/j.atherosclerosis.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, Mohlenkamp S. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–95. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 20.Alvey NJ, Pedley A, Rosenquist KJ, Massaro JM, O’Donnell CJ, Hoffmann U, Fox CS. Association of fat density with subclinical atherosclerosis. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovascular imaging. 2013;6:762–71. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann U, Truong QA, Fleg JL, Goehler A, Gazelle S, Wiviott S, Lee H, Udelson JE, Schoenfeld D, Romicat, II Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. American heart journal. 2012;163:330–8. 338 e1. doi: 10.1016/j.ahj.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puchner SB, Lu MT, Mayrhofer T, Liu T, Pursnani A, Ghoshhajra BB, Truong QA, Wiviott SD, Fleg JL, Hoffmann U, Ferencik M. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology. 2015;274:693–701. doi: 10.1148/radiol.14140933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, Keaney JF, Jr, Lipinska I, Meigs JB, Kathiresan S, O’Donnell CJ, Fox CS, Benjamin EJ. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–45. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, Brady TJ, Pomerantsev E, Ludwig J, Flachskampf FA, Wicky S, Jang IK, Daniel WG. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842–7. doi: 10.1016/j.jacc.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J, Shimada K, Yoshiyama M. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovascular imaging. 2013;6:448–57. doi: 10.1016/j.jcmg.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Otsuka F, Stolzmann P, Scheffel H, Ferencik M, Kriegel MF, Seifarth H, Virmani R, Hoffmann U. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovascular imaging. 2012;5:1243–52. doi: 10.1016/j.jcmg.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 31.van Velzen JE, de Graaf FR, de Graaf MA, Schuijf JD, Kroft LJ, de Roos A, Reiber JH, Bax JJ, Jukema JW, Boersma E, Schalij MJ, van der Wall EE. Comprehensive assessment of spotty calcifications on computed tomography angiography: comparison to plaque characteristics on intravascular ultrasound with radiofrequency backscatter analysis. J Nucl Cardiol. 2011;18:893–903. doi: 10.1007/s12350-011-9428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nature clinical practice Cardiovascular medicine. 2005;2:536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 33.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. Journal of cardiothoracic surgery. 2014;9:2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, Sata M. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1077–84. doi: 10.1161/ATVBAHA.112.300829. [DOI] [PubMed] [Google Scholar]
- 35.Maurovich-Horvat P, Kallianos K, Engel L, Szymonifka J, Fox CS, Hoffmann U, Truong QA. Influence of pericoronary adipose tissue on local coronary atherosclerosis as assessed by a novel MDCT volumetric method. Atherosclerosis. 2011;219:151–7. doi: 10.1016/j.atherosclerosis.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorter PM, Lindert AS, Vos AM, Meijs MF, Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kälsch H, Seibel RM, Erbel R, Möhlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–9. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Vos AM, Prokop M, Roos CJ, Meijs MF, Schouw YT, Rutten A, Gorter PM, Cramer M, Doevendans PA, Rensing BJ, Bartelink M, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. European Heart Journal. 2008;29:777–83. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 39.Longenecker CT, Jiang Y, Yun CH, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, Bezerra HG, McComsey GA. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. International journal of cardiology. 2013;168:4039–45. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristoffersen US, Lebech AM, Wiinberg N, Petersen CL, Hasbak P, Gutte H, Jensen GB, Hag AM, Ripa RS, Kjaer A. Silent ischemic heart disease and pericardial fat volume in HIV-infected patients: a case-control myocardial perfusion scintigraphy study. PloS one. 2013;8:e72066. doi: 10.1371/journal.pone.0072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–30. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 42.Rajani R, Shmilovich H, Nakazato R, Nakanishi R, Otaki Y, Cheng VY, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Min JK, Berman DS, Dey D. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high-risk coronary plaque features assessed by coronary CT angiography. J Cardiovasc Comput Tomogr. 2013;7:125–32. doi: 10.1016/j.jcct.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrigan TP, Nair D, Schoenhagen P, Curtin RJ, Popovic ZB, Halliburton S, Kuzmiak S, White RD, Flamm SD, Desai MY. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J. 2009;30:362–71. doi: 10.1093/eurheartj/ehn605. [DOI] [PubMed] [Google Scholar]
- 44.Nakazato R, Rajani R, Cheng VY, Shmilovich H, Nakanishi R, Otaki Y, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Wong ND, Shaw LJ, Budoff M, Rozanski A, Berman DS, Dey D. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis. 2012;220:139–44. doi: 10.1016/j.atherosclerosis.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Fox CS, Hickson D, Sarpong D, Ekunwe L, May WD, Hundley GW, Carr JJ, Taylor HA. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes care. 2010;33:1635–9. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:40. doi: 10.1186/1532-429X-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen O, Sharma A, Ahmad I, Bourji N, Nestoiter K, Hua P, Hua B, Ivanov A, Yossef J, Klem I, Briggs WM, Sacchi TJ, Heitner JF. Correlation between pericardial, mediastinal, and intrathoracic fat volumes with the presence and severity of coronary artery disease, metabolic syndrome, and cardiac risk factors. European heart journal cardiovascular Imaging. 2014 doi: 10.1093/ehjci/jeu145. [DOI] [PubMed] [Google Scholar]
- 48.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circulation Cardiovascular imaging. 2010;3:559–66. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shmilovich H, Dey D, Cheng VY, Rajani R, Nakazato R, Otaki Y, Nakanishi R, Slomka PJ, Thomson LE, Hayes SW, Friedman JD, Gransar H, Wong ND, Shaw LJ, Budoff M, Rozanski A, Berman DS. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. The American journal of cardiology. 2011;108:1680–5. doi: 10.1016/j.amjcard.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovascular imaging. 2010;3:352–60. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, Murabito JM, Hoffmann U, Fox CS. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. The Journal of clinical endocrinology and metabolism. 2015;100:227–34. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, Ohba K, Matsubara J, Sumida H, Nagayoshi Y, Sakamoto K, Utsunomiya D, Awai K, Jinnouchi H, Matsuzawa Y, Yamashita Y, Asada Y, Kimura K, Umemura S, Ogawa H. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213:649–55. doi: 10.1016/j.atherosclerosis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clement K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


