Abstract
The heart’s extraordinary metabolic flexibility allows it to adapt to normal changes in physiology in order to preserve its function. Alterations in the metabolic profile of the heart have also been attributed to pathological conditions such as ischemia and hypertrophy; however, research during the past decade has established that cardiac metabolic adaptations can precede the onset of pathologies. It is therefore critical to understand how changes in cardiac substrate availability and use trigger events that ultimately result in heart dysfunction. This review examines the mechanisms by which the heart obtains fuels from the circulation or from mobilization of intracellular stores. We next describe experimental models that exhibit either an increase in glucose use or a decrease in FA oxidation, and how these aberrant conditions affect cardiac metabolism and function. Finally, we highlight the importance of alternative, and relatively under investigated, strategies for the treatment of heart failure.
Introduction
The heart, the powerhouse of the body and the organ with the greatest energy requirement, is remarkably flexible when it comes to substrate use; it is this metabolic flexibility that confers the heart’s remarkable resiliency. The adult heart has the ability to switch energy sources as dictated by substrate availability, hormonal status, and physiological conditions [1, 2]. Sixty to 90% of the energy used by the adult fasting heart is provided by the mitochondrial oxidation of long-chain fatty acids (FAs), with the remainder supplied by oxidation of glucose, lactate and ketone bodies [3]. In contrast, fuel preference in fetal hearts is determined by the low oxygen environment, in which the oxidation of carbohydrate substrate is more energetically efficient per mole of oxygen consumed. Although the preferred cardiac metabolic substrate during embryonic development is glucose, lipid metabolism is also crucial. RXRα knockout embryos, in which expression of multiple lipid metabolic target proteins is downregulated, display malformations and developmental delays and die from cardiac failure in utero [4], suggesting a correlation between energy homeostasis and signaling in the fetal heart. Maintaining cardiac lipid homeostasis is crucial throughout life, however, and alterations in lipid metabolism in the adult heart may underlie the contractile dysfunction associated with senescence. Thus, aging hearts display increased levels of saturated FAs in membrane phospholipids, a feature that has been associated with decreased membrane fluidity and transport [5]. In addition, reduced mitochondrial content of cardiolipin and alterations in cardiolipin FA composition observed in both aging hearts and the genetic disorder, Barth syndrome, have been linked to disturbances of the electron transport chain assembly and activity, decreased ATP production and increased formation of reactive oxygen species (ROS), and defective protein import and mitophagy, which ultimately lead to cardiovascular dysfunction [6].
Shortly after birth, the heart undergoes a major switch from the use of glucose and lactate towards the use of lipids [7, 8]. This switch in substrate use that occurs as the heart transitions to the adult stage is not permanent; healthy adult hearts retain the ability to revert to the fetal metabolic program in response to physiological stress [9]. While maintaining metabolic flexibility is essential for normal cardiovascular function, a reversion to fetal metabolism is also observed in failing hearts [10]. Thus, left ventricular hypertrophy (LVH), systemic hypertension, and myocardial ischemia are accompanied by increases in glucose use [11]. The loss of cardiac metabolic flexibility in favor of increased FA use is also detrimental to heart function, as elevated FA oxidation is observed in metabolic heart disease and diabetes [11]. Alterations in substrate use due to mutations in metabolic pathways that result in myocardial energy shortage and/or lack of crucial membrane components (e.g., cardiolipin deficiency in Barth syndrome [12]) can lead to cardiac hypertrophy and dysfunction, whereas an imbalance between FA uptake and oxidation can cause excess lipid accumulation, lipotoxicity and associated cardiac pathologies; pertinent studies will be summarized in the following sections. Thus, it remains unclear whether the shift in cardiac energy substrate is a cause or consequence of pathologic events, and whether this shift is beneficial or deleterious to cardiac function. Moreover, our understanding of the mechanisms that induce this switch and the regulatory level at which these changes occur remains limited. In this review we strive to summarize the abundant literature depicting both sides of the coin. The reader will note that we have cited mainly primary references; in doing so, we aimed to take a fresh look at the original findings and integrate them with novel concepts arising from more recent reports in order to further our understanding of this highly debated issue.
Cardiac substrate uptake and metabolism
Cardiac mechanical performance is preferentially sustained by the oxidation of FAs and glucose; however, lactate becomes important as a major metabolic substrate during exercise [13], and liver-derived ketones may also be used as cardiac fuel during fasting [14]. Differences in cardiac substrate use are also species-dependent, with isolated murine hearts relying primarily on ketone bodies (34%) and lactate (24%) for energy when exposed to a physiological mixture of glucose, glycerol, lactate, pyruvate, acetoacetate, and long-chain FAs in the presence of albumin [15]; it is unlikely, however, that acetoacetate and lactate are major substrates when VLDL-TAG, the favored suppler of FAs, is present [16, 17]. Thus, the heart has been described as a “true omnivore”- that is, functioning optimally when oxidizing different substrates simultaneously [18]. The availability and transport of a substrate into cardiomyocytes therefore becomes the rate-limiting step that determines substrate selection, and metabolism of the imported substrate will limit the use of other energy sources.
In the immediate postprandial state in rodents fed a low-fat diet, when non-esterified FA levels are low and circulating concentrations of glucose and insulin increase, exogenous glucose becomes the cardiac fuel of choice [18]. The uptake of extracellular glucose into cardiomyocytes occurs along a steep concentration gradient and, due to the hydrophilic nature of the substrate, must be facilitated by the transmembrane glucose transporters GLUT4 and GLUT1 [19]. These transporters are translocated from intracellular stores to the sarcolemma via vesicular trafficking and in response to insulin and contraction [20], stimuli which activate independent signaling pathways. Insulin stimulation of cardiac glucose uptake occurs via phosphatidylinositol-3 kinase (PI3K) [21, 22], whereas contraction increases the intracellular AMP/ATP ratio, which activates AMP-activated kinase (AMPK) [23]. GLUT4, the insulin-sensitive transporter, is responsible for the sharp rise in cardiac glucose uptake after insulin stimulation or following a sudden increase in heart work [22, 24]; moreover, AMPK activation can also increase cardiac glucose uptake via GLUT4 translocation [25]. GLUT1 has generally been regarded as the basal cardiac glucose transporter due to its predominantly sarcolemmal location; however, both insulin and contraction signaling can trigger the recruitment of additional GLUT1 from intracellular pools to the sarcolemma [21, 26, 27], although this recruitment has only a minor effect on insulin-stimulated glucose uptake [28]. Increased presence of glucose transporters on the plasma membrane also occurs in experimental models of ischemia [29–31], as well as in mice with decreased cardiac FA activation caused by cardiomyocyte-specific knockout of long-chain acyl-CoA synthetase isoform-1 (Acsl1) [32]. Consequently, each of these models exhibits a net increase in the velocity of glucose import and metabolism. Internalized glucose is rapidly phosphorylated to glucose-6-phosphate and used primarily for glycolysis, although incorporation of glucose into glycogen also occurs, particularly in human myocardium [33], in hypertrophied and ischemic hearts [34, 35], under conditions in which lactate is the predominant substrate [36, 37], and during fasting [38]. Moreover, and in agreement with metabolic substrate preference, glycogen synthesis is high in fetal and newborn hearts, in which glycogen occupies 30% of total cardiomyocyte volume [39]. Differential cardiac metabolism of carbohydrate may also determine its cellular fate, since the heart converts excess extracellular glucose into lactate in the presence of glycogen, which, when available, is hydrolyzed to glucosyl moieties that are preferentially oxidized [40].
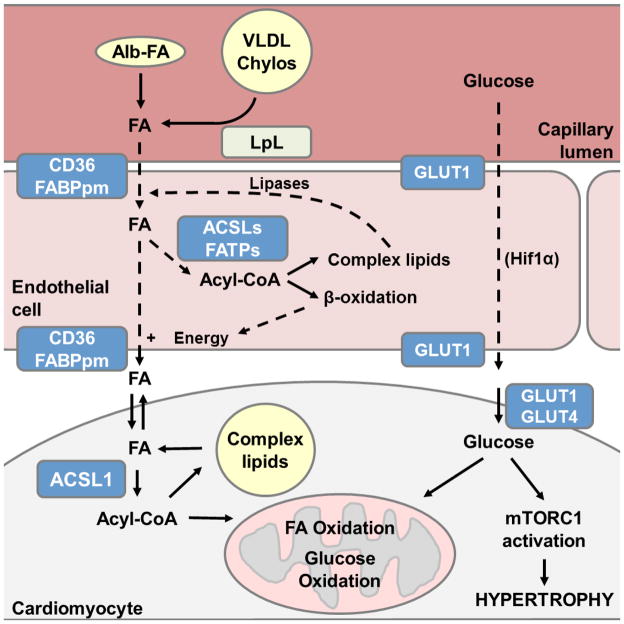
The import and oxidation of FAs becomes critical under fasting conditions when blood glucose is low and FAs are released from adipose stores into the circulation. In the fed state, however, cardiomyocytes acquire their lipid substrates almost exclusively from the lipoprotein lipase (LpL)-mediated hydrolysis of the triacylglycerol (TAG) carried in circulating lipoproteins; only a minor contribution comes from non-esterified FAs bound to plasma albumin [16, 17]. LpL is located on the luminal membrane of endothelial cells [41–43], and although different mechanisms of transendothelial FA trafficking have been proposed [44–46], the process by which exogenous FAs move across an endothelial cell to its basolateral membrane remains unknown (Fig. 1). Also unknown is how FAs are then transported into the subendothelial space in order to reach the cardiomyocyte sarcolemma. Despite the ability of amphipathic FAs to partition into biological membranes via passive diffusion [47], transport of FAs into cardiomyocytes is thought to occur via a protein-facilitated process like that of glucose, in which recruitment of protein transporters to the sarcolemma plays a major regulatory role [20, 48]. Four proteins have been implicated in the uptake of FAs into cardiomyocytes: two plasma membrane proteins, the FA translocase FAT/CD36 and the FA binding protein (FABPpm) [49], and two very-long-chain acyl-CoA synthetases (ACSVL), ACSVL4 and ACSVL2, otherwise known as FA transport proteins FATP1 [50] and FATP6 [51], respectively. The presence of the ACSVLs on intracellular organelle membranes (ER, mitochondria, and lipid droplets) rather than the plasma membrane suggests that they enhance transport by vectorial acylation, i.e. activation, of exogenous FAs to acyl-CoAs [52, 53]. In this regard, ACSL1 may also play a major role in FA import, given that it accounts for > 90% of total long-chain ACS activity in cardiomyocytes [54]. Like glucose transport, FA uptake into cardiomyocytes is stimulated by both insulin and contraction. While the effect of insulin on FA uptake is modest (1.5-fold for FAs [55] vs 2- to 14-fold for glucose [21, 22, 24, 56, 57]), the resulting increase in FA import is significant and CD36-dependent [55]. Contraction induces cardiac FA uptake via two related but distinct mechanisms involving the intracellular energy-sensing enzyme AMPK, the AMPK-mediated translocation of CD36 to the sarcolemma [58, 59], and the AMPK-mediated inhibition of acetyl-CoA carboxylase, which diminishes malonyl-CoA levels and thereby de-inhibits carnitine palmitoyltransferase I (CPT-1) [60], the enzyme responsible for the first step in mitochondrial FA import. The combined effect of contraction-mediated AMPK activation thus leads to an increased uptake of exogenous FAs that are preferentially channeled towards mitochondrial β-oxidation. Due to the potential toxicity of high intracellular concentrations of free FAs, exogenous FAs are rapidly activated upon entry by ACS-mediated esterification to acyl-CoA [61]. This activation step serves to effectively trap FAs within cardiomyocytes for their further metabolism, and may provide a potential regulatory step in determining their downstream metabolic fate. In line with this concept, ACSL1 preferentially targets FAs towards oxidation and away from TAG synthesis in heart [54, 62]. CD36 has also been implicated in the compartmentalization of exogenous FAs in skeletal myocytes [63]. Overexpression of CD36 in C2C12 myotubes and the consequent stimulation of FA transport does not invariably result in TAG accumulation. CD36-overexpressing myotubes incubated with exogenous palmitate accumulate TAG, whereas exposing the same cells to oleate results in the channeling of incoming FAs to a lipase-accessible TAG pool, thereby enhancing TAG turnover and preventing TAG accumulation. These data suggest that CD36-mediated transport itself could determine the metabolic fate of specific imported FAs, perhaps through the intrinsic properties of the distinct FA species that elicit differential interactions with CD36 and/or downstream cellular processes. Similarly, inhibiting cardiac LpL activity by either genetic manipulation or the use of pharmacological inhibitors results in defective lipid droplet formation even when FA uptake is increased [64], indicating that LpL maintains cardiac lipid homeostasis by enhancing the cycling of exogenous FAs into intracellular TAG stores. This finding challenges long-standing assumptions that cardiomyocytes have little capacity for TAG accumulation, or that exogenous non-esterified FAs constitute their major source of oxidative substrate. A requirement for FA cycling through an endogenous TAG pool prior to hydrolysis for cardiac β-oxidation is demonstrated by the adipose triglyceride lipase (ATGL) deficient model and in isolated hearts. Mice with a global ATGL-deficient background exhibit massive cardiac TAG accumulation and develop a lethal cardiomyopathy [65] that can be reversed by cardiac-specific overexpression of ATGL [66]. Furthermore, isolated wild-type hearts perfused with a physiological mixture of substrates show that 60% of exogenous FAs are incorporated into TAG, while 40% of these FAs proceed directly to oxidation when mechanical demand is low [15]. This preference for FAs derived from intracellular lipid droplets is further enhanced by PPARα-overexpression, suggesting that it is mediated by PPARα [66, 67].
Figure 1.
Proposed role of endothelial metabolism in regulating cardiac substrate use. The metabolism of exogenous FA and glucose within endothelial cells may determine FA availability at the cardiomyocyte sarcolemma, thereby regulating cardiac substrate selection and cardiomyocyte energy metabolism. Albumin (Alb)-bound fatty acids (FAs) derived from the circulation and those hydrolyzed by lipoprotein lipase (LpL) at the cell surface enter endothelial cells assisted by the membrane proteins CD36 and FABPpm, a mechanism enhanced by acyl-CoA synthetase (ACS)-mediated vectorial acylation. ACS-mediated activation of FAs has additional roles: the generation of energy via FA β-oxidation for FA transport, or the esterification of FAs to complex lipids for storage and subsequent hydrolysis and vesicular or other transendothelial trafficking to the basolateral membrane for export. Glucose transport through endothelial cells requires both HIF1α and GLUT1, but the underlying mechanism has not been elucidated. Solid lines indicate published data supports this mechanism, whereas dashed arrows are used to denote an unknown mechanism.
In addition to substrate transport and intracellular location of energy stores, cardiac fuel selection is regulated by metabolites derived from the degradation of specific substrates. Thus, the cardiac metabolism of glucose leads to increased acetyl-CoA production from pyruvate and subsequent malonyl-CoA inhibition of CPT-1, ultimately decreasing FA β-oxidation [60, 68, 69]. Conversely, both the acetyl-CoA and NADH products of FA oxidation can allosterically activate pyruvate dehydrogenase kinase (PDK) [70] and directly inhibit pyruvate dehydrogenase (PDH) activity [71, 72], thereby reducing PDH flux and glucose oxidation. In addition, the Krebs cycle metabolism of the FA-derived acetyl-CoA to citrate blocks glycolysis by allosterically inhibiting phosphofructokinase 1 [73]. Thus, FA oxidation inhibits glucose oxidation, glycolysis and glucose uptake [26, 74].
Cardiac glucose metabolism: a double-edged sword?
The benefits of increased cardiac glucose use for obesity-related cardiomyopathy or heart failure remains a controversial topic [34, 54, 62, 75, 76]. Analysis of several rodent models suggests that left ventricular hypertrophy (LVH) precedes the metabolic changes that result in increased glucose use and reduced FA oxidation [35, 40, 75]. On the other hand, increased reliance on glucose for cardiac energy production has been linked to the development of LVH and impaired cardiac function [54, 62, 77–80], suggesting that enhanced glucose use precedes the onset of pathology. See Table 1 for a summary of all the models described.
Table 1.
Models of Altered Cardiac Glucose Metabolism and Implications for Cardiovascular Function
| Model | Effect on Cardiac Metabolism | Functional Outcome | References | |
|---|---|---|---|---|
| Cardioprotective effects of glucose | GLUT1 overexpression | Decreased FA oxidation Increased glucose and lactate use |
None Protective in the context of pressure-induced LVH, ischemic stress, or PPARα deficiency during high workload |
[83] [84] [85] |
| PPARα knockout | 3-fold increase in glucose and lactate use Decreased lipid droplet accumulation 80% reduction in LpL and CD36 mRNA upon fasting Decreased ATP synthesis during high workload |
None under basal conditions Impaired contractile function when subjected to elevated workload, reversed by GLUT1 overexpression |
[85] [64] |
|
| Cardiac GLUT4 knockout | 3-fold increase in glucose transport Lack of insulin-stimulated glucose uptake Accelerated ATP depletion during ischemia |
Compensated hypertrophy in basal conditions Severe systolic and diastolic dysfunction in the context of ischemia |
[28] [81] |
|
| Reduced FAO capacity | Global and heart-specific ACSL1 knockout |
≥90% decrease in FA oxidation 8-fold increase in glucose uptake Reversion to fetal metabolic program |
mTOR-responsive hypertrophy Diastolic dysfunction |
[30] [54] [72] |
| CPT1b+/− CPT1b−/− |
Increased glucose use upon TAC Myocardial lipid accumulation - |
80% mortality after TAC Cardiac hypertrophy and premature mortality at basal conditions |
[138] [139] |
|
| LCAD deficiency (mice) | Impaired FA oxidation Cardiac TAG accumulation |
Severe hypertrophy Cardiomyopathy |
[132] [144] |
|
| Increased reliance on glucose | Global ATGL knockout | Massive myocardial TAG accumulation 54% increase in cardiac glucose uptake Increased glucose use upon fasting |
Severe impairment of mitochondrial function Lethal cardiomyopathy Both reversed by cardiac-specific ATGL overexpression or treatment with PPARα agonist |
[65] [66] |
| Pressure overload | Increased glucose uptake Impaired FAO, increased glucose oxidation Reversion to fetal metabolic program Increased G6P accumulation |
Impaired mitochondrial respiratory capacity Progressive hypertrophy and systolic dysfunction |
[77] [78] [79] |
|
| Failing human hearts | Reversion to fetal metabolic program Increased G6P accumulation |
Heart failure | [10] [77] |
|
| Ex vivo working hearts perfused with glucose | Obligate glucose use Mismatch between glucose uptake and oxidation Reversion to fetal metabolic program G6P accumulation, mTORC1 activation |
ER stress Decreased cardiac power and output |
[77] [78] [79] |
|
The importance of glucose metabolism in the maintenance of cardiac function under stress conditions is underscored by studies of mice with a heart-specific genetic ablation of insulin-stimulated glucose uptake. Mice deficient in cardiac GLUT4 (G4H−/−) lack insulin-stimulated glucose uptake, and develop morphological alterations and compensated hypertrophy [28]. An observed increase in basal cardiac glucose metabolism is likely responsible for the preservation of contractile function in these hearts under normal conditions; however, G4H−/− hearts subjected to ischemia-associated stress develop severe systolic and diastolic dysfunction due to accelerated ATP depletion [81]. Similarly, mice with a cardiac-specific insulin receptor knockout (CIRKO) are defective in heart glucose transport and oxidation. CIRKO mice develop cardiac dysfunction during pressure overload-induced hypertrophy, possibly as a result of insufficient energy production [82]. Thus, an adaptive shift in cardiac substrate selection towards the preferential oxidation of carbohydrates can be beneficial in an acute setting, and is advantageous in specific models of cardiomyopathy. For instance, cardiac-specific overexpression of GLUT1 protects against pressure-induced LVH or ischemic stress in the aging heart [83, 84]. Increased glucose uptake and oxidation via cardiac GLUT1 overexpression is also beneficial in the context of PPARα deficiency (PPARα−/−) [85]. Metabolic remodeling towards increased glucose use in PPARα−/− hearts is sufficient for maintaining energy homeostasis and cardiac function under basal conditions, but not when the heart is subjected to an elevated workload; however, cardiac-specific GLUT1 overexpression can rescue this defect. Downregulation of PPARα and PGC-1α, the master regulators of FA oxidation and mitochondrial biogenesis, is widely observed in hypertrophied and failing hearts [86–88]; reductions in the expression of CPT-1 and medium-chain acyl-CoA dehydrogenase (MCAD) have also been reported [86, 88, 89]. The metabolic switch to glucose oxidation in experimental models of hypertrophy is therefore presumed to be secondary to the impaired supply of FA. Thus, the improvement in cardiac function resulting from increased glucose use in the context of the specific cardiomyopathies described could be attributed to enhanced energy production efficiency rather than to glucose metabolism per se. However, these beneficial effects appear to be short-lived, as demonstrated by studies in hypertrophied myocardium [90] and observations in patients with dilated cardiomyopathy [91]. Such studies indicate that, in the long-term, the adaptive switch in cardiac metabolism towards increased glycolysis is not sufficient to meet the energetic demands of the diseased heart.
In contrast to the models described above in which enhanced glucose use is protective, obligate cardiac use of glucose due to genetic modification or forced glucose uptake is directly linked to the development of hypertrophy. For example, isolated beating hearts perfused with glucose exhibit metabolic changes that precede and mediate the onset of decreased cardiac power and output, suggestive of contractile dysfunction [77]. In addition, our work in models of altered cardiac FA oxidation resulting from either global or cardiomyocyte-specific ACSL1 deficiency suggests that it is the shift to glycolysis that promotes a reversion to the fetal metabolic program, the development of cardiac hypertrophy, and diastolic dysfunction [32, 54, 62]. Similar effects have been observed in hypertensive humans, in which glucose metabolic remodeling precedes the development of LVH [80]. Forced glucose uptake is also associated with LVH and cardiac failure in humans [92, 93]. This occurs with the administration of the tyrosine kinase inhibitor sunitinib, which increases cardiac glucose uptake and use in mice, together with the activation of the fetal gene program that is also observed in failing human hearts [94]. Taken as a whole, these results are compelling indicators that reliance on glucose is detrimental to cardiac function.
Glucose, mTORC1, and cardiac hypertrophy
A potential cause for the development of LVH and cardiac dysfunction in models of increased cardiac glucose use is the activation of mTORC1 (mechanistic target of rapamycin, complex 1), a major regulator of cell growth and proliferation [95]. The mTORC1 pathway is essential for embryonic heart development and postnatal adaptive cardiomyocyte growth and function [96]; however, derangements in mTORC1 signaling result in the development of LVH [54, 62, 97, 98]. Activated mTORC1 and subsequent phosphorylation of its target, S6 kinase (S6K), is observed in several models of cardiac hypertrophy, including spontaneous hypertensive rats [99], thyroid hormone-induced cardiac hypertrophy [100], and exercise-induced cardiac hypertrophy [101]. The mTORC1-S6K axis is potently activated by glutamine in neonatal cardiomyocytes [102] and by leucine in the adult heart [103]; the link between glucose and mTORC1 activation is less well established. In ex vivo working hearts, hemodynamic stress alone can result in increased glucose use and mTORC1-responsive cardiac remodeling [104]. Moreover, phosphorylation of glucose and the concomitant accumulation of glucose-6-phosphate (G6P) is required and sufficient to elicit mTORC1 activation both in vivo and ex vivo [77, 79, 105]. Increased glucose uptake, mTORC1 activation, and G6P accumulation are also observed in ACSL1-deficient hearts [32, 54, 62], showing that this process occurs in vivo. Mechanical unloading in failing human hearts results in decreased G6P levels, further implicating cardiac glucose metabolism as a cause of load-induced mTORC1 activation [77].
The regulation of mTORC1 is complex. Among other nodes, mTORC1 activity is inhibited by the intracellular energy sensor AMPK [106]. Although AMPK is itself activated by an increased AMP/ATP ratio indicative of low cellular energy levels, AMPK levels are reduced in isolated rat hearts that have been subjected to an elevated workload, despite marked increases in glucose uptake and mTORC1 activation [77]. Similarly, the amount of activated AMPK is significantly reduced in Acsl1-deficient ventricles, even though the myocardial AMP/ATP ratio remains normal [54]. These studies suggest that an upregulation of glucose metabolism is sufficient to maintain energy homeostasis, or alternatively, that AMPK activation is reversed by high glucose flux. Although decreases in AMPK phosphorylation suggest that AMPK deinhibits mTORC1 in these models, mTORC1 activation may yet be unrelated to AMPK activity. In support of this notion, a recent study suggests that AMPK does not regulate carbohydrate-mediated S6K phosphorylation, the usual indicator of mTORC1 activation [105]. In addition, mTORC1 stimulation of physiological and pathological hypertrophy appears to be mediated by eukaryotic translation initiation factor 4E-BP [107] rather than S6K [108]. Finally, we cannot exclude the possibility that mTORC1 regulation might be mediated via unknown glucose-responsive effectors. Of note, cardiac hypertrophy is not induced by expression of constitutively active mTORC1 or by overexpression of wild-type mTORC1 [109, 110]. These findings suggest that mTORC1 activation alone may not be sufficient to elicit hypertrophy, but that coordinated modulation and integration of multiple pathways must exist.
Glucose uptake and N-acetyl-D-glucosamine protein modification
Although the bulk of exogenous glucose is channeled towards oxidation and, to a lesser extent, towards glycogen synthesis, other pathways of glucose metabolism have been described in cardiomyocytes. The importance of these accessory glycolytic pathways on cardiac physiology is beginning to be appreciated, with N-acetyl-D-glucosamine modification (O-GlcNAcylation) gaining traction as a potential therapeutic target due to the fact that only two enzymes control it: O-GlcNAc transferase (OGT), catalyzing the O-linked attachment of N-acetyl-D-glucosamine to serine and threonine residues of nuclear, cytoplasmic and mitochondrial proteins; and O-GlcNAcase (OGA), mediating N-acetyl-D-glucosamine removal [111–113]. Protein glycosylation via this process requires flux through multiple metabolic pathways, and, thus, O-GlcNAcylation has been postulated to function as a nutrient/metabolic sensor [114]; however, its role in cardiac function has remained relatively unknown. The last decade has seen an emergence of studies delineating the link between increased cardiac glucose uptake, O-GlcNAcylation and cardiac hypertrophy. Experimental models of pressure-induced hypertrophy indicate that elevated glucose uptake results in increased flux through the hexosamine biosynthetic pathway [115]. Consistent with this notion, glucose toxicity and insulin resistance correlate with increased cardiac protein O-GlcNAcylation [116, 117]. Additionally, O-GlcNAc synthesis is required for the transcriptional activation and progression of cardiac hypertrophy [118], and cardiac-specific knockout of OGT and the concomitant decrease in O-GlcNAc levels prevent transaortic constriction (TAC)-induced hypertrophy [115, 119]. Conversely, protein O-GlcNAcylation is cardioprotective in the context of ischemic stress [120]. The paradoxical effects of O-GlcNAcylation on cardiac hypertrophy and function can be explained by the diversity of cellular processes that can be controlled through this pathway. Thus, direct glucose-mediated regulation of protein function via O-GlcNAcylation of mitochondrial proteins leads to decreased mitochondrial oxygen consumption and ATP production rates [121], thereby contributing to cardiac dysfunction. On the other hand, glucose may exert its regulatory effects in a more indirect way through the O-linked glycosylation of specific transcription factors, eliciting a transcriptional response that leads to a reversion to the fetal metabolic program [115]. Finally, glucose-induced O-GlcNAcylation may provide the missing link between glucose uptake and AMPK-independent mTORC1 regulation in the development of hypertrophy and cardiac dysfunction, perhaps by direct modification and activation of mTORC1, or through the inhibition of mTORC1 phosphorylation at specific residues.
Decreased FA oxidation in cardiac function: friend or foe?
Increased cardiac TAG accumulation in human obesity-related cardiomyopathy has been associated with structural remodeling such as left ventricular and atrial hypertrophy, and functional changes like impaired myocardial contractility [122, 123]. Animal studies have recapitulated these findings in models of diet-induced obesity [124], in the genetic obesity ob/ob and db/db mouse models [124–127], in mice with cardiac-specific overexpression of ACSL1 [128] or FATP1 [129], and in mice with global ATGL deficiency [65]. Thus, decreasing the uptake and use of FAs in heart has been proposed as a potential strategy to prevent obesity-linked cardiomyopathies. Although decreasing cardiac use of FAs through inhibition of CPT-1a [130] or FA oxidation [131] is an interesting approach, data from human deficiencies in FA oxidation and animal models with impaired FA oxidative capacity have shown that a substantial decrease in FA oxidation results in cardiac hypertrophy [54, 62, 132–135]. Initial studies of short-term CPT-1 inhibition by oxfenicine treatment in mice suggested that this manipulation protects against high fat diet (HFD)-induced insulin resistance [136]; however, prolonged HFD feeding causes lipid accumulation and impaired insulin signaling [137], effectively reversing the beneficial effects of short-term CPT-1 inhibition. Partial CPT-1b deletion (CPT1b+/−) results in 80% mortality from congestive heart failure after TAC [138], while homozygous CPT-1b knockout mice display cardiac hypertrophy and premature mortality under basal conditions [139], illustrating the importance of FA oxidation for normal heart function. As described in previous sections, abolishing FA oxidation in ACSL1-deficient hearts leads to increased glucose uptake and mTORC1-mediated hypertrophy [54, 62]. Manifestations of impaired cardiac FA oxidation capacity caused by deficiency of very-long-chain acyl-CoA dehydrogenase (VLCAD) in humans include myocardial TAG accumulation and the development of hypertrophic or dilated cardiomyopathy [133, 135]. Rodents, which express both long chain acyl-CoA dehydrogenase (LCAD) and VLCAD [140], show no effect of LCAD or VLCAD deficiency under normal conditions, despite the presence of cardiac hypertrophy; however compensation fails upon fasting, cold exposure, and as a function of age, resulting in energy depletion and decreased cardiac performance [141–144]. Decreased FA oxidation capacity under conditions of normal or elevated FA availability likely results in the channeling of exogenous FA into storage and a concomitant decrease in cardiac performance, arguing against therapies that seek to decrease FA oxidation in failing hearts. In addition, the resultant increased concentrations of intracellular FA may lead to aberrant cardiac signaling and altered acetylation and palmitoylation of proteins. Numerous elegant studies on myocardial lipid accumulation, lipotoxicity and cardiac dysfunction have been published; for an extended overview on cardiac lipid accumulation and the role of FA in cardiac signaling and protein modification, the reader is referred to other articles in this issue. On the other hand, HFD interventions are advantageous in preventing and ameliorating cardiac dysfunction in several models of heart failure [145–147], and enhanced FA use does not necessarily alter cardiac function under basal conditions [148], further challenging the notion that enhanced use of FA is detrimental to cardiovascular health.
Perspectives
Decreased oxidation of FA, the preferred energetic substrate in the adult heart, results in increased glucose uptake and use. Although this switch in cardiac substrate use has been postulated to be beneficial in specific models of cardiomyopathy, increased glucose use may also precede the onset of hypertrophy. Suggestions for therapies to treat heart disease have focused on compensating the energy deficit in failing hearts by altering cardiac metabolism; however, a shift towards glucose use could generate permanent changes in protein function and gene expression via mechanisms that have not been fully elucidated. For instance, the uncoupling of glycolysis and glucose oxidation in this context allows excess glucose to be channeled into alternative glycolytic pathways. Thus, increased glucose flux through the hexosamine biosynthetic pathway results in O-GlcNAcylation activation, which can directly impact cardiac performance by modifying mitochondrial proteins [121]. More importantly, the glycosylation of transcription factors when O-GlcNAc levels are high has been postulated to be the central mechanism by which glucose induces a permanent return to the fetal metabolic program [94, 115]. Driving enhanced use of glucose also has consequences that extend beyond altered myocardial energetics or genetic reprogramming, such as changes in membrane composition [149] and mTORC1-mediated inhibition of autophagy [150]. On the other hand, decreased cardiac lipid metabolism can cause TAG to accumulate in cardiomyocytes and lead to aberrant lipid signaling [66], the production of ROS, ER stress, and impaired mitochondrial function. Thus, preserving cardiac metabolic flexibility is crucial in order to prevent, and perhaps even reverse, cardiovascular disease. Future efforts should therefore be directed towards understanding what controls the availability of cardiac substrates, and how this availability can be regulated to balance substrate supply and use under specific conditions without altering overall cardiac metabolism.
In this regard, the development of cardiovascular therapies demands a better understanding of the role of the endothelium in cardiac substrate selection. Although the facilitated uptake of glucose and FAs at the cardiomyocyte surface has been studied extensively, the precise mechanisms that underlie nutrient flow through the subvascular compartment remains unknown. Endothelial cells occupy a strategic position between circulating metabolic substrates in the blood and cardiomyocytes, raising the intriguing possibility that uptake and metabolism within the endothelium may play a major role in determining the rate of substrate transport to cardiomyocytes, thereby controlling downstream cardiac substrate availability and use. In support of this theory, endothelial cells have been shown to regulate glucose uptake by the brain and heart [151]; however, the precise mechanism by which glucose is transported through the heart endothelium is yet to be elucidated. In this respect, communication between cardiomyocytes and endothelial cells would be essential for efficient regulation of substrate delivery. Indeed, a recent study has identified cardiomyocyte-derived exosomes loaded with glucose transporters and glycolytic machinery components as key in allowing for a rapid response from the endothelium in the coordinated regulation of glucose uptake by the heart [152]. Similar mechanisms may govern cardiac FA uptake and use. A recent report showed that endothelial cell expression of CD36 and LpL is regulated by the Meox2/Tcf15 transcriptional complex, facilitating FA uptake and transport across heart endothelial cells, and thereby affecting cardiac contractility [153]. Transendothelial FA trafficking may involve CD36, the FA binding proteins FABP4 and FABP5 [154], and the membrane-associated proteins FATP3 and FATP4, whose ACS activity enhances FA uptake [52, 53]. This raises the question as to whether ACSLs may play a similar, and perhaps coordinated, role in FA transport by endothelial cells. Supporting this idea, overexpressed FATP1 and ACSL1 interact functionally in yeast [155], and, when coexpressed in NIH 3T3 cells, synergistically increase FA import rates [156]. Given the distinct tissue expression and cellular locations of the different FATP/ACSVL and ACSL isoforms, it has been postulated that these enzymes may coordinately traffic FAs into discrete metabolic pools, acting in concert to confer selectivity and specificity to FA use by distinct metabolic pathways [157]. We posit that ACSL-mediated activation of FA in endothelial cells could perform a dual role: the generation of energy via β-oxidation to power FA transport, and the esterification of FA to TAG for storage or vesicular transendothelial trafficking to the endothelial cell basolateral membrane for export (Fig. 1). Regulation of FA transport through the endothelium via activation by ACSL or FATP/ACSVL could modulate FA availability for β-oxidation in cardiomyocytes and ultimately control cardiac metabolic efficiency. Endothelial cell lipid metabolism therefore warrants serious investigation.
Highlights.
The heart adapts to normal changes in physiology by switching metabolic substrates.
Altered cardiac metabolism has also been identified under pathological conditions.
Increased glucose use may precede the onset of various pathologies.
Decreased fatty acid oxidation does not ameliorate dysfunction in multiple models.
Alternative strategies investigating transendothelial fuel transport are warranted.
Acknowledgments
This work was supported by grants from the National Institutes of Health, DK56599 and DK59935.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Lozano P, Smith SM, Perkins G, Kubalak SW, Boss GR, Sucov HM, Evans RM, Chien KR. Energy deprivation and a deficiency in downstream metabolic target genes during the onset of embryonic heart failure in RXRalpha−/− embryos. Development. 1998;125:533–544. doi: 10.1242/dev.125.3.533. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Jimenez JA, Bordoni A, Lorenzini A, Rossi CA, Biagi PL, Hrelia S. Linoleic acid metabolism in primary cultures of adult rat cardiomyocytes is impaired by aging. Biochem Biophys Res Commun. 1997;237:142–145. doi: 10.1006/bbrc.1997.7101. [DOI] [PubMed] [Google Scholar]
- 6.Shen Z, Ye C, McCain K, Greenberg ML. The Role of Cardiolipin in Cardiovascular Health. Biomed Res Int. 2015;2015:891707. doi: 10.1155/2015/891707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher DJ. Oxygenation and metabolism in the developing heart. Semin Perinatol. 1984;8:217–225. [PubMed] [Google Scholar]
- 8.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 9.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 11.Carvajal K, Moreno-Sanchez R. Heart metabolic disturbances in cardiovascular diseases. Arch Med Res. 2003;34:89–99. doi: 10.1016/S0188-4409(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 12.Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJ, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Commun. 2000;279:378–382. doi: 10.1006/bbrc.2000.3952. [DOI] [PubMed] [Google Scholar]
- 13.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972;15:289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- 15.Stowe KA, Burgess SC, Merritt M, Sherry AD, Malloy CR. Storage and oxidation of long-chain fatty acids in the C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Lett. 2006;580:4282–4287. doi: 10.1016/j.febslet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. J Clin Invest. 1960;39:717–723. doi: 10.1172/JCI104088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab. 2003;284:E331–339. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- 18.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 19.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 21.Fischer Y, Thomas J, Sevilla L, Munoz P, Becker C, Holman G, Kozka IJ, Palacin M, Testar X, Kammermeier H, Zorzano A. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–7092. doi: 10.1074/jbc.272.11.7085. [DOI] [PubMed] [Google Scholar]
- 22.Ramrath S, Tritschler HJ, Eckel J. Stimulation of cardiac glucose transport by thioctic acid and insulin. Horm Metab Res. 1999;31:632–635. doi: 10.1055/s-2007-978811. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Donthi RV, Huisamen B, Lochner A. Effect of vanadate and insulin on glucose transport in isolated adult rat cardiomyocytes. Cardiovasc Drugs Ther. 2000;14:463–470. doi: 10.1023/a:1007876703644. [DOI] [PubMed] [Google Scholar]
- 25.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler TJ, Fell RD, Hauck MA. Translocation of two glucose transporters in heart: effects of rotenone, uncouplers, workload, palmitate, insulin and anoxia. Biochim Biophys Acta. 1994;1196:191–200. doi: 10.1016/0005-2736(94)00211-8. [DOI] [PubMed] [Google Scholar]
- 27.Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y. The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology. 2001;142:5267–5276. doi: 10.1210/endo.142.12.8555. [DOI] [PubMed] [Google Scholar]
- 28.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler TJ. Translocation of glucose transporters in response to anoxia in heart. J Biol Chem. 1988;263:19447–19454. [PubMed] [Google Scholar]
- 30.Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- 31.Doria-Medina CL, Lund DD, Pasley A, Sandra A, Sivitz WI. Immunolocalization of GLUT-1 glucose transporter in rat skeletal muscle and in normal and hypoxic cardiac tissue. Am J Physiol. 1993;265:E454–464. doi: 10.1152/ajpendo.1993.265.3.E454. [DOI] [PubMed] [Google Scholar]
- 32.Schisler JC, Grevengoed TJ, Pascual F, Cooper DE, Ellis JM, Paul DS, Willis MS, Patterson C, Jia W, Coleman RA. Cardiac energy dependence on glucose increases metabolites related to glutathione and activates metabolic genes controlled by mechanistic target of rapamycin. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris DL, Craig JC. Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest. 1985;76:1819–1827. doi: 10.1172/JCI112174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- 35.Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. J Mol Cell Cardiol. 1999;31:493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- 36.Depré C, Veitch K, Hue L. Role of fructose 2,6-bisphosphate in the control of glycolysis. Stimulation of glycogen synthesis by lactate in the isolated working rat heart. Acta Cardiol. 1993;48:147–164. [PubMed] [Google Scholar]
- 37.Laughlin MR, Taylor J, Chesnick AS, Balaban RS. Nonglucose substrates increase glycogen synthesis in vivo in dog heart. Am J Physiol. 1994;267:H219–223. doi: 10.1152/ajpheart.1994.267.1.H217. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Nguyen VT, Taegtmeyer H. Feeding and fasting determine postischemic glucose utilization in isolated working rat hearts. Am J Physiol. 1991;260:H542–548. doi: 10.1152/ajpheart.1991.260.2.H542. [DOI] [PubMed] [Google Scholar]
- 39.Shelley HJ. Carbohydrate Reserves in the Newborn Infant. Br Med J. 1964;1:273–275. doi: 10.1136/bmj.1.5378.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodwin GW, Ahmad F, Taegtmeyer H. Preferential oxidation of glycogen in isolated working rat heart. J Clin Invest. 1996;97:1409–1416. doi: 10.1172/JCI118561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien KD, Ferguson M, Gordon D, Deeb SS, Chait A. Lipoprotein lipase is produced by cardiac myocytes rather than interstitial cells in human myocardium. Arterioscler Thromb. 1994;14:1445–1451. doi: 10.1161/01.atv.14.9.1445. [DOI] [PubMed] [Google Scholar]
- 42.Davies BS, Beigneux AP, Barnes RH, 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies BS, Goulbourne CN, Barnes RH, 2nd, Turlo KA, Gin P, Vaughan S, Vaux DJ, Bensadoun A, Beigneux AP, Fong LG, Young SG. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res. 2012;53:2690–2697. doi: 10.1194/jlr.M031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goto K, Iso T, Hanaoka H, Yamaguchi A, Suga T, Hattori A, Irie Y, Shinagawa Y, Matsui H, Syamsunarno MR, Matsui M, Haque A, Arai M, Kunimoto F, Yokoyama T, Endo K, Gonzalez FJ, Kurabayashi M. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J Am Heart Assoc. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, Goulbourne CN, Tatar A, Turlo K, Wu D, Beigneux AP, Grovenor CR, Fong LG, Young SG. High-resolution imaging of dietary lipids in cells and tissues by NanoSIMS analysis. J Lipid Res. 2014;55:2156–2166. doi: 10.1194/jlr.M053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose H, Hennecke T, Kammermeier H. Sarcolemmal fatty acid transfer in isolated cardiomyocytes governed by albumin/membrane-lipid partition. J Mol Cell Cardiol. 1990;22:883–892. doi: 10.1016/0022-2828(90)90119-m. [DOI] [PubMed] [Google Scholar]
- 48.Abumrad N, Harmon C, Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- 49.Luiken JJ, Turcotte LP, Bonen A. Protein-mediated palmitate uptake and expression of fatty acid transport proteins in heart giant vesicles. J Lipid Res. 1999;40:1007–1016. [PubMed] [Google Scholar]
- 50.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 51.Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278:16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- 52.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–278. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 53.Füllekrug J, Ehehalt R, Poppelreuther M. Outlook: membrane junctions enable the metabolic trapping of fatty acids by intracellular acyl-CoA synthetases. Front Physiol. 2012;3:401. doi: 10.3389/fphys.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, Coleman RA. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–1262. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 56.Fischer Y, Kamp J, Thomas J, Popping S, Rose H, Carpene C, Kammermeier H. Signals mediating stimulation of cardiomyocyte glucose transport by the alpha-adrenergic agonist phenylephrine. Am J Physiol. 1996;270:C1211–1220. doi: 10.1152/ajpcell.1996.270.4.C1211. [DOI] [PubMed] [Google Scholar]
- 57.Luiken JJ, van Nieuwenhoven FA, America G, van der Vusse GJ, Glatz JF. Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: involvement of sarcolemmal proteins. J Lipid Res. 1997;38:745–758. [PubMed] [Google Scholar]
- 58.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 59.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355:204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 60.Lopaschuk GD. Malonyl CoA control of fatty acid oxidation in the diabetic rat heart. Adv Exp Med Biol. 2001;498:155–165. doi: 10.1007/978-1-4615-1321-6_21. [DOI] [PubMed] [Google Scholar]
- 61.Groot PH, Scholte HR, Hulsmann WC. Fatty acid activation: specificity, localization, and function. Adv Lipid Res. 1976;14:75–126. doi: 10.1016/b978-0-12-024914-5.50009-7. [DOI] [PubMed] [Google Scholar]
- 62.Paul DS, Grevengoed TJ, Pascual F, Ellis JM, Willis MS, Coleman RA. Deficiency of cardiac acyl-CoA synthetase-1 induces diastolic dysfunction, but pathologic hypertrophy is reversed by rapamycin. Biochim Biophys Acta. 2014;1841:880–887. doi: 10.1016/j.bbalip.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastie CC, Hajri T, Drover VA, Grimaldi PA, Abumrad NA. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes. 2004;53:2209–2216. doi: 10.2337/diabetes.53.9.2209. [DOI] [PubMed] [Google Scholar]
- 64.Trent CM, Yu S, Hu Y, Skoller N, Huggins LA, Homma S, Goldberg IJ. Lipoprotein lipase activity is required for cardiac lipid droplet production. J Lipid Res. 2014;55:645–658. doi: 10.1194/jlr.M043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 66.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banke NH, Wende AR, Leone TC, O’Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awan MM, Saggerson ED. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem J. 1993;295(Pt 1):61–66. doi: 10.1042/bj2950061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 70.Orfali KA, Fryer LG, Holness MJ, Sugden MC. Long-term regulation of pyruvate dehydrogenase kinase by high-fat feeding. Experiments in vivo and in cultured cardiomyocytes. FEBS Lett. 1993;336:501–505. doi: 10.1016/0014-5793(93)80864-q. [DOI] [PubMed] [Google Scholar]
- 71.Cooper RH, Randle PJ, Denton RM. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975;257:808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- 72.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976;154:327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes, and starvation. Nature. 1963;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- 74.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Tian R. Transcriptional regulation of energy substrate metabolism in normal and hypertrophied heart. Curr Hypertens Rep. 2003;5:454–458. doi: 10.1007/s11906-003-0052-7. [DOI] [PubMed] [Google Scholar]
- 77.Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc. 2013;2:e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 79.Zhong M, Alonso CE, Taegtmeyer H, Kundu BK. Quantitative PET imaging detects early metabolic remodeling in a mouse model of pressure-overload left ventricular hypertrophy in vivo. J Nucl Med. 2013;54:609–615. doi: 10.2967/jnumed.112.108092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamirani YS, Kundu BK, Zhong M, McBride A, Li Y, Davogustto GE, Taegtmeyer H, Bourque JM. Noninvasive detection of early metabolic left ventricular remodeling in systemic hypertension. Cardiology. 2016;133:157–162. doi: 10.1159/000441276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 82.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 83.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 84.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 85.Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 86.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sano M, Wang SC, Shirai M, Scaglia F, Xie M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, Taffet GE, Hamamori Y, Michael LH, Craigen WJ, Schneider MD. Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J. 2004;23:3559–3569. doi: 10.1038/sj.emboj.7600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 89.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 90.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 91.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–3278. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 92.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasinoff BB, Patel D, O’Hara KA. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol Pharmacol. 2008;74:1722–1728. doi: 10.1124/mol.108.050104. [DOI] [PubMed] [Google Scholar]
- 94.Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, Taegtmeyer H. A PKM2 signature in the failing heart. Biochem Biophys Res Commun. 2015;459:430–436. doi: 10.1016/j.bbrc.2015.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol. 2007;47:443–467. doi: 10.1146/annurev.pharmtox.47.120505.105359. [DOI] [PubMed] [Google Scholar]
- 96.Zhang P, Shan T, Liang X, Deng C, Kuang S. Mammalian target of rapamycin is essential for cardiomyocyte survival and heart development in mice. Biochem Biophys Res Commun. 2014;452:53–59. doi: 10.1016/j.bbrc.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuzman JA, O’Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148:3477–3484. doi: 10.1210/en.2007-0099. [DOI] [PubMed] [Google Scholar]
- 101.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 102.Xia Y, Wen HY, Young ME, Guthrie PH, Taegtmeyer H, Kellems RE. Mammalian target of rapamycin and protein kinase A signaling mediate the cardiac transcriptional response to glutamine. J Biol Chem. 2003;278:13143–13150. doi: 10.1074/jbc.M208500200. [DOI] [PubMed] [Google Scholar]
- 103.Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 104.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S, Guthrie PH, Chan SS, Haq S, Taegtmeyer H. Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart. Cardiovasc Res. 2007;76:71–80. doi: 10.1016/j.cardiores.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 107.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol. 2004;24:6231–6240. doi: 10.1128/MCB.24.14.6231-6240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, Bu G, Li W, Boyle DW, Rubart M, Field LJ, Abraham R, Liechty EA, Shou W. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 112.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990;265:2563–2568. [PubMed] [Google Scholar]
- 113.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 114.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Biol. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cooksey RC, Hebert LF, Jr, Zhu JH, Wofford P, Garvey WT, McClain DA. Mechanism of hexosamine-induced insulin resistance in transgenic mice overexpressing glutamine:fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology. 1999;140:1151–1157. doi: 10.1210/endo.140.3.6563. [DOI] [PubMed] [Google Scholar]
- 117.Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, Shi Y. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006;55:93–101. [PubMed] [Google Scholar]
- 118.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2012;302:H2122–2130. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Ma J, Liu T, Wei AC, Banerjee P, O’Rourke B, Hart GW. O-GlcNAcomic profiling identifies widespread O-linked beta-N-acetylglucosamine modification (O-GlcNAcylation) in oxidative phosphorylation system regulating cardiac mitochondrial function. J Biol Chem. 2015;290:29141–29153. doi: 10.1074/jbc.M115.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kasper EK, Hruban RH, Baughman KL. Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. Am J Cardiol. 1992;70:921–924. doi: 10.1016/0002-9149(92)90739-l. [DOI] [PubMed] [Google Scholar]
- 123.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D’Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ge F, Hu C, Hyodo E, Arai K, Zhou S, Lobdell Ht, Walewski JL, Homma S, Berk PD. Cardiomyocyte triglyceride accumulation and reduced ventricular function in mice with obesity reflect increased long chain fatty acid uptake and de novo fatty acid synthesis. J Obes. 2012;2012:205648. doi: 10.1155/2012/205648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, Sempokuya T, Alegria L, Bugger H, Abel ED. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–1434. doi: 10.2337/db10-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 127.Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, Culver B, Ren J. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol. 2006;188:25–36. doi: 10.1677/joe.1.06241. [DOI] [PubMed] [Google Scholar]
- 128.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 130.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci. 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 131.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 132.Cox KB, Liu J, Tian L, Barnes S, Yang Q, Wood PA. Cardiac hypertrophy in mice with long-chain acyl-CoA dehydrogenase or very long-chain acyl-CoA dehydrogenase deficiency. Lab Invest. 2009;89:1348–1354. doi: 10.1038/labinvest.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Melegh B, Bene J, Mogyorosy G, Havasi V, Komlosi K, Pajor L, Olah E, Kispal G, Sumegi B, Mehes K. Phenotypic manifestations of the OCTN2 V295X mutation: sudden infant death and carnitine-responsive cardiomyopathy in Roma families. American journal of medical genetics Part A. 2004;131:121–126. doi: 10.1002/ajmg.a.30207. [DOI] [PubMed] [Google Scholar]
- 134.Rupp H, Jacob R. Metabolically-modulated growth and phenotype of the rat heart. European heart journal. 1992;13(Suppl D):56–61. doi: 10.1093/eurheartj/13.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- 135.Exil VJ, Roberts RL, Sims H, McLaughlin JE, Malkin RA, Gardner CD, Ni G, Rottman JN, Strauss AW. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ Res. 2003;93:448–455. doi: 10.1161/01.RES.0000088786.19197.E4. [DOI] [PubMed] [Google Scholar]
- 136.Kim T, He L, Johnson MS, Li Y, Zeng L, Ding Y, Long Q, Moore JF, Sharer JD, Nagy TR, Young ME, Wood PA, Yang Q. Carnitine Palmitoyltransferase 1b Deficiency Protects Mice from Diet-Induced Insulin Resistance. J Diabetes Metab. 2014;5:361. doi: 10.4172/2155-6156.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim T, Moore JF, Sharer JD, Yang K, Wood PA, Yang Q. Carnitine Palmitoyltransferase 1b Deficient Mice Develop Severe Insulin Resistance After Prolonged High Fat Diet Feeding. J Diabetes Metab. 2014;5 doi: 10.4172/2155-6156.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126:1705–1716. doi: 10.1161/CIRCULATIONAHA.111.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16:757–760. doi: 10.1111/dom.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chegary M, Brinke H, Ruiter JP, Wijburg FA, Stoll MS, Minkler PE, van Weeghel M, Schulz H, Hoppel CL, Wanders RJ, Houten SM. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta. 2009;1791:806–815. doi: 10.1016/j.bbalip.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tucci S, Flogel U, Hermann S, Sturm M, Schafers M, Spiekerkoetter U. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(-/-)) mice. Biochim Biophys Acta. 2014;1842:677–685. doi: 10.1016/j.bbadis.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 142.Bakermans AJ, Geraedts TR, van Weeghel M, Denis S, Joao Ferraz M, Aerts JM, Aten J, Nicolay K, Houten SM, Prompers JJ. Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging. 2011;4:558–565. doi: 10.1161/CIRCIMAGING.111.963751. [DOI] [PubMed] [Google Scholar]
- 143.Xiong D, He H, James J, Tokunaga C, Powers C, Huang Y, Osinska H, Towbin JA, Purevjav E, Balschi JA, Javadov S, McGowan FX, Jr, Strauss AW, Khuchua Z. Cardiac-specific VLCAD deficiency induces dilated cardiomyopathy and cold intolerance. Am J Physiol Heart Circ Physiol. 2014;306:H326–338. doi: 10.1152/ajpheart.00931.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA, O’Brien WE, Wood PA. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci USA. 1998;95:15592–15597. doi: 10.1073/pnas.95.26.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 146.Chess DJ, Khairallah RJ, O’Shea KM, Xu W, Stanley WC. A high-fat diet increases adiposity but maintains mitochondrial oxidative enzymes without affecting development of heart failure with pressure overload. Am J Physiol Heart Circ Physiol. 2009;297:H1585–1593. doi: 10.1152/ajpheart.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 148.Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, Finck BN, Kelly DP. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–11162. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grevengoed TJ, Martin SA, Katunga L, Cooper DE, Anderson EJ, Murphy RC, Coleman RA. Acyl-CoA synthetase 1 deficiency alters cardiolipin species and impairs mitochondrial function. J Lipid Res. 2015;56:1572–1582. doi: 10.1194/jlr.M059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grevengoed TJ, Cooper DE, Young PA, Ellis JM, Coleman RA. Loss of long-chain acyl-CoA synthetase isoform 1 impairs cardiac autophagy and mitochondrial structure through mechanistic target of rapamycin complex 1 activation. FASEB J. 2015;29:4641–4653. doi: 10.1096/fj.15-272732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang Y, Lei L, Liu D, Jovin I, Russell R, Johnson RS, Di Lorenzo A, Giordano FJ. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1alpha-dependent function. Proc Natl Acad Sci USA. 2012;109:17478–17483. doi: 10.1073/pnas.1209281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 153.Coppiello G, Collantes M, Sirerol-Piquer MS, Vandenwijngaert S, Schoors S, Swinnen M, Vandersmissen I, Herijgers P, Topal B, van Loon J, Goffin J, Prosper F, Carmeliet P, Garcia-Verdugo JM, Janssens S, Penuelas I, Aranguren XL, Luttun A. Meox2/Tcf15 heterodimers program the heart capillary endothelium for cardiac fatty acid uptake. Circulation. 2015;131:815–826. doi: 10.1161/CIRCULATIONAHA.114.013721. [DOI] [PubMed] [Google Scholar]
- 154.Iso T, Maeda K, Hanaoka H, Suga T, Goto K, Syamsunarno MR, Hishiki T, Nagahata Y, Matsui H, Arai M, Yamaguchi A, Abumrad NA, Sano M, Suematsu M, Endo K, Hotamisligil GS, Kurabayashi M. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol. 2013;33:2549–2557. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zou Z, Tong F, Faergeman NJ, Borsting C, Black PN, DiRusso CC. Vectorial acylation in Saccharomyces cerevisiae. Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. J Biol Chem. 2003;278:16414–16422. doi: 10.1074/jbc.M210557200. [DOI] [PubMed] [Google Scholar]
- 156.Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res. 2006;47:665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- 157.Jia Z, Pei Z, Maiguel D, Toomer CJ, Watkins PA. The fatty acid transport protein (FATP) family: very long chain acyl-CoA synthetases or solute carriers? J Mol Neurosci. 2007;33:25–31. doi: 10.1007/s12031-007-0038-z. [DOI] [PubMed] [Google Scholar]