Abstract
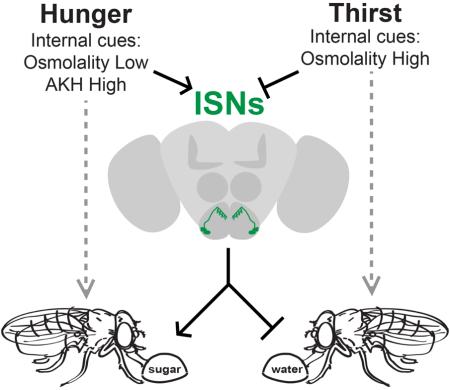
Hunger and thirst are ancient homeostatic drives for food and water consumption. Although molecular and neural mechanisms underlying these drives are currently being uncovered, less is known about how hunger and thirst interact. Here, we use molecular genetic, behavioral, and anatomical studies in Drosophila to identify four neurons that modulate food and water consumption. Activation of these neurons promotes sugar consumption and restricts water consumption, whereas inactivation promotes water consumption and restricts sugar consumption. By calcium imaging studies, we show that these neurons are directly regulated by a hormone signal of nutrient levels and by osmolality. Finally, we identify a hormone receptor and an osmolality-sensitive ion channel that underlie this regulation. Thus, a small population of neurons senses internal signals of nutrient and water availability to balance sugar and water consumption. Our results suggest an elegant mechanism by which interoceptive neurons oppositely regulate homeostatic drives to eat and drink.
Graphical abstract
Introduction
To achieve homeostasis, animals must regulate consumption of external nutrients based on internal metabolic needs. Remarkably, animals whose nervous systems differ dramatically in organization exhibit some of the same homeostatic consumption behaviors, such as orally consuming food or water in response to starvation or dehydration. While these similarities suggest conserved underlying mechanisms, how nervous systems regulate consumption in a manner that reflects internal state remains an open question.
A key requirement for homeostatic regulation is the ability to sense internal nutrient abundance and either promote consumption in nutrient-deprived states or inhibit consumption in nutrient-replete states. In mammals, a major site of internal nutrient sensing is the hypothalamus, a conserved forebrain region that senses nutrients and coordinates behavioral responses to changes in their abundance (Sternson, 2013). Neurons in the hypothalamus include direct sensors of circulating sugars like glucose, as well as sensors of metabolic cues, like insulin, ghrelin, and glucagon. The hypothalamus also contains neurons whose activity is regulated by extracellular osmolality and are therefore sensitive to internal water abundance (Bourque, 2008). However, how the nervous system uses information encoded by hypothalamic sensors to regulate consumption of food and water remains unresolved.
Like mammals, the fruit fly Drosophila melanogaster regulates consumption of food and water depending on internal metabolic state. Although flies lack a direct homolog of the hypothalamus, neural populations in the Drosophila brain function as internal nutrient sensors, including glucose, fructose, and amino acid sensors that regulate feeding decisions (Dus et al., 2015; Bjordal et al., 2014; Miyamoto et al., 2012). Flies also regulate water consumption based on internal water abundance (Dethier, 1976), although internal sensors underlying this behavior have not previously been characterized. Thus, mammals and insects both regulate food and water consumption based on internal metabolic state, and in many instances accomplish this regulation by similar mechanisms. However, the neurons and molecules that regulate homeostatic consumption remain incompletely understood. In particular, mechanisms that coordinate the consumption of different essential nutrients, such as sugar or water, have been largely unexplored.
Here we report findings from two behavioral screens for neurons that regulate food or water consumption in Drosophila. Surprisingly, these screens independently identified the same four neurons as regulators of both food and water consumption. The neurons are located in the subesophageal zone (SEZ), a key relay for feeding regulation in the fly brain, and we name them Interoceptive SEZ Neurons (ISNs). Using genetic tools, behavioral assays, and calcium imaging, we show that ISNs are sensitive both to an internal signal of nutrient deprivation, the glucagon-like peptide adipokinetic hormone (AKH), and an internal signal of water abundance, extracellular osmolality. We identify the G-protein coupled receptor, adipokinetic hormone receptor (AKHR), and a conserved TRPV channel, Nanchung (Nan), as underlying responses to AKH and osmolality, respectively. Finally, we show that ISNs oppositely regulate sugar and water consumption, suggesting that they function to restore internal homeostasis. The convergence of internal signals of nutrient and water availability onto interoceptive neurons suggests an unexpected principle by which the nervous system might coordinate homeostatic behaviors.
Results
A behavioral screen for neurons that regulate feeding
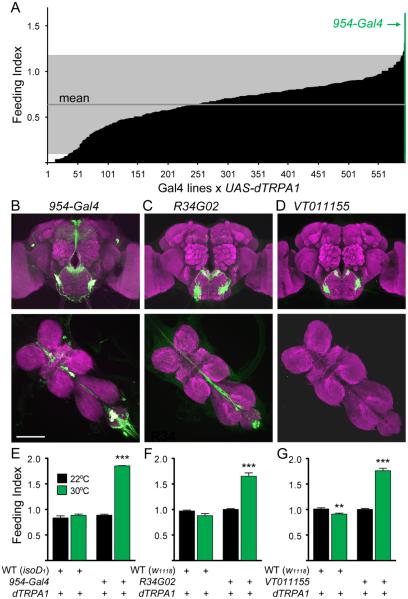
To identify neurons that regulate feeding, we transiently activated candidate neurons with the heat-activated cation channel, dTRPA1, and determined the effect on feeding in adult flies. Consumption was monitored by scoring the amount of blue dye in the abdomens of flies with access to 200mM sucrose containing blue dye for 30 minutes. Approximately 600 Gal4 lines from the InSite collection (Gohl et al., 2011) with expression in the central nervous system were crossed to flies carrying the UAS-dTRPA1 transgene (Hamada et al., 2008), allowing for Gal4-dependent neural activation. Fed flies were tested for sucrose consumption during heat-induced depolarization of Gal4-expressing neurons (Figure 1A; Table S1 reports transgenic flies used in this study). Four lines exhibited dramatically increased feeding, with the 954-Gal4 line showing the strongest consumption.
Figure 1. Identification of neurons that promote sucrose consumption.
A. Behavioral screen for flies that overconsume sucrose. Gal4 lines were crossed to UAS-dTRPA1 for heat-inducible neural activation and tested for sucrose consumption at 30°C under non-deprived conditions (n=5-30 flies/line). Gray marks 2 standard deviations from the mean.
B-D. (B) Expression of 954-Gal4, UAS-mCD8::GFP (green) in brain (top) and ventral nerve cord (middle), (C) R34G02 expression, (D) VT011155 expression. Scale 100 μm.
E-G. (E) Sucrose consumption of isoD1 (WT), UAS-dTRPA1 flies or 954-Gal4, UAS-dTRPA1 flies at 30°C (upon neural activation) and 22°C (control). (F) R34G02 sucrose consumption phenotype, (WT = w1118), (G) VT011155 sucrose consumption phenotype, (WT = w1118). n=5-8 vials, 15-20 flies/vial, mean ± SEM, **p<0.01, ***p<0.001, t-tests (23°C vs 32°C) with Holm-Sidak correction.
See Figure S1 for additional experiments to identify the neurons that promote sucrose consumption. See Table S1 for fly genotypes for all experiments.
Four neurons in the 954-Gal4 line promote ingestive behaviors to sucrose
To identify neurons causal for increased feeding in the 954-Gal4 line, we began by characterizing its expression pattern. In the central brain, 954-Gal4 drove UAS-mCD8::GFP expression in neurons of the pars intercerebralis, dorsal lateral protocerebrum, subesophageal zone (SEZ), and ventral nerve cord (VNC) (Figure 1B). To identify which neurons contribute to the feeding phenotype of the 954-Gal4 line, we employed an intersectional approach using the Gal4 inhibitor Gal80 to restrict Gal4 expression to smaller neural populations. The Tshirt-Gal80 transgene (Clyne and Miesenbock, 2008) blocked Gal4 expression in the VNC but did not eliminate increased consumption upon dTRPA1 activation of 954-Gal4 neurons, demonstrating that VNC neurons are not required (Figure S1A). Next, we identified one line, 149-Gal80 (Gordon, M.D., unpublished), that eliminated SEZ GFP expression without affecting pars intercerebralis or dorsal lateral protocerebrum expression (Figure S1B). This transgene eliminated the increased feeding phenotype in the 954-Gal4 line, arguing that 954-Gal4 SEZ neurons are necessary for increased consumption.
We screened existing Gal4 collections (Jenett et al., 2012; Dickson, B.J., unpublished) to identify lines that exhibit Gal4 expression in these SEZ neurons and identified two Gal4 lines, R34G02 and VT011155, that labeled neurons that resembled the 954-Gal4 SEZ cluster. The R34G02 line also drove expression in a pair of VNC neurons and abdominal ganglia projections (Figure 1C). Remarkably, the VT011155 line exclusively labeled the SEZ neurons (Figure 1D). Both lines exhibited the feeding phenotype identified in 954-Gal4 (Figure 1E-G). In addition, activation of 954-Gal4, R34G02 or VT011155 neurons with UAS-dTRPA1 increased rates of proboscis extension to sugar stimuli, a non-ingestive behavior that flies exhibit to an appetitive taste stimulus (Figure S1C). We generated a R34G02-LexA line and performed double labeling experiments with the Gal4 lines to test for co-expression and found that 954-Gal4, VT011155, and R34G02-Gal4 are all co-expressed in the same two SEZ neurons per hemisphere, with 954-Gal4 also showing expression in two additional SEZ neurons per hemisphere (Figure S1D, E). These findings demonstrate that activation of four neurons in the SEZ causes increased feeding and promotes proboscis extension in well-fed animals. We name these interoceptive SEZ neurons (ISNs) and the VT011155 line which specifically labels them ISN-Gal4.
ISNs directly respond to AKH and are indirectly inhibited by insulin
Pre- and post-synaptic sites on ISNs overlap in the dorsal SEZ (Figure S1F). ISNs might therefore be components of feeding sensorimotor circuits, or they may modulate activity in these circuits in response to internal cues. To distinguish between these models, we tested whether ISNs respond to sensory detection of taste compounds. Tastants were applied to the proboscis while monitoring activity in ISNs by GCaMP5G or GCaMP6s calcium imaging in live flies (Akerboom et al., 2012; Harris et al., 2015). Although stimulation with sucrose, water, or the bitter compound denatonium triggered sensory neuron responses, no activation was seen in ISNs (Figure S2), indicating that the ISNs are unlikely to report detection of taste compounds.
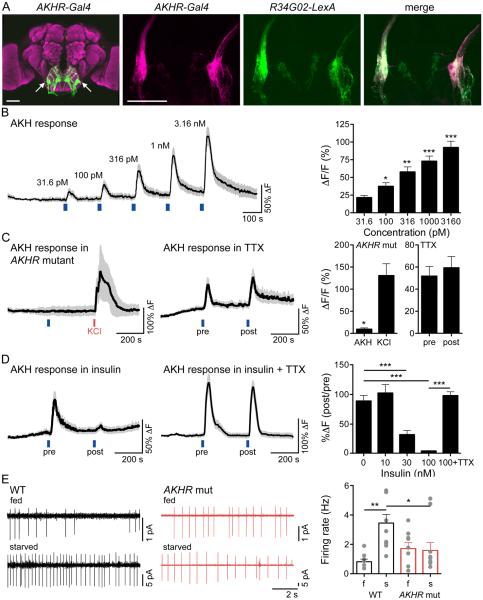
An alternative hypothesis is that these neurons encode information about hunger state. As hormones often signal metabolic status, we tested whether existing hormone receptor Gal4 lines marked the ISNs. One line, adipokinetic hormone receptor (AKHR)-Gal4 (Bharucha et al., 2008) exhibited strong labeling of ISNs, confirmed by double labeling with R34G02-LexA, suggesting these neurons may be regulated by AKH (Figure 2A).
Figure 2. ISNs respond to AKH and are inhibited by insulin.
A. (left) Expression of AKHR-Gal4, UAS-mCD8::GFP in brain. (second) AKHR-Gal4, UAS-mCD8::RFP in SEZ, (third) R34G02-LexA, lexAop-mCD8::GFP in SEZ, (right) overlay. Scale 50 μm.
B. (left) GCaMP5G increase in ISNs in response to AKH (31.6 pM, 100 pM, 316 pM, 1 nM, 3.16 nM) applied at the blue lines. n=7, mean (black line) ± SEM (grey). (right) Maximum GCaMP5G increase (%ΔF/F) to AKH, mean ± SEM, same data as trace. Repeated-measures one way ANOVA followed by holm-sidak post hoc test, comparing to 31.6 pM response. *p<0.05, **p<0.01, ***p<0.001.
C. (left) GCaMP5G increase in ISNs to 1 nM AKH (blue line) or 30 mM KCl (red line) in the AKHR mutant. n=5, mean ± SEM. (middle) GCaMP5G increase in ISNs to 316 pM AKH (blue line) pre- or post-TTX (100 nM) application. n=8, mean ± SEM. (right) GCaMP5G increase (%ΔF/F) to AKH in the AKHR mutant (t-test, *p<0.05) or pre- or post-TTX application (t-test, ns). Mean ± SEM, same data as traces.
D. (left) GCaMP5G increase in ISNs to 316 pM AKH (blue line) pre- or post-insulin (100 nM) application. n=11, mean ± SEM. (middle) GCaMP5G increase in ISNs to 316 pM AKH (blue line) pre- or post-insulin (100 nM) and TTX (100 nM) application. n=10, mean ± SEM. (right) GCaMP5G change (%ΔF). Mean ± SEM, insulin concentrations in the absence of TTX: One-way ANOVA with Dunnet’s post hoc test comparing to 0 nM Insulin, ***p<0.001. Effect of TTX: t-test (100nM Insulin vs. 100nM insulin + TTX), ***p<0.001, same data as traces plus additional insulin concentrations.
E. (left) Example cell-attached recordings from AKHR+ SEZ neurons in AKHR mutant or wild type flies, fed (f) or starved (s) 24H, and (right) summary graph. n = 6-11, mean +/− SEM, one-way ANOVA, Tukey Post Hoc, **p<0.01, *p<0.05. See Figure S2, showing that ISNs are not taste-responsive.
AKH is a peptide hormone that is synthesized exclusively by neurosecretory cells in the corpus cardiacum and secreted into the circulating hemolymph, where it acts in a similar manner to mammalian glucagon. AKH secretion is stimulated under low nutrient conditions, which in turn leads to lipolysis, glycogenolysis, and release of sugar and lipid nutrients into the hemolymph from the fat body, the primary site of nutrient storage in Drosophila (Kim and Rulifson, 2004; Lee and Park, 2004). The endocrine role of AKH in regulation of insect metabolism is established, and this hormone is well positioned to signal nutrient status to the brain (Bharucha et al., 2008).
To directly test whether AKH modulates ISNs, calcium levels in these neurons were monitored by GCaMP5G fluorescence upon AKH perfusion in a dissected brain preparation (Figure 2B). Brief pulses of AKH produced rapid, robust, and dose-dependent GCaMP5G fluorescence increases with picomolar to nanomolar AKH concentrations, the physiological range of AKH measured in locust hemolymph (Candy, 2002). The ISNs of animals lacking AKHR did not respond to AKH, verifying that the AKH-induced responses were a result of activation of AKHR (Figure 2C).
In principle, the AKH-induced response might be cell-autonomous (AKH might directly bind to AKHR on ISNs to increase calcium) or non-autonomous (AKH might bind a receptor on other neurons that increase ISN activity via synaptic transmission). To test whether AKH directly modulates ISN activity, we applied the voltage-gated sodium channel blocker tetrodotoxin (TTX) to inhibit action potentials. ISNs responded to AKH even in the presence of TTX (Figure 2C), arguing that AKH directly activates ISNs.
AKH plays a role analogous to glucagon in signaling nutrient depletion and promoting release of stored nutrients. Insulin plays an opposing role, signaling nutrient abundance and promoting storage of circulating nutrients. Given the opposing endocrine roles of insulin and AKH, we tested whether insulin might regulate ISNs in a manner opposite to AKH. Insulin application alone did not induce a calcium response in ISNs (not shown). To test whether insulin affects the ability of ISNs to respond to AKH, dissected brains were perfused with two spaced pulses of AKH, separated by a 3-minute perfusion with insulin (Figure 2D). Insulin reduced the response of ISNs to AKH in a concentration-dependent manner. To test whether insulin acts cell-autonomously, TTX was applied to eliminate action potentials and non-autonomous effects. We found that insulin no longer reduced AKH responses in the presence of TTX (Figure 2D). This argues that ISNs do not directly sense insulin, but receive synaptic input from other neurons that provide an inhibitory drive onto ISNs. These studies demonstrate that ISNs are capable of receiving and integrating inputs reflecting both low and high nutrient levels, directly responding to AKH and receiving inhibition from an insulin-sensitive pathway.
To directly test whether ISNs report physiological need, we reasoned that the activity in ISNs would likely be different in starved versus fed animals. We monitored ISN activity by cell-attached electrophysiological recordings in the living fly under fed or starved conditions. ISN activity decreased in the fed state and increased in the starved state, and this state-dependent activity of ISNs was absent in the AKHR mutant (Figure 2E). These experiments show that the ISNs are modulated in the living animal based on nutritional state and that this requires AKHR.
A behavioral screen for molecules that regulate water consumption
Our studies indicate that ISNs respond to signals of internal energy state and that their activity is sufficient to promote feeding, separating metabolic and neural functions of AKH. In a different behavioral screen to identify molecules that regulate water consumption, we ultimately identified additional molecules that are expressed in ISNs, regulate their activity, and provide unexpected insight into their function.
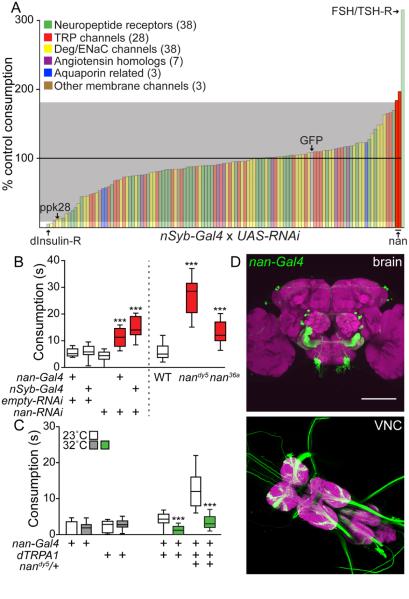
With the goal of identifying molecules that might signal thirst or water satiety in Drosophila, we screened a panel of 94 candidate ion channels and neuropeptide receptors for water consumption defects following RNAi knockdown with the pan-neuronal nSynaptobrevin-Gal4 (nSyb-Gal4) line. Flies were placed in less than 20% relative humidity for 2 hours, which specifically increased water consumption (Lin et al., 2014) (Figure 3A). Upon pan-neuronal RNAi expression, the majority of RNAi lines showed behavior similar to GFP RNAi controls (109% control consumption). As expected, RNAi to PPK28, an ion channel expressed in sensory neurons and essential for water taste detection (Cameron et al., 2010; Chen et al., 2010), reduced water consumption (12% control consumption). In addition, RNAi against a number of genes reproducibly increased or decreased water consumption compared to sibling controls. These included the insulin-like receptor (5% control consumption) and the follicle stimulating hormone receptor (315% control consumption), which have been implicated in water homeostasis in insects (Liu et al., 2015; Paluzzi et al., 2014; Sellami et al., 2011).
Figure 3. Identification of nanchung and its role in water consumption.
A. Behavioral screen for genes regulating water consumption. UAS-RNAi lines were crossed to nSyb-Gal4. 10 RNAi and 10 sibling control flies were assayed/RNAi line. Average water consumption time in RNAi group versus sibling control was used to calculate % control consumption. Gray marks 2 standard deviations from mean. Two nan RNAi lines (BSC# 31674 and 31925) are highlighted in red. Behavioral data for nan RNAi BSC# 31925 are shown unless noted.
B. Water consumption in nan RNAi and nan mutant backgrounds. For all box plots, whiskers = 10th to 90th percentile, box = 25th to 75th percentile, and line in box = median. n=28-60 flies, one-way ANOVA, Tukey Post Hoc: for RNAi experiments (***p<0.001), and for mutant experiments (***p<0.001).
C. Activating nan-Gal4 neurons affects water consumption. n=20-63 flies, t-test, Holm-Sidak correction, (23°C vs 32°C per genotype), ***p<0.001.
D. nan-Gal4, UAS-mCD8::GFP expression in the brain (top) and VNC (bottom). Scale 100 μm.
Among the candidates with no known role in water consumption behavior was the TRPV family member, Nanchung (Nan) (184% and 197% control consumption, independent RNAi lines). Nan is a non-selective cation channel that participates in Drosophila proprioception, hearing, and hygrosensation (Gong et al., 2004; Kim et al., 2003; Liu et al., 2007; Zhang et al., 2013). Interestingly, TRPV channels function as osmosensors in C. elegans and mammals and likely play a role in water consumption regulation in mice (Colbert et al., 1997; Liedtke and Friedman, 2003; Liedtke et al., 2003). We therefore chose to further investigate the role of Nan in Drosophila water consumption.
nanchung is required to restrict water consumption
To examine whether nan-expressing neurons regulate water consumption, we tested the behavioral response of nan mutants as well as animals expressing nan RNAi specifically in nan-Gal4 neurons, using described lines (Kim et al., 2003). Water consumption time was measured in single flies following two hours of acute desiccation (Figure 3B). Both nan36a and nandy5 mutants as well as nan-Gal4, UAS-nan RNAi flies consumed significantly more water than controls, demonstrating that nanchung is necessary to restrict water consumption.
To examine how activity in nanchung neurons influences water consumption, we inducibly activated these neurons with dTRPA1. Flies expressing UAS-dTRPA1 in nan-Gal4 neurons reduced water consumption upon dTRPA1 activation. Transient activation of nan neurons was also sufficient to rescue elevated water consumption observed in nandy5 heterozygotes (Figure 3C), suggesting that nanchung neurons function to restrict water consumption.
Nanchung neurons in the SEZ are osmosensitive
The expression pattern of nanchung has been described previously using nan-Gal4 (Kim et al., 2003; Liu et al., 2007) (Figure 3D). These studies found expression in chordotonal organs of the legs and antennae, as well as in a small number of putative interneurons in the higher brain. Because nan has been proposed to detect water vapor in antennal neurons, we tested whether antennae were required for dTRPA1-mediated activation of nan neurons to reduce water consumption in nandy5 heterozygotes and found no role for antennae (not shown). These data argue that Nanchung’s function as an antennal sensor of water vapor is independent of its role in water consumption.
An alternative possibility is that Nanchung functions in central brain neurons labeled by nan-Gal4 to regulate water consumption. Nan belongs to a class of mechanosensitive TRPV channels and is sufficient to confer osmosensitivity to cultured mammalian cells (Kim et al., 2003). Moreover, TRPV channels have been proposed to confer osmosensitivity to central neurons in the mammalian brain (Bourque, 2008; Liedtke and Friedman, 2003). We therefore tested whether central neurons labeled by the nan-Gal4 line were directly osmosensitive.
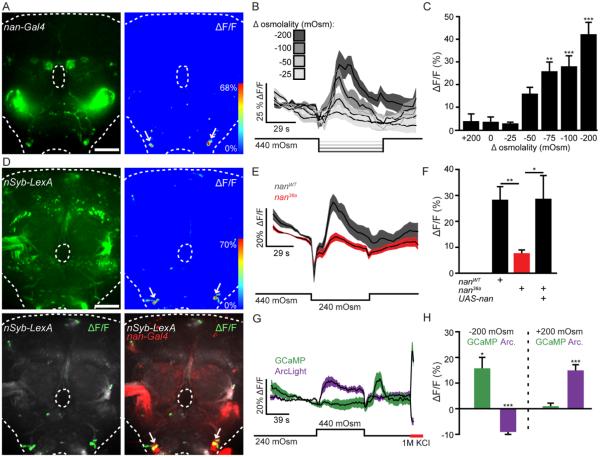
nan-Gal4, UAS-GCaMP6s brains were perfused with artificial hemolymph (AHL) of different osmolalities while GCaMP6s fluorescence was monitored (Chen et al., 2013). Decreasing osmolality caused robust calcium responses from bilaterally symmetric nan-Gal4 neurons in the ventrolateral SEZ (Figure 4A). These responses were dose-dependent and observed following osmolality decreases but not increases (Figure 4B, C). To test whether these responses are specific to nan SEZ neurons, brains expressing GCaMP6s with the pan-neuronal driver nSyb-LexA were perfused with low osmolality solution. Again, strong calcium responses were observed only in the SEZ neurons labeled by nan-Gal4 (Figure 4D; Figure S3). Moreover, Nan is necessary for the osmolality responses, as calcium responses to low osmolality were significantly decreased in a nan36a mutant background and were rescued with a UAS-nan transgene (Kim et al., 2003) (Figure 4E, F).
Figure 4. Nanchung SEZ neurons respond to osmolality.
A. nan-Gal4, UAS-GCaMP6s expression in brain (left) and example ΔF/F heat-map of the same brain (right) upon an extracellular osmolality decrease of 200mOsm/kg. Scale 50 μm.
B. ΔF/F traces (mean ± SEM) for osmolality decreases of 25, 50, 100, and 200 mOsm/Kg (n=9-12 brains/condition).
C. Maximum ΔF/F responses (mean ± SEM) for osmolality changes. n=7-12 brains/condition, One way ANOVA, Dunnet’s Post Hoc to mock (0 mOsm change) **p<0.01, ***p<0.001, data from B plus additional osmolalities.
D. (top left) nSyb-LexA, lexAop-GCaMP6s expression in brain. (top right) ΔF/F heat-map to an osmolality decrease of 200mOsm/kg in the same brain. (bottom left) ΔF/F response (green) overlayed on nSyb-LexA, lexAop-GCaMP6s expression (grey). (bottom right) ΔF/F response (green) overlayed on nSyb-LexA, lexAop-GCaMP6s (grey) and nan-Gal4 expression (red) in same brain. Scale 50 μm.
E. ΔF/F traces upon osmolality decreases of 200 mOsm/Kg in nan36a mutant or wild type (WT) flies. n=28-33. E-H: data are mean ± SEM.
F. ΔF/F graphs for WT, mutant, and rescue flies (UAS-nan; nan36a). n=33, 28, and 12. one-way ANOVA,Tukey Post Hoc, *p<0.05, **p<0.01, data from E plus rescue.
G. ΔF/F traces to 200 mOsm/Kg osmolality decreases in flies expressing UAS-GCaMP6s (green, n=9 brains) or UAS-ArcLight (magenta, n=11 brains) in ISNs.
H. ΔF/F graphs for GCaMP6s and ArcLight from F. One sample t-tests for difference from theoretical mean of 0.0, *p<0.05, ***p<0.001.
See also Figure S3, showing quantification of whole brain responses.
These data indicate that nan SEZ neurons are uniquely capable of responding to low extracellular osmolality with calcium increases. Because GCaMP6s preferentially reports calcium increases over decreases, we used the genetically encoded voltage sensor ArcLight (Cao et al., 2013) to test whether nan SEZ neuron activity is bidirectionally regulated by osmolality changes. Consistent with GCaMP6s imaging, decreasing extracellular osmolality decreased ArcLight fluorescence, indicating depolarization. In addition, we found that increasing extracellular osmolality significantly increased ArcLight fluorescence in these neurons, indicating hyperpolarization (Figure 4G, H). Taken together, these data suggest that nan SEZ neurons report bidirectional extracellular osmolality changes in the brain.
The ISNs express nanchung and AKHR and respond to osmolality and AKH
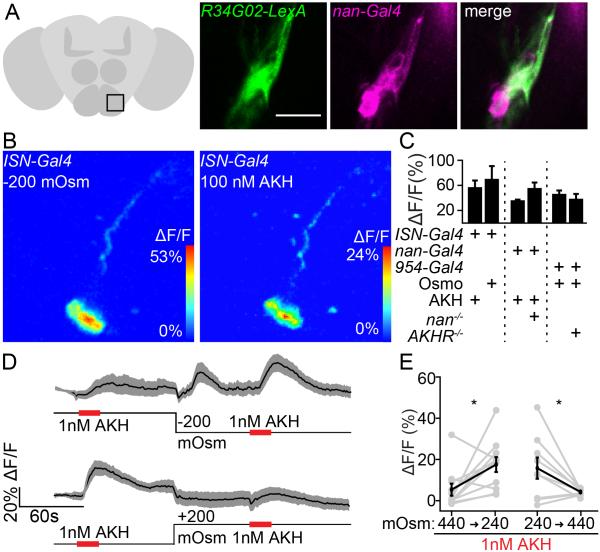
During the course of these studies, we noted that the osmosensitive nan SEZ neurons had a similar spatial location and morphology as the ISNs that express AKHR, suggesting that they might be the same neurons. We tested this by examining overlap between nan-Gal4 neurons and the ISNs, marked by R34G02-LexA, and indeed found nan-Gal4 labels the ISNs as well as two additional SEZ neurons per hemisphere (Figure 5A). Consistent with this overlap, the ISNs responded to low osmolality and to AKH (Figure 5B, C). Responses were detected in neurites and cell bodies (Figure S3C, D). The AKHR-negative, Nan-positive neurons did not respond to osmolality or contribute to consumption behavior (Figure S4), arguing that the ISNs are a unique class of neurons that respond to osmolality and AKH. Thus, two screens for neurons regulating different homeostatic behaviors independently identified the ISNs.
Figure 5. Nanchung SEZ neurons are ISNs.
A. Co-expression of R34G02-LexA and nan-Gal4 in ISNs. Zoom shows one nan-Gal4 cluster. Scale 20 μm.
B. (left) Low osmolality response and (right) AKH response in the same cell.
C. maximum ΔF/F graphs (mean ± SEM) to 200 mOsm/kg osmolality decreases (Osmo) or 1 nM AKH in control or mutant backgrounds. n=5-10 brains, t-test Holm-Sidak correction, ns.
D. ΔF/F traces (mean ± SEM) to 1 nM AKH in 440 mOsm/kg (high) or 240 mOsm/Kg (low) extracellular osmolality and graph of ΔF/F responses. Top: n=9 brains. Bottom: n = 11 brains.
E. Quatification of ΔF/F graphs from D , mean ± SEM. brains, paired t-test, *p<0.05.
See also Figure S4, showing that the AKHR-, Nan+ neurons do not respond to osmolality.
ISNs are responsive to both osmolality and the hormone AKH. To examine how ISNs might integrate these two signals, we first asked whether Nan might be required for ISNs to sense AKH, and whether AKHR might be required for ISNs to sense osmolality. We therefore monitored osmolality responses in AKHR mutants and AKH responses in nan mutants (Figure 5C). By calcium imaging, loss of AKHR did not affect the ability of ISNs to respond to osmolality, nor did loss of Nan affect the AKH response. All imaging experiments were performed under controlled osmolality and AKH conditions, as monitoring GCaMP activity by necessity requires removing cuticle and exposing the brain to artificial hemolymph. Thus, these experiments indicate that each input increases ISNs activity via an independent molecular mechanism, but do not directly monitor interactions between ISN inputs.
We therefore asked whether ISN activity resulting from changes in one input, osmolality, might affect the ability of ISNs to respond to another input, AKH. To test this, we monitored calcium responses to AKH in ISNs of brains perfused with high or low extracellular osmolality. We found that high extracellular osmolality significantly reduced AKH responses by an average of 70% (Figure 5D, E). These results argue that ISNs sense extracellular AKH and osmolality via independent molecular mechanisms, AKHR and Nanchung, but that these two inputs regulate a common output, ISN activity.
Starvation reduces Drosophila hemolymph osmolality
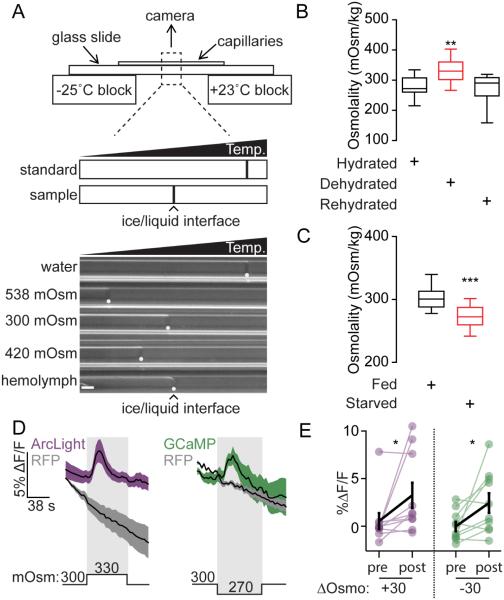
AKH levels increase with starvation and activate ISNs, consistent with its action as a hunger signal that drives feeding. Low extracellular osmolality also activates ISNs, suggesting that low osmolality might also act as an internal signal of nutrient deprivation. Flies starved for one day have approximately 75% lower hemolymph sugar levels (Na et al., 2013), and we hypothesized that this reduction in sugar levels might reduce hemolymph osmolality. To test this, we measured the hemolymph osmolality of single fed or starved flies with a temperature gradient osmometer (Arav and Rubinsky, 1994) (Figure 6A).
Figure 6. Drosophila hemolymph osmolality decreases during starvation.
A. Top: Temperature (Temp.) gradient osmometer. Distance between sample and standard ice/liquid interfaces was used to calculate sample osmolality. Bottom: Freezing interfaces (white dots) from standards and single fly hemolymph. Scale 0.05 mm.
B. Desiccation affects hemolymph osmolality. n=14-17, one way ANOVA, Tukey’s Post Hoc, **p<0.01. Dehydrated: 6 hours at <20% RH. Hydrated: 6 hours in a humid box. Rehydrated: water was administered as described in methods.
C. Starvation affects hemolymph osmolality. n=14, t-test, ***p<0.001.
D. ΔF/F traces (mean ± SEM) to 30 mOsm/Kg osmolality decreases or increases in flies expressing UAS-GCaMP6s (green, n=10 brains) or UAS-ArcLight (magenta, n=10 brains) in ISNs.
E. ΔF/F graphs (mean ± SEM) for GCaMP6s and ArcLight from D. Wilcoxon matched-pairs signed rank test, *p<0.05.
To confirm that we could detect physiological changes in osmolality, we measured hemolymph osmolality of single flies placed in dry (<20% RH) or humid (>80% RH) environments for 6-8 hours. Consistent with previous studies (Albers and Bradley, 2004), desiccation increased hemolymph osmolality by an average of 55 mOsm/kg. In addition, hemolymph osmolality of desiccated flies returned to control levels five minutes after water consumption (Figure 6B).
To test the effect of starvation on hemolymph osmolality, hemolymph was collected from single flies that were either fed or starved for 24 hours with access to water. We found that hemolymph osmolality of starved flies was lower than that of well-fed animals by ~30 mOsm/kg (Figure 6C). Importantly, physiological changes in osmolality of the magnitude observed following starvation were sufficient to elicit responses from ISNs in imaging preparations (Figure 6D, E). Thus, both low extracellular osmolality and increased AKH abundance may be starvation signals that increase ISN activity and promote feeding.
ISNs oppositely regulate sugar and water consumption
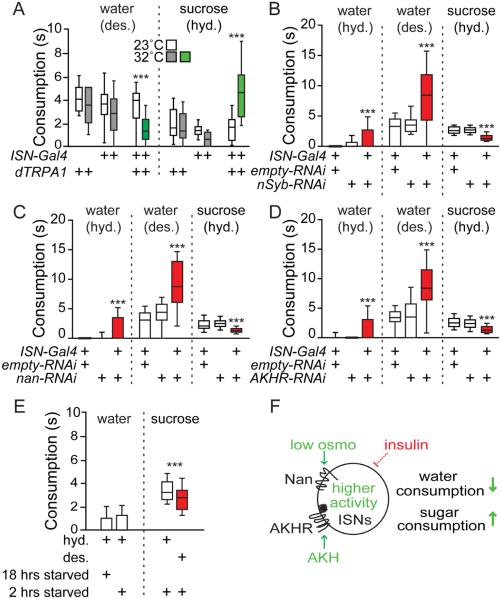
Taken together, our data suggest a model in which ISNs are sensitive to internal signals for both water and sugar abundance, and are sufficient to modulate both water and sugar consumption. To directly ask how ISN activity impacts sugar and water consumption, we drove dTRPA1 expression with ISN-Gal4, which exclusively labels ISNs. Consistent with our previous observations, ISN-Gal4, UAS-dTRPA1 flies avidly consumed sucrose upon TRP activation at 32°C, but not at 23°C. In contrast, these flies exhibited markedly reduced water consumption at 32°C but not at 23°C (Figure 7A).
Figure 7. ISNs promote sucrose consumption and inhibit water consumption.
A. ISN activation with dTRPA1 alters water and 1M sucrose consumption. Desiccated (des.), hydrated (hyd.). n=30-76 flies. Water consumption: ***p<0.001, t-tests (23°C vs 32°C) with Holm-Sidak correction.
B. ISN silencing with nSyb RNAi affects water and sugar consumption. n=38-57 flies. B-D: one-way ANOVA, Tukey’s Post Hoc, ***p<0.001.
C. ISN-specific nan RNAi affects water and sugar consumption. n=37-67 flies.
D. ISN-specific AKHR RNAi affects water and sugar consumption. n=22-70 flies.
E. Desiccation affects sugar consumption in wild type flies. n = 84-101, ***p<.001, t-test (hyd. vs. des.).
F. Low osmolality (osmo) and high AKH increase ISN activity, which promotes sugar consumption and inhibits water consumption. Insulin indirectly inhibits ISNs. High osmo and low AKH reduce ISN activity to promote water consumption and inhibit sugar consumption. See Figure S5 for additional behavioral experiments.
To test the effect of inhibiting ISN activity on water and sugar consumption, we used ISN-Gal4 to selectively drive expression of RNAi against nSynaptobrevin (nSyb), which is required for synaptic transmission. Flies expressing nSyb RNAi in ISNs increased water consumption relative to controls by almost twofold. In contrast, expression of nSyb RNAi in ISNs decreased sucrose intake by an average of 44% (Figure 7B). Thus, neural activity in ISNs oppositely regulates sugar and water consumption behaviors: increased ISN activity both promotes sugar and restricts water consumption, whereas decreased activity promotes water and restricts sugar consumption.
To ask whether nanchung and AKHR are important for the ability of ISNs to regulate food and water intake, we expressed nan RNAi or AKHR RNAi selectively in ISNs and examined the effect on water and sucrose consumption. Flies expressing either of two independent nan RNAi constructs in ISNs increased water consumption and decreased sucrose consumption relative to controls, consistent with phenotypes observed when silencing ISNs (Figure 7C, S5A-C). Flies expressing nan RNAi under the control of nSyb-Gal4 and nan-Gal4 drivers produced the same reciprocal effects on water and sucrose consumption (Figure S5D, E). Like flies expressing nan RNAi, flies expressing AKHR RNAi in ISNs also increased water intake and decreased sucrose intake relative to controls (Figure 7D). The nan and AKHR RNAi data is consistent with the notion that both AKHR and osmolality contribute to the activation of ISNs. The loss of either signal decreased activity in ISNs, leading to loss-of-function phenotypes similar to those observed when silencing ISNs with nSyb RNAi.
The finding that the ISNs sense signals of hunger and thirst, AKH and osmolality, and oppositely regulate sugar and water consumption, suggests that hunger and thirst may be competing drives under some conditions. To test this, we examined whether there were consumption differences in flies with competing needs. Under conditions when flies were not thirsty, there was no drive to consume water and this was not affected by starvation state (Figure 7E). However, under conditions when flies were mildly starved (2 hours), we found that sucrose consumption was reduced in thirsty flies compared to water-sated flies (Figure 7E). These data argue that under conditions of competing needs, there is a balance between water and sucrose consumption. As the relative weight of these needs changes, the balance would be predicted to change as well.
Discussion
In this study, we uncover a neural mechanism that coordinates two essential homeostatic behaviors: sugar and water consumption. This coordination is achieved by two neurons per SEZ hemisphere of the Drosophila brain, the ISNs, which are sensitive to internal signals for both hunger and thirst and whose activity oppositely regulates sugar and water consumption (Figure 7F). The antagonistic manner in which ISNs couple these behaviors suggests a regulatory principle by which animal nervous systems might promote internal osmotic and metabolic homeostasis.
Four neurons oppositely regulate sugar and water consumption in Drosophila
Low internal osmolality and high AKH are signals of water satiety and hunger, respectively. ISN activity increases both in the presence of low extracellular osmolality and AKH. We find that increasing ISN activity promotes sugar consumption and reduces water consumption. Conversely, high internal osmolality and low AKH are signals of thirst and food satiety. ISN activity decreases and AKH responses are reduced in the presence of high extracellular osmolality or insulin. Decreasing ISN activity increases water consumption and reduces sugar consumption.
How do ISNs achieve opposite regulation of a single behavior, consumption, in a manner that depends on the substance being consumed? One possibility is that the downstream targets of ISNs include interneurons involved in the behavioral response to water and sugar taste. This model predicts that increased ISN activity promotes the ability of sugar taste interneurons to drive consumption while inhibiting the ability of water taste interneurons to do so. It may be possible to test hypotheses about the neural circuits in which ISNs participate through the use of large scale calcium imaging.
Molecules for sensing internal hunger and thirst cues
ISNs regulate sugar and water consumption in a manner that appropriately reflects internal hunger and thirst states. Here, we show that two genes, AKHR and nanchung, are expressed in ISNs and function to confer sensitivity to these states.
AKHR is a G-protein coupled receptor expressed in the fat body and the brain that has been well characterized in the context of insect metabolic regulation (Bharucha et al., 2008; Candy, 2002). The ligand for this receptor, AKH, is secreted into the hemolymph by specialized neurosecretory cells in the corpus cardiacum (Kim and Rulifson, 2004), where it acts under conditions of food deprivation. Here, we identify a role for AKH in regulating the activity of four interneurons in the SEZ, the ISNs, and show that this activity promotes sugar consumption. AKH abundance in the hemolymph therefore promotes feeding via the ISNs. Manipulating AKHR exclusively in the ISNs provided a means to separate the metabolic and neural effects of AKH, uncovering a role for AKH in the nervous system.
Sensors for internal hemolymph osmolality have not previously been described. Here, we find that the non-selective cation channel Nanchung is expressed in ISNs and is required for their responses to low osmolality. Although we do not know if Nan is the direct osmosensor in ISNs, previous studies found that Nan confers low osmolality responses when expressed in heterologous cells (Kim et al., 2003), consistent with this notion. Nan family members of the TRPV4 family have been shown to participate in osmosensation in C. elegans and mammals (Colbert et al., 1997; Liedtke and Friedman 2003; Liedtke et al., 2003), suggesting an ancient and conserved function. Nanchung participates in sensory detection of mechanical stimuli in Drosophila, including proprioception, audition, and low humidity sensing (Gong et al., 2004; Kim et al., 2003; Liu et al., 2007; Zhang et al., 2013). It is interesting that the same molecule that is involved in external sensory detection of mechanical stimuli also participates in internal detection of osmolality, a mechanical stimulus. Similar molecular re-tooling has recently been described for the GR43a gustatory receptor, which acts as a sensory receptor to monitor fructose in the environment and as an internal sensor monitoring circulating fructose levels in brain hemolymph (Miyamoto et al., 2012).
In the mammalian brain, osmosensitive neurons are generally found in areas which lack a blood-brain barrier. The blood-brain barrier of Drosophila expresses multiple aquaporins and may potentially regulate hemolymph osmolality (Limmer et al., 2014). Whether changes in hemolymph osmolality are regulated by the blood-brain barrier to impact ISN activity is an interesting question for future study.
Coupling of sugar and water consumption behaviors as a mechanism for homeostasis
ISNs oppositely regulate the behavioral responses to hunger and thirst states. How might this type of coordination be adaptive? One possibility is suggested by the fact that sugar and water consumption perturb internal osmotic homeostasis in opposite directions. In Drosophila and mammals, sugar consumption leads to increased blood-sugar levels and increased blood osmolality. Conversely, water consumption leads to lowered blood osmolality. Our studies show that ISNs are sensitive to extracellular osmolality, and that they oppositely regulate sugar and water consumption. Under high osmotic conditions, decreased ISN activity promotes water consumption, reducing internal osmolality. Under low osmotic conditions, increased ISN activity promotes sucrose consumption, increasing internal osmolality. Thus, ISNs may monitor internal osmolality to reciprocally regulate sugar and water consumption to restore homeostasis.
Reciprocal regulation of food and water consumption has been reported in both classical and recent rodent studies. For example, increasing bloood osmolality promotes water consumption and inhibits food consumption in rats, whereas decreasing osmolality has the opposite effect (Gutman and Krausz, 1969). In addition, ghrelin, a key internal signal for hunger in mammals, is sufficient not only to promote feeding but also to inhibit water consumption in rats (Mietlicki et al., 2009). Thus, vertebrates and invertebrates may share mechanisms for coupling water and sugar consumption in a manner that promotes homeostasis. In Drosophila, the convergence of internal signals onto the ISNs provides a mechanism to weigh homeostatic deviations and drive consumption to restore balance.
Other neurons in the Drosophila brain process homeostatic needs for water and sugar separately. For example, water reward and sugar reward are processed by different subsets of mushroom body input neurons, likely independent of gustatory sensory activation (Burke et al., 2012; Lin et al., 2014; Liu et al., 2012). Neuropeptide F, small Neuropeptide F, and dopamine are all signals of nutrient deprivation that promote nutrient intake (Hergarden et al., 2012; Inagaki et al., 2012; Inagaki et al., 2014; Lee et al., 2004; Marella et al., 2012). Circulating glucose and fructose in the hemolymph also report the nutritional state and alter feeding behavior by direct activation of a few central neurons (Miyamoto et al., 2012; Dus et al., 2015). The ISNs are unique in that they detect multiple internal state signals and use this information to weigh competing needs. In addition to parallel, independent pathways for eating and drinking, this study demonstrates the existence of a pathway that couples these drives.
Experimental Procedures
Additional details are available in Extended Experimental Procedures.
Blue Dye Consumption experiments
Flies (15-20/vial) were transferred from food to filter paper soaked in 200mM sucrose and blue dye. After feeding animals were scored a 0 (no dye in abdomen), 1 (less than half of the abdomen was blue), or 2 (half or more of the abdomen contained dye). The scores of all flies in one vial were averaged and considered one trial.
Proboscis Extension Response Assays
PER was performed as described (Marella et al., 2012), except that each animal was considered a data point, and was categorized as responding 0, 1, 2, or 3 times.
Temporal Consumption Assays
Assays were performed as described (Pool et al., 2014). Animals were presented with a taste stimulus ten times and total consumption time was monitored. To generate thirsty flies, flies were placed in a sealed chamber with ~250g CaSO4 (Drierite, stock# 23001) for 2 hours, unless otherwise noted.
Immunohistochemistry
Immunohistochemistry was performed as described (Marella et al., 2012).
Calcium and voltage imaging
GCaMP5G or GCaMP6s imaging of taste responses was performed as described (Harris et al., 2015). Stacks of 14-20 Z-slices were collected at approximately 0.3 Hz. For GCaMP5G or GCaMP6s imaging of AKH or osmolality responses, the brain was removed in ice-cold calcium and magnesium-free artificial hemolymph-like solution (AHL), transferred to a perfusion chamber with room temperature AHL, and immobilized with tungsten wire. Image analysis was performed in ImageJ.
Hemolymph osmolality measurements
An osmometer was assembled and used as described (Arav and Rubinsky, 1994), with minor modifications detailed in Extended Experimental Procedures.
Electrophysiology
Extracellular recordings were performed in live animals as described (Pool et al., 2014).
Statistical Analyses
Student’s t-test, with Sidak correction for multiple comparisons, was used to compare two groups. ANOVA followed by Tukey’s post hoc test was used to compare three or more groups. ANOVA followed by Dunnet’s post hoc test was used to compare multiple responses of varying stimuli.
Supplementary Material
Highlights.
Four interoceptive neurons oppositely regulate sugar and waterconsumption
These neurons detect a peptide hormone that signals nutrient deprivation
These neurons directly sense changes in osmolality through an ion channel
These neurons report homeostatic changes and drive consumption to restore balance
Acknowledgments
The Scott lab provided comments on the manuscript. Dr. B. Rubinksy provided materials for osmolality measurements. This work was supported by a grant from the NIH (R01 DK09874; K.S.) and an NSF predoctoral fellowship (N.J.).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
N.J. performed the RNAi screen and characterized the role of Nanchung. B.M. performed the neural activation screen and characterized the role of AKHR. N.J. and B.M performed calcium imaging, behavioral, and osmolality measurement studies. K.M. performed electrophysiology. N.J., B.M., and K.S. wrote the manuscript and K.S. supervised the study.
References
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers MA, Bradley TJ. Osmotic regulation in adult Drosophila melanogaster during dehydration and rehydration. J. Exper. Biol. 2004;207:2313–2321. doi: 10.1242/jeb.01024. [DOI] [PubMed] [Google Scholar]
- Arav A, Rubinsky B. Temperature gradient osmometer and anomalies in freezing temperatures. Am. J. Physiol. 1994;267:1646–1652. doi: 10.1152/ajpregu.1994.267.6.R1646. [DOI] [PubMed] [Google Scholar]
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exper. Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordal M, Arquier N, Kniazeff J, Pin JP, Leopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nature Reviews. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy DJ. Adipokinetic hormones concentrations in the haemolymph of Schistocerca gregaria, measured by radioimmunoassay. Insect Biochem. and Mol. Biol. 2002;32:1361–1367. doi: 10.1016/s0965-1748(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Cao G, Platisa J, Pieribone VA, Raccuglia D, Knust M, Nitabach M. Genetically targetted optical electrophysiology in intact neural circuits. Cell. 2013;144:904–913. doi: 10.1016/j.cell.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for Drosophila gustatory water reception. J. Neurosci. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Harvard University Press; Cambridge, MA: 1976. [Google Scholar]
- Dus M, Lai JS, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, Suh GS. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin CC, Potter CJ, Clandinin TR. A versatile in vivo system for directed dissection of gene expression patterns. Nature Methods. 2011;8:231–237. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman Y, Krausz M. Regulation of food and water intake in rats as related to plasma osmolarity and volume. Physiol. and Behav. 1969;4:311–313. [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DT, Kallman BR, Mullaney BC, Scott K. Representations of Taste Modality in the Drosophila Brain. Neuron. 2015;86:1449–1460. doi: 10.1016/j.neuron.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergarden AC, Taylor TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc. Nat. Acad. Sci. USA. 2012;109:3967–3972. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jagadish S, Barne G, Ishimoto H, Ben-Tabou de-Leon S, Wong AM, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc. Nat. Acad. Sci. USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Nat. Acad. Sci. USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmer S, Weiler A, Volkenhoff A, Babatz F, Klämbt C. The Drosophila blood-brain barrier: Development and function of a glial epithelium. Front Neurosci. 2014;14:365. doi: 10.3389/fnins.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, David O, Chandra V, Talbot C, Huetteroth W, Waddell S. Neural correlates of water reward in thirsty Drosophila. Nat. Neurosci. 2014;17:1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Plaçais P-Y, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo J, Carlsson MA, Nässel DR. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. J. Comp. Neurol. 2015;523:1840–1863. doi: 10.1002/cne.23768. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki EG, Nowak EL, Daniels D. The effect of ghrelin on water intake during dipsogenic conditions. Physiol. and Behav. 2009;96:37–43. doi: 10.1016/j.physbeh.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genetics. 2013;9:e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluzzi JP, Vanderveken M, O'Donnell MJ. The heterodimeric glycoprotein hormone, GPA2/GPB5, regulates ion transport across the hindgut of the adult mosquito, Aedes aegypti. PloS ONE. 2014;9:e86386. doi: 10.1371/journal.pone.0086386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool AH, Kvello P, Mann K, Cheung SK, Gordon MD, Wang L, Scott K. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83:164–77. doi: 10.1016/j.neuron.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami A, Agricola HJ, Veenstra JA. Neuroendocrine cells in Drosophila melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. Gen. Comp. Endocrinol. 2011;170:582–588. doi: 10.1016/j.ygcen.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77:810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Jan LY, Jan YN. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc. Nat. Acad. Sci. USA. 2013;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.