Abstract
Cell reprogramming to pluripotency is an inefficient process and various approaches have been devised to improve the yield of induced pluripotent stem cells. However, the effect of biophysical factors on cell reprogramming is not well understood. Here we showed that, for the first time, dynamic culture with orbital shaking significantly improved the reprogramming efficiency in adherent cells. Manipulating the viscosity of the culture medium suggested that the improved efficiency is mainly attributed to convective mixing rather than hydrodynamic shear stress. Temporal studies demonstrated that the enhancement of reprogramming efficiency required the dynamic culture in the middle but not early phase. In the early phase, fibroblasts had a high proliferation rate, but as the culture became over-confluent in the middle phase, expression of p57 was upregulated to inhibit cell proliferation and consequently, cell reprogramming. Subjecting the over confluent culture to orbital shaking prevented the upregulation of p57, thus improving reprogramming efficiency. Seeding cells at low densities to avoid over-confluency resulted in a lower efficiency, and optimal reprogramming efficiency was attained at a high seeding density with dynamic culture. Our findings provide insight into the underlying mechanisms of how dynamic culture condition regulate cell reprogramming, and will have broad impact on cell engineering for regenerative medicine and disease modeling.
Keywords: Induced pluripotent stem cell (iPSC), Cell reprogramming, Cell proliferation
1. Introduction
Cell reprogramming is a major advancement in the field of cell biology and cell engineering. The forced expression of the transcription factors Oct4, Klf4, Myc, and Sox2 (OSKM) reprograms somatic cells into induced pluripotent stem cells (iPSCs) [1][2] which can be used for disease modeling and regenerative medicine applications. The efficiency of this process is relatively low, ranging between 0.1% and 10% for most somatic cell types[3]. To date, extensive work has been done to improve efficiency through further genetic manipulation[4–7] or addition of chemicals[8–12].
Manipulating the biophysical environment of the cells can also improve reprogramming efficiency. Culturing fibroblasts on microtopography can modulate their epigenetic states to improve reprogramming efficiency by as much as 4 folds[13]. Subjecting the culture to hypoxic condition can also improve efficiency, although the underlying mechanism is not yet elucidated[14]. By using a cell suspension culture, higher yield of iPSCs can be obtained over time[15]. Culturing fibroblasts on soft hydrogel can also improve efficiency by promoting mesenchymal to epithelial transition during the early phase of reprogramming[16]. Such biophysical approaches demonstrate the potential of adopting this paradigm in improving reprogramming efficiency. However, in most cases, the underlying mechanisms are not well understood.
When we investigated the effects of dynamic culture conditions on adherent reprogramming cells, we found a significant increase in reprogramming efficiency. We further demonstrated that the dynamic culture condition prevented the cell cycle arrest in the middle phase of reprogramming by suppressing the expression of cell cycle inhibitor p57.
2. Materials and Methods
2.1 Fibroblast isolation, cell culture and reprogramming
Skin fibroblasts were derived from transgenic mice that carried doxycycline-inducible OSKM genes (stock no 011011, Jackson Lab). The four reprogramming factors, OSKM, were expressed from the collagen type 1 gene locus upon induction with doxycycline[17]. 1 day post-partum mice were sacrificed by decapitation with a pair of sharp scissors. The skin was peeled off and floated on 0.05% freshly thawed trypsin overnight and the dermis was separated from the epidermis next day. The dermis was cut up and digested in 200U/ml collagenase II and 0.1% Trypsin at 37 °C for 30 minutes. The digesting mixture was mixed with fetal bovine serum (FBS) to quench the digestion enzyme activity and spun down. The pellet was plated on gelatin coated dish and expanded for 2 days in MEF medium (DMEM with 10% FBS and 1% penicillin/streptomycin). Thereafter, the culture was trpysinized, passed through a 40 μm filter and frozen down into aliquots in a medium containing 10% dimethyl sulfoxide (DMSO) and 90% FBS.
For cell reprogramming experiment, fibroblasts were seeded into 6 well plates in MEF medium. Unless otherwise stated, cells were seeded at a density of 3000 cells/cm2. The next day, medium was replaced with reprogramming medium (Knockout™ DMEM, 10% Knockout Serum Replacement™, 5% FBS, 1% penicillin/streptomycin, 1% Glutamax, 1000 U/ml LIF and 2μg/ml doxycycline) to induce the expression of OSKM.
For dynamic culture, the culture plates were placed on an orbital shaker in the incubator and, unless noted otherwise, agitated at 100 rounds per minute (rpm). At the indicated times, doxycycline was removed from the reprogramming medium to stop the exogenous expression of OSKM, with all other medium components retained. For all experiments, media was changed once every 2–3 days.
Reprogrammed iPSC colonies with three dimensional dome-like morphology and clear boundary were picked on day 20 and expanded in reprogramming medium for 4 passages. To form embryoid bodies (EBs), the iPSCs were suspended in 20 μl hanging drops at 2000 cells per drop for 3 days in EB medium (same as reprogramming medium but with LIF removed). The embryoid bodies were then plated on gelatin coated plate and cultured for an additional 9 days in EB medium to allow further spontaneous differentiation before fixation and staining.
2.2 Immunofluorescence staining and microscopy
For immunostaining, cells were fixed in 4% paraformaldehyde for 15 minutes, and permeabilized with 0.5% Triton-X 100 for 15 minutes. Cells were stained with the respective primary and secondary antibodies, and imaged with a Zeiss AxioObserver epi-fluorescent microscope. Refer to supplementary table 1 for list of primary antibodies. Secondary antibodies were Alexa Fluor 488 or Alexa Fluor 546 (ThermoFisher Scientific).
To determine cell proliferation rate, EDU staining was performed by using Click-iT® EdU Alexa Fluor® 488 Imaging Kit (ThermoFisher Scientific). Samples were pulsed with 10 μM EDU for 30 minutes. Subsequent steps were performed according to manufacturer’s instructions.
2.3 Quantification of reprogramming efficiency
At the indicated time, culture was fixed and immunostained for iPSC markers Nanog and SSEA1. Stained samples were kept in phosphate buffered saline (PBS) and loaded onto ImageXpress Micro High-Content Analysis System (Molecular Devices) for automated whole well imaging at 4X magnification. The images were stitched using the MetaXpress Analysis Software (Molecular Devices) and loaded in Photoshop for colony counting. A transparent layer was created and positive colonies were marked and covered with a circular brush. The layer was then saved as a separate image and the number of circular marks were counted using ImageJ. Reprogramming efficiency was obtained by dividing the number of Nanog+ positive colonies by the total number of cells seeded at the beginning of the experiments.
For flow cytometry analysis, the culture was first treated with 500U/ml collagenase II dissolved in plain Knockout™ DMEM for 15 minutes to partially digest the ECM, followed by trypsin treatment. The detached cells was triturated with progressively smaller needles to obtain a single cell suspension before incubation with StainAlive™ SSEA1 antibody (Stemgent) for 30 minutes. The suspension was spun down and washed with PBS two times before being analyzed with Guava (Merck Millipore).
2.4 Reverse transcription-quantitative polymerase chain Reaction (RT-qPCR)
At the indicated time, culture was lysed with Trizol (ThermoFisher Scientific) and RNA was extracted following the manufacturer’s instruction. RNA concentration was quantified with Nanodrop 1000 (Thermo Scientific) and equal amount was loaded for cDNA synthesis using Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific). cDNA was then loaded into 96 well PCR plate with primers and Maxima SYBR Green qPCR Master Mix (ThermoFisher Scientific). Primer information is listed in Supplementary Table 2. Thermal cycling and data acquisition was performed on iQ5 system (Biorad). GAPDH used as housekeeping gene for nomalization. Data was analyzed with ΔΔCt method.
2.5 Western blotting analysis
To determine p57 expression, cells were lysed with a lysis buffer containing 20 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 10 mM NaF along with protease inhibitors (phenylmethyl sulphonyl fluoride, Na3VO4 and leupeptin). Lysates were centrifuged and the supernatants removed and quantified by Direct Detect® Infrared Spectrometer (Merck Millipore). For analysis of YAP/TAZ and β-Catenin localization, nuclear and cytoplasmic lysates were prepared with NucBuster™ Protein Extraction Kit (Merck Millipore) according to the manufacturer’s instructions.
Equal amounts of proteins were separated with SDS–PAGE and then transferred to polyvinylidene fluoride membranes. Membranes were blocked in either denatured 5% BSA (when staining for phosphorylated proteins) or 3% non-fat milk and then incubated with primary antibodies. Refer to supplementary table 1 for list of antibodies. Next, membranes were incubated with HRP-conjugated IgG secondary antibodies (Santa Cruz Biotechnologies) for one hour. Protein bands were visualized by using Western Lightning Plus-Enhanced Chemiluminescence Substrate (Perkin Elmer Life & Analytical Sciences).
2.6 Measurement of viscosity, shear stress and mixing rate
Dextran was added to manipulate the viscosity of the culture medium. Measured amount of dextran was sterilized by incubation in boiling water bath for 30 minutes. The viscosity of medium with and without dextran at 37°C was measured with rheometer (MCR300, Anton Paar, Ashland, VA). 50 mm parallel plate at a gap height of 0.5 mm was used. A humidity chamber was placed around the sample to prevent dehydration. The lower plate temperature was regulated with a Peltier heating element connected to a recirculating water bath. Medium was sheared at different rates and the torque measurement was used to calculate viscosity by the equation:
| equation 1 |
where M is torque, h is distance between plates, Ω is rate of rotation and R is radius of plate.
The average shear stress τ at the bottom of the culture dishes on the orbital shaker was determined by the equation:
| equation 2 |
where R is the radius of the dish, ρ is fluid density, η is dynamic viscosity and f is orbiting frequency [18].
To measure mixing rate, a spot of blue dye (3 μl) was added to the medium and the time taken for the spot to become completely homogenous with the medium recorded.
2.7 siRNA transfection
800 pmol of siRNA in 1ml DMEM and 20μl Lipofectamin 2000 (Invitrogen) in 1ml DMEM were incubated separately for 5 minutes and then mixed and incubated together for another 20 minutes at room temperature. 500μl of the siRNA-lipid complex was added to the reprogramming medium in each well (total of 4 wells), and the medium was replaced after 6 hrs. Transfection was performed every 48 hours for a total of 5 times. The following siRNAs were used: p57 siRNA (pool of 37621A-C, Santa Cruz Biotechnology); control siRNA (D-001810-10-20, Dharmacon)
3. Results
3.1 Dynamic culture improved reprogramming efficiency
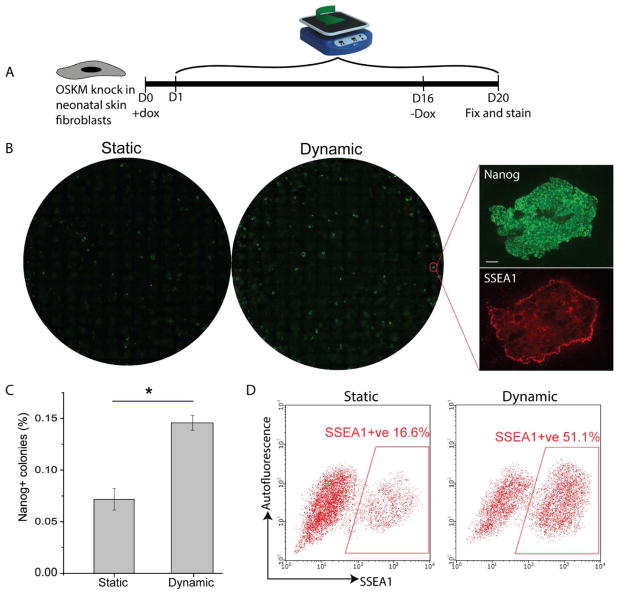
We utilized neonatal skin fibroblasts with doxycycline-inducible OSKM genes for our experiments. The cells were seeded at 3000 cell/cm2 and allowed to attach overnight before reprogramming medium containing doxycycline was added. Orbital shaking was started the day after. Doxycycline was withdrawn on day 16 and cultures were fixed on day 20 and counted for the number of Nanog+ colonies (Fig. 1A). Dynamic culture significantly increased the reprogramming efficiency by 2 fold (Fig. 1B, 1C). In addition, we also ran flow cytometry analysis for SSEA1, a surface marker of iPSCs, to quantify efficiency at individual cell level. Consistently, the percentage of SSEA1+ cells was increased by 3 fold for dynamic culture (Fig. 1D).
Fig 1.
The effect of dynamic culture on cell reprogramming efficiency. A. Schematics of experiment. OSKM expression was induced from day 1 to day 16, and withdrawn from day 16 onwards. Dynamic culture was applied from day 1 onwards. B. Whole well image of Nanog+ colonies for static and dynamic culture. Insets are exemplified high magnification views. Scale bar denotes 100 μm. C. Quantification of reprogramming efficiencies for static and dynamic culture. The number of Nanog+ colonies was normalized by initial seeding density to calculate the efficiency. (*p<0.05. n=6.) D. Quantification of the percentage of SSEA1+ cells by flow cytometry to measure the reprogramming efficiency at individual cell level.
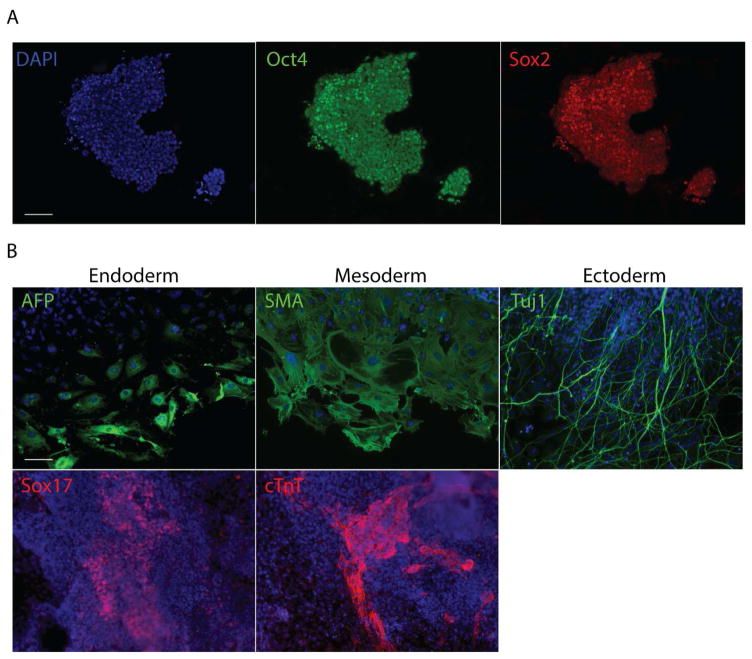
iPSCs colonies obtained under dynamic culture were picked on day 20, expanded for 4 passages and then stained for Oct4 and Sox2 (Fig. 2A). Embryoid bodies formed with the expanded iPSCs were allowed to spontaneously differentiate. Differentiated cells stained positive for markers from the 3 germ layers (Fig. 2B), indicating that the iPSCs obtained under dynamic culture were pluripotent. We also compared the doubling times for iPSCs derived under dynamic and static culture, and found them to be similar (Supplementary table 3). To assess the long term self-renewal and pluripotency of the iPSCs obtained under dynamic culture, we cultured them for a total of 28 days after picking. After 28 days, they stained positive for pluripotency markers Nanog, SSEA1 and Oct4 (Supplementary figure 1A) and formed 3 germ layers upon spontaneous differentiation (Supplementary figure 1B), indicating that they maintained their self-renewal capability and pluripotency long term.
Fig 2.
Characterization of the pluripotency of iPSCs. A. Picked iPSC colonies were stained for Oct4 and Sox2. B. iPSCS derived under dynamic culture were used to form EBs and allowed to spontaneously differentiate. Differentiated cells were stained for markers from the 3 germ layers. AFP is alpha fetoprotein, SMA is α-smooth muscle actin and cTnT is cardiac troponin T. Scale bars denote 100 μm.
To assess if mechanical shear stress exerted on cells by dynamic culture could cause chromosomal aberrations through, for instance, inducing DNA double-stranded break, we stained the reprogramming culture with antibody targeting γH2AX. H2AX is a variant of the histone H2A family, which in response to DNA double-strand breaks becomes phosphorylated into the form known as γH2AX[19]. On day 8, we observed no positive staining in nascent colonies in both dynamic and static culture (Supplementary figure 2A). On day 20, we observed some staining for γH2AX in reprogrammed Nanog positive colonies in both dynamic and static culture (Supplementary figure 2B). This positive staining could be attributed to a basal level of γH2AX in iPSCs, as has been reported[20]. More importantly, the intensity of γH2AX staining was not significantly different between dynamic and static culture (Supplementary figure 2B), indicating that dynamic culture did not cause additional double stranded breaks.
3.2 Time course study revealed the optimal period for dynamic culture
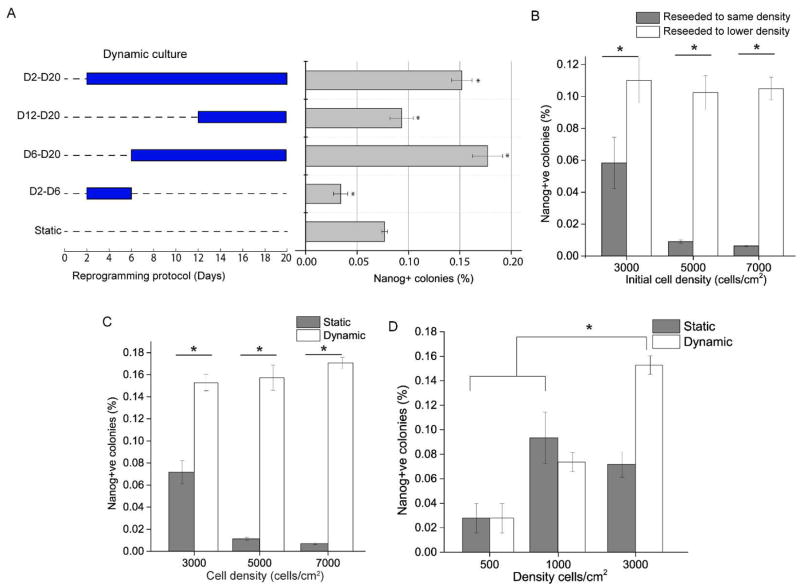
We next sought to establish the optimal period of dynamic culture to improve reprogramming efficiency. Applying shaking during the early phase (D2–D6) inhibited reprogramming, and during the late phase (D12–D20) resulted in no significant change. The greatest improvement was obtained when shaking was applied from day 6 onwards (Fig. 3A). Close examination of cell culture showed that day 6 was when the reprogramming culture became confluent. We therefore hypothesized that from this time point onwards, the confluence of the cells had a negative effect on reprogramming, and that dynamic culture ameliorated some of these effects to improve reprogramming efficiency.
Fig 3.
Effects of cell seeding density and dynamic culture on cell reprogramming. A. Orbital shaking applied at different time points as indicated by the graph on the left, with the corresponding reprogramming efficiency indicated on the right. (*p<0.05. n=3.) B. Reprogramming efficiencies after reseeding cells on day 6. Fibroblasts were initially seeded at different densities and, on day 6, either reseeded to a lower density of 10000/cm2 (grey bars) or reseeded without any adjustment in cell density (white bars). (*p<0.05. n=3.) C. Reprogramming efficiencies for static and dynamic culture at different initial seeding densities. Dynamic culture was started on day 6 onwards. (*p<0.05. n=3.) D. Cells were seeded at low seeding densities (500 and 1000/cm2), and reprogrammed under static or dynamic culture (starting at day 6). (*p<0.05. n=3.)
3.3 Dynamic culture ameliorated negative effect of over-confluence on reprogramming
First we sought to establish the effect of cell density on reprogramming. We seeded cells at 3000, 5000 and 7000/cm2 and added doxycycline for 16 days and fixed cells on day 20. Reprogramming efficiency dropped significantly as seeding density increased (Fig. 3B). We then determined whether the negative effect of higher seeding density on reprogramming efficiency was manifested only after the culture became confluent. We replated the reprogramming culture at day 6 to a lower density (10000/cm2) to see if reprogramming efficiency was improved. Indeed, after being relieved of their confluent niche, reprogramming efficiency of the cells was significantly improved to the same level for all 3 initial seeding densities (Fig. 3B). To rule out the possibility that reseeding in itself affects reprogramming efficiency, we also trypsinized and reseeded all of the cells without adjusting the cell density. As a result, reprogramming efficiency was comparatively much lower (Fig. 3B). Therefore, we concluded that the negative effect of higher seeding densities on reprogramming manifested after the density of the culture has grown beyond a certain threshold.
Next, we tested if dynamic culture applied post-confluence could then rescue reprogramming efficiency at the three high seeding densities. Simply, we started orbital shaking from day 6 onwards for the different cell densities and quantified Nanog+ colonies at the end. Indeed, reprogramming efficiencies were significant improved for all three seeding densities (Fig. 3C).
We then asked the question: At a sufficiently low seeding density, would reprogramming efficiency be higher and dynamic culture not have any effect anymore? To answer this, we seeded cells at 500 and 1000/cm2, induced them with doxycycline at day 0 and applied orbital shaking from day 6 onwards. Results showed that dynamic culture did not affect reprogramming efficiency at these low densities (Fig. 3D). Additionally, the efficiencies were several folds lower than high density dynamic culture. One explanation is that cell-cell communications such as juxtacrine signaling important for reprograming might take place less effectively at lower seeding densities[21].
3.4 Mixing rather than shear stress conferred the beneficial effect of dynamic culture
Cells subjected to dynamic culture experience both convective mixing and hydrodynamic shear stress compared to static culture. Convective mixing could enhance the transport of cytokines, nutrients and metabolic products to regulate cell signaling, and oscillatory hydrodynamic shear stress can trigger various mechanotransduction pathways in cells, some of which could directly or indirectly affect the reprogramming process. We thus sought to find out which of these two phenomena contributed to the beneficial effect of dynamic culture.
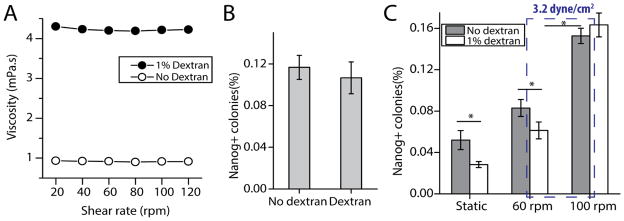
Our approach was to use dextran to adjust the viscosity of the medium so that we could apply similar magnitude of shear stress with different rates of mixing. By increasing viscosity, we obtained the same magnitude of shear stress at a lower frequency of orbit. We added 1% high molecular weight dextran (5 million to 60 million Da.) to the culture medium to increase the viscosity of the medium from 0.912 mPa.s to 4.21 mPa.s, as measured by rheometry (Fig. 4A). Addition of dextran did not alter the Newtonian property of the medium as viscosity remained constant across different shear rates (Fig. 4A). To ensure that dextran in itself did not significantly affect the reprogramming process, we reprogrammed cells seeded at 1000/cm2 with and without dextran under static condition, and we observed a similar reprogramming efficiency (Fig. 4B). The size and morphology of the colonies were indistinguishable between the two conditions (Data not shown).
Fig 4.
The effect of mixing and shear stress on cell reprogramming. A. Rheometer measurement of viscosity against shear rate for media with and without 1% dextran. B. Reprogramming efficiencies for media with and without dextran. Fibroblasts were seeded at 1000/cm2 and reprogrammed under static culture condition. C. Reprogramming efficiency for fibroblasts seeded at 3000/cm2 for various conditions. Dotted box indicates the two experimental conditions with the same magnitude of shear stress but different mixing rate. (*p<0.05. n=3)
We then reprogrammed cells seeded at 3000/cm2 statically and dynamically at 60 rpm and 100 rpm, with and without dextran (Fig. 4C). Under static condition, the reprogramming efficiency was slightly lower for dextran medium than no-dextran medium (Fig. 4C), although this was not observed for cells seeded at 1000/cm2 (Fig. 4B). At these two rotation rates, shear stress exerted by dextran medium at 60 rpm (DM 60) and no-dextran medium at 100 rpm (NDM 100) is both equivalent to 3.2 dyne/cm2 according to equation 2 (Materials and Methods). On the other hand, rate of convective mixing is lower for DM 60 than NDM 100 as the higher viscosity and lower rate of agitation would lead to less bulk motion of the medium. We confirmed this by measuring the time it took for a spot of blue dye to mix completely for DM 60 and NDM 100. DM 60 took 311 seconds, while NDM 100 took 21 seconds, a difference of 15 folds. If shear stress is the dominant attributable factor, we would expect efficiencies to be similar between these 2 conditions. If mixing is the dominant factor, efficiency would be lower for DM 60. The results showed that the reprogramming efficiency was significantly lower for DM 60, indicating that mixing is the dominant factor (Fig. 4C). At 100 rpm, reprogramming efficiency was similar for the media with and without dextran, even though shear stress was different (7.0 dyne/cm2 for dextran and 3.2 dyne/cm2 for no-dextran). This suggests that at 100 rpm, the rate of mixing is sufficiently high even for a medium with higher viscosity to enhance reprogramming efficiency.
3.5 Effect of dynamic culture on mechanosensitive proteins YAP and β-catenin
To explore the underlying mechanisms of how dynamic culture enhanced reprogramming, we examined the effects of dynamic culture on mechanosensitive signaling molecules YAP and β-Catenin. YAP is a transcriptional coactivator in the Hippo pathway and plays crucial roles in embryonic stem cell self-renewal and iPSCs reprogramming[22]. It translocates to the cytosol and becomes inactivated when cell density become too high, but can be reactivated by mechanical cues[23]. β-catenin is a transcriptional coactivator in the Wnt pathway, and its activation and translocation into nucleus are critical in the later stage of reprogramming [24][25]. β-catenin itself has also been found to be responsive to shear stress [26]. Importantly, both proteins have been implicated in contact inhibition and cell cycle arrest [27][28][29].
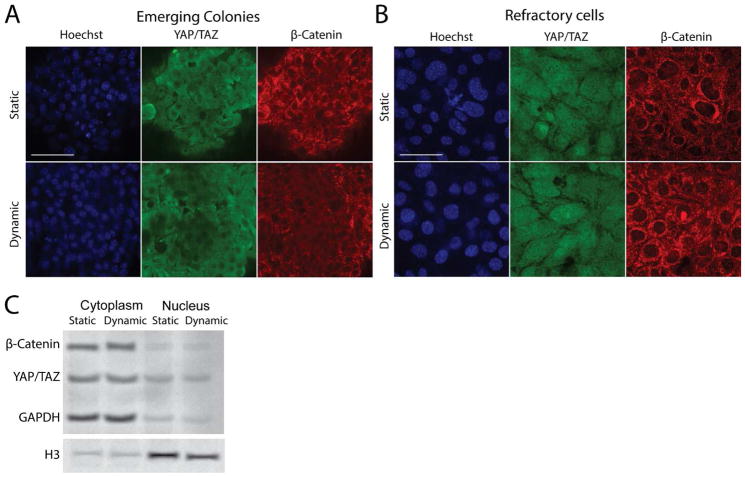
We started dynamic culture on day 6 for 48 hours before fixing and staining. We observed two main categories of cells in the reprogramming culture as described in previous literature: Emerging colonies and refractory cells (Fig 5A,B). Confocal image showed that localization of YAP and β-catenin was indistinguishable between the static and dynamic culture for both categories of cells (Fig. 5A,B). In addition, we performed Western blotting analysis of the bulk nuclear and cytoplasmic fractions and the results substantiated this observation (Fig. 5C). As an interesting side note, YAP was restricted to the cytoplasm for the emerging colonies, but showed both cytoplasm and nuclear localization in refractory cells (Fig. 5A,B).
Fig 5.
Effect of dynamic culture on signaling molecules. A, B. Confocal microscope images for emerging colonies and refractory cells on day 8 for static and dynamic culture. Dynamic culture has been applied for 48 hrs. Scale bar denotes 100 μm. C. Western blotting analysis of cytoplasmic and nuclear fractions for samples in A and B.
3.6 Effect of dynamic culture on reprogramming efficiency was mediated by p57 expression level
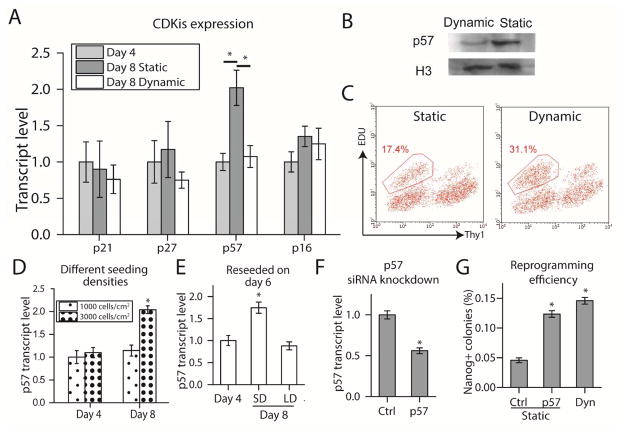
We further explored other pathways that may be regulated by dynamic culture. We know that a high proliferation rate is required for reprogramming [30], and that proliferation rate is inhibited when density becomes too high [31]. Therefore, we measured the transcript level of cyclin dependent kinase inhibitors (CDKi) from the Cip/Kip (p21, p57, p27) and Ink4 (p16) family for the static and dynamic culture on days 4 and 8 with RT-qPCR. For the dynamic culture sample, orbital shaking was applied from day 6 onwards. Of all the CDKis, mRNA level of p57 was upregulated 2-fold for the static culture from day 4 to day 8, but not so for the dynamic culture (Fig. 6A). Protein level as measured by Western blot also confirmed this observation (Fig. 6B).
Fig 6.
Effect of static and dynamic culture on cell cycle. A. Transcript level quantification with RT-qPCR for various Cyclin Dependent Kinase Inhibitors. (*p<0.05. n=3.) B. Western blot for p57 to verify A at protein level. C. Flow cytometry for EDU staining on day 8. D. p57 expression of static reprogramming cultures over time at different seeding densities. (*p<0.05, compared to 3000 cells/cm2, Day 4. n=3.) E. Reprogramming culture initially seeded at 3000cells/cm2 was reseeded to lower density (LD) of 10000 cells/cm2 or same density (SD) on day 6 and RT-qPCR was performed on day 8. (*p<0.05, compared to Day 4. n=3.) F. p57 siRNA was transfected into reprogramming culture on day 6 and RT-qPCR was performed on day 8. (*p<0.05, n=3) G. Reprogramming efficiency of cultures transfected with p57 siRNA. (*p<0.05, compared to static, non-targeting control. n=3.)
We then further determined whether cell proliferation rate for static culture was lower. Specifically, we examined cells that are poised to undergo reprogramming. Expression level of Thy1 can be used as an early negative marker of reprogramming. Cells negative for this marker after four-factor induction are poised to undergo successful reprogramming [32]. Cells on day 8 were pulsed with EDU for 30 minutes before being fixed, stained and subjected to flow cytometry. The Thy1- fraction showed a lower proportion of EDU staining for static culture, implying that proliferation rate was indeed inhibited (Fig. 6C).
We next examined what caused p57 expression to be upregulated from day 4 to day 8. We hypothesized that the overconfluency of the culture from day 6 onwards promoted cell cycle arrest and upregulation of p57. We carried out 2 experiments to test this hypothesis. In the first experiment, on day 4 and day 8, we quantified p57 expression level of reprogramming cultures that were initially seeded at 1000 and 3000 cells/cm2, and observed upregulation of p57 for 3000 cells/cm2 but not 1000 cells/cm2 (Fig. 6D). This is in line with the observation that culture seeded at 1000 cells/cm2 did not become confluent until day 12 or so, whereas culture seeded at 3000 cells/cm2 became confluent from day 6 onwards. In the second experiment, we trypsinized and reseeded either a portion of the reprogramming cells on day 6 to a lower density or all of the cells to the same density as before. Cells seeded to a lower density did not upregulate p57 while those seeded to the same density did. Results from both experiments indicated that the upregulation of p57 in reprogramming cells was caused by the culture becoming over confluent.
Overconfluence of the culture led to upregulation of p57, and dynamic culture prevented it. Therefore, we hypothesize that the effect of dynamic culture on reprogramming efficiency was mediated through p57 regulation. To test this hypothesis, we performed siRNA knockdown of p57 in the static reprogramming culture to see if we could recapitulate the effect of dynamic culture. We transfected static reprogramming culture from day 6 onwards with either p57 siRNA or non-targeting control every 48 hours until day 14. 48 hours after the first transfection, we quantified p57 expression by RT-qPCR and confirmed that p57 transcript level was knockdown by about 50% (Fig 6F), which was the same extent of downregulation by dynamic culture (Fig 6A). By day 20, the reprogramming efficiency for the static culture with p57 siRNA knockdown was improved to the same level as that for dynamic culture (Fig 6G), indicating that downregulation of p57 with siRNA knockdown recapitulated the effect of dynamic culture.
3.7 Dynamic culture synergize with small molecules to improve reprogramming efficiency
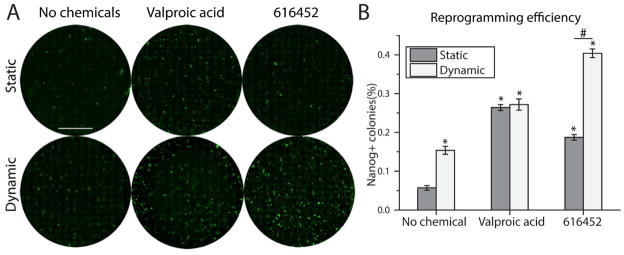
Addition of small molecules to the reprogramming media can improve reprogramming efficiency (reviewed in [33]). In particular, valproic acid, a HDAC inhibitor, and 616452, an Alk5 inhibitor, have been reported to improve efficiency significantly by at least several folds[34–36]. The exact mechanisms by which these chemicals improved reprogramming efficiency were not elucidated. We hypothesized that if these chemicals act via biological pathways that are orthogonal to p57 regulation, dynamic culture will have an additive or synergistic effect with them. We added 2 mM of valproic acid and 1 μM of 616452 to the reprogramming media for the first 16 days during the doxycycline induction phase, and withdrew the chemicals together with doxycycline from day 16 onwards. Dynamic culture was started from day 6 onwards. Dynamic culture alone without any chemicals added improved efficiency by 2.5 folds (Fig 7A, B). Valproic acid improved efficiency by 4.5 folds under both static and dynamic culture (Fig 7A, B). 616452 improved efficiency by 3 folds under static culture, but by 7 folds under dynamic culture (Fig 7A, B). Therefore, dynamic culture did not have any synergistic effect with valproic acid, but synergized with 616452 to improve efficiency multiplicatively.
Fig 7.
Dynamic culture synergized with 616452 to improve reprogramming efficiency. A. Representative whole well images of reprogrammed culture stained with Nanog. Scale bar denotes 10 mm. B. Quantification of Nanog positive colonies for different treatments. (*p<0.05, compared to static with no chemicals. n=3. #p<0.05.)
Discussion
Previous work has demonstrated the importance of cell proliferation on reprogramming[37][30][38][39] although the underlying reasons have not been elucidated yet. One possibility is that the extensive nuclear changes occurring during cell division allow the epigenome to be modified for the core-transcriptional circuitry of pluripotency to be established. For instance, it has been suggested that the large-scale remodeling of three dimensional chromatin architecture occurring during a brief period in early G1 phase may provide a window of opportunity to influence cellular identity in response to extracellular cues[40]. Another possibility is that the chromatin is in a less condensed state immediately following DNA replication, and this allows the Yamanaka factors to form transcription complexes at otherwise inaccessible pluripotency genes loci and subsequently turn on their expression[41]. During the initial phase of reprogramming, all cells undergo a dramatic increase in proliferation rate[42], and the culture would inevitably become over-confluent at some point. We demonstrate that this causes an upregulation of p57 and inhibits reprogramming through the inhibition of cell proliferation. By subjecting the culture to orbital shaking, upregulation of p57 is prevented and reprogramming efficiency is dramatically improved. Expression level of p57 has been directly implicated in reprogramming efficiency[43]. Reseeding the culture to a lower density upon reaching confluency could also improve efficiency, but not to the same extent as dynamic culture, suggesting that the process of reseeding in itself is detrimental, as has been described before[44].
One question is then whether low seeding density could avoid over-confluency and thus obviate the need for dynamic culture in improving reprogramming efficiency. However, we demonstrated that this is not the case as reprogramming efficiency for sparsely seeded cells cultured statically is lower than more densely seeded cells cultured dynamically. Previous work has shown that E-cadherin mediated cell to cell contact is critical for reprogramming [21]. This and perhaps other juxtacrine signaling critical to reprogramming cannot occur effectively when seeding density is low. Under static condition, increasing seeding density allow such signaling processes to take place but also introduces the problem of over-confluency and inhibition of cell proliferation. By combining high seeding density with dynamic culture, we demonstrate that optimal reprogramming efficiency is obtained. Reprogramming efficiency is not improved by dynamic culture for sparsely seeded cells. Confluency was never reached for 500 cells/cm2, thus explaining why dynamic culture did not improve efficiency at this seeding density since it exerts its beneficial effect only post confluence. Confluence was reached for 1000/cm2 typically after day 12 and by then, cells probably have undergone sufficient number of divisions to be reprogrammed successfully.
To decouple the effect of convective mixing from hydrodynamic shear stress, we applied similar shear stress at different shear rates by adjusting the viscosity of the medium with dextran. Our results suggest that the beneficial property of dynamic culture is mainly attributed to mixing. However, the highest shear stress applied for all the experimental conditions was 7 dyne/cm2, which might not be high enough to stimulate the mechanotransduction pathways that affect reprogramming. Improving the transport rate of solutes through convective mixing has two effects: first, more efficient removal of metabolic wastes and inhibitory cytokines from the cells; secondly, more efficient supply of nutrients to the cells. Either or both of these could contribute to maintaining cell proliferation rate post-confluence and improving successful reprogramming outcome.
In general, apart from direct cell to cell contact[45], contact inhibition could possibly be mediated by the local buildup or depletion of soluble factors[46], in which case dynamic culture could abolish these local gradients simply by bulk mixing. It will be interesting to study if this effect of dynamic culture on cell proliferation via p57 regulation manifests beyond the reprogramming context. If so, dynamic culture can be broadly applied to the expansion of adherent cell culture for regenerative medicine purposes.
We compared the effect of dynamic culture to small molecules boosters of reprogramming and test for synergistic effects between the two. Dynamic culture did not synergize with valproic acid, suggesting that both of them could be acting along related pathways to improve efficiency. For instance, it has been reported that valproic acid prevented senescence in reprogramming cells possibly by downregulating p16 and p21 expression[34]. The functions of p57, p16 and p21 overlap to some degree in that they all inhibit the activity of Cyclin Dependent Kinases 4 and 6[47–49]. Thus, by downregulating p16 and p21, valproic acid could be inducing the same effect as downregulation of p57 by dynamic culture. On the other hand, dynamic culture synergized with 616452 to improve reprogramming efficiency. This suggested that p57 upregulation still posed as a barrier to reprogramming in 616452 treated culture, and dynamic culture helped remove this barrier. In fact, it was noted that 616452 treatment resulted in higher cell number during the starting phase of reprogramming, thus possibly exacerbating overconfluency and p57 upregulation[35]. Thus, dynamic culture could be mitigating this undesirable side effect, thus further enhancing the effect of 616452 treatment on reprogramming efficiency.
4. Conclusion
Over confluency in reprogramming cultures inhibit cell proliferation and subsequently reprogramming efficiency. Dynamic culture could ameliorate this heretofore unaddressed phenomenon to easily and effectively improve reprogramming efficiency.
Supplementary Material
Supplementary Figure 1: Pluripotency at day 28
A) Picked iPSC colonies from dynamic culture were cultured for 28 days and stained for pluripotency markers. B) iPSCs from A) were subjected to EB formation and spontaneous differentiation. 3 germ layers were formed.
Supplementary Figure 2: A) Reprogramming culture under dynamic and static conditions were fixed and stained for Nanog and γH2AX on day 8. Nascent colonies were imaged. Scale bar denotes 100 μm. B) Reprogrammed culture was fixed and stained on day 20. Images are shown on the left. Scale bar denotes 100 μm. Scatter plot of γH2AX intensity is shown on the right.
Supplementary Table 1: Antibody information
Supplementary Table 2: Primer sequences
Supplementary Table 3: Doubling time
Acknowledgments
This work is supported in part by grant from the National Institute of Health (EB012240 and HL083900 to S. Li) and fellowship from the Agency for Science, Technology and Research (to J. Sia). The authors would like to thank M. West at the CIRM/QB3 Shared Stem Cell Facility of UC Berkeley, W. C. Huang and Z. Huang for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 3.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 6.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.di Stefano B, Collombet S, Graf T. C/EBPalpha poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature. 2013;1:8. doi: 10.1038/nature12885. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Nur O, Brumbaugh J, Verheul C, Apostolou E, Pruteanu-Malinici I, Walsh RM, et al. Small molecules facilitate rapid and synchronous iPSC generation. Nat Methods. 2014;11:1170–1176. doi: 10.1038/nmeth.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, et al. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal SE, Amlani B, Chen T, Tsirigos A, Stadtfeld M. Combinatorial modulation of signaling pathways reveals cell-type-specific requirements for highly efficient and synchronous iPSC reprogramming. Stem Cell Reports. 2014;3:574–584. doi: 10.1016/j.stemcr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods. 2012;9:509–516. doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi B, Park K, Kim J, Ko K, Kim J, Han DK, et al. Stiffness of Hydrogels Regulates Cellular Reprogramming Efficiency Through Mesenchymal-to-Epithelial Transition and Stemness Markers. Macromol Biosci. 2015 doi: 10.1002/mabi.201500273. [DOI] [PubMed] [Google Scholar]
- 17.Carey BW, Markoulaki S, Beard C, Hanna J, Jaenisch R. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat Methods. 2010;7:56–59. doi: 10.1038/nmeth.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JMD, Chakraborty A, Sharp MK, Berson RE. Spatial and temporal resolution of shear in an orbiting petri dish. Biotechnol Prog. 2011;27:460–5. doi: 10.1002/btpr.507. [DOI] [PubMed] [Google Scholar]
- 19.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 20.Turinetto V, Orlando L, Sanchez-Ripoll Y, Kumpfmueller B, Storm MP, Porcedda P, et al. High basal γH2AX levels sustain self-renewal of mouse embryonic and induced pluripotent stem cells. Stem Cells. 2012;30:1414–1423. doi: 10.1002/stem.1133s. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, et al. E-Cadherin-Mediated Cell–Cell Contact Is Critical for Induced Pluripotent Stem Cell Generation. Stem Cells. 2010;28:1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- 22.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aulicino F, Theka I, Ombrato L, Lluis F, Cosma MP. Temporal perturbation of the Wnt signaling pathway in the control of cell reprogramming is modulated by TCF1. Stem Cell Reports. 2014;2:707–720. doi: 10.1016/j.stemcr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo Y, Chang T, Hsu W, Zhou J, Lee H, Hui-Chun Ho J, et al. Oscillatory Shear Stress Mediates Directional Reorganization of Actin Cytoskeleton and Alters Differentiation Propensity of Mesenchymal Stem Cells. Stem Cells. 2015;33:429–442. doi: 10.1002/stem.1860. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of β-catenin is regulated by cell density. Biochem Biophys Res Commun. 2002;292:195–199. doi: 10.1006/bbrc.2002.6625. [DOI] [PubMed] [Google Scholar]
- 29.Orford K, Orford CC, Byers SW. Exogenous expression of β-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855–868. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna J, Saha K, Pando B, Van Zon J, Lengner CJ, Creyghton MP, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoker MGP, Rubin H. Density dependent inhibition of cell growth in culture. 1967 doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- 32.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Li W, Laurent T, Ding S. Small molecules, big roles -- the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci. 2012;125:5609–5620. doi: 10.1242/jcs.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhai Y, Chen X, Yu D, Li T, Cui J, Wang G, et al. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp Cell Res. 2015;337:61–67. doi: 10.1016/j.yexcr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Maherali N, Hochedlinger K. Tgfβ Signal Inhibition Cooperates in the Induction of iPSCs and Replaces Sox2 and cMyc. Curr Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koche RP, Smith ZD, Adli M, Gu H, Ku M, Gnirke A, et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz S, Panopoulos AD, Herrerias A, Bissig KD, Lutz M, Berggren WT, et al. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr Biol. 2011;21:45–52. doi: 10.1016/j.cub.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert DM. Cell fate transitions and the replication timing decision point. J Cell Biol. 2010;191:899–903. doi: 10.1083/jcb.201007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolffe AP. Implications of DNA replication for eukaryotic gene expression. J Cell Sci. 1991;99:201–206. doi: 10.1242/jcs.99.2.201. [DOI] [PubMed] [Google Scholar]
- 42.Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo S, Zi X, Schulz VP, Cheng J, Zhong M, Koochaki SHJ, et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell. 2014;156:649–662. doi: 10.1016/j.cell.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pour M, Pilzer I, Rosner R, Smith ZD, Meissner A, Nachman I. Epigenetic predisposition to reprogramming fates in somatic cells. EMBO Rep. 2015:e201439264. doi: 10.15252/embr.201439264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, et al. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc Natl Acad Sci. 2014;111:17188–17193. doi: 10.1073/pnas.1419554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 48.Serrano M, Lee H-W, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a Locus in Tumor Suppression and Cell Mortality. Cell. 1996;85:27–37. doi: 10.1016/S0092-8674(00)81079-X. [DOI] [PubMed] [Google Scholar]
- 49.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Pluripotency at day 28
A) Picked iPSC colonies from dynamic culture were cultured for 28 days and stained for pluripotency markers. B) iPSCs from A) were subjected to EB formation and spontaneous differentiation. 3 germ layers were formed.
Supplementary Figure 2: A) Reprogramming culture under dynamic and static conditions were fixed and stained for Nanog and γH2AX on day 8. Nascent colonies were imaged. Scale bar denotes 100 μm. B) Reprogrammed culture was fixed and stained on day 20. Images are shown on the left. Scale bar denotes 100 μm. Scatter plot of γH2AX intensity is shown on the right.
Supplementary Table 1: Antibody information
Supplementary Table 2: Primer sequences
Supplementary Table 3: Doubling time