Abstract
Long-term potentiation (LTP) and long-term depression (LTD) are two distinct forms of synaptic plasticity that have been extensively characterized at the Schaffer collateral-CA1 (SC-CA1) synapse and the mossy fiber (MF)-CA3 synapse within the hippocampus, and are postulated to be the molecular underpinning for several cognitive functions. Deficits in LTP and LTD have been implicated in the pathophysiology of several neurological and psychiatric disorders. Therefore, there has been a large effort focused on developing an understanding of the mechanisms underlying these forms of plasticity and novel therapeutic strategies that improve or rescue these plasticity deficits. Among many other targets, the metabotropic glutamate (mGlu) receptors show promise as novel therapeutic candidates for the treatment of these disorders. Among the eight distinct mGlu receptor subtypes (mGlu1-8), the mGlu1,2,3,5,7 subtypes are expressed throughout the hippocampus and have been shown to play important roles in the regulation of synaptic plasticity in this brain area. However, development of therapeutic agents that target these mGlu receptors has been hampered by a lack of subtype-selective compounds. Recently, discovery of allosteric modulators of mGlu receptors has provided novel ligands that are highly selective for individual mGlu receptor subtypes. The mGlu receptors modulate the multiple forms of synaptic plasticity at both SC-CA1 and MF synapses and allosteric modulators of mGlu receptors have emerged as potential therapeutic agents that may rescue plasticity deficits and improve cognitive function in patients suffering from multiple neurological and psychiatric disorders.
Keywords: Allosteric, hippocampus, long term potentiation, long term depression, mGlu, mGluR, mossy fiber, SC-CA1
1. INTRODUCTION
1.1. Circuitry within the Hippocampus
The hippocampus has long been considered as a primary brain region responsible for several important cognitive functions, such as learning and memory [1, 2]. The hippocampal network is highly dynamic and has the capacity to undergo changes in synaptic efficacy in an activity-dependent manner. These forms of synaptic plasticity observed within the hippocampal circuitry are thought to be critical for many major functions of the hippocampus. Short-term plasticity (STP), long-term potentiation (LTP), and long-term depression (LTD) are the most commonly investigated forms of synaptic plasticity within the hippocampus [1].
The hippocampus can be divided into three anatomical sub regions referred to as the dentate gyrus (DG), Cornus Ammonis 1 (CA1), and Cornus Ammonis 3 (CA3) [1, 2]. Synaptic plasticity is most commonly studied in two major intra-hippocampal pathways. The mossy fiber (MF) pathway consists of projections from DG granule neurons to area CA3, while the projections from CA3 pyramidal neurons compose the Schaffer collaterals that innervate area CA1 pyramidal neurons (SC-CA1) [1, 3]. The SC-CA1 synapse is the most commonly studied synapse within the hippocampal circuit as it is readily accessible for studies in isolation in the intact brain as well as in acute brain slices. The MF pathways are comprised of excitatory projections from the dentate gyrus granule cells that synapse onto either CA3 pyramidal cells or inhibitory interneurons [3]. The name ‘mossy’ was coined by Ramón y Cajal in 1894 because of the numerous and characteristic projections these neurons make, giving a moss-like appearance [3]. Granule cell axons from the dentate gyrus have more than one terminal type, forming both large mossy fiber boutons innervating the CA3 pyramidal neurons and small, filopodial extensions which emanate from these boutons that innervate the stratum lucidum interneurons of CA3 [4-6]. Unlike the SC-CA1 synapse, the MF synapses are less extensively characterized pathway owing to their overall complexity in anatomy as well as in physiology.
In addition to the intra-hippocampal connections described above, the hippocampus receives strong cortical input from the entorhinal cortex via the perforant pathway [7]. Traditionally, the perforant path connects most strongly to the DG via either the medial or lateral perforant path; however, there is also evidence that connections from the entorhinal cortex can project directly to areas CA3 and CA1 [7]. The majority of connections arise from layer II of the entorhinal cortex, which have been shown to project both to the DG and area CA3; however, a small number of additional connections can also arise from the deep layers of the entorhinal cortex [7]. The most heavily studied projections, those from the entorhinal cortex to the DG, are known to undergo synaptic plasticity, which plays a critical role in regulating the strength and timing of perforant path connections [7, 8]. In addition, there is also evidence that connections from the entorhinal cortex to area CA3 can also undergo synaptic plasticity [9]. As both of these projections have been discussed in recent reviews [7-9], the focus of this discussion will be on the intra-hippocampal connections. It should be noted, however, that the contribution of the perforant path to general information flow through the hippocampus is critical and, additionally, that the ability of those connections to undergo synaptic plasticity is important for the overall strength and timing of entorhinal cortex input to the hippocampus.
1.2. Expression Patterns of Metabotropic Glutamate Receptors in the Hippocampus
Glutamate is the primary excitatory neurotransmitter in the hippocampal circuit and acts on two distinct types of receptors. The ionotropic glutamate receptors are ligand-gated ion channels responsible for fast synaptic transmission and consist of three family members: α-amino-3-hydroxy-5-methyl-4-isoxazoleproplionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate receptors [10, 11]. All three types of receptors are activated by glutamate binding and flux positively charged ions into the cell, resulting in depolarization. NMDA receptors are distinct from AMPA and kainate receptors due to the requirement for a co-incident relief of a Mg2+ block in response to depolarization for activation. As such, AMPA and kainate receptors tend to function primarily under basal transmission conditions while NMDA receptors become active under strong synaptic activation.
The second group of glutamate receptors termed the metabotropic glutamate receptors (mGlu receptors), are 7 transmembrane spanning, G-protein coupled receptors (GPCRs) that signal through second messenger systems and indirectly gate ion channels. The mGlu receptors can be further divided into three distinct sub-groups based on sequence homology and G protein coupling. Group I mGlu receptors, including mGlu1 and mGlu5, are coupled to Gq and signal through protein kinase C (PKC) activation and increases in intracellular Ca2+ [12-17]. They are expressed at a variety of synapses throughout the brain, including the hippocampus. At the SC-CA1 synapse, both mGlu1 and mGlu5 are expressed postsynaptically on CA1 pyramidal cells [18, 19]. At the mossy fiber (MF)-CA3 synapse, mGlu1 and mGlu5 are expressed on the dendrites and dendritic spines of CA3 pyramidal cells [20].
Group II mGlu receptors include Gi/o-coupled mGlu2 and mGlu3. Activation of these receptors leads to reductions in cAMP levels, decreases in the conductance of N-type Ca2+ channels, and activation of other signaling pathways [21-26]. The expression of group II receptors is most prominent in the dentate gyrus and at the MF-CA3 synapse; however, there is evidence of mGlu3 receptor expression on astrocytes within area CA1 [27-29]. At the MF-CA3 synapse, mGlu2 is the predominant group II receptor and is expressed on presynaptic MF boutons [30].
Group III mGlu receptors are also Gi/o-coupled and include mGlu4, mGlu6, mGlu7, and mGlu8. With the exception of retinally-restricted mGlu6 [31], all of the other group III receptors are expressed at varying levels in the hippocampus. mGlu4 is expressed axonally on CA3 afferents at extremely low levels but there is no functional evidence for its contribution to transmission at the CA3-CA1 (SC-CA1) synapse [32-34]. mGlu8 is expressed presynaptically on CA3 afferents in neonatal animals; however, functional expression of the receptor disappears with age [32, 35]. In contrast, mGlu7 expression is low at SC-CA1 synapses in neonatal animals and increases with age [32, 36]. In adult animals, mGlu7 is expressed presynaptically at asymmetrical synapses within the synaptic cleft in CA3 [37, 38]. At MF synapses, mGlu7 is also expressed presynaptically on MF afferents synapsing onto stratum lucidum inhibitory interneurons [30].
The varying distribution of the mGlu receptors within these two hippocampal areas indicates that they may each play unique roles in the regulation of transmission, especially in the induction of long-lasting changes in synaptic strength. As such, the development of drug-like molecules that target individual mGlu receptor subtypes could have unique effects on one hippocampal region over another and may result in overall net increases or decreases in information flow throughout the hippocampal circuitry. This change in information flow can be critical to the improvement of cognitive or memory processing functions associated with a variety of neurodevelopmental and psychiatric diseases.
1.3. Allosteric Modulators Provide mGlu Receptor Subtype Selectivity
The traditional approach to the development of tool compounds targeting mGlu receptors was to identify and optimize compounds that bind to the orthosteric site, or glutamate binding site, on the receptor. This strategy has been useful but has not provided selective ligands for individual mGlu receptor subtypes due to the high conservation of the orthosteric site among all mGlu receptors [39]. Allosteric modulators, on the other hand, bind to a site on the receptor that is distinct from the orthosteric binding site; this has provided greater selectivity of compounds for one mGlu receptor subtype over other family members. There are several classes of allosteric modulators that have been developed, including both positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs). PAMs bind to a receptor and potentiate the effect of the endogenous ligand whereas NAMs inhibit the effects of the endogenous ligand in a non-competitive manner. The development and use of these compounds in pre-clinical animal models have been extremely successful [40-42]. In the following sections, we will describe work using both orthosteric and allosteric compounds targeting mGlu receptors to identify the precise roles that each mGlu receptor subtype plays in various forms of synaptic plasticity. Table 1 provides a list and abbreviations of orthosteric and allosteric ligands at mGlu receptors that have been used in these studies. Additionally, we will discuss various neuro- developmental, neurodegenerative, and psychiatric disorders for which deficits or alterations in hippocampal plasticity have been observed. Finally, we will summarize important advances in the use of novel, selective, allosteric modulators to improve hippocampal synaptic plasticity deficits.
Table 1. Abbreviations of orthosteric and allosteric compounds.
| Abbreviation | Chemical Name |
|---|---|
| ADX71743 | (+)-6-(2,4-dimethylphenyl)-2-ethyl-6,7-dihydrobenzo[d]oxazol-4(5H)-one |
| AIDA | (RS)-1-Aminoindan-1,5-dicarboxylic acid |
| AP5 | (2R)-amino-5-phosphonovaleric acid |
| ATPA | (RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid |
| CDPPB | 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide |
| CPCCOEt | 7-(Hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester |
| CTEP | 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine |
| cyclohexamine | 4-[(2R)-2-[(1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl]piperidine-2,6-dione |
| DCG-IV | (2S,2’R,3’R)-2-(2’,3’-dicarboxycyclopropyl)-glycine |
| DCPG | (S)-3,4-dicarboxyphenylglycine |
| DHPG | (S)-3,5-dihydroxyphenylglycine |
| DL-AP3 | DL-2-Amino-3-phosphonopropionic acid |
| EGLU | (2S)-α-ethylglutamic acid |
| JNJ16259685 | (3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanon |
| L-AP4 | L-2-amino-4-phosphonobutyric acid |
| LSP4-2022 | (2S)-2-amino-4-4-(carboxymethoxy)-phenylhydroxymethyl-hydroxyphosphorylbutanoic acid |
| LY341495 | (2S)-2-amino-2-[(1S,2S)-2-carboxcycloprop-1-yl]-3-(xanth-9-yl) propanoic acid |
| LY367385 | (S)-(+)-alpha-amino-4-carboxy-2-methylbenzene-acetic acid |
| LY395756 | (1S,2S,4R,5R,6S)-rel-2-amino-4-methylbicyclo[3.1.0]hexane-2,6-dicarboxylic acid |
| MCCG | α-methyl-cyclopropyl glycine |
| MCPG | (RS)-α-methyl-4-carboxyphenylglycine |
| MK-801 | [5R,10S]-[+]-5-methy-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine |
| MMPIP | 6-(4-methoxyphenyl)-5-methyl-3-pyridin-4-ylisoxazonolo[4,5-c]pyridine-4(5H)-one |
| MPEP | 2-Methyl-6-(phenylethynyl)pyridine hydrochloride |
| MSOP | (RS)-α-Methylserine-O-phosphate |
| MSOPPE | (RS)-α-methylserine-O-phosphate monophenyl-phosphoryl ester |
| PHCCC | (-)-N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide |
| VU-29 | 4-Nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide |
| VU0155094 | Methyl 4-(3-(2-((4-acetamidophenyl)thio)acetyl)-2,5-dimethyl-1H-pyrrol-1-yl)benzoate |
| VU0422288 | N-(3-chloro-4-((5-chloropyridin-2-yl)oxy)phenyl)picolinamide |
| (1S,3S)-ACPD | (1S,3S)-1-aminocyclopentane-1,3-dicarboxylic acid |
| 4CPG | (R)-4-carboxyphenylglycine |
2. synaptic plasticity at schaffer collateral-ca1 synapses
2.1. Long-term Potentiation
Several forms of LTP have been described at the SC-CA1 synapse. The most commonly studied form of LTP at this synapse is a potentiation induced by applying high frequency stimulation (HFS) to the axon fibers from CA3 [43]. This results in a long-lasting form of LTP, which has been used as the basis for many studies to date. This form of LTP is known to be frequency-dependent, as stimulation of axon fibers at 100 Hz for 1 second leads to a robust LTP, whereas stimulation at 3 Hz results in a robust LTD, and stimulation at 10 Hz does not result in any change in synaptic strength [44]. HFS-induced LTP is dependent upon NMDA receptor activation, postsynaptic increases in intracellular calcium, and depolarization of CA1 pyramidal cells [45-48]. After the discovery of HFS-induced LTP, a second form of LTP was identified, which is induced by theta burst stimulation (TBS) [49]. This paradigm more closely mimics the oscillation patterns of firing CA3 neurons observed in vivo [49]; thus, it may be more physiologically relevant compared to HFS paradigms. As many of the mechanisms underlying HFS and TBS-induced LTP overlap, they will be discussed together [50, 51].
Fig. 1 summarizes the roles of mGlu receptors in regulating synaptic plasticity at the SC-CA1 synapse. Postsynaptic group I (mGlu1 and mGlu5) receptors are thought to be involved in the induction and maintenance of both forms of LTP at the SC-CA1 synapse. To date, very few studies have been performed that directly investigate the contribution of mGlu1 activation to LTP at this synapse [52-54]; however, one study has found that application of the mGlu1 antagonist, LY367385, prevented the induction of LTP at SC-CA1 synapses when the antagonist was applied prior to HFS, but not after HFS (three trains of 100 Hz stimulation) [54]. Additionally, Aiba et al. reported that slices from mGlu1 knockout mice respond with substantially reduced levels of LTP at SC-CA1 when stimulated using HFS (five trains of 100 Hz stimulation); this phenotype was accompanied by modest deficits in a contextual fear conditioning assay, a measure of hippocampal-mediated learning and memory [52]. However, a separate study failed to observe LTP deficits at SC-CA1 in mGlu1 knockouts using HFS consisting of one train of 100 Hz stimulation [53]. In vitro, several studies indicate that mGlu1 activation increases the number of functional NMDA receptors on the postsynaptic membrane [55, 56]. In addition, in Xenopus oocytes, activation of mGlu1 results in a synaptosomal-associated protein 25 (SNAP-25)-mediated exocytosis of NMDA receptors [55]. mGlu1 stimulation also leads to the activation of transient receptor potential channel 1 (TRPC1) cation channels, which are responsible for a slow, inward current present in cerebellar purkinje cells; a similar mechanism for the induction of LTP may also be at play in the hippocampus [57]. mGlu1 activation also leads to increases in intracellular calcium and depolarization of CA1 pyramidal cells, all of which could facilitate LTP induction [58]. Thus, taken together, these results suggest that, while mGlu1 may participate in regulation of LTP, this receptor is not necessary for the induction or maintenance of LTP at SC-CA1.
Fig. (1).
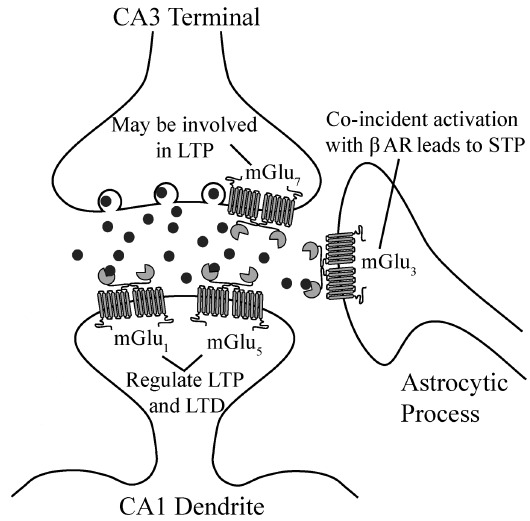
Summary of expression and role of mGlu receptors in synaptic plasticity at SC-CA1 synapses. Afferents projecting from CA3 pyramidal cells synapse onto the dendrites of CA1 pyramidal neurons. mGlu7 is expressed presynaptically and its activation may be involved in regulating LTP. Both mGlu1 and mGlu5 are expressed postsynaptically on CA1 pyramidal neurons, where they are known to regulate both LTP and LTD. mGlu3 is expressed on astrocytes within area CA1 and is involved in the induction of a short term depression also reliant on activation of β adrenergic receptors. Abbreviations: LTP- long term potentiation, LTD- long term depression, β AR- β adrenergic receptors, STP- short term plasticity.
The second member of the group I mGlu receptor family is mGlu5, which has been extensively characterized as a major regulator of LTP at SC-CA1 [59, 60]. Genetic deletion of mGlu5 [61, 62] or application of the mGlu5 selective antagonist MPEP [60] can reduce induction of LTP (using both TBS and 3 trains of 100 Hz stimulation) at the SC-CA1 synapse [63, 64], while the mGlu1/5 agonist DHPG primes LTP induction using either one train of 100 Hz stimulation or a sub-maximal TBS induction paradigm (5 bursts of 100 Hz, 200 ms inter-burst interval) [65, 66]. mGlu5 activation leads to potentiation of NMDA receptor currents due to a coupling mechanism that involves scaffolding through both postsynaptic density protein 95 (PSD-95) and Homer [58, 67]. This may contribute to the regulation of LTP by mGlu5. In addition, recent studies suggest that TBS-induced activation of mGlu5 can induce a form of “metaplasticity” that is mediated by release of endocannabinoids (eCBs) from CA1 pyramidal cells and eCB activation of CB1 receptors to induce depression of transmission at inhibitory synapses onto CA1 pyramidal cells [68-70]. This disinhibition, combined with the ability of mGlu5 to potentiate NMDA receptor currents, could be a major component of the role of mGlu5 in “priming” SC-CA1 synapses for induction of LTP reported in earlier studies [65, 66].
The persistent portion of LTP is dependent upon de novo protein synthesis [71-73] and is also regulated by activation of mGlu5 [63]. Therefore, activation of mGlu5 potentiates both the induction and maintenance of LTP at SC-CA1 synapses. Indeed, a threshold-level of LTP induced with one train of TBS can be significantly potentiated when slices are pre-treated with mGlu5 PAMs [74, 75]. This pre-treatment results in an increase in short term plasticity, termed post-tetanic potentiation (PTP), as well as the induction of LTP [74]. Taken together, mGlu5 plays an important role in regulating both the induction and maintenance of LTP at SC-CA1, and compounds that potentiate mGlu5 function may also help to restore LTP in settings where SC-CA1 plasticity is compromised.
The expression of group II receptors at SC-CA1 is extremely low [27, 76, 77]. There is a distinct lack of mGlu2 expression at the SC-CA1 synapse and mGlu3 expression is restricted to astrocytes in area CA1 [27, 77]. Antagonism of mGlu2 and mGlu3 using the orthosteric antagonist, MCCG, does not block LTP (induced using eight trains of 200 Hz stimulation) at this synapse, suggesting that activation of these receptors is not necessary for induction of LTP [78]. In contrast, agonism of mGlu2 and mGlu3 using the orthosteric agonist, (1S,3S)-ACPD, prevents the formation of LTP but retains much of the PTP using the same induction paradigm listed above [78]. The authors suggest that activation of group II mGlu receptors may increase the threshold for LTP induction by reducing presynaptic glutamate release; however, this hypothesis has not been directly tested at the SC-CA1 synapse [78]. Additionally, activation of group II mGlu receptors with concentrations of the group II agonist, DCG-IV, that are selective for these receptors does not affect basal excitatory transmission at SC-CA1, and the direct effects of DCG-IV on LTP induction has not been investigated [79]. However, when group II mGlu receptors are co-activated with a Gs coupled-receptor, such as a β-adrenergic receptor (βAR), a profound depression of synaptic transmission results [80, 81]. These effects are due to synergistic signaling of group II mGlu receptors and βARs on astrocytes, resulting in release of adenosine and subsequent activation of A1 adenosine receptors on SC terminals [29]. This mechanism is thought to be mediated by mGlu3, as it is the only group II mGlu receptor subtype localized on astrocytes throughout the brain, including in CA1 [77]. Given the recent evidence indicating that astrocytes play an important role in neuronal network function, including the induction of LTP [82], mGlu3 may play an important role in this unique form of associative plasticity at this synapse.
The group III receptors, which consist of mGlu4, mGlu6, mGlu7, and mGlu8, are also presynaptic Gi/o-coupled receptors that traditionally function as autoreceptors to decrease neurotransmitter release [83]. Among these receptors, mGlu7 and mGlu8 are the only members of this group that have been shown to be functional at SC-CA1 synapses [32, 34]. mGlu6 is expressed almost exclusively in the retina [31] and evidence for expression of functional mGlu4 at SC-CA1 synapses has not been found [26, 32, 34, 84]. Group III receptors are expressed presynaptically on synaptic terminals from CA3 where their activation results in a reduction in glutamatergic transmission [32, 34, 79, 85, 86]. Reductions in transmission have primarily been shown using the group III orthosteric agonist, L-AP4. L-AP4 activates all group III receptors over a wide range of concentrations, with rank order potency (from most potent to least potent) of mGlu4>mGlu8>>mGlu7 [32, 87]. As such, when concentrations of L-AP4 are used that should selectively activate mGlu4, no reduction in glutamatergic transmission is observed [32]. Additionally, the mGlu4-selective PAM, PHCCC, fails to potentiate the effects of L-AP4 at this synapse [32]. In neonatal animals, there is strong evidence that mGlu8 is the only group III receptor subtype present at SC-CA1 synapses; however, there is a developmental switch that occurs where mGlu7 expression replaces mGlu8 in adulthood [32, 88]. Due to this change in receptor expression, there have been no studies to date that have investigated the role of mGlu8 in LTP in adult animals. It will be interesting, however, to determine if perhaps it plays a role in LTP early in development.
Finally, despite clear evidence that mGlu7 is expressed presynaptically at SC-CA1 synapses in adult animals, few studies have directly investigated the role of this receptor in LTP [37, 89, 90]. One study in mGlu7 knockout mice demonstrated that there was a deficit in PTP and short-term potentiation (STP), but no effect on LTP (induced using one train of 100 Hz stimulation) [89]. This suggests that mGlu7 may play a more important role in short-term, but not long-term, plasticity at this synapse. Additional evidence for mGlu7-modulated control of STP comes from studies of basal synaptic transmission [34]. Application of the group III agonist, LSP4-2022, with and without two distinct group III PAMs, VU0155094 and VU0422288, results in a decrease in transmission at SC-CA1 synapses with a concomitant increase in paired-pulse ratio (PPR) [34]. Additionally, application of L-AP4 also results in a depression of transmission at the SC-CA1 synapse, which is reversed by a novel mGlu7-selective NAM, ADX71743 [85]. These studies indicate that transmission at SC-CA1 can be reduced by activation of presynaptic mGlu7. There have also been numerous studies in vivo indicating that mGlu7 knockout mice or mice treated with a selective mGlu7 NAM, MMPIP, show deficits in behavioral tasks that rely on proper hippocampal plasticity [91-95]. Interpretation of data using MMPIP, however, is hampered by the fact that this compound displays context-dependent pharmacology [86]; despite robust NAM activity in certain in vitro cellular assays, MMPIP fails to block L-AP4-mediated depression of synaptic transmission at SC-CA1, whereas ADX71743 robustly blocks depression of transmission [85, 86]. Therefore, mGlu7 could potentially play an important role in the induction of LTP, yet progress in this area has been delayed due to a lack of compounds that can differentiate mGlu7 from the other group III receptors.
2.2. Long-term Depression
There are three distinct forms of mGlu receptor-modulated LTD that are commonly studied at SC-CA1 synapses. The first is a chemical LTD which is induced by application of the group I orthosteric agonist, DHPG [96-98]. DHPG-LTD is NMDA receptor-independent; however, it relies on rapid extracellular signal-regulated kinase (ERK)-dependent dendritic messenger RNA (mRNA) translation without new transcription [74, 97-99]. Several studies suggest that this mechanism of LTD involves changes in both post and presynaptic function. Indeed, DHPG application results in a lasting reduction in postsynaptic AMPA receptor expression [100] as well as a reduction in presynaptic glutamate release [101]. The second form of LTD is mediated by synaptically-released glutamate and is induced by applying a low-frequency stimulation (900 stimuli delivered at 1 Hz, LFS) [44]. This LTD is developmentally regulated, as a robust form of LTD is observed in young rats (aged 12-20 days), whereas this LTD is absent in adult animals [102] and is also dependent upon de novo postsynaptic protein synthesis [54]. Additionally, a third form of LTD can be induced by applying 900 paired stimulations at 1 Hz (PP-LFS) [97, 103, 104]. This LTD persists at all ages; however, the requirement for NMDA receptor activation seems to change with age. In young animals (younger than 50 day old rats), NMDA receptor antagonists prevent induction of LTD; in contrast, LTD can still be induced even in the presence of the NMDA receptor antagonist, AP5, in adult animals (rats aged 12-15 weeks) [102]. There is, however, a form of PP-LFS that is still NMDA receptor-dependent in adult animals. This can be achieved by adjusting the paired-pulse interval (PPI) from 50 to 200 ms. Additionally, these separate forms of LTD (LFS, PP-LFS) occur via different downstream mechanisms, as the induction of one cannot be occluded by prior saturation of the other [102].
There is strong evidence that the different forms of LTD present at SC-CA1 synapses are differentially regulated by group I mGlu receptors [54, 74, 98]. There is evidence that mGlu1 and mGlu5 play distinct roles in the regulation of CA1 cell excitability [58]. mGlu1 activation is responsible for increasing postsynaptic intracellular Ca2+ concentrations and depolarization of CA1 cells, whereas mGlu5 activation results in the suppression of a Ca2+-activated potassium current and direct potentiation of NMDA receptors [58]. In addition, there are clear differences in the roles of these receptors in regulation of LTD at SC-CA1 synapses [54]. For example, antagonism of mGlu1 using LY367385 prior to induction of LTD using LFS results in a complete blockade of LTD [54]. This effect of mGlu1 antagonism was specific to the induction phase of LTD, as application of an mGlu1 antagonist after LFS does not prevent LTD [54]. This finding points to a specific role for mGlu1 in regulating the induction, rather than the maintenance, of LFS-LTD. In contrast, selective antagonism of mGlu5 with MPEP results in blockade of LFS-LTD regardless of whether the NAM is applied before or after LFS [54], indicating that there is a mechanistic difference in the ability of mGlu1 and mGlu5 to activate downstream signaling pathways necessary for LFS-LTD. It is interesting to note, however, that application of the mGlu5 PAM, VU-29, does not potentiate LFS-LTD [74]. One explanation for this phenomenon could be that LFS induction requires saturating recruitment of mGlu5 for induction, such that a PAM provides no further efficacy of LTD induction. In contrast to LFS-LTD, application of VU-29 results in a potentiation of PP-LFS LTD and DHPG-LTD when a sub-maximal concentration of DHPG (25 µM) is used [74]. These results suggest that mGlu5 PAMs maintain the proper form and direction of synaptic plasticity depending upon the stimulation paradigm.
Few studies to date have found evidence for a direct role of group II receptors at SC-CA1 synapses using subtype-selective compounds in the modulation of LTD [105, 106]. One study found that the non-selective group I/II mGlu receptor antagonist, 4CPG, and the group II antagonists MSOPPE and EGLU, blocked LFS-LTD induced in vivo in awake, behaving rats [105]. Additionally, activation of group II receptors using 5 µM DCG-IV, along with concomitant elevations in cyclic guanosine monophosphate (cGMP), leads to a weak LTD at SC-CA1 synapses [106]. However, the requirement for the use of 5 µM DCG-IV to observe this effect does not rule out off-target activity at other receptors, as this concentration is sufficient to activate NMDA receptors [107, 108]. Specific involvement of group II receptors in common forms of LTD discussed earlier has not been directly studied and both mGlu2 and mGlu3 have been shown to be involved in the induction of LTD in other brain regions [109-112]. Thus, it will be important to investigate the involvement of these receptors in various forms of LTD using newer generation, selective antagonists of these receptors.
As described earlier, the primary group III receptor expressed at SC-CA1 synapses in adult animals is mGlu7 [32, 34, 88]. Due to the lack of sub-type selective compounds, direct investigation of mGlu7 in LTD has not been performed. There are several reports, however, indicating that antagonism of group III mGlu receptors blocks LTD at SC-CA1 synapses [113]. When considered with anatomical expression patterns and functional studies at SC-CA1, it is possible that these effects can be attributed to mGlu7 [32, 34, 37, 38, 85, 86]. Additionally, one study found that intracerebroventricular (ICV) injection of L-AP4 resulted in a slow-onset chemically-induced LTD observed in vivo in area CA1 [114]. However, despite these studies, direct investigation using selective compounds targeting mGlu7 have not been performed and, as such, the precise involvement of mGlu7 in the regulation of LTD has not been determined.
3. SYNAPTIC PLASTICITY AT MOSSY FIBER-CA3 SYNAPSES
Due to the difficulty of studying this synapse in isolation, hippocampal MF synapses have been studied far less extensively relative to SC-CA1 synapses [6, 115]. However, similar to the SC-CA1 synapse, the MF synapse can undergo various forms of short-term and long-term plasticity. Since excitatory transmission at this synapse is mediated by glutamate, activation of mGlu receptors localized both at presynaptic and postsynaptic sites has also been heavily implicated in the induction and expression of MF synaptic plasticity (see Fig. 2). In this section, we will review the different forms of plasticity that have been described at these synapses, with emphasis on regulation by the mGlu receptors.
Fig. (2).
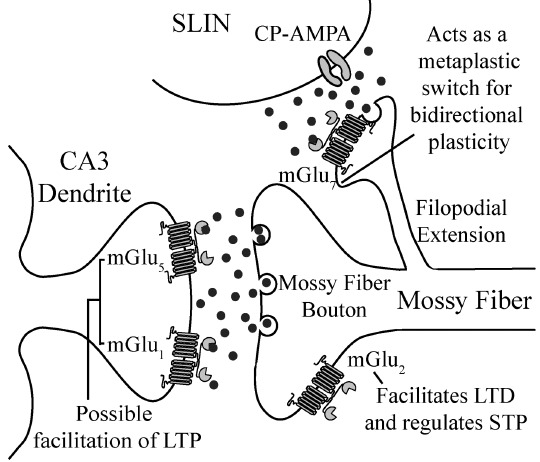
Summary of expression and role of mGlu receptors in synaptic plasticity at MF synapses. Projections from dentate gyrus granule cells (termed mossy fibers) form connections with both CA3 pyramidal cells and interneurons. mGlu2 is expressed on large, mossy fiber boutons which innervate CA3 pyramidal cells, where it is thought to facilitate induction of LTD and regulate short term plasticity (both frequency facilitation and paired-pulse facilitation). Both mGlu1 and mGlu5 are expressed postsynaptically on CA3 pyramidal cells and play a potential role in facilitation of LTP. mGlu7 is expressed presynaptically at MF-SLIN synapses that express calcium-permeable AMPA receptors postsynaptically, where it acts as a metaplastic switch for bidirectional plasticity. Abbreviations: LTP- long term potentiation, LTD- long term depression, SLIN- stratum lucidum interneuron, STP- short term plasticity, CP-AMPA- calcium-permeable AMPA receptors.
3.1. Short-term Plasticity
Properties of synaptic plasticity at the MF synapses depend on the postsynaptic target [116]. MF-pyramidal neuron synapses characteristically show uniquely large paired-pulse facilitation (PPF) [117]. This is a distinct form of STP which is not observed at other hippocampal or cortical synapses, including SC-CA1. This PPF is at least two times greater in magnitude than the PPF observed at commissural/associational (C/A) synapses [5]. These synapses are distinct inputs to the CA3 area originating from other CA3 cells either from the same or opposite hemisphere [5]. MF synapses also exhibit strong frequency facilitation, as increasing stimulation frequency results in a significant increase in synaptic strength [118]. Unlike the C/A synapses, frequency facilitation at MF synapses is observed at inter-stimulus intervals (ISI) as long as 40 seconds and is generally much greater in magnitude [5]. In contrast, MF-interneuron synapses show much smaller PPF or even exhibit synaptic depression in response to paired-pulse stimulation [116] due to a higher neurotransmitter basal release probability [119, 120]. In addition, the PTP observed after LTP induction is significantly prolonged when compared to SC-CA1 synapses [5, 121].
Several factors have been implicated in regulating STP at the MF synapses, including Ca2+, A1 adenosine receptors, and kainate receptors [122-125]. Additionally, mGlu receptors also play an important role in STP at MF synapses. Presynaptic group II mGlu receptors located specifically at MF-pyramidal neuron synapses act as autoreceptors and have been implicated in regulating PPF and frequency facilitation [6, 126]. It is proposed that activation of group II mGlu receptors suppresses glutamate release by inhibiting Ca2+ channels via a membrane-delimited mechanism [6, 126]. Indeed, DCG-IV (1 µM) significantly reduces presynaptic Ca2+ transients at the MF-pyramidal neuron synapse while simultaneously reducing synaptic transmission [126]. Additionally, electron microscopy immunostaining reveals that mGlu2 is the predominant subtype found in the presynaptic locus [30] and is thought to be the mGlu receptor mediating regulation of short-term plasticity.
mGlu2 receptors, however, are located at the pre-terminal zone on MF afferents [30]. Due to their remote location, LFS may not result in sufficient glutamate release to activate these receptors [127]. In support of this hypothesis, non-selective mGlu receptor antagonists have no effect on baseline excitatory postsynaptic currents (EPSCs) evoked in CA3 pyramidal cells by MF stimulation with LFS (0.5 Hz) [128]. However, an enhancement of synaptic transmission is observed in the presence of mGlu receptor antagonists when MF afferents are stimulated at a higher frequency (1 Hz) [128]. This inhibitory action of presynaptic mGlu receptors is not limited to the activated synapse but also affects neighboring MF-pyramidal cell synapses [129]. Thus, group II mGlu receptors can act as a brake to limit the magnitude of frequency facilitation as well as inhibit surrounding synapses [6]. Importantly, there are possible species differences in the role that mGlu receptors play in the regulation of MF-pyramidal neuron synapses.
In addition to group II mGlu receptors, group III mGlu receptors have also been identified at the MF-pyramidal neurons of guinea pig, but not in other rodents [130, 131]. Whole cell recording of CA3 pyramidal neurons in guinea pigs reveals that activation of group III mGlu receptors by L-AP4 selectively depresses MF, but not C/A, transmission [117]. With respect to the MF-stratum lucidum interneuron synapses that are physiologically distinct in terms of their plasticity characteristics, localization of mGlu7 has been confirmed using mGlu subtype specific antibodies and electron microscopy [30, 37, 90]. However, the specific physiological role of these receptors at this synapse has not been elucidated. It is thought that mGlu2 and mGlu7 primarily act as negative feedback regulators of STP at the MF-pyramidal neuron synapses; yet, the involvement of other mGlu receptors, specifically mGlu1 and mGlu5, requires further investigation.
3.2. Long-term Potentiation
Like SC-CA1 synapses, MF synapses also exhibit activity-dependent, long-term changes in synaptic strength (e.g., LTP). However, there are fundamental differences in the mechanisms governing such LTP at this synapse. Several forms of LTP have been described, including an NMDA-dependent, NMDA-independent, and a chemical form of LTP. The most commonly reported form of LTP in the MF pathway is induced by HFS (100 Hz for 1s/0.5s or 25 Hz for 5s) [6]. In contrast to SC-CA1, HFS-LTP in this pathway is independent of NMDA receptor activation [121, 132]. Although NMDA receptor expression [133] and evoked NMDA receptor currents [134] are observed at MF synapses, the expression of this NMDA receptor-independent form of LTP is exclusively presynaptic. This is due to a characteristic increase in neurotransmitter release probability, as evident from changes in PPR [121, 135, 136], as well as from quantal analysis involving analysis of coefficient of variation (CV), failure rate, and miniature synaptic events [136-138].
The mechanisms governing LTP induction at MF synapses and the role of mGlu receptors have yet to be definitively resolved. Despite extensive pharmacological and genetic studies, there are conflicting reports on the role of mGlu1 and mGlu5 receptors in MF-LTP. Early reports of mGlu receptor involvement in MF-LTP came from use of the mGlu receptor antagonist, DL-AP3, which blocks HFS-LTP (induced by two trains of 100 Hz for 1 second), at this synapse [139]. Concurrently, the mGlu receptor agonist, ibotenate, induces LTP even in absence of any tetanic stimulation [139]. Additionally, MCPG also blocks this NMDA receptor-independent HFS-LTP (induced by one train of 100 Hz for 1 second) [140]. Group I mGlu receptors may modulate MF-LTP by triggering a delayed rise in postsynaptic Ca2+ via IP3-mediated release from internal stores [141]. Competitive and non-competitive group I mGlu receptor antagonists such as AIDA, MCPG, and CPCCOEt reversibly block the rise in postsynaptic Ca2+ observed during MF tetanic stimulation in presence of the ionotropic glutamate receptor blocker, kynurenate, and also prevent the induction of MF-LTP [141]. Furthermore, mGlu1 knockout mice display impaired HFS-LTP (induced by one train of 100 Hz for 1 second) at the MF synapses but not at the C/A synapses [53]. In contrast, mGlu5 knockout mice have no deficits in MF HFS-LTP (induced by one train of 100 Hz for 0.5 seconds) [62]. However, there is evidence that both mGlu1 and mGlu5 may act synergistically to regulate MF-LTP [142]. As such, the nonselective group I antagonist, MCPG, blocks MF HFS-LTP (induced by one train of 100 Hz for 1 second). Consistent with the MCPG result, a combination of LY367385 (mGlu1 antagonist) and MPEP (mGlu5 NAM), also block this form of MF HFS-LTP; in contrast, either antagonist alone is ineffective. Interestingly, co-application of both an mGlu1 and mGlu5 antagonist decreases Ca2+ entry into individual presynaptic MF boutons, thus providing a mechanism by which group I mGlu receptors can modulate induction of MF-LTP [142].
In spite of this evidence, there are several conflicting reports which suggest that there is no involvement of group I mGlu receptors in MF-LTP. For example, some studies have reported that group I mGlu receptor antagonists have no effect on MF-LTP (induced by one train of 100 Hz for 1 second) [143-145]. Additionally, others have reported that mGlu1 knockout mice do not show any deficit in this form of MF HFS-LTP [143]. Similarly, some studies suggest that the rise in postsynaptic Ca2+ downstream of group I mGlu receptor activation is unnecessary for 100 Hz-induced MF LTP [121, 138, 145-147]. This discrepancy may be due to several issues, including the use of non-selective pharmacological agents, differences in animal ages, and differences in the tetanus protocol used for inducing MF-LTP. Thus, future studies controlling for these variables and utilizing recently discovered subtype selective pharmacological agents will be critical in definitively determining the role of group I mGlu receptors in MF-LTP.
Interestingly, group I mGlu receptors are involved in inducing a chemical form of LTP at this synapse [142]. Although activation of group I mGlu receptors alone with DHPG does not induce LTP, potentiation of MF synaptic responses can occur when DHPG is co-applied with an ineffective concentration of the kainate receptor agonist, ATPA. Importantly, this potentiation occurs in presence of AP5, indicating that, similar to tetanus-induced MF-LTP, NMDA-independent LTP can be induced by a synergistic activation of group I mGlu receptors and kainate receptors. This chemical form of NMDA-independent LTP is not blocked by application of either MPEP or LY367385 alone, indicating that activation of either mGlu1 or mGlu5, along with kainate receptors, is sufficient to induce chemical LTP. Finally, chemical LTP was also found to be dependent on Ca2+ released from intracellular stores. The ryanodine receptor blocker, ryanodine, completely blocks the induction of chemical LTP and also results in an increase in Ca2+ signaling in presynaptic MF boutons [142]. The results of these studies also help to explain some of the discrepancies related to mGlu receptor involvement in MF-LTP induced by tetanus. First, they show that activation of either mGlu1 or mGlu5 is sufficient for LTP and provides a possible explanation for the lack of effect of MF-LTP deficits in mGlu1 or mGlu5 knockout mice. Second, they show that group I mGlu receptors may affect presynaptic Ca2+ pools in MF boutons. Although no direct mechanism for this phenomenon is known, it is possible that it could occur either by activation of presynaptically located group I mGlu receptors or via activation of postsynaptic group I mGlu receptors, leading to the production of retrograde messengers. This might account for the discrepancy observed in certain studies where chelation of postsynaptic Ca2+ is not sufficient to block MF-LTP [121, 138, 145-147].
An additional form of NMDA-independent and mGlu receptor-dependent LTP has also been reported at MF synapses. This LTP at MF synapses is induced by a combination of postsynaptic membrane hyperpolarization and paired pulse low frequency stimulation [148]. It is dependent upon mGlu receptor activation, as MCPG application blocks induction, and is also dependent upon postsynaptic elevation in Ca2+ levels [148]. Thus, the current evidence provides a strong basis for the involvement of group I mGlu receptors in regulation of the induction of NMDA-independent MF-LTP by stimulating the release of internal Ca2+ from the presynaptic MF boutons and/or postsynaptic CA3 neurons.
Recently, an NMDA-dependent form of LTP has been described at the MF-pyramidal neuron synapse [149, 150]. Delivery of a short tetanus (24 stimuli at 25 Hz) potentiates NMDA receptor-mediated EPSCs but has no effect on AMPA receptor-mediated EPSCs [149, 150]. This potentiation of NMDA transmission is input-specific, as it is only observed at MF synapses and not at C/A synapses. Interestingly, this form of LTP is mediated postsynaptically and is dependent on Ca2+ released from inositol triphosphate (IP3)-sensitive stores [149, 150]. It can also be blocked by the MPEP but not the mGlu1 NAM, CPCCOEt. Thus, this NMDA-dependent MF-LTP requires co-activation of mGlu5 and NMDA receptors [149]. The role of other mGlu receptors in this form of LTP has not yet been elucidated and further studies are required to investigate the involvement of other subtypes.
The MF-LTP described above is primarily based on investigations of the MF-CA3 pyramidal neuron synapses. In contrast, much less is known about LTP at MF-interneuron synapses. There is evidence, however, that plasticity at these synapses is governed by a distinct set of mechanisms that are separate from what is observed at the MF-pyramidal neuron synapses. For example, HFS (induced by three trains of 100 pulses at 100 Hz repeated every 10 seconds) of the MF induces LTP in CA3 pyramidal neurons and in CA3 stratum lacunosome-moleculare (L-M) interneurons [151, 152]; however, it induces LTD in CA3 stratum lucidum interneurons (SLIN) (see next section), suggesting that there is target cell-dependent plasticity within the MF-CA3 circuit [153]. The hebbian form of LTP observed in L-M interneurons is dependent on mGlu1 [151], and mGlu1-mediated Ca2+ signaling is thought to control the polarity of MF plasticity at these synapses [151]. HFS induces LTP in control conditions, whereas HFS in the presence of the mGlu1 antagonist, LY367385, results in LTD [151]. This phenomenon may be specific to mGlu1; however, the role of other mGlu receptor subtypes in LTP induction of the MF-interneuron synapses has not been investigated.
3.3. Long-term Depression
MF-CA3 pyramidal neuron synapses also exhibit LTD. Repetitive LFS, such as 1Hz for 15 min, induces LTD at this synapse [127, 142, 154]. This form of LFS-LTD is NMDA receptor-dependent at other synapses; however, the mechanism governing this form of plasticity at the MF-CA3 pyramidal neuron synapse is fundamentally different [6]. In this case, LFS-induced LTD at MF synapses is heavily dependent upon mGlu2 receptor activation. For example, LFS-LTD is lost in mGlu2 knockout mice, despite normal histological features, basal synaptic transmission properties, and LTP induced by HFS [127]. MF-LTD is independent of NMDA receptor activation and relies on a presynaptic expression pattern characterized by a reduction of neurotransmitter release [154]. This form of LTD is also blocked by application of MCPG [154], thus reiterating the role of mGlu receptors in this form of MF-LTD. Further studies suggest that mGlu2 activation leads to decreases in cAMP levels and PKA activation [155], followed by a rise in presynaptic Ca2+ [154, 155], which in turn leads to the subsequent expression of MF-LTD. Additionally, pharmacological activation of mGlu2 alone using DCG-IV is sufficient to induce LTD at this synapse, corroborating the role of mGlu2 in MF-LTD [110]. Similarly, application of the mGlu2 agonist, LY395756, also induces significant LTD of the MF-pyramidal neuron synapses. Interestingly, this form of LTD can be reversed by application of the group II mGlu receptor antagonist, LY341495, applied after LTD expression, indicating that mGlu2 may remain active even during the MF-LTD and long after the exogenous agonist has washed out [110].
In addition to the conventional form of MF-pyramidal neuron LTD, there is also evidence for target cell-specific LTD at MF-interneuron synapses. Interestingly, the same HFS protocol (three-four trains of 100 Hz for 1 second repeated every 10 seconds) that induces LTP at MF-pyramidal neuron synapses leads to a presynaptically expressed form of LTD at MF-SLIN synapses [116, 156-158]. Unlike the LTP at MF-pyramidal neuron synapses, tetanus-induced LTD at MF-SLIN synapses is independent of adenylyl cyclase activation [157], indicating that there is anatomical segregation of distinct biochemical signaling cascades to functionally divergent presynaptic terminals of the same afferent [4, 159]. There are also two different forms of tetanus-induced MF-SLIN LTD which depend on specific postsynaptic architecture of AMPA receptors in the SLINs. A small portion of SLINs, primarily expressing GluR2-containing, Ca2+ impermeable, AMPA receptors, exhibit the more common postsynaptic form of LTD, which is dependent on Ca2+ influx through NMDA receptors and subsequent internalization of AMPA receptors [156, 160, 161]. On the other hand, a major subgroup of SLINs expressing GluR2-lacking Ca2+ permeable AMPA receptors exhibit a presynaptically expressed NMDA-independent LTD following tetanic stimulation (three trains of 100 pulses at 100 Hz repeated every 10 seconds) of the MF afferents [156, 161]. Several studies have indicated that this form of LTD is mediated by mGlu7, which is strongly expressed in the MF stratum lucidum [30, 153]. Accordingly, application of the group III antagonist, MSOP, prevents NMDA-independent MF-SLIN LTD without altering basal transmission [158]. Moreover, application of L-AP4 at a concentration high enough to activate mGlu7, but not the group II agonist, DCG-IV, induces a chemical form of LTD at the MF-SLIN synapses that shares the presynaptic expression profile observed for tetanus-induced LTD [157, 158]. Interestingly, this form of chemical LTD is absent when presynaptic stimulation is interrupted during L-AP4 treatment [158] or when postsynaptic Ca2+ influx is blocked [156, 158], indicating that this group III mGlu receptor-mediated LTD is hebbian in nature. In further studies, it has been shown that MF-SLIN LTD relies on mGlu7-mediated PKC signaling and subsequent reduction in the activity of P/Q-type voltage gated Ca2+ channels [158, 162].
The MF-SLIN synapses also show bidirectional metaplasticity, where the polarity of tetanus-induced-plasticity depends upon the recent history of the synapse. Therefore, if MF-SLIN synapses first undergo L-AP4-induced LTD, a subsequent tetanic stimulation reverses this depression by persistently enhancing neurotransmitter release [158]. It is hypothesized that mGlu7 plays an important role in this bidirectional plasticity and acts as a ‘metaplastic switch’ [153]. Indeed, exogenous agonist treatment can lead to mGlu7 internalization from MF-SLIN synapses [158, 159] and the absence of mGlu7 in the presynaptic terminal can be the deciding factor for the direction of tetanus induced synaptic plasticity observed. However, the special emphasis on mGlu7 is entirely based on the combination of histological localization of this subtype and the use of nonselective group III mGlu receptor agonists and antagonists. Further studies need to be performed using more selective compounds, such as the recently discovered mGlu7 NAM, ADX71743 [85], to reinforce the involvement of this subtype among the group III mGlu receptors as the primary candidate in regulating MF-interneuron plasticity.
4. APPLICATIONS OF MGLU RECEPTOR ALLOSTERIC MODULATORS TO NEURODEVELOPMENTAL AND PSYCHIATRIC DISEASES
There are several psychiatric and neurodevelopmental disorders that are associated with deficits in hippocampal synaptic plasticity, including autism spectrum disorders, schizophrenia, and Alzheimer’s disease [76, 163-172]. Additionally, these deficits occur in forms of synaptic plasticity that are known to be modulated or mediated by mGlu receptors, as discussed in the above sections. As such, several efforts have been undertaken to develop novel allosteric modulators targeting various mGlu receptors with the hope of restoring such synaptic plasticity deficits and thus identifying novel therapeutics for these disorders.
Several monogenic autism spectrum disorders are associated with deficits in hippocampal plasticity and cognitive function [163, 164, 166, 168, 169, 171-179]. Specifically, synaptic plasticity deficits have been reported that are associated with Fragile X syndrome, Tuberous Sclerosis, Rett Syndrome, and human chromosome 16p11.2 microdeletion. Fragile X syndrome is a X-linked disorder associated with trinucleotide repeat expansion in the 5’ untranslated region of the Fragile X mental retardation 1 (FMR1) gene, which leads to decreased expression of Fragile X mental retardation protein (FMRP) [173, 174, 178]. As FMRP is responsible for repressing translation of mRNAs in the brain that are associated with downstream signaling of mGlu1 and mGlu5 activation, loss of FMRP leads to excessive protein synthesis and results in enhanced mGlu receptor-mediated LTD at SC-CA1 synapses [166]. Excitingly, it has been found that antagonists or NAMs of mGlu5, such as MPEP and CTEP, are efficacious in reducing enhanced DHPG-LTD and reversing some of the phenotypic deficits observed in FMRP knockout mice [180, 181]. There is also additional evidence that mGlu1 NAMs may also be efficacious as treatments for Fragile X syndrome [181]. Indeed, one study found that antagonism of mGlu1 using the NAM, JNJ16259685, was also sufficient to decrease Fragile X syndrome phenotypes in animal models, such as marble burying and pre-pulse inhibition [181]. The direct effects of mGlu1 NAMs on DHPG-induced LTD have not yet been studied; however, this would be important to understand the contribution of mGlu1 activity to hippocampal function.
A second monogenic form of autism, termed Tuberous Sclerosis, has also been suggested to involve changes in mGlu5 function in the hippocampus [164]. As opposed to FMRP knockout mice, genetic deletion of or the introduction of inactivating mutations into tuberous sclerosis complex (Tsc) 1 or 2 results in hyper activation of the mammalian Target of Rapamycin (mTOR) [182]. Genetic deletion of Tsc2 results in reduced DHPG-LTD and PP-LFS LTD with no change in the induction of LFS-LTD at SC-CA1 synapses [164]. Because Tsc2 KO mice display opposite electro- physiological phenotypes to FMRP KO mice, this indicates that mGlu5 PAMs could be efficacious in restoring proper levels of LTD in these animals [164]. Indeed, application of the mGlu5 PAM, CDPPB, resulted in an enhancement of DHPG-LTD [164].
A third form of monogenic autism is the X-linked disorder Rett Syndrome, which results from loss-of-function mutations in the transcription factor, methyl CpG binding protein 2 (Mecp2) [176]. Knockout or mutation of the Mecp2 gene in mice results in specific deficits in SC-CA1 plasticity. Specifically, Mecp2 knockout and mutant mice display a significant decrease in PPR, suggesting that there are deficits in presynaptic regulation of neurotransmitter release [163]. These mice also display reduced TBS and HFS-induced LTP [163, 168, 172]. Interestingly, while deficits in the induction of PP-LFS LTD are observed, DHPG-LTD remains intact [168].
MECP2-Duplication syndrome is another form of monogenic autism which results from the duplication, rather than the loss, of the MECP2 gene [183, 184]. Despite genetically opposing levels of Mecp2, mouse models of Mecp2 duplication syndrome display similar synaptic plasticity deficits [169], and these mice display deficits in HFS-LTP at SC-CA1 [169]. However, it is interesting to note that these mice display an increase in PPR, which is the opposite effect observed in Mecp2 knockout mice [163, 169]. This could potentially indicate that Mecp2 functions to regulate an aspect of synaptic transmission such that opposing effects on STP are observed between Mecp2 knockout and Mecp2 Duplication; however, both of these changes would be sufficient to cause a deficit in LTP.
Finally, human chromosome 16p11.2 microdeletion is another genetic form of autism associated with deficits in hippocampal synaptic plasticity [171, 175, 177, 179]. Mice with a genetic deletion mimicking this microdeletion display alterations in DHPG-LTD and PP-LFS LTD at SC-CA1 synapses [171]. While no deficit in the magnitude of LTD is observed when compared to wild-type mice, in both cases, the persistent phase of the LTD is no longer protein-synthesis dependent, as treatment with the protein synthesis inhibitor, cyclohexamine, prevents the late phase of LTD in wild-type but not mutant mice [171]. No deficits were observed in TBS-LTP or LFS-LTD, perhaps indicating that this microdeletion results in changes specifically associated with the mechanisms involved in DHPG- and PP-LFS LTD.
In addition to autism spectrum disorders, there is also evidence for hippocampal synaptic plasticity deficits in animal models of schizophrenia [167, 170]. For example, the reelin knockout mouse is a model of schizophrenia that mimics many of the cognitive phenotypes observed in patients as evidenced by deficits in contextual fear conditioning and the Morris water maze tasks [170]. In addition, these mice also display deficits in TBS-LTP and LFS-LTD [170]. A second model of schizophrenia, in which rodents are treated for 7 days with the NMDA receptor open channel blocker MK-801, also display severe cognitive impairments in spatial working memory [167]. Additionally, this is associated with a profound deficit in HFS-induced LTP [167]. While the reelin knockout mouse model displays a deficit in LFS-LTD, no deficit was observed in the sub-chronic MK-801 model [167, 170]. Taken together, both of these models, while different in their mechanism of induction, result in similar deficits in LTP in the hippocampus, perhaps indicating that they arise from a common mechanism.
Finally, Alzheimer’s disease is the most common form of dementia, and is characterized by memory loss and a decline in cognitive function [185]. Hallmarks of the disorder include the accumulation of amyloid β (Aβ) and progressive neurodegeneration [185]. A prominently studied rodent model of Alzheimer’s disease is the CK-p25 mouse model [76]. In this model, there is an over-expression of the protein p25, which is a truncated version of p35 generated from enzymatic conversion [76]. p35 normally functions as a regulator of the serine/threonine kinase, Cdk5 [76]. In contrast to p35, p25 activity leads to hyper-activation of cyclin-dependent kinase 5 (Cdk5) and results in neurodegeneration [165]. As such, prolonged over-expression of p25 leads to long-lasting Cdk5 activity, which results in cognitive impairments and significant deficits in HFS-LTP at SC-CA1 synapses [165].
Unlike SC-CA1 synaptic plasticity, very little is known about the contribution of MF synaptic plasticity to behavioral dysfunctions observed in neurological disorders. There is even debate on the importance of long-term plasticity in this pathway, with views speculating that there is a more important role for short-term plasticity in this pathway than LTP and LTD [186]. However, emerging reports suggest that both forms of plasticity in the MF pathway may have significance in learning and memory and can have implications for cognitive defects observed in neurological and neuropsychiatric disorders. In freely moving rats, exploration of a novel environment results in facilitation of LTP at MF synapses, whereas exploration of large spatial landmarks results in facilitation of LTD at MF-CA3 pyramidal neuron synapses [187]. Data from mGlu1 knockout mice show that impairment in MF-LTP, but not SC-CA1 LTP, results in a gross deficit in reference memory during performance in the Morris water maze task [53]. Additionally, infusion of DCG-IV in area CA3 in vivo, which is implicated in disruption of LTD and STP at MF synapses, leads to an inhibition of contextual fear memory in rodents [188]. Taken together, these results suggest an important, and possibly independent, role for MF synaptic plasticity in spatial learning and memory. Furthermore, recent evidence also suggests that MF plasticity may be disrupted in specific neurodegenerative disorders [189]. The R6/2 and complexin II knockout mouse models of Huntington’s disease, which display spatial learning deficits similar to those observed in Huntington’s patients, also exhibit selective impairments in MF-LTP with no changes in basal synaptic transmission in the CA3 region [189]. Similarly, a transgenic Alzheimer’s disease mouse model that age-dependently overproduces Aß peptide display a significant loss of short-term frequency facilitation at MF synapses without changes in the basic properties of synaptic transmission [190]. In addition, HFS NMDA receptor-independent LTP was absent at MF synapses in these transgenic mice [190]. If MF LTP is dependent on group I mGlu receptors, then either mGlu1 or mGlu5 PAMs might be rendered as possible candidates to reverse such deficits, potentially providing evidence for therapeutic efficacy in improving cognitive function in Huntington’s and Alzheimer’s patients. Taken together, these studies provide convincing evidence that plasticity at MF synapses has equally important physiological and functional implications as SC-CA1 synapses with indications that impaired MF synaptic plasticity could be a pathophysiological feature characteristic of or underlying certain neurological disorders. As mGlu receptors play a central role in regulating synaptic plasticity at this synapse, selective activation of mGlu receptors may be a promising strategy to rescue deficits in MF plasticity in specific disease states.
In summary, there are multiple neurodegenerative, neurodevelopmental, and psychiatric diseases that are associated with cognitive impairment and deficits in hippocampal synaptic plasticity. While some have clear ties to mGlu receptor function, for example, Fragile X syndrome, many of the others have not been rigorously investigated [174]. As such, it will be important to determine if modulation of mGlu receptor function can ameliorate hippocampal plasticity deficits in these animal models. Additionally, many novel, highly selective tool compounds have recently been developed, which makes it possible to rigorously test the function of individual mGlu receptor subtypes [85, 112, 191-194]. While mGlu5 PAMs and NAMs have been well-studied in the literature, there are far fewer mGlu1 PAMs and NAMs available for study [75, 191, 192, 195-199]. More recently, novel, selective mGlu1 PAMs have been developed that are highly selective for mGlu1 over other mGlu receptors [192]. Additionally, some novel mGlu1 NAMs have also been reported [192, 193]. Together, these tool compounds will allow for an investigation into the precise role that mGlu1 plays in the induction of LTP and LTD.
There are also two new reports describing the discovery of a selective mGlu3 NAM and an mGlu2 NAM, both of which show promise as tool compounds for both in vitro and in vivo use [112, 194]. These two compounds will finally allow for the careful investigation of mGlu2 vs. mGlu3 in order to tease apart distinct functions governed by each receptor. Finally, the discovery of selective group III compounds has been by far the biggest challenge in the field. For the study of hippocampal plasticity, compounds that selectively target mGlu7 and mGlu8 would be most useful. Despite the availability of the selective mGlu8 agonist, DCPG, the development of selective agonists for mGlu7 has not been as successful. The discovery of LSP4-2022, a group III orthosteric agonist, provides selectivity for mGlu7 over mGlu8 [200]. This compound, however, is still much more potent at mGlu4 over mGlu7 and mGlu8, [200] which makes its use at synapses expressing all of the group III receptors limited. Additionally, two novel group III PAMs have been reported and have shown to be efficacious in decreasing glutamatergic transmission at SC-CA1 synapses when used in combination with LSP4-2022 [34]; again, these compounds are not selective for mGlu7. Finally, the development of ADX71743 provides a novel mGlu7 NAM for use both in in vitro and in vivo studies [85]; it is important to note that ADX71743 can potentially be used in combination with nonselective compounds to isolate an mGlu7-specific component to a given response.
5. CONCLUSION
The involvement of specific mGlu receptors in distinct forms of synaptic plasticity suggests that modulation of mGlu receptors may be efficacious in treating cognitive impairments associated with several neurodevelopmental, neurodegenerative, and psychiatric disorders. It is interesting that, at both SC-CA1 and MF synapses, regulation of plasticity seems to be dominated by only a few mGlu receptors. At SC-CA1 synapses, the majority of evidence indicates that mGlu5 plays a pivotal role in the induction and maintenance of both LTP and LTD [59, 60, 63, 74, 98]. Importantly, it has been shown that mGlu5 PAMs have the ability to bi-directionally potentiate synaptic plasticity [74]. This is a key finding, as it indicates that, regardless of the direction of plasticity, potentiation of mGlu5 could rescue deficits without disrupting the ratio between potentiation and depression. Additionally, the role of mGlu5 in MF-LTP is, if at all, to potentiate MF-CA3 pyramidal cell LTP [141]. On a network level, this could indicate that potentiation of mGlu5 might result in a potentiation of CA3 afferent activity into area CA1 and lead to a net increase in activity in CA1.
Based on expression patterns, there is a distinct separation of the group II receptors between SC-CA1 and MF synapses [27, 77]. At SC-CA1, mGlu3 plays a unique role in a coincident form of transient depression that relies on both glutamatergic and noradrenergic signaling in astrocytes [28, 29]. However, the relevance of this phenomenon to plasticity at this synapse is not clear at this point. In contrast to mGlu3, mGlu2 does not play a role in the regulation of synaptic plasticity at SC-CA1 [110, 154, 155]. Instead, mGlu2 activation leads to LTD at MF-CA3 pyramidal cell synapses, which, from a network perspective, could reduce the drive into and out of CA3. Theoretically, as this form of LTD occurs via a presynaptic mechanism, this could indicate that mGlu2 activation may trigger an LTD and result in decreased output from CA3 in response to increased drive from DG granule cells.
Finally, there is clear involvement of mGlu7 in mediating a bidirectional switch between LTP and LTD at MF synapses. Activation of mGlu7 in the CA3 interneurons during MF-HFS leads to LTD in the interneurons, [153, 157, 159, 162], which in turn may facilitate the induction and/or maintenance of HFS mediated LTP observed in the CA3 pyramidal cells. This target-specific HFS mediated plasticity seems to lead to a net disinhibition of CA3 pyramidal cells, resulting in increased drive out of CA3. Thus, there is a clear link between the involvement of mGlu receptors in the mechanisms required to induce or maintain several forms of plasticity throughout the hippocampus. However, several aspects still require extensive future studies to further tease apart the intricate regulation of SC-CA1 and MF plasticity by the different mGlu receptor subtypes. One interesting aspect that requires further attention is the developmental regulation of hippocampal plasticity by mGlu receptors. There is already evidence showing that a developmental switch exists in the regulation of mGlu dependent LTD in the SC-CA1 synapses [201]. In addition, it is well-known that a developmental switch occurs at SC-CA1 synapses between the expression of mGlu8 and mGlu7, with mGlu8 being the predominant group III mGlu receptor expressed in neonatal animals and mGlu7 expression occurring in adulthood [32]. Thus it will be interesting to investigate whether such developmental nuances exist in other mGlu receptor dependent plasticity mechanisms in SC-CA1 and MF synapses.
Additionally, as there have been many reports linking plasticity deficits to cognitive impairments in various psychiatric and neurodegenerative, and neurodevelopmental diseases, there has been progress to develop novel, selective ligands, including allosteric modulators, as new therapeutic treatment options for these disorders. Several mGlu receptors have a long history of involvement in autism and schizophrenia, whereas investigation into others is just beginning. The use of new and novel ligands targeting members of the mGlu receptor family is anticipated to provide insight into the function of these receptors in synaptic plasticity and diseases.
ACKNOWLEDGEMENTS
All authors have contributed to the writing and editing of the manuscript.
CONFLICT OF INTEREST
CMN and PJC receive research support that includes salary support from Bristol Myers Squibb and Astra Zeneca and are inventors on multiple composition of matter patents protecting allosteric modulators of GPCRs. This work was supported by U01 MH87965, R37 NS31373, U19 MH97056, R01 MH73676, and R01 MH62646 to PJC, R21 MH102548, a treatment grant from Autism Speaks, and a Basic Research Grant from Rettsyndrome.org to CMN, and R01 NS65867 to ZX. RKS was supported by a Weatherstone Predoctoral Fellowship from Autism Speaks and the Howard Hughes Medical Institute Certificate Program in Molecular Medicine (HHMI CPMM) through Vanderbilt University. AGW was supported by a postdoctoral fellowship through the PhRMA Foundation.
REFERENCES
- 1.Andersen P. The hippocampus book. Oxford, New York: Oxford University Press; 2007. [Google Scholar]
- 2.Schultz C., Engelhardt M. Anatomy of the hippocampal formation. Front Neurol. Neurosci. 2014;34:6–17. doi: 10.1159/000360925. [DOI] [PubMed] [Google Scholar]
- 3.Cajal R.y. The Croonian Lecture: The Minute Structure of the Nervous Centres. Br. Med. J. 1732;1894(1):543. [PMC free article] [PubMed] [Google Scholar]
- 4.Acsady L., Kamondi A., Sik A., Freund T., Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 1998;18(9):3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henze D.A., Urban N.N., Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98(3):407–427. doi: 10.1016/S0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 6.Nicoll R.A., Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 7.Witter M.P. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog. Brain Res. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- 8.Poschel B., Stanton P.K. Comparison of cellular mechanisms of long-term depression of synaptic strength at perforant path-granule cell and Schaffer collateral-CA1 synapses. Prog. Brain Res. 2007;163:473–500. doi: 10.1016/S0079-6123(07)63026-X. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove K.E., Galvan E.J., Barrionuevo G., Meriney S.D. mGluRs modulate strength and timing of excitatory transmission in hippocampal area CA3. Mol. Neurobiol. 2011;44(1):93–101. doi: 10.1007/s12035-011-8187-z. [DOI] [PubMed] [Google Scholar]
- 10.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyklicky V., Korinek M., Smejkalova T., Balik A., Krausova B., Kaniakova M., Lichnerova K., Cerny J., Krusek J., Dittert I., Horak M., Vyklicky L. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014;63(Suppl. 1):S191–S203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 12.Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992;267(19):13361–13368. [PubMed] [Google Scholar]
- 13.Schoepp D.D., Goldsworthy J., Johnson B.G., Salhoff C.R., Baker S.R. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J. Neurochem. 1994;63(2):769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- 14.Schoepp D.D., Salhoff C.R., Wright R.A., Johnson B.G., Burnett J.P., Mayne N.G., Belagaje R., Wu S., Monn J.A. The novel metabotropic glutamate receptor agonist 2R,4R-APDC potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5-dihydroxyphenylglycine: evidence for a synergistic interaction between group 1 and group 2 receptors. Neuropharmacology. 1996;35(12):1661–1672. doi: 10.1016/S0028-3908(96)00121-9. [DOI] [PubMed] [Google Scholar]
- 15.Sladeczek F., Pin J.P., Recasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- 17.Toms N.J., Jane D.E., Tse H.W., Roberts P.J. Characterization of metabotropic glutamate receptor-stimulated phosphoinositide hydrolysis in rat cultured cerebellar granule cells. Br. J. Pharmacol. 1995;116(7):2824–2827. doi: 10.1111/j.1476-5381.1995.tb15932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraguti F., Conquet F., Corti C., Grandes P., Kuhn R., Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J. Comp. Neurol. 1998;400(3):391–407. doi: 10.1002/(SICI)1096-9861(19981026)400:3%3C391:AID-CNE8%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Gereau R.W., Conn P.J. Roles of specific metabotropic glutamate receptor subtypes in regulation of hippocampal CA1 pyramidal cell excitability. J. Neurophysiol. 1995;74(1):122–129. doi: 10.1152/jn.1995.74.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Lujan R., Nusser Z., Roberts J.D., Shigemoto R., Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996;8(7):1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 21.Genazzani A.A., Casabona G., L'Episcopo M.R., Condorelli D.F., Dell'Albani P., Shinozaki H., Nicoletti F. Characterization of metabotropic glutamate receptors negatively linked to adenylyl cyclase in brain slices. Brain Res. 1993;622(1-2):132–138. doi: 10.1016/0006-8993(93)90811-Z. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y., Tanabe Y., Aramori I., Masu M., Shimamoto K., Ohfune Y., Nakanishi S. Agonist analysis of 2-(carboxycyclopropyl) glycine isomers for cloned metabotropic glutamate receptor subtypes expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1992;107(2):539–543. doi: 10.1111/j.1476-5381.1992.tb12780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S.R., Lovinger D.M., McCool B.A., Lewis D.L. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14(5):1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 24.McCool B.A., Pin J.P., Brust P.F., Harpold M.M., Lovinger D.M. Functional coupling of rat group II metabotropic glutamate receptors to an omega-conotoxin GVIA-sensitive calcium channel in human embryonic kidney 293 cells. Mol. Pharmacol. 1996;50(4):912–922. [PubMed] [Google Scholar]
- 25.Prezeau L., Manzoni O., Homburger V., Sladeczek F., Curry K., Bockaert J. Characterization of a metabotropic glutamate receptor: direct negative coupling to adenylyl cyclase and involvement of a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. USA. 1992;89(17):8040–8044. doi: 10.1073/pnas.89.17.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohishi H., Shigemoto R., Nakanishi S., Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1993;335(2):252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 28.Winder D.G., Conn P.J. Roles of metabotropic glutamate receptors in glial function and glial-neuronal communication. J. Neurosci. Res. 1996;46(2):131–137. doi: 10.1002/(SICI)1097-4547(19961015)46:2%3C131:AID-JNR1%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Winder D.G., Ritch P.S., Gereau R.W.t., Conn P.J. Novel glial-neuronal signalling by coactivation of metabotropic glutamate and beta-adrenergic receptors in rat hippocampus. J.Neurosci. Res. 1996;46(2):131–137. doi: 10.1113/jphysiol.1996.sp021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigemoto R., Kinoshita A., Wada E., Nomura S., Ohishi H., Takada M., Flor P.J., Neki A., Abe T., Nakanishi S., Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17(19):7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima Y., Iwakabe H., Akazawa C., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993;268(16):11868–11873. [PubMed] [Google Scholar]
- 32.Ayala J.E., Niswender C.M., Luo Q., Banko J.L., Conn P.J. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacology. 2008;54(5):804–814. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferraguti F., Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326(2):483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 34.Jalan-Sakrikar N., Field J.R., Klar R., Mattmann M.E., Gregory K.J., Zamorano R., Engers D.W., Bollinger S.R., Weaver C.D., Days E.L., Lewis L.M., Utley T.J., Hurtado M., Rigault D., Acher F., Walker A.G., Melancon B.J., Wood M.R., Lindsley C.W., Conn P.J., Xiang Z., Hopkins C.R., Niswender C.M. Identification of Positive Allosteric Modulators VU0155094 (ML397) and VU0422288 (ML396) Reveals New Insights into the Biology of Metabotropic Glutamate Receptor 7. ACS Chem. Neurosci. 2014;5(12):1221–1237. doi: 10.1021/cn500153z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corti C., Restituito S., Rimland J.M., Brabet I., Corsi M., Pin J.P., Ferraguti F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur. J. Neurosci. 1998;10(12):3629–3641. doi: 10.1046/j.1460-9568.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 36.Bradley S.R., Rees H.D., Yi H., Levey A.I., Conn P.J. Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J. Neurochem. 1998;71(2):636–645. doi: 10.1046/j.1471-4159.1998.71020636.x. [DOI] [PubMed] [Google Scholar]
- 37.Bradley S.R., Levey A.I., Hersch S.M., Conn P.J. Immuno- cytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J. Neurosci. 1996;16(6):2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto N., Hori S., Akazawa C., Hayashi Y., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J. Biol. Chem. 1994;269(2):1231–1236. [PubMed] [Google Scholar]
- 39.Mitri C., Parmentier M.L., Pin J.P., Bockaert J., Grau Y. Divergent evolution in metabotropic glutamate receptors. A new receptor activated by an endogenous ligand different from glutamate in insects. J. Biol. Chem. 2004;279(10):9313–9320. doi: 10.1074/jbc.M310878200. [DOI] [PubMed] [Google Scholar]
- 40.Conn P.J., Lindsley C.W., Meiler J., Niswender C.M. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat. Rev. Drug Discov. 2014;13(9):692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin S., Niswender C.M. Progress toward advanced understanding of metabotropic glutamate receptors: structure, signaling and therapeutic indications. Cell. Signal. 2014;26(10):2284–2297. doi: 10.1016/j.cellsig.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartzkroin P.A., Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975;89(1):107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- 44.Dudek S.M., Bear M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. USA. 1992;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collingridge G.L., Kehl S.J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunwiddie T., Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J. Physiol. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malinow R., Miller J.P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- 48.Morris R.G., Anderson E., Lynch G.S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 49.Larson J., Wong D., Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 50.Larson J., Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232(4753):985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- 51.Larson J., Lynch G. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441(1-2):111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- 52.Aiba A., Chen C., Herrup K., Rosenmund C., Stevens C.F., Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79(2):365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 53.Conquet F., Bashir Z.I., Davies C.H., Daniel H., Ferraguti F., Bordi F., Franz-Bacon K., Reggiani A., Matarese V., Conde F., et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372(6503):237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 54.Neyman S., Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur. J. Neurosci. 2008;27(6):1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan J.Y., Skeberdis V.A., Jover T., Zheng X., Bennett M.V., Zukin R.S. Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J. Neurosci. 2001;21(16):6058–6068. doi: 10.1523/JNEUROSCI.21-16-06058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skeberdis V.A., Lan J., Opitz T., Zheng X., Bennett M.V., Zukin R.S. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40(7):856–865. doi: 10.1016/S0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.J., Kim Y.S., Yuan J.P., Petralia R.S., Worley P.F., Linden D.J. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426(6964):285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- 58.Mannaioni G., Marino M.J., Valenti O., Traynelis S.F., Conn P.J. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001;21(16):5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balschun D., Manahan-Vaughan D., Wagner T., Behnisch T., Reymann K.G., Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learn. Mem. 1999;6(2):138–152. [PMC free article] [PubMed] [Google Scholar]
- 60.Manahan-Vaughan D., Braunewell K.H. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb. Cortex. 2005;15(11):1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- 61.Jia Z., Lu Y., Henderson J., Taverna F., Romano C., Abramow-Newerly W., Wojtowicz J.M., Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn. Mem. 1998;5(4-5):331–343. [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y.M., Jia Z., Janus C., Henderson J.T., Gerlai R., Wojtowicz J.M., Roder J.C. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 1997;17(13):5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francesconi W., Cammalleri M., Sanna P.P. The metabotropic glutamate receptor 5 is necessary for late-phase long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1022(1-2):12–18. doi: 10.1016/j.brainres.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 64.Shalin S.C., Hernandez C.M., Dougherty M.K., Morrison D.K., Sweatt J.D. Kinase suppressor of Ras1 compartmentalizes hippocampal signal transduction and subserves synaptic plasticity and memory formation. Neuron. 2006;50(5):765–779. doi: 10.1016/j.neuron.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Cohen A.S., Abraham W.C. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J. Neurophysiol. 1996;76(2):953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- 66.Raymond C.R., Thompson V.L., Tate W.P., Abraham W.C. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J. Neurosci. 2000;20(3):969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hermans E., Challiss R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359(Pt 3):465–484. doi: 10.1042/bj3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chevaleyre V., Castillo P.E. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38(3):461–472. doi: 10.1016/S0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 69.Xu J., Antion M.D., Nomura T., Kraniotis S., Zhu Y., Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J. Neurosci. 2014;34(50):16762–16773. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Younts T.J., Chevaleyre V., Castillo P.E. CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J. Neurosci. 2013;33(34):13743–13757. doi: 10.1523/JNEUROSCI.0817-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deadwyler S.A., Dunwiddie T., Lynch G. A critical level of protein synthesis is required for long-term potentiation. Synapse. 1987;1(1):90–95. doi: 10.1002/syn.890010112. [DOI] [PubMed] [Google Scholar]
- 72.Frey U., Krug M., Reymann K.G., Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452(1-2):57–65. doi: 10.1016/0006-8993(88)90008-X. [DOI] [PubMed] [Google Scholar]
- 73.Stanton P.K., Sarvey J.M. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J. Neurosci. 1984;4(12):3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ayala J.E., Chen Y., Banko J.L., Sheffler D.J., Williams R., Telk A.N., Watson N.L., Xiang Z., Zhang Y., Jones P.J., Lindsley C.W., Olive M.F., Conn P.J. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34(9):2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noetzel M.J., Gregory K.J., Vinson P.N., Manka J.T., Stauffer S.R., Lindsley C.W., Niswender C.M., Xiang Z., Conn P.J. A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol. Pharmacol. 2013;83(4):835–847. doi: 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohshima T., Ward J.M., Huh C.G., Longenecker G. Veeranna; Pant, H.C.; Brady, R.O.; Martin, L.J.; Kulkarni, A.B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA. 1996;93(20):11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohishi H., Shigemoto R., Nakanishi S., Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 78.Breakwell N.A., Rowan M.J., Anwyl R. (+)-MCPG blocks induction of LTP in CA1 of rat hippocampus via agonist action at an mGluR group II receptor. J. Neurophysiol. 1998;79(3):1270–1276. doi: 10.1152/jn.1998.79.3.1270. [DOI] [PubMed] [Google Scholar]
- 79.Gereau R.W., Conn P.J. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J. Neurosci. 1995;15(10):6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gereau R.W., Conn P.J. Potentiation of cAMP responses by metabotropic glutamate receptors depresses excitatory synaptic transmission by a kinase-independent mechanism. Neuron. 1994;12(5):1121–1129. doi: 10.1016/0896-6273(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 81.Gereau R.W., Winder D.G., Conn P.J. Pharmacological differentiation of the effects of co-activation of beta-adrenergic and metabotropic glutamate receptors in rat hippocampus. Neurosci. Lett. 1995;186(2-3):119–122. doi: 10.1016/0304-3940(95)11300-L. [DOI] [PubMed] [Google Scholar]
- 82.Ota Y., Zanetti A.T., Hallock R.M. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural.Plast. 2013;2013:185463. doi: 10.1155/2013/185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Losonczy A., Somogyi P., Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J. Neurophysiol. 2003;89(4):1910–1919. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- 84.Kristensen P., Suzdak P.D., Thomsen C. Expression pattern and pharmacology of the rat type IV metabotropic glutamate receptor. Neurosci. Lett. 1993;155(2):159–162. doi: 10.1016/0304-3940(93)90697-J. [DOI] [PubMed] [Google Scholar]
- 85.Kalinichev M., Rouillier M., Girard F., Royer-Urios I., Bournique B., Finn T., Charvin D., Campo B., Le Poul E., Mutel V., Poli S., Neale S.A., Salt T.E., Lutjens R. ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. J. Pharmacol. Exp. Ther. 2013;344(3):624–636. doi: 10.1124/jpet.112.200915. [DOI] [PubMed] [Google Scholar]
- 86.Niswender C.M., Johnson K.A., Miller N.R., Ayala J.E., Luo Q., Williams R., Saleh S., Orton D., Weaver C.D., Conn P.J. Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol. Pharmacol. 2010;77(3):459–468. doi: 10.1124/mol.109.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosemond E., Wang M., Yao Y., Storjohann L., Stormann T., Johnson E.C., Hampson D.R. Molecular basis for the differential agonist affinities of group III metabotropic glutamate receptors. Mol. Pharmacol. 2004;66(4):834–842. doi: 10.1124/mol.104.002956. [DOI] [PubMed] [Google Scholar]
- 88.Baskys A., Malenka R.C. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J. Physiol. 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bushell T.J., Sansig G., Collett V.J., van der Putten H., Collingridge G.L. Altered short-term synaptic plasticity in mice lacking the metabotropic glutamate receptor mGlu7. ScientificWorldJournal. 2002;2:730–737. doi: 10.1100/tsw.2002.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shigemoto R., Kulik A., Roberts J.D., Ohishi H., Nusser Z., Kaneko T., Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381(6582):523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 91.Callaerts-Vegh Z., Beckers T., Ball S.M., Baeyens F., Callaerts P.F., Cryan J.F., Molnar E., D'Hooge R. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J. Neurosci. 2006;26(24):6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goddyn H., Callaerts-Vegh Z., Stroobants S., Dirikx T., Vansteenwegen D., Hermans D., van der Putten H., D'Hooge R. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol. Learn. Mem. 2008;90(1):103–111. doi: 10.1016/j.ejphar.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 93.Hikichi H., Murai T., Okuda S., Maehara S., Satow A., Ise S., Nishino M., Suzuki G., Takehana H., Hata M., Ohta H. Effects of a novel metabotropic glutamate receptor 7 negative allosteric modulator, 6-(4-methoxyphenyl)-5-methyl-3-pyridin-4-ylisoxazonolo[4,5-c]pyridin-4(5H)-one (MMPIP), on the central nervous system in rodents. Eur. J. Pharmacol. 2010;639(1-3):106–114. doi: 10.1016/j.ejphar.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 94.Holscher C., Schmid S., Pilz P.K., Sansig G., van der Putten H., Plappert C.F. Lack of the metabotropic glutamate receptor subtype 7 selectively impairs short-term working memory but not long-term memory. Behav. Brain Res. 2004;154(2):473–481. doi: 10.1016/j.bbr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Holscher C., Schmid S., Pilz P.K., Sansig G., van der Putten H., Plappert C.F. Lack of the metabotropic glutamate receptor subtype 7 selectively modulates Theta rhythm and working memory. Learn. Mem. 2005;12(5):450–455. doi: 10.1101/lm.98305. [DOI] [PubMed] [Google Scholar]
- 96.Fitzjohn S.M., Kingston A.E., Lodge D., Collingridge G.L. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology. 1999;38(10):1577–1583. doi: 10.1016/S0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- 97.Huber K.M., Kayser M.S., Bear M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 98.Huber K.M., Roder J.C., Bear M.F. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J. Neurophysiol. 2001;86(1):321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 99.Palmer M.J., Irving A.J., Seabrook G.R., Jane D.E., Collingridge G.L. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36(11-12):1517–1532. doi: 10.1016/S0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 100.Snyder E.M., Philpot B.D., Huber K.M., Dong X., Fallon J.R., Bear M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4(11):1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 101.Fitzjohn S.M., Palmer M.J., May J.E., Neeson A., Morris S.A., Collingridge G.L. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J. Physiol. 2001;537(Pt 2):421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kemp N., McQueen J., Faulkes S., Bashir Z.I. Different forms of LTD in the CA1 region of the hippocampus: role of age and stimulus protocol. Eur. J. Neurosci. 2000;12(1):360–366. doi: 10.1046/j.1460-9568.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 103.Kemp N., Bashir Z.I. NMDA receptor-dependent and -independent long-term depression in the CA1 region of the adult rat hippocampus in vitro. Neuropharmacology. 1997;36(3):397–399. doi: 10.1016/S0028-3908(96)90015-5. [DOI] [PubMed] [Google Scholar]
- 104.Kemp N., Bashir Z.I. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38(4):495–504. doi: 10.1016/S0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 105.Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J. Neurosci. 1997;17(9):3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santschi L.A., Zhang X.L., Stanton P.K. Activation of receptors negatively coupled to adenylate cyclase is required for induction of long-term synaptic depression at Schaffer collateral-CA1 synapses. J. Neurobiol. 2006;66(3):205–219. doi: 10.1002/neu.20213. [DOI] [PubMed] [Google Scholar]
- 107.Uyama Y., Ishida M., Shinozaki H. DCG-IV, a potent metabotropic glutamate receptor agonist, as an NMDA receptor agonist in the rat cortical slice. Brain Res. 1997;752(1-2):327–330. doi: 10.1016/S0006-8993(97)00079-6. [DOI] [PubMed] [Google Scholar]
- 108.Wilsch V.W., Pidoplichko V.I., Opitz T., Shinozaki H., Reymann K.G. Metabotropic glutamate receptor agonist DCG-IV as NMDA receptor agonist in immature rat hippocampal neurons. Eur. J. Pharmacol. 1994;262(3):287–291. doi: 10.1016/0014-2999(94)90743-9. [DOI] [PubMed] [Google Scholar]
- 109.Johnson K.A., Niswender C.M., Conn P.J., Xiang Z. Activation of group II metabotropic glutamate receptors induces long-term depression of excitatory synaptic transmission in the substantia nigra pars reticulata. Neurosci. Lett. 2011;504(2):102–106. doi: 10.1016/j.neulet.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lodge D., Tidball P., Mercier M.S., Lucas S.J., Hanna L., Ceolin L., Kritikos M., Fitzjohn S.M., Sherwood J.L., Bannister N., Volianskis A., Jane D.E., Bortolotto Z.A., Collingridge G.L. Antagonists reversibly reverse chemical LTD induced by group I, group II and group III metabotropic glutamate receptors. Neuropharmacology. 2013;74:135–146. doi: 10.1016/j.neuropharm.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 111.Otani S., Daniel H., Takita M., Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J. Neurosci. 2002;22(9):3434–3444. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker A.G., Wenthur C.J., Xiang Z., Rook J.M., Emmitte K.A., Niswender C.M., Lindsley C.W., Conn P.J. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altinbilek B., Manahan-Vaughan D. Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory. Eur. J. Neurosci. 2007;26(5):1166–1172. doi: 10.1111/j.1460-9568.2007.05742.x. [DOI] [PubMed] [Google Scholar]
- 114.Naie K., Gundimi S., Siegmund H., Heinemann U., Manahan-Vaughan D. Group III metabotropic glutamate receptor-mediated, chemically induced long-term depression differentially affects cell viability in the hippocampus. Eur. J. Pharmacol. 2006;535(1-3):104–113. doi: 10.1016/j.ejphar.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 115.Claiborne B.J., Xiang Z., Brown T.H. Hippocampal circuitry complicates analysis of long-term potentiation in mossy fiber synapses. Hippocampus. 1993;3(2):115–121. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- 116.Toth K., Suares G., Lawrence J.J., Philips-Tansey E., McBain C.J. Differential mechanisms of transmission at three types of mossy fiber synapse. J. Neurosci. 2000;20(22):8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salin P.A., Scanziani M., Malenka R.C., Nicoll R.A. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc. Natl. Acad. Sci. USA. 1996;93(23):13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung M.W., McNaughton B.L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 119.Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J. Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lawrence J.J., Grinspan Z.M., McBain C.J. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J. Physiol. 2004;554(Pt 1):175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zalutsky R.A., Nicoll R.A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248(4963):1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 122.Contractor A., Swanson G., Heinemann S.F. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29(1):209–216. doi: 10.1016/S0896-6273(01)00191-X. [DOI] [PubMed] [Google Scholar]
- 123.Fedele D.E., Gouder N., Guttinger M., Gabernet L., Scheurer L., Rulicke T., Crestani F., Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128(Pt 10):2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 124.Moore K.A., Nicoll R.A., Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA. 2003;100(24):14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmitz D., Mellor J., Nicoll R.A. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291(5510):1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 126.Kamiya H., Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J. Physiol. 1999;518(Pt 2):497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yokoi M., Kobayashi K., Manabe T., Takahashi T., Sakaguchi I., Katsuura G., Shigemoto R., Ohishi H., Nomura S., Nakamura K., Nakao K., Katsuki M., Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273(5275):645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 128.Scanziani M., Salin P.A., Vogt K.E., Malenka R.C., Nicoll R.A. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385(6617):630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 129.Vogt K.E., Nicoll R.A. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc. Natl. Acad. Sci. USA. 1999;96(3):1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lanthorn T.H., Ganong A.H., Cotman C.W. 2-Amino-4-phosphonobutyrate selectively blocks mossy fiber-CA3 responses in guinea pig but not rat hippocampus. Brain Res. 1984;290(1):174–178. doi: 10.1016/0006-8993(84)90750-9. [DOI] [PubMed] [Google Scholar]
- 131.Manzoni O.J., Castillo P.E., Nicoll R.A. Pharmacology of metabotropic glutamate receptors at the mossy fiber synapses of the guinea pig hippocampus. Neuropharmacology. 1995;34(8):965–971. doi: 10.1016/0028-3908(95)00060-J. [DOI] [PubMed] [Google Scholar]
- 132.Harris E.W., Cotman C.W. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci. Lett. 1986;70(1):132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe M., Fukaya M., Sakimura K., Manabe T., Mishina M., Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur. J. Neurosci. 1998;10(2):478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- 134.Weisskopf M.G., Nicoll R.A. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376(6537):256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- 135.Staubli U., Larson J., Lynch G. Mossy fiber potentiation and long-term potentiation involve different expression mechanisms. 1990. [DOI] [PubMed]
- 136.Xiang Z., Greenwood A.C., Kairiss E.W., Brown T.H. Quantal mechanism of long-term potentiation in hippocampal mossy-fiber synapses. J. Neurophysiol. 1994;71(6):2552–2556. doi: 10.1152/jn.1994.71.6.2552. [DOI] [PubMed] [Google Scholar]
- 137.Lopez-Garcia J.C., Arancio O., Kandel E.R., Baranes D. A presynaptic locus for long-term potentiation of elementary synaptic transmission at mossy fiber synapses in culture. Proc. Natl. Acad. Sci. USA. 1996;93(10):4712–4717. doi: 10.1073/pnas.93.10.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tong G., Malenka R.C., Nicoll R.A. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16(6):1147–1157. doi: 10.1016/S0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- 139.Ito I., Sugiyama H. Roles of glutamate receptors in long-term potentiation at hippocampal mossy fiber synapses. Neuroreport. 1991;2(6):333–336. doi: 10.1097/00001756-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 140.Bashir Z.I., Bortolotto Z.A., Davies C.H., Berretta N., Irving A.J., Seal A.J., Henley J.M., Jane D.E., Watkins J.C., Collingridge G.L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 141.Yeckel M.F., Kapur A., Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat. Neurosci. 1999;2(7):625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nistico R., Dargan S.L., Amici M., Collingridge G.L., Bortolotto Z.A. Synergistic interactions between kainate and mGlu receptors regulate bouton Ca signalling and mossy fibre LTP. Sci. Rep. 2011;1:103. doi: 10.1038/srep00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsia A.Y., Salin P.A., Castillo P.E., Aiba A., Abeliovich A., Tonegawa S., Nicoll R.A. Evidence against a role for metabotropic glutamate receptors in mossy fiber LTP: the use of mutant mice and pharmacological antagonists. Neuropharmacology. 1995;34(11):1567–1572. doi: 10.1016/0028-3908(95)00115-M. [DOI] [PubMed] [Google Scholar]
- 144.Manzoni O.J., Weisskopf M.G., Nicoll R.A. MCPG antagonizes metabotropic glutamate receptors but not long-term potentiation in the hippocampus. Eur. J. Neurosci. 1994;6(6):1050–1054. doi: 10.1111/j.1460-9568.1994.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 145.Mellor J., Nicoll R.A. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat. Neurosci. 2001;4(2):125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 146.Katsuki H., Kaneko S., Tajima A., Satoh M. Separate mechanisms of long-term potentiation in two input systems to CA3 pyramidal neurons of rat hippocampal slices as revealed by the whole-cell patch-clamp technique. Neurosci. Res. 1991;12(3):393–402. doi: 10.1016/0168-0102(91)90070-F. [DOI] [PubMed] [Google Scholar]
- 147.Langdon R.B., Johnson J.W., Barrionuevo G. Posttetanic potentiation and presynaptically induced long-term potentiation at the mossy fiber synapse in rat hippocampus. J. Neurobiol. 1995;26(3):370–385. doi: 10.1002/neu.480260309. [DOI] [PubMed] [Google Scholar]
- 148.Sokolov M.V., Rossokhin A.V., M.K. A, Gasparini S., Berretta N., Cherubini E., Voronin L.L. Associative mossy fibre LTP induced by pairing presynaptic stimulation with postsynaptic hyperpolarization of CA3 neurons in rat hippocampal slice. Eur. J. Neurosci. 2003;17(7):1425–1437. doi: 10.1046/j.1460-9568.2003.02563.x. [DOI] [PubMed] [Google Scholar]
- 149.Kwon H.B., Castillo P.E. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57(1):108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rebola N., Lujan R., Cunha R.A., Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57(1):121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 151.Galvan E.J., Calixto E., Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J. Neurosci. 2008;28(52):14042–14055. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Galvan E.J., Cosgrove K.E., Mauna J.C., Card J.P., Thiels E., Meriney S.D., Barrionuevo G. Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons. J. Neurosci. 2010;30(8):2844–2855. doi: 10.1523/JNEUROSCI.5269-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pelkey K.A., McBain C.J. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J. Physiol. 2008;586(6):1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kobayashi K., Manabe T., Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273(5275):648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 155.Tzounopoulos T., Janz R., Sudhof T.C., Nicoll R.A., Malenka R.C. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21(4):837–845. doi: 10.1016/S0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 156.Lei S., McBain C.J. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33(6):921–933. doi: 10.1016/S0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- 157.Maccaferri G., Toth K., McBain C.J. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279(5355):1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- 158.Pelkey K.A., Lavezzari G., Racca C., Roche K.W., McBain C.J. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46(1):89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 159.Pelkey K.A., Yuan X., Lavezzari G., Roche K.W., McBain C.J. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52(1):108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 160.Bischofberger J., Jonas P. TwoB or not twoB: differential transmission at glutamatergic mossy fiber-interneuron synapses in the hippocampus. Trends Neurosci. 2002;25(12):600–603. doi: 10.1016/S0166-2236(02)02259-2. [DOI] [PubMed] [Google Scholar]
- 161.Lei S., McBain C.J. Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J. Neurosci. 2004;24(9):2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pelkey K.A., Topolnik L., Lacaille J.C., McBain C.J. Compartmentalized Ca(2+) channel regulation at divergent mossyfiber release sites underlies target cell-dependent plasticity. Neuron. 2006;52(3):497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 163.Asaka Y., Jugloff D.G., Zhang L., Eubanks J.H., Fitzsimonds R.M. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 2006;21(1):217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 164.Auerbach B.D., Osterweil E.K., Bear M.F. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fischer A., Sananbenesi F., Pang P.T., Lu B., Tsai L.H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48(5):825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 166.Huber K.M., Gallagher S.M., Warren S.T., Bear M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Manahan-Vaughan D., Wildforster V., Thomsen C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1). Eur. J. Neurosci. 2008;28(7):1342–1350. doi: 10.1111/j.1460-9568.2008.06433.x. [DOI] [PubMed] [Google Scholar]
- 168.Moretti P., Levenson J.M., Battaglia F., Atkinson R., Teague R., Antalffy B., Armstrong D., Arancio O., Sweatt J.D., Zoghbi H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 2006;26(1):319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Na E.S., Nelson E.D., Adachi M., Autry A.E., Mahgoub M.A., Kavalali E.T., Monteggia L.M. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J. Neurosci. 2012;32(9):3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiu S., Korwek K.M., Pratt-Davis A.R., Peters M., Bergman M.Y., Weeber E.J. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol. Learn. Mem. 2006;85(3):228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 171.Tian D., Stoppel L.J., Heynen A.J., Lindemann L., Jaeschke G., Mills A.A., Bear M.F. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 2015 doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weng S.M., McLeod F., Bailey M.E., Cobb S.R. Synaptic plasticity deficits in an experimental model of rett syndrome: long-term potentiation saturation and its pharmacological reversal. 2011. [DOI] [PubMed]
- 173.Bear M.F. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav. 2005;4(6):393–398. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- 174.Bear M.F., Huber K.M., Warren S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 175.Golzio C., Willer J., Talkowski M.E., Oh E.C., Taniguchi Y., Jacquemont S., Reymond A., Sun M., Sawa A., Gusella J.F., Kamiya A., Beckmann J.S., Katsanis N. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485(7398):363–367. doi: 10.1038/nature11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27(3):322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 177.Horev G., Ellegood J., Lerch J.P., Son Y.E., Muthuswamy L., Vogel H., Krieger A.M., Buja A., Henkelman R.M., Wigler M., Mills A.A. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. USA. 2011;108(41):17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.O'Donnell W.T., Warren S.T. A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 179.Portmann T., Yang M., Mao R., Panagiotakos G., Ellegood J., Dolen G., Bader P.L., Grueter B.A., Goold C., Fisher E., Clifford K., Rengarajan P., Kalikhman D., Loureiro D., Saw N.L., Zhengqui Z., Miller M.A., Lerch J.P., Henkelman R.M., Shamloo M., Malenka R.C., Crawley J.N., Dolmetsch R.E. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Reports. 2014;7(4):1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Michalon A., Sidorov M., Ballard T.M., Ozmen L., Spooren W., Wettstein J.G., Jaeschke G., Bear M.F., Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thomas A.M., Bui N., Perkins J.R., Yuva-Paylor L.A., Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl.) 2012;219(1):47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 182.Feliciano D.M., Lin T.V., Hartman N.W., Bartley C.M., Kubera C., Hsieh L., Lafourcade C., O'Keefe R.A., Bordey A. A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits. Int. J. Dev. Neurosci. 2013;31(7):667–678. doi: 10.1016/j.ijdevneu.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luikenhuis S., Giacometti E., Beard C.F., Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci. USA. 2004;101(16):6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramocki M.B., Tavyev Y.J., Peters S.U. The MECP2 duplication syndrome. Am. J. Med. Genet. A. 2010;152A(5):1079–1088. doi: 10.1002/ajmg.a.33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52(1):3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 186.Urban N.N., Henze D.A., Barrionuevo G. Revisiting the role of the hippocampal mossy fiber synapse. Hippocampus. 2001;11(4):408–417. doi: 10.1002/hipo.1055. [DOI] [PubMed] [Google Scholar]
- 187.Hagena H., Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex. 2011;21(11):2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Daumas S., Ceccom J., Halley H., Frances B., Lassalle J.M. Activation of metabotropic glutamate receptor type 2/3 supports the involvement of the hippocampal mossy fiber pathway on contextual fear memory consolidation. Learn. Mem. 2009;16(8):504–507. doi: 10.1101/lm.1418309. [DOI] [PubMed] [Google Scholar]
- 189.Gibson H.E., Reim K., Brose N., Morton A.J., Jones S. A similar impairment in CA3 mossy fibre LTP in the R6/2 mouse model of Huntington's disease and in the complexin II knockout mouse. Eur. J. Neurosci. 2005;22(7):1701–1712. doi: 10.1111/j.1460-9568.2005.04349.x. [DOI] [PubMed] [Google Scholar]
- 190.Witton J., Brown J.T., Jones M.W., Randall A.D. Altered synaptic plasticity in the mossy fibre pathway of transgenic mice expressing mutant amyloid precursor protein. Mol. Brain. 2010;3:32. doi: 10.1186/1756-6606-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cho H.P., Engers D.W., Venable D.F., Niswender C.M., Lindsley C.W., Conn P.J., Emmitte K.A., Rodriguez A.L. A novel class of succinimide-derived negative allosteric modulators of metabotropic glutamate receptor subtype 1 provides insight into a disconnect in activity between the rat and human receptors. ACS Chem. Neurosci. 2014;5(7):597–610. doi: 10.1021/cn5000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cho H.P., Garcia-Barrantes P.M., Brogan J.T., Hopkins C.R., Niswender C.M., Rodriguez A.L., Venable D.F., Morrison R.D., Bubser M., Daniels J.S., Jones C.K., Conn P.J., Lindsley C.W. Chemical modulation of mutant mGlu1 receptors derived from deleterious GRM1 mutations found in schizophrenics. ACS Chem. Biol. 2014;9(10):2334–2346. doi: 10.1021/cb500560h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lovell K.M., Felts A.S., Rodriguez A.L., Venable D.F., Cho H.P., Morrison R.D., Byers F.W., Daniels J.S., Niswender C.M., Conn P.J., Lindsley C.W., Emmitte K.A. N-Acyl-N'-arylpiperazines as negative allosteric modulators of mGlu1: identification of VU0469650, a potent and selective tool compound with CNS exposure in rats. Bioorg. Med. Chem. Lett. 2013;23(13):3713–3718. doi: 10.1016/j.bmcl.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wenthur C.J., Morrison R., Felts A.S., Smith K.A., Engers J.L., Byers F.W., Daniels J.S., Emmitte K.A., Conn P.J., Lindsley C.W. Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl) ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM). J. Med. Chem. 2013;56(12):5208–5212. doi: 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen Y., Nong Y., Goudet C., Hemstapat K., de Paulis T., Pin J.P., Conn P.J. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol. Pharmacol. 2007;71(5):1389–1398. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- 196.Rodriguez A.L., Tarr J.C., Zhou Y., Williams R., Gregory K.J., Bridges T.M., Daniels J.S., Niswender C.M., Conn P.J., Lindsley C.W., Stauffer S.R. Identification of a glycine sulfonamide based non-MPEP site positive allosteric potentiator (PAM) of mGlu5. 2010 [PubMed] [Google Scholar]
- 197.Turlington M., Noetzel M.J., Bridges T.M., Vinson P.N., Steckler T., Lavreysen H., Mackie C., Bartolome-Nebreda J.M., Conde-Ceide S., Tong H.M., Macdonald G.J., Daniels J.S., Jones C.K., Niswender C.M., Conn P.J., Lindsley C.W., Stauffer S.R. Discovery and SAR of a novel series of metabotropic glutamate receptor 5 positive allosteric modulators with high ligand efficiency. Bioorg. Med. Chem. Lett. 2014;24(15):3641–3646. doi: 10.1016/j.bmcl.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Varnes J.G., Marcus A.P., Mauger R.C., Throner S.R., Hoesch V., King M.M., Wang X., Sygowski L.A., Spear N., Gadient R., Brown D.G., Campbell J.B. Discovery of novel positive allosteric modulators of the metabotropic glutamate receptor 5 (mGlu5). Bioorg. Med. Chem. Lett. 2011;21(5):1402–1406. doi: 10.1016/j.bmcl.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 199.Zhou Y., Chun A., Gogliotti R.D., Dawson E.S., Vinson P.N., Niswender C.M., Noetzel M.J., Rook J.M., Bridges T.M., Daniels J.S., Jones C., Conn P.J., Lindsley C.W., Stauffer S.R. Pure Positive Allosteric Modulators (PAMs) of mGlu5 with Competitive MPEP-Site Interaction. 2010 [PubMed] [Google Scholar]
- 200.Goudet C., Vilar B., Courtiol T., Deltheil T., Bessiron T., Brabet I., Oueslati N., Rigault D., Bertrand H.O., McLean H., Daniel H., Amalric M., Acher F., Pin J.P. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 2012;26(4):1682–1693. doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- 201.Nosyreva E.D., Huber K.M. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 2005;25(11):2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


