Abstract
Background:
Video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) are the prime modalities for the evaluation of small bowel (SB) Crohn’s disease (CD). Mucosal inflammation on VCE is quantified using the Lewis score (LS). Diffusion-weighted (DW) magnetic resonance imaging (MRI) allows for accurate assessment of SB inflammation without administration of intravenous contrast material. The Magnetic Resonance Index of Activity (MaRiA) and the Clermont index are quantitative activity indices validated for contrast-enhanced MRE and DW-MRE, respectively. The aim of this study was to compare the quantification of distal SB inflammation by VCE and MR-related activity indices.
Methods:
Patients with known quiescent SB CD were prospectively recruited and underwent MRE and VCE. LS, MaRIA and Clermont scores were calculated for the distal SB.
Results:
Both MRI-based indices significantly correlated with the LS and the Clermont index (r = 0.50, p = 0.001 and r = 0.53, p = 0.001, respectively). Both MaRIA and Clermont scores were significantly lower in patients with mucosal healing (LS < 135). The area under the curve (AUC) with both MR scores was moderate for prediction of any mucosal inflammation (LS ⩾ 135) and excellent for prediction of moderate-to-severe inflammation (LS ⩾ 790) (0.71 and 0.74 versus 0.93 and 0.91 for MaRIA and Clermont score, respectively).
Conclusions:
Modest correlation between VCE- and MRE-based quantitative indices of inflammation in patients with quiescent SB CD was observed. Between-modality correlation was higher in patients with endoscopically severe disease. DW-MRE gauged by Clermont score was at least as accurate as contrast-enhanced MRE for quantification of SB inflammation.
Keywords: Crohn’s disease, diffusion-weighted MRI, magnetic resonance enterography, videocapsule endoscopy
Introduction
The treatment paradigm in Crohn’s disease (CD) has shifted from targeting symptoms towards reducing inflammatory activity and achieving mucosal healing, as mucosal healing was repeatedly demonstrated to be associated with improved short- and long-term outcomes [Schnitzler et al. 2009; Baert et al. 2010]. Unfortunately, in the colon, evaluation of mucosal healing necessitates repeated endoscopies, associated with risks and inconveniency to patients. Evaluation of small bowel (SB) inflammation by endoscopy is even more challenging. The poor correlation between clinical symptom-based indices such as the Crohn’s disease activity index (CDAI) with endoscopic and biochemical disease activity has been extensively demonstrated [Peyrin-Biroulet et al. 2014]. Inflammatory biomarkers such as C-reactive protein (CRP) and fecal calprotectin (FCP) provide a valuable insight into the inflammatory burden of the disease. Nonetheless, the accuracy of both for the assessment of mucosal healing is limited. Approximately 30% of CD patients do not present with elevated CRP levels even during clinical relapse [Kopylov et al. 2014]. FCP is strongly correlated with colonic inflammation, but its accuracy in isolated SB disease [Costa et al. 2003, 2005; Koulaouzidis et al. 2011; Laharie et al. 2011] is limited.
Video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) are both leading modalities for SB assessment in patients with suspected and established CD [Annese et al. 2013]. VCE has been established as an accurate modality for CD diagnosis, with diagnostic yield superior to that of CT enterography, ileocolonoscopy SB follow-through [Dionisio et al. 2010] and possibly MRE [Jensen et al. 2011; Wiarda et al. 2012]. SB inflammation on VCE can be quantified using two diagnostic scores, the Lewis score (LS) [Gralnek et al. 2008] and capsule endoscopy Crohn’s disease activity index (CECDAI) [Gal et al. 2008]. Both were recently validated for monitoring of inflammatory activity in SB [Cotter et al. 2014; Hall et al. 2014]. The utility of MRE for quantitative evaluation of SB inflammation in CD is established [Rimola et al. 2009, 2011; Ordas et al. 2014]; a novel MRE-based index [Magnetic Resonance Index of Activity (MaRIA) score] demonstrated significant correlation with the Crohn’s Disease Endoscopic Index of Activity (CDEIS) [Rimola et al. 2011; Ordas et al. 2014]. Diffusion-weighted (DW)-MRE yields comparable performance and does not require intravenous contrast material [Hordonneau et al. 2014]. The DW-MRE-based Clermont index, which incorporates the apparent diffusion coefficient (ADC), was recently validated in distal SB CD and was strongly correlated with MaRIA and the Simple Endoscopic Score for CD (SES-CD) [Caruso et al. 2014]. However, neither MaRIA nor the Clermont score were ever correlated with VCE-derived markers of endoscopic activity that may potentially provide a more accurate assessment of SB mucosal inflammation.
The aims of this study were to evaluate the correlation of MRE- and VCE-derived activity indices in the distal SB, and to evaluate the correlation of these indices with inflammatory biomarker levels.
Methods
Patient population
This was a prospective observational study. The study population included adult (>18 years) CD patients with known SB disease in remission or experiencing mild disease symptoms, as determined by a CDAI of <220. All patients were in corticosteroid-free remission for 3–24 months and were treated with a stable medication dose (60 days for thiopurines and methotrexate, 60 days for infliximab, 30 days for adalimumab and for 5-ASA agents).
Patients were excluded if they were unable to understand or provide informed consent, had severe comorbidities such as liver, kidney neurologic, metabolic or cardiorespiratory disorders not controlled at the time of enrollment, had difficulty in swallowing, history of aspirations or dysphagia, claustrophobia, implanted metal objects or cardiac pacemaker precluding performance of MRI, or had known or suspected intestinal obstruction or severe stricture.
All patients signed an informed consent and the study was approved by the institutional ethics review board.
Study procedure
MRE scans
All patients underwent MRE upon enrollment. All MRE examinations were performed using a 1.5 T GE Optima MR450w scanner with GEM Suite (GE Healthcare) with oral and intravenous contrast. MR image acquisition was performed using a previously described protocol [Shrot et al. 2011]. SB distention was obtained by using oral contrast: 360 ml of Osmitrol 20% diluted in 1.5 liter of water. Patients were instructed to drink four doses of 465 ml every 15 minutes an hour before undergoing the MRE examination. During the last 15 minutes, patients received via infusion 150 ml of saline containing 0.5 mg of glucagon in a slow drip. The protocol for MR image acquisition is outlined in online Supplementary Table 1. Axial and coronal LAVA (liver acquisition with volume acquisition) sequences were acquired before and 40 seconds after intravenous administration of gadolinium (0.5 mmol/ml by 0.2 ml/kg). A segmental MaRIA score was calculated using the following formula: MaRIA = 1.5 × wall thickness (mm) + 0.02 × relative contrast enhancement + 5 × edema + 10 × ulcers [Rimola et al. 2009]. Similarly, a segmental Clermont score was calculated using the following formula: Clermont = −1.32 × ADC (10−3 mm2/s) + 1.646 × wall thickness (mm) + 8.306 × ulcers + 5.613 × edema + 5.039 [Buisson et al. 2013]. For the purpose of MaRIA and Clermont score calculation, the distal SB was defined as the distal 20 cm of the terminal ileum. A board-certified abdominal radiologist with 10 years of experience in reading MRE reviewed all MRE examinations
Capsule endoscopy studies
A patency capsule (PC) test (Given Imaging, Yokneam, Israel) was performed in all patients with active SB disease detected on MRE. If no active SB disease was detected by MRE, a PC study was not performed. If the PC was not eliminated from the SB within 30 hours, the patients were withdrawn from the study. In patients with isolated SB CD (L1 by the Montreal classification), SB-III capsule (Given Imaging, Yokneam, Israel) was used. In patients with SB and colonic CD (L3), a colonic capsule (PillCam colonic capsule 2, Given Imaging, Yokneam, Israel) was administered; in these patients, the SB data was reviewed and scored as described for the SB capsule.
The preparation for VCE included intake of clear fluids only for 24 hours prior to the procedure and a 12-hour overnight fast. For a colonic capsule study, a split-dose PEG preparation was used. An additional fluid bolus was given after 2 hours from ingestion of the capsule in order to facilitate SB transit. All images were reviewed using the RAPID 8 software (Given Imaging, Yokneam, Israel). The adaptive frame rate mode was activated to ensure visualization of the entire SB. Mucosal inflammation was quantified using the LS [Gralnek et al. 2008]. Mucosal healing was defined as LS < 135, mild-to-moderate inflammation as LS of 135–790, and moderate-to-severe inflammation as LS ⩾ 790 [Gralnek et al. 2008]. LS was calculated automatically (RAPID Reader, Given Imaging, Yokneam, Israel) for SB-III capsule, and manually for the colonic capsule. The inflammatory activity in the distal SB (third tertile) was correlated with the MR-derived scores.
Inflammatory biomarkers
FCP levels were measured using the Quantum blue calprotectin kit (BÜHLMANN Laboratories AG, Basel, Switzerland). The reported value range is 30–300 μg/g. Levels >100 μg/g were considered positive. CRP levels were considered elevated if >5 mg/l.
The study was approved by the institutional ethics review board.
Statistical analysis
Descriptive statistics were presented as means ± standard deviations for continuous variables and percentages for categorical variables. Categorical variables were analyzed by chi square/Fisher’s exact test and continuous variables by Student t test/Mann–Whitney U test. We evaluated the correlation of elevated biomarkers (FCP, CRP and combination) with SB mucosal healing as obtained by VCE and MRE. Sensitivity, specificity, negative predictive value (NPV) and positive predictive values (PPV) as well as Spearman’s rank (r) correlation were calculated. Correlation r values <0.3 were considered as weak-to-low correlation, 0.3–0.49 as low-to-moderate, 0.5–0.69 as moderate, and ⩾0.7 as strong correlation [Fleiss, 1981]. A two-tailed p value <0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve was constructed for correlation of MaRIA score presence of any mucosal inflammation (LS ⩾ 135) and moderate-to-severe inflammation (LS > 790), and area under the curve (AUC) was calculated. An AUC of 0.6–0.7 was considered poor, and 0.9–1 as excellent. Overall agreement between the modalities for detection of small bowel ulcers and stenosis was evaluated using kappa statistics. The analysis was performed using IBM SPSS statistic (Version 20.0; Armonk, NY, USA).
Results
Patient population
Eighty one patients were enrolled and underwent MRE. One patient was excluded after an episode of severe abdominal pain following ingestion of oral contrast material for MRE; two additional patients were withdrawn from the study following a clinical relapse prior to VCE. After exclusion of patients with a high risk of capsule retention d/t severe stricturing disease detected by MRE (2 patients) and patients who failed PC test (16 patients) or have withdrawn their consent (4 patients), 56 remaining patients underwent a diagnostic VCE. All VCE examinations were complete (the capsule reached the cecum or toilet). MR-based scores in the distal SB could not be calculated for the distal SB in 3/56 patients because of motion artifacts and inadequate contrast filling. The clinical and demographic characteristic of these patients are detailed in Table 1.
Table 1.
Patient clinical and demographic characteristics.
| N | % | ||
|---|---|---|---|
| Male/female | 30/26 | 53.6/46.4 | |
| Age at diagnosis (years) | 26±11 | ||
| Disease duration (years) | 6±5 | ||
| Clinical remission | 52 | 92.3% | |
| Smoking status | current | 11 | 19.6% |
| never smoked | 36 | 64.3% | |
| past smoking | 9 | 16.1% | |
| Previous abdominal surgery | 8 | 14.2 | |
| segmental small bowel resection | 7 | 12.5 | |
| Ileocecal resection | 1 | 1.8 | |
| Perianal disease | 13 | 23.2 | |
| Current treatment | 45 | 80.4% | |
| None | 11 | 19.6 | |
| thiopurine | 25 | 44.6% | |
| Anti-TNF | 21 | 37.5% | |
| Combined anti-TNF+thiopurine | 7 | 12.5% | |
TNF, tumor necrosis factor.
Detection of inflammation by VCE and MRE
Active inflammation in the distal SB was detected by VCE in 38/56 (67.8%). Moderate-to-severe inflammation (LS > 790) was detected in 8/56 (14.3%), mild-to-moderate inflammation (LS = 135–790) LS in 30 (53.6%), and no disease/mucosal healing (LS < 135) in 18/56 (30.2%) of the patients, respectively. Active inflammation was detected in the SB by MRE in 42/56 (75%). The overall between-modality agreement was low-to-moderate (K = 0.41, p = 0.02). The mean LS in the distal SB was 380 ± 570.
Correlation of the MaRIA score with the Lewis score
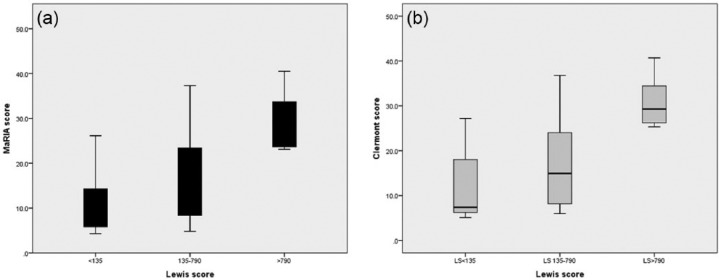
The mean Maria score in the distal SB was 16.3 ± 10.3. The mean MaRIA score was significantly lower in patients with endoscopic mucosal healing in the distal SB (18 versus 38 patients, 7.9 ± 1.9 versus 10.8 ± 1.8, p = 0.007). The mean MaRIA score in patients with moderate-to-severe SB inflammation was significantly higher than that of patients with milder inflammation (8 versus 48 patients, 16.1 ± 9.9 versus 29.6 ± 6.3,p = 0.0001) (Figure 1(a)). Significant correlation was demonstrated between the MaRIA score and LS (r = 0.50, p = 0.0001).
Figure 1.
Correlation of degree of small bowel inflammation (Lewis score categories) with the Magnetic Resonance Index of Activity (MaRIA) (a) and Clermont (b) score.
The AUC for MaRIA score for detection of any active inflammation in the distal SB (LS ⩾ 135) was 0.71 (p = 0.017). The optimal accuracy for prediction of mucosal inflammation by VCE corresponded to MaRIA score of 7.6 (sensitivity 82.9%, specificity 58.6%). The AUC for detection of moderate-to-severe inflammation (LS ⩾ 790) was 0.93 (p = 0.0001) with MaRIA score of 22.6, associated with a sensitivity of 100% and specificity of 88.9%.
Correlation of the Clermont score with the Lewis score
The mean Clermont score was 18.9 ± 10.8. The Clermont score significantly correlated with the LS (r = 0.53, p = 0.001). The mean Clermont score was significantly lower in patients with mucosal healing in the distal SB (18 versus 38 patients, 6.6 ± 10.5 versus 12.1 ± 8.1, p = 0.005). The mean Clermont score in patients with moderate-to-severe SB inflammation was significantly higher compared with that of patients with mild inflammation (8 versus 48 patients, 16.6 ± 9.4 versus 30.7 ± 5.4, p = 0.0001) (Figure 1(b)).
The AUC for the Clermont score for detection of any active inflammation in the distal SB (LS ⩾ 135) was 0.74 (p = 0.006). The optimal accuracy for prediction of mucosal inflammation by VCE corresponded to a Clermont score of 8.1 (sensitivity 82.9%, specificity 58.8%). The AUC for detection of moderate-to-severe inflammation (LS ⩾ 790) was 0.91 (p = 0.0001) with Clermont score of 25.2, associated with a sensitivity of 100% and specificity of 88.9%.
Correlation with inflammatory biomarkers
FCP levels were moderately correlated with both MR indices (Table 2). The correlation was weaker for LS in the distal SB. CRP weakly correlated with MR indices; no correlation between CRP and distal LS was observed. The MaRIA and Clermont scores highly correlated.
Table 2.
Correlation of inflammatory biomarkers with VCE- and MRE-based activity indices.
| MaRIA | Clermont | LS | CRP | FCP | ||
|---|---|---|---|---|---|---|
| MaRIA | r | 1 | 0.991 | 0.501 | 0.296 | 0.453 |
| p | 0.000 | 0.000 | 0.024 | 0.001 | ||
| Clermont score | r | 0.991 | 1 | 0.533 | 0.265 | 0.450 |
| p | 0.000 | 0.000 | 0.044 | 0.001 | ||
| LS | r | 0.501 | 0.533 | 1 | 0.024 | 0.359 |
| p | 0.000 | 0.000 | 0.865 | 0.007 | ||
| CRP | r | 0.296 | 0.265 | 0.024 | 1 | 0.176 |
| p | 0.024 | 0.044 | 0.865 | 0.194 | ||
| FCP | r | 0.453 | 0.450 | 0.359 | 0.176 | 1 |
| p | 0.001 | 0.001 | 0.007 | 0.194 |
VCE, video capsule endoscopy; MRE, magnetic resonance enterography; MaRIA, Magnetic Resonance Index of Activity; LS, Lewis score; FCP, fecal calprotectin; CRP, C-reactive protein.
Detection of ulcers by VCE and MRE
Ulcers in the distal SB were detected by VCE in 37/56 (66.1%) of the patients. In patients with distal SB ulcers detected by VCE, MRE detected ulcers in 10 (sensitivity 28.6%) patients; in 8 additional patients, ulcers detected by MRE where not confirmed by VCE (specificity 55.6%). The positive (PPV) and negative (NPV) predictive values of MRE for detection of distal SB ulcers was 55.5% and 30.1%, respectively. Overall between-modality agreement for detection of SB ulcers was very weak (κ = 0.13). In patients with moderate-to-severe distal SB inflammation, the accuracy of MRE for detection of ulcers was somewhat improved (3/7, sensitivity 42.8%, specificity 100%).
Safety
One patient had a symptomatic PC retention manifesting with abdominal pain and vomiting. The patient was treated with corticosteroids with subsequent resolution of the symptoms within 2 days. An additional patient had an episode of severe abdominal pain following ingestion of oral contrast material for MRE. No adverse effects related to VCE ingestion or VCE retentions occurred in this study.
Discussion
The results of our study demonstrate correlation between quantitative characteristics of SB inflammation as demonstrated by VCE and MRE. Both modalities allow for accurate visualization of the SB and provide valuable clinical information for diagnosis and monitoring of SB CD [Cotter et al. 2014; Ordas et al. 2014]. Although the overall accuracy of MRE and VCE is comparable, the features of the data yielded by both are quite different and the degree of correlation was modest. While VCE provides subtle details of mucosal appearance, MRE is useful for demonstration of intraluminal inflammation and edema (manifested by bowel wall thickness and attenuation), in addition to extraluminal complications such as abscesses and fistulae. These different capabilities were reflected by our results, as ulcers detected by VCE were reported by MRE in less than 1/3 of the patients. Moreover, VCE is more sensitive than MRE for detection of proximal SB disease (not evaluated in this study as MRI indices do not include the proximal segments). Despite the poor agreement between the modalities for detection of SB ulcers, there was a fair correlation in the quantitative assessment of disease severity between MRE and VCE. Interestingly, the accuracy of detection of ulcers by MRE was higher in patients with severe inflammation on VCE. In patients with mild-to-moderate inflammation, the accuracy of MRE for detection of endoscopically active disease was significantly more limited. Our results suggest that severe inflammation in the small bowel will be accurately detected by both modalities, while in patients with mild-to-moderate mucosal inflammation MRE is likely to underestimate the inflammatory burden. These findings underscore the limitations of MRE for detection of mild disease, which is likely to be limited to the mucosal layer and not involve the deeper layers of the intestinal wall or significantly alter the vascular patterns.
Our study is the first to correlate MR-derived indices with VCE. Importantly, correlation of the MaRIA score results and VCE-derived indices was comparable with previous reports for ileocolonoscopic inflammation scores [Ordas et al. 2014; Caruso et al. 2014]. Moreover, there was a statistically significant difference in the mean MaRIA and Clermont scores in patients with mucosal healing, mild and moderate-to-severe inflammation. Interestingly, the MaRIA score associated with absence of ulcers on ileocolonoscopy (mucosal healing) as reported by Ordas and colleagues (MaRIA score of 7) [Ordas et al. 2014] was similar to our results. The performance of the MaRIA and Clermont scores was moderate for prediction of any mucosal inflammation, and excellent for prediction of moderate to severe inflammation. These results suggest that in patients with more significant disease there is a higher concordance of the inflammatory features depicted by both modalities. As reported previously [Kopylov et al. 2015a, 2015b], inflammatory biomarkers do not reflect the full extent of the SB inflammation, as manifested by the moderate correlation of FCP with VCE and MRE scores. To date, the clinical significance of mild mucosal inflammation diagnosed by capsule endoscopy is unclear. The majority of the currently available data linking mucosal inflammation to long-term prognosis refers to colonic inflammation [Schnitzler et al. 2009; Baert et al. 2010; Peyrin-Biroulet et al. 2014], and to the best of our knowledge there is no currently available data to clearly support this paradigm for SB mucosal healing. Further prospective studies are merited in order to establish whether SB mucosal healing should guide therapeutic changes in patients with SB CD.
In concordance with previous studies [Buisson et al. 2013; Hordonneau et al. 2014], DW-MR was at least as accurate as contrast-enhanced MRE, while allowing for accurate quantitative diagnosis without administration of intravenous contrast material. Intravenous gadolinium is associated with rare but serious adverse effects such as hypersensitivity reactions and nephrogenic systemic sclerosis [Rose and Choi, 2015]. The correlation between both MRE scores was excellent, as described previously [Buisson et al. 2013, Hordonneau et al. 2014].
Our study has several limitations. Primarily, the study cohort included only patients in clinical remission. Even though some degree of inflammatory activity was detected in the majority of patients, it is possible that inclusion of patients with clinically active disease may have yielded different results. Importantly, we may expect an even higher concordance between the modalities in patients with clinically active disease, as in our cohort patients with significant disease detected by VCE were more likely to have corresponding MRE findings. An additional limitation is the utilization of a modified MaRIA score. The original score [Rimola et al. 2009] includes segmental subscores accumulated for sections of the colon and the distal ileum. In our cohort, only a minority of patients had active colonic disease, and ileocolonoscopic surveillance was not performed. Henceforth we calculated and used for correlation only the segmental score for the distal ileum. In addition, we did not calculate the MaRIA score for the proximal SB, due to a significantly diminished diagnostic performance of MRE in this segment [Dionisio et al. 2010], a particular limitation being the diminished ability to visualize fine details of the proximal SB mucosa, such as ulcerations that are an inherent component of both scores. Neither MaRIA nor Clermont score were calculated or validated in the proximal SB in previous studies.
In addition, it is impossible to perform an accurate estimation of bowel length using VCE, and the segments of the SB are separated by transit time, without a constant correlation between time and distance. For the purpose of the analysis, we used the distal third of the SB (last 33% of the SB transit time) for the LS.
In patients with active colonic disease we performed a pan enteric evaluation with the colonic capsule. The technical specifications of the PillCam Colon 2 capsule are similar to these of the SB-III (except for an additional camera and a higher speed of the adaptive frame rate). PillCam Colon 2 capsule setup allows for evaluation of the entire SB in a very similar way to that of the SB capsule. Several centers recently reported their successful experience with the colonic capsule for evaluation of the entire intestinal tract in patients with CD [Boal Carvalho et al. 2015; D’haens et al. 2015; Hall et al. 2015]
Finally, our study cohort was composed of patients in clinical remission, with a relatively small proportion of patients with significant endoscopic inflammation. Our results might have been different in patients with clinically active or endoscopically more severe inflammation in the SB.
In conclusion, our study demonstrated a significant correlation between VCE- and MRE-based quantitative indices of inflammation in patients with quiescent SB CD. Despite several modality-specific limitations, both VCE and MRE provided an accurate and comprehensive evaluation of the SB, and were able to detect ongoing inflammation in a majority of patients with clinically quiescent disease. The concordance between the modalities was substantially better in patients with significant SB inflammation. DW MRE was at least as accurate as contrast-enhanced MRE for quantification of SB inflammation. The accuracy of these inflammatory indices for prediction of future relapses and long-term complications merits evaluation in future prospective studies.
Supplementary Material
Acknowledgments
We would like to thank Along Lang, MD, Dan Carter, MD, Amir Waizbard, MD and Eyal Shachar, MD for their contribution to the study.
Footnotes
Funding: The study was partially supported by a generous grant from the Leona M. and Harry B. Helmsley Charitable Trust.
Conflict of interest statement: Uri Kopylov, Eyal Klang, Doron Yablecovitch, Adi Lahat, Sandra Neuman, Nina Levhar, Tomer Greener, Benjamin Avidan, Henit Yanai, Batya Weiss and Marianne M. Amitai have no conflicts to declare. Iris Dotan has received advisory boards/lectures/research support from Janssen, AbbVie, MSD, Takeda, Ferring, Falk Pharma, Rafa, Genentech, Pfizer, Given Imaging, Teva and Protalix. Shomron Ben-Horin has received research support and/or consultancy fees from MSD, AbbVie, Janssen, CellTrion and Takeda. Rami Eliakim has received advisory board/lecture fees from Given Imaging.
Contributor Information
Uri Kopylov, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer 52621, Israel.
Eyal Klang, Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Doron Yablecovitch, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Adi Lahat, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Benjamin Avidan, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Sandra Neuman, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Nina Levhar, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Tomer Greener, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Noa Rozendorn, Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Arkadi Beytelman, Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Henit Yanai, IBD Center, Department of Gastroenterology and Liver Diseases, Tel Aviv Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Iris Dotan, IBD Center, Department of Gastroenterology and Liver Diseases, Tel Aviv Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Yehuda Chowers, Rambam Health Care Campus, Haifa, Israel; Bruce Rappaport School of Medicine, Technion Israel Institute of Technology, Haifa, Israel.
Batya Weiss, Edmond and Lily Safra Children’s Hospital, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Shomron Ben-Horin, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Marianne M. Amitai, Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
Rami Eliakim, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel.
References
- Annese V., Daperno M., Rutter M., Amiot A., Bossuyt P., East J., et al. (2013) European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 7: 982–1018. [DOI] [PubMed] [Google Scholar]
- Baert F., Moortgat L., Van Assche G., Caenepeel P., Vergauwe P., De Vos M., et al. (2010) Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 138: 463–468; quiz e410–461. [DOI] [PubMed] [Google Scholar]
- Boal Carvalho P., Rosa B., Dias De Castro F., Moreira M., Cotter J. (2015) PillCam Colon 2 in Crohn’s disease: a new concept of pan-enteric mucosal healing assessment. World J Gastroenterol 21: 7233–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson A., Joubert A., Montoriol P., Da Ines D., Hordonneau C., Pereira B., et al. (2013) Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther 37: 537–545. [DOI] [PubMed] [Google Scholar]
- Caruso A., D’inca R., Scarpa M., Manfrin P., Rudatis M., Pozza A., et al. (2014) Diffusion-weighted magnetic resonance for assessing ileal Crohn’s disease activity. Inflamm Bowel Dis 20: 1575–1583. [DOI] [PubMed] [Google Scholar]
- Costa F., Mumolo M., Bellini M., Romano M., Ceccarelli L., Arpe P., et al. (2003) Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Digest Liver Dis 35: 642–647. [DOI] [PubMed] [Google Scholar]
- Costa F., Mumolo M., Ceccarelli L., Bellini M., Romano M., Sterpi C., et al. (2005) Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 54: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter J., Dias De, Castro F., Magalhaes J., Joao Moreira M., Rosa B. (2014) Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy, in press. [DOI] [PubMed] [Google Scholar]
- D’haens G., Lowenberg M., Samaan M., Franchimont D., Ponsioen C., Van Den Brink G., et al. (2015) Safety and feasibility of using the second-generation PillCam colon capsule to assess active colonic Crohn’s disease. Clin Gastroenterol Hepatol 13: 1480–1486.e1483. [DOI] [PubMed] [Google Scholar]
- Dionisio P., Gurudu S., Leighton J., Leontiadis G., Fleischer D., Hara A., et al. (2010) Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 105: 1240–1248; quiz 1249. [DOI] [PubMed] [Google Scholar]
- Fleiss J. (1981) Statistical Methods for Rates and Proportions (2nd edn). New York: John Wiley & Sons. [Google Scholar]
- Gal E., Geller A., Fraser G., Levi Z., Niv Y. (2008) Assessment and validation of the new Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI). Digest Dis Sci 53: 1933–1937. [DOI] [PubMed] [Google Scholar]
- Gralnek I., Defranchis R., Seidman E., Leighton J., Legnani P., Lewis B. (2008) Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 27: 146–154. [DOI] [PubMed] [Google Scholar]
- Hall B., Holleran G., Chin J., Smith S., Ryan B., Mahmud N., et al. (2014) A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis, in press. [DOI] [PubMed] [Google Scholar]
- Hall B., Holleran G., Mcnamara D. (2015) Pillcam Colon 2((C)) as a pan-enteroscopic test in Crohn’s disease. World J Gastrointest Endosc 7: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordonneau C., Buisson A., Scanzi J., Goutorbe F., Pereira B., Borderon C., et al. (2014) Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 109: 89–98. [DOI] [PubMed] [Google Scholar]
- Jensen M., Kjeldsen J., Rafaelsen S., Nathan T. (2011) Diagnostic accuracies of MR enterography and CT enterography in symptomatic Crohn’s disease. Scand J Gastroenterol 46: 1449–1457. [DOI] [PubMed] [Google Scholar]
- Kopylov U., Adi Lahat D., Neuman S., Levhar N., Greener T., Klang E., et al. (2015a) Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy and imaging. Am J Gastroenterol, in press. [DOI] [PubMed] [Google Scholar]
- Kopylov U., Nemeth A., Koulaouzidis A., Makins R., Wild G., Afif W., et al. (2015b) Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 21: 93–100. [DOI] [PubMed] [Google Scholar]
- Kopylov U., Rosenfeld G., Bressler B., Seidman E. (2014) Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis, in press. [DOI] [PubMed] [Google Scholar]
- Koulaouzidis A., Douglas S., Rogers M., Arnott I., Plevris J. (2011) Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol 46: 561–566. [DOI] [PubMed] [Google Scholar]
- Laharie D., Mesli S., El Hajbi F., Chabrun E., Chanteloup E., Capdepont M., et al. (2011) Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther 34: 462–469. [DOI] [PubMed] [Google Scholar]
- Ordas I., Rimola J., Rodriguez S., Paredes J., Martinez-Perez M., Blanc E., et al. (2014) Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 146: 374–382.e371. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Reinisch W., Colombel J., Mantzaris G., Kornbluth A., Diamond R., et al. (2014) Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the Sonic Trial. Gut 63: 88–95. [DOI] [PubMed] [Google Scholar]
- Rimola J., Ordas I., Rodriguez S., Garcia-Bosch O., Aceituno M., Llach J., et al. (2011) Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 17: 1759–1768. [DOI] [PubMed] [Google Scholar]
- Rimola J., Rodriguez S., Garcia-Bosch O., Ordas I., Ayala E., Aceituno M., et al. (2009) Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 58: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Rose T., Jr, Choi J. (2015) Intravenous imaging contrast media complications: the basics that every clinician needs to know. Am J Med, in press. [DOI] [PubMed] [Google Scholar]
- Schnitzler F., Fidder H., Ferrante M., Noman M., Arijs I., Van Assche G., et al. (2009) Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- Shrot S., Konen E., Hertz M., Amitai M. (2011) Magnetic resonance enterography: 4 years experience in a tertiary medical center. Isr Med Assoc J 13: 172–177. [PubMed] [Google Scholar]
- Wiarda B., Heine D., Mensink P., Stolk M., Dees J., Hazenberg H., et al. (2012) Comparison of magnetic resonance enteroclysis and capsule endoscopy with balloon-assisted enteroscopy in patients with obscure gastrointestinal bleeding. Endoscopy 44: 668–673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.