Abstract
There is ample empirical evidence to support the notion that the biological impacts of estrogen extend beyond the gonads to other bodily systems, including the brain and behavior. Converging preclinical findings have indicated a neuroprotective role for estrogen in a variety of experimental models of cognitive function and brain insult. However, the surprising null or even detrimental findings of several large clinical trials evaluating the ability of estrogen-containing hormone treatments to protect against age-related brain changes and insults, including cognitive aging and brain injury, led to hesitation by both clinicians and patients in the use of exogenous estrogenic treatments for nervous system outcomes. That estrogen-containing therapies are used by tens of millions of women for a variety of health-related applications across the lifespan has made identifying conditions under which benefits with estrogen treatment will be realized an important public health issue. Here we provide a summary of the biological actions of estrogen and estrogen-containing formulations in the context of aging, cognition, stroke, and traumatic brain injury. We have devoted special attention to highlighting the notion that estrogen appears to be a conditional neuroprotectant whose efficacy is modulated by several interacting factors. By developing criteria standards for desired beneficial peripheral and neuroprotective outcomes among unique patient populations, we can optimize estrogen treatments for attenuating the consequences of, and perhaps even preventing, cognitive aging and brain injury.
Keywords: Estrogen, Neuroprotection, Aging, Cognition, Stroke, Traumatic brain injury
1. Introduction: the role of estrogen beyond the gonads
Our current understanding of the biological role of the sex hormone estrogen and more broadly, the field of women’s health, has its origins in the avian testis. Indeed, in 1849, the physiologist Arnold Adolf Berthold reported on his findings regarding the anatomical and behavioral consequences of testicular removal and transplantation in roosters (Berthold, 1849). In an elegantly designed experiment, Berthold subjected cockerels to full or partial testicular removal. Among animals in which both testes were excised, physiology and behavior was markedly altered in that “they were not aggressive, they fought other cockerels rarely and in a half-hearted manner, and developed the monotone voice of the capon”. Yet, in birds that had only one testis removed or in castrated birds who received testicular transplantation, he noted that behavior remained indiscernible from that of a normal rooster, with these birds still crowing, fighting, and displaying the “usual reactions to hens”. Given that the transplanted testes did not always re-establish nerve connections within the rooster, Berthold attributed these findings to “some productive function of the testes. . . by their action on the blood stream, and then by corresponding reaction of the blood on the entire organism, of which, it is true, the nervous system represents a considerable part.” Thus, whether Berthold realized the impact of his discovery or not, this experiment provided some of the first empirical support for the influence of sex hormones on the body and brain.
Characterizing the physiological impacts of estrogen is as important today as it was in Berthold’s era. Nearly half of the global population is female, and sex-specific shifts in endogenous hormone levels related to cyclicity, pregnancy, and menopause are associated with differences in cognitive performance as well as altered risk for, and outcome from, neurological insults (Kimura, 2002; Kittner et al., 1996; Lisabeth and Bushnell, 2012; Workman et al., 2012). Further, today tens of millions of women use estrogen-containing treatments for many reasons ranging from menstrual cycle regulation to contraception to the amelioration of symptoms associated with the menopausal transition (Hersh et al., 2004; Jones et al., 2012). Although numerous reports note a multitude of beneficial neuroprotective effects of estrogens (reviewed in Acosta et al., 2013; Arevalo et al., 2015; Brown, 2009; Luine, 2014; Simpkins and Singh, 2008), the use of estrogenic compounds is controversial. Indeed, the known increased risk of stroke associated with oral contraceptive (OC) use coupled with the surprising null or even detrimental findings of the large, double-blind, placebo-controlled Women's Health Initiative (WHI) clinical trial regarding the risk for adverse outcomes among post-menopausal women taking hormone therapy (HT) led to hesitation by both clinicians and patients in the use of exogenous estrogen-containing treatments for brain-related outcomes (Kittner et al., 1996; Manson et al., 2013). Thus, there is a pressing medical need to understand the conditions under which estrogens exert neuroprotection.
Here, we review the literature regarding the nature of neuroprotection by estrogen and estrogen-containing compounds among females. We limited this discussion to studies in which human participants, rodent subjects, or in vitro cultures were utilized to assess the effects of ‘estrogen’ within the context of cognition, stroke, and traumatic brain injury (TBI). Our strategy for selection of published articles for citation in the current review is defined by the inclusion of studies in which a finding was first demonstrated, as well as seminal papers describing key caveats within a given field of research. In areas of research where the number of published studies is limited (for example, the cognitive impacts of estrone; E1), we made efforts to include all work conducted. Whenever possible, references included are primary research articles although we have also directed readers to several thorough and key reviews exhaustively addressing topics beyond the scope of the current discussion. For instance, neuroprotective actions of estrogens, and the complex mechanisms underlying these effects, have been documented in several domains of neurological function, injury, and disease (Chakrabarti et al., 2014), such as the experimental autoimmune encephalomyelitis model of multiple sclerosis (Offner and Polanczyk, 2006), spinal cord injury (Elkabes and Nicot, 2014) and Parkinson's Disease (Smith and Dahodwala, 2014), to name just a few. The beneficial effects of estrogens in these domains are noteworthy and we direct the reader to several key reviews on each of these important subjects.
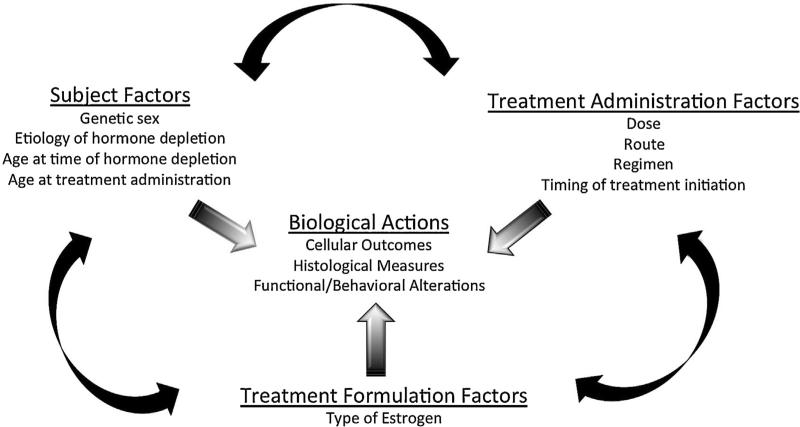
As well, the majority of exogenous estrogen-containing therapies given to women also include a progestin. Progestins have known actions in the nervous system, many of which can be beneficial (Brinton et al., 2008). However, mounting evidence suggests that the addition of a progestin may in fact attenuate, obviate, or even reverse the beneficial actions of estrogens when administered together (Acosta et al., 2013). Indeed, many factors likely influence the impact of estrogen + progestin combination HTs including the type of progestin administered (natural progesterone versus synthetic versions), the age of the organism at time of treatment, and the duration between hormone depletion and subsequent treatment (Singh and Su, 2013). A detailed discussion of the neuroactive effects of progesterone, either alone or in combination with estrogen, is beyond the scope of this review and has been extensively discussed elsewhere. The authors direct interested readers to several excellent reviews on this subject (Deutsch et al., 2013; Wei and Xiao, 2013).
Of note, in many previously published reports, the term estrogen has been used indiscriminately and interchangeably to refer to both the large hormonal group or a specific molecule. As highlighted by Blaustein (2008), the importance of precision in hormone nomenclature is critical. For instance, until recently, the vast majority of studies assessing the neuroprotective effects of estrogen in animals utilized 17 beta-estradiol (17βE2). Yet, for the woman, a wide variety of hormone treatment options exist ranging from ethinyl estradiol-based OCs to 17βE2-based vaginal creams to Premarin®-based menopausal HTs (conjugated equine estrogens; CEE). As will be discussed in detail below, not all estrogens impart the same neurobiological effects and many of these estrogens and estrogen-containing compounds have yet to be comprehensively evaluated for their unique neurobiological actions. Thus, for the purposes of this review, when the term estrogen is used, we refer to the broad class of natural and synthetic estrogen-like molecules that have estrogenic activity at the various estrogen receptors. As well, whenever possible, when referencing work in which exogenous estrogen treatment is administered, the specific estrogen used in each study cited will be listed.
2. The estrogen molecule, its receptors, and its biological actions in the body and brain
Steroid hormones are synthesized from cholesterol through a variety of chemical reactions and can exert important physiological effects throughout the lifespan. 17βE2 is the most potent naturally-circulating estrogen, followed by E1 and estriol (E3), in order of receptor affinity (Kuhl, 2005; Sitruk-Ware, 2002). These and other hormones play crucial roles in the modulation of central nervous system (CNS) substrates and the behaviors they regulate. Indeed, endogenous hormones can have organizational physiological effects, operationally defined as permanent changes in tissue which occur early in life (either during prenatal development or in the initial days of the post-natal period); these organizational effects cannot be reversed by hormone depletion (Arnold, 2009). As well, later in life, activational effects of endogenous sex steroids and/or exogenous hormone administration can transiently impact the structure and function of these organized neural substrates and pathways. It has been suggested that both organizational and activational hormone actions account for the well-documented sex differences in cognitive outcomes and sex-specific risks for, and consequences of, diseased/injured brain states (Kimura, 2002; Manwani and McCullough, 2011). Although hormone levels can vary greatly across the menstrual cycle and some of these variations have been associated with cycle stage-specific alterations in cognitive performance and damage following brain injury, the ratio of circulating 17βE2:E1 is generally considered to be 1:1 during the reproductive years of adulthood (Rannevik et al., 1995). A notable exception to this is during pregnancy, in which E3 produced by the placenta is the predominant circulating estrogen; levels decline rapidly in the post-partum period (Neves-e-Castro, 1975). As women age, they experience menopause, a transition from reproductive capability to reproductive senescence (Timiras et al., 1995). The menopausal transition, typically occurring during the fifth decade of life, is characterized by depleted ovarian follicles, declines in naturally circulating levels of sex hormones, such as estrogens and progesterone, and a dysregulation of gonadotropin feedback loops marked by increasing levels of follicular stimulating hormone and luteinizing hormone (Rannevik et al., 1995). During this time, the ratio of circulating estrogen levels shifts such that E1 is the principle circulating estrogen (Rannevik et al., 1995). As a result of these changing hormone levels, menopause is accompanied by hot flashes, urogenital atrophy, cognitive decline (specifically learning and memory), changes in risk for neurodegenerative diseases, worsened outcomes following brain trauma, and other symptoms that reduce quality of life (Freedman, 2002; Sherwin and Henry, 2008). These consequences of the menopausal transition become important when considering that life expectancy has increased over the past century, but the age of spontaneous menopause has not changed (Hawkes, 2003). This means that women now spend a larger proportion of life in this post-menopausal, hypoestrogenic state associated with numerous negative physiological and neurological consequences. As well, the size of the aging female population is growing. By the year 2050, 90 million people are projected to be over 65 years of age (US Census, 2008). Given that women tend to outlive men, over half of this large aging population will be women. Thus, understanding the physiological impacts of female sex hormones is, and will continue to be, a crucial public health issue.
Many of the diverse biological effects of estrogen are mediated by ligand interactions with two classical nuclear estrogen receptors (ER), ER-alpha (ERα) and ER-beta (ERβ). Both ERs are members of the nuclear receptor superfamily (Peterson, 2000) but they differ in their chromosomal localizations and ligand-binding domains (Gustafsson, 1999). Discovered in uterine tissue (Jensen and Jacobson, 1962; Toft and Gorski, 1966) and cloned in 1986 (Greene et al., 1986), ERα was the first nuclear ER that demonstrated binding specificity for 17βE2 and was thought to be the sole ER with which all estrogens interacted. However, 10 years later, the discovery of a second nuclear ER in a cDNA library from rat prostate, ERβ, added clarity, and perhaps more complexity, to our understanding of the pharmacology and physiology of ER function (Kuiper et al., 1996). Currently, six ERβ splice variants have been reported in the brain and other tissues. Intriguingly, the ERβ1 isoform exhibits a neuroprotective role, functioning as a tumor suppressor, while the ERb2 isoform appears to function in a dominant negative role to initiate oncogenesis (Böttner et al., 2014; Dey et al., 2015; Handa et al., 2012). Both ERα and ERβ regulate the physiological actions of estrogens primarily through the classical genomic signaling pathways, through which binding of an estrogenic ligand to ERα or ERβ in the cytoplasm promotes translocation of the ligand-receptor complex to the nucleus to serve as a transcription factor via binding to estrogen response elements (EREs) at gene promoters. Studies over the past decade have also revealed important physiological roles for another ER, G-protein coupled ER 1 (GPER1; previously known as GPR30), in the brain and periphery. In contrast to ERα and ERβ, ligand binding to GPER1 occurs exclusively at the membrane and mediates several of the rapid, nongenomic signaling actions of estrogens (Prossnitz and Barton, 2014).
The pleiotropic actions of estrogens are amplified by the complex and diverse pattern of ER distribution throughout the brain and periphery. Traditionally, ERs have been associated with organs and tissues such as the uterus, ovaries, breast, hypothalamus, and pituitary and participate in the classical reproductive functions. As well, numerous nonreproductive functions for ERs have been identified in several other bodily tissues and organ systems including brain, cardiovascular tissue, bone, immune cells, and liver (Kuiper et al., 1997). Within the brain, ER subtypes are found in cognitive brain regions associated with learning and memory, such as the amygdala, cerebral cortex, hippocampus, and basal forebrain (Shughrue et al., 1997; Shughrue et al., 2000). ERs have also been identified in nearly all cell types found in the CNS, including neurons, astrocytes, microglia, oligodendrocytes, endothelial cells, and vascular smooth muscle cells. The physiological actions of each ER are not mutually exclusive, as converging data suggest the existence of both distinct and overlapping complex biological roles for each receptor subtype. Indeed, the physiological mechanisms with which endogenous and/or exogenous estrogens can impart their effects are diverse as estrogens participate in numerous modes of signal transduction. That estrogens can be synthesized de novo or via the action of aromatase in distinct brain regions further exemplifies the complexity of estrogenic signaling cascades (Li et al., 2014). The modes of ER signaling have significantly expanded beyond the traditional view of ERα and ERβ as transcription factors to include: rapid effects at the membrane on signal transduction pathways ligand, ligand-independent signaling, and receptor binding to non-traditional ligands (Deroo and Korach, 2006). Specific mechanisms of action for ERs in stroke, TBI, and cognition will be addressed later in this review (see Section 4).
3. Use of rodent models to assess estrogenic actions in the nervous system
Within the context of aging and age-related hormone depletion, given the many parallels between humans and this animal model, from the basic science perspective the middle-aged, ovariectomized (Ovx) rodent is the gold standard. For instance, the effects of aging among women and rodents for a variety of insults including cognitive aging and brain injury are homologous. Indeed, cognitive aging occurs spontaneously in both species (Berchtold and Cotman, 2009) and stroke or stroke model neuropathology is similar in both humans and rodents (Durukan and Tatlisumak, 2007). Further, the physiological and cognitive consequences following surgical hormone depletion are also similar in both species. For instance, following oophorectomy in women, in which the ovaries are excised, circulating levels of sex hormones including 17βE2 and testosterone decline (Laughlin et al., 2000). Similarly, following surgical ovary-removal in rats, circulating estrogens and progesterone fall to low levels (Wise and Ratner, 1980). In both species, the sudden loss of ovarian hormones is also associated with memory impairments (Acosta et al., 2013; Henderson and Sherwin, 2007; Rocca et al., 2011). Further, stroke risk is higher among oophorectomized women who underwent surgical menopause prior to 40 years of age relative to women who underwent natural menopause during their early fifties (Baba et al., 2010), and stroke outcome is poorer among Ovx rodents compared to intact controls, at least in young adult animals (Leon et al., 2012). It is noteworthy that the Ovx methodological approach dramatically reduces levels of numerous other sex hormones including progesterone (Wise and Ratner, 1980), creating a background of minimal estrogen and progesterone hormone levels with which to assess the unique impacts of hormone loss or treatment. However, Ovx also initiates dramatic shifts in circulating gonadotropin levels (Wise and Ratner, 1980), an important consideration in the interpretation of findings using this model.
Despite the similarity in ovarian hormone profiles following surgical ovary removal in women and rats, an important limitation of this model is that most women do not undergo surgically-induced menopause. Indeed, the majority of women experience transitional menopause, in which follicles deplete and hormone levels change over many years, while only small portion of women experience oophorectomy (Timiras et al., 1995). This begs the question, why do we not use the intact aging female rat as a model of human menopause? The answer to this stems from differences in the mechanisms and the trajectory of spontaneous, transitional reproductive senescence in the two species. In women, the depletion of the ovarian follicles ultimately induces reproductive senescence (Neal-Perry et al., 2010). Conversely, in female rodents, the ovaries remain capable of reproduction given that transfer of an ovary from an aged donor rat to a young female recipient can still result in the support of normal cyclicity and the maintenance of viable pregnancies (Peng and Huang, 1972). Instead, the proposed mechanism of female rodent reproductive senescence is dysregulation of the hypothalamic-pituitary-gonadal axis to respond to 17βE2 positive feedback. Indeed, Selmar Aschheim's 1964 seminal findings revealed that transplant of a young adult ovary into an aged rats failed to restore cyclicity (Neal-Perry et al., 2010). Similarly, transplant of hypothalamic nuclei from an aged rat into a young recipient disrupted normal cyclicity in these younger animals (Peng and Huang, 1972). This age-related hypothalamic dysregulation results in highly irregular cycles consisting of either constant estrus or persistent diestrus states, characterized by moderate levels of 17βE2 and high levels of progesterone, respectively (Lu et al., 1979; Wise and Ratner, 1980). Therefore, although evidence suggests that alterations in hypothalamic-pituitary-gonadal axis feedback mechanisms are early mediators of the menopausal transition in both women and rodents (Neal-Perry et al., 2010), the fundamental differences in follicular content and ovarian functional capacity during aging limit the utility of the ovary-intact aging rodent in studies investigating the aging process. However, new rodent models of transitional human menopause have been recently developed that selectively deplete ovarian follicles (Mayer et al., 2005) and have important implications for the field of women's health (see Section 7.2).
4. Mechanisms of estrogen neuroprotection
Estrogens have been shown to impact a number of distinct cell types, neuronal signaling cascades, and nervous system substrates associated with cognitive aging, injury, and disease (Prokai and Simpkins, 2007). Thus the mechanism behind estrogen's neuro-protective effects is most likely a multifactorial combination of diverse neurobiological and signaling impacts. As well, the source of estrogen may play an important role in neuroactivity and protection from injury as estrogens are derived not only from ovary in the periphery but also can be synthesized from cholesterol de novo or via the action of aromatase in various brain regions, such as the hippocampus, and by several neural cell types, including neurons and astrocytes (Li et al., 2014). It has been hypothesized that much of estrogen's protective actions in the brain following injury may be due to not to peripherally-derived estrogens but to estrogens synthesized within the CNS (Fester and Rune, 2014; Zhang et al., 2014), and this hypothesis has been reviewed elsewhere (Arevalo et al., 2015). The major neuroactive effects of estrogens are discussed here.
4.1. Cerebral microvasculature and blood–brain barrier
Compromised cerebral microvascular function impairs the integrity of the blood–brain barrier (BBB). The cerebral microvasculature, and by extension, the BBB, is a major target of estrogen action. ERα, ERβ, and GPER1 are expressed on brain endothelial cells, although most of the physiological activity of 17βE2 is mediated through ERα (Duckles and Krause, 2011; Spary et al., 2009). Estrogens exert multiple protective actions at the cerebrovasculature by increasing vasodilation, decreasing vascular inflammation, and enhancing mitochondrial function. Acting through both genomic and non-genomic mechanisms, 17βE2 also increases expression of endothelial nitric oxide synthase (eNOS) which promotes increased vasodilation through enhanced nitric oxide availability in cerebral tissues (Duckles and Krause, 2011).
The protective actions of 17βE2 in brain endothelial cells is extended to the BBB, the dynamic interface that permits the passage of small molecules and limits entry of immune cells and larger inflammatory molecules into the brain parenchyma. The permeability of the BBB may be modulated through both ERα and ERβ (Bake and Sohrabji, 2004; Brown et al., 2010; Cipolla et al., 2009). 17βE2 also plays an important role in mediating leukocyteendothelial interations. It decreases messenger ribonucleic acid (mRNA) expression of the proinflammatory endothelial molecules e-selectin, ICAM-1, and VCAM-1 (Nakagami et al., 2010). Further, tight junction (TJ) proteins are critical for maintaining the structure and integrity of the vascular endothelial membrane in capillaries but play a less important role at postcapillary venules (Bechmann et al., 2007). The TJ proteins occludin and claudin-5 are also regulated by 17βE2 at the mRNA and protein level (Bake et al., 2009; Burek et al., 2010).
4.2. Mitochondrial function
several lines of evidence support a potential role of mitochondria in the neuroprotective effects of estrogens (Simpkins and Dykens, 2008). Long ago, estrogen was shown to bind to components of the mitochondria, including the F0/F1 ATPase (Zheng and Ramirez, 1999a,b) and more recently, we have shown ER localization to the mitochondria (Yang et al., 2004). Estrogens also influence anti-apoptotic proteins (Nilsen and Brinton, 2003; Pike, 1999; Singer et al., 1998; Wise et al., 2000; Yang et al., 2004; Zhao et al., 2004), which act on mitochondria such that they increase the production of adenosine triphosphate (ATP) under conditions of cellular stress (Wang et al., 2001, 2003a, 2006). With inhibition of ATP production by 3-nitropropionic acid (3NPA), a succinate dehydrogenase inhibitor that uncouples oxidative phosphorylation, estrogens reduce ATP decline. Similarly, H2O2 caused a dose- and time-dependent decline in ATP production (Wang et al., 2003b, 2006) by compromising mitochondrial oxidative phosphorylation. 17βE2 ameliorated the H2O2-induced decline in cellular ATP (Wang et al., 2003b, 2006). More recently, we assessed the effects of estrogens on the inhibition of mitochondrial function induced by β-amyloid oligomers (Sarkar et al., 2015). We demonstrated that oligomeric β-amyloid caused a fission of mitochondria, slowed their movement, and reduced oxidative phosphorylation; all of these effects of oligomeric β-amyloid were ameliorated by 17βE2. Under glutamate stimulation, estrogens enhance Ca2+ flux into cells (Nilsen et al., 2002; Zhao et al., 2004), an effect that may be involved in estrogen's ability to increase memory function through an N-methyl-d-aspartate (NMDA) receptor-mediated mechanism (Diaz Brinton, 2001; Foy et al., 1999). Estrogens also potentiate Ca2+ influx through L-type Ca2+ channels (Sarkar et al., 2008). However at high glutamate stimulation, estrogens reduce mitochondrial influx of Ca2+ (Nilsen and Brinton, 2003; Nilsen et al., 2002; Wang et al., 2006).
Estrogens also protect mitochondria Ca2+ from other stressors. 3NPA caused a rapid and profound increase in cytosolic Ca2+ concentrations (Wang et al., 2001). Estrogens reduced the influx of Ca2+ into the cytosol and mitochondria as a result of 3NPA treatment. Similarly, cytosolic and mitochondrial Ca2+ levels were reduced by 17βE2 when H2O2 was used as a pro-oxidant (Wang et al., 2006). Since sustained increases in mitochondrial Ca2+ impair oxidative phosphorylation, these observations indicate that the Ca2+ modulating effects of estrogens protects ATP production, and as a result, neuronal viability. The role of estrogenic mitochondrial actions in neuroprotection can be determined by the correlation between the potency of compounds in assays of mitoprotection and neuroprotection. A strong correlation between these two parameters supports the role of mitochondria in neuroprotection. We tested the correlation between the neuroprotective activity of estrogens and Δψm collapse induced by Ca2+ loading in a neuronal cell line. Ten estrogen analogs with neuroprotective potency (ED50) of 20 nM to 8.6 μM were compared (Dykens, 1995). The correlation between ED50 values for neuroprotection and the ED50 values for Ca2+-induced Δψm collapse were highly correlated (r2 = 0.73, Spearman r = −0.9387, p < 0.0001) (Dykens et al., 2003), suggesting a strong relationship between these two parameters.
4.3. Anti-inflammatory actions
The neuroprotective and anti-inflammatory actions of estrogens in early ischemic stroke and TBI are not mutually exclusive. The pleiotropic effects of estrogens and ERs across multiple cell types within the brain makes it difficult to dissociate these mechanisms from each other. Nevertheless, it is clear that physiological concentrations of 17βE2 exhibit dramatic anti-inflammatory activity in the CNS when administered to young mice. Whether the anti-inflammatory effects of 17βE2 persist following reproductive senescence (in rodents) or menopause (in humans) is under intense investigation. The majority of studies suggest that these effects do not persist in older female rodents (Leon et al., 2012; Sohrabji et al., 2013b; Strom et al., 2011). In addition to the loss of the anti-inflammatory properties of 17βE2 in young, Ovx mice, recent studies strongly suggest that the anti-inflammatory effects of 17βE2 are lost during a period of prolonged hypoestrogenicity in middle-aged (Suzuki et al., 2007a), or reproductively senescent (Selvamani and Sohrabji, 2010a; Sohrabji et al., 2013a), mice. 17βE2 also suppresses a systemic post-stroke immunosuppression phenotype in animal models that closely mimics a peripheral immunosuppressive phenotype seen in human patients (Ritzel et al., 2013; Zhang et al., 2010).
Several excellent reviews address cell type-specific anti-inflammatory mechanisms of 17βE2 in microglia (Habib and Beyer, 2015; Vegeto et al., 2008), astrocytes (Acaz-Fonseca et al., 2014), endothelial cells (Sohrabji et al., 2013a), and oligodendrocytes (Arevalo et al., 2010) during neurological injury. In young and middle-aged preclinical animal models of stroke, 17βE2 inhibits the activation of the pro-inflammatory transcription factor, nuclear factor-κβ, which induces transcription of numerous cytokines such as tumor necrosis factor-α (TNFα), chemokine ligand 2 (CCL2), interleukin-6 (Vegeto et al., 2008). In contrast to its effects on eNOS in the brain cerebral microvasculature, 17βE2 also decreases expression of inducible NOS (iNOS), which produces nitric oxide as part of the innate inflammatory response (Garry et al., 2015). Some of the anti-inflammatory actions of 17βE2 in ischemic stroke are derived, in part, through interations with iNOS. In the permanent (pMCAO) middle cerebral artery occlusion (MCAO) model of ischemic stroke, Ovx iNOS null mice exhibited smaller infarct volumes than their wild type (WT) counterparts (Brown et al., 2008) but Ovx mice were afforded no additional protection with 17βE2 replacement (Brown et al., 2008; Park et al., 2006). More recent studies suggest that 17βE2 administration prior to transient MCAO (tMCAO) suppresses activation of the inflammasome, a multiprotein intracellular complex that coordinates the innate immune response, in male mice (Slowik and Beyer, 2015).
4.4. Free-radical scavenging
Estrogens exert anti-oxidant effects (Ayres et al., 1996; Miller et al., 1996; Mooradian, 1993; Romer et al., 1997a,b; Sawada et al., 1998; Tang et al., 1996) but are comparatively poor scavengers of reactive oxygen species (ROS). For neurons exposed to H2O2, 17βE2 is ineffective in reducing cellular ROS levels as measured by general ROS dyes. However, 17βE2 is effective in preventing the production of ROS induced by 3NPA treatment (Wang et al., 2006). The observation that estrogens are potent in preventing ROS production led us to investigate their role in inhibition of lipid peroxidation. In neuroprotection assays in vitro, we showed that estrogens interact with the abundant aqueous soluble anti-oxidant, glutathione (Green et al., 1998; Gridley et al., 1998). Also since estrogens have a log P of about 3, they reside in the membrane component of cells (Liang et al., 2001), where they could prevent oxidation of phospholipids. Estrogens then may interrupt lipid peroxidation chain reactions using a major source of cellular reducing potential, such as glutathione or nicotinamide adenine dinucleotide phosphate (NADPH). We described estrogen conversion to a quinol product that was able to be reduced back to the parent estrogen in the presence of NADPH (Prokai et al., 2003a,b).
4.5. Synaptic and structural plasticity
Estrogen has known impacts on measures of plasticity within the CNS and this may represent an important mechanism by which estrogens can impact cognitive function. For instance, dendritic spine morphology and number are known to change following learning or long-term potentiation (LTP; Bliss et al., 2007); Woolley and colleagues first established that shifts in endogenous estrogen levels across the estrous cycle impacted dendritic architecture complexity in the cornu ammonis 1 (CA1) region of the hippocampus (Woolley et al., 1990). This same group later showed that hormone loss reduced spine number, and subsequent treatment with 17βE2 reversed this loss (Woolley and McEwen, 1992), an effect that was mediated by an NMDA receptor-dependent mechanism (Woolley and McEwen, 1994; Woolley et al., 1997), further supporting the ability of estrogen to modify hippocampal structure. As well, enhanced LTP has been noted in cycling females during the proestrous stage, when estrogen levels are high (Good et al., 1999; Warren et al., 1995), and chronic 17βE2 treatment attenuates the disruptive effect of hormone depletion on long-term depression (Day and Good, 2005). Further, estrogen appears to modulate LTP through interaction with ERβ (Liu et al., 2008), which are localized within hippocampal axons, dendrites, and dendritic spines (Milner et al., 2001, 2005). More specifically, Liu et al. (2008) demonstrated that there were significant increases among synaptic plasticity marker proteins PSD-95 and GluR1 in Ovx wild type animals that received the ERβ agonist, WAY-200070. Interestingly, these changes were not shown among Ovx WT mice that received the ERα agonist nor in ERβ knockout (KO) mice. As well, hippocampal slices treated with WAY-200070 enhanced LTP when slices were from WT but not ERβ KO, female mice.
In addition to modifying synaptic architecture within a set of neurons, it appears that estrogen can modify structural plasticity to influence brain function. Neurogenesis, the process of creating new neurons, is indispensable in early brain development and to adult brain function. Previously, neurogenic activity was thought to be limited to early critical periods of neuronal development and 17βE2 appears to have a role in this process. Indeed, 17βE2 promotes neuritogenesis in developing hippocampal neurons via a GPER-dependent mediated mechanism (Ruiz-Palmero et al., 2013). However, numerous laboratories have clearly demonstrated that neurogenesis also occurs in the subventricular zone (SVZ) and dentate gyrus (DG) in rodent and human adult brain (reviewed in Aimone et al., 2014). In the adult brain, sex hormones have been found to influence the number of new neurons present in the hippocampal formation (Gould, 2007). Seminal findings from the Gould laboratory noted dramatic fluctuations in proliferation of progenitor cells with changes in endogenous estrogen levels across the cycle (Tanapat et al., 1999). Hormone depletion significantly reduces proliferating neuron number and 17βE2 treatment can reverse this if administered near the time of Ovx; this appears to occur through both genomic ER subtypes (Barha et al., 2009b; Ormerod et al., 2003; Suzuki et al., 2007b; Tanapat et al., 2005).
4.6. Impacts on the cholinergic neurotransmitter system
Estrogens may alter cognitive outcomes by their actions on distinct neurotransmitter systems known to be involved with cognition. The basal forebrain cholinergic system is important for learning and memory and is susceptible to age-related changes (for review, see Gibbs, 2010). For example, in aged female rats with working memory impairments, less choline acetyltransferase (ChAT) protein activity was found in the basal forebrain, relative to younger counterparts (Luine and Hearns, 1990), suggesting that lower levels of ChAT activity are associated with worse memory performance during aging. 17βE2 seems to beneficially impact the basal forebrain cholinergic system, as well as cognitive performance. In adult Ovx rats, 17βE2 treatment increased ChAT protein activity and ChAT immunoreactive cell counts in distinct basal forebrain subregions (Gibbs, 1997). Further, evidence from Gibbs’ laboratory suggests that not only did 17βE2 enhance memory performance but that the beneficial effects of 17βE2 treatment on cognition require a functioning basal forebrain cholinergic system. Indeed, 17βE2 was ineffective at improving cognition in animals with basal forebrain lesions, and enhanced memory only in nonlesion controls (Gibbs, 2002, 2007). Cholinergic projections to hippocampus are also involved with the memory enhancing effects of 17βE2 (Fader et al., 1998, 1999; Packard, 1998). In addition to 17βE2, other estrogenic formulations are known to impact the cholinergic system. Indeed, CEE treatment in middle-aged Ovx rats increased basal forebrain ChAT-immunoreactive neuron counts and concomitantly aided spatial memory and retention (Acosta et al., 2009b). Yet, interestingly, the primary circulating estrogen following CEE treatment, E1, which impaired memory performance, also failed to impact basal forebrain ChAT-positive cell counts (Engler-Chiurazzi et al., 2012). Thus, these findings suggest that the basal forebrain cholinergic system may be a crucial component of the cognitive neuroprotection afforded by some, but not all, estrogens.
4.7. Cellular maintenance and survival
Neurotrophins may be one mechanism of estrogen-induced neuroprotection or mnemonic changes. Survival and maintenance of neurons are dependent upon neurotrophins, including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF; Davies, 1996; Granholm, 2000). Age-related neurotrophin changes have been reported in animal models, and NGF and BDNF have been associated with cognitive function (Bimonte et al., 2003; Granholm, 2000; Hall et al., 2000; Kesslak et al., 1998). 17βE2 treatment significantly impacts neurotrophin systems in young and aged Ovx rats, increasing neurotrophin and its receptor mRNA levels in basal forebrain, frontal cortex, and hippocampus (McMillan et al., 1996; Pan et al., 1999; Singh et al., 1995; Sohrabji et al., 1995) as well as elevating NGF and BDNF protein levels in cognitive brain regions (Bimonte-Nelson et al., 2004a,b). Moreover, there is mounting evidence from the Sohrabji group that estrogen imparts neuroprotective action via interations with insulin-like growth factor (IGF) receptor (Sohrabji and Williams, 2013). Indeed, in young animals, blockade of IGF receptors obviates the protective actions of 17βE2 against endothelin-1 (ET-1) induced stroke damage (Selvamani and Sohrabji, 2010b). Interestingly, it appears that protective actions of IGF are dependent on an estrogen rich hormonal milieu as among aged animals, IGF was only effective at reducing infarct volume in Ovx animals that had been treated with 17βE2, but not in Vehicle treated animals. As well, it has been hypothesized that both estrogen and microglia-derived IGF act synergistically to promote cellular health in the context of the injured brain (Sohrabji and Williams, 2013).
In addition to modulating the growth factor system, 17βE2 is also known to modulate cell death cascades (Alkayed et al., 2001; Zhang and Bhavnani, 2006). Apoptosis and necrosis are the two primary mechanisms of neuronal cell death during neurological injury, although it is likely that other cell death mechanisms including autophagy are equally important (Gabryel et al., 2012). The anti-apoptotic effects of estrogens during ischemic stroke and TBI are well characterized in preclinical animal models. Numerous laboratories have shown that physiological concentrations of 17βE2 exert anti-apoptotic actions in the infarct penumbra but not in the infarct core, demonstrating that 17βE2 protects against delayed cell death but not immediate cell death in ischemic injury. Most apoptotic mechanisms implicated in stroke and TBI are either caspase-dependent or caspase-independent. Early studies showed that low levels of 17βE2 upregulate the cell survival factor bcl-2, an upstream inhibitor of caspases, in ischemic injury (Alkayed et al., 2001; Dubal et al., 1999). Data from several labs suggests that one anti-apoptotic mechanism utilized by 17βE2 is the suppression of activated caspase-3 during cerebral ischemia (Dubal et al., 2006; Harms et al., 2001; Jover et al., 2002; Rau et al., 2003; Soustiel et al., 2005). The specific pathways leading to caspase-3 activation are complex. The actions of caspase-3, a prototypical effector caspase, are regulated by the actions of two initiator caspases, caspase-8 and caspase-9. Caspase-8 activation is a hallmark of the extrinsic death receptor-mediated pathway, while caspase-9 activation is a hallmark of the intrinsic mitochondrial-cytochrome death pathway (Budihardjo et al., 1999; Zhang et al., 2004). Studies from the McCullough laboratory have implicated an important sex-specific mechanism for caspase-mediated effects of estrogens in ischemic stroke. Interestingly, caspase-dependent cell death mechanisms predominate in female mice while caspase-independent mechanisms are preferentially utilized in male mice (Koellhoffer and McCullough, 2013; Liu et al., 2009).
5. Estrogen and cognitive neuroprotection
5.1. What is cognition and how is it assessed in the human versus the rodent?
Cognition is an exceedingly large umbrella term used to describe the higher order neural processing and behavioral output that occurs within an organism in response to a given stimulus. Some of these responses may be simple, almost reflexive (such as the aversive avoidance withdrawal), and can be tested with ease in multiple experimental models spanning the entirety of the evolutionary totem pole. However, many responses are exceptionally complex and require the coordinated efforts of several neural systems in order to generate an appropriate behavioral output. Given the high degree of homology and translatability between the everyday memory demands and mnemonic processing of humans and lower species, tests of learning and memory are commonly employed in preclinical evaluations of hormone effects on cognition. The demonstration of learned content by an organism is contingent upon a variety of factors including the perception of, and attention to, the information/stimulus to be learned, the consolidation of this information from short-term to long-term memory, and the eventual retrieval and recall of the necessary information when required. Estrogens are known to impact multiple aspects of this complex mnemonic system.
A number of tests with a high degree of human translational validity are employed in preclinical studies assessing learning and memory in rodents (Rodriguiz and Wetsel, 2006). Tests assessing hippocampal-dependent spatial navigation memory, a form of declarative memory that involves the ability to learn to utilize and remember distal landmarks that are associated with obtaining a reward and/or avoiding an aversive stimulus within a complex environment (Eichenbaum, 2000), are common in the field of rodent cognition. The ability to accurately navigate through space is crucial for the survival of all organisms, and performance on these tasks has been shown to be similar in both humans and rodents (for example, see Mennenga et al., 2014). Many tests, such as the Morris water maze (MM), assess hippocampal-dependent spatial reference memory (Morris et al., 1982), memory for information that remains consistent across time (Olton, 1979). As well, the spatial working memory system facilitates memory for information that changes across time, and must be updated, manipulated, and kept in a readily available state (Baddeley, 2010; Jarrard et al., 1984; Olton, 1979). In addition, tests of novelty recognition are often employed given their relative ease of implementation and established use in the field (Ennaceur, 2010). Finally, fear-mediated memory can be assessed through the use of painful stimuli associated with a certain context, such as the active/passive avoidance, or contextual fear conditioning, paradigms (Maren, 2001). Many of these mnemonic processes are known to decline with increasing age and are impacted by estrogens in both species (Rodefer and Baxter, 2007), making them useful for the evaluation of estrogen effects in an aging preclinical model.
5.2. Effects of endogenous estrogens on cognitive function
Among humans, several lines of evidence support the impact of estrogen on cognition. Pronounced and reliable sexual dimorphisms in cognitive performance on specific functional domains established unique roles for the distinct sex hormones in cognition (reviewed in Kimura, 2002). For instance, men exhibit a clear advantage in visuospatial memory whereas women outperform men in tasks of verbal fluency and verbal memory (Bleecker et al., 1988; Galea and Kimura, 1993; Vandenberg and Kuse, 1978). Interestingly, cognitive performance among women is not static but fluctuates dramatically with changing levels of endogenous estrogens. For instance, women experiencing their menses, when estrogen levels are low, tend to display better performance on tasks typically associated with a male advantage (Hampson, 1990). Similarly, peri- and post-menopausal women show impaired memory scores, especially when compared to pre-menopausal women (Farrag et al., 2002; Greendale et al., 2010), with abrupt hormone loss via surgical oophorectomy prior to the age of natural menopause exacerbating cognitive decline (Nappi et al., 1999; Rocca et al., 2007). As well, some, but not all, studies note an association between worse memory performance and lower circulating estrogen levels among aging women (Lebrun et al., 2005; Wolf and Kirschbaum, 2002; but see Almeida et al., 2005; Barrett-Connor and Goodman-Gruen, 1999; Henderson et al., 2013). Thus, converging data suggest that endogenous hormones can impact mnemonic function and indicate that low levels of circulating estrogens is not optimal for cognition. Mirroring the results of experiments in humans, findings from the animal literature also support the notion that estrogen impacts cognitive outcomes. Males outperform females on tasks of spatial navigation memory (Luine and Rodriguez, 1994) and fluctuations in endogenous circulating sex hormone levels among females may moderate this effect. Some rodent studies have reported more accurate reference memory performance on the MM among female animals in estrus (Warren and Juraska, 1997), a phase of the estrous cycle when circulating 17βE2 levels tend to be low relative to other stages (Lerner et al., 1990). Yet, enhanced reference memory performance has also been found during the proestrous phase when circulating estrogen levels surge (Frick and Berger-Sweeney, 2001). Still others have reported no alterations in working and reference memory performance across any estrous cycle stage (Berry et al., 1997; Stackman et al., 1997). Further supporting the notion of hormone-related changes in cognitive performance, age-related declines in spatial memory tend to emerge between 12 and 18 months of age (Markowska, 1999) when female rodents undergo the estropausal transition associated with increases in circulating gonadotropins, declines in estrogen levels, and dysregulation of progesterone and androgens (Lu et al., 1979). As is the case in women, surgical depletion of endogenous circulating sex hormones via Ovx in the rodent is associated with impairments on cognitive outcomes when compared to intact, cycling controls (Bimonte and Denenberg, 1999; Daniel et al., 1999; Feng et al., 2004; Talboom et al., 2008; Wallace et al., 2006).
5.3. Cognitive effects of exogenous estrogen treatments
Additional support for the beneficial effect of estrogen comes from findings of studies in which exogenous estrogen administration appears to ameliorate the cognitive deficits associated with hormone loss in both humans and rodents. In an elegantly designed study, Sherwin (1988) noted maintained verbal memory scores among women who received 17βE2 HT following surgical removal of the ovaries while those women who received vehicle following oophorectomy surgery showed declined performance across time. As well, treatment of post-menopausal women with Alzheimer's Disease with transdermal 17βE2 improved performance on tasks of attention and verbal memory (Asthana et al., 1999). Other findings, including case studies (Ohkura et al., 1995), non-randomized quasi-experimental designs (Carlson and Sherwin, 1998) and small double-blind, placebo controlled studies (Campbell and Whitehead, 1977), also support a protective role for the most commonly-prescribed estrogen-containing menopausal treatment, CEE. However, several notable reports have found null or even detrimental cognitive effects following the administration of HT. For instance, findings from the now controversial WHI Memory Study (WHIMS) showed that CEE treatment yielded a non-significant increased incidence of probable dementia and mild cognitive impairment in women 65 and over (Espeland et al., 2004; Shumaker et al., 2004). Further, there was an elevated probable dementia risk, and no effect on mild cognitive impairment, in women with an intact uterus and ovaries taking Prempro® (CEE + medroxyprogesterone acetate (MPA); Shumaker et al., 2003). Thus, disagreement of clinical findings regarding the neuroprotection afforded by estrogen-containing treatments has resulted in uncertainty among medical practitioners and patients regarding the effectiveness of estrogen for protection against cognitive aging and dementia.
17βE2 is the most commonly tested estrogen for cognition in the rodent, and a plethora of evidence supports the notion that treatment with this estrogen enhances learning and memory (Table 1). In young adult animals, studies assessing the effects of treatment with 17βE2 following Ovx note beneficial effects on tasks of novel object recognition and object location memory (Gresack and Frick, 2004; Lewis et al., 2008; Luine et al., 2003), aversive avoidance memory (Foster et al., 2003; Simpkins et al., 1997a; Singh et al., 1994), and spatial working and reference memory (Bimonte and Denenberg, 1999; Daniel et al., 1997; El-Bakri et al., 2004; Gibbs, 1999; Hruska and Dohanich, 2007; Luine and Rodriguez, 1994; Talboom et al., 2008). Interestingly, the effects of exogenous administration of estrogens seem to be modulated, at least in part, by the age of the animal at the time of treatment. Indeed, converging data suggest that cognitive responsiveness to estrogen stimulation declines with age (Foster et al., 2003; Gresack et al., 2007; Talboom et al., 2008). For instance, the same dose of 17βE2 treatment that effectively enhanced performance on the MM among 4 and 16 month old, Ovx rats was generally ineffective in 24 month olds (Talboom et al., 2008). It is known that ER distribution changes with age in both aging women and Ovx rats (Adams et al., 2002; Mehra et al., 2005; Waters et al., 2011; Yamaguchi-Shima and Yuri, 2007) and it has been hypothesized that changes in ER ratios during aging may account for this reduced receptivity to estrogen treatment (Foster, 2012). However, some studies still report benefits of 17βE2 administration in aged rodents (Frick et al., 2002; Markowska and Savonenko, 2002), suggesting that other factors may interact to influence the realization of cognitive benefits with 17βE2 (see Section 7).
Table 1.
Effects of exogenous estrogen treatment on cognition in ovariectomized female rodents.a
| Estrogen | Reference | Age (months) | Treatment regimen | Dose | Cognitive outcome | Cognitive effect |
|---|---|---|---|---|---|---|
| 17βE2 | Luine et al., 2003 | 2 | Acute injection | 15 μg/kg | OR, OP | Enhanced |
| Daniel and Dohanich, 2001 | 2 | Acute injection | 10 μg | RAM | Enhanced | |
| Gresack and Frick, 2004 | 6 | Cyclic daily injections | 0.1 mg/kg | Water RAM, OR | No impact | |
| 0.2 mg/kg | Water RAM, OR | Enhanced | ||||
| Simpkins et al., 1997a,b | Adultb | Continuousc | Pellet | 2-way AA, MM | Enhanced | |
| Bimonte and Denenberg, 1999 | 2 | Continuousc | 10 mm capsule (0.025 in ID) | Water RAM | No impact | |
| 2 × 10mm capsules (0.025 in ID) | Water RAM | Enhanced | ||||
| Gibbs, 1999 | 5 | Continuousc | 3 mm capsule (0.058 in ID) | DMP + scopolamine challenge | Enhanced | |
| Daniel et al., 1997 | 2 | Continuousc | 5 mm capsule (0.058 in ID) | RAM | Enhanced | |
| Talboom et al., 2008 | 4 | Continuous | 0.25 mg; pellet | MM | Enhanced | |
| 16 | Continuous | 0.25 mg; pellet | MM | Enhanced | ||
| 24 | Continuousc | 0.25 mg; pellet | MM | Minor enhancement | ||
| Daniel et al., 2006 | 12 | Continuousc | 5 mm capsule (0.058 in ID) at time of Ovx | RAM | Enhanced | |
| 5 mm capsule (0.058 in ID) | RAM | No impact | ||||
| 5 months post-Ovx | ||||||
| CEE | Barha and Galea, 2013 | Adultb | Cyclic daily injections | 10μg/0.10ml | RAM | Impaired |
| 20 μg/0.10ml | RAM | No impact | ||||
| Walf and Frye, 2008 | 13 | Acute injection | 0.625 mg/kg | OR | Enhanced | |
| Acosta et al., 2009a,b | 13 | Cyclic daily injectionsd | 10 μg/day | MM, DMS | Enhanced | |
| 20μg/day | MM, DMS | Enhanced | ||||
| 30μg/day | MM, DMS | Enhanced | ||||
| Engler-Chiurazzi et al., 2011 | 13 | Continuous | 12 μg/day; pump | MM, DMS | Impaired | |
| 24 μg/day; pump | Water RAM, DMS | Enhanced | ||||
| 36 μg/day; pump | Water RAM, DMS | Enhanced | ||||
| E1 | Barha et al., 2009a,b | Adultb | Acute injection | 0.30 μg/.10ml | Contextual Conditioned Fear Response | No impact |
| 1 μg/.10ml | Contextual Conditioned Fear Response | Impaired | ||||
| 10 μg/0.1 ml | Contextual Conditioned Fear Response | No impact | ||||
| McClure et al., 2013 | Adultb | Cyclic daily injections | 10 μg/0.1 ml | MM | Impaired | |
| Engler-Chiurazzi et al., 2012 | 13 | Continuous | 2.6 μg/day; pump | DMS | No impact | |
| 4.0 μg/day; pump | DMS | No impact | ||||
| 8.0μg/day; pump | DMS | Impaired | ||||
| 17αE2 | Luine et al., 2003 | 2 | Acute injection | 15 μg/kg | OR, OP | Enhanced |
| Barha et al. 2009 | Adultb | Acute injection | 0.30 μg/.10ml | Contextual conditioned fear response | Enhanced | |
| 1 μg/.10ml | Contextual conditioned fear response | Impaired | ||||
| 10 μg/0.1 ml | Contextual conditioned fear response | Impaired |
Note: All outcomes described are compared to Ovx animals given vehicle.
See list of abbreviations for tests used.
Weights, but not ages, were provided.
Subcutaneous pellets or Silastic capsules were used. Doses listed, when provided in each reference, were based on approximate circulating estrogen levels after release. ID refers to inner diameter.
Injection regimen consisted of two days of treatment followed by two days off.
One strategy to optimize estrogenic HTs for cognitive outcomes has been through the methodical modulation of ER stimulation. Findings from several studies have indicated a role for specific ERs in memory performance. For instance, Rissman et al. (2002) found that 17βE2 impaired learning on the MM in ERβ KO mice compared to estrogen-treated WT controls. Similarly, ERβ KO mice given 17βE2 were impaired on the Y-maze, exhibiting a lower percentage of trials without an error than WTs and ERα KO mice receiving the same treatment (Liu et al., 2008), further supporting the requirement of ERβ, and but not ERα, for 17βE2-induced spatial memory enhancements. Interestingly, other findings highlight the importance of ERα in memory function. Foster et al. (2008) used a lentiviral vector to restore ERα expression in adult Ovx, ERα KO mice, finding that increased ERα expression in these animals enhanced spatial reference memory MM performance compared to that of ERα KO controls. Studies using selective ER modulators (SERMs) as tools to evaluate the impact of ER stimulation in young adult rats have also imparted mixed mnemonic effects. For instance, there is disagreement regarding the impact of SERMs on object memory, with some studies reporting that both propylpyrazole triol (PPT; ERα agonist) and diarylpropionitrile (DPN; ERβ agonist) enhance performance, and others reporting that either DPN or PPT, but not both, impart benefits (Frye et al., 2007; Jacome et al., 2010; Walf et al., 2006). As well, findings on spatial memory tasks are also inconsistent. For example, on the MM, DPN benefitted, while PPT failed to impact, spatial reference memory performance (Rhodes and Frye, 2006). Conversely, PPT, DPN, and 17βE2 each enhanced spatial working memory performance on the delayed match to position (DMP) task (Hammond et al., 2009). Among middle aged animals, PPT was associated with delayed alternation impairments (Neese et al., 2010), even though Witty et al. (2012) found that lentiviral-induced increases in hippocampal ERα expression benefitted radial arm maze (RAM) working memory. Thus, the conflicting findings from these studies indicate that the relationship between memory outcomes, ERs, ER ligand stimulation, and aging is complex and requires further investigation to uncover clinical applications for ER-targeted interventions.
6. Estrogen neuroprotection in brain injury
6.1. Estrogen and ischemia
Afflicting nearly 795,000 people per year and associated with an annual total cost of 34 billion US dollars, stroke is the fifth leading cause of death and the leading cause of long-term disability in the United States. Similarly, stroke is the second-leading cause of death worldwide, accounting for 11% of all deaths globally (Mozaffarian et al., 2015). Broadly defined, stroke is a failure of the supply of oxygen and glucose to neurological tissues. Accounting for 87% of stroke cases, ischemic stroke is caused by a blockage of a cerebral artery, resulting in a loss of blood flow to the brain area supplied by that artery. The phenotype of functional consequences can be diverse and the success of medical recanalization interventions, such as chemical or mechanical endovascular therapy, are important predictors of outcome. While surgeons typically employ a variety of tools to physically disrupt or remove a clot, only one pharmacological option exists, recombinant tissue plasminogen activator (tPA) (Mozaffarian et al., 2015; Rouchaud et al., 2011). Although proven effective, the therapeutic window of tPA administration is exceptionally short, due to the unacceptable increased risk of cerebral hemorrhage when given after that time point (Gurman et al., 2015).
6.1.1. Clinical studies
Sex differences in stroke are well documented. Although younger women have a lower incidence of ischemic stroke than young adult men, the sex difference shifts in older cohorts such that post-menopausal women have an equivalent or higher incidence of stroke than their age-matched male counterparts (Reeves et al., 2008). Women are affected by 40,000 more strokes annually than men, and represent approximately 56% of the 6.8 million stroke survivors in the United States. In addition, women are more likely to be older at the time of first stroke and have a lower quality of life post-stroke than their male counterparts, even after adjusting for other sex-specific variables (reviewed in Bushnell and McCullough, 2014; Gibson, 2013). Indeed, results of the Framingham Heath Study indicated that women were older at the time of stroke, have more comorbid disease at time of stroke, and tend to have more severe strokes with worse outcomes (Petrea et al., 2009). Of the many modifiable risk factors for stroke, including hypertension, history of smoking, diabetic status, and physical activity, the primary non-modifiable risk factor for stroke is increased age (Goldstein et al., 2011). Another non-modifiable risk factor for stroke women is the onset of menopause. The stroke risk for women doubles approximately 10 years post-menopause. Menopause is also associated with an increased risk of the modifiable risk factors listed above. Thus, large shifts in endogenous sex hormone levels, as well as prior use of estrogen-containing therapies, appear to be additional risk factors that contribute to the female disadvantage in stroke risk (Lisabeth and Bushnell, 2012).
Few clinical studies have examined the relationship between HT and stroke risk as a primary outcome. Most clinical studies that have investigated the relationship between HT and cardiovascular disease have focused on prevention of coronary heart disease rather than stroke, and the overall evidence suggested no benefit of HT on stroke risk (Henderson and Lobo, 2012; Paganini-Hill, 2001). The Women's Estrogen for Stroke Trial (WEST) addressed stroke risk as the primary outcome in women with an intact uterus who had a history of prior ischemic stroke or transient ischemic attack. Results from the WEST showed that 17βE2-based HT had no effect on recurrent stroke after a 2.8 year follow-up (Viscoli et al., 2001). In contrast, stroke risk was a secondary outcome in the WHI. The CEE-alone trial of the WHI reported an increased risk (30%) of ischemic, but not hemorrhagic, stroke in women who received CEE compared to placebo. This risk corresponds to an additional nine cases of stroke per 10,000 person-years of HT use (Anderson et al., 2004; Henderson and Lobo, 2012; Hendrix et al., 2006).
Over the past 10 years since the termination of the WHI, several studies have re-examined the major findings of the WHI to focus on the group of women in the WHI who were 50–59 years of age when they enrolled in the WHI, an age group that represents the time frame of the menopausal transition. In one secondary analysis of the WHI, Roussow and colleagues showed that the youngest group of women in the CEE arm of the study did not have an increased risk of stroke (Rossouw et al., 2007). In the WHIMS-Y study, CEE administration to women aged 50–55 at the beginning of the study sustained neither risk nor benefit to cognitive function (Espeland et al., 2013). Current clinical guidelines recommend against the use of any kind of HT for primary or secondary prevention of stroke. The guidelines also emphasize that signifi-cant gaps persist in our understanding of the benefits and harms of HT, particularly with younger women who are in the early peri-menopausal and post-menopausal periods (Bushnell and McCullough, 2014; Bushnell et al., 2014).
6.1.2. Preclinical studies
The search for therapeutic agents to treat stroke remains elusive. A greater appreciation of the gender biology of stroke in preclinical studies will support this endeavor. In spite of the abundant epidemiological and physiological evidence for a sexual dimorphism in stroke, most preclinical studies in stroke have been performed in young, male rodents. We and others participated in a National Institute of Neurological Disorders and Stroke-sponsored workshop in 2006 to summarize the research gaps pertaining to stroke risk, with an emphasis on clinical and preclinical estrogen studies (Bushnell et al., 2006). Following the early termination of the CEE arm of the WHI in 2004, basic researchers sought to identify the reasons for the discrepancies between clinical and preclinical studies (Anderson et al., 2004). While much experimental evidence from rodent and non-human primate animal models of stroke, as well as in vitro models of ischemic injury, overwhelmingly demonstrated that estrogenic compounds were neuroprotective, the early termination of the estrogen-only arm of the WHI suggested otherwise.
Thus, it is critical that investigators identify the circumstances under which estrogen is beneficial and when it is harmful. Two of the basic science research recommendations from this workshop pertained to sex steroids: (1) define which stimuli precipitate stroke events, as well as the influence of sex steroids on these processes, and (2) delineate the mechanism by which sex steroids and their mimetics act as neurovascular protectants in both stroke and neurodegenerative disease models. Incorporating the physiological complexity of sex steroids like estrogen into clinically-relevant, age-appropriate animal models of stroke is paramount for implementation of a successful therapeutic agent for treatment of ischemic or hemorrhagic stroke. We review critical variables in rodent models of stroke that have contributed to our current perspectives on estrogen-mediated neuroprotection. Representative studies are summarized in Table 2. Since many preclinical studies were performed in rodents, this section will be limited to mouse and rat models of ischemic stroke. The reader is referred to the following reviews for a discussion of animal models of stroke in other species including nonhuman primates (Casals et al., 2011; Cook and Tymianski, 2012) and estrogen action in animal models of ischemic stroke (Carswell et al., 2010).
Table 2.
Effects of exogenous estrogen treatment on ischemic stroke outcome in ovariectomized female rodents.a
| Estrogen | Reference | Age (mo) |
Injury mechanism | Treatment regimen |
Dose | Stroke outcome |
Effect |
|---|---|---|---|---|---|---|---|
| 17βE2 | Simpkins et al., 1997a,b | Adultb | tMCAO 40 min + 24 h reperfusion | Acute injection 24 h pre-injury | 1 mg/kg | Infarct volume | Decreased |
| Dubal et al., 1998 | Adult | pMCAO, 24h | Continuous | 180 μg/ml; capsule | Infarct volume Ctx, Str | Region-dependent decrease | |
| 1 mg/ml; capsule | Infarct volume Ctx, Str | Region-dependent decrease | |||||
| Rusa et al., 1999 | Adult | tMCAO 120min + 22 h reperfusion | Continuous | 25 μg; pellet 100 μg; pellet |
Infarct volume Ctx Infarct volume Ctx |
Decreased No impact |
|
| Zhang et al., 1998 | Adultb | tMCAO 40min + 24 h reperfusion | Acute injection 40 min post-injury | 1 mg/kg | Infarct volume Ctx Mortality |
Decreased Decreased |
|
| Wang et al., 1999 | Adultb | tMCAO 30 min + 72 h reperfusion | Cyclic daily injections 2 wks pre-injury | 0.1 mg/kg | Percent cell loss Str, Hippo | Decreased | |
| Neurological score | Decreased | ||||||
| Yang et al., 2000 | Adultb | pMCAO | Acute injection 0.5-4 h post-injury | 100 μg/kg | Infarct volume | Decrease, up to 4 h post-injury | |
| Dubal et al., 2001 | Adultb | pMCAO, 24h | Continuous | 180 μg/ml; capsule | Infarct volume Ctx, Str, Hippo | Region-dependent decrease | |
| Horsburgh et al., 2002 | Transient global ischemia, 17 min | Continuous | 25 μg; capsule 250 μg; capsule |
Infarct volume Infarct volume |
Decreased Decreased |
||
| Pellet | Infarct volume | Decreased | |||||
| Li et al., 2004 | 2-3 | tMCAO 90 min + 2-6wk reperfusion | Continuous | 180 μg/ml; capsule | Cylinder Test | Improved | |
| Gordon et al., 2005 | pMCAO | Continuous | 25 μg; capsule | Infarct volume | Increased | ||
| Continuous | 250 μg; capsule | Infarct volume | Increased | ||||
| Continuous | Pellet | Infarct volume | Increased | ||||
| Miller et al., 2005 | 21 days | Transient global ischemia 10 min | Continuous | Pellet | Neuronal number Hippo | Increased | |
| Bingham et al., 2005 | 3 | pMCAO, electrocoagulation 24 h | Continuous | 0.025 mg; pellet | Neuronal perikaryal damage | Increased | |
| 0.25 mg; pellet | Neuronal perikaryal damage | Increased | |||||
| Selvamani and Sohrabji, 2010a,b | 6-7 | Endothelin-induced MCAO (ET-1), 7 days | Continuous | 1.0 mg; pellet | Infarct volume Ctx, Str | Region-dependent decrease | |
| 10-12 | Endothelin-induced MCAO (ET-1), 7 days | Continuous | 1.0 mg; pellet | Infarct volume Ctx, Str | Increased | ||
| Liu et al., 2012 | 17-22 | tMCAO 90 min + reperfusion | Continuous | 180 μg/ml; pellet | Infarct volume Ctx, Str | Decreased | |
| Acute | 180 μg/ml; pellet | Infarct volume Ctx, Str | No impact | ||||
| Leon et al., 2012 | 18 | tMCAO + tPA reperfusion | Continuous | 1.5 mg; pellet | Infract volume Ctx, Str | Increased | |
| Functional recovery | No impact | ||||||
| Strom et al., 2013 | Adultb | tMCAO 60 min+ 72 h reperfusion | Continuous | 180 μg/ml; capsule 180 μg/ml; capsule |
Infarct volume Functional recovery |
No impact No impact |
|
| 50,000 μg/ml; capsule | Infarct volume | No impact | |||||
| 50,000 μg/ml; capsule | Functional recovery | No impact | |||||
| E1 | Perez et al., 2005a,b | Adultb | pMCAO | Acute injection, pre-injury | 100 μg/kg | Infarct volume | Decreased |
| CEE | Rusa et al., 1999 | Adult | tMCAO 120 min + 22 h reperfusion | Acute injection 1 h pre-injury | 1 mg/kg | Infarct volume Ctx | None |
| Littleton-Kearney et al., 2005 | 2-3 | tMCAO 120min + 22 h reperfusion | Consumed from diet 2 mo pre-injury | 0.625 mg/day | Infarct volume Ctx, Subctx | Decreased | |
| 17αE2 | Simpkins et al., 1997a,b | Adultb | tMCAO 40 min + 24 h reperfusion | Acute injection 24 h pre-injury | 1 mg/kg; capsule | Infarct volume | Decreased |
| ZYC3c | Liu et al., 2002 | Adultb | tMCAO 60 min+ 24 h reperfusion | Acute injection 2 h pre-injury | 100 μg/kg | Infarct volume | Decreased |
| Ent-E2 | Green et al., 2001 | Adultb | tMCAO 60 min+ 24 h reperfusion | Acute injection 2 h pre-injury | 100 μg/kg | Infarct volume | Decreased |
Note: All outcomes described are compared to Ovx, injured animals given vehicle.
See list of abbreviation for brain regions and stroke models.
Weights, but not ages, were provided.
ZYC3 refers to 2-adamantyl-estra-1,3,5(10)-trien-3-ol-17-one.
One of the most important factors in preclinical stroke research is the selection of the experimental model to ensure a reproducible injury. MCAO is the most common experimental stroke model employed in rodents, using an intraluminal filament to occlude the middle cerebral artery, thereby resulting in the loss of approximately 50–75% blood flow to the cortex or striatum (Longa et al., 1989; Macrae, 2011). In pMCAO, the filament remains lodged in the artery for the duration of experimental stroke (Bingham et al., 2005; Dubal et al., 1998; Dubal et al., 2001; Perez et al., 2005a,b). Conversely, in a tMCAO model, blood flow to the brain is blocked by the filament for a specific period of time, generally 30–120 min, and is then removed. Filament removal allows re-entry of blood into the artery and also results in reperfusion injury. Thus, pMCAO primarily assesses the effect of estrogen on the injury due to loss of blood flow, while tMCAO assesses the effect of estrogen on injury due to loss of blood flow and reperfusion injury. Fewer studies have employed global models of tMCAO, which are characterized by brief (less than 20 min) periods that occlude blood flow to both hemispheres (Horsburgh et al., 2002; Miller et al., 2005). An alternative MCAO model employs injection of the vasoconstrictive peptide, ET-1; this method occludes blood flow to 30–50% of normal and results in a delayed hypoperfusion (Biernaskie et al., 2001). This model has been widely used to demonstrate the loss of estrogen's neuroprotective effects in aged, reproductively senescent rats (Lewis et al., 2012; Selvamani and Sohrabji, 2010a,b). Similarly, Leon and colleagues (Leon et al., 2012) also showed a deleterious effect of estrogen in aged rats using a tMCAO model coupled with the tPA during reperfusion. In contrast, few laboratories have demonstrated a beneficial effect of estrogen in aged rats (Liu et al., 2012). Occlusion via electrocoagulation has been used less frequently in experimental ischemic stroke, with more neurodamaging effects of estrogen reported in this model than in others (Bingham et al., 2005; Carswell et al., 2004; Gordon et al., 2005). The distinctions among experimental stroke models, however subtle, are critically important when evaluating the efficacy of estrogenic action in neuroprotection.
An additional critical difference between preclinical and clinical studies to assess estrogen-mediated neuroprotection in ischemic stroke is the study endpoint or outcome. As shown in Table 2, infarct volume or quantification of neuronal cell loss is the most common endpoint in preclinical studies. In general, these studies have demonstrated that continuous treatment with 17βE2 reduces infarct volume in the cortex and to a lesser degree in the striatum, with limited, if any protection in the hippocampus (Dubal et al., 1998; Dubal et al., 2001; Rusa et al., 1999; Simpkins et al., 1997b). In contrast, the common endpoint for clinical studies is functional or disease outcomes. Comparatively, a smaller number of preclinical studies have employed acute functional endpoints or mortality as endpoints, whereas the benchmark for therapeutic efficacy in humans is either survival or long-term recovery of motor function, memory, or cognition. The effects of estrogen on functional outcome after ischemic stroke are unclear and under-studied, as we identified a limited number of studies that reported any effects of estrogen on either short-term (Strom et al., 2013; Wang et al., 1999; Zhang et al., 1998) or long-term functional outcomes (Li et al., 2004). The appropriate design of preclinical studies to uncover the mechanisms that determine whether estrogen promotes long-term functional recovery following experimentally-induced stroke is essential for elucidating the biological underpinnings of estrogen-mediated neuroprotection.
We were one of the first laboratories to demonstrate estrogen-mediated neuroprotection in rodents (Simpkins et al., 1997b), and many laboratories have expanded upon these findings over the past twenty years. The age of animals, animal strain, estrogen treatment regimen, and estrogen dose are all critical factors that have contributed to the discrepancies between clinical and preclinical findings. overall, the majority of studies on estrogen and neuroprotection have employed the tMCAO model while restricting blood flow at various times within a 90 min time frame, with administration of varying doses and formulations of estrogen either before injury, at the time of injury, prior to reperfusion, and after reperfusion. It is critical that we elucidate, using clinically relevant models of ischemic stroke, the circumstances under which estrogens are neuroprotective and when they are neuro-damaging in ischemic stroke, as well as neurodegenerative disease. The Stroke therapy Academic Industry Roundtable (STAIR) preclinical recommendations provide an excellent foundation for sound experimental design coupled with transparent reporting of study results. The recommendations of particular relevance to the study of estrogen-mediated neuroprotection urge investigators to: (1) employ both acute and long-term histological and functional endpoints and (2) use both permanent and transient occlusion models, and (3) define the window of therapeutic efficacy (Fisher et al., 2009). We urge investigators to implement these criteria in an effort to provide continued clarity to basic scientists, clinicians, and, most importantly, the millions of women who desire to use HT to improve their quality of life.
6.2. Estrogen and traumatic brain injury
TBI is defined as an injury induced by an external force which results in altered brain function or pathology, such as the presence of clinical symptoms of amnesia, loss of consciousness, etc. (Menon et al., 2010). Although the mechanisms can be diverse (blunt force, gun shot or other penetrative object, explosive blast, etc.), primary TBI is most commonly associated with shearing and contusive damage to neural tissues due to an external force and includes ischemic hypoxia, hematoma, edema, diffuse axonal injury and contusion (reviewed in Maas et al., 2008). The initiation of several pathological cascades results in a diffuse secondary TBI, the consequences of which may not be observable for many days, weeks, or even years following the initial injury. Causes of secondary injury commonly include dysregulation of excitatory neurotransmitters, apoptosis and necrosis, initiation of the inflammatory cascade, disruption of the BBB and cerebral blood flow (CBF), altered energy metabolism, and free radical production. Thus, the pathophysiology of TBI is associated with numerous detrimental neurobiological consequences.
In addition to the devastating pathological processes associated with this condition, the human impact of TBI is high. Each year, an estimated 1.7 million people sustain a TBI and of those, approximately 53,000 will die (Coronado et al., 2011). Among the survivors, the primary and secondary neuropathological alterations are associated with a variety of detrimental consequences including cognitive decline, locomotor impairments, and psychological problems, all of which can have profound negative impacts on daily living and quality of life. It is known that TBI is associated with young adulthood, especially among males, as it is major cause of death in this age group (Maas et al., 2008). As of 2005, there were an estimated 3.17 million adults in the United States suffering from extended, and in some cases life-long, disability associated with a TBI, making these insults extremely cost-burdensome on the healthcare system (Zaloshnja et al., 2008). In fact, total direct and indirect estimated annual costs (including costs associated with missed work and lost productivity) for the treatment of TBI have been estimated to be as high as 76.1 billion dollars (Ma et al., 2014). Unfortunately, the incidence of TBI is rising, given that it is becoming increasingly associated with aging as the large Baby Boomer population enters senescence and become at higher risk for neurotrauma-inducing falls (Roozenbeek et al., 2013). Thus, taken together, TBI represents an urgent medical need; characterizing risk factors and developing interventions for these patients will become an increasingly important direction for research.
A significant risk factor for TBI, and therefore potential treatment option, may be genetic sex and the associated circulating sex-specific hormones. Indeed, preclinical observations of a female advantage in severity of TBI and functional recovery have been reported (Roof et al., 1993; Wagner et al., 2002). Evidence suggests that shifts in the hormonal milieu are associated with changes in risk/outcome. Protection from TBI in the rodent has been reported in proestrous phase (Maghool et al., 2013) when estrogen levels are increasing, although not all studies have replicated this effect (Wagner et al., 2004). Further supporting this hypothesis is the finding that the protective female advantage on outcome measures, such as a reduction of BBB permeability and cerebral edema, was attenuated or even completely absent in Ovx-induced surgically hormone-deplete rats (Bramlett and Dietrich, 2001; Suzuki et al., 2004). Exogenous administration of 17βE2 either in the weeks preceding insult or within minutes to hours after injury appears to ameliorate the detrimental impacts of hormone deficiency (Khaksari et al., 2011; O'Connor et al., 2005; Roof and Hall, 2000), the effects of which have been shown to be mediated through a variety of mechanisms including GPER1 and the classical genomic ERα and ERβ pathways as shown via the use of SERMs (Asl et al., 2013; Day et al., 2013; Khaksari et al., 2013). Despite these promising findings, not all studies report a beneficial effect of estrogen treatment among female animals (Bruce-Keller et al., 2007; Lebesgue et al., 2006). For instance, in Ovx mice, neither acute (10 μg/kg single intraperitoneal injection) nor chronic (180ug/ml subcutaneous Silastic® capsule) 17βE2 given prior to undergoing a controlled cortical impact TBI attenuated cortical and hippocampal cell loss nor impacted microglia reactivity (Bruce-Keller et al., 2007). Taken together, in general, findings from these studies support the hypothesis that estrogens can exert neuroprotective benefits in TBI but further work is needed to clarify the conditions in which these benefits will occur (Table 3).
Table 3.
Effects of exogenous estrogen treatment on traumatic brain injury in ovariectomized female rodents.
| Estrogen | Reference | Age (months) | Injury mechanism | Treatment regimen | Dose | Outcome | Effect |
|---|---|---|---|---|---|---|---|
| 17βE2 | Roof and Hall, 2000 | Adulta | Impact acceleration (500g from 1.5 m) | Cyclic daily injections | 0.1 mg/kg | Cortical blood flow | Protection |
| O'Connor et al., 2005 | 2 | Impact acceleration (450g from 2m) | Acute injection | 33.3 μg/kg | Cerebral edema Blood-brain barrier permeability |
Protection Protection |
|
| Roof et al., 1993 | 3 | Controlled cortical impact (2.25 m/s, 2 mm depth) | Continuous | 5 mm capsule | Cerebral edema | No impact | |
| Bruce-Keller et al., 2007 | 2-3 | Controlled cortical impact (3.5 m/s, 1 mm depth) | Acute injection | 10 μg/kg | Cortical tissue sparing Hippocampal neuronal injury |
No impact No impact |
|
| Microglial activation | No impact | ||||||
| 2-3 | Controlled cortical impact (3.5 m/s, 1 mm depth) | Continuous | 10 mm | Cortical tissue sparing Hippocampal neuronal injury |
No impact No impact |
||
| Microglial activation | No impact | ||||||
| Petrone et al., 2014 | Adult | Controlled cortical impact (3.0 m/s, 1.2 mm depth) | Acute injection | 100 μg/kg | Cortical apoptosis | Protection | |
| Lebesgue et al., 2006 | Adulta | Lateral fluid percussion (2.4-2.9 atm pulse) | Continuous | 0.1 mg; pellet | Hippocampal apoptosis Hippocampal DNA damage |
No impact No impact |
|
| MWM | No impact | ||||||
| Mortality | Worsened |
Note: All outcomes described are compared to Ovx, injured animals given vehicle.
Weights, but not ages, were provided.
Similarly, the findings with regards to TBI at the bedside are also controversial. While some studies note a protective effect of female sex (Berry et al., 2009; Groswasser et al., 1998), many clinical reports have failed to find a sex-difference in post-TBI outcome (Coimbra et al., 2003; Magnotti et al., 2008; Slewa-Younan et al., 2004) and others have suggested that outcome among females was actually worse compared to male patients (Farace and Alves, 2000). For example, even after controlling for a variety of subject-level confounding factors, female patients were found to have higher post-concussive symptoms at three months following mild TBI (Bazarian et al., 2010). While the male-dominated adulthood sex difference in TBI rate diminishes among elderly populations (Pentland et al., 1986), the interactive effect of age appears to play an important role in outcomes for women. Reproductive status is thought to mediate this effect. For instance, many of the studies in which a detrimental effect of female sex on TBI was found were conducted in populations of adult to middle-aged, and thus reproductively capable, women (Bazarian et al., 2010; Coimbra et al., 2003). Farin et al. (2003) reported that women (ages 15–79) had a greater frequency of brain swelling and intracranial hypertension than men. Yet when specific age-groups were compared, the detrimental effect was observed only in women younger than 51 while rates among aged men and women were similar. In addition, Berry et al. (2009) found women in age brackets of 45–54 years and those older than 55 years of age (presumably corresponding to the peri- and post-menopausal periods, respectively), but not women younger than 45, had reduced mortality following moderate to severe TBI as compared to males in this age-group. However, other studies have reported worse outcomes in older populations of women (Gan et al., 2004; Pentland et al., 1986). Unfortunately, neither reproductive status nor use of estrogen-containing treatments such OCs, HTs, etc., were directly assessed (for example, by way of assessing circulating hormone levels, survey of last menstrual period, or self-report of menopausal symptomology) in any of the aforementioned studies and therefore, hormone status cannot be directly associated with differences among these studies. Thus, the relationship between biological sex and outcome in regards to TBI cannot be easily predicted by sex hormones and controversy as to estrogen's neuroprotective role remains. Results of the RESCUE-TBI Phase II clinical trial (NCT00973674; www.clinicaltrials.gov), which will evaluate the potential beneficial effects of acute intravenous CEE treatment on short-term mortality and neurological outcomes following TBI, will begin to clarify this important issue.
7. Factors that influence the realization of neuroprotective effects of estrogen
The evidence presented thus far suggests substantial support of estrogen as a neuroprotective agent in several domains of brain function and injury. However, clinical and preclinical findings of detrimental neurological effects following HT suggest that the story of estrogen's impacts on the nervous system is not a simple one. Instead estrogen can be thought of as a conditional neuroprotectant that, when administered, is associated with a multitude of beneficial impacts but whose biological actions are dictated by several modulating factors that can substantially alter the realization of these beneficial effects. We have directly addressed a set of these factors in the following subsections.
7.1. Etiology of hormone depletion
Evidence suggests that the nature of the menopausal transition, either spontaneous/transitional (also referred to as natural) or surgical, can influence cognitive outcomes during aging. Studies in women have reported that cognitive performance is worse among women who underwent oophorectomy at or before the time of spontaneous menopause (Nappi et al., 1999; Rocca et al., 2007). This notion has also been described in work with aging rodents. Indeed, the Bimonte-Nelson team has capitalized on a novel approach to hormone depletion in animals (Acosta et al., 2009a, 2010). Characterized by Loretta Mayer et al. (2002), the toxin vinylcyclohexene diepoxide (VCD) selectively depletes ovarian primordial and primary follicles, resulting in a hormone environment associated with increased gonadotropins, high circulating androstenedione, and very low levels of estrogens and progesterone, a state that more similarly parallels the physiological milieu of a naturally menopausal woman (Mayer et al., 2005). This transitional ‘menopause’ was associated with cognitive deficits on tests of spatial working memory in middle-aged female rats (Acosta et al., 2009a). Among these animals, higher levels of circulating androstenedione were correlated with worse memory performance. This impairment was ameliorated by surgical removal of the follicle-deplete ovary via Ovx. Animals that underwent transitional hormone depletion followed by surgical removal of the depleted ovaries also outperformed animals that underwent rapid hormone loss via Ovx surgery.
As well, the effects of estrogen treatment may also differ depending on menopause etiology. Findings from the WHI noted that different estrogen formulations given to women depending upon whether their menopause was transitional (CEE + MPA) or surgical (CEE alone) resulted in different effects on adverse outcomes such as stroke risk and probable dementia (Manson et al., 2013). From a preclinical perspective, Acosta et al. (2010) noted that CEE only benefitted memory performance in animals that had undergone Ovx; CEE treatment among VCD-induced gradual hormone depleted animals actually impaired memory performance among middle-age female rodents. Taken together, these findings suggest that etiology of menopause can have important impacts on age-related changes in cognition and response to injury; this factor should be considered in the context of administration of exogenous estrogenic treatments.
An additional important factor with regards to this issue is that patient age at the time of hormone depletion also may play a role in health outcomes following menopause (Shuster et al., 2010). Indeed, women with surgical menopause tend to be younger than transitional menopausal women and cognitive detriments are associated with younger age at the time of surgical hormone loss (Nappi et al., 1999; Rocca et al., 2007). Some studies have even failed to detect differences in cognitive change among women undergoing hysterectomy with or without bilateral oophorectomy when compared to similarly aged women undergoing transitional menopause (Kok et al., 2006; Kritz-Silverstein and Barrett-Connor, 2002). Additional support for the impact of age on hormone depletion effects stems from rodent work suggesting that Ovx is detrimental for memory in young adult animals, beneficial in aged animals (Bimonte and Denenberg, 1999; Bimonte-Nelson et al., 2003), and transitions from detrimental to beneficial during the reproductive senescence period (Engler et al., 2007). Further work teasing apart the role for menopause etiology and age at which menopause occurs is warranted.
7.2. Critical window for estrogenic intervention
For many decades, converging evidence from observational and small, randomized trials suggests that menopausal HT could protect against brain aging and cognitive decline (Sherwin and Henry, 2008). Results of the large WHI were eagerly anticipated and expected to validate the plethora of preclinical and clinical reports noting benefits with estrogen-containing HTs. Yet, surprisingly, the WHI reported an increased risk for ischemic stroke, declines in global cognitive function, and a doubling in the risk of probable dementia among women taking CEE + MPA (Rapp et al., 2003; Rossouw et al., 2002; Shumaker et al., 2003). Among women taking unopposed CEE, there was an increased risk of stroke, a non-significant 49% increased risk of probable dementia, and suggestive evidence for impaired global cognitive function (Anderson et al., 2004; Espeland et al., 2004; Shumaker et al., 2004). Importantly, these women were at least 65 years of age at the time of evaluation for cognitive outcomes. The publication of these controversial findings generated substantial speculation as to the perplexing lack of neuroprotection. One prominent hypothesis was that of the ‘critical window of opportunity’ for the realization of beneficial outcomes of estrogenic intervention. Similarly, the ‘healthy cell’ theory proposed by Dr. Roberta Brinton suggested that reductions in risk for age-related memory changes with estrogen-containing treatments were more likely to be observed among younger, cognitively uncompromised, healthy patients as opposed to those women with underlying age-related neuropathology (Brinton, 2008). Indeed, analyses of WHI data stratified by age suggested that HT had fewer adverse outcomes among younger menopausal women, who initiated treatment near the time of menopause (Manson et al., 2013; Rossouw et al., 2002). Findings of a critical window of opportunity for estrogenic intervention are mirrored in small observational and randomized clinical studies in women (Maki, 2006). Indeed, among women who received bilateral oophorectomy prior to age of spontaneous menopause, those that were treated with estrogen therapy until age 50 did not show cognitive decline relative to a referent group of women (Rocca et al., 2007). Similarly, Dumas et al. (2008) reported that estrogen is capable of counteracting the memory impairing effect of cholinergic challenge but only when administered near the time of menopause. As well, in women who initiated a short-term regimen (2–3 years) of 17βE2-based HT near the time menopause, risk for cognitive decline was reduced when performance was assessed at age 64 (Bagger et al., 2005). It is noteworthy that this short-term treatment regimen was as effective at protecting cognitive function as estrogen treatments lasting many years in duration. However, the estrogen formulation used in the WHI was CEE, either alone or in combination with MPA. The WHI Memory Study of Younger Women (WHIMS-Y) failed to note memory benefits with CEE even when initiated near the time of menopause (Espeland et al., 2013). As well, the Kronos Early Estrogen Prevention Cognitive and Affective Study (KEEPS) did not detect differences on global cognitive outcomes (as measured by the Mini Mental Status Exam) among women randomized to receive estrogen + progestin combination treatment (either oral CEE, or transdermal 17βE2, +micronized progesterone) as compared to placebo (Gleason et al., 2015). Other factors, such as type of progestin included in the HT formulation, differed between these studies; the impact of these other factors are hotly debated and discussed in the sections detailed here as well as in several key reviews (Rocca et al., 2014; Singh and Su, 2013). Nevertheless, results of an additional clinical trial assessing the critical window of menopausal HT initiation, the Early Versus Late Intervention Trial with Estradiol (ELITE; NCT00114517; www.clinicaltrials.gov) are eagerly anticipated and will hopefully clarify this complicated hypothesis.
Evidence from rodent literature also supports the notion that beneficial neuroprotective effects of estrogen treatment will be realized if initiated near the time of hormone depletion (Daniel, 2013; Maki, 2006). For instance, in an elegantly designed study of middle-aged rats, 17βE2 was only protective against cognitive decline on the water RAM when initiated at the time of, but not five months following, Ovx (Daniel et al., 2006). Other animal studies support the existence of a critical period for estrogenic intervention following hormone removal (Gibbs, 2000; Vedder et al., 2014), even if the duration of estrogen treatment is short (Rodgers et al., 2010). This reduction in cognitive benefit following long delays between hormone depletion and 17βE2 intervention is associated with an attenuated ability of 17βE2 to increase hippocampal spine density, a failure to improve LTP, and brain region specific changes in ERα expression (Bohacek and Daniel, 2009; McLaughlin et al., 2008; Smith et al., 2010). Indeed, mounting evidence implicates age-related changes in expression and/or function of ERα in the reduced neurological responsivity to estrogen and represents a possible target for extending the therapeutic window of estrogen intervention (Foster, 2012). Taken together, there is sufficient preclinical and clinical data to support the possible existence of a critical window for HT initiation following hormone depletion. However, it is as yet unclear whether a critical window exists for CEE, highlighting the possibility that factors associated with the estrogenic formulation may also influence the neuroactive actions of estrogen-containing HTs.
7.3. Estrogen type
7.3.1. Conjugated equine estrogen therapy
The majority of studies reporting beneficial effects of estrogen within the context of cognition, stroke, and TBI utilized the most potent circulating estrogen, 17βE2 (Maki, 2012; Sherwin and Henry, 2008). Yet, 17βE2 is not a prevalent component of the most common menopausal HTs used by women. CEE, a purified pregnant mare urine complex of estrogens first developed by Wyeth, is the most widely used estrogen-based menopausal HT in North America (Hersh et al., 2004). Although primarily composed of E1 sulfate and containing only trace amounts of 17βE2, CEE is a mixture of at least 10 estrogen sulfates, many of which are unique to horses and have yet to be evaluated for their neuroprotective impacts in a human or rodent model (Kuhl, 2005). After metabolism, the biologically active hormones in circulation are primarily E1 and the more potent 17βE2, as well as equine-specific estrogens such as equilin and delta8,9-dehydroestrone (Bhavnani, 2003; Kuhl, 2005). Despite CEE being an effective treatment for relieving the negative vasomotor symptoms and vaginal atrophy of menopause (Freedman, 2002), clinical and preclinical findings of CEE's neuroprotective effects are inconclusive. Model-specific effects have been noted. Among women, in an excellent review by Sherwin and Henry (2008), the authors note that studies in which menopausal HT improved cognitive performance measures utilized 17βE2, while CEE was the principal HT used by women in those studies reporting null or negative effects. Cell culture models suggest that CEE can be neuroprotective. Specifically, CEE enhances neuronal growth and increases neuronal survival after experimentally-induced insult in vitro (Brinton et al., 2000; Diaz Brinton et al., 2000). The results from a limited number of preclinical studies assessing the effects of CEE in experimental stroke in vivo suggest that CEE may, but not always, reduce infarct volume (Littleton-Kearney et al., 2005; Rusa et al., 1999). One study noted no differences in post-stroke lesion volume, and thus no protection, among Ovx female rats given an acute CEE injection 30 min before MCAO (Rusa et al., 1999). However, it is worth noting that these studies were carried out in young, healthy rodents. CEE has also been shown to impact cognition in the middle-aged Ovx rat. CEE administered subcutaneously via an acute injection (Walf and Frye, 2008), via chronic cyclical injections (Acosta et al., 2009a,b), or at higher doses administered via continuous release from osmotic pumps (Engler-Chiurazzi et al., 2011), enhances object memory and spatial navigation memory. Yet, detrimental effects have also been reported. Indeed, in young adult Ovx rats, although daily injections of CEE increased hippocampal neurogenesis, this treatment was associated with memory impairments on the RAM (Barha and Galea, 2013). In this same study, CEE also increased numbers of new neurons; this increase correlated with worse maze performance among treated groups. Further, we have reported that CEE dose-dependently impaired working memory performance in middle-aged, Ovx rats, an effect possibly related to the ratio of circulating estrogens (Engler-Chiurazzi et al., 2011). Taken together, the findings regarding the neurobiological impact of CEE are inconsistent across several experimental models.
7.3.2. Feminizing estrogenic components of conjugated equine estrogen
Feminizing estrogen components of CEE appear to exert their own neuroactive, yet not always neuroprotective, effects. E1 sulfate is the principle component of CEE and following treatment with CEE to peri- and post-menopausal women, and middle-aged, Ovx rats, circulating levels of E1 increase (Acosta et al., 2009a,b; Yasui et al., 1999). In addition, prior to menopause, endogenous E1 circulates in approximately a 1:1 ratio with 17βE2 (Rannevik et al., 1995). However, during the menopausal transition, levels of 17βE2 decline to a greater extent than do levels of E1, changing the circulating E1 to 17βE2 ratio to 2:1 (Rannevik et al., 1995). Thus, this large change in the circulating ratio of E1 to 17βE2 both due to aging and to HTs may have a significant impact on cognitive ability and neuroprotection and makes quantifying the neurobiological impact of this estrogen clinically relevant. In vitro work suggests protective effects with E1 treatment against cellular insult (Zhao and Brinton, 2006). However, for most measures in which other estrogenic CEE components (e.g., equilin and delta8,9-dehydroestrone) were neuroprotective, E1 was less effective (Brinton et al., 1997; Zhao and Brinton, 2006). In an in vivo behaving rodent, detrimental effects are observed. Indeed, unlike 17βE2 or another CEE component, delta8,9-dehydroestrone, E1 dose-dependently failed to impact, and at some doses even impaired, aversive memory in the contextual fear conditioning task and spatial working memory among adult and middle-aged Ovx rats (Barha et al., 2009a; Engler-Chiurazzi et al., 2012; Talboom et al., 2010). Further, while chronic 17βE2 increased basal forebrain ChAT-immunoreactive neurons and number of bromodeoxyuridine (BrdU)-labeled neurons in the DG, substrates underlying memory performance, chronic E1 does not impart these same changes (Engler-Chiurazzi et al., 2012; McClure et al., 2013). Thus, taken together, evidence suggests that E1 is not an optimal neuroprotectant nor an ideal component of menopausal HT and highlights that the type of estrogen contained in a HT formulation should be of primary consideration in the design of future treatments.
7.3.3. Non-feminizing estrogens
The use of non-feminizing estrogens holds promise as an alternative for the development of novel HTs (Petrone et al., 2014). 17 alpha-estradiol (17αE2) binds to each ER with a substantially lower affinity than does 17βE2 (Kuiper et al., 1997) and likely does not stimulate peripheral reproductive tissues. In vitro, 17αE2 is as protective against the toxic effects of serum deprivation (Green et al., 1997) and oxidative stress as 17βE2 (Green et al., 2001). Further, 17αE2 is synthesized in the brain and thought to underlie the rapid membrane receptor-mediated actions of estrogen (Toran-Allerand et al., 2005). 17αE2 protects against ischemic insult (Simpkins et al., 1997b), reduces Alzheimer's Disease-associated β-amyloid levels in transgenic mice (Levin-Allerhand et al., 2002), and improves cognitive outcomes including object, reference, and context fear memory (Barha et al., 2009a; Inagaki et al., 2010; Luine et al., 2003; Rhodes and Frye, 2006). These enhancing effects may be mediated by its demonstrated ability to increase DG cell proliferation (Barha et al., 2009b) and/or to increase hippocampal spine density (MacLusky et al., 2005). In some disease states, 17αE2 may even be more neuroprotective than its stereoisomer. Using a model of neonatal brain damage, McClean and Nuñez (2008) note that 17αE2, but not 17βE2, attenuated memory impairments and reductions in hippocampal cell volume following muscimol treatment. Thus, the findings regarding beneficial effects of 17αE2 suggest that feminizing ligand-ER interations may not be a pre-requisite for estrogen neuroprotection.
More broadly, the identification of the molecular characteristics necessary for the neuroprotective effects of 17βE2 represent an important strategy in the optimization of current and future HT options. Indeed, it is the phenolic structure of the estrogen molecule that is essential for the realization of protection against oxidative stress and serum deprivation (Behl et al., 1997; Green et al., 1997). Modifications to the molecular structure of endogenous parent estrogens, E1 or 17βE2, to minimize the detrimental stimulatory estrogenic actions in uterus and breast while simultaneously imparting neuroprotective effects have yielded promising results (Yi et al., 2011). In general, A-ring modifications such as the addition of bulky alkyl groups at the 2- and 4-carbon positions can enhance neuroprotection following a variety of in vitro insults (Perez et al., 2005a,b) as well as following cerebral ischemia in vivo (Perez et al., 2005a). Importantly, in these models, such modifications are more potent neuroprotectants than the parent 17βE2 molecule yet also substantially reduce binding to and activity at the ER. These findings suggest that these novel estrogen analogs impart their neuroprotective effects via their anti-oxidant activity or other mechanisms independent of the classical nuclear cascade (Perez et al., 2005b), representing a novel and effective therapeutic intervention approach for the treatment of menopausal symptoms and the prevention of injury-induced neuropathology.
7.4. Estrogen dose, mode of administration, and regimen
In addition to the type of estrogen formulation selected, factors associated with the way in which the estrogenic treatment is administered are important clinical considerations; variations in estrogen administration can have meaningful impacts on the realization of neuroprotection. Indeed, several studies suggest that there is an optimal dose range for cognitive outcomes in rodents (Barha et al., 2009a; Holmes et al., 2002; Inagaki et al., 2010; MacLusky et al., 2005). As well, converging evidence suggests that the mode of treatment regimen (acute/cyclic versus chronic/continuous) can influence the effectiveness of the estrogen formulation. For instance, acute or intermittent injection regimens of 17βE2 impart neuroprotection in models of TBI and stroke and can enhance memory on multiple behavioral tasks (Bimonte-Nelson et al., 2006; Gresack and Frick, 2004; Luine et al., 2003; O’Connor et al., 2005; Roof and Hall, 2000). However, as shown in Tables 1–3, studies utilizing chronic treatment via continuous administration regimens do not always report beneficial effects. Differences in ER expression, with cyclic estrogen treatment facilitating ER recycling, and continuous estrogen treatment down-regulating ERs, indicate divergent neural mechanisms of action for distinct modes of treatment administration that likely impact the neurobiological effects of estrogens (Blaustein, 1993; Brown et al., 1996; Kassis and Gorski, 1981; Rosser et al., 1993). However, differences in methodological approach, such as injury model used, timing of estrogen treatment relative to assessment, or outcomes/cognitive tests evaluated, could also account for lack of consistent beneficial estrogenic effects between studies. More work to clarify the unique impact of each of these factors is warranted.
7.5. Genetic sex
The recent change in National Institutes of Health funding guidelines to require the consideration as sex as a biological variable in new grant proposals highlights the importance of systematic investigation of sex differences in response to neuroprotective interventions. As such, a brief discussion of the actions of estrogens in the male is warranted. As has been shown among females, 17βE2 treatment in males can impact cognitive outcomes. For instance, continuous administration of 17βE2 enhanced delayed memory retention among adult and aged male rats (Luine and Rodriguez, 1994) and acute post-training 17βE2 injected directly into hippocampus enhanced reference memory MM performance (Packard et al., 1996). The protective effects of estrogen treatment in the context of brain injury also extend to males. Indeed, although castration of adult male rats did not alter stroke injury following reversible MCAO, treatment of 17βE2 to castrated animals reduced cortical and caudate infarct volume (Toung et al., 1998 but see Hawk et al., 1998). As well, some of the earliest reports of estrogen use in rodent models of TBI suggested that 17βE2 could attenuate infract size and improve CBF of male animals (Emerson et al., 1993; O'Connor et al., 2005; Roof and Hall, 2000). Other estrogens and estrogen formulations also show promising neuroprotective effects in males. A single intravenous injection of CEE during reperfusion following MCAO reduced infarct volume (Toung et al., 1998) and increased CBF recovery (McCullough et al., 2001). Further, both CEE (Chen et al., 2009; Soustiel et al., 2005) and E1 (Gatson et al., 2012) significantly reduced insult-induced lesion size and apoptosis following TBI in young adult male rats. As of yet, there is a paucity of reports examining the neuroprotective effects of CEE or E1 in both males and females following stroke or TBI. Investigations regarding the impacts of these unique estrogen types and formulations in both sexes are crucial to attaining a comprehensive understanding of the utility of estrogen as a neuroprotective agent (see Section 7.3).
8. Summary, conclusions, and future directions: from testes to today
As has been discussed in the preceding sections, there is ample empirical evidence to support the notion that sex hormones impact body and brain. As was shown in Berthold's caponized roosters, the loss of these hormones can have dramatic behavioral alterations. Since that time, the knowledge regarding the intricacies of reproductive control by the endocrine system and the interations between this system and other body structures, including the brain, has advanced substantially, leading to the growth of the women's health field and to the development of therapeutic interventions to modulate the physiological effects of shifts in hormonal milieu, such as those occurring during the menopausal transition. Some of these interventions have numerous beneficial impacts not only on symptomatic relief but may also provide protection against cognitive aging and brain trauma. Yet, sufficient evidence suggesting null or even detrimental neurological effects of some estrogen-containing formulations has led to uncertainty among basic scientists, clinicians, and patients regarding the use of estrogen-containing treatments for neuro-protection and rescue of the damaged brain.
Deciphering the complexities of this important public health issue has proved a significant challenge for the field given the diversity of models, formulations, administration regimens, and outcome measures used to evaluate estrogenic action. Converging evidence presented here has identified a number of factors that impact the realization of beneficial effects of estrogenic treatments (Fig. 1). These factors can be divided into three broad categories that include: (1) subject factors, (2) treatment administration factors, and (3) treatment formulation factors. It is noteworthy that none of these factors can be considered in isolation as they likely interact to exacERβate or attenuate the actions of each other. As well, consistency and specificity of biological outcome measures across studies are crucial for a cohesive understanding of the actions of a particular estrogen-containing treatment. For example, administration of CEE, a formulation with mixed findings regarding its impact on memory, may not be sufficient to alter cognitive performance of a healthy perimenopausal woman. However, detrimental cognitive impacts of CEE could be compounded when it is administered to an elderly woman many years removed from spontaneous menopause. Further, these detriments may be especially prominent if the CEE treatment were administered chronically via non-optimal route or at an inappropriate dosage. Finally, these impacts may not be global but instead specific to certain performance domains. Thus, the actions of estrogen within the normal, aging, and injured brain are diverse, conditional, and contingent on a number of factors. Developing criteria standards for desired beneficial peripheral and neuroprotective outcomes while simultaneously avoiding detrimental stimulatory actions among unique patient populations will be an important future direction for the optimization of estrogen treatments within the context of cognitive aging and brain injury.
Fig. 1.
Estrogen as a conditional neuroprotectant. Estrogen acts as a neuroprotectant whose biological actions are modulated by subject factors, treatment administration factors, and treatment formulation factors.
Acknowledgements
The writing of this review was supported by the National Institutes of Health (JWS = P20 GM109098, P01 AG022550, and P01 AG027956; CMB = K01 NS081015). This work was also supported U54 GM109492. This content is solely the responsibility of the authors.
Abbreviations
- 3NPA
3-nitropropionic acid
- 17αE2
17 alpha-estradiol
- 17βE2
17 beta-estradiol
- ATP
adenosine triphosphate
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- BrdU
bromodeoxyuridine
- CA
Cornu ammonis
- CBF
cerebral blood flow
- CCL2
chemokine ligand 2
- CEE
conjugated equine estrogens
- ChAT
choline acetyltransferase
- CNS
central nervous system
- DG
dentate gyrus
- DMP/DMS
delayed match to position/delayed match to sample
- DPN
diarylpropionitrile
- E1
estrone
- E3
estriol
- ELITE
Early Versus Late Intervention Trial with Estradiol
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptor
- ERα
estrogen receptor-α
- ERβ
estrogen receptor-β
- ERE
Estrogen Response Element
- ET-1
endothelin-1
- GPER1
G protein-coupled ER 1 (or GPR30)
- HT
hormone therapy
- IGF
insulin-like growth factor
- iNOS
inducible nitric oxide synthase
- KEEPS
Kronos Early Estrogen Prevention Cognitive and Affective Study
- KO
knock out
- LTP
long term potentiation
- MCAO
middle cerebral artery occlusion
- MM
morris water maze
- MPA
medroxyprogesterone acetate
- mRNA
messenger RNA
- NGF
nerve growth factor
- NADPH
nicotinamide adenine dinucleotide phosphate
- NMDA
N-methyl-d-aspartate
- OC
oral contraceptive
- Ovx
ovariectomy
- pMCAO
permanent MCAO
- Premarin®
CEE
- Prempro®
CEE + MPA
- PPT
propylpyrazole triol
- RAM
radial arm maze
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- SERMs
Selective Estrogen Receptor Modulators
- STAIR
stroke therapy academic industry roundtable
- SVZ
subventricular zone
- TBI
traumatic brain injury
- TJ
tight junction
- tMCAO
transient MCAO
- TNFa
tumor necrosis factor alpha
- tPA
tissue plasminogen activator
- VCD
vinylcyclohexene diepoxide
- WEST
Women's Estrogen for Stroke Trial
- WHI
Women's Health Initiative
- WHIMS
WHI Memory Study
- WHIMS-Y
WHIMS of Younger Women
- WT
wild type
References
- Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM. Role of astrocytes in the neuroprotective actions of 17b-estradiol and selective estrogen receptor modulators. Mol. Cell. Endocrinol. 2014;389:48–57. doi: 10.1016/j.mce.2014.01.009. http://dx.doi.org/10.1016/j.mce.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: models, mazes, and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. http://dx.doi.org/10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CWS, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009a;150:4248–4259. doi: 10.1210/en.2008-1802. http://dx.doi.org/10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm. Behav. 2009b;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. http://dx.doi.org/10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson H. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151:3795–3804. doi: 10.1210/en.2010-0055. http://dx.doi.org/10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WGM, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-a in the hippocampus of female rats. J. Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J. Neurosci. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager N, Vasikaram S, Leedman P, Flicker L. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. Am. J. Geriatr. Psychiatry. 2005;13:142–149. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Arevalo M-A, Azcoitia I, Garcia-Segura L. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. http://dx.doi.org/10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. 50th Anniv. Publ. Phoenix, Goy, Gerall Young 1959 Organ. Eff. Horm. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. http://dx.doi.org/10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asl SZ, Khaksari M, Khachki AS, Shahrokhi N, Nourizade S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. J. Neurosurg. 2013;119:353–361. doi: 10.3171/2013.4.JNS121636. http://dx.doi.org/10.3171/2013.4.JNS121636. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–678. doi: 10.1016/s0306-4530(99)00020-7. http://dx.doi.org/10.1016/S0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Ayres S, Tang M, Subbiah MT. Estradiol-17beta as an antioxidant: some distinct features when compared with common fat-soluble antioxidants. J. Lab. Clin. Med. 1996;128:367–375. doi: 10.1016/s0022-2143(96)80008-4. [DOI] [PubMed] [Google Scholar]
- Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41. http://dx.doi.org/10.1097/gme.0-b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Curr. Biol. 2010;20:R136–R140. doi: 10.1016/j.cub.2009.12.014. http://dx.doi.org/10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Bake S, Friedman JA, Sohrabji F. Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins. Microvasc. Res. 2009;78:413–424. doi: 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17b-estradiol differentially regulates blood–brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LAM. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2009a;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. The hormone therapy, Premarin, impairs hippo-campus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol. Aging. 2013;34:986–1004. doi: 10.1016/j.neurobiolaging.2012.07.009. http://dx.doi.org/10.1016/j.neurobiolaging.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LAM. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J. Neuroendocrinol. 2009b;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. http://dx.doi.org/10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J. Am. Geriatr. Soc. 1999;47:1289–1293. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma. 2010;27:527–539. doi: 10.1089/neu.2009.1068. http://dx.doi.org/10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood–brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’H F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure–activity relationship. Mol. Pharmacol. 1997;51:535–541. http://dx.doi.org/10.1124/mol.51.4.535. [PubMed] [Google Scholar]
- Berchtold NC, Cotman CW. Bizon JL, Woods A, editors. Normal and pathological aging: from animals to humans. Anim. Model. Hum. Cogn. Aging. Humana Press, a part of Springer Science + Business Media, LLC. 2009 http://dx.doi.org/10.1007/978-1-59745-422-3_1.
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav. Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J. Trauma Inj. Infect. Crit. Care. 2009;67:950–953. doi: 10.1097/TA.0b013e3181ba3354. http://dx.doi.org/10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- Berthold A. The transplantation of testes. 1849:399–401. Texts Doc. [Google Scholar]
- Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn. Reson. Med. 2001;46:827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17beta-oestradiol after permanent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2005;25:414–420. doi: 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. Proc. 11th Int. Congr. Horm. Steroids 7th Int. Congr. Horm. Cancer ICHS ICHC. 2003;2002(85):473–482. doi: 10.1016/s0960-0760(03)00220-6. http://dx.doi.org/10.1016/S0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. http://dx.doi.org/10.1016/S0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol. Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004a;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm A-C. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur. J. Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. http://dx.doi.org/10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm A-CE. Ovarian hormones and cognition in the aged female rat: I, Long-term, but not short-term, ovariectomy enhances spatial performance. Behav. Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. http://dx.doi.org/10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm A-CE. Ovarian hormones and cognition in the aged female rat: II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav. Neurosci. 2004b;118:707–714. doi: 10.1037/0735-7044.118.4.707. http://dx.doi.org/10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. An estrogen by any other name . Endocrinology. 2008;149:2697–2698. doi: 10.1210/en.2008-0396. http://dx.doi.org/10.1210/en.2008-0396. [DOI] [PubMed] [Google Scholar]
- Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am. J. Physiol. Cell Physiol. 1993;264:C1367–C1387. doi: 10.1152/ajpcell.1993.264.6.C1367. [DOI] [PubMed] [Google Scholar]
- Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA. Age-related sex differences in verbal memory. J. Clin. Psychol. 1988;44:403–411. doi: 10.1002/1097-4679(198805)44:3<403::aid-jclp2270440315>3.0.co;2-0. http://dx.doi.org/10.1177/0192513X12437708. [DOI] [PubMed] [Google Scholar]
- Bliss T, Collingridge G, Morris R. Synaptic plasticity in the hippocampus. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; New York, NY: 2007. pp. 343–747. [Google Scholar]
- Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J. Neuroendocrinol. 2009;21:640–647. doi: 10.1111/j.1365-2826.2009.01882.x. http://dx.doi.org/10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Böttner M, Thelen P, Jarry H. Estrogen receptor beta: tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2014;139:245–251. doi: 10.1016/j.jsbmb.2013.03.003. http://dx.doi.org/10.1016/j.jsbmb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. http://dx.doi.org/10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease. Maturitas. 2000;34(Suppl.):S35–S52. doi: 10.1016/s0378-5122(00)00107-9. http://dx.doi.org/10.1016/S0378-5122(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Proffitt P, Tran J, Luu R. Equilin, a principal component of the estrogen replacement therapy Premarin, increases the growth of cortical neurons via an NMDA receptor-dependent mechanism. Exp. Neurol. 1997;147:211–220. doi: 10.1006/exnr.1997.6619. http://dx.doi.org/10.1006/exnr.1997.6619. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Rapid Actions Steroid Horm. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. http://dx.doi.org/10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin. Reprod. Med. 2009;27:240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Dela Cruz CD, Yang E, Wise PM. Inducible nitric oxide synthase and estradiol exhibit complementary neuroprotective roles after ischemic brain injury. Exp. Neurol. 2008;210:782–787. doi: 10.1016/j.expneurol.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors {alpha} and {beta}. Endocrinology. 2010;151:4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Dimayuga FO, Reed JL, Wang C, Angers R, Wilson ME, Dimayuga VM, Scheff SW. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma. 2007;24:203–215. doi: 10.1089/neu.2006.0163. [DOI] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Burek M, Arias-Loza PA, Roewer N, Förster CY. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2010;30:298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, Stern B, Herrington D, Ford-Lynch G, Gorelick P, James A, Brown CM, Choi E, Bray P, Newby LK, Goldstein LB, Simpkins J. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke. 2006;37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- Bushnell C, McCullough L. Stroke prevention in women: synopsis of the 2014 American Heart Association/American Stroke Association guideline. Ann. Intern. Med. 2014;160:853–857. doi: 10.7326/M14-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR, Research C, for, H.B.P. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clin. Obstet. Gynaecol. 1977;4:31–47. [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23:583–603. doi: 10.1016/s0306-4530(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J. Cereb. Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Farr TD. Complexities of oestrogen in stroke. Clin. Sci. 2010;118:375–389. doi: 10.1042/CS20090018. [DOI] [PubMed] [Google Scholar]
- Casals JB, Pieri NCG, Feitosa MLT, Ercolin ACM, Roballo KCS, Barreto RSN, Bressan FF, Martins DS, Miglino MA, Ambrósio CE. The use of animal models for stroke research: a review. Comp. Med. 2011;61:305–313. [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Estrogen receptor agonists for attenuation of neuroinflammation and neuro-degeneration. Brain Res. Bull. 2014;109C:22–31. doi: 10.1016/j.brainresbull.2014.09.004. http://dx.doi.org/10.1016/j.brain-resbull.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chang CY, Chang HK, Chen WC, Lin MT, Wang JJ, Chen JC, Chang FM. Premarin stimulates estrogen receptor-alpha to protect against traumatic brain injury in male rats. Crit. Care Med. 2009;37:3097–3106. doi: 10.1097/CCM.0b013e3181bc7986. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Godfrey JA, Wiegman MJ. The effect of ovariectomy and estrogen on penetrating brain arterioles and blood–brain barrier permeability. Microcirculation. 2009;16:685–693. doi: 10.3109/10739680903164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra R, Hoyt DB, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no!. J. Trauma Inj. Infect. Crit. Care. 2003;54:689–700. doi: 10.1097/01.TA.0000058314.31655.5F. http://dx.doi.org/10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9:371–379. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm. Neurotrauma Prot. Degener. Plast. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. http://dx.doi.org/10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA-receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neuro. 2001;21(7):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. http://dx.doi.org/10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Davies AM. The neurotrophic hypothesis: where does it stand? Philos. Trans. R. Soc. London B: Biol. Sci. 1996;351:389–394. doi: 10.1098/rstb.1996.0033. http://dx.doi.org/10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- Day M, Good M. Ovariectomy-induced disruption of long-term synaptic depression in the hippocampal CA1 region in vivo is attenuated with chronic estrogen replacement. Neurobiol. Learn. Mem. 2005;83:13–21. doi: 10.1016/j.nlm.2004.06.009. http://dx.doi.org/10.1016/j.nlm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Day NL, Floyd CL, D'Alessandro LT, Hubbard WJ, Chaudry IH. 17bestradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1. J. Neurotrauma. 2013;30:1531–1541. doi: 10.1089/neu.2013.2854. http://dx.doi.org/10.1089/neu.2013.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. doi: 10.1172/JCI27987. http://dx.doi.org/10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone's role in neuroprotection, a review of the evidence. Brain Res. 2013;1530:82–105. doi: 10.1016/j.brainres.2013.07.014. http://dx.doi.org/10.1016/j.brainres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Dey P, Velazquez-Villegas LA, Faria M, Turner A, Jonsson P, Webb P, Williams C, Gustafsson J-Å, Ström AM. Estrogen receptor b2 induces hypoxia signature of gene expression by stabilizing HIF-1a in prostate cancer. PLOS ONE. 2015;10:e0128239. doi: 10.1371/journal.pone.0128239. http://dx.doi.org/10.1371/journal.pone.0128239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Brinton R. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's Disease: recent insights and remaining challenges. Learn. Memory. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu H-P. The women's health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol. Aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. http://dx.doi.org/10.1016/S0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Mechanisms of cerebrovascular protection: oestrogen, inflammation and mitochondria. Acta Physiol. 2011;203:149–154. doi: 10.1111/j.1748-1716.2010.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm. Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. http://dx.doi.org/10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. http://dx.doi.org/10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Dykens JA. Mitochondrial radical production and mechanisms of oxidative excitotoxicity. In: Davies KJA, Ursini F, editors. The Oxygen Paradox. Cleup Press (U. of Padova); 1995. pp. 453–467. [Google Scholar]
- Dykens JA, Simpkins JW, Wang J, Gordon K. Polycyclic phenols, estrogens and neuroprotection: a proposed mitochondrial mechanism. Exp. Gerontol. 2003;38:101–107. doi: 10.1016/s0531-5565(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000;1:41–50. doi: 10.1038/35036213. http://dx.doi.org/10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J. Cell. Mol. Med. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. http://dx.doi.org/10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabes S, Nicot AB. Sex steroids and neuroprotection in spinal cord injury: a review of preclinical investigations. Exp. Neurol. 2014;259:28–37. doi: 10.1016/j.expneurol.2014.01.008. http://dx.doi.org/10.1016/j.expneurol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Emerson CS, Headrick JP, Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. http://dx.doi.org/10.1016/0006-8993(93)90778-L. [DOI] [PubMed] [Google Scholar]
- Engler EB, Zay C, Alexander G, Bimonte-Nelson H. Society for Neuroscience. San Diego, CA: 2007. Ovarian hormone loss and cognitive decline at multiple timepoints during aging; correlations with gonadotropins. unpublished data. [Google Scholar]
- Engler-Chiurazzi E, Talboom JS, Braden BB, Tsang CWS, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson H. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm. Behav. 2012;62:1–9. doi: 10.1016/j.yhbeh.2012.04.004. http://dx.doi.org/10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson H. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol. Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. http://dx.doi.org/10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, Lane D, Wactawski-Wende J, Stefanick ML, Li W, Resnick SM. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern. Med. 2013;173 doi: 10.1001/jamainternmed.2013.7727. http://dx.doi.org/10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol. Learn. Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. http://dx.doi.org/10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol. Biochem. Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. http://dx.doi.org/10.1016/S0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J. Neurosurg. 2000;93:539–545. doi: 10.3171/jns.2000.93.4.0539. http://dx.doi.org/10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- Farin A, Deutsch R, Biegon A, Marshall LF. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurg. 2003;98:32–36. doi: 10.3171/jns.2003.98.1.0032. http://dx.doi.org/10.3171/jns.2003.98.1.0032. [DOI] [PubMed] [Google Scholar]
- Farrag AF, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement. Geriatr. Cogn. Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang J. Long-term effects of melatonin or 17b-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. http://dx.doi.org/10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2014;1621:1–8. doi: 10.1016/j.brainres.2014.10.033. http://dx.doi.org/10.1016/j.brainres.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. http://dx.doi.org/10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockoutmice. Mol. Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. http://dx.doi.org/10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Freedman MA. Quality of life and menopause: the role of estrogen. J. Women's Health. 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav. Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. http://dx.doi.org/10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. http://dx.doi.org/10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryel B, Kost A, Kasprowska D. Neuronal autophagy in cerebral ischemia—a potential target for neuroprotective strategies? Pharmacol. Rep. 2012;64:1–15. doi: 10.1016/s1734-1140(12)70725-9. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Kimura D. Sex differences in route-learning. Pers. Individ. Dif. 1993;14:53–65. http://dx.doi.org/10.1016/0191-8869(93)90174-2. [Google Scholar]
- Gan BK, Lim JHG, Ng IHB. Outcome of moderate and severe traumatic brain injury amongst the elderly in Singapore. Ann. Acad. Med. Singap. 2004;33:63–67. [PubMed] [Google Scholar]
- Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT. The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp. Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Liu M-M, Abdelfattah K, Wigginton JG, Smith S, Wolf S, Simpkins JW, Minei JP. Estrone is neuroprotective in rats after traumatic brain injury. J. Neurotrauma. 2012;29:2209–2219. doi: 10.1089/neu.2011.2274. http://dx.doi.org/10.1089/neu.2011.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr. Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. http://dx.doi.org/10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm. Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. http://dx.doi.org/10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm. Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. http://dx.doi.org/10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol. Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. http://dx.doi.org/10.1016/S0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm. Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. http://dx.doi.org/10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. http://dx.doi.org/10.1016/S0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibson CL. Cerebral ischemic stroke: is gender important? J. Cereb. Blood Flow Metab. 2013;33:1355–1361. doi: 10.1038/jcbfm.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur. J. Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. http://dx.doi.org/10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HV. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Gould E. Structural plasticity. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; New York, NY: 2007. pp. 343–747. [Google Scholar]
- Granholm A-C. Oestrogen and nerve growth factor – neuroprotection and repair in Alzheimer's disease. Expert Opin. Investig. Drugs. 2000;9:685–694. doi: 10.1517/13543784.9.4.685. http://dx.doi.org/10.1517/13543784.9.4.685. [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW. 17a-estradiol exerts neuroprotective effects on SK-N-SH Cells. J. Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience. 1998;84:7–10. doi: 10.1016/s0306-4522(97)00595-2. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang S-H, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. http://dx.doi.org/10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Wight RG, Huang M-H, Avis N, Gold EB, Joffe H, Seeman T, Vuge M, Karlamangla AS. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. Am. J. Epidemiol. 2010;171:1214–1224. doi: 10.1093/aje/kwq067. http://dx.doi.org/10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol. Learn. Mem. 2007;88:393–408. doi: 10.1016/j.nlm.2007.07.015. http://dx.doi.org/10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley KE, Green PS, Simpkins JW. A novel, synergistic interaction between 17 beta-estradiol and glutathione in the protection of neurons against beta-amyloid 25-35-induced toxicity in vitro. Mol. Pharmacol. 1998;54:874–880. doi: 10.1124/mol.54.5.874. [DOI] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Gurman P, Miranda OR, Nathan A, Washington C, Rosen Y, Elman NM. Recombinant tissue plasminogen activators (rtPA): a review. Clin. Pharmacol. Ther. 2015;97:274–285. doi: 10.1002/cpt.33. http://dx.doi.org/10.1002/cpt.33. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor beta—a new dimension in estrogen mechanism of action. J. Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. http://dx.doi.org/10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015;146:3–14. doi: 10.1016/j.jsbmb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat. Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. http://dx.doi.org/10.1002/(SICI)1097-0258(20000615/30)19:11/12<1555::AID-SIM445>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm. Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. http://dx.doi.org/10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15:97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor β in adult brain function. J. Neuroendocrinol. 2012;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. http://dx.doi.org/10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apototic versus necrotic neurodegeneration. J. Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. http://dx.doi.org/10.1016/S0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Lobo RA. Hormone therapy and the risk of stroke: perspectives 10 years after the Women's Health Initiative trials. Climacteric. 2012;15:229–234. doi: 10.3109/13697137.2012.656254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- Henderson VW, John, St. JA, Hodis HN, McCleary CA, Stanczyk FZ, Karim R, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognition, mood, and physiological concentrations of sex hormones in the early and late post-menopause. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20290–20295. doi: 10.1073/pnas.1312353110. http://dx.doi.org/10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stafford RS, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. http://dx.doi.org/10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav. Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. http://dx.doi.org/10.1037/0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J. Cereb. Blood Flow Metab. 2002;22:1189–1195. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1–42) and ibotenic acid. Horm. Behav. 2007;52:297–306. doi: 10.1016/j.yhbeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav. 2010 doi: 10.1016/j.yhbeh.2010.05.013. http://dx.doi.org/10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERb agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol. Learn. Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. http://dx.doi.org/10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog. Horm. Res. 1962;18:387. [Google Scholar]
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995 National Health Statistics Reports. 2012;(60) [PubMed] [Google Scholar]
- Jover T, Tananka H, Calderone A, Oguro K, Bennett MVL, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. 2002 doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis JA, Gorski J. Estrogen receptor replenishment. Evidence for receptor recycling. J. Biol. Chem. 1981;256:7378–7382. [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav. Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. http://dx.doi.org/10.1037/0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Khaksari M, Keshavarzi Z, Gholamhoseinian A, Bibak B. The effect of female sexual hormones on the intestinal and serum cytokine response after traumatic brain injury: different roles for estrogen receptor subtypes. Can. J. Physiol. Pharmacol. 2013;91:700–707. doi: 10.1139/cjpp-2012-0359. http://dx.doi.org/10.1139/cjpp-2012-0359. [DOI] [PubMed] [Google Scholar]
- Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can. J. Physiol. Pharmacol. 2011;89:31–40. doi: 10.1139/y10-103. http://dx.doi.org/10.1139/Y10-103. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex hormones influence human cognitive pattern. Neuroendocrinol. Lett. 2002;23:67–77. [PubMed] [Google Scholar]
- Kittner SJ, Stern BJ, Feeser BR, Hebel JR, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N. Engl. J. Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. http://dx.doi.org/10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl. Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, van der Schouw TY, Grobbee DE, Wadsworth MEJ, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13 doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E. Hysterectomy, oophorectomy, and cognitive function in older women. J. Am. Geriatr. Soc. 2002;50:55–61. doi: 10.1046/j.1532-5415.2002.50008.x. http://dx.doi.org/10.1046/j.1532-5415.2002.50008.x. [DOI] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8:3–63. doi: 10.1080/13697130500148875. http://dx.doi.org/10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-Å. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. http://dx.doi.org/10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. http://dx.doi.org/10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, LeBold DG, Surles NO, Morales DM, Etgen AM, Zukin RS, Saatman KE. Effects of estradiol on cognition and hippocampal pathology after lateral fluid percussion brain injury in female rats. J. Neurotrauma. 2006;23:1814–1827. doi: 10.1089/neu.2006.23.1814. http://dx.doi.org/10.1089/neu.2006.23.1814. [DOI] [PubMed] [Google Scholar]
- Lebrun CEI, Van Der Schouw YT, De Jong FH, Pols HAP, Grobbee DE, Lamberts SWJ. Endogenous oestrogens are related to cognition in healthy elderly women. Clin. Endocrinol. (Oxf) 2005;63:50–55. doi: 10.1111/j.1365-2265.2005.02297.x. http://dx.doi.org/10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology. 2012;153:3386–3393. doi: 10.1210/en.2011-1859. http://dx.doi.org/10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner SP, Meredith S, Thayne WV, Butcher RL. Age-related alterations in follicular development and hormonal profiles in rats with 4-day estrous cycles. Biol. Reprod. 1990;42:633–638. doi: 10.1095/biolreprod42.4.633. http://dx.doi.org/10.1095/biolreprod42.4.633. [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Smith JD. Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J. Nutr. Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol. Aging. 2012;33:1123, e1–16. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav. Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. http://dx.doi.org/10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer's disease. Mol. Cell. Endocrinol. 2014;389:13–21. doi: 10.1016/j.mce.2013.12.018. http://dx.doi.org/10.1016/j.mce.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liang Y, Belford S, Tang F, Prokai L, Simpkins JW, Hughes JA. Membrane fluidity effects of estratrienes. Brain Res. Bull. 2001;54:661–668. doi: 10.1016/s0361-9230(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. http://dx.doi.org/10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton-Kearney MT, Klaus JA, Hurn PD. Effects of combined oral conjugated estrogens and medroxyprogesterone acetate on brain infarction size after experimental stroke in rat. J. Cereb. Blood Flow Metab. 2005;25:421–426. doi: 10.1038/sj.jcbfm.9600052. [DOI] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J. Neuroendocr. 2012;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. http://dx.doi.org/10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang SH, Perez E, Yi KD, Wu SS, Eberst K, Prokai L, Prokai-Tatrai K, Cai ZY, Covery DF, Day AL, Simpkins JW. Neuroprotective effects of a novel non-receptor-binding estrogen analogue: in vitro and in vivo analysis. Stroke. 2002;10:2485–2491. doi: 10.1161/01.str.0000030317.43597.c8. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SSC. Chronological changes in sex steroid, gonadotropin and prolactin secretion in aging female rats displaying different reproductive states. Biol. Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. http://dx.doi.org/10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Luine V, Hearns M. Spatial memory deficits in aged rats: contributions of the cholinergic system assessed by ChAT. Brain Res. 1990;523:321–324. doi: 10.1016/0006-8993(90)91507-d. http://dx.doi.org/10.1016/0006-8993(90)91507-D. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav. Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function: past, present and future. Horm. Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. http://dx.doi.org/10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. http://dx.doi.org/10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the united states: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 95. 2014:986–995.e1. doi: 10.1016/j.apmr.2013.10.032. http://dx.doi.org/10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed]
- Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. http://dx.doi.org/10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. http://dx.doi.org/10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Macrae IM. Preclinical stroke research—advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011;164:1062–1078. doi: 10.1111/j.1476-5381.2011.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghool F, Khaksari M, Siahposht Khachki A. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. http://dx.doi.org/10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Magnotti LJ, Fischer PE, Zarzaur BL, Fabian TC, Croce M. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J. Am. Coll. Surg. 2008;206:984–991. doi: 10.1016/j.jamcollsurg.2007.12.038. http://dx.doi.org/10.1016/j.jamcollsurg.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Maki PM. Minireview: Effects of different HT formulations on cognition. Endocrinology. 2012;153:3564–3570. doi: 10.1210/en.2012-1175. http://dx.doi.org/10.1210/en.2012-1175. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. http://dx.doi.org/10.1016/j.neuro-science.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell L, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. The Women's Health Initiative hormone therapy trials: update and overview of health outcomes during the intervention and post-stopping phases. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. http://dx.doi.org/10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Women's Heal. 2011;7:319–339. doi: 10.2217/whe.11.22. http://dx.doi.org/10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance in the estrous cycle. J. Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J. Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler. Thromb. Vasc. Biol. 2005;25:1910–1916. doi: 10.1161/01.ATV.0000175767.46520.6a. http://dx.doi.org/10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod. Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. http://dx.doi.org/10.1016/S0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- McClean J, Nuñez JL. 17α-Estradiol is neuroprotective in male and female rats in a model of early brain injury. Exp. Neurol. 2008;210:41–50. doi: 10.1016/j.expneurol.2007.09.027. http://dx.doi.org/10.1016/j.expneurol.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure RES, Barha CK, Galea LAM. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm. Behav. 2013;63:144–157. doi: 10.1016/j.yhbeh.2012.09.011. http://dx.doi.org/10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. http://dx.doi.org/10.1161/01.STR.32.3.796. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm. Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. http://dx.doi.org/10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J. Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. http://dx.doi.org/10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Mennenga SE, Baxter LC, Grunfeld IS, Brewer GA, Aiken LS, Engler-Chiurazzi EB, Camp BW, Acosta JI, Braden BB, Schaefer KR, Gerson JE, Lavery CN, Tsang CWS, Hewitt LT, Kingston ML, Koebele SV, Patten KJ, Ball BH, McBeath MK, Bimonte-Nelson HA. Navigating to new frontiers in behavioral neuroscience: traditional neuropsychological tests predict human performance on a rodent-inspired radial-arm maze. Front. Behav. Neurosci. 2014;8:294. doi: 10.3389/fnbeh.2014.00294. http://dx.doi.org/10.3389/fnbeh.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. http://dx.doi.org/10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Miller CP, Jirkovsky I, Hayhurst DA, Adelman SJ. In vitro antioxidant effects of estrogens with a hindered 3-OH function on the copper-induced oxidation of low density lipoprotein. Steroids. 1996;61:305–308. doi: 10.1016/0039-128x(95)00234-h. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor immuno-reactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. http://dx.doi.org/10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–371. http://dx.doi.org/10.1002/1096-9861(20010115)429:3<355::AID-CNE1>3.0.CO;2-#. [PubMed] [Google Scholar]
- Mooradian AD. Antioxidant properties of steroids. J. Steroid Biochem. Mol. Biol. 1993;45:509–511. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics, C., Stroke Statistics, S. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappy G. Memory functioning at menopause: impact of age in ovarectomized women. Gynecol. Obstet. Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Nakagami F, Nakagami H, Osako MK, Iwabayashi M, Taniyama Y, Doi T, Shimizu H, Shimamura M, Rakugi H, Morishita R. Estrogen attenuates vascular remodeling in Lp(a) transgenic mice. Atherosclerosis. 2010;211:41–47. doi: 10.1016/j.atherosclerosis.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: an update. Maturitas. 2010;67:34–38. doi: 10.1016/j.maturitas.2010.04.016. http://dx.doi.org/10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm. Behav. 2010;58:878–890. doi: 10.1016/j.yhbeh.2010.08.017. http://dx.doi.org/10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-e-Castro M. The possible role of high estriol levels in pregnancy. Med. Hypotheses. 1975;1:132–134. doi: 10.1016/0306-9877(75)90054-7. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- O'Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. http://dx.doi.org/10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann. N. Y. Acad. Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. http://dx.doi.org/10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Olton DS. Mazes, maps, and memory. Am. Psychol. 1979;34:583–596. doi: 10.1037//0003-066x.34.7.583. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT-Y, Galea LAM. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J. Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. http://dx.doi.org/10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm. Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. http://dx.doi.org/10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav. Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. http://dx.doi.org/10.1037/0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas. 2001;38:243–261. doi: 10.1016/s0378-5122(01)00167-0. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson TB. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc. Soc. Exp. Biol. Med. (New York, N.Y.) 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J. Cereb. Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Peng MT, Huang HH. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil. Steril. 1972;23:535–542. doi: 10.1016/s0015-0282(16)39131-2. [DOI] [PubMed] [Google Scholar]
- Pentland B, Jones PA, Roy CW, Miller JD. Head injury in the elderly. Age Ageing. 1986;15:193–202. doi: 10.1093/ageing/15.4.193. [DOI] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang S-H, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005a;1038:216–222. doi: 10.1016/j.brainres.2005.01.026. http://dx.doi.org/10.1016/j.brainres.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Perez E, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of estratriene analogs: structure–activity relationships and molecular optimization. Drug Dev. Res. 2005b;66:78–92. [Google Scholar]
- Peterson CM. Estrogen and progesterone receptors: an overview from the year 2000. J. Soc. Gynecol. Investig. 2000;7:S3–S7. doi: 10.1016/s1071-5576(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framing-ham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone AB, Gatson JW, Simpkins JW, Reed MN. Non-feminizing estrogens: a novel neuroprotective therapy. Mol. Cell. Endocrinol. 2014;389:40–47. doi: 10.1016/j.mce.2013.12.017. http://dx.doi.org/10.1016/j.mce.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J. Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova A, Perez E, Liu R, Simpkins J. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Natl. Acad. Sci. U. S. A. 2003a;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Simpkins JW. Quinol-based metabolic cycle for estrogens in rat liver microsomes. Drug Metab. Dispos. 2003b;31:701–704. doi: 10.1124/dmd.31.6.701. [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure–nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol. Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. http://dx.doi.org/10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014;389(1–2):71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. doi: 10.1016/0378-5122(94)00869-9. http://dx.doi.org/10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J. Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Böttner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J. Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol. Learn. Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. http://dx.doi.org/10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson J-A. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. http://dx.doi.org/10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm. Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. http://dx.doi.org/10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shusterd LT. Oophorectomy, estrogen, and dementia: a 2014 update. Mol. Cell. Endocrinol. 2014;18:1199–1216. doi: 10.1016/j.mce.2014.01.020. http://dx.doi.org/10.1016/j.mce.2014.01.020.Oophorectomy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. http://dx.doi.org/10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Baxter MG. Neuropsychology of cognitive aging in rodents. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton, FL: 2007. Chapter 3. [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. http://dx.doi.org/10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. CRC Press; Boca Raton, FL: 2006. Chapter 12. [PubMed] [Google Scholar]
- Romer W, Oettel M, Droescher P, Schwarz S. Novel “scavestrogens” and their radical scavenging effects, iron-chelating, and total antioxidative activities: delta 8,9-dehydro derivatives of 17 alpha-estradiol and 17 beta-estradiol. Steroids. 1997a;62:304–310. doi: 10.1016/s0039-128x(96)00224-3. [DOI] [PubMed] [Google Scholar]
- Romer W, Oettel M, Menzenbach B, Droescher P, Schwarz S. Novel estrogens and their radical scavenging effects, iron-chelating, and total anti-oxidative activities: 17 alpha-substituted analogs of delta 9(11)-dehydro-17 beta-estradiol. Steroids. 1997b;62:688–694. doi: 10.1016/s0039-128x(97)00068-8. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. http://dx.doi.org/10.1016/0006-8993(93)91526-X. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. http://dx.doi.org/10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Rosser M, Chorich L, Howard E, Zamorano P, Mahesh VB. Changes in rat uterine estrogen receptor messenger ribonucleic acid levels during estrogenand progesterone-induced estrogen receptor depletion and subsequent replenishment. Biol. Reprod. 1993;48:89–98. doi: 10.1095/biolreprod48.1.89. http://dx.doi.org/10.1095/biolreprod48.1.89. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Rouchaud A, Mazighi M, Labreuche J, Meseguer E, Serfaty JM, Laissy JP, Lavallee PC, Cabrejo L, Guidoux C, Lapergue B, Klein IF, Olivot JM, Abboud H, Simon O, Schouman-Claeys E, Amarenco P. Outcomes of mechanical endovascular therapy for acute ischemic stroke: a clinical registry study and systematic review. Stroke. 2011;42:1289–1294. doi: 10.1161/STROKEAHA.110.599399. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palmero I, Hernando M, Garcia-Segura LM, Arevalo M-A. G protein-coupled estrogen receptor is required for the neuritogenic mechanism of 17bestradiol in developing hippocampal neurons. Mol. Cell. Endocrinol. 2013;372:105–115. doi: 10.1016/j.mce.2013.03.018. http://dx.doi.org/10.1016/j.mce.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. http://dx.doi.org/10.1161/01.STR.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Huang R-Q, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. http://dx.doi.org/10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Jun S, Simpkins JW. Estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERb AKAP and Drp1. Brain Res. 2015;1616:101–111. doi: 10.1016/j.brainres.2015.04.059. http://dx.doi.org/10.1016/j.brainres.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J. Neurosci. Res. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol. Aging. 2010a;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 2010b;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front. Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. http://dx.doi.org/10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. http://dx.doi.org/10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. http://dx.doi.org/10.1016/S0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. http://dx.doi.org/10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. http://dx.doi.org/10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or earlymenopause: long-term health consequences. Predict. Chronic Dis. Midlife. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. http://dx.doi.org/10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuro-protection. Brain Res. Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am. J. Med. 1997a;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997b;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Singh M. More than a decade of estrogen neuroprotection. Leon Thal Symp. Prev. Dement. 2008;4:S131–S136. doi: 10.1016/j.jalz.2007.10.009. http://dx.doi.org/10.1016/j.jalz.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation result in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocr. Soc. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone and neuroprotection. Horm. Neurotrauma Prot. Degener. Plast. 2013;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. http://dx.doi.org/10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R. Progestogens in hormonal replacement therapy: new molecules, risks, and benefits. Menopause. 2002;9:6–15. doi: 10.1097/00042192-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE. Sex differences in injury severity and outcome measures after traumatic brain injury1. Arch. Phys. Med. Rehabil. 2004;85:376–379. doi: 10.1016/j.apmr.2003.05.007. http://dx.doi.org/10.1016/j.apmr.2003.05.007. [DOI] [PubMed] [Google Scholar]
- Slowik A, Beyer C. Inflammasomes are neuroprotective targets for sex steroids. J. Steroid Biochem. Mol. Biol. 2015;153:135–143. doi: 10.1016/j.jsbmb.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. http://dx.doi.org/10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Exp. Neurol. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. http://dx.doi.org/10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Bake S, Lewis DK. Age-related changes in brain support cells: implications for stroke severity. Neurochem. Int. 2013a;63:291–301. doi: 10.1016/j.neuint.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RCG, Dominique Toran-Allerandt AC. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Neurobiology. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Selvamani A, Balden R. Revisiting the timing hypothesis: biomarkers that define the therapeutic window of estrogen for stroke. Horm. Behav. 2013b;63:222–230. doi: 10.1016/j.yhbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Williams M. Stroke neuroprotection: oestrogen and insulin-like growth factor-1 interactions and the role of microglia. J. Neuroendocrinol. 2013;25:1173–1181. doi: 10.1111/jne.12059. http://dx.doi.org/10.1111/jne.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J. Neurotrauma. 2005;22:345–352. doi: 10.1089/neu.2005.22.345. http://dx.doi.org/10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J. Chem. Neuroanat. 2009;38:185–196. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol. Learn. Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. http://dx.doi.org/10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Strom JO, Ingberg E, Theodorsson E, Theodorsson A. Effects of high and low 17beta-estradiol doses on focal cerebral ischemia: negative results. Sci. Rep. 2013;3:3111. doi: 10.1038/srep03111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Mechanisms of estrogens' dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int. J. Mol. Sci. 2011;12:1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U. S. A. 2007a;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 2007b;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Ruenes G, Dietrich WD. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Engler-Chiurazzi EB, Whiteaker P, Simard AR, Lukas R, Acosta JI, Prokai L, Bimonte-Nelson HA. A component of Premarin® enhances multiple cognitive functions and influences nicotinic receptor expression. Horm. Behav. 2010;58:917–928. doi: 10.1016/j.yhbeh.2010.09.002. http://dx.doi.org/10.1016/j.yhbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. http://dx.doi.org/10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. http://dx.doi.org/10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Tang M, Abplanalp W, Ayres S, Subbiah MT. Superior and distinct antioxidant effects of selected estrogen metabolites on lipid peroxidation. Metabolism. 1996;45:411–414. doi: 10.1016/s0026-0495(96)90212-7. [DOI] [PubMed] [Google Scholar]
- Timiras P, Quay W, Vernadakis A, editors. Hormones and Aging. CRC Press; New York: 1995. [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc. Natl. Acad. Sci. U. S. A. 1966;55:1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand C, Tinnikov AA, Singh RJ, Nethrapalli IS. 17α-Estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. http://dx.doi.org/10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. http://dx.doi.org/10.1161/01.STR.29.8.1666. [DOI] [PubMed] [Google Scholar]
- US Census Bureau Projections of the Population of Selected Age Groups and Sex for the United States: 2010 to 2050 (NP2008-T2) 2008 [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Percept. Mot. Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. http://dx.doi.org/10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Bredemann TM, McMahon LL. Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiol. Aging. 2014;35:2183–2192. doi: 10.1016/j.neurobiolaging.2014.04.004. http://dx.doi.org/10.1016/j.neurobiolaging.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocr. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. http://dx.doi.org/10.1016/S0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Zafonte RD, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. http://dx.doi.org/10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–792. doi: 10.1097/WNR.0b013e3282fe209c. http://dx.doi.org/10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Rhodes a.M.E., Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. http://dx.doi.org/10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. http://dx.doi.org/10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 2001;77:804–811. doi: 10.1046/j.1471-4159.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Santizo R, Baughman VL, Pelligrino DA, Iadecola C. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke. 1999;30:630–637. doi: 10.1161/01.str.30.3.630. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, Simpkins JW. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol. Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. http://dx.doi.org/10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez EJ, Zhang X, Simpkins JW. Estrogens protect human SK-N-SH neuroblastoma cells against H2O2. Toxic. Soc. Neurosci. Abstr. 2003a;29:635. [Google Scholar]
- Wang X, Simpkins JW, Dykens JA, Cammarata PR. Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability. Invest. Ophthalmol. Vis. Sci. 2003b;44:2067–2075. doi: 10.1167/iovs.02-0841. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. http://dx.doi.org/10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav. Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WGM, Lou WYW, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. http://dx.doi.org/10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Xiao G. The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects. Acta. Pharmacol. Sin. 2013;34:1485–1490. doi: 10.1038/aps.2013.160. http://dx.doi.org/10.1038/aps.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW. Estradiol is a neuroprotective factor in in vivo and in vitro models of brain injury. J. Neurocytol. 2000;29:401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations old and young rats. Neuroendocrinology. 1980;30:15–19. doi: 10.1159/000122968. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland S, Daniel JM. Increasing hippo-campal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS ONE. 2012;7:e51385. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm. Behav. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J. Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav. Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. http://dx.doi.org/10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr., Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;3:745–749. doi: 10.1161/01.str.31.3.745. [DOI] [PubMed] [Google Scholar]
- Yasui T, Yamada M, Kinoshita H, Uemura H, Yoneda N, Irahara M, Aono T, Sunahara S, Mito Y, Kurimoto F, Hata K. Combination of automatic HPLC-RIA method for determination of estrone and estradiol in serum. J. Clin. Lab. Anal. 1999;13:266–272. doi: 10.1002/(SICI)1098-2825(1999)13:6<266::AID-JCLA3>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Perez E, Yang S, Liu R, Covey DF, Simpkins JW. The assessment of non-feminizing estrogens for use in neuroprotection. Wind. Oppor. Menopause Estrogens Brain. 2011;1379:61–70. doi: 10.1016/j.brainres.2010.11.058. http://dx.doi.org/10.1016/j.brainres.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. http://dx.doi.org/10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yin W, Chen J. Apoptosis in cerebral ischemia: executional and regulatory signaling mechanisms. Neurol. Res. 2004;26:835–845. doi: 10.1179/016164104X3824. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, Vadlamudi RK, Brann DW. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol. 2014;389:84–91. doi: 10.1016/j.mce.2013.12.019. http://dx.doi.org/10.1016/j.mce.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving cal-pain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49. doi: 10.1186/1471-2202-7-49. http://dx.doi.org/10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton R. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin(R)) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wu T-W, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. http://dx.doi.org/10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J. Steroid Biochem. Mol. Biol. 1999a;68:65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Rapid inhibition of rat brain mitochondrial proton F0F1-ATPase activity by estrogens: comparison with Na+ K+-ATPase of porcine cortex. Eur. J. Pharmacol. 1999b;368:95–102. doi: 10.1016/s0014-2999(99)00012-6. http://dx.doi.org/10.1016/S0014-2999(99)00012-6. [DOI] [PubMed] [Google Scholar]