Abstract
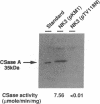
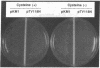
Cysteine synthase (CSase) [O-acetyl-L-serine acetate-lyase (adding hydrogen sulfide), EC 4.2.99.8] catalyzes the formation of L-cysteine, the key step in sulfur assimilation in plants, from O-acetyl-L-serine and hydrogen sulfide. We report here the isolation and characterization of cDNA clones encoding cysteine synthase from spinach (Spinacia oleracea L.). Internal peptide sequences were obtained from V8 protease-digested fragments of purified CSase. A lambda gt10 cDNA library was constructed from poly(A)+ RNA of young green leaves of spinach. Screening with two synthetic mixed nucleotides encoding the partial peptide sequences revealed 19 positively hybridized clones among 2 x 10(5) clones. Nucleotide sequence analysis of two independent cDNA clones revealed a continuous open reading frame encoding a polypeptide of 325 amino acids with a calculated molecular mass of 34,185 Da. Sequence comparison of the deduced amino acids revealed 53% identity with CSases of Escherichia coli and Salmonella typhimurium. Sequence homology was also observed with other metabolic enzymes for amino acids in bacteria and yeast and with rat hemoprotein H-450. A bacterial expression vector was constructed and could genetically complement an E. coli auxotroph that lacks CSases. The accumulation of functionally active spinach CSase in E. coli was also demonstrated by immunoblotting and assaying enzymatic activity. Southern hybridization analysis showed the presence of two to three copies of the cDNA sequence in the genome of spinach. RNA blot hybridization suggested constitutive expression in leaves and roots of spinach.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byrne C. R., Monroe R. S., Ward K. A., Kredich N. M. DNA sequences of the cysK regions of Salmonella typhimurium and Escherichia coli and linkage of the cysK regions to ptsH. J Bacteriol. 1988 Jul;170(7):3150–3157. doi: 10.1128/jb.170.7.3150-3157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasSarma S., Tischer E., Goodman H. M. Plant glutamine synthetase complements a glnA mutation in Escherichia coli. Science. 1986 Jun 6;232(4755):1242–1244. doi: 10.1126/science.2871626. [DOI] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Frisch D. A., Tommey A. M., Gengenbach B. G., Somers D. A. Direct genetic selection of a maize cDNA for dihydrodipicolinate synthase in an Escherichia coli dapA- auxotroph. Mol Gen Genet. 1991 Aug;228(1-2):287–293. doi: 10.1007/BF00282478. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Qian J. H., Satoh S., Kokudo S., Ikegami R., Hamaoka T. Studies on the induction of tolerance to alloantigens. II. The generation of serum factor(s) able to transfer alloantigen-specific tolerance for delayed-type hypersensitivity by portal venous inoculation with allogeneic cells. J Immunol. 1986 Apr 15;136(8):2763–2768. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Band L., Shimotsu H. Nucleotide sequence of the Bacillus subtilis tryptophan operon. Gene. 1985;34(2-3):169–177. doi: 10.1016/0378-1119(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Hirano H., Watanabe T. Microsequencing of proteins electrotransferred onto immobilizing matrices from polyacrylamide gel electrophoresis: application to an insoluble protein. Electrophoresis. 1990 Jul;11(7):573–580. doi: 10.1002/elps.1150110708. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Inoue Y. A new photosystem II reaction center component (4.8 kDa protein) encoded by chloroplast genome. FEBS Lett. 1988 Dec 5;241(1-2):99–104. doi: 10.1016/0014-5793(88)81039-1. [DOI] [PubMed] [Google Scholar]
- Levy S., Danchin A. Phylogeny of metabolic pathways: O-acetylserine sulphydrylase A is homologous to the tryptophan synthase beta subunit. Mol Microbiol. 1988 Nov;2(6):777–783. doi: 10.1111/j.1365-2958.1988.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Lunn J. E., Droux M., Martin J., Douce R. Localization of ATP Sulfurylase and O-Acetylserine(thiol)lyase in Spinach Leaves. Plant Physiol. 1990 Nov;94(3):1345–1352. doi: 10.1104/pp.94.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Noji M., Ohmori S., Imai Y., Murakoshi I. Integration and expression of a rabbit liver cytochrome P-450 gene in transgenic Nicotiana tabacum. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7041–7045. doi: 10.1073/pnas.88.16.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirko A., Hryniewicz M., Hulanicka D., Böck A. Sulfate and thiosulfate transport in Escherichia coli K-12: nucleotide sequence and expression of the cysTWAM gene cluster. J Bacteriol. 1990 Jun;172(6):3351–3357. doi: 10.1128/jb.172.6.3351-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]