Abstract
BACKGROUND
Red blood cell (RBC) transfusion thresholds have yet to be examined in large randomized trials in hematologic malignancies. This pilot study in acute leukemia uses a restrictive compared to a liberal transfusion strategy.
STUDY DESIGN AND METHODS
A randomized (2:1) study was conducted of restrictive (LOW) hemoglobin (Hb) trigger (7 g/dL) compared to higher (HIGH) Hb trigger (8 g/dL). The primary outcome was feasibility of conducting a larger trial. The four requirements for success required that more than 50% of the eligible patients could be consented, more than 75% of the patients randomized to the LOW arm tolerated the transfusion trigger, fewer than 15% of patients crossed over from the LOW arm to the HIGH arm, and no indication for the need to pause the study for safety concerns. Secondary outcomes included fatigue, bleeding, and RBCs and platelets transfused.
RESULTS
Ninety patients were consented and randomly assigned to LOW to HIGH. The four criteria for the primary objective of feasibility were met. When the number of units transfused was compared, adjusting for baseline Hb, the LOW arm was transfused on average 8.0 (95% confidence interval [CI], 6.9–9.1) units/patient while the HIGH arm received 11.7 (95% CI, 10.1–13.2) units (p = 0.0003). There was no significant difference in bleeding events or neutropenic fevers between study arms.
CONCLUSION
This study establishes feasibility for trial of Hb thresholds in leukemia through demonstration of success in all primary outcome metrics and a favorable safety profile. This population requires further study to evaluate the equivalence of liberal and restrictive transfusion thresholds in this unique clinical setting.
Nearly all patients with cancer experience some degree of anemia, either from the primary disease or from the effects of its treatment.1 In solid tumor malignancies, disease does not typically involve the marrow, and patients may require only a few red blood cell (RBC) units during the course of their chemotherapy. In contrast, patients with hematologic malignancies, especially the acute leukemias, typically have marrow involvement and the space-occupying leukemia cells prevent normal hematopoiesis, often resulting in profound cytopenias that require both RBC and platelet (PLT) transfusion support. In appropriate patients, high-dose chemotherapy is applied to treat the leukemia and the effects of this therapy are thus superimposed on an already dysfunctional marrow. Chemotherapeutic agents induce anemia by directly impairing hematopoiesis, including synthesis of RBC precursors in the marrow. Both malignant and healthy stem cells are affected by the chemotherapy, and even after the malignant cells are killed, it can take weeks for healthy cells to reconstitute the marrow. Consequently, leukemia patients are uniquely affected by both their disease and its treatment, resulting in a universal requirement for both RBC and PLT transfusions.
The number of RBC units required to support a leukemia patient through induction therapy has been reported in wide ranges from 30 to 60 units during the first 2 months of therapy2 but has been decreasing over time likely due to increased attention to transfusion burdens. Current practice in our center and most other major comprehensive leukemia programs utilizes a hemoglobin (Hb) transfusion trigger of 8 to 8.5 g/dL or higher, often with 2 units transfused when triggered.
Historically, RBC transfusions have been utilized to obtain relatively high Hb targets (e.g., ≥9–10 g/dL) in hematologic malignancy patients with the hope of increasing oxygen delivery, improving organ function, and decreasing patient fatigue. Increasing data in other clinical settings suggest that a lower Hb transfusion threshold (7–8 g/dL) is associated with identical or even lower mortality rates compared to a higher Hb transfusion threshold (9–10 g/dL). Prospective randomized trials supporting lower transfusion thresholds have been completed or planned in high-risk orthopedic surgery patients,3 critically ill adult4 and pediatric intensive care unit patients,5 patients with acute gastrointestinal bleeding,6 cardiac surgical patients,7 and stem cell transplant recipients.8 These studies have mainly shown that a restrictive strategy of RBC transfusion is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill medical and surgical patients. However, no trials with this restrictive approach have been conducted in the oncologic setting with patients receiving active chemotherapy with the goal of feasibility. Webert and colleagues9 did conduct a pilot study in leukemia and transplant patients where they compared an augmented RBC threshold against the standard with the goal of assessing the feasibility of a larger trial investigating the effect of Hb on bleeding risk in thrombocytopenic patients. Since it is known that acute leukemia patients have the added complication of accompanying thrombocytopenia this concept of restricting RBCs must be studied further, as there is the concern that anemia may promote increased bleeding with fewer available RBCs to push PLTs to the vessel wall.10 Furthermore, there is the additional challenge of managing transfusions in leukemia patients whose care is often divided between the inpatient and outpatient settings as they receive therapy and then await marrow recovery.
Acute leukemia patients, despite their unique challenges, represent a worthwhile population in which to employ rigorous clinical trial procedures to address the issue of transfusion thresholds. Here we review the outcomes in a randomized pilot study using a restrictive transfusion strategy, in which patients received single-unit RBC transfusions with Hb transfusion trigger of 7 g/dL, compared to a more standard 8 g/dL trigger to determine the feasibility of conducting a larger clinical trial.
MATERIALS AND METHODS
Setting
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins is a tertiary referral center for oncology, specifically patients with acute leukemias. Approximately 125 new acute leukemia patients are treated as inpatients annually.
Human subjects protection
This study was approved by the institutional review board (IRB) at Johns Hopkins. All patients signed an IRB-approved informed consent for their randomization and for their data to be obtained in accordance with the Declaration of Helsinki. All data collected were stored and secured in an IRB-approved database.
Intervention
Figure 1 outlines the study design. The randomization was done using a 2:1 group assignment ratio, with two patients assigned to the restrictive (LOW) Hb trigger (7 g/dL) and one patient assigned to the higher (HIGH) Hb trigger (8 g/dL), to ensure that enough patients were assigned to the restrictive trigger to determine the feasibility of a larger trial. There were no changes to the trial after it commenced.
Fig. 1.
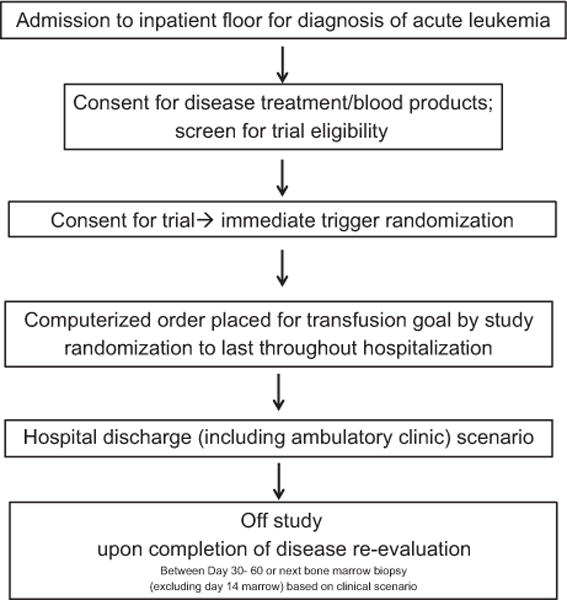
Trial procedure.
The primary objective was the feasibility of conducting a larger randomized trial, which was defined a priori as achieving the following four criteria: 1) more than 50% of the eligible patients consented, 2) more than 75% of the patients randomized to the 7 g/dL arm tolerated the transfusion trigger, 3) fewer than 15% of patients crossed over from the lower transfusion threshold arm to the higher transfusion threshold arm, and 4) no indications for the need to pause the study for safety concerns. The tolerability of the LOW arm was defined as a lack of patient or physician desire for the patient to be transfused at a goal higher than the preset 7 g/dL. Crossover from the higher transfusion threshold arm to the lower transfusion threshold arm was not assessed for feasibility as the higher transfusion threshold of 8 g/dL was the current standard at our institution and was not to be deviated from outside the setting of the study.
The secondary outcomes included fatigue, bleeding, response to therapy, vital status on Day 60, length of hospital stay (days), and finally the number of units of RBCs and PLTs transfused per patient. Fatigue was assessed by a numeric 10-point fatigue scale that patients reported to staff where 0/10 was no fatigue, 5/10 was moderate fatigue, and 10/10 was worst possible fatigue and graded as the National Cancer Institute Fatigue Scale.11 Bleeding was graded using the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Response to therapy was evaluated by the Cheson criteria12 with addition of flow and molecular features. Vital status on Day 60 was censored when disease was reevaluated with marrow examination after therapy. The patients were assessed for bleeding and fatigue daily by the treating providers and documented in the daily progress notes. This was prospectively planned at the start of the trial and is the standard protocol for these patients at our institution. The bedside nurses as well as the physicians are required to document bleeding and fatigue daily. There were no adjudicators.
The criteria for inclusion included all acute leukemia patients (acute myeloid leukemia [AML], acute lymphoblastic leukemia/lymphoma, acute promyelocytic leukemia [APL], treatment-related myeloid neoplasm, high-grade myelodysplastic syndrome) more than 18 years of age admitted to the inpatient leukemia services with plans for inpatient myelosuppressive chemotherapy with standard of care or clinical protocol regimens (this included induction for patients with low-risk APL). All patients met clinical criteria for receipt of induction treatment. The criteria for recipient ineligibility included acute coronary syndrome (as defined by active chest pain, dynamic electrocardiogram changes, troponin greater than 2.5), known active blood loss with hemodynamic instability, receiving erythropoietin-stimulating agents before admission, or a documented wish against transfusion for personal or religious beliefs. No patients were excluded on the basis of sex, racial, or ethnic background.
The random-number sequence was generated using computer software (JMP Version 9.0, SAS Institute). Treatment assignment was done with a 2:1 ratio, for the LOW:HIGH Hb trigger groups, respectively. Blocking was used to specify a 2:1 ratio of treatment groups for each group of 18 consecutive patients. Sealed opaque sequentially numbered envelopes were opened upon determination of inclusion for each patient in the trial. The randomization sequence and creation and numbering of the envelopes was performed by an investigator who did not enroll or consent patients for the trial.
The blood components for these oncology patients were all prepared per institutional standard procedure from our blood bank. All RBC units were leukoreduced and irradiated and prepared in additive solutions. All PLTs were single-donor apheresis collections that were leukoreduced and irradiated.
Statistical analysis
Feasibility criteria were summarized with proportions and 95% confidence intervals (CIs). Safety outcomes and transfusion variables were compared between study arms with either the Wilcoxon-Mann-Whitney test for continuous factors or the chi-square or Fisher’s exact tests for categorical variables. An analysis of covariance model was used to compare the number of RBC units transfused between the study arms, adjusting for baseline Hb.
Hb values before and after each transfusion were compared by study arm. To account for the correlation among the multiple pre and post values obtained from the same patient, generalized estimating equations were used (assuming a compound symmetry correlation structure) for model estimation and hypothesis testing of mean differences based on the chi-square statistic. Specifically, we modeled the vector of Hb values among patients as a function of study arm, pre- or posttransfusion, and the interaction of the two. Particular comparisons of interest included overall differences between the HIGH and LOW threshold arms, differences between arms before transfusion, and after transfusions. Statistical analyses were performed using computer software (R 3.0, www.r-project.org) and all p values reported are two sided.
RESULTS
Figure 2 is a flow diagram showing the number of patients eligible, approached, and consented, as well as their disposition after randomization. Between April 15, 2014, and July 23, 2015, there were 162 patients eligible for this study, of which 112 were approached for consent. Approachability was only limited by trial personnel availability to consent. Patients newly admitted to the leukemia service to undergo intensive induction chemotherapy were the included denominator throughout. Ninety patients of the 112 approached (80.4%; 95% CI, 71.78%–87.26%) gave consent, and all of the consented patients were randomized, 60 to the LOW transfusion threshold arm and 30 to the HIGH arm. The accrual rate was approximately six patients per month. One patient randomized to the LOW arm was not treated on study as the patient withdrew consent before any transfusions performed. All 89 patients randomly assigned and treated on the study protocol were included in the analysis. The patients who were approached and declined cited reasons for refusal as lack of willingness to participate in a clinical trial (seven patients), refusal for randomization (12 patients), and expressed concern of withholding of standard of care transfusion threshold (three patients).
Fig. 2.
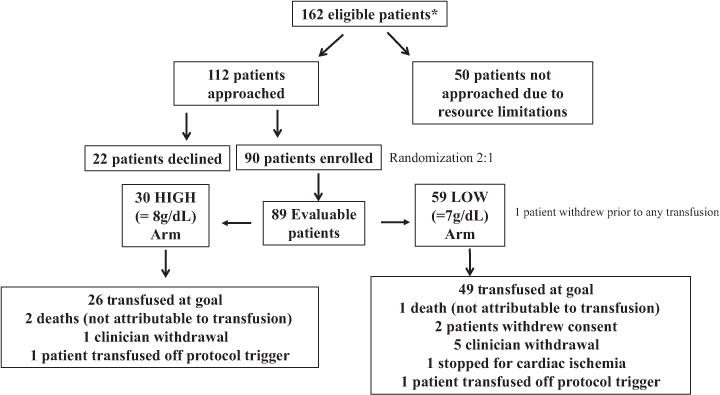
Patient distribution. *All 162 patients were consecutive, newly admitted acute leukemia patients eligible by preset criteria.
The four criteria for the primary objective were met. For consent and tolerability, the lower bounds of the 95% CIs for the estimates were above the benchmark of 50 and 75% set in the protocol. The crossover criterion was met as well, but the CI did include the benchmark of 15%. The study was not paused for safety concerns.
Baseline data are summarized in Table 1 by study arm. Age, sex, diagnoses, Eastern Cooperative Oncology Group performance status, white blood cell (WBC) counts, and PLT counts were comparable across the two study arms. The period of time over which patients were on study during which RBC units were transfused (consent until the time of disease reevaluation) was also similar between the two groups. In the LOW threshold arm the median on study duration was 5.9 weeks and in the HIGH threshold arm it was 6.1 weeks (Wilcoxon p = 0.45). Baseline Hb levels were somewhat lower in the LOW threshold group, a median of 8.3 g/dL compared to 8.9 g/dL in the HIGH group (Wilcoxon p = 0.03).
TABLE 1.
Patient characteristics*
| Characteristic | LOW (n=59) | HIGH (n=30) | p value |
|---|---|---|---|
| Age (years) | 56 (45.5–67) | 62.5 (55.2–67.8) | 0.23 |
| Sex male (%) | 33 (46) | 16 (47) | 0.94 |
| Diagnosis (%) | 0.44 | ||
| AML (tAML, sAML, CML blast crisis) | 50 (85) | 23 (77) | |
| ALL-B cell | 6 (10) | 5 (17) | |
| ALL-T cell | 1 (2) | 2 (7) | |
| APL | 2 (3) | 0 (0) | |
| ECOG performance status | 0.87 | ||
| 0 | 39 (66) | 19 (63) | |
| 1 | 19 (32) | 10 (33) | |
| 2 | 1 (2) | 1(3) | |
| WBC count at presentation (×109/L) | 4.5 (1.3–20.8) | 5.2 (1.5–17.8) | 0.85 |
| Hb at presentation (g/dL) | 8.3 (7.5–8.9) | 8.9 (8.1–9.2) | 0.03 |
| PLT count at presentation (×109/L) | 58 (32.5–89.5) | 70.5 (44–97.2) | 0.43 |
Data are reported as frequency (%) or as median (interquartile range), unless otherwise reported.
ALL = acute lymphoblastic leukemia/lymphoma; CML = chronic myelogenous leukemia; ECOG = Eastern Cooperative Oncology Group; SAML = secondary AML; tAML = treatment-related AML.
Evaluability was 100% in the HIGH arm and 98% (95% CI, 91.06%–99.96%) in the LOW arm. There were two protocol deviations, one per arm, where patients were transfused before having reached their preset trigger accidentally. The only program-related unacceptable toxicity occurred in the LOW arm. Both patient and clinician decisions to withdraw from study were slightly higher in the LOW arm: patient decision two of 59 (3.4%; 95% CI, 0.41%–11.71%) versus zero of 30 (0%; 95% CI, NA–11.57%), and clinician decision five of 59 (8.5%; 95% CI, 2.81%–18.68%) versus one of 30 (3.3%; 95% CI, 0.08%–17.22%). The two patient reasons for withdrawal of consent were both noted as decreased performance status or fatigue that they believed would improve after transfusion to a higher Hb. Upon subsequent query, both patients believed that they did feel better off the trial. The clinician withdrawals of consent were an inpatient fall attributed to anemia resulting in a head laceration (one patient), sepsis and goal of improved perfusion with higher Hb (two patients), inability to follow trial trigger due to extensive alloantibodies and the requirement to transfusion only when blood was available (one patient), and a decreased patient performance status or fatigue perceived by the provider as related to anemia (one patient).
One death occurred in the LOW arm and two in the HIGH arm. No deaths were attributed to the study procedures but were instead attributed to induction mortality related to underlying disease or complication of chemotherapy that was previously known. Overall, the proportion of patients completing the study was similar: 49 of 59 (83.1%; 95% CI, 71.03%–91.56%) for the LOW arm and 26 of 30 (86.7%; 95% CI, 69.28%–96.24%) for the HIGH (Fisher’s exact, p = 0.77). The incidence of crossover was also similar in the two study arms: seven of 59 (11.9%; 95% CI, 4.91%–22.93%) in the LOW arm and two of 30(6.7%; 95% CI, 0.82%–22.07%) in the HIGH (chi-square, p = 0.44).
Transfusion outcomes, safety, and other secondary endpoints are summarized in Table 2. The number of RBC units transfused was significantly different between study arms. As expected, patients in the LOW threshold arm received fewer RBC units, a median of 8 (6–11) compared to a median of 10 (8–12) in the HIGH threshold arm (Wilcoxon p = 0.01). When the mean number of RBC units transfused was compared between arms of the study, adjusting for baseline Hb, the LOW arm was transfused 8.0 (95% CI, 6.9–9.1) units per patient while the HIGH arm patients received 11.7 (95% CI, 10.1–13.2) units for an estimated difference (LOW minus HIGH) of −3.7 (95% CI, −5.6 to −1.7) units per patient, analysis of covariance p = 0.0003. Fifty-three of 59 patients (89.8%; 95% CI, 79.17, 96.18%) on the LOW arm were able to tolerate this restrictive Hb trigger.
TABLE 2.
Patient results*
| LOW (n=59) | HIGH (n=30) | p value | |
|---|---|---|---|
| RBC transfusions (units) | |||
| Median | 8 (6–11) | 10 (8–12) | 0.01 |
| Mean | 8.2 (4.2) | 11.3 (5.4) | 0.01 |
| PLT transfusions in transfusion episodes | 9 (5.5–12.5) | 9 (7–12) | 0.81 |
| Mean corpuscle Hb concentration (posttransfusion) | 33.6 (1.4) | 33.2 (2) | 0.91 |
| Bleeding events by grade (%) | 0.82 | ||
| 0 | 40 (68) | 19 (30) | |
| 1 | 10 (17) | 6 (20) | |
| 2 | 4 (7) | 3 (10) | |
| 3 | 3 (5) | 2 (7) | |
| 4 | 2 (3) | 0 (0) | |
| Length of inpatient stay (days) | 35.5 (31.2–43.8) | 36 (29.2–44) | 0.53 |
| Fatigue Scale Score | 4.8 (4–5.2) | 4.5 (3.6–5) | 0.32 |
| Episodes of neutropenic fever (%) | 0.60 | ||
| 0 | 15 (25) | 9 (30) | |
| 1 | 23 (39) | 13 (43) | |
| 2 | 13 (22) | 5 (17) | |
| 3 | 6 (10) | 3 (3) | |
| 4 | 3 (3) | 3 (3) | |
| 5 | 0 (0) | 3 (3) | |
| Disease response after therapy (%) | 0.42 | ||
| Complete response | 25 (42) | 17 (57) | |
| Minimal residual disease by flow cytometry | 11 (19) | 2 (7) | |
| Minimal residual disease by molecular features | 4 (7) | 2 (7) | |
| Partial response with transfusion dependence | 5 (8) | 2 (7) | |
| Treatment failure | 14 (24) | 6 (20) | |
| Not evaluable | 0 (0) | 3 (3) |
Data are reported as frequency (%) or as median (interquartile range) or mean (SD).
We examined study Hb values for mean differences between study arm and before and after transfusion. Both study arm and time of measurement were significant factors (p < 0.0001). Generalized estimating equations tests of contrasts, between arms irrespective of time, between post- and pretransfusion irrespective of study arm, as well as between arms adjusting for time and between time points adjusting for arm, were significant with the HIGH arm mean always higher and the posttransfusion mean always higher (p < 0.0001). The lowest values were seen in the LOW threshold arm before transfusion, with a mean of 6.8 g/dL (95% CI, 6.79–6.85 g/dL), and the highest in the HIGH arm after transfusion, with a mean of 8.6 g/dL (95% CI, 8.44–8.71 g/dL). The LOW threshold mean Hb after transfusion was the same as the mean Hb in the HIGH arm before transfusion: 7.7 g/dL (95% CI, 7.6–7.7 g/dL) and 7.7 (95% CI, 7.6–7.8 g/dL), respectively. This demonstrated a difference in achieved Hb between the two arms of nearly 1 to 7.7 g/dL in the LOW threshold arm compared to 8.6 g/dL in the HIGH. The mean corpuscle Hb concentration after transfusion in the LOW arm was 33.6 ± 1.4 and 33.2 ± 2 in the HIGH arm.
Neither the frequency of bleeding, 32 and 37% in the LOW and HIGH arms, respectively, nor the distribution of bleeding grades was significantly different between study arms. The median fatigue scores were similar, 4.8 and 4.5 for the LOW and HIGH groups, respectively, as were frequency distributions of episodes of neutropenic fever (see Table 2).
In the LOW threshold arm, 19% of patients survived past Day 60, 5% died before this, and 76% were censored as alive. In the HIGH therapy arm 23% were alive on Day 60, 10% had died before Day 60, and 67% were censored as alive.
DISCUSSION
Here we demonstrate that, in a single institution, both patients and physicians tolerate randomization between Hb transfusion thresholds in cytopenic acute leukemia patients receiving myelosuppressive chemotherapy. This pilot study suggests that a larger randomized clinical trial in the same population will be possible. In our feasibility study of transfusion thresholds, all four criteria defining the success of the pilot study were achieved. For consent and tolerability, the lower bounds of the 95% CIs for the estimates were above the benchmarks set. The crossover criterion was met as well, but the CI did include the benchmark of 15%. The study was not paused for safety concerns and there was no signal for harm in either transfusion threshold group. The patients in the LOW arm received fewer transfusions and did not experience higher fatigue scores or more bleeding events in this current trial. It should be noted that when considering the potential drawbacks of the lower Hb threshold, increased fatigue was a primary concern among patients and their physicians, but our preliminary findings suggest no significant difference in fatigue. On average 3 fewer RBC units per patient were transfused in the LOW arm (an approximate 20% reduction in RBC requirements).
Given the patient population of those with leukemia admitted for induction there were relatively few patients who refused the study and no one who did not meet the eligibility criteria as these patients do not often present with bleeding and hemodynamic instability or coronary ischemia as demonstrated by elevated troponins. This suggests that the challenges to broader patient recruitment will likely be limited to patient and physician preference which was also relatively easily overcome in this small pilot. The reasons for clinical and patient withdrawal as described above will remain a challenge for a larger study but our hope would be that this reassuring pilot study will allow a larger trial to proceed with equipoise.
Current practice in most major comprehensive cancer centers utilizes Hb transfusion triggers of 8 g/dL or higher. Cancer- and chemotherapy-induced anemia guidelines from the National Comprehensive Cancer Network are broad with large ranges for transfusion triggers in asymptomatic anemia of Hb 7 to 9 g/dL and if symptomatic, then 8 to 10 g/dL. However, these recommendations and practices are based on little data. It is possible that the use of a higher Hb transfusion threshold may contribute to worse outcomes in acute leukemia. Studies in critically ill and cardiac surgery patients have suggested an increased infectious risk13 with a liberal transfusion strategy using higher Hb targets, which may be especially relevant in neutropenic patients. Overly aggressive transfusion practices may also contribute to volume overload and subsequent pulmonary edema and respiratory failure, both of which are common during induction chemotherapy (e.g., with cytarabine and known capillary leak syndrome). Additionally, after surviving induction, many leukemia patients will proceed to bone marrow transplantation and require additional transfusion support during a second period of aplasia. Iron overload and resultant toxicity is a real concern in these patients and may be mitigated by limiting the initial transfusions during induction. On the other hand, it should be noted that none of the critical care or surgical transfusion studies discussed above examined transfusion thresholds in patients with concomitant thrombocytopenia requiring PLT transfusions. There is the theoretical concern that thrombocytopenic patients require higher Hb and hematocrits to avoid critical bleeding events.10 Furthermore, the development of RBC alloimmunization and a resulting increase in cost and complexity of transfusion is also a concern; studies in patients with myelodysplastic syndrome and chronic myelomonocytic leukemia found that alloimmunization increased with the number of RBC transfusions and was associated with a significant incidence of complex immunization.14 Additionally, there is evidence that increased transfusions in cancer patients (all tumor types, predominantly solid tumors) are associated with increased mortality (odds ratio, 1.34; 95% CI, 1.29–1.38),15 although it is unclear whether observation is related to more advanced disease or the adverse effects of transfusions such as increased infections. Finally, resource utilization is an important consideration. If the transfusion reduction data from this pilot are extrapolated to the nearly 55,000 acute leukemia patients treated per year in the United States, approximately 165,000 units of RBCs could be saved yearly using the lower transfusion threshold. The acquisition cost for a unit of leukoreduced RBCs is between $200 and $250 in the United States, but the total cost, including overhead, of bringing a unit of blood from the donor to the recipient, is fourfold higher than the acquisition cost.16,17 This reduction could result in significantly conserved health care costs and resources.
The 2009 Transfusion Medicine State-of-the-Science Symposium from the National Heart, Lung, and Blood Institute concluded that RBC transfusion and/or blood conservation management RBC transfusion trigger strategies should be investigated further with the goal of improved overall outcomes in different patient populations (e.g., acute leukemias).18 Acute leukemia patients represent a reasonable population in which to employ rigorous clinical trial procedures to address this issue. Much of our understanding of PLT transfusion management for oncology patients was derived from clinical trials involving patients with acute leukemia,19,20 suggesting how beneficial trials can be in this patient population.
To build on the current pilot data in a larger trial, the next steps should involve the study of transfusion triggers in acute leukemia in a multi-institutional fashion. While we have demonstrated feasibility with this limited pilot study, the ideal transfusion threshold for patients receiving chemotherapy for acute leukemia remains unknown. In response to the data in nononcologic populations and in an attempt to curtail inappropriate and potentially injurious transfusions, accreditation agencies such as the AABB and The Joint Commission have encouraged patient blood management programs to promote restrictive transfusion practices.13,21–24 In the leukemia patient population, which is necessarily committed to multiple transfusions over the treatment course, it seems prudent to assess if reducing transfusions is possible in the trial setting. The likely next step in a randomized trial should narrow the study population to only include AML so that the therapy is more homogeneous. Further randomized trials with robust methodology are required to determine the optimal transfusion strategy for such patients with objectives beyond feasibility, including overall survival and a more detailed assessment of morbid events such as hospital-acquired infections, overall costs, and efficacy. A large multicenter trial of likely more than 1000 patients and perhaps a greater separation of the Hb transfusions triggers will be required. Suggestions to enhance detection of a treatment effect include comparisons of 7 g/dL versus 9 g/dL as has been done in other populations to gain a greater distinction between the treatment cohorts. Additionally, there could be benefits of stratifying by age given the bimodal age presentations of AML patients. There could be a difference in older patients who have either had an antecedent hematologic disorder or have previously undiagnosed ischemic heart disease—differences that could not be discerned in this pilot trial.
Certain limitations should be recognized in the current study. Although the sample size was too small to determine some differences in all outcomes, for the purposes of a pilot and feasibility study, we feel that we were able to accomplish our primary aims with the number of patients studied. Second, the study, by its nature, was not blinded and both the patients and their providers were aware of the treatment assignment groups. Lack of blinding could theoretically have influenced some outcome measures. For example, the fatigue scores may have been falsely low in the LOW group, resulting in an overestimation of fatigue difference between groups; however, the similarity in fatigue scores suggests this potential bias was not a concern. Finally, there was initial inherent bias among nurses and physicians who were concerned about withholding transfusions from patients who need them, which may have increased the incidence of crossovers from the LOW to the HIGH group. This bias, however, appeared to decrease over time, suggesting that the change in practice to a restrictive transfusion strategy is one that can be accomplished with clinical providers adapting well to the change.
This study is unique due to the inherent challenges of the patient population in the context of disease and therapy as well as known biases at play in the hematologic malignancies population. Patients and clinicians were amenable to the study. This population requires an independent study since the previous studies of primary surgical or intensive care patients demonstrating the equivalence of liberal and restrictive transfusion thresholds were not conducted in patients with thrombocytopenia, nor in patients with prolonged illnesses. A larger clinical trial is imperative and would be highly innovative research evaluating blood transfusion, one of the most commonly used treatments available for decades, in patients with acute leukemia. Such a trial would have the potential to improve outcomes and reduce costs, which in turn increased the value of care rendered to our patients.
Acknowledgments
We are grateful to the patients who agreed to be a part of this study and the nurses (especially Karen Mackey) and doctors who actively participated in the study and the clinical care of these patients.
This work was supported by a grant from the Society for the Advancement of Blood Management (SABM) sponsored by Haemonetics Corp. (Braintree, MA; to AED).
ABBREVIATIONS
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- IRB
institutional review board
Footnotes
This is trial NCT02086773 on ClinicalTrials.gov.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
References
- 1.Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. Oncologist. 2009;14(Suppl 1):43–56. doi: 10.1634/theoncologist.2009-S1-43. [DOI] [PubMed] [Google Scholar]
- 2.Cannas G, Fattoum J, Raba M, et al. Transfusion dependency at diagnosis and transfusion intensity during initial chemotherapy are associated with poorer outcomes in adult acute myeloid leukemia. Ann Hematol. 2015;94:1797–806. doi: 10.1007/s00277-015-2456-2. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 8.Tay J, Tinmouth A, Fergusson D, et al. Transfusion of red cells in hematopoietic stem cell transplantation (TRIST): study protocol for a randomized controlled trial. Trials. 2011;12:207. doi: 10.1186/1745-6215-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webert KE, Cook RJ, Couban S, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion. 2008;48:81–91. doi: 10.1111/j.1537-2995.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 10.Escolar G, Garrido M, Mazzara R, et al. Experimental basis for the use of red cell transfusion in the management of anemic-thrombocytopenic patients. Transfusion. 1988;28:406–11. doi: 10.1046/j.1537-2995.1988.28588337325.x. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanz C, Nomdedeu M, Belkaid M, et al. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53:710–5. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shander A, Hofmann A, Gombotz H, et al. Estimating the cost of blood: past, present, and future directions. Best Pract Res Clin Anaesthesiol. 2007;21:271–89. doi: 10.1016/j.bpa.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 18.Josephson CD, Glynn SA, Kleinman SH, et al. A multidisciplinary “think tank”: the top 10 clinical trial opportunities in transfusion medicine from the National Heart, Lung, and Blood Institute-sponsored 2009 state-of-the-science symposium. Transfusion. 2011;51:828–41. doi: 10.1111/j.1537-2995.2010.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.[No authors listed]; Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 21.Spahn DR, Vamvakas EC. Is best transfusion practice alone best clinical practice? Blood Transfus. 2013;11:172–4. doi: 10.2450/2012.0283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vamvakas EC. Reasons for moving toward a patient-centric paradigm of clinical transfusion medicine practice. Transfusion. 2013;53:888–901. doi: 10.1111/j.1537-2995.2012.03825.x. [DOI] [PubMed] [Google Scholar]
- 23.Zalpuri S, Middelburg RA, van de Watering L, et al. Association vs. causality in transfusion medicine: understanding multivariable analysis in prediction vs. etiologic research. Transfus Med Rev. 2013;27:74–81. doi: 10.1016/j.tmrv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Hopewell S, Omar O, Hyde C, et al. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ Open. 2013;3:e002154. doi: 10.1136/bmjopen-2012-002154. [DOI] [PMC free article] [PubMed] [Google Scholar]


