Highlight
Recent research shows that sugar availability triggers bud outgrowth. This paper further demonstrates that the effect of sucrose involves changes in the hormonal network related to bud outgrowth, and identifies potential hormones involved in sugar control.
Key words: Auxin, bud burst, cytokinins, Rosa sp., shoot branching, strigolactones, sugar, sugar signalling.
Abstract
Sugar has only recently been identified as a key player in triggering bud outgrowth, while hormonal control of bud outgrowth is already well established. To get a better understanding of sugar control, the present study investigated how sugar availability modulates the hormonal network during bud outgrowth in Rosa hybrida. Other plant models, for which mutants are available, were used when necessary. Buds were grown in vitro to manipulate available sugars. The temporal patterns of the hormonal regulatory network were assessed in parallel with bud outgrowth dynamics. Sucrose determined bud entrance into sustained growth in a concentration-dependent manner. Sustained growth was accompanied by sustained auxin production in buds, and sustained auxin export in a DR5::GUS-expressing pea line. Several events occurred ahead of sucrose-stimulated bud outgrowth. Sucrose upregulated early auxin synthesis genes (RhTAR1, RhYUC1) and the auxin efflux carrier gene RhPIN1, and promoted PIN1 abundance at the plasma membrane in a pPIN1::PIN1-GFP-expressing tomato line. Sucrose downregulated both RwMAX2, involved in the strigolactone-transduction pathway, and RhBRC1, a repressor of branching, at an early stage. The presence of sucrose also increased stem cytokinin content, but sucrose-promoted bud outgrowth was not related to that pathway. In these processes, several non-metabolizable sucrose analogues induced sustained bud outgrowth in R. hybrida, Pisum sativum, and Arabidopsis thaliana, suggesting that sucrose was involved in a signalling pathway. In conclusion, we identified potential hormonal candidates for bud outgrowth control by sugar. They are central to future investigations aimed at disentangling the processes that underlie regulation of bud outgrowth by sugar.
Introduction
Bud outgrowth is a highly controlled process by which the plant can adjust its development to environmental conditions. Among the internal regulators affecting this process, auxin (indole-3-acetic acid, IAA) produced by the young apical leaves was suggested to be responsible for the inhibition of lower axillary buds during apical dominance a long time ago (Thimann and Skoog, 1934; Cline, 1991; Domagalska and Leyser, 2011). Recently, less emphasis has been given to the role of auxin during apical dominance; rather, the involvement of sugar has been investigated. Indeed, after decapitation in pea, Morris et al. (2005) showed that initial bud outgrowth occurred prior to changes in auxin content in the adjacent stem tissues. Mason et al. (2014) support a theory in which loss of the shoot tip would remove a large sink for sugar and induce rapid distribution of sugar over long distances, which would be responsible for initial bud outgrowth. It is well established that bud outgrowth occurs along with a large induction of sugar metabolism and transport within buds (Marquat et al., 1999; Decourteix et al., 2008; Bonhomme et al., 2010; Girault et al., 2010; Henry et al., 2011; Rabot et al., 2012). Moreover, defoliation experiments (Mitchell, 1953; Kebrom et al., 2010) and more recently the use of the wheat tiller inhibition mutant, in which sugars are diverted to highly elongating internodes (Kebrom et al., 2012), suggest the control of bud outgrowth by sugar availability within the plant. Mason et al. (2014) demonstrated a causal relationship between sugar availability and bud outgrowth in planta: external sugar supply was sufficient to trigger bud outgrowth in non-decapitated pea plants. Similarly, a supply of sugar is necessary to trigger the outgrowth of buds in vitro (Henry et al., 2011; Rabot et al., 2012; Mason et al., 2014). In this process, sucrose was suggested not only to play a trophic role, but also to act as a signalling entity, because palatinose, a non-metabolizable sucrose analogue, is able to trigger bud outgrowth (Rabot et al., 2012). Although these findings highlight the importance of sugar as a trigger of bud outgrowth, the mechanisms underlying the induction of bud outgrowth by sugar are yet to be elucidated.
Much work has been done to unravel the inhibitory effect of auxin on bud outgrowth (Thimann and Skoog, 1934; Ferguson and Beveridge, 2009; Waldie et al., 2010; Domagalska and Leyser, 2011). This effect is indirect, since apically derived auxin does not enter the buds (Hall and Hillman, 1975; Prasad et al., 1993) and therefore two leading models, involving second messengers and the process of auxin canalization, have been proposed. The auxin canalization-based model is based on Sachs’ auxin transport model (Sachs, 1981; Bennett et al., 2014). In this model, axillary buds are activated when the auxin initially flowing out of the bud is sufficient to trigger the establishment of a polar auxin transport canal connecting it to the auxin stream in the stem (Li and Bangerth, 1999; Domagalska and Leyser, 2011). The continual flow of auxin produced at the apex prevents axillary buds on the same axis from exporting their own auxin, thereby maintaining apical dominance. The establishment of polar auxin transport involves a regulatory positive feedback loop between the directional auxin flow and the polarization of the efflux facilitator PIN-FORMED proteins (PINs) at the plasma membrane in the direction of the initial flow (Bennett et al., 2014). Strigolactones would act upstream of auxin by stimulating PIN removal from the plasma membrane, thus reducing the ability of the bud to create its own polar auxin transport (Shinohara et al., 2013). In the second messenger-based model, auxin flow in the main stem negatively regulates the synthesis of cytokinins (Sachs and Thimann, 1967; Shimizu-Sato et al., 2009) and positively regulates the levels of strigolactones (Brewer et al., 2009), which act antagonistically on buds by inducing and inhibiting their outgrowth, respectively (Hayward et al., 2009; Dun et al., 2012). Within buds, the antagonistic effect of cytokinins and strigolactones is notably integrated by BRANCHED1 (BRC1), a transcription factor mainly expressed in non-growing axillary buds and with knock-out mutants that exhibit a highly branched phenotype (Aguilar-Martínez et al., 2007; Braun et al., 2012; Dun et al., 2012).
In contexts other than bud outgrowth, several studies report that sugars control the biosynthesis, transport, or signalling of certain hormones, including auxin and cytokinins (Mishra et al., 2009; LeClere et al., 2010; Arrom and Munné-Bosch, 2012b; Sairanen et al., 2012). In Arabidopsis thaliana roots, a genome-wide expression profiling study showed that glucose upregulated YUCCA2, an auxin biosynthesis gene, and two members of the auxin efflux gene family including PIN1 (Mishra et al., 2009). Moreover, sugars stimulated auxin biosynthesis by upregulating ZmYUCC1 in developing maize kernels (LeClere et al., 2010) or AtTAA and AtYUCCA8 in Arabidopsis seedlings (Stewart Lilley et al., 2012). Several genes involved in cytokinin metabolism are also regulated by sugars in Arabidopsis (Kushwah and Laxmi, 2013). Glucose upregulates the cytokinin biosynthesis gene IPT3, and has a differential effect on the expression of cytokinin catabolism-related genes: it upregulates CKX4 and downregulates CKX5. Unlike for auxin and cytokinins, no report is currently available about the relationships between sugars and strigolactones, although we know that their biosynthesis and signalling pathways are under the control of phosphate and nitrogen (Czarnecki et al., 2013; Sun et al., 2014).
The objective of the present study was to determine whether sucrose and its signalling component modulate early hormonal homeostasis during bud outgrowth in Rosa hybrida. To monitor sugar availability for buds, we cultivated bud-bearing stem segments in vitro and supplied them with different sugar conditions, including different sucrose concentrations and non-metabolizable sucrose analogues (Chatfield et al., 2000; Henry et al., 2011; Rabot et al., 2012). To assess the sequence of events, we monitored the temporal patterns of bud outgrowth and hormonal state. We used several techniques to characterize hormonal state, including the determination of hormone levels and gene expression, and imaging of reporter genes. This latter technique required the use of specific mutants of other species (tomato and pea). We thus demonstrated that sucrose availability modulates the entrance of buds into sustained growth, and that this effect is conserved across species. This impact is preceded by an early modification of hormonal homeostasis. We also identified some components of the hormonal network as potential candidates explaining the regulation of bud outgrowth by sucrose.
Materials and methods
Plant culture, in vitro cultivation of axillary buds and growth analysis
For the experiments on R. hybrida L. ‘Radrazz’, cuttings from cloned mother plants were grown in a greenhouse where the temperature was maintained around 22°C. Extra light was supplied by high-pressure sodium vapour lamps below 200W m–2. Water and mineral nutrients were provided by sub-irrigation for 10min day–1. Nodes from the median part of the stem were harvested on single-axis plants when the floral bud was visible, as previously described (Girault et al., 2010).
For the experiment on A. thaliana (L.) Heynh., wild-type (WT) Columbia-0 was used. Seeds were sown and stratified for 48h at 7°C, then plants were grown in a growth chamber with a 16h day length at a temperature of 20/18°C (day/night). After 6 weeks of culture, first and second nodes bearing 1-mm-long buds were harvested on secondary flowering branches.
For the experiments on Pisum sativum L., the W6 22593 genotype was used for the WT, and DR5::GUS DSB2024, containing an auxin-inducible promoter fused with the β-glucuronidase reporter (DeMason and Polowick, 2009), was used to visualize auxin export. Plants were sown and grown in the growth chamber in the same conditions as for the Arabidopsis experiments, except stratification, which was not performed on this species. The third basal leaf-bearing node of single-axis plants was harvested when the fourth leaf was totally expanded.
For the experiment on Solanum lycopersicum L., the ‘Money Maker’ genotype was used as the WT and the pAtPIN1::AtPIN1-GFP-expressing line (Bayer et al., 2009) was used to visualize PIN1 localization within bud stem. Plants were sown and grown in the growth chamber in the same conditions as for the Arabidopsis experiments, except stratification, which was not performed on this species. The second basal leaf-bearing node of single-axis plants was harvested when the plants were about 15cm long (3 to 4-week-old plants).
Once harvested, 1.5-cm stem segments were grown in vitro on classical solid MS medium (Duchefa) (1% gelose, aubygel) supplemented with different sucrose concentrations or different non-metabolizable analogues (palatinose, glucose[1→6]fructose; turanose, glucose[1→3]fructose; melibiose, galactose[1→6]glucose; and lactulose, galactose[1→4]fructose). These sugar analogues were initially used at 80mM for rose (Loreti et al., 2000) and then at 30mM for herbaceous plants (pea and Arabidopsis), which was sufficient to induce bud outgrowth. In all experiments, mannitol was used as an osmotic control, as it is not metabolized by R. hybrida (Henry et al., 2011) and is non-toxic for bud outgrowth (Supplementary Figure S1). In vitro excised buds were grown in a growth chamber (Strader) with a 16-h day length at a temperature of 23/20°C (day/night).
For the work shown in Fig. 3, buds were treated with 1-N-naphthylphthalamic acid (NPA), an auxin transport inhibitor, by deposition, on a bud, of a drop of unsolidified mixture containing 1% gelose, 1% PEG, 0.01% Tween-20, and 0.2% DMSO supplemented with 1mM NPA. The control corresponded to the same mixture without NPA.
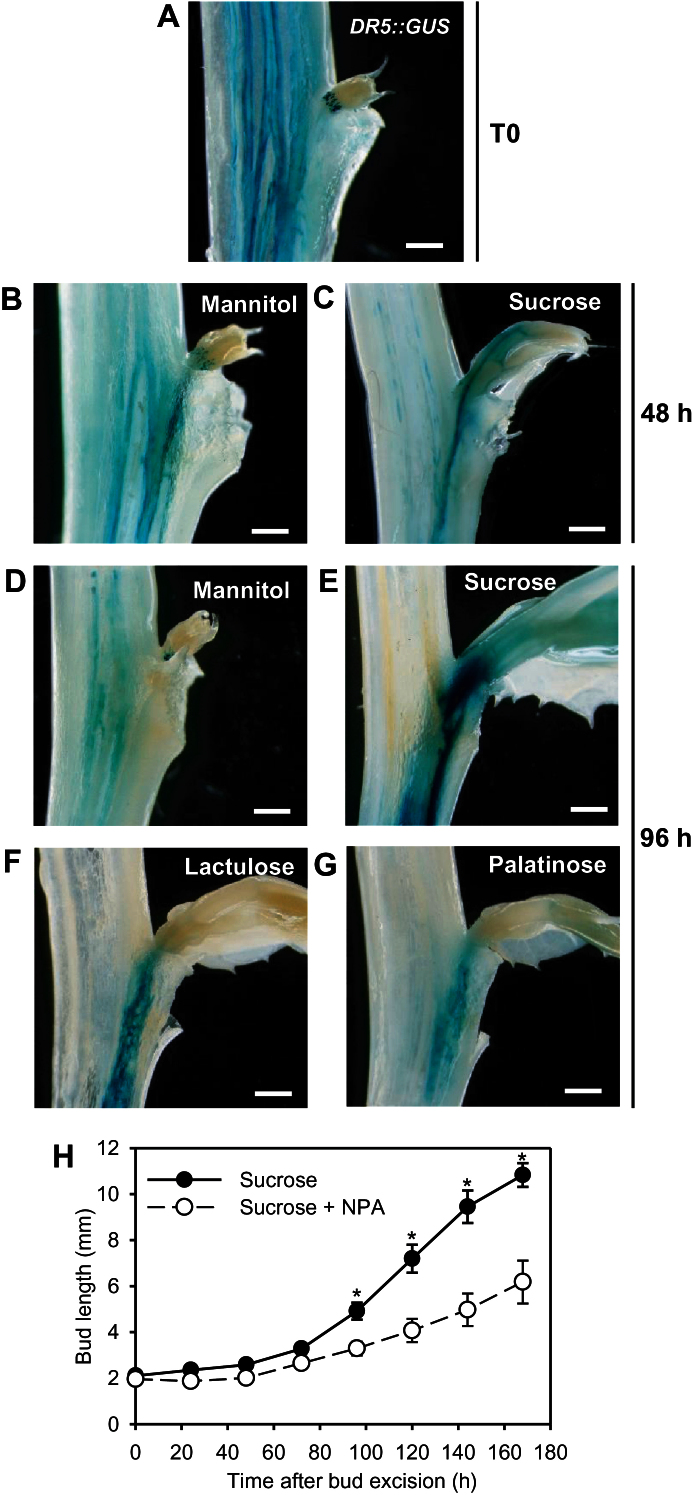
Fig. 3.
GUS staining in bud-bearing stem sections of the DR5::GUS-expressing pea line (A) before treatment (T0); or grown for 48h with 100mM (B) mannitol or (C) sucrose; or grown for 96h with 100mM (D) mannitol or (E) sucrose or 80mM (F) lactulose or (G) palatinose. (H) Elongation of buds grown with 30mM sucrose and treated with a drop of gelose on buds containing 1% PEG, 0.01% Tween-20, and 0.2% DMSO supplemented or not with 1mM NPA. (A–G) Images are representative of five replicates; (H) mean ± SE of eight replicates. Asterisks indicate significant differences between the treatment conditions. White bars represent 1mm.
For the work shown in Fig. 6, synthetic cytokinin 6-benzylaminopruine (BAP) and inhibitors of cytokinin synthesis (lovastatine; Hartig and Beck, 2005) and signalling (LGR-991 and PI-55; Nisler et al., 2010), were added in the growth medium at 10 µM.
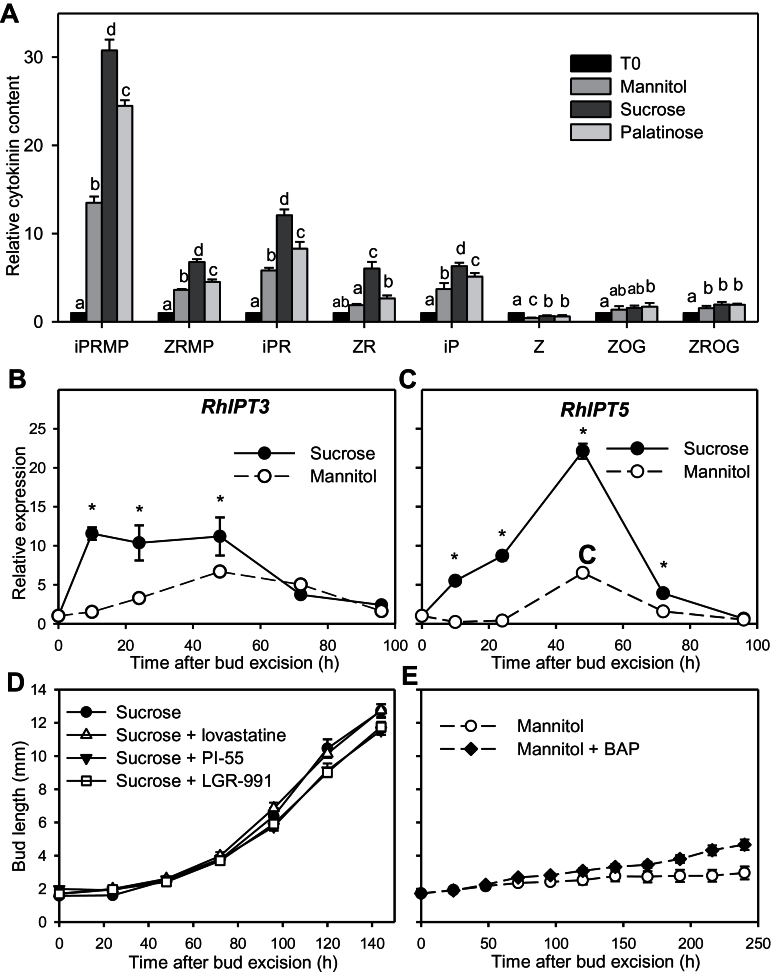
Fig. 6.
(A) Nodal stem content in isopentenyl adenine riboside 5′-monophosphate (iPRMP), zeatin riboside 5′-monophosphate (ZRMP), isopentenyl adenine riboside (iPR), zeatin riboside (ZR), isopentenyl adenine (iP), zeatin (Z), Z O-glucoside (ZOG), and ZR O-glucoside (ZROG) for intact plants at the FBV stage (T0) or grown for 24h on 100mM mannitol, 100mM sucrose, or 30mM palatinose; contents are expressed relative to T0. Relative expression is also shown of (B) RhIPT3 and (C) RhIPT5 in nodal stem sections grown in vitro with 100mM mannitol or 100mM sucrose up to 96h after their excision. Also elongation of buds grown with (D) 30mM sucrose alone or with 10 µM lovastatine, PI-55, or LGR-991; and (E) with 30mM mannitol alone or with 10 µM 6-benzylaminopurine (BAP). Data are mean ± SE of three measurements on a pool of 60 buds (A–C) and 10 replicates (D, E). Asterisks and letters indicate significant differences between the different treatments for each time point.
Once in vitro, buds were imaged daily and their elongation was quantified as described in the Supplementary material and in Supplementary Figure S2.
Free IAA content analysis
For each sample, 5mg of frozen tissue was extracted with 50mM phosphate buffer containing an internal standard of [2H5] IAA at a concentration of 100fmol mg–1 of plant material. The extract was subjected to solid-phase extraction as described previously (Pencík et al., 2009) and free IAA was analysed three times by ultra-high performance chromatography coupled with tandem mass spectrometry.
Cytokinin content analysis
For each sample, 10mg of freeze-dried powder was extracted with 0.8ml of acetone/water/acetic acid (80/19/1, v/v/v), and 10 stable-labelled isotopes (OLChemIm) were used as internal standards and added as follows: 1ng of 2H5-t-Z7G (trans-zeatin-7-glucoside), 1ng of 2H5-t-Z9G (trans-zeatin-9-glucoside), 1ng of 2H5-t-ZOG (trans-zeatin O-glucoside), 1ng of 15N-t-Z (trans-zeatin), 1ng of 2H5-t-ZROG (trans-zeatin riboside O-glucoside), 1ng of 2H5-t-ZR (trans-zeatin riboside), 1ng of 2H6-iPRMP (isopentenyl adenosine monophosphate), 1ng of 2H6-iP (isopentenyl adenine), 1ng of 2H5-t-ZRMP (trans-zeatin riboside monophosphate), 0.1ng of 15N-iPR (isopentenyl adenosine). The extract was vigorously shaken for 1min, sonicated for 1min at 25 Hz, shaken for 10min at 4°C in a Thermomixer (Eppendorf), and then centrifuged (8000g, 4°C, 10min). The supernatants were collected, and the pellets were re-extracted twice with 0.4ml of the same extraction solution, then vigorously shaken (1min) and sonicated (1min, 25 Hz). After centrifugation, the three supernatants were pooled and dried (final volume 1.6ml).
Each dry extract was dissolved in 140 µl of acetonitrile/water (50/50, v/v), filtered, and analysed using a Waters Acquity ultra performance liquid chromatograph coupled to a Waters Xevo Triple quadrupole mass spectrometer TQD (UPLC-ESI-MS/MS). The compounds were separated on a reverse-phase column (Uptisphere C18 UP3HDO, 100×2.1mm × 3 µm particle size; Interchim, France) using a flow rate of 0.4ml min–1 and a binary gradient: (i) acetic acid, 0.1% in water (v/v); and (ii) acetonitrile, with 0.1% acetic acid. The solvent gradient was applied as follows [t (min), % A]: (0, 95%), (12, 40%), (13, 0%), (16, 95%); the column temperature was 40°C. Mass spectrometry was conducted in electrospray and Multiple Reaction Monitoring (MRM) scanning mode, in negative ion mode. Relevant instrumental parameters were set as follows: capillary 1.5kV (negative mode); source block and desolvation gas temperatures 130 and 500°C, respectively. Nitrogen was used to assist the cone and desolvation (150 and 800 l h–1, respectively); argon was used as the collision gas at a flow of 0.18ml min–1. The parameters used for MRM quantification of the different hormones are shown in Supplementary Table S1A.
The amounts of ZOG and ZROG were expressed as a ratio of standard peak areas (2H5-t-ZOG 287 > 225 and 2H5-t-ZROG 519 > 225) per unit dry weight, due to impurities contained in the samples. These matrix impurities co-eluted with the ZROG or ZOG peak.
Samples were reconstituted in 140 µl of 50/50 acetonitrile/H2O (v/v) per ml of injected volume. The limit of detection (LOD) and limit of quantification (LOQ) were extrapolated for each hormone from calibration curves and samples using the Quantify module of MassLynx software, version 4.1. The LODs and LOQs are listed in Supplementary Table S1B.
Gene molecular cloning
5′ and 3′ cDNA ends were amplified using GeneRacer technology (Life Technologies), and then full-length cDNAs of each gene were cloned. The amplified fragments were cloned into the pGEM-T-easy vector (Promega) and transfected into Escherichia coli JM109 (Promega). Plasmids were purified using NucleoSpin Plasmid mini prep (Macherey-Nagel) and sequenced (GATC Biotech, Germany). Gene identity was determined based on their homology with Arabidopsis gene sequences and the presence of putative conserved domains in the corresponding peptide sequence (Supplementary Table S2). RwMAX1/2/3/4 and RhPP2A were identified in previous studies (Klie and Debener, 2011; Djennane et al., 2014)
Quantification of gene expression
Total RNAs from ground frozen samples were extracted from buds using an RNA NucleoSpin kit (Macherey-Nagel). Genomic DNA was removed by incubating RNAs with DNase (Biolabs) for 10min at 37°C (1 µl of DNase for 10 µg of RNA). The reaction was stopped by adding EDTA at a final concentration of 5mM followed by 10min at 75°C. The absence of contamination by genomic DNA was checked by PCR using a specific primer designed against an intron region of the RhGAPDH gene (Girault et al., 2010; Henry et al., 2011). cDNAs were obtained by reverse transcription performed on 1 µg of RNA using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed with SYBR Green Supermix (Biorad) using cDNA as a template, with the following programme: 2min at 50°C, 10min at 95°C, then 40 cycles of 15 s at 95°C and 60 s at 60 °C. The primers used for the qRT-PCR are given in Supplementary Table S3. Specific sets of primers were selected according to their melting curves. Fluorescence detection was performed using a Chromo4 Real-time PCR detector (Biorad). Quantification of relative gene expression was determined using RhSAND1 expression as an internal control (Henry et al., 2011; Rabot et al., 2012).
Confocal laser-scanning microscopy and GFP quantification
Longitudinal hand sections of in vitro-cultivated bud-bearing stem segments of pAtPIN1::AtPIN1-GFP-expressing tomato line were observed using a confocal laser scanning microscope (NIKON Eclipe Ti) with a 20× water immersion objective (excitation wavelength 488nm, emission spectra between 500 and 550nm). Laser power remained unchanged throughout the experiment. Quantification of the GFP signal was performed on 2D images using ImageJ software. Representative micrographs are given in Supplementary Figure S4. Integrated density of grey was determined on the 10 most intensely polarized plasma membrane poles of cells for each sample. The results are the means of three to four for replicates for each condition.
GUS staining and light microscopy
Bud-bearing stem segments of garden pea were harvested in 85% (v/v) ice-cold acetone, rinsed in distilled water, infiltrated with a GUS-staining solution (Na2HPO4, 68mM; NaH2PO4, 32mM; 0.2% Triton X-100; potassium ferrocyanide, 0.5mM; potassium ferricyanide, 0.5mM; and 0.5mg ml–1 of 5-bromo-4-chloro-3-indolyl-D-glucuronic acid) under vacuum and incubated at 37°C overnight. The samples were then cleared with ethanol:ethyl acetate 3:1 (v:v) prepared one week before and observed under a binocular microscope (Leica).
Statistical analyses
Statistical analyses were done using the Rcmdr package of R software for Windows (R Development Core Team, 2011). One-way ANOVA (α = 0.05) was run to test for the effects of sugar conditions on bud outgrowth. Significant differences are indicated by different letters or asterisks directly on the figures.
Results
Impact of sucrose on bud outgrowth dynamics
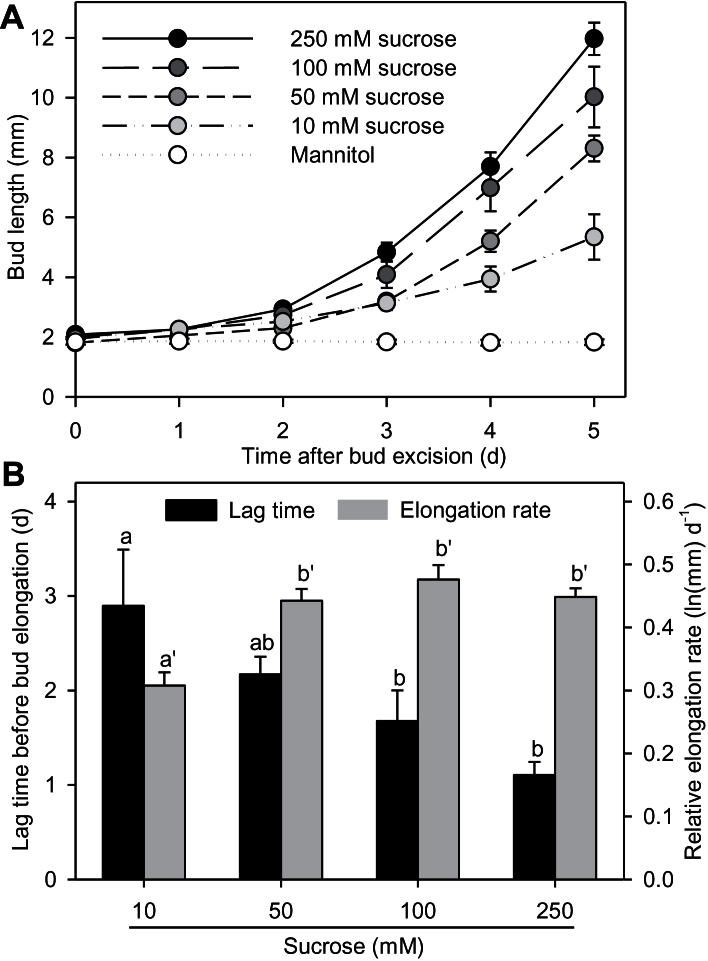
In vitro cultivated buds elongated within the first 6 days with all the sucrose concentrations tested, whereas no sustained bud outgrowth was observed in the presence of mannitol, used as an osmotic control (Fig. 1A). Initial slow growth was detected visually with mannitol, just after stem excision from the plant (data not shown), but could not be quantified from bud pictures. Sucrose stimulated bud elongation in a concentration-dependent manner. After 5 days of incubation, the average length of the buds kept on 10mM sucrose was 5.3mm, but was more than doubled (12.0mm) on 250mM sucrose. With sucrose, bud outgrowth showed a first phase of slow elongation, followed by a phase of rapid elongation. Fitting a two-phase exponential function on bud elongation (Supplementary Figure S2) revealed that increasing sucrose concentration gradually shortened the duration before the phase of rapid growth (2.9 and 1.1 days with 10 and 250mM sucrose, respectively; Fig. 1B). Moreover, at 10mM, the elongation rate of the rapid growth phase was reduced [(0.31 ln(mm) day−1] compared to higher concentrations [about 0.45 ln(mm) day−1], but there was no significant difference between concentrations above 50mM. These results demonstrate that sucrose is necessary for the transition between slow and rapid growth and modulates the timing of the transition in a concentration-dependent manner.
Fig. 1.
(A) Bud elongation. (B) Estimated lag times before elongation and relative elongation rates of buds cultivated in vitro. Experiments were conducted with 100mM mannitol or 10, 50, 100, or 250mM sucrose. Data are mean ± SE of 10 replicates. Letters indicate significant differences between means.
Impact of non-metabolizable sucrose analogues on bud outgrowth
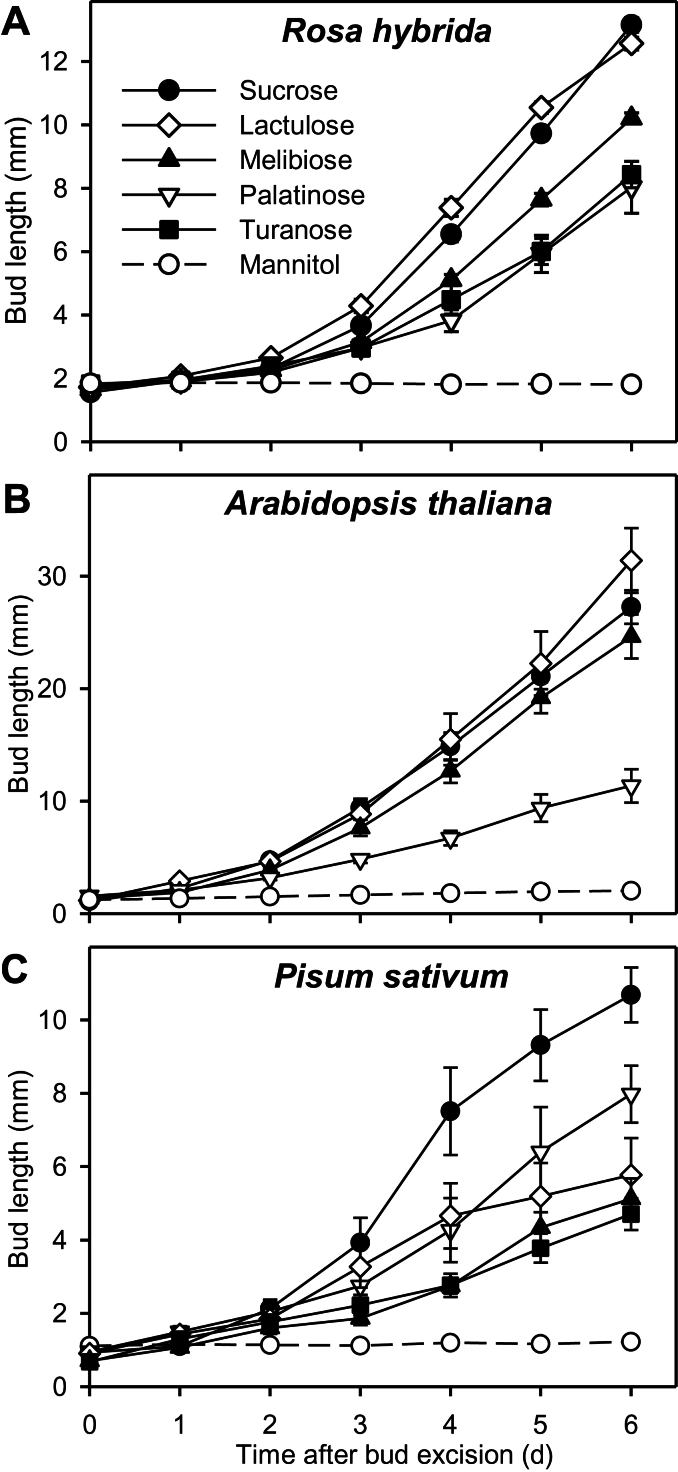
In order to confirm the previously suggested signalling role played by sucrose during bud outgrowth (Rabot et al., 2012), the effect of several non-metabolizable sucrose analogues was investigated on in vitro-cultivated buds. In R. hybrida, buds grew out with all sucrose analogues (Fig. 2A). Lactulose yielded the same bud elongation pattern as sucrose (13mm after 6 days). In contrast, after 6 days, buds were shorter with melibiose (10mm), palatinose, and turanose (8mm) than with sucrose or lactulose.
Fig. 2.
Elongation of buds grown with (A) 80mM mannitol, sucrose or non-metabolizable sucrose analogues (lactulose, melibiose, turanose, and palatinose) for R. hybrida, or with 30mM sucrose or 30mM non-metabolizable sucrose analogues for (B) Arabidopsis and (C) P. sativum. Data are mean ± SE of 8–10 replicates.
Similarly, buds of Arabidopsis and P. sativum also grew out in the presence of sucrose or sucrose analogues but not in presence of mannitol (Fig. 2B, C). In Arabidopsis, palatinose triggered weaker bud elongation (11.4mm after 6 days) than sucrose, lactulose, and melibiose (27, 31, and 25mm, respectively, after 6 days). In pea, all the sucrose analogues had a weaker effect than sucrose (6, 8, and 5mm with melibiose, palatinose, and turanose, respectively, compared to 11mm with sucrose, after 6 days). These data suggest that sucrose can play a signalling role in the processes governing bud entrance into rapid growth and that this effect is conserved across species.
Impact of sucrose on auxin export from buds.
It is well known that an active bud exports its own auxin in the stem (Li and Bangerth, 1999; Domagalska and Leyser, 2011). The positive impact of sucrose on the establishment of an auxin transport canal between bud and stem was checked using a DR5::GUS-expressing pea line containing an auxin-inducible promoter fused to the β-glucuronidase reporter gene (DeMason and Polowick, 2009). On intact plants (Fig. 3A, T0), the apex-derived auxin flux in the stem was visible but no clear flux was detected between bud and stem. 48h after bud-bearing stem excision from the plant, an auxin transport canal was visible between bud and stem with both mannitol and sucrose, but the blue precipitate was more intense with sucrose (Fig. 3B, C). At 96h, this canal had consistently disappeared with mannitol but had intensified with sucrose (Fig. 3D, E). Similarly to sucrose, lactulose and palatinose showed a sustained auxin canal between bud and stem (Fig. 3F, G), suggesting a possible signalling role for sucrose in this process.
The results above demonstrate that the ability of sucrose to sustain bud outgrowth is related to its ability to sustain auxin export. To test whether auxin export is limiting in the effect of sucrose on bud outgrowth, we used NPA, an inhibitor of auxin transport, specifically applied on the bud. Bud elongation was slowed down in the presence of sucrose + NPA compared to sucrose alone (Fig. 3H). At 7 days, bud length was about 43% less with NPA than without NPA, indicating that auxin export from the bud is limiting for sustained growth in the presence of sucrose.
Impact of sucrose on auxin concentration and synthesis in buds
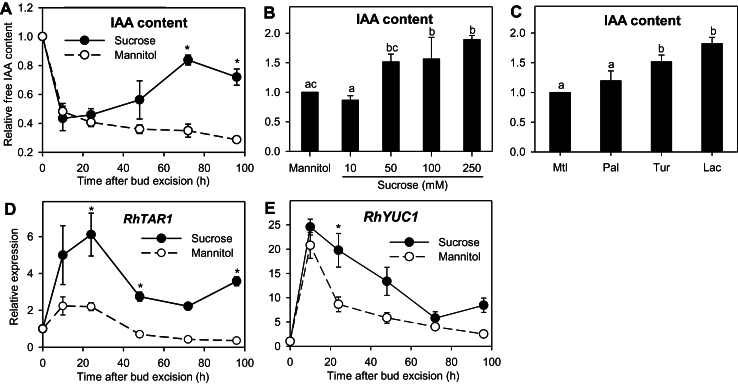
Sustained auxin export from buds implies an ability of the buds to synthesize their own auxin and transport it into the stem. In the first 10h, auxin levels dropped by a large amount in buds incubated with sucrose or mannitol (–57 and –52%, respectively; Fig. 4A). This level remained low for the buds kept on mannitol throughout the whole incubation period, while it gradually accumulated in the buds grown with sucrose, reaching its maximum level at 72h (+95% compared to 10h). The effect of sucrose on auxin accumulation was concentration dependent (Fig. 4B). Increasing sucrose to 50 or 100mM increased bud auxin content at 48h by about 56% compared to mannitol. The increase reached 89% with 250mM sucrose. Turanose and lactulose also increased the auxin content at 48h compared to mannitol (+51 and +82%, respectively; Fig. 4C), suggesting a possible signalling role of sucrose in this process.
Fig. 4.
(A) Relative IAA content (relative to T0 value) in buds of R. hybrida grown in vitro with 100mM sucrose or 100mM mannitol for 96h. (B, C) Relative IAA content (relative to mannitol value) in buds grown for 48h with (B) 100mM mannitol or a range of sucrose concentrations (10, 50, 100, or 250mM), or (C) 100mM mannitol (Mtl) or 80mM sucrose analogues: palatinose (Pal), turanose (Tur), or lactulose (Lac). (D, E) Relative expression of (D) RhTAR1 and (E) RhYUC1. Data are mean ± SE of three measurements on a pool of 60 buds. Asterisks and letters indicate significant differences between the different treatments for each time-point.
Auxin is mainly synthesized from tryptophan through the indole-3-pyruvic acid (IPA) pathway (Mashiguchi et al., 2011; Zhao, 2012) in two enzymatic steps implying the involvement of TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 and related proteins (TAA1 and TARs) and YUCCA flavin monooxygenase-like proteins (Zhao, 2012; Ljung, 2013). To test whether auxin accumulation in sucrose-fed buds is related to a stimulation of auxin synthesis within the bud, we investigated the effect of sucrose on certain genes implied to be involved in auxin biosynthesis on the basis of their sequence: RhTAR1 and RhYUC1 (Supplementary Table S2). As for auxin content, the expression level of these genes was mainly stimulated in the buds supplied with sucrose compared to mannitol (Fig. 4D, E). Higher expression with sucrose compared with mannitol occurred as early as 10h for RhTAR1 (25- vs 21-fold) and 24h for RhYUC1 (6- vs 2-fold), and was maintained over the 96h period studied. The general temporal pattern of expression was close for the two genes, with an induction peak at 10h, followed by a decrease until 72h.
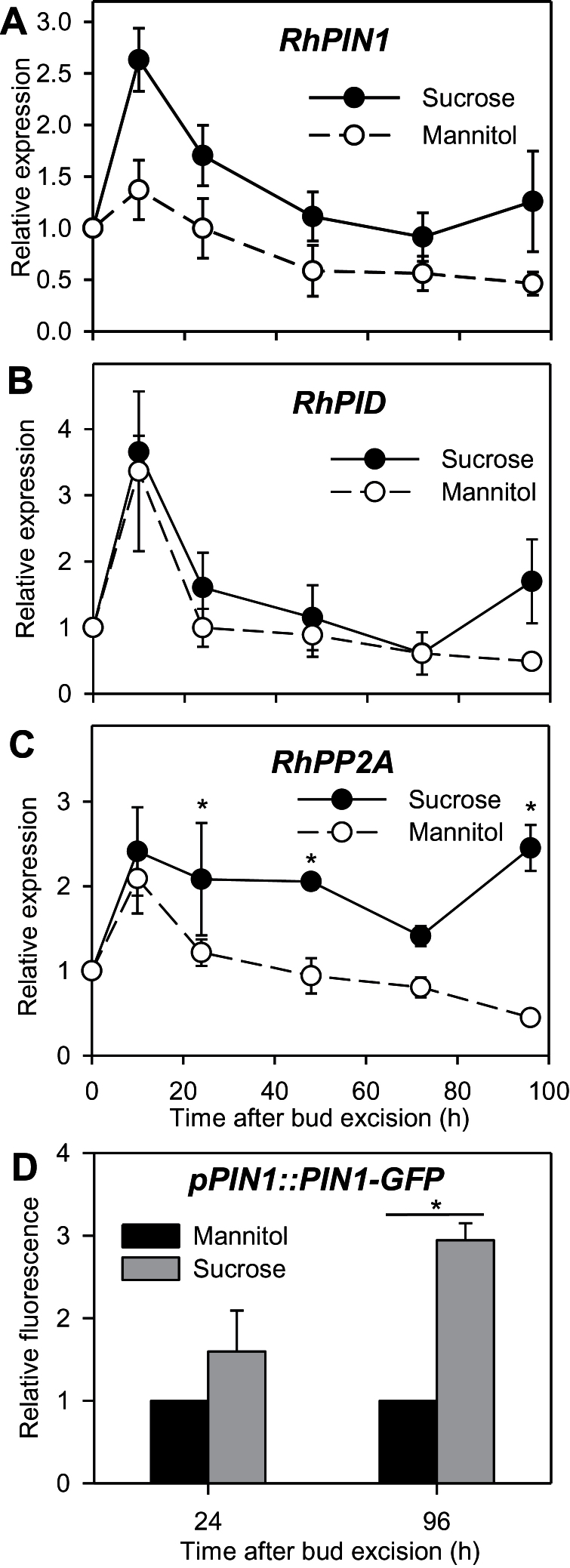
Impact of sucrose on PIN abundance and polarization in buds
Intercellular auxin export is mainly ensured by PIN-FORMED efflux carrier proteins (Petrášek and Friml, 2009). Their targeting to a pole of the cell is antagonistically mediated by the PINOID serin/threonin protein kinase (PID) and the PROTEIN PHOSPHATASE 2A (PP2A) (Michniewicz et al., 2007; Zhang et al., 2010). The transcripts of RhPIN1, putatively encoding an AtPIN1-like auxin efflux carrier (Supplementary Table S2), rapidly accumulated in the buds within the first 10h (a 3-fold increase; Fig. 5), and were 2-fold higher with sucrose than with mannitol after 10h. The transcript levels of RhPIN1 then decreased. RhPID and RhPP2A expression also peaked after 10h (4- and 2-fold increases, respectively) with mannitol and sucrose. However, upregulation with sucrose compared to mannitol only occurred late in the outgrowth process, after 96h for RhPID and after 24h for RhPP2A.
Fig. 5.
Relative expression of (A) RhPIN1, (B) RhPID, and (C) RhPP2A in buds grown in vitro for 96h with 100mM mannitol or sucrose. (D) Relative fluorescence of the GFP signal at the polarized pole of the cells in bud stems of the pPIN1::PIN1-GFP-expressing tomato line grown for 24h or 96h with 100mM mannitol or sucrose. Data are mean ± SE of three measurements on a pool of 60 buds (A–C) and four replicates (D). Asterisks indicate significant differences between the different treatments for each time point.
To check whether the presence of sucrose resulted in the targeting of PINs to the plasma membrane in the bud, we assessed the amount of PIN1 polarized in buds grown with sucrose or mannitol in the pAtPIN1::AtPIN1-GFP tomato line (Money Maker background). As in R. hydrida, sustained bud outgrowth in this tomato line was promoted by sucrose but not by mannitol (Supplementary Figure S3). A GFP signal localized at the basal pole of the cells was detected with both sugar treatments (Supplementary Figure S4). The quantification of GFP signal indicated that there was no significant difference between sucrose and mannitol at 24h, but that it was 3-fold higher with sucrose than with mannitol at 96h (Fig. 5D).
Impact of sucrose on cytokinin synthesis and accumulation in stem tissues
To test the involvement of cytokinins in sucrose-stimulated bud outgrowth, we quantified their accumulation in stem sections cultivated in vitro between 0 and 24h (which is before rapid bud growth) with mannitol, sucrose, or palatinose (Fig. 6A). Active cytokinins are derived from intermediate forms and can be converted into inactive conjugated forms (Sakakibara, 2006). The three types of sugars induced an accumulation of cytokinin intermediate forms (iPRMP, ZRMP, iPR, and ZR) and an active form (iP), while conjugated inactive forms (ZOG and ZROG) accumulated more modestly and zeatin, another active form, did not accumulate. Interestingly, cytokinin accumulation with mannitol was lower than with sucrose, and slightly lower than with palatinose. This demonstrates that cytokinins accumulate before rapid bud growth in the presence of sucrose.
The rate-limiting step of cytokinin biosynthesis is catalysed by enzymes of the ISOPENTENYL TRANSFERASE (IPT) family (Sakakibara, 2006; Miyawaki et al., 2006). As a complement to cytokinin quantification at 24h, we quantified the relative expression of R. hybrida Isopentenyl Transferase3 and 5 (RhIPT3 and RhIPT5) in the stem segments (Fig. 6B and C). These genes code for proteins that share high homology with the cytokinin-biosynthesis enzymes of Arabidopsis AtIPT3 and AtIPT5, respectively (Supplementary Table S2). During the first 48h, sucrose promoted the expression of RhIPT3 and RhIPT5 compared to mannitol. In response to sucrose, expression of RhIPT3 rapidly increased just after stem excision, within the first 10h (12-fold increase), whereas in response to mannitol, it increased progressively and to a lesser extent than sucrose (7-fold increase). RhIPT5 transcripts accumulated more progressively than RhIPT3 with sucrose to reach their maximum value at 48h (22-fold increase), but strongly compared with mannitol (7-fold). After 48h, the expression level of RhIPT3 and RhIPT5 dropped to the same level with sucrose and mannitol. These results suggest that sucrose promoted cytokinin accumulation in the stems at least through a rapid upregulation of RhIPT3 and RhIPT5.
To determine whether the impact of sucrose on bud outgrowth could be mediated by cytokinins, 10 µM solutions of different inhibitors of cytokinin synthesis (lovastatine) and perception (PI-55, LGR-991) were added to the growth medium in the presence of 30mM sucrose (Fig. 6D). On the whole, bud elongation was insensitive to these inhibitors. No difference was observed in the elongation of buds cultivated with sucrose or sucrose plus cytokinin inhibitors during the first 72h. At 96h, bud length became lower in the presence of PI-55 and LGR-991, but differences between treatments were slight and occurred well after buds entered rapid growth. Moreover, supplying 10 µM BAP, a synthetic cytokinin, to medium with mannitol did not trigger sustained bud outgrowth (Fig. 6E). These results suggest that the observed promoted effect of sucrose on rapid bud growth is not mediated by cytokinins.
Impact of sucrose on strigolactone signalling genes
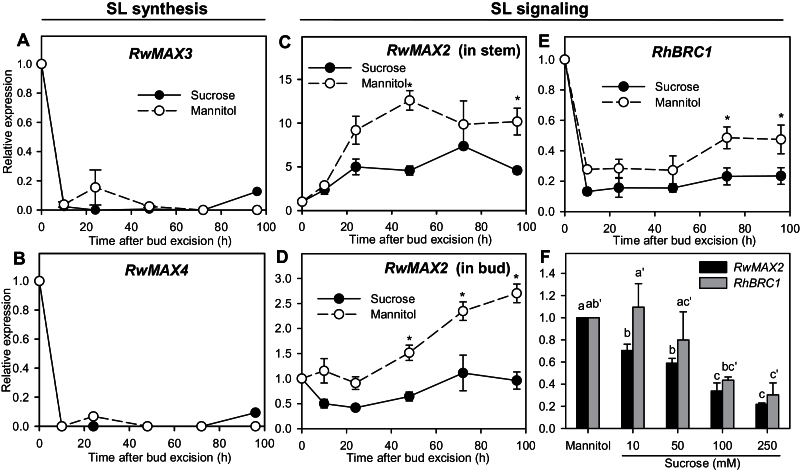
To test the involvement of strigolactones in sucrose-stimulated bud outgrowth, we quantified the expression of genes putatively implied in strigolactone synthesis and signalling. Strigolactones are produced by a sequential cleavage of a carotenoid precursor that involves different CAROTENOID CLEAVAGE DIOXYGENASE enzymes (CCD7/MAX3/RMS5, CCD8/MAX4/RMS1) (Lin et al., 2009; de Saint Germain et al., 2013). Strigolactone perception is notably mediated by an F-box protein encoded by RMS4/MAX2/D3 (Stirnberg et al., 2002, 2007; Hamiaux et al., 2012). The expression of RwMAX3 and RwMAX4, putatively involved in strigolactone synthesis, dropped abruptly after excision to become undetectable after 10h and then remained very low whether the buds were cultivated with sucrose or mannitol (Fig. 7A, B). The expression of RwMAX2 was repressed with sucrose compared to mannitol in both stems and buds (Fig. 7C, D). In stems, RwMAX2 expression increased within the first 24h with both sucrose and mannitol, but to a lesser extent with sucrose (5-fold increase) than with mannitol (9-fold increase). Afterwards, RwMAX2 expression in stems remained stable for both treatments. In buds, RwMAX2 expression dropped (0.5-fold) within the first 24h for sucrose while it remained stable for mannitol. This initial downregulation of RwMAX2 was negatively related to sucrose concentration and its expression was 5-fold lower with 250mM sucrose than with mannitol (Fig. 7F). After 24h, RwMAX2 expression in buds increased to reach, at 96h, a value close to that observed at 0h with sucrose and a value 3-fold higher with mannitol.
Fig. 7.
Relative expression of (A) RwMAX3, (B) RwMAX4, and (C) RwMAX2 in nodal stems grown for 96h with 100mM mannitol or sucrose; relative expression of (D) RwMAX2, and (E) RhBRC1 in buds grown for 96h with 100mM mannitol or sucrose; and (F) relative expression of RwMAX2 and RhBRC1 in buds grown for 24h with 100mM mannitol or 10, 50, 100, or 250mM sucrose. Data are mean ± SE of three replicates. Asterisks indicate significant differences between sucrose and mannitol for each time point. Letters indicate significant differences between means for each gene.
Strigolactones modulate BRC1 expression, a gene coding for a transcription factor that inhibits bud outgrowth and with expression well related to bud outgrowth state (Aguilar-Martínez et al., 2007; Braun et al., 2012). Similarly to RwMAX2, RhBRC1 expression in buds was repressed overall with sucrose compared to mannitol (Fig. 7E). During the first 10h, irrespective of the sugar tested, RhBRC1 expression showed a strong drop, which was slightly stronger with sucrose than with mannitol (8- and 4-fold, respectively). Thereafter, RhBRC1 expression remained quite stable until 48h and then, with mannitol only, slightly re-increased. RhBRC1 expression at 24h was negatively related to sucrose concentration, exhibiting much lower values with 100 or 250mM sucrose compared to mannitol, 10, or 50mM sucrose (Fig. 7F).
Discussion
Sucrose promotes sustained bud outgrowth
Bud outgrowth can be divided into three stages: a dormancy stage, a transition stage, and a sustained growth stage (Stafstrom and Sussex, 1992; Devitt and Stafstrom, 1995; Cline, 1997; Dun et al., 2006). Depending on the conditions, buds in the transition stage will either go back to dormancy or enter sustained growth (Waldie et al., 2010). We clearly observed two growth periods for R. hybrida buds after their excision from the bud-bearing stem segments and their transfer in vitro: an initial period of slow growth, which indicates that buds are not in a dormant stage, followed by a phase of rapid elongation in response to sucrose supply (Fig. 1). There was a progressive delay in buds entering rapid elongation when sucrose availability decreased. With mannitol, used as the ‘no sugar’ control, buds stopped their growth after the initial slow growth phase. These results support the hypothesis that sugar availability controls the entrance of buds into sustained growth. This need for sugar is conserved across species, as demonstrated in Arabidopsis and P. sativum (Fig. 2). Our results complement those showing that early bud release after decapitation is also controlled by an increase in sugar availability in pea (Mason et al., 2014). For in vitro-cultivated R. hybrida buds, we observed a rapid degradation of starch reserves in the stem parenchyma (Supplementary Figure S5A) after excision of bud-bearing stem segments from the plant. Starch degradation may increase sugar availability for buds and in turn lead to their initial slow growth. Alternatively, initial slow bud growth could be related to the high auxin drop in the stem observed as early as 10h after stem excision (Supplementary Figure S6).
Sucrose acts as a trophic and signalling entity in bud outgrowth
The role of sucrose cannot be restricted to a trophic role since different non-metabolizable sucrose analogues induced bud elongation in R. hybrida, Arabidopsis, and P. sativum in vitro (Fig. 2). Bud elongation was not always similar between sucrose and the analogue tested, probably due to different conformations of analogues, as previously observed with barley embryos (Loreti et al., 2000) and in tobacco cell suspensions (Atanassova et al., 2003). By contrast to sucrose, none of the sucrose analogues increased the dry weight of the bud-bearing stem segments, demonstrating that none of them were metabolized (Supplementary Figure S5B), which is in line with previous studies (Loreti et al., 2000; Atanassova et al., 2003). Moreover, the analogues were not absorbed by RhSUC2 (Supplementary Table S4; Rabot et al., 2012), a sucrose transporter preferentially expressed in R. hybrida during bud outgrowth (Henry et al., 2011). Such an absence of analogue import into cells rules out their potential degradation by vacuolar invertases and sucrose synthases, as reported with palatinose in sugarcane cells (Wu and Birch, 2011). Finally, the effect of analogues was unlikely to be an indirect result of an impact on starch reserve mobilization or auxin depletion from the stem, as starch and auxin decreases in the stem were similar among treatments with and without analogues (Supplementary Figure S5A and C). These findings and others, discussed below, are consistent with the fact that sugars can serve, at least partly, as signalling molecules in the control of bud outgrowth.
Sucrose promotes sustained auxin synthesis within buds and auxin export from bud to stem. It is well known that bud outgrowth occurs along with auxin export out of the bud (Morris, 1977; Li and Bangerth, 1999). We checked this for sucrose- or sucrose analogue-promoted bud outgrowth using the auxin-inducible DR5::GUS-expressing pea line (Fig. 3A–G). We observed a sustained auxin canal between bud and stem with sucrose or sucrose analogues, but not with mannitol. Interestingly, we noted initial auxin export just after excising the bud-bearing stem segments, irrespective of the presence of sucrose or mannitol. Consistently, we noted a substantial auxin drop in buds within the first 10h after stem excision with sucrose or mannitol (Fig. 4A). Such a drop could be explained as follows: stem excision from the plant is followed by a rapid auxin drop in the stem (Supplementary Figure S6), initiating the creation of a canal of active auxin transport between bud and stem by positive feedback between auxin directional flux and PIN polarization in the direction of the flux (Bennett et al., 2014). In line with this idea, the genes responsible for PIN polarization, RhPID and RhPP2A, were rapidly induced after stem excision with sucrose or mannitol (Fig. 5B, C).
The maintenance of auxin export with sucrose or its non-metabolizable analogues was accompanied by the capacity of the bud to synthesize auxin. After the initial auxin drop due to excision, buds supplied with either sucrose or non-metabolizable analogues were able to re-accumulate auxin, in contrast to buds grown with mannitol (Fig. 4A). Stimulation of auxin synthesis by sugars has been reported in a variety of developing plant organs including kernel embryos (LeClere et al., 2010), as well as Arabidopsis roots (Mishra et al., 2009), seedlings (Sairanen et al., 2012), and hypocotyls (Stewart Lilley et al., 2012). RhTAR1 and RhYUC1, two genes involved in the indole-3-pyruvic acid pathway, the main auxin biosynthesis pathway (Mashiguchi et al., 2011), showed a higher expression with sucrose compared with mannitol; this began early, before any visible effect on auxin re-accumulation in buds (Fig. 4D, E). This induction of auxin synthesis genes by sucrose could be direct via the WRKY transcription factor (TF) from R. hybrida, SUCROSE SIGNALLING1 (RhSUSI1). Indeed, this orthologue of the sucrose-induced TF SUSIBA2 in barley (Sun et al., 2003) was both induced by sucrose in the bud and able to bind to the promoter of RhTAR1 (Supplementary Figure S7), suggesting that sucrose could induce RhTAR1 in an RhSUSI1-dependent manner. For buds cultivated with mannitol, we observed an initial peak in auxin biosynthesis gene expression, as with sucrose, but expression values progressively decreased to become even lower than those observed with intact plants. This initial peak may be related to the release of sucrose and hexoses from the breakdown of stem starch reserves, as well as to auxin depletion in the stem after excision (Supplementary Figures S5 and S6).
Besides auxin synthesis, the maintenance of an auxin canal with sucrose was also highly related to the capacity of buds to upregulate PIN1, an auxin efflux carrier involved in polar auxin transport and auxin canalization (Wisniewska et al., 2006; Bennett et al., 2014). RhPIN1 showed an early higher expression with sucrose compared with mannitol, which was maintained throughout the 96-h period studied (Fig. 5A). Consistent with this, glucose highly upregulates AtPIN1 and AtPIN2 in Arabidopsis roots (Mishra et al., 2009) and sucrose upregulates AtPIN7 and downregulates AtIAA3, a negative regulator of AtPIN7 in Arabidopsis shoots (Stewart Lilley et al., 2012). RhPP2A, which is required for the posttranslational mechanisms leading to the polarization of PIN proteins at the basal pole of the cells (Michniewicz et al., 2007; Grunewald and Friml, 2010), was also upregulated with sucrose. However, we did not observe sucrose-mediated RhPP2A upregulation as early as RhPIN1 upregulation (Fig. 5C), suggesting that RhPP2A may be involved in the maintenance of the canal rather than in its initial establishment. Consistently, in pPIN1::PIN1-GFP-expressing tomato, sucrose triggered a higher GFP signal than mannitol at the basal pole of cells in bud stem tissues during the sustained bud outgrowth period (after 96h; Fig. 5D). These findings suggest that sucrose could promote the establishment of polar auxin transport within the axillary bud by controlling PIN protein synthesis and/or PIN targeting to the plasma membrane. Such regulation could be the result of the negative impact of sucrose on the strigolactone pathway (discussed below), which has been shown to deplete PIN proteins from the plasma membrane (Shinohara et al., 2013). Moreover, it cannot be excluded that the effect of sucrose on PIN1 polarization is the consequence of an increase of the auxin flow between bud and stem due to stimulation of auxin synthesis.
Altogether, these findings support the hypothesis that the sustained bud outgrowth observed in response to sucrose involves an early stimulation and the maintenance of processes responsible for auxin synthesis and transport. By applying NPA, an inhibitor of auxin transport, specifically on buds we further demonstrated that auxin export from the bud is limiting in sucrose-promoted sustained bud outgrowth (Fig. 3H). Similarly, NPA application on pea buds of decapitated plants was able to inhibit sustained bud outgrowth, but not earlier phases (Brewer et al., 2009; Mason et al., 2014), suggesting that auxin transport in bud is not critical in bud release following decapitation, although it may be crucial for ongoing bud outgrowth (Dun et al., 2006; Ferguson and Beveridge, 2009).
Sucrose promotes sustained growth in a cytokinin-independent manner
Cytokinins are involved in stimulation of bud outgrowth in different species including rosebush (Sachs and Thimann, 1967; Bredmose et al., 2005; Shimizu-Sato et al., 2009). Our results demonstrate that sucrose upregulates cytokinin biosynthesis in stem tissues and that a disaccharide signalling pathway can be at least partially involved. Indeed, compared to mannitol, sucrose and palatinose induced an overaccumulation of intermediate and active forms of cytokinins in R. hybrida stems very early in the outgrowth process, and the overexpression of RhIPT3 and RhIPT5, two cytokinin biosynthesis-related genes (Fig. 6A–C). The effect of sugar on cytokinin production has previously only been reported for Lilium floral tissues (Arrom and Munné-Bosch, 2012a) and Arabidopsis seedlings (Kushwah and Laxmi, 2013). The effect of sucrose on the expression of RhIPT3 and RhIPT5 started after stem excision and was maintained over the first 48h during the phase of slow growth (Fig. 1B). Thereafter, their expression levels dropped (Fig. 6B, C), as previously observed with PsIPT1 and PsIPT2 in P. sativum stems upon decapitation (Tanaka et al., 2006). This may involve a feedback loop in which auxin derived from outgrowing buds after 48h represses cytokinin biosynthesis, as suggested by the expression pattern of the cytokinin-catabolizing R. hybrida CYTOKININ OXIDASE/DEHYDROGENASE1, RhCKX1 (Supplementary Figure S8), an auxin-inducible gene in stems (Shimizu-Sato et al., 2009). RhCKX1 expression was low in the first 48h following excision and subsequently increased only in the presence of sucrose.
Beside sugars, cytokinin biosynthesis is well known to be repressed by auxin in different species (Tanaka et al., 2006; Minakuchi et al., 2010), while it is induced by nitrogen in Arabidopsis (Takei et al., 2004). This makes cytokinin production in the nodal stem a good potential integrator of the nutrient and auxin status in the regulation of bud outgrowth. However, we report here that cytokinins are not necessarily involved in sucrose-promoted bud outgrowth in our system. Indeed, bud outgrowth with sucrose was not inhibited by different cytokinin synthesis and perception inhibitors (Fig. 6D). Moreover, the addition of BAP, a synthetic cytokinin, in the medium did not trigger rapid growth of buds grown with mannitol, indicating that cytokinins are not limiting in our system (Fig. 6E). Similarly, cytokinins applied directly to axillary buds or the overexpression of cytokinin biosynthesis genes does not always induce bud outgrowth in other species (King and Van Staden, 1988; Medford et al., 1989).
Sucrose downregulates MAX2 and BRC1, two genes involved in strigolactone signal transduction
Strigolactones are involved in the inhibition of bud outgrowth and have been suggested to be second messengers of auxin (Brewer et al., 2009; Waldie et al., 2010, 2014). In buds, the expression of the strigolactone synthesis-related genes, RwMAX3 and RwMAX4, was not modulated by sucrose (Fig. 7A, B), but it dropped a lot within the first 10h, probably related to auxin depletion following stem excision. In contrast, RwMAX2, a key regulatory gene in the signal transduction of strigolactones, was repressed early and in a concentration-dependent manner by sucrose (Fig. 7C, D, F). Strigolactone perception through RwMAX2 could thus be one of the ways whereby sucrose promotes sustained bud outgrowth. MAX2 was also reported to be involved in bud outgrowth regulation by light. In sorghum, inhibition of bud outgrowth in the phyB mutant or by FR treatment was related to high SbMAX2 expression in buds (Kebrom et al., 2010). In Rosa, darkening of the distal part of the shoot triggered a strong increase of RwMAX2 expression in darkened buds (Djennane et al., 2014).
BRC1 appears as a common target for different signals controlling bud outgrowth, including strigolactones and cytokinins, and thus as an essential component of bud outgrowth control (Aguilar-Martínez et al., 2007; Braun et al., 2012; Dun et al., 2012). The expression of R. hybrida BRANCHED1, RhBRC1, in buds was repressed early with sucrose compared to mannitol, and with high sucrose concentrations (100, 250mM) compared with low concentrations (10, 50mM; Fig. 7E, F). Accordingly, Mason et al. (2014) observed that artificially increasing sucrose levels in pea plants repressed BRC1 expression early. In our conditions, RhBRC1 expression appeared more sensitive to stem excision than to external sucrose supply. Indeed, the expression showed an initial strong drop whether the buds were cultivated with sucrose or mannitol. This drop may be related to the fact that stem excision increased sucrose and hexoses from breakdown of stem starch reserves, or to the auxin depletion from the stem, another variable known indirectly to control BRC1 (Chen et al., 2013) (Supplementary Figures S7 and S8).
In conclusion, our study demonstrates that sucrose, recently shown to control initial bud release, also regulates the entrance of buds into sustained growth. We identified several hormonal components of the bud outgrowth regulatory network that were affected early by sucrose availability before the effect of sucrose was visible on bud outgrowth, suggesting their possible involvement in the control of sustained bud growth (summarized in Supplementary Figure S9). This study on isolated buds provides basic information on which further investigations could focus for understanding the mechanisms whereby sugars control bud outgrowth and therefore their role in the control of bud outgrowth patterns in planta.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Table S1. Parameters used for cytokinin content analysis.
Supplementary Table S2. Putative identity and functions of the genes cloned in this study.
Supplementary Table S3. Primers used for qRT-PCR.
Supplementary Table S4. Impact of sucrose analogues on RhSUC2 transport capacity in yeast.
Supplementary Figure S1. Elongation time course of buds grown with sucrose or sucrose plus mannitol, showing mannitol toxicity.
Supplementary Figure S2. Observed and fitted elongation time courses for buds.
Supplementary Figure S3. Impact of sucrose on tomato bud elongation.
Supplementary Figure S4. Micrographs of the DR5::GFP-expressing tomato bud stems.
Supplementary Figure S5. Metabolization of sucrose analogues in R. hybrida.
Supplementary Figure S6. Auxin depletion in stem segments.
Supplementary Figure S7. Relative expression of RhSUSI with sucrose or mannitol.
Supplementary Figure S8. Relative expression of RhCKX1.
Supplementary Figure S9. Schematic representation of the impact of sucrose on the hormonal regulation of bud outgrowth.
Supplementary material. Additional experimental details.
Acknowledgements
We wish to thank Patricia Polowick for providing the pea lines, Cris Kuhlemeier for providing the tomato lines, Lukas Spíchal for providing cytokinin inhibitors, and Catherine Rameau and Christine Beveridge for valuable discussions. We also thank C. Bouffard, A. Lebrec, B. Dubuc, N. Brouard, and M. Laffaire for providing plant material; and L. Ogé, F. Braud, M. Juchaux, F. Simonneau, and the IMAC platform for their technical help and assistance.
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. The Plant Cell 19, 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrom L, Munné-Bosch S. 2012a. Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Science 188–189, 41–47. [DOI] [PubMed] [Google Scholar]
- Arrom L, Munné-Bosch S. 2012b. Hormonal changes during flower development in floral tissues of Lilium. Planta 236, 343–354. [DOI] [PubMed] [Google Scholar]
- Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos-Thévenot P, Delrot S. 2003. Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiology 131, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C. 2009. Integration of transport-based models for phyllotaxis and midvein formation. Genes and Development 23, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Hines G, Leyser O. 2014. Canalization: what the flux? Trends in Genetics 30, 41–48. [DOI] [PubMed] [Google Scholar]
- Bonhomme M, Peuch M, Ameglio T, Rageau R, Guilliot A, Decourteix M, Alves G, Sakr S, Lacointe A. 2010. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiology 30, 89–102. [DOI] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot J-P, et al. 2012. The pea TCP transcription factor PsBRC1 acts downstream of Strigolactones to control shoot branching. Plant Physiology 158, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredmose N, Kristiansen K, Nørbæk R, Christensen LP, Hansen-Møller J. 2005. Changes in concentrations of cytokinins (CKs) in root and axillary bud tissue of miniature rose suggest that local CK biosynthesis and zeatin-type CKs play important roles in axillary bud growth. Journal of Plant Growth Regulation 24, 238–250. [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. 2009. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiology 150, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. 2000. The hormonal regulation of axillary bud growth in Arabidopsis. The Plant Journal 24, 159–169. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou X, Xi L, Li J, Zhao R, Ma N, Zhao L. 2013. Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema × grandiflora cv. Jinba). PloS ONE 8, e61717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. 1991. Apical dominance. The Botanical Review 57, 318–358. [Google Scholar]
- Cline M. 1997. Concepts and terminology of apical dominance. American Journal of Botany 84, 1064–1064. [PubMed] [Google Scholar]
- Czarnecki O, Yang J, Weston DJ, Tuskan GA, Chen J-G. 2013. A dual role of strigolactones in phosphate acquisition and utilization in plants. International Journal of Molecular Sciences 14, 7681–7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourteix M, Alves G, Bonhomme M, et al. 2008. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: their potential role in early events of growth resumption. Tree Physiology 28, 215–224. [DOI] [PubMed] [Google Scholar]
- DeMason DA, Polowick PL. 2009. Patterns of DR5::GUS expression in organs of pea (Pisum sativum). International Journal of Plant Sciences 170, 1–11. [Google Scholar]
- De Saint Germain A, Bonhomme S, Boyer F-D, Rameau C. 2013. Novel insights into strigolactone distribution and signalling. Current Opinion in Plant Biology 16, 583–589. [DOI] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP. 1995. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Molecular Biology 29, 255–265. [DOI] [PubMed] [Google Scholar]
- Djennane S, Hibrand-Saint Oyant L, Kawamura K, et al. 2014. Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant, Cell and Environment 37, 742–757. [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews: Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, Ferguson BJ, Beveridge CA. 2006. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiology 142, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Beveridge CA. 2009. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiology 149, 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault T, Abidi F, Sigogne M, Pelleschi-Travier S, Boumaza R, Sakr S, Leduc N. 2010. Sugars are under light control during bud burst in Rosa sp. Plant, Cell and Environment , 1339–1350. [DOI] [PubMed] [Google Scholar]
- Grunewald W, Friml J. 2010. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. The EMBO Journal 29, 2700–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Hillman JR. 1975. Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. timing of bud growth following decapitation. Planta 123, 137–143. [DOI] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. 2012. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Current biology 22, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E. 2005. Assessment of lovastatin application as tool in probing cytokinin-mediated cell cycle regulation. Physiologia Plantarum 125, 260–267. [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. 2009. Interactions between auxin and strigolactone in shoot branching control. Plant Physiology 151, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Rabot A, Laloi M, Mortreau E, Sigogne M, Leduc N, Lemoine R, Sakr S, Vian A, Pelleschi-Travier S. 2011. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant, Cell and Environment 34, 1776–1789. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Hays DB, Finlayson SA. 2010. Vegetative axillary bud dormancy induced by shade and defoliation signals in the grasses. Plant Signaling and Behavior 5, 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W. 2012. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiology 160, 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RA, Van Staden J. 1988. Differential responses of buds along the shoot of Pisum sativum to isopentenyladenine and zeatin application. Plant Physiology and Biochemistry 26, 253–259. [Google Scholar]
- Klie M, Debener T. 2011. Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrida). BMC Research Notes 4, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwah S, Laxmi A. 2013. The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant, Cell and Environment , 235–253. [DOI] [PubMed] [Google Scholar]
- LeClere S, Schmelz EA, Chourey PS. 2010. Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiology 153, 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bangerth F. 1999. Autoinhibition of indoleacetic acid transport in the shoots of two‐branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiologia Plantarum 106, 415–420. [Google Scholar]
- Lin H, Wang R, Qian Q, et al. 2009. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. The Plant Cell 21, 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. 2013. Auxin metabolism and homeostasis during plant. Development 140, 943–950. [DOI] [PubMed] [Google Scholar]
- Loreti E, Alpi A, Perata P. 2000. Glucose and disaccharide-sensing mechanisms modulate the expression of alpha-amylase in barley embryos. Plant Physiology 123, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquat C, Vandamme M, Gendraud M, Pétel G. 1999. Dormancy in vegetative buds of peach: relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Scientia Horticulturae 79, 151–162. [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, et al. 2011. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108, 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences, USA 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. 1989. Alterations of edogenous ctokinins in tansgenic pants using a chimeric isopentenyl transferase gene. The Plant Cell 1, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, et al. 2007. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056. [DOI] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, et al. 2010. FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant and Cell Physiology 51, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BS, Singh M, Aggrawal P, Laxmi A. 2009. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4, e4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ. 1953. Influence of light and temperature on the growth of ryegrass (Lolium spp.). Physiologia Plantarum 6, 21–46. [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA 103, 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. 1977. Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136, 91–96. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA. 2005. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiology 138, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisler J, Zatloukal M, Popa I, Dolezal K, Strnad M, Spíchal L. 2010. Cytokinin receptor antagonists derived from 6-benzylaminopurine. Phytochemistry 71, 823–830. [DOI] [PubMed] [Google Scholar]
- Pencík A, Rolcík J, Novák O, Magnus V, Barták P, Buchtík R, Salopek-Sondi B, Strnad M. 2009. Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 80, 651–655. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Friml J. 2009. Auxin tansport routes in plant development. Development 136, 2675–2688. [DOI] [PubMed] [Google Scholar]
- Prasad TK, Li X, Abdel-Rahman AM, Hosokawa Z, Cloud NP, Lamotte CE, Cline MG. 1993. Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil? Annals of Botany 71, 223–229. [Google Scholar]
- Rabot A, Henry C, Ben Baaziz K, et al. 2012. Insight into the role of sugars in bud burst under light in the rose. Plant and Cell Physiology 53, 1068–1082. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2013. R: A language and environment for statistical computing . Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues. Advances in Botanical Research 9, 151–262. [Google Scholar]
- Sachs T, Thimann KV. 1967. The role of auxins and cytokinins in the release of buds from dominance. American Journal of Botany 54, 136. [Google Scholar]
- Sairanen I, Novák O, Pěnčík A, Ikeda Y, Jones B, Sandberg G, Ljung K. 2012. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. The Plant Cell 24, 4907–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. 2006. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology 57, 431–449. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H. 2009. Auxin-cytokinin interactions in the control of shoot branching. Plant Molecular Biology 69, 429–435. [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O. 2013. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology 11, e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom JP, Sussex IM. 1992. Expression of a ribosomal protein gene in axillary buds of pea seedlings 1. Plant Physiology 100, 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL. 2012. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiology 160, 2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Ottoline Leyser HM. 2007. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. The Plant Journal 50, 80–94. [DOI] [PubMed] [Google Scholar]
- Stirnberg P, Sande K van de, Leyser HMO. 2002. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C. 2003. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. The Plant Cell 15, 2076–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tao J, Liu S, Huang S, Chen S, Xie X, Yoneyama K, Zhang Y, Xu G. 2014. Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. Journal of Experimental Botany 65, 6735–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H. 2004. AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant and Cell Physiology 45, 1053–1062. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. 2006. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. The Plant Journal 45, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. 1934. On the inhibition of bud development and other functions of growth substance in Vicia Faba. Proceedings of the Royal Society of London: B 114, 317–339. [Google Scholar]
- Waldie T, Hayward A, Beveridge CA. 2010. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant Molecular Biology 73, 27–36. [DOI] [PubMed] [Google Scholar]
- Waldie T, McCulloch H, Leyser O. 2014. Strigolactones and the control of plant development: lessons from shoot branching. The Plant Journal 79, 607–622. [DOI] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J. 2006. Polar PIN localization directs auxin flow in plants. Science 312, 883. [DOI] [PubMed] [Google Scholar]
- Wu L, Birch RG. 2011. Isomaltulose is actively metabolized in plant cells. Plant Physiology 157, 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nodzyński T, Pěnčík A, Rolčík J, Friml J. 2010. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proceedings of the National Academy of Sciences, USA 107, 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. 2012. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant 5, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.