Abstract
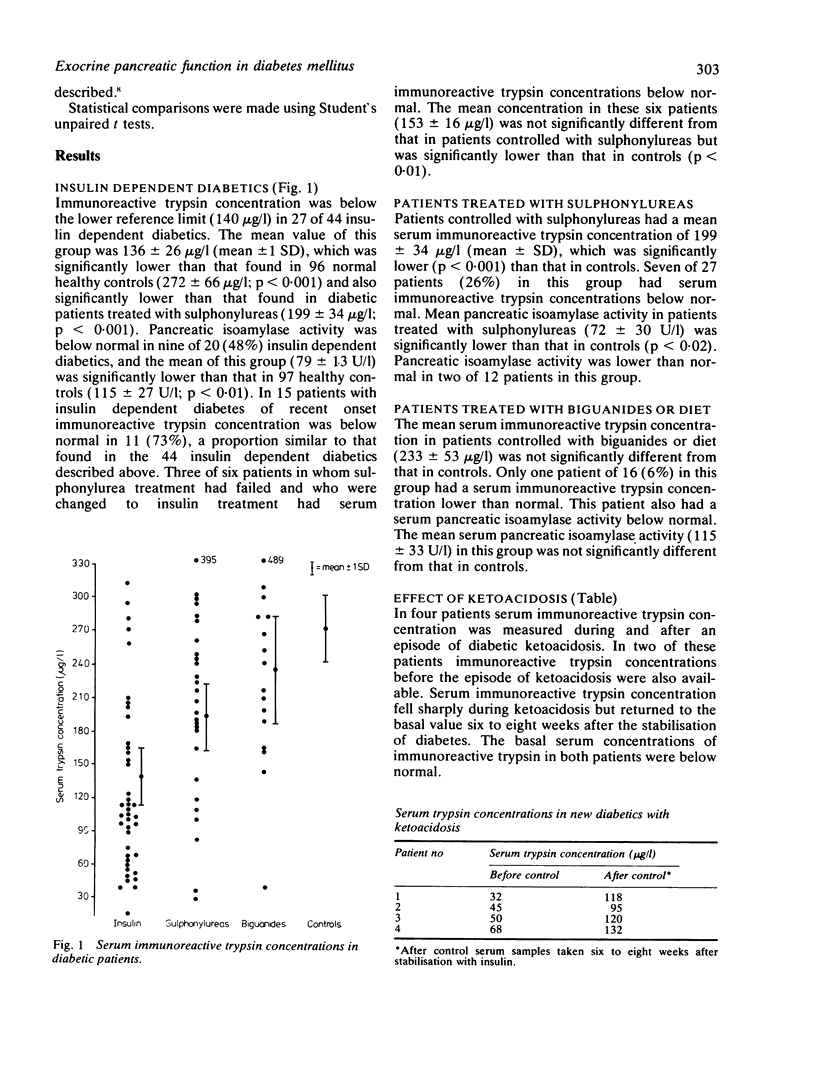
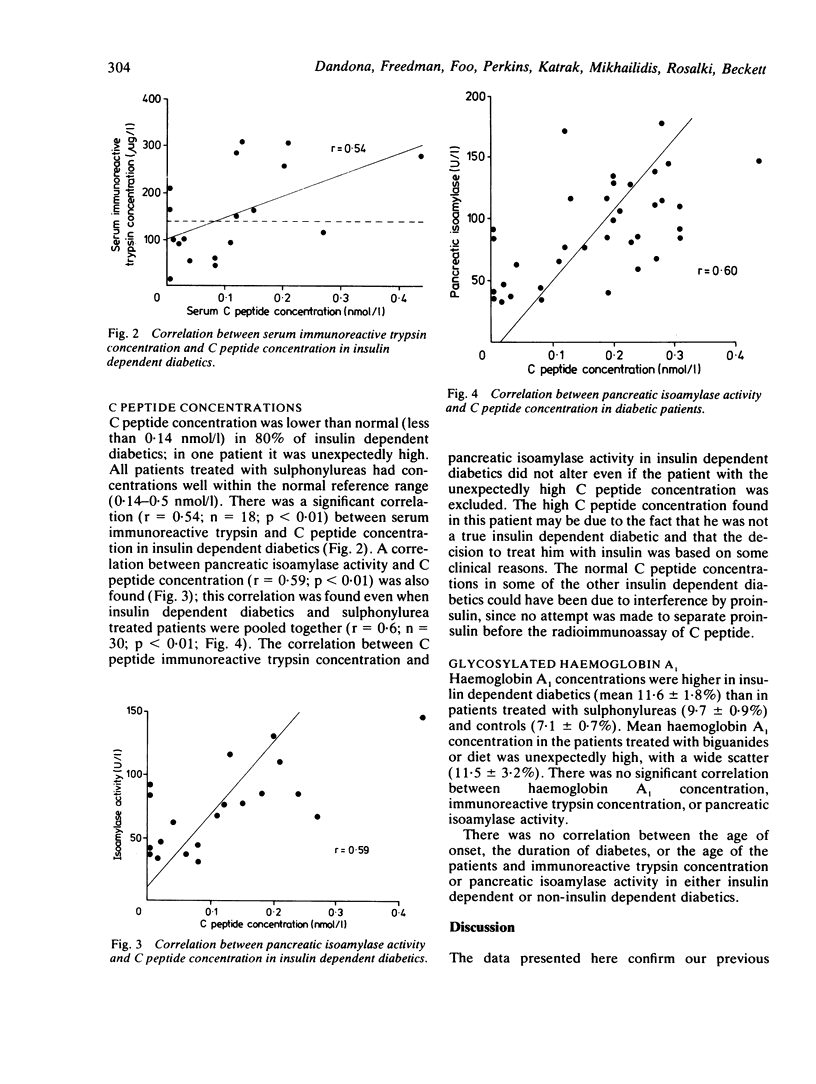
An investigation of serum immunoreactive trypsin concentration and pancreatic isoamylase activity in patients with diabetes mellitus has shown that exocrine pancreatic deficit is maximal in insulin dependent diabetics, intermediate in those controlled with sulphonylureas, and absent in patients controlled with biguanides or diet or both. A significant correlation between the serum concentrations of both these pancreatic enzymes and C peptide was found. Serum pancreatic enzyme concentrations were not related to glycosylated haemoglobin concentrations, the dosage of insulin, or the age of onset of diabetes. The concentration of immunoreactive trypsin was found to be low in most of the insulin dependent diabetics in whom this enzyme was measured at the time of the clinical onset of diabetes. Thus exocrine pancreatic deficit in diabetes closely parallels the endocrine beta cell deficit and occurs concurrently with, or antedates, the clinical presentation of type I diabetes. It is therefore possible that in type I diabetes similar mechanisms are entailed in the pathogenesis of impaired endocrine and exocrine pancreatic function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Barnes A. J., Bloom S. R. Hypotrypsinaemia in diabetes mellitus. Clin Chim Acta. 1979 Oct 1;97(2-3):213–216. doi: 10.1016/0009-8981(79)90418-2. [DOI] [PubMed] [Google Scholar]
- Dandona P., Elias E., Beckett A. G. Serum trypsin concentrations in diabetes mellitus. Br Med J. 1978 Oct 21;2(6145):1125–1125. doi: 10.1136/bmj.2.6145.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P., Freedman D., Moorhead J. F. Glycosylated haemoglobin in chronic renal failure. Br Med J. 1979 May 5;1(6172):1183–1184. doi: 10.1136/bmj.1.6172.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P., Hodson M. E., Batten J. C. beta Cell reserve in cystic fibrosis patients and heterozygotes. J Clin Pathol. 1983 Jul;36(7):790–792. doi: 10.1136/jcp.36.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P., Hodson M., Bell J., Ramdial L., Beldon I., Batten J. C. Serum immunoreactive trypsin in cystic fibrosis. Thorax. 1981 Jan;36(1):60–62. doi: 10.1136/thx.36.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P., Hussain M. A., Varghese Z., Politis D., Flynn D. M., Hoffbrand A. V. Insulin resistance and iron overload. Ann Clin Biochem. 1983 Mar;20(Pt 2):77–79. doi: 10.1177/000456328302000203. [DOI] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Pek S., Chance R. E. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- Foo Y., Rosalki S. B., Ramdial L., Mikhailidis D., Dandona P. Serum isoamylase activities in diabetes mellitus. J Clin Pathol. 1980 Nov;33(11):1102–1105. doi: 10.1136/jcp.33.11.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier B. M., Saunders J. H., Wormsley K. G., Bouchier I. A. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976 Sep;17(9):685–691. doi: 10.1136/gut.17.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B., Vinik A. I., Valtysson G., Zoghlin G. Truncal vagotomy abolishes the somatostatin response to insulin-induced hypoglycemia in man. J Clin Endocrinol Metab. 1981 Apr;52(4):823–825. doi: 10.1210/jcem-52-4-823. [DOI] [PubMed] [Google Scholar]
- Grossman M. I. Candidate hormones of the gut. I. Introduction. Gastroenterology. 1974 Oct;67(4):730–731. [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hussain M., Dandona P., Fedail S. S., Ramdial L., Flynn D., Hoffbrand A. V. Serum immunoreactive trypsin in beta-thalassaemia major. J Clin Pathol. 1981 Sep;34(9):970–971. doi: 10.1136/jcp.34.9.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglee D., De Albarran R., Katrak A., Freedman D. B., Beckett A. G., Dandona P. Low pancreatic lipase in insulin-dependent diabetics. J Clin Pathol. 1983 Feb;36(2):200–202. doi: 10.1136/jcp.36.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglee D., Katrak A., Hoffbrand A. V., Dandona P. Pancreatic lipase in serum of patients with beta-thalassemia major. Clin Chem. 1983 Nov;29(11):2003–2004. [PubMed] [Google Scholar]
- Junglee D., Penketh A., Katrak A., Hodson M. E., Batten J. C., Dandona P. Serum pancreatic lipase activity in cystic fibrosis. Br Med J (Clin Res Ed) 1983 May 28;286(6379):1693–1694. doi: 10.1136/bmj.286.6379.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol. 1976 Oct;261(3):505–521. doi: 10.1113/jphysiol.1976.sp011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Sankaran H., Wong K. Y., Williams J. A., Goldfine I. D. Insulin receptors in isolated mouse pancreatic acini. Biochem Biophys Res Commun. 1978 Sep 29;84(2):293–299. doi: 10.1016/0006-291x(78)90169-9. [DOI] [PubMed] [Google Scholar]
- Magid E., Horsing M., Rune S. J. On the quantitation of Iso-amylases in serum and the diagnostic value of serum pancreatic type amylase in chronic pancreatitis. Scand J Gastroenterol. 1977;12(5):621–627. doi: 10.3109/00365527709181344. [DOI] [PubMed] [Google Scholar]
- Mikhailidis D. P., Foo Y., Ramdial L., Kirk R. M., Rosalki S. B., Dandona P. Pancreatic exocrine function after truncal and highly selective vagotomy. J Clin Pathol. 1981 Sep;34(9):963–964. doi: 10.1136/jcp.34.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. A direct staining technique for amylase isoenzyme demonstration. J Clin Pathol. 1970 May;23(4):373–374. doi: 10.1136/jcp.23.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell W. S., Mitchell C. J., Hamilton I., Leek J. P., Kelleher J. Clinical value of serum immunoreactive trypsin concentration. Br Med J (Clin Res Ed) 1981 Nov 28;283(6304):1429–1432. doi: 10.1136/bmj.283.6304.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrha J., Stepán J., Havránek T., Skrha F., Herfort K., Sramkova J., Pav J. Isoamylases in diabetes mellitus. Diabetologia. 1981 Feb;20(2):129–133. doi: 10.1007/BF00262015. [DOI] [PubMed] [Google Scholar]


