Abstract
Purpose
The opioid epidemic is a public health threat with consequences affecting newborns. Neonatal Abstinence Syndrome (NAS) is a constellation of withdrawal symptoms resulting primarily from in utero opioid exposure. The purpose of this study is to examine NAS and drug-specific trends in West Virginia (WV), where rurality-related issues are largely present.
Methods
The 2007–2013 WV Health Care Authority, Uniform Billing Data were analyzed for 119,605 newborn admissions with 1,974 NAS diagnoses. NAS (ICD9-CM 779.5) and exposure diagnostic codes for opioids, hallucinogens, and cocaine were utilized as incidence rate (IR) per 1,000 live births.
Findings
Between 2007 and 2013, NAS IR significantly increased from 7.74 to 31.56 per 1,000 live births per year (Z: −19.10, P < .0001). During this time period, opioid exposure increased (Z: −9.56, P < .0001), while cocaine exposure decreased (Z: 3.62, P = .0003). In 2013, the southeastern region of the state had the highest NAS IR of 48.76 per 1,000 live births. NAS infants were more likely to experience other clinical conditions, longer hospital stay, and be insured by Medicaid.
Conclusions
Statewide NAS IR increased four-fold over the study period, with rates over 3 times the national annual averages. This alarming trend is deleterious for the health of WV mother-child dyads and it strains the state’s health care system. Therefore, WV has a unique need for prenatal public health drug treatment and prevention resources, specifically targeting the southeastern region. Further examination of maternal drug-specific trends and general underutilization of neonatal exposure ICD-9-CM codes is indicated.
Keywords: geographic variation, neonatal abstinence syndrome, opioid use, prenatal drug abuse, rural health
With the increase in opioid prescribing and prescription overdose deaths, it is evident that an opioid epidemic has emerged in the United States (US).1 This epidemic has far reaching consequences that has affected one of the most vulnerable populations, pregnant women and infants. Neonatal abstinence syndrome (NAS) is a constellation of signs and symptoms of withdrawal that occurs in newborns as a result of illegal or prescription in utero drug exposure.2 NAS-like symptoms are characterized as central nervous system, gastrointestinal, respiratory, and autonomic disturbances.2,3 Specific signs and symptoms include: irritability, feeding difficulties, excessive sucking and/or crying, hyperactive reflexes, sleep problems, vomiting, diarrhea, and sometimes seizures. NAS most commonly results from antepartum opiate use, but the diagnosis has occurred in the context of other illicit and prescription drugs.4 The syndrome is diagnosed in 55% to 94% of newborns exposed to opioids in utero, and it is commonly a comorbid diagnosis with other conditions such as low birthweight, preterm birth, and intrauterine growth retardation.3 In addition, the total hospital charges for NAS reached $1.5 billion in 2012, with 81% of costs charged to respective state Medicaid programs.5
From 2000 to 2009, maternal opiate use during pregnancy increased from 1.19 to 5.63 per 1,000 hospital births, and from 2000 to 2012, the incidence of NAS in the US increased nearly 500% from 1.2 to 5.8 per 1,000 hospital births per year.5,6 In 2012, nationwide geographic variations in NAS showed that the largest rates occurred in the East South Central parts of the country (Kentucky, Tennessee, Mississippi, and Alabama) with 16.2 per 1,000 live births.5 Minimal research has addressed NAS and neonatal in utero substance exposure in rural settings, as granularity of the geographic national variation in NAS study findings do not include specific state and sub-state rates.
Although substance use in pregnancy is a concern nationwide, the state of West Virginia (WV) faces distinct challenges due to issues related to rurality, poverty,7 and drug use. WV is ranked as the third most rural state, with a population of 950,184 residing in 2010 Census Bureau-defined rural areas.8 Rural areas are defined as all population, housing, and territory encompassing less than 2,500 people.8 In addition, it is the only state completely immersed within the Appalachian region.9 WV has the highest age-adjusted death rate from drug poisoning in the country (36.3 per 100,000 population) and the third-highest prescribing rate of opioid analgesics (137.6 per 100 people).10 In terms of neonatal substance exposure, a 2009 study that analyzed umbilical cord tissue samples from 8 birthing hospitals located across WV showed that 19.2% of infants were antenatally exposed to licit/illicit drugs and alcohol, excluding nicotine.11
Substance use during pregnancy is more common among women residing in rural areas.12–15 Rural pregnant women have increased rates of prescription opiate, benzodiazepine, and injection drug use compared to pregnant women from urban areas.13 In addition to increased drug use, pregnant women with opioid use disorders are faced with societal stigmas and lowered accessibility to substance abuse treatment in rural areas where lack of resources and greater disparities exist.16–19
With the increase in antepartum opiate use, NAS diagnosis, and hospital utilization, NAS is a major public health concern. There is no WV statewide or regional estimate of the incidence of NAS, thus the purpose of the current study is to outline the scope of the problem in the primarily rural state of WV. The primary objectives were to examine state- and region-level NAS and drug-specific trends between 2007 and 2013, as well as to present infant patient characteristics associated with NAS and hospital-level NAS rates for WV birthing hospitals.
Methods
Data Source and Identification of Sample
A serial cross-sectional analysis was conducted with data from the WV Health Care Authority (HCA), Uniform Billing Database (UB). The claims data represented 35 hospitals and were collected at the conclusion of every year from 2007 to 2013. The HCA provided de-identified hospital inpatient discharges of WV resident newborn admissions that were delivered in-state, excluding stillborn (International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) code 779.9) and HIV diagnoses. Specifically, patient-level geographical locations were taken from ZIP codes of hospital patients’ home addresses and newborn admissions referred to admission for delivery as well as readmissions or hospital transfers. Multiple birth pregnancies were counted as separate live births. The UB data reflect the final amount billed/charged on the newborn’s claim and not the total cost of delivery; therefore, a health care expenditure analysis was not conducted.
Infants born in hospitals that delivered 10 newborns or fewer within the 7-year period were excluded from the study (ie, 5 hospitals and 15 newborns). None of the newborns that were excluded due to place of delivery were diagnosed with NAS. In addition, the excluded hospitals were proportionally distributed across the state. This exclusion helped eliminate bias, as these infants could have been diagnosed and treated differently than those delivered at registered birthing centers.
Newborns were also excluded from the study if they were diagnosed with any of the following conditions (n=758): intraventricular hemorrhage (ICD9-CM, 772.1x), periventricular leukomalacia (ICD9-CM, 779.7), necrotizing enterocolitis (ICD9-CM, 777.5x), spontaneous intestinal perforation (ICD9-CM, 777.6), or bronchopulmonary dysplasia (ICD9-CM, 770.7). These newborns were likely to have prolonged length of stay in the hospital and were more likely to have received opiate medication resulting in iatrogenic NAS. Likely iatrogenic NAS cases were excluded from the study because the etiology of withdrawal differs from antenatal exposed NAS.6 Total excluded cases equal less than 1% of the baseline population (ie, 0.99% of 120,378 observations).
Outcome
NAS incidence rate (IR) per 1,000 live births was the outcome of interest. NAS (yes, no) was defined via the presence of an ICD9-CM 779.5: “Drug withdrawal syndrome in newborn” diagnosis (diagnostic fields 2–18). NAS IR includes infants treated non-pharmacologically as well as those treated pharmacologically (ie, with morphine or methadone as the first-line regimen). The denominator for “live births” was derived from the patient hospital admission type 4, or “newborn” admission. Type of admission was coded according to official UB-04 data specifications from the National Uniform Billing Committee. This method was favored over use of V30x principal diagnostic codes because infants could possibly experience premature discharge from the hospital prior to the onset of withdrawal symptoms.3,20,21 Therefore, NAS could be diagnosed outside of the original hospital admission (ie, at time of delivery).
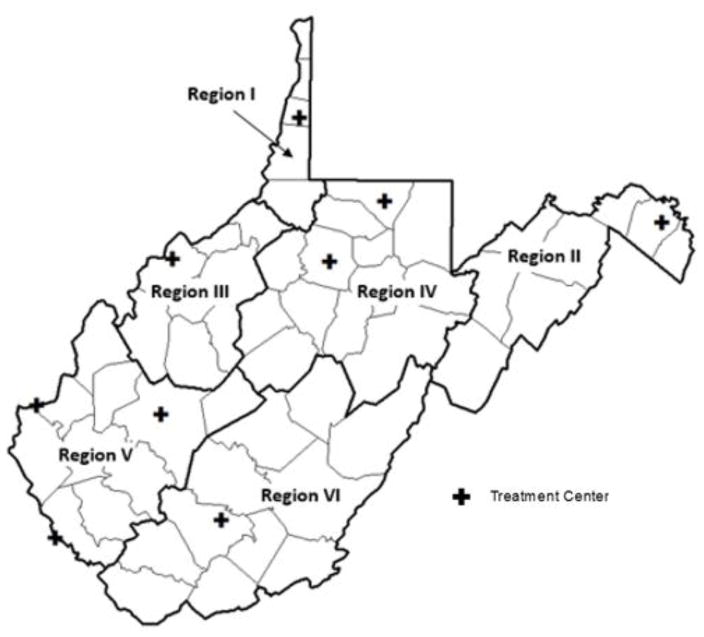
In order to comply with the federal Health Insurance Portability and Accountability Act (HIPPA) privacy standards, the data were aggregated by 6 geographic sub-state regions predefined by the Substance Abuse and Mental Health Services Administration (SAMHSA) 2008–2010 NSDUH report (Figure 1). The geographic presence of substance use-related issues varied greatly within each state. Therefore, SAMHSA created predefined sub-state regions to more accurately measure and address the public health problem.22 West Virginia sub-state regions were defined as county groupings that covered the entire state via a collaboration between state substance abuse agency and SAMHSA representatives.22
Figure 1.
SAMSHA West Virginia Region and Opioid Treatment Program Center Map
Neonatal substance exposure diagnosis (yes, no) was the secondary outcome of interest. The ICD9-CM codes associated with noxious substances affecting infants and breast feeding children (760.7x) were captured to identify the type and magnitude of exposure. These diagnoses represent exposure to narcotics, including heroin and prescription opioid analgesics (760.72), hallucinogens (760.73), and cocaine (760.75). Exposure codes are recorded at the discretion of the health care provider/medical coder and represent a combination of maternal self-report and maternal/neonatal drug screen results.
Descriptive Variables
Descriptive patient-level characteristics associated with substance use and obstetric/neonatal health outcomes were analyzed. These variables included the newborn’s gender, hospital length of stay, clinical conditions, in utero substance exposure, and mother’s insurance type. Seizures, adverse respiratory symptoms, and feeding difficulties are clinical conditions associated with NAS diagnosis.2,3,23 Diagnoses and respective ICD9-CM codes, seizures (779.0, 780.3), respiratory symptoms (769.x, 770.x), and feeding difficulties (779.3) were coded to better describe the sample. Mother’s insurance type was a 4-category variable that encompassed Medicaid, Medicare, PEIA (WV Public Employees Insurance Agency), and Other (eg, commercial companies and self-payers). In addition to the outcome, neonatal substance exposure codes (ie, ICD9-CM 760.72, 760.73, and 760.75) were described within the presence of a NAS diagnosis. These exposures are presented as separate binary variables (ie, diagnosed versus not diagnosed), as newborns could have polysubstance exposure.
Statistical Analysis
NAS IRs were calculated using the number of NAS diagnoses as the numerator and the respective number of live births in the denominator and multiplied by 1,000 to obtain IRs per 1,000 live births by region and year. This measure of infant morbidity captures newly occurring NAS diagnoses, which exhibit short duration and occur only once during infancy.24 Therefore, each newborn’s person time at risk was equal to 1 unit (ie, the number of annual live births). Statewide data were analyzed first and then the data were further broken down into region-specific outcomes.
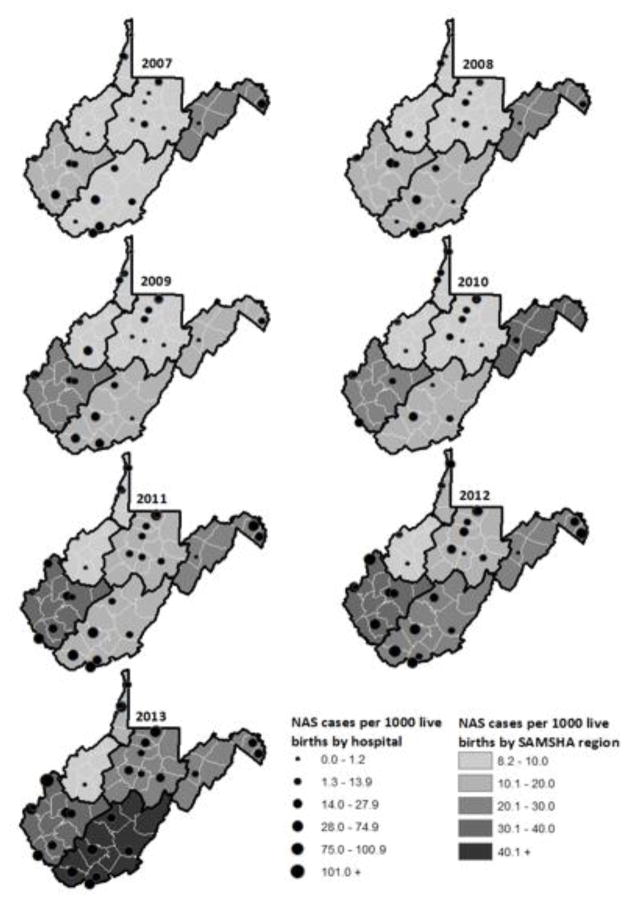
The outcome was visually demonstrated via annual comparative WV region maps utilizing ArcGIS 10.2.25 NAS IRs were stratified, in order to be representative of the SAMHSA 6 WV sub-state regions (Figure 1).22 Stratified by birthing hospitals, the hospital-level NAS IRs were represented by the diameter of circles on the GIS regional map (Figure 2).
Figure 2.
West Virginia Regional and Hospital Neonatal Abstinence Syndrome Incidence Rate per 1,000 Live Births.
To assess overall differences in proportions of newborns with and without NAS by year, the Mantel-Haenszel Chi-square (χ2MH) test was stratified by both region and hospital.26 Additionally, the Cochran-Armitage test (Z) for trend was utilized to test for linear trends in the proportion of NAS diagnosis from 2007 to 2013. Differences among delivery characteristics (ie, neonatal length of stay, insurance type, and clinical diagnoses) and NAS diagnosis were tested using the Pearson Chi-square (χ2) test for categorical characteristics and the Wilcoxon Ranked-Sum test (z) for continuous characteristics. An a priori 2-sided type 1 error of 5% was considered statistically significant. Statistical analyses were conducted using SAS® 9.4 (SAS Institute Inc., Cary, North Carolina). Ethical approval for the current study was obtained from the Institutional Review Board at the academic institution of the authors.
Results
NAS and Exposure
Overall, the current study analyzed 119,605 live births with 1,974 NAS diagnoses (ie, a total of 16.5 per 1,000 live births) (Figure 2). The number of NAS cases diagnosed at time of birth (ie, the number of NAS cases with a V30x principal diagnosis) were 1,942 or 98.4% of the total NAS cases. With regard to rates over time, between 2007 and 2013 the statewide rate of newborns diagnosed with NAS significantly increased from 7.74 to 31.57 per 1,000 live births per year (Z: −19.10, P < .0001). There were statistically significant differences in the magnitude of the trend in NAS IR by region; region 1 (Z: −4.73, P < .0001), region 3 (Z: −2.41, P = .016), region 4 (Z: −10.51, P < .0001), region 5 (Z: −8.00, P < .0001), and region 6 (Z: −18.77, P < .0001) significantly increased over time. In 2013, the southeastern region of the state (ie, region 5) had the highest NAS IR of 48.76 per 1,000 live births. There was no significant trend of NAS diagnoses in region 2 (Z: −0.002, P = .999). After adjusting for regional variation, there was an overall significant increase in NAS diagnoses from 2007 to 2013 (χ2MH=429.78, 6 d.f., P < .0001).
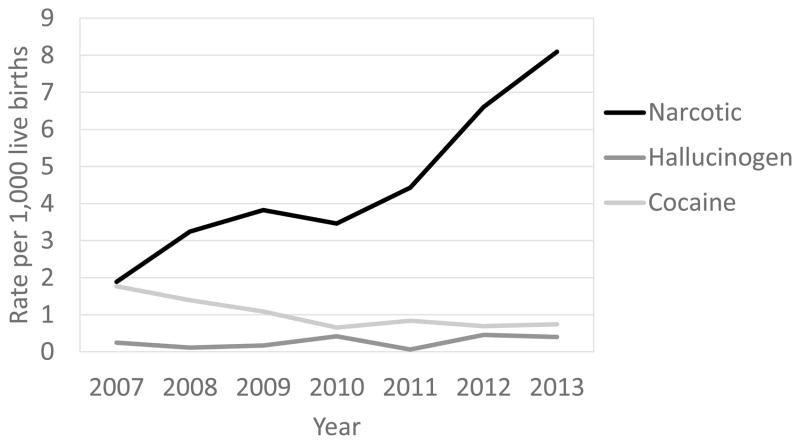
Between 2007 and 2013, the statewide rate of newborns diagnosed with neonatal substance exposure per 1,000 live births significantly increased from 1.89 to 8.09 for narcotics (Z: −9.56, P < .0001) (Figure 3). Region-specific narcotic-exposed diagnoses significantly increased over time in regions 1 (Z: −5.30, P < .0001), 2 (Z: −4.24, P < .0001), 3 (Z: −2.02, P = .04), 4 (Z: −8.46, P < .0001), and 6 (Z: −2.52, P = .01). Neonatal exposure diagnoses did not differ over time in region 5 (Z: 0.84, P = .40). After controlling for region differences, the NAS IR trend was statistically significant (χ2MH=99.01, 6 d.f., P < .0001).
Figure 3.
Number of inpatient hospitalizations resulting from expsoure to noxious substances, West Virginia, 2007–2013.
During this time period the diagnosis of cocaine exposure decreased (Z: 3.62, P = .0003) from 1.77 to 0.74 per 1,000 live births. Region-specific cocaine-exposed diagnoses significantly decreased over time in regions 5 (Z: 2.49, P = .013) and 6 (Z: 3.02, P = .003). These diagnoses did not significantly decrease between 2007 and 2013 for regions 1 (Z: 0.27, P = .786), 2 (Z: 1.20, P = .229), 3 (Z: 1.46, P = .144), and 4 (Z: 0.733, P = .4636). After controlling for regional variation, cocaine diagnosed exposure decreased over time (χ2MH=17.77, 6 d.f., P = .0007). Diagnosis of hallucinogenic agent exposure did not significantly change over time (Z: −1.61, P = .11).
Controlling for year, the amount of NAS cases differed significantly by birthing hospital (n=30, χ2MH=906.67, 29 d.f., P < .0001). Between 2007 and 2013, the total hospital rate of NAS diagnoses ranged from zero to 55.7 per 1,000 live births. During the study period, the 3 level III neonatal intensive-care units (NICUs) in WV delivered a total of 11.97, 29.55, and 20.45 NAS diagnosed infants per 1,000 live births, respectively.
Characteristics by NAS Diagnosis
Infants with a primary or secondary NAS diagnosis were more likely to exhibit respiratory issues (χ2: 366.01, 1 d.f., P < .0001), feeding difficulties (χ2: 95.38, 1 d.f., P < .0001), and seizures (χ2: 92.50, 1 d.f., exact P < .0001), compared to newborns without a NAS diagnosis. These babies were also statistically more likely to be diagnosed with narcotics (χ2: 903.56, 1 d.f., exact P < .0001), hallucinogens (v: 11.77, 1 d.f., exact P = .0156), and cocaine (χ2: 113.53, 1 d.f., exact P < .0001) exposures than newborns without NAS. Comparatively, newborns diagnosed with NAS had a longer length of stay (z: 57.77, 1 d.f., P < .0001) and were more likely to have Medicaid insurance (χ2: 753.01, 3 d.f., P < .0001) (Table 1) than non-NAS newborns.
Table 1.
West Virginia Delivery Characteristics by Neonatal Abstinence Syndrome: HCA,UB 2007–2013
| Characteristics | Total | NAS | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Total sample | 119,605 | 1974 (1.65%) | 117,631 (98.35%) | |
| Male | 60,923 (50.94%) | 1053 (53.34%) | 59,870 (50.90%) | .0967 a |
| Neonatal substance exposure | ||||
| Narcotics | < .0001 a | |||
| Yes | 543 (0.45%) | 98 (4.96%) | 445 (0.38%) | |
| No | 119,062 (99.55%) | 1957 (99.14%) | 117,526 (99.91%) | |
| Hallucinogenic agentsd | .0156 b | |||
| Yes | 32 (0.03%) | -- | -- | |
| No | 119,573 (99.97%) | 1957 (99.14%) | 117,526 (99.91%) | |
| Cocaine | < .0001 b | |||
| Yes | 122 (0.10%) | 17 (0.86%) | 105 (0.09%) | |
| No | 119,483 (99.90%) | 1957 (99.14%) | 117,526 (99.91%) | |
| Presence of neonatal conditions | ||||
| Respiratory diagnoses | 10,060 (8.41%) | 400 (20.26%) | 9660 (8.21%) | < .001 a |
| Feeding difficulty | 872 (0.73%) | 51 (2.58%) | 821 (0.70%) | < .001 a |
| Seizure | 158 (0.13%) | 18 (0.91%) | 140 (0.12%) | < .0001 b |
| Neonatal hospital length of stay, Median (IQR) | 2 (2, 3) | 8 (4, 18) | 2 (2, 3) | < .001 c |
| Insurance | < .001 a | |||
| Medicaid | 65,502 (54.77%) | 1680 (85.11%) | 63,822 (54.26%) | |
| Medicared | 97 (0.08%) | -- | -- | |
| Other | 45,898 (38.37%) | 277 (14.03%) | 45,621 (38.78%) | |
| PEIA | 8108 (6.78%) | 16 (0.81%) | 8092 (6.88%) | |
HCA, Health Care Authority; UB, Uniform Billing Database; NAS, Neonatal Abstinence Syndrome; TCHG, Total hospital charge; IQR, Interquartile range; PEIA, Public Employees Insurance Agency
Pearson Chi-square
Two-sided Fisher’s exact test
Wilcoxon Ranked Sum
Variable is not stratified via NAS diagnosis in order to comply with the HCA UB data user agreement of not reporting data less than or equal to 10 counts per cell.
Conclusions
NAS and Exposure
The current study was the first to assess the incidence rate (IR) of NAS throughout the predominantly rural state of WV. Between 2007 and 2013, WV NAS rates increased over 4-fold. Although the upward trend was expected, the increase of annual NAS rates was over 3-fold in WV compared to national estimates between 2009 and 2012.5,6 In 2009, WV’s NAS IR was 3.5 times as high as nationwide rates (3.4 vs 11.8 NAS per 1,000 live births).6 The same year, Ohio reported a NAS rate of about 5 cases per 1,000 live births and Vermont found a NAS and opioid exposure rate of 24.2 per 1,000 live births.27,28 Vermont’s rate was likely higher because it utilized a different case definition (ie, all opioid-exposed infants and/or infants diagnosed with NAS).27 Heterogeneous case definitions can result in faulty comparative estimates that may lead to inappropriate distribution of resources. This example highlights the importance of national standardized diagnostic criteria. In 2010, WV’s NAS IR was over 3 times as high as nationwide estimates (14.7 vs 4.8 NAS per 1,000 live births).5 In 2011, WV’s statewide NAS IR was 16.9 per 1,000 live births, which was higher than the national average (5.0) and various state-specific rates in Tennessee (8.5), Kentucky (13.2) and Florida (7.52) in the same year.5,29
Between 2007 and 2013, the current study showed the rate of neonatal substance exposure diagnosis increased over 4-fold for narcotics while neonatal cocaine exposure decreased over 2-fold and hallucinogens remained stable. Further examination of a potential shift in maternal drug use is indicated. Although national estimates of neonatal substance exposure via the ICD9-CM diagnostic codes 760.7x are not currently available, Ohio reported similar patterns to WV between 2004 and 2011 of neonatal narcotic, cocaine, and hallucinogen substance exposure.28 In addition, nationwide data during this time period showed that opioid-related overdose deaths and substance abuse treatment admission discharges vastly increased, while these estimates decreased for cocaine-related deaths and treatment admissions.30,31 A drastic increase in the environmental availability of prescription narcotics (ie, increased prescriptions written and dispensed, greater social acceptability for non-medical use, and pharmaceutical marketing) could explain the increase in neonatal opioid and other noxious substance exposure resulting in the quadrupled NAS rates.
Geographic and Time Trend Variations
In the present study, NAS rates differed by WV residential sub-state SAMHSA regions, with the highest occurring in the southeastern part of the state. This finding is congruent with other data, as age-adjusted death rates due to drug poisoning per 100,000 WV residents was the highest in the south between 1999 and 2009.32 In addition, other characteristics associated with substance use and obstetric/neonatal health outcomes were found to be higher among residents from the respective area, compared to the rest of the state. According to the 2010 WV Vital Statistics, the highest percentages of low birthweight babies, mothers less than 18 years of age at delivery, no prenatal care, and congenital anomalies occurred among residents from the southeastern regions of WV.9 This area also had the highest prevalence of nonmedical use of pain relievers (12 or older) and drug/narcotic-related arrest.22,33
Compounding the challenges, treatment facilities are unevenly available throughout WV. Throughout the study period, there were 9 WV federally funded opioid maintenance therapy (OMT) facilities that accepted pregnant women for the recommended OMT treatment, of which only 1 was located in the southeastern area of WV (Figure 1).34 Although OMT can result in NAS, the therapy is preferred over continued illegal substance use in order to improve obstetric and neonatal outcomes via decreased maternal illicit behaviors, improved prenatal care, and elimination of acute intoxication and withdrawal during pregnancy.23,35 Therefore, higher regional occurrence of adverse obstetric and neonatal health outcomes could be linked to the lack of available treatment options for this vulnerable population.35,36
An examination of time trends by region showed that incidence of NAS rates first rose in the Eastern Panhandle and during subsequent years rose throughout WV, with the highest IR in the southern regions of the state. The high initial NAS rate in the Eastern Panhandle may explain why NAS IR trends were not statistically significant in region 2. Although statistically significant, the NAS IR growth in region 3 was not substantial because many high-risk women residing on the Ohio-WV border likely delivered their newborn at a closer level 3 NICU in Ohio. These women would not be captured in the data set, as they did not deliver in-state. Aside from the inherent risk factors mentioned above, this statewide trend could have resulted from potential drug trafficking routes and increased medical awareness. The Office of National Drug Control Policy has identified 11 southern WV counties (9 located in region 6 and 2 in region 5) as high-intensity drug trafficking areas due to the Appalachian drug abuse problem.37
Geographically, the US 19 corridor is also a high-intensity drug trafficking area because of the flow of prescription pills from Florida throughout Appalachia.38 Additionally, WV is located in the middle of drug trafficking routes on major highways connecting the West and Southwest regions to the profitable East Coast markets.39
A heightened medical awareness may also increase rates of NAS diagnoses over time. There could be more accurate diagnosis in areas around major medical centers and hospitals located on the Ohio, Maryland, and Virginia borders. Although provider awareness of NAS and its signs/symptoms could contribute to the increasing trend, the opioid epidemic literature, along with the current study finding of increased neonatal substance exposure, suggest increased rates are a result of actual use during the perinatal period.
Along with regional differences, NAS IR varied by hospital. High-risk patients are more likely to deliver at the closest hospital capable of dealing with fetal and/or maternal complications. During the study period, the level III NICU hospitals, those capable of treating NAS-diagnosed infants, had comparatively high NAS cases per 1,000 live births. These hospitals were located in regions 6 and 4, potentially resulting in more accessibility to patients residing in those regions. There was no clear pattern of regional differences among diagnosed neonatal substance use exposure. In addition, NAS rates were greater than substance exposure rates, thus they did not mirror each other. These findings indicate WV greatly underutilizes neonatal substance exposure codes (ie, the 760.7x ICD9-CM codes). NAS-related reporting and surveillance would be improved if practitioners diagnosed every NAS infant with respective exposure codes. Reporting improvements would also occur if policy recommendations included adding NAS to WV’s list of reportable diseases, implemented an interoperable insurance/hospital system, and created a statewide standardized surveillance system to track NAS.40
NAS Associated Impact
The significant associations between NAS and neonatal clinical conditions (ie, respiratory issues, feeding difficulties, and seizures), as well as NAS and substance exposure (ie, narcotics, hallucinogens, and cocaine), are consistent with existing research.3,27,41 As expected, infants diagnosed with NAS were also more likely to be covered by Medicaid and have a longer hospital length of stay.5,6,42 The average hospital length of stay for NAS infants in WV was 12.7 days (a median of 8 with an interquartile range of 4 to 18), shorter than the average national estimate of 16 days. In contrast, the WV average length of stay for newborns not diagnosed with NAS was 2.86 days (a median of 2 with an interquartile range of 2 to 3), equivalent to the US average of 3 days.6
As highlighted by associated neonatal comorbidity and longer hospital length of stay, the impact of NAS to WV is substantial. Addressing this high cost and medical-resource-intensive public health issue involves a multi-faceted approach to policy, interventions, and evaluation of systematic efforts. Rural pregnant women with substance use disorders have unique treatment needs14; therefore, future research is needed to identify optional substance use treatment and potential barriers to treatment access for this population.
From the primary prevention standpoint, it is important that efforts are made to deter opioid misuse during the preconception period. Increased patient-provider discussions regarding the risks and potential benefits of opiate use during pregnancy can help minimize the emerging public health threat of NAS.43 Patient-provider discussions can serve as an opportunity to reduce the amount of antenatal opiate use and unintended pregnancies via screening for substance use, educating women of childbearing potential about associated maternal and infant health risks, and encouraging appropriate use of contraceptive devices to avoid fetal substance exposure.29 Additional prevention measures include prescribing clinicians’ use of the state prescription drug monitoring program and required counsel about risk of opiate use during pregnancy to women of childbearing age who are prescribed narcotics.29
Limitations
There are inherent limitations to using hospital discharge data. The HCA-UB data was created for payment use; therefore it is restrictive in its use to research a statewide health condition. Hospital charges might not translate to actual costs because charges are often negotiated by payers, and costs can be separated via mother and infant claims; thus a cost analysis was not conducted. In addition, hospital billing practices could change over time. Overall, population-based surveillance is dependent on accurate and homogenous statewide coding from documenting a condition in the medical records to being coded in the discharge abstract. Furthermore, hospital billing data may underreport the diagnosis of NAS and neonatal substance exposure, especially when not all newborns are drug screened and exposure status is based on maternal self-report.5,6,27,44 It may be difficult to draw conclusions based on under reporting of neonatal in utero exposure diagnoses, particularly if there was regional variation in accuracy of reporting.
Statewide identifiable data were unavailable; therefore, hospital admissions/discharges rather than individual patients were the units of observation. Although unlikely, NAS discharges may result in duplication when the same patient (ie, a newborn) has multiple hospital admissions. This would potentially underestimate NAS rates because it could increase the total population (ie, denominator), while the NAS cases (numerator) would remain constant as this condition is likely to be diagnosed at the first hospital admission. In fact, the current study found 98.4% of the NAS cases were diagnosed at time of birth (ie, first hospital admission). Although innate data source limitations exist, the HCA-UB is retrospectively the best approach to obtaining a statewide NAS rate of WV residents because it is currently the only data source that captures all insurance types. Strictly using Medicaid data would overestimate NAS rates, as it would mostly capture the at-risk population.44
Conclusion
The current study provides further public health justification to increase efforts aimed at reversing the NAS-associated burden within rural areas. Newborns with NAS were more likely to present other clinical conditions, experience longer hospital length of stay, and be insured by Medicaid. West Virginia statewide neonatal diagnostic rates of both NAS and narcotic exposure have greatly increased. Public health interventions and treatment programs for this vulnerable population are especially important in the southeastern region of WV. Inherent limitations of currently available data to study NAS also need to be addressed. Health care providers and medical coders should better utilize the neonatal substance exposure codes and provide standardized patient-level identified data for future public health research and quality improvement initiatives.
Implications of the study findings can be applicable outside of WV because if NAS rates continue to rise in Appalachia other rural states might face similar issues. Women residing in rural areas are more likely to use opiate substances during pregnancy and live in medically underserved areas compared to their urban counterparts.13,45 This is problematic as rural states generally are less equipped to handle the emerging public health threat of NAS.46 Therefore, surveillance and early identification of an emerging issue should be encouraged to better prepare preventative and treatment efforts.
Acknowledgments
Funding: This work was supported by the National Institute of General Medical Sciences, NIH grant U54GM104942 (DLL).
Footnotes
Disclosures: The authors of this work have no conflicts of interest to report.
References
- 1.Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012 Jul;15(3 Suppl):Es9–Es38. [PubMed] [Google Scholar]
- 2.American College of Obstetrics and Gynecology (ACOG) Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012 May;119(5):1070–1076. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 3.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–e560. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 4.Jansson LM, Velez ML. Infants of drug-dependent mothers. Pediatr Rev. 2011 Jan;32(1):5–12. doi: 10.1542/pir.32-1-5. quiz 12–13. [DOI] [PubMed] [Google Scholar]
- 5.Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):650–655. doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal Abstinence Syndrome and Associated Health Care Expenditures United States, 2000–2009. JAMA: Journal of the American Medical Association. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 7.Trust for America’s Health. [Accessed December 15, 2014];Key Health Data About West Virginia. Available at: http://www.healthyamericans.org/states/?stateid=WV#section=4,year=2014,code=hrsamch.
- 8.United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. [Accessed October 15, 2015];Geographic Concepts. 2015 Feb 09; Available at: https://www.census.gov/geo/reference/ua/uafaq.html.
- 9.Appalachian Regional Commission. The Appalachian Region. [Accessed December 10, 2014];Map of the Appalachian Region. Available at: http://www.arc.gov/appalachian_region/mapofappalachia.asp.
- 10.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014 Jul 4;63(26):563–568. [PMC free article] [PubMed] [Google Scholar]
- 11.Stitely ML, Calhoun B, Maxwell S, Nerhood R, Chaffin D. Prevalence of drug use in pregnant West Virginia patients. The West Virginia Medical Journal. 2010;106(4 Spec):48–52. [PubMed] [Google Scholar]
- 12.Burns L, Black E, Powers JR, et al. Geographic and maternal characteristics associated with alcohol use in pregnancy. Alcohol Clin Exp Res. 2011 Jul;35(7):1230–1237. doi: 10.1111/j.1530-0277.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- 13.Shannon LM, Havens JR, Hays L. Examining differences in substance use among rural and urban pregnant women. Am J Addict. 2010 Nov-Dec;19(6):467–473. doi: 10.1111/j.1521-0391.2010.00079.x. [DOI] [PubMed] [Google Scholar]
- 14.Jumah NA, Graves L, Kahan M. The management of opioid dependence during pregnancy in rural and remote settings. Cmaj. 2015 Jan 6;187(1):E41–E46. doi: 10.1503/cmaj.131723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson R, Bolisetty S, Ingall C. The profile of substance-using pregnant mothers and their newborns at a regional rural hospital in New South Wales. The Australian & New Zealand Journal Of Obstetrics & Gynaecology. 2001;41(4):415–419. doi: 10.1111/j.1479-828x.2001.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 16.Bailey BA, Cole LK. Rurality and birth outcomes: findings from southern appalachia and the potential role of pregnancy smoking. J Rural Health. 2009;25(2):141–149. doi: 10.1111/j.1748-0361.2009.00210.x. [DOI] [PubMed] [Google Scholar]
- 17.Lander LR, Marshalek P, Yitayew M, Ford D, Sullivan CR, Gurka KK. Rural healthcare disparities: challenges and solutions for the pregnant opioid-dependent population. W V Med J. 2013 Jul-Aug;109(4):22–27. [PubMed] [Google Scholar]
- 18.Hart LG, Salsberg E, Phillips DM, Lishner DM. Rural health care providers in the United States. J Rural Health. 2002;18(Suppl):211–232. doi: 10.1111/j.1748-0361.2002.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 19.Lisonkova S, Sheps SB, Janssen PA, Lee SK, Dahlgren L, Macnab YC. Birth outcomes among older mothers in rural versus urban areas: a residence-based approach. J Rural Health. 2011;27(2):211–219. doi: 10.1111/j.1748-0361.2010.00332.x. [DOI] [PubMed] [Google Scholar]
- 20.Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009 Jan-Feb;5(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Wexelblatt SL, Ward LP, Torok K, Tisdale E, Meinzen-Derr JK, Greenberg JM. Universal Maternal Drug Testing in a High-Prevalence Region of Prescription Opiate Abuse. Journal of Pediatrics. 2015;166(3):582–586. doi: 10.1016/j.jpeds.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Accessed August 11, 2014];The NSDUH Report: Substance Use Disorders in Substate Regions: 2008 to 2010. 2012 Sep 18; Available at: http://archive.samhsa.gov/data/2k12/NSDUH113/SR113StateSubUseDisorder2012.pdf.
- 23.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010 Dec 9;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams M, Alexander G, Kirby R, Wingate M. Perinatal Epidemiology for Public Health Practice. New York, NY: Springer Science+Buisness Media, LLC; 2009. [Google Scholar]
- 25.ArcGIS Desktop: Release 10.2 [computer program] Redlands, CA: Environmental Systems Research Institute; 2013. [Google Scholar]
- 26.Stokes M, Davis C, Koch G. Categorical Data Analysis Using SAS®. 3. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 27.Livingston S. [Accessed July 6, 2015];Neonates Exposed to Opioids in Vermont: Vermont Uniform Hospital Discharge Data Set. 2015 Jun; Available at: http://www.healthyamericans.org/states/?stateid=WV#section=4,year=2014,code=hrsamch.
- 28.Massatti R, Falb M, Yors A, Potts L, Beeghly C, Starr S. [Accessed January 12, 2015];Neonatal abstinence syndrome and drug use among pregnant women in Ohio, 2004–2011. 2013 Nov; Available at: http://www.healthy.ohio.gov/~/media/HealthyOhio/ASSETS/Files/injury%20prevention/NAS%20Report%20FINAL.ashx.
- 29.Association of State and Territorial Health Officials (ASTHO) Neonatal abstinence syndrome: How states can help advance the knowledge base for primary prevention and best practices of care. Arlington, VA: ASTHO; 2014. Retrieved from www.astho.org/prevention/nas-neonatal-abstinence-report/ [Google Scholar]
- 30.National Institute of Health (NIH), National Institue on Drug Abuse (NIDA) [Accessed December 18, 2014];Overdose Death Rates. Available at: http://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 31.Purdue Healthcare Advisors. [Accessed January 12, 2015];2012 West Virginia State Health Profile: Shaping safe and healthy communities. 2013 May; Available at: http://www.dhhr.wv.gov/publichealthquality/statepublichealthassessment/Documents/2012%20State%20Health%20Profile%20Final%20May%202013.pdf.
- 32.Rossen LM, Khan D, Warner M. Trends and geographic patterns in drug-poisoning death rates in the U.S., 1999–2009. Am J Prev Med. 2013 Dec;45(6):e19–e25. doi: 10.1016/j.amepre.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West Virginia Department of Justice and Community Services. [Accessed December 18, 2014];West Virginia Incident-Based Reporting System (WVIBRS) 2015 Available at http://www.djcs.wv.gov/ORSP/SAC/Pages/WVIBRS.aspx.
- 34.Substance Abuse and Mental Health Services Administration. [Accessed December 18, 2014];Opioid Treatment Program Directory. Available at: http://dpt2.samhsa.gov/treatment/directory.aspx.
- 35.Meyer M, Benvenuto A, Howard D, et al. Development of a substance abuse program for opioid-dependent nonurban pregnant women improves outcome. J Addict Med. 2012 Jun;6(2):124–130. doi: 10.1097/ADM.0b013e3182541933. [DOI] [PubMed] [Google Scholar]
- 36.Beardsley K, Wish ED, Fitzelle DB, O’Grady K, Arria AM. Distance traveled to outpatient drug treatment and client retention. J Subst Abuse Treat. 2003 Dec;25(4):279–285. doi: 10.1016/s0740-5472(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 37.Office of National Drug Control Policy. Appalachia High-Intensity Drug Trafficking Areas. [Accessed January 6, 2015];High-Intensity Drug Trafficking Areas. Available at: https://www.ncjrs.gov/ondcppubs/publications/enforce/hidta2001/appl-fs.html.
- 38.Executive Office of the President, Office of National Drug Control Policy. [Accessed January 6, 2015];High Intensity Drug Trafficking Areas Program Report to Congress. 2011 Jun; Available at https://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/hidta_2011.pdf.
- 39.Drug Enforcement Administration (DEA) [Accessed January 6, 2015];West Virginia DEA Resources. Available at: http://www.drugenforcementedu.org/west-virginia/
- 40.Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW. Implementation of a statewide surveillance system for neonatal abstinence syndrome - Tennessee, 2013. MMWR Morb Mortal Wkly Rep. 2015 Feb 13;64(5):125–128. [PMC free article] [PubMed] [Google Scholar]
- 41.Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014 Aug;134(2):e547–e561. doi: 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell M, Nassar N, Leonard H, et al. Increasing prevalence of neonatal withdrawal syndrome: population study of maternal factors and child protection involvement. Pediatrics. 2009 Apr;123(4):e614–e621. doi: 10.1542/peds.2008-2888. [DOI] [PubMed] [Google Scholar]
- 43.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009 Feb;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev. 2007 Sep;26(5):487–492. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]
- 45.National Rural Health Association. [Accessed October 20, 2015];What’s different about rural health care? Available at: http://www.ruralhealthweb.org/go/left/about-rural-health/what-s-different-about-rural-health-care.
- 46.Carpender SK, Campbell PH, Quiram BJ, Frances J, Artzberger JJ. Urban Evacuations and Rural America: Lessons Learned from Hurricane Rita. Public Health Reports. 2006 Nov-Dec;121(6):775–779. doi: 10.1177/003335490612100620. [DOI] [PMC free article] [PubMed] [Google Scholar]