Abstract
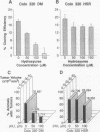
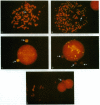
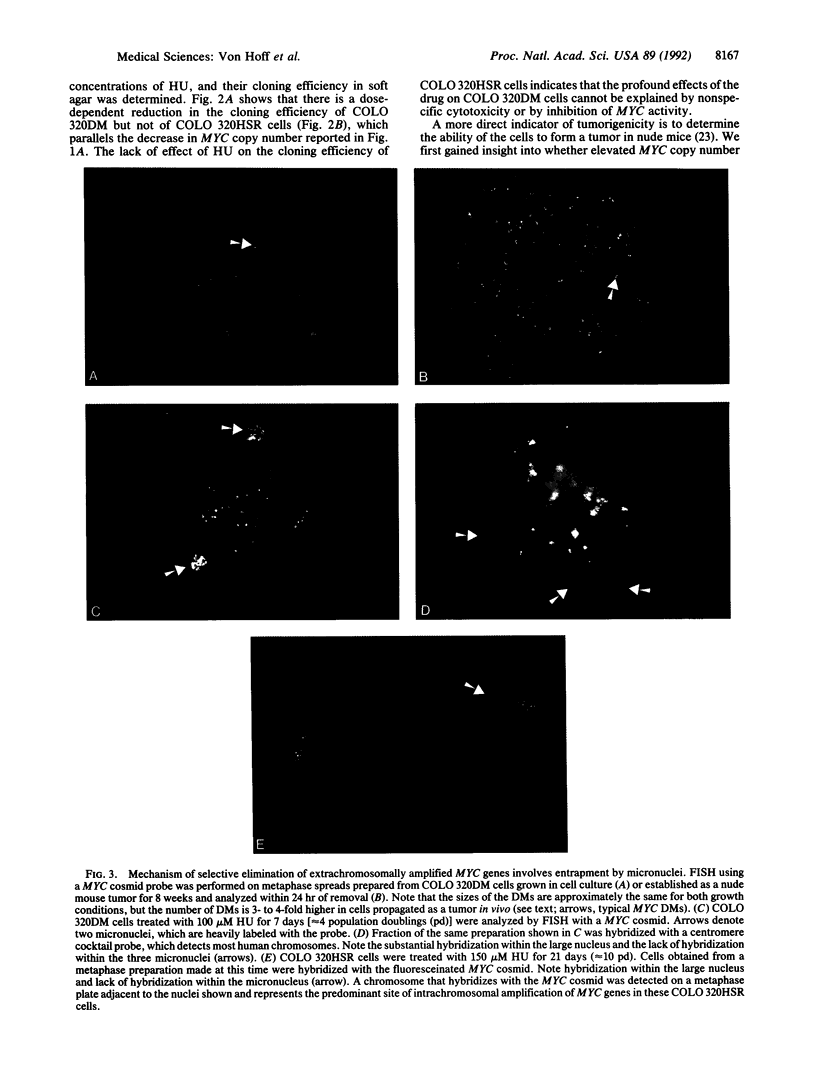
Oncogene amplification has been observed in a broad spectrum of human tumors and has been associated with a poor prognosis for patients with several different types of malignancies. Importantly, at biopsy, the amplified genes localize to acentric extrachromosomal elements such as double-minute chromosomes (DMs) in the vast majority of cases. We show here that treatment of several human tumor cell lines with low concentrations of hydroxyurea accelerates the loss of their extrachromosomally amplified oncogenes. The decreases in MYC copy number in a human tumor cell line correlated with a dramatic reduction in cloning efficiency in soft agar and tumorigenicity in nude mice. No effect on gene copy number or tumorigenicity was observed for a closely related cell line containing the same number of chromosomally amplified MYC genes. One step involved in the accelerated loss of extrachromosomal elements is shown to involve their preferential entrapment of DMs within micronuclei. The data suggest that agents that accelerate the loss of extrachromosomally amplified genes could provide valuable tools for moderating the growth of a large number of human neoplasms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P., Scherthan H., Zankl H. Use of a centromere-specific DNA probe (p82H) in nonisotopic in situ hybridization for classification of micronuclei. Genes Chromosomes Cancer. 1990 May;2(1):59–62. doi: 10.1002/gcc.2870020111. [DOI] [PubMed] [Google Scholar]
- Benner S. E., Wahl G. M., Von Hoff D. D. Double minute chromosomes and homogeneously staining regions in tumors taken directly from patients versus in human tumor cell lines. Anticancer Drugs. 1991 Feb;2(1):11–25. doi: 10.1097/00001813-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Berns E. M., Klijn J. G., van Putten W. L., van Staveren I. L., Portengen H., Foekens J. A. c-myc amplification is a better prognostic factor than HER2/neu amplification in primary breast cancer. Cancer Res. 1992 Mar 1;52(5):1107–1113. [PubMed] [Google Scholar]
- Boise L. H., Grant S., Westin E. H. Altered expression of cell cycle related genes including c-myb in a subclone of HL-60 that exhibits reversible differentiation. Cell Growth Differ. 1992 Jan;3(1):53–61. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Eisner J. M., Casey B. J. Malpractice, informed consent, and the use of low osmolality contrast media. Conn Med. 1988 Feb;52(2):87–91. [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Imaizumi M., Breitman T. R. Changes in c-myc, c-fms, and N-ras proto-oncogene expression associated with retinoic acid-induced monocytic differentiation of human leukemia HL60/MRI cells. Cancer Res. 1988 Dec 1;48(23):6733–6738. [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Loss and stabilization of amplified dihydrofolate reductase genes in mouse sarcoma S-180 cell lines. Mol Cell Biol. 1981 Dec;1(12):1084–1093. doi: 10.1128/mcb.1.12.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A., Levan G. Have double minutes functioning centromeres? Hereditas. 1978;88(1):81–92. doi: 10.1111/j.1601-5223.1978.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Levan G., Mandahl N., Bengtsson B. O., Levan A. Experimental elimination and recovery of double minute chromosomes in malignant cell populations. Hereditas. 1977;86(1):75–90. doi: 10.1111/j.1601-5223.1977.tb01214.x. [DOI] [PubMed] [Google Scholar]
- Levan O., Levan A. Specific chromosome changes in malignancy: studies in rat sarcomas induced by two polycyclic hydrocarbons. Hereditas. 1975;79(2):161–198. doi: 10.1111/j.1601-5223.1975.tb01475.x. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Mark J. Rous sarcomas in mice: the chromosomal progression during early in vivo transplantation. Hereditas. 1970;65(1):59–81. doi: 10.1111/j.1601-5223.1970.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Miller B. M., Zitzelsberger H. F., Weier H. U., Adler I. D. Classification of micronuclei in murine erythrocytes: immunofluorescent staining using CREST antibodies compared to in situ hybridization with biotinylated gamma satellite DNA. Mutagenesis. 1991 Jul;6(4):297–302. doi: 10.1093/mutage/6.4.297. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., McGill J. R., Zabel B. U. In situ hybridization of metaphase and prometaphase chromosomes. Methods Enzymol. 1987;151:279–292. doi: 10.1016/s0076-6879(87)51024-2. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Schimke R. T., Brown P. C., Kaufman R. J., McGrogan M., Slate D. L. Chromosomal and extrachromosomal localization of amplified dihydrofolate reductase genes in cultured mammalian cells. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):785–797. doi: 10.1101/sqb.1981.045.01.097. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Snapka R. M., Varshavsky A. Loss of unstably amplified dihydrofolate reductase genes from mouse cells is greatly accelerated by hydroxyurea. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7533–7537. doi: 10.1073/pnas.80.24.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Buick R. N., Olson S., Horns R. C., Jr, Schimke R. T. Cytologic evidence for gene amplification in methotrexate-resistant cells obtained from a patient with ovarian adenocarcinoma. J Clin Oncol. 1984 Jan;2(1):8–15. doi: 10.1200/JCO.1984.2.1.8. [DOI] [PubMed] [Google Scholar]
- Trent J., Meltzer P., Rosenblum M., Harsh G., Kinzler K., Mashal R., Feinberg A., Vogelstein B. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci U S A. 1986 Jan;83(2):470–473. doi: 10.1073/pnas.83.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDevanter D. R., Piaskowski V. D., Casper J. T., Douglass E. C., Von Hoff D. D. Ability of circular extrachromosomal DNA molecules to carry amplified MYCN proto-oncogenes in human neuroblastomas in vivo. J Natl Cancer Inst. 1990 Dec 5;82(23):1815–1821. doi: 10.1093/jnci/82.23.1815. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Clark G. M., Stogdill B. J., Sarosdy M. F., O'Brien M. T., Casper J. T., Mattox D. E., Page C. P., Cruz A. B., Sandbach J. F. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983 Apr;43(4):1926–1931. [PubMed] [Google Scholar]
- Von Hoff D. D., Forseth B., Clare C. N., Hansen K. L., VanDevanter D. Double minutes arise from circular extrachromosomal DNA intermediates which integrate into chromosomal sites in human HL-60 leukemia cells. J Clin Invest. 1990 Jun;85(6):1887–1895. doi: 10.1172/JCI114650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D. D., Needham-VanDevanter D. R., Yucel J., Windle B. E., Wahl G. M. Amplified human MYC oncogenes localized to replicating submicroscopic circular DNA molecules. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4804–4808. doi: 10.1073/pnas.85.13.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D. D., Waddelow T., Forseth B., Davidson K., Scott J., Wahl G. Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res. 1991 Dec 1;51(23 Pt 1):6273–6279. [PubMed] [Google Scholar]
- Wani M. A., Snapka R. M. Drug-induced loss of unstably amplified genes. Cancer Invest. 1990;8(6):587–593. doi: 10.3109/07357909009018925. [DOI] [PubMed] [Google Scholar]
- Wani M. A., Strayer J. M., Snapka R. M. Hypersensitivity to low level cytotoxic stress in mouse cells with high levels of DHFR gene amplification. Anticancer Drugs. 1990 Oct;1(1):67–75. doi: 10.1097/00001813-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Watson P. H., Pon R. T., Shiu R. P. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer Res. 1991 Aug 1;51(15):3996–4000. [PubMed] [Google Scholar]
- Windle B., Draper B. W., Yin Y. X., O'Gorman S., Wahl G. M. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991 Feb;5(2):160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]
- Yoshida M. C., Wada M., Satoh H., Yoshida T., Sakamoto H., Miyagawa K., Yokota J., Koda T., Kakinuma M., Sugimura T. Human HST1 (HSTF1) gene maps to chromosome band 11q13 and coamplifies with the INT2 gene in human cancer. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4861–4864. doi: 10.1073/pnas.85.13.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]