Significance
Tuberculosis is an ancient human disease that continues to affect millions of people worldwide. A crucial component of the origins of the tuberculosis bacterium remains a mystery: What were the conditions that precipitated its emergence as an obligate transmissible human pathogen? Here, we identify a connection between the emergence of tuberculosis and another major event in human prehistory, namely the discovery of controlled fire use. Our results have serious and cautionary implications for the emergence of new infectious diseases—feedback between cultural innovation and alteration of living conditions can catalyze unexpected changes with potentially devastating consequences lasting thousands of years.
Keywords: tuberculosis, pathogen evolution, cultural evolution, epidemiology, mathematical modeling
Abstract
Tuberculosis (TB) is caused by the Mycobacterium tuberculosis complex (MTBC), a wildly successful group of organisms and the leading cause of death resulting from a single bacterial pathogen worldwide. It is generally accepted that MTBC established itself in human populations in Africa and that animal-infecting strains diverged from human strains. However, the precise causal factors of TB emergence remain unknown. Here, we propose that the advent of controlled fire use in early humans created the ideal conditions for the emergence of TB as a transmissible disease. This hypothesis is supported by mathematical modeling together with a synthesis of evidence from epidemiology, evolutionary genetics, and paleoanthropology.
Human pathogen surveys (1, 2) have revealed trends in the emergence of novel human infectious diseases; among the identified drivers, an overarching theme is change. For instance, altered human–environment interactions, changes to subsistence practices and social adaptations, diversity in human population health, and microbial adaptation have all been linked to instances of novel infections (3–7). Understanding the timing and context of emergence is therefore critical to identifying the origins of human infectious diseases.
A range of transitions in human ecological history have coincided with major changes to patterns of infectious disease in human populations (4–7). The beginnings of agriculture and livestock husbandry, or the Neolithic period, from around 10,000 y ago led to dramatic changes in human–pathogen interactions (4–7). Humans share almost 150 enzootic pathogens with domesticated animals, all of which, except dogs, were domesticated during the Neolithic (7). The subsequent rise of dense and sedentary populations supported by agriculture, extensive ecological disruptions, and high contact rates with domesticated animals and wild pest species such as rodents are thought to have provided new opportunities for pathogens to cross species boundaries and to have supported more virulent “crowd” infections such as measles and smallpox (3–7). Although the Neolithic period was probably not the first ecological transition with consequences for human–pathogen coevolution (4), it is widely regarded to have been the most wide-reaching epidemiological transition in human history (4–7).
However, another more ancient cultural shift might have affected human evolution even more dramatically: the advent of the controlled use of fire. Early hominins might have used fire in an ad hoc manner by at least 1.0 million years ago (8), but the controlled use of fire arose more recently, perhaps only 300,000–400,000 y ago (9). Since Darwin’s time (10), fire has been viewed as one of the most important human cultural innovations. Today, it is recognized as a preeminent example of cultural niche construction whereby humans, through their use of fire, altered their selective environment and the conditions of their own evolution (9, 11).
Besides providing warmth and light, fire would have extended the breadth and quality of foods consumed by early humans and supported geographic range expansions. Fire would have allowed large-scale manipulation of the environment through burning of vegetation, improved food storage, and advanced techniques of tool manufacture (12–14). Fire would also have aided defense against predators—from microorganisms through to mammals—and in the control of pest species including insects (14). These effects likely had far-reaching implications for early humans, including changes to activity patterns (12–15), social organization (15, 16), reproductive behavior (15), cognitive evolution (15), and perhaps even life history (12–15). We suggest that the controlled use of fire also had a major effect on human host–pathogen relationships, not just in sterilizing food (12), but also in unexpected ways relating to cultural changes triggered by fire use and physiological effects experienced by early humans exposed to campfire smoke. We hypothesize that these changes, caused by controlled fire use, precipitated the emergence of the Mycobacterium tuberculosis complex (MTBC), the causative agent of the new infectious disease: tuberculosis (TB). This hypothetical process of TB emergence is represented schematically in Fig. 1.
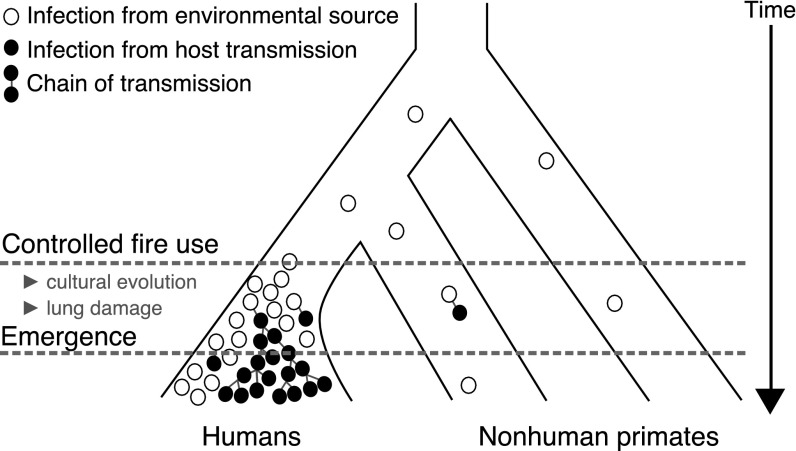
Fig. 1.
A scenario for the evolutionary emergence of MTBC in humans. The open circles indicate sporadic mycobacterial infections from an environment source, whereas the filled circles indicate new infections attributable to transmission between individual hosts. Direct transmission of mycobacterial infection was presumably rare in ancient populations until ideal conditions appeared for the evolutionary emergence of MTBC. We hypothesize that the controlled use of fire in early humans precipitated the emergence of tuberculosis as a transmissible disease. The time axis is not to scale.
Results
To evaluate our hypothesis, we first review a range of evidence from evolutionary genetics, epidemiology, and paleoanthropology to reveal the current state of knowledge on the emergence of TB in early human populations and the possibility of a link between this emergence and the advent of the controlled use of fire. In light of this synthesis of evidence, we then consider quantitatively the rise in the risk of the evolutionary emergence of TB attributable to controlled fire use.
The Evolutionary Origins of TB.
The age of the association between humans and MTBC remains controversial: some studies estimate the most recent common ancestor (MRCA) of MTCB existed less than 6,000 y ago (17–19), whereas others have obtained a figure of around 70,000 y ago (20–22). Regardless of which estimate is more accurate, TB is likely to be older in humans than these inferred ages. These coalescent estimates are based on extant lineages and do not necessarily provide the age of the first strain to infect humans; selective sweeps, population bottlenecks, and simply genetic drift can all make the MRCA younger than the origin of a species (23). It is unlikely, however, that TB was a transmissible disease before the separation of the human lineage from other apes. Although other mammals, including chimpanzees, can be infected with TB, there is no evidence of any cospeciation between nonhuman primates and mycobacteria (24).
The archaeological and molecular evidence taken together favors an early emergence of MTBC originating in Africa and a long-term association with modern humans on this continent. For instance, all strains adapted to animal hosts are derived from human strains (23), ruling out the possibility that TB was first a bovine disease which crossed over to humans during the Neolithic period. Whole-genome draft sequencing has identified deep lineages in Africa that are excellent candidates for representing the original ecotype of the MTBC (25) and African lineages show high diversity compared with other regions (26). Cases of MTBC in pre-Columbian human remains from Peru (27) and Chile (28) and in a 17,500 y old Bison bone discovered in the United States (29) further suggest that MTBC is ancient and crossed the Bering Strait during the Late Pleistocene. Contrasting evidence from MTBC genomes isolated from Peruvian human remains suggests that sea mammals were a source of TB in the Americas (18), although this evidence does not preclude the possibility of preexisting MTBC strains. Further support for ancient origins of TB is found in the form of characteristic lesions on Paleolithic human remains (30) and from ancient DNA isolates (31).
Nontuberculous mycobacteria are ubiquitous in the environment and account for the majority of species within the genus Mycobacterium (32). Such organisms can infect humans opportunistically, particularly in immunocompromised individuals and patients with inflammatory lung diseases (33, 34). For instance, Mycobacterium abscessus is a major respiratory pathogen in patients with cystic fibrosis who have profoundly impaired local immune protective mechanisms (35). Although they are not generally thought to establish person-to-person chains of transmission, clusters of closely related strains of M. abscessus have been isolated from patients with cystic fibrosis, suggesting such transmission can occur, although the mechanism may be indirect (34, 36).
Comparative analysis indicates that MTBC diverged from a related nontuberculous mycobacterial species before arising as a clonal expansion from a generalist ancestral mycobacterial population shared by the smooth tubercle bacilli Mycobacterium canettii (37, 38). This ancestor of MTBC, which might have resembled Mycobacterium kansasii (38), presumably lived in the environment and caused sporadic disease before it acquired the ability to transmit between hosts (22, 38). Patients infected with M. kansasii present very similarly to TB and respond to antituberculous antibiotics (39), although M. kansasii is not transmissible in humans and has significantly reduced in vivo fitness (38).
Conditions for Evolutionary Emergence.
For an environmental species to become an endemic human pathogen, the species must undergo a series of evolutionary transformations (5, 6). Exposure of a host to a microbe requires sufficient overlap of their habitats. Evolutionary transformations then allow more complex interactions to arise where the potential pathogen becomes capable of infecting humans and then of transmitting between hosts. Endemic persistence, however, requires the basic reproduction number in human populations (the expected number of secondary cases produced by a typical case in a completely susceptible host population) to be greater than unity. The emergence of transmissible TB, therefore, would have been a rare and unlucky event in which an opportunistic and poorly transmitting environmental Mycobacterium evolved the ability to transmit over short chains of infection until its reached the critical value of unity (40).
Today, MTBC causes mostly latent infection in human hosts, although a small fraction of these latent infections reactivate to the disease state before the hosts die (41). It is not known whether the MTBC precursor also caused latent infection or whether latency evolved subsequent to its emergence (42). Either way, for TB to emerge as a transmissible disease, its etiological agent had to overcome the obstacle of a low . The occurrence of any of the risk factors for infectious disease emergence (see Introduction) brought about by a transition in human culture or behavior would have facilitated this process (5, 6). Such a transition would necessarily have occurred well before the existence of the MRCA of MTBC and affected the rate of pathogen exposure or the extent of human susceptibility to infection. The transition might also have provided novel opportunities for between-host transmission events and/or supported within-host adaptation of the pathogen (40). The prehistoric transition of controlling fire, we argue, satisfies these criteria for influencing the emergence of MTBC in human populations.
Cultural Impact of Fire Use.
The earliest convincing evidence for human control of fire predates both estimates for the MRCA of MTBC (21), as well as the oldest molecular dates (43), and fossil remains for anatomically modern humans (44) by considerable margins. The current consensus is that fire was first made by Homo heidelbergensis (8), a species widely regarded to be the last common ancestor of the Neanderthals and anatomically modern humans (45). The regular use of fire would have constructed a new cultural niche for hominins with potentially profound implications for the evolution of H. heidelbergensis and, ultimately, anatomically modern humans through genetic and ecological inheritance (12–15, 46).
One important change experienced by these early fire-makers that, we argue, influenced the sporadic transmission of and susceptibility to pulmonary mycobacterial infection, would have been the addition of new contexts for human social interactions. For instance, fire would have provided light to allow social and subsistence activities to occur beyond daylight hours and food being brought to a central site for cooking and preservation, and fire maintenance would all have increased interactions and physical contacts among individuals (16, 47). Early humans in these new contexts would necessarily have had greatly increased inhalation of smoke from fires used for these purposes but also by fires used to repel insects and other pests and possibly even for communication (48). Indeed, the presence of small microcharcoal fragments in 300,000- to 400,000-y-old dental calculus samples taken from human remains at Qesem Cave is suggestive of accidental smoke inhalation from hearth fires (49).
Although fire must also have been used at open-air sites, most Pleistocene evidence for controlled fire use from the archaeological record derives from caves (8, 9). The biased record of fire use results, in large part, from geological and taphonomic factors, with cave sediments being more conducive to the preservation of charcoal, ash, and burnt bone. Temporary shelters were also built by hunter–gatherers across the globe and were undoubtedly one of the keys to the successful occupation of wide-ranging environments by modern humans. Sheltered spaces such as caves and built structures are inherently social spaces (16, 47), and camp fires act as a natural focal point for social gatherings (11). Therefore, early fire makers occupying poorly ventilated spaces were likely to have been especially vulnerable to inhalation of smoke from fires.
Physiological Impact of Fire Use.
A large proportion of biomass smoke constituents are in the inhalable size range (e.g., carbon monoxide, aldehydes, nitric oxide, and particulate matter), and many of these constituents incite chronic inflammatory and destructive changes in the respiratory system (50). Early hunter–gatherer communities burning biomass fuels in poorly ventilated spaces were, therefore, vulnerable to a wide range of unprecedented adverse health effects (51).
Metaanalysis has revealed positive associations between smoking tobacco and domestic use of biomass fuels with increased risk of MTBC infection and TB disease (52). Possible mechanisms suggested for these associations include the impairment of respiratory defenses against mycobacteria, because even a brief exposure of animals to wood smoke can alter bacterial clearance and macrophage-mediated local immunity (53). This association is likely to be attributable to the critical role played by the local immune response (both innate and adaptive) in protective immunity against MTBC infection, much more so than humoral adaptive immunity (54). The process of developing protective immunity against MTBC infection is largely coordinated within the lungs and lymphatics through, for instance, the mucociliary escalator and macrophage function, processes that may be particularly impaired by smoke exposure and in patients with inflammatory lung diseases (53–55).
Several respiratory diseases associated with tissue destruction and impaired local immunity are also well-established to be associated with TB and other mycobacterial infections (56–60). The best characterized of these conditions include chronic obstructive pulmonary disease (COPD), bronchiectasis, and silicosis, whereas the association between nontuberculous mycobacterial disease and cystic fibrosis is discussed above in The Evolutionary Origins of TB. The high risk of TB and other mycobacterial infections in patients with COPD is particularly well characterized because of the high prevalence of smoking in modern societies (56, 57), although indoor biomass burning remains a major causative factor globally (52, 61, 62). Bronchiectasis is another obstructive lung disease that is significantly more common in populations exposed to wood smoke (63) and is similarly associated with an increased risk of mycobacterial infection (58). Meanwhile, mycobacterial infection is also a risk factor for the development and progression of these diseases (64), indicating that there is likely to be a feedback mechanism supporting the persistence of all three conditions in humans.
The pathogenesis of smoke exposure is closely related to that of silicosis, which is caused by the inhalation of silica particles that damage alveolar macrophages and cause inflammation and fibrogenesis (65). Individuals exposed to aerosolized silica, even without silicosis, have a dramatically increased risk of mycobacterial infection (59). The individual-level relative risk for silicosis and silica-associated TB also persists after exposure has ceased and is dose-dependent (60, 66). Although biomass burning is not predominantly silica exposure, these principles may apply to other inorganic inhaled particles.
Model of TB Emergence.
To test the logic in our hypothesis that the advent of the controlled use of fire could have significantly affected the probability of TB emergence, we considered quantitatively the rise in the risk of the evolutionary emergence of TB attributable to the use of fire. We developed a mathematical model, building on existing theory of the evolutionary emergence of pathogens (40), to ask how the ancestor of MTBC could evolve such that its basic reproductive number reached unity. The model assumes that can increase because of two mechanisms: (i) by adaptive evolution during chains of transmission and (ii) by the effects of habitual fire use, namely increased host-susceptibility attributable to biomass smoke-induced lung damage and greater between-host transmission opportunities brought about by a developing human social culture. The model also assumes that host-susceptibility remains otherwise unchanged through time (this assumption is relaxed in an extended model presented in SI Appendix) and allows slow exponential growth of early human populations before expansion out of Africa (67), although the effect of this assumption on the results was minor.
We calculated the cumulative probability over time of TB emergence as an endemic human pathogen. This quantity increases with time and the rate of infections attributable to host exposure to MTBC’s ancestral natural reservoir. The cumulative probability also depends on the probability that a chain of transmission evolves an greater than unity, and the probability that an evolved pathogen does not undergo stochastic extinction.
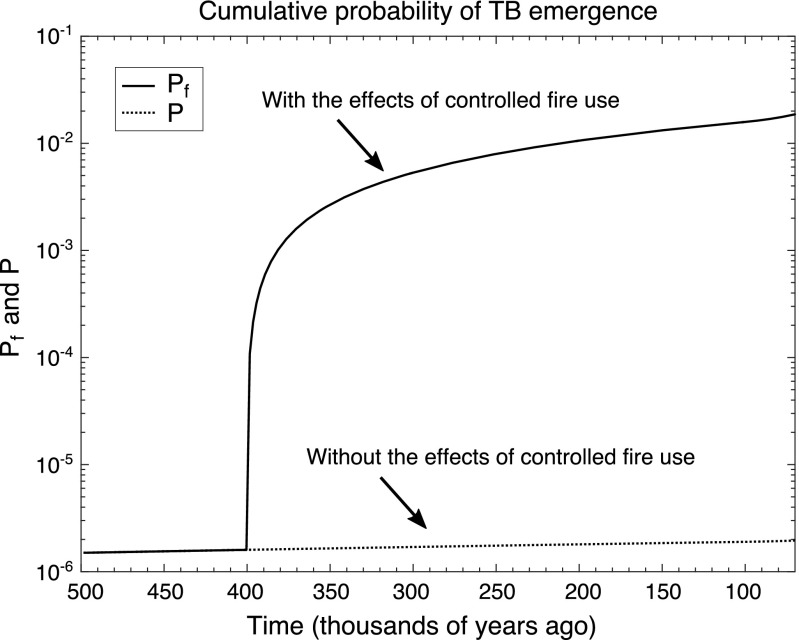
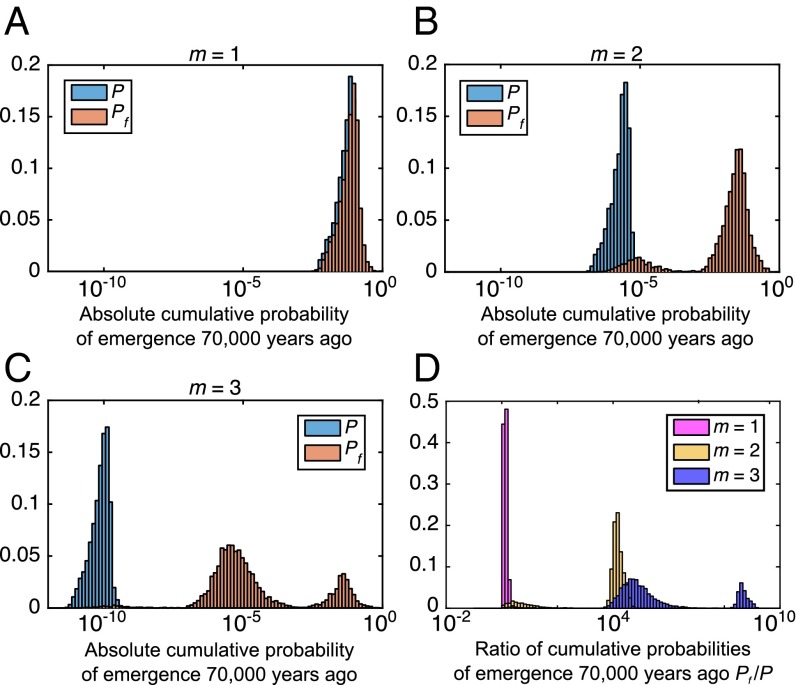
Under our model, controlled fire use increased the cumulative probability that TB emerged by several orders of magnitude compared with the case where TB emerges without fire (Fig. 2). This result holds when we consider the cumulative probability of TB emerging up to and including both estimates for the time since the MRCA of MTBC of 70,000 y ago (Fig. 2) and 6,000 y ago (SI Appendix, Fig. S2) and is robust to variation in model parameters including the number of mutations required for the ancestor of MTBC to evolve an greater than unity from the characteristic of the natural reservoir (Fig. 3). As the number of mutations required for to exceed unity increases, so too does the influence controlled fire use has on the probability of emergence, whereas the cumulative probability quickly decreases with and without fire use. Nevertheless, it is likely that only a few mutations were needed before TB could emerge, because as few as three mutations can significantly attenuate the virulence of Mycobacterium tuberculosis in human cells (68) and recombination at one locus can increase the virulence of M. canettii in cellular and animal infection models (69). Although unrelated to mycobacteria, it is also noteworthy that five mutations are required for avian influenza to acquire the ability of airborne transmission in ferrets (70).
Fig. 2.
Model results of the change in the cumulative probability of TB emergence attributable to the advent of controlled use of fire 400,000 y ago. Here, we assume that two mutations are required for the environmental mycobacteria to evolve to have an . For more details, see SI Appendix, Figs. S1 and S2 and Table S1.
Fig. 3.
Sensitivity analysis of the cumulative probability of TB emergence 70,000 y ago attributable to the advent of controlled use of fire. The varied input parameters include the characteristic of the environmental reservoir of the MTBC precursor, the factor L that was increased because of fire use, the time of onset of controlled fire use, the time of onset of human population growth, and the rate α of population growth and the early human population size . (A–C) The frequency distributions of the absolute cumulative probabilities of TB emergence by 70,000 y ago with fire (orange) and without fire P (blue). (D) Frequency distribution of the ratio of cumulative probabilities of the emergence of TB by 70,000 y ago . The three alternative scenarios shown here represent the cases where one (), two (), and three () mutations are required for the environmental mycobacteria to evolve to have an . For more details and additional results, see SI Appendix, Figs. S3 and S4 and Table S2.
Discussion
Here, we have argued that the extensive changes to human ecology and unprecedented physiological consequences brought about by the controlled use of fire in the Pleistocene created ideal conditions for the emergence of TB. It is possible that during this period of significant ecological and social change, range extensions leading to the consumption of novel food sources and altered energy requirements increased exposure of early humans to the natural reservoir of ancestral MTBC, likely the soil. This increased exposure brought about an increasing number of infections and stuttering transmission chains, both of which provided new opportunities for within-host adaptive evolution. Coupled with increasing host-susceptibility to mycobacterial infection attributable to biomass smoke-induced lung damage and the increased opportunities for transmission brought about by the developing social culture that fire use encouraged, we hypothesize that the MTBC precursor evolved an greater than unity relatively quickly, almost guaranteeing MTBC's emergence as a specialized human pathogen.
An alternative hypothesis is that the emergence of MTBC predates hominins and MTBC infection spanned the species divide. However, this hypothesis is unlikely to be true because there is no evidence for coevolution of MTBC and humans before ∼70,000 y ago (21). Another alternative is that MTBC is the result of an ancient zoonotic transfer from wildlife to early humans. Although this hypothesis cannot be ruled out, comparative genomic analyses suggest that all known strains that cause TB in animals diverged from major human strains (23). Therefore, such a hypothetical ancient nonhuman host is either extinct or remains to be identified. Furthermore, close, regular contacts with an infected host would be required to cause zoonosis (71), which is unlikely before humans began herding and farming. Nevertheless, our model results suggest that a species jump to humans was much more likely following the advent of the controlled use of fire. It is also possible that MTBC emerged in the Pleistocene without any influence of fire use. If so, this emergence was an extremely unlucky event for early humans in the absence of compounding factors magnifying host-susceptibility and opportunities for transmission.
One effect of fire that potentially conflicts with our hypothesis is the possible reduction in rates of malnutrition attributable to fire increasing the supply of food. For instance, cooking detoxified and likely increased the digestibility of a range of foods, thereby increasing the diversity of the food available (12). Because malnutrition is a risk factor for TB (72), improved nutrition owing to fire would have alleviated the risk of opportunistic infection to some extent. However, the ubiquity of the signs of metabolic disease in recent hunter–gatherer and Pleistocene skeletons suggests nutritional disorders remained relatively common (73, 74).
If humans and not other mammals were the original host of MTBC (22), our hypothesis offers a possible explanation: the persistence of the MTBC precursor, we argue, was only feasible in human populations because humans were the only species capable of controlling fire and therefore were especially vulnerable to pulmonary mycobacterial infection. Hence, humans were the only species able to support opportunistic infection at a sufficient rate for the MTBC precursor to evolve transmissibility and strategies to evade the host immune response.
Our proposal for the origins of TB and the generality of the mathematical model results (40) serve to illustrate how a series of events triggered by just one change to our way of life can pave the way for the establishment of a novel human infectious disease, one that ultimately transforms into the world’s leading cause of death attributable to a single bacterial pathogen (41). If our hypothesis is correct, fire use would be a remarkable example of technology presenting itself as a double-edged sword. Benefits of fire overwhelmingly outweigh the negatives, but the risks of technological innovation include the possibility of novel persistent infectious diseases (4).
Materials and Methods
Model of Infectious Disease Emergence.
Our model of TB emergence in early human hunter–gather populations builds on expressions derived in a previous study quantifying the role of evolution in the emergence of infectious diseases (40). In that study (40), the probability that a chain of transmission initiated by sporadic infection from an opportunistic pathogen evolves an greater than 1 was calculated as
| [1] |
where is the basic reproductive number characteristic of the natural reservoir of the pathogen, is the basic reproductive number of the evolved strain after i mutation events, m is the number of mutation events required for , and μ is the mutation rate. When more than one mutation is required for a strain to attain an in the host population, we define the fitness landscape on which evolution occurs to be
| [2] |
Here, we assume that the fitness effect of mutations is always additive. The parameter z controls the curvature of the fitness landscape. When , the of new strains increases by a constant amount with each mutation. When z is large, early mutations have a minimal effect on the fitness of a new strain, whereas later mutations cause large changes to . The opposite occurs when z is small. Recombination is not considered explicitly in the model, but the effect of accelerated evolution attributable to recombination can be explored by choosing and a small m (SI Appendix, Fig. S2B).
Emergence requires the strain to evolve an and also for the evolved strain to avoid extinction attributable to stochastic effects. Hence, the probability of emergence after a single infection is
| [3] |
and the mean number of introductions of the pathogen into the human population until emergence occurs is simply . We use this expression to determine the cumulative probability of MTBC emerging in early human populations up to a point in time t. If the average per capita rate of infection attributable to host contact with the pathogen’s natural reservoir is C, and is the census host-population size after t years, then we can consider the emergence of the precursor of MTBC as an inhomogeneous Poisson process with rate parameter . In this case, the cumulative probability of the emergence of MTBC after t years is
| [4] |
To account for slow exponential growth of early human populations before the expansion out of Africa, we define the census population size to be
| [5] |
This definition assumes that humans underwent two phases of growth whereby an ancestral phase of constant population size is followed by exponential population expansion at a rate α during the late Pleistocene (67). We do not assume an association between the technological innovation of fire and demographic growth.
Here, host-susceptibility is held constant through time. In the SI Appendix, we relax this assumption and allow exposure to the pathogen to, over time, decrease host-susceptibility because of an increase in frequency of protective alleles against intracellular infection (75). The effect of this additional assumption is to increase the mean number of introductions until emergence, thereby delaying emergence. In the model, we set the time to be two million years ago, which is consistent with estimates for the age of the progenitor species of MTBC (37).
TB Emergence Following the Advent of the Controlled Use of Fire.
We assume that the of the precursor of MTBC was elevated following the advent of the controlled use of fire because of two mechanisms: increased host-susceptibility attributable to biomass smoke-induced lung damage and greater between-host transmission opportunities brought about by the developing human culture. In particular, we assume that habitual fire use caused the reproductive numbers of precursor strains to increase by a factor L. Now, the number of mutations necessary until is and the probability that a chain of transmission evolves an greater than 1 following the advent of fire is
| [6] |
Assuming , the probability of MTBC emergence after a single infection becomes
| [7] |
and the mean number of introductions of the pathogen into the human population until emergence occurs reduces to . Hence, if is the time of onset of widespread controlled fire use, then the cumulative probability of the emergence of MTBC after t years is
| [8] |
To understand how the model parameters are otherwise influencing these quantities, we plot pevolve, , P(t) and Pf(t) for a range of values of m, L, and z in SI Appendix, Fig. S2.
Sensitivity Analysis.
A sensitivity analysis of the cumulative probability of MTBC emergence 70,000 y ago (P and ) was performed on the model with (SI Appendix, Fig. S3), (Fig. 3), and (SI Appendix, Fig. S4); 5,000 points in the parameter space were sampled based on the Latin Hypercubic Sampling method (76). The varied input parameters included , L, , , α, and . Details of the distributions from which the input parameter variables were sampled are provided in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
This work was supported by the Australian Research Council through Grants FT140100398 (to M.M.T.) and FT12100168 (to D.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603224113/-/DCSupplemental.
References
- 1.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser MJ, Kirschner D. The equilibria that allow bacterial persistence in human hosts. Nature. 2007;449(7164):843–849. doi: 10.1038/nature06198. [DOI] [PubMed] [Google Scholar]
- 4.McMichael AJ. Environmental and social influences on emerging infectious diseases: Past, present and future. Philos Trans R Soc Lond B Biol Sci. 2004;359(1447):1049–1058. doi: 10.1098/rstb.2004.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447(7142):279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolhouse M, Gaunt E. Ecological origins of novel human pathogens. Crit Rev Microbiol. 2007;33(4):231–242. doi: 10.1080/10408410701647560. [DOI] [PubMed] [Google Scholar]
- 7.Weiss RA. The Leeuwenhoek Lecture 2001. Animal origins of human infectious disease. Philos Trans R Soc Lond B Biol Sci. 2001;356(1410):957–977. doi: 10.1098/rstb.2001.0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berna F, et al. Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc Natl Acad Sci USA. 2012;109(20):E1215–E1220. doi: 10.1073/pnas.1117620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roebroeks W, Villa P. On the earliest evidence for habitual use of fire in Europe. Proc Natl Acad Sci USA. 2011;108(13):5209–5214. doi: 10.1073/pnas.1018116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin C. The Descent of Man and Selection in Relation to Sex. John Murray; London: 1871. [Google Scholar]
- 11.Attwell L, Kovarovic K, Kendal J. Fire in the Plio-Pleistocene: The functions of hominin fire use, and the mechanistic, developmental and evolutionary consequences. J Anthropol Sci. 2015;93:1–20. doi: 10.4436/JASS.93006. [DOI] [PubMed] [Google Scholar]
- 12.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N. The raw and the stolen. Cooking and the ecology of human origins. Curr Anthropol. 1999;40(5):567–594. [PubMed] [Google Scholar]
- 13.Wrangham R, Carmody R. Human adaptation to the control of fire. Evol Anthropol. 2010;19(5):187–199. [Google Scholar]
- 14.Marlowe FW. Hunter-gatherers and human evolution. Evol Anthropol. 2005;14(2):54–67. [Google Scholar]
- 15.Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evol Anthropol. 2000;9(4):156–185. [Google Scholar]
- 16.Wiessner PW. Embers of society: Firelight talk among the Ju/′hoansi Bushmen. Proc Natl Acad Sci USA. 2014;111(39):14027–14035. doi: 10.1073/pnas.1404212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepperell CS, et al. The role of selection in shaping diversity of natural M. tuberculosis populations. PLoS Pathog. 2013;9(8):e1003543. doi: 10.1371/journal.ppat.1003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bos KI, et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature. 2014;514(7523):494–497. doi: 10.1038/nature13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay GL, et al. Eighteenth-century genomes show that mixed infections were common at time of peak tuberculosis in Europe. Nat Commun. 2015;6:6717. doi: 10.1038/ncomms7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2012;367(1590):850–859. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comas I, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45(10):1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galagan JE. Genomic insights into tuberculosis. Nat Rev Genet. 2014;15(5):307–320. doi: 10.1038/nrg3664. [DOI] [PubMed] [Google Scholar]
- 23.Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV. Myths and misconceptions: The origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7(7):537–544. doi: 10.1038/nrmicro2165. [DOI] [PubMed] [Google Scholar]
- 24.Coscolla M, et al. Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg Infect Dis. 2013;19(6):969–976. doi: 10.3201/eid1906.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blouin Y, et al. Significance of the identification in the Horn of Africa of an exceptionally deep branching Mycobacterium tuberculosis clade. PLoS One. 2012;7(12):e52841. doi: 10.1371/journal.pone.0052841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comas I, et al. Population genomics of Mycobacterium tuberculosis in Ethiopia contradicts the virgin soil hypothesis for human tuberculosis in Sub-Saharan Africa. Curr Biol. 2015;25(24):3260–3266. doi: 10.1016/j.cub.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salo WL, Aufderheide AC, Buikstra J, Holcomb TA. Identification of Mycobacterium tuberculosis DNA in a pre-Columbian Peruvian mummy. Proc Natl Acad Sci USA. 1994;91(6):2091–2094. doi: 10.1073/pnas.91.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arriaza BT, Salo W, Aufderheide AC, Holcomb TA. Pre-Columbian tuberculosis in northern Chile: Molecular and skeletal evidence. Am J Phys Anthropol. 1995;98(1):37–45. doi: 10.1002/ajpa.1330980104. [DOI] [PubMed] [Google Scholar]
- 29.Rothschild BM, et al. Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present. Clin Infect Dis. 2001;33(3):305–311. doi: 10.1086/321886. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy KAR, Deraniyagala SU. Fossil remains of 28,000-year-old hominids from Sri Lanka. Curr Anthropol. 1989;30(3):394–399. [Google Scholar]
- 31.Hershkovitz I, et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS One. 2008;3(10):e3426. doi: 10.1371/journal.pone.0003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falkinham JO., 3rd Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107(2):356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- 33.Simpson GL, Raffin TA, Remington JS. Association of prior nocardiosis and subsequent occurrence of nontuberculous mycobacteriosis in a defined, immunosuppressed population. J Infect Dis. 1982;146(2):211–219. doi: 10.1093/infdis/146.2.211. [DOI] [PubMed] [Google Scholar]
- 34.Bryant JM, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet. 2013;381(9877):1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85(2):229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 36.Aitken ML, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med. 2012;185(2):231–232. doi: 10.1164/ajrccm.185.2.231. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez MC, et al. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 2005;1(1):e5. doi: 10.1371/journal.ppat.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, et al. Insights on the emergence of Mycobacterium tuberculosis from the analysis of Mycobacterium kansasii. Genome Biol Evol. 2015;7(3):856–870. doi: 10.1093/gbe/evv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans SA, Colville A, Evans AJ, Crisp AJ, Johnston ID. Pulmonary Mycobacterium kansasii infection: Comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax. 1996;51(12):1248–1252. doi: 10.1136/thx.51.12.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426(6967):658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization . Global Tuberculosis Report 2015. WHO; Geneva: 2015. [Google Scholar]
- 42.Chisholm RH, Tanaka MM. The emergence of latent infection in the early evolution of Mycobacterium tuberculosis. Proc Biol Sci. 2016;283(1831):20160499. doi: 10.1098/rspb.2016.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez FL, Watkins JC, Hammer MF. Neandertal origin of genetic variation at the cluster of OAS immunity genes. Mol Biol Evol. 2013;30(4):798–801. doi: 10.1093/molbev/mst004. [DOI] [PubMed] [Google Scholar]
- 44.McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433(7027):733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 45.Stringer C. Evolution: What makes a modern human. Nature. 2012;485(7396):33–35. doi: 10.1038/485033a. [DOI] [PubMed] [Google Scholar]
- 46.Boyd R, Richerson PJ. Culture and the Evolutionary Process. Univ of Chicago Press; Chicago: 1988. [Google Scholar]
- 47.Stiner MC, Gopher A, Barkai R. Hearth-side socioeconomics, hunting and paleoecology during the late Lower Paleolithic at Qesem Cave, Israel. J Hum Evol. 2011;60(2):213–233. doi: 10.1016/j.jhevol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Scherjon F, Bakels C, MacDonald K, Roebroeks W. Burning the land. Curr Anthropol. 2015;56(3):299–326. [Google Scholar]
- 49.Hardy K, et al. Dental calculus reveals potential respiratory irritants and ingestion of essential plant-based nutrients at Lower Palaeolithic Qesem Cave Israel. Quat Int. 2015;398:129–135. [Google Scholar]
- 50.Larson TV, Koenig JQ. Wood smoke: Emissions and noncancer respiratory effects. Annu Rev Public Health. 1994;15(1):133–156. doi: 10.1146/annurev.pu.15.050194.001025. [DOI] [PubMed] [Google Scholar]
- 51.Naeher LP, et al. Woodsmoke health effects: A review. Inhal Toxicol. 2007;19(1):67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 52.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fick RB, Jr, Paul ES, Merrill WW, Reynolds HY, Loke JS. Alterations in the antibacterial properties of rabbit pulmonary macrophages exposed to wood smoke. Am Rev Respir Dis. 1984;129(1):76–81. doi: 10.1164/arrd.1984.129.1.76. [DOI] [PubMed] [Google Scholar]
- 54.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19(1):93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 55.Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J. 1999;13(5):1177–1188. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- 56.Inghammar M, et al. COPD and the risk of tuberculosis--A population-based cohort study. PLoS One. 2010;5(4):e10138. doi: 10.1371/journal.pone.0010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andréjak C, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262. doi: 10.1136/thoraxjnl-2012-201772. [DOI] [PubMed] [Google Scholar]
- 58.Chu H, Zhao L, Xiao H, Zhang Z, Zhang J. Prevalence of nontuberculosis mycobacterial in patients with bronchiectasis: A meta-analysis. Arch Med Sci. 2014;10(4):661–668. doi: 10.5114/aoms.2014.44857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbett EL, et al. Risk factors for pulmonary mycobacterial disease in South African gold miners. A case-control study. Am J Respir Crit Care Med. 1999;159(1):94–99. doi: 10.1164/ajrccm.159.1.9803048. [DOI] [PubMed] [Google Scholar]
- 60.Sonnenberg P, et al. Risk factors for pulmonary disease due to culture-positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. Eur Respir J. 2000;15(2):291–296. doi: 10.1034/j.1399-3003.2000.15b12.x. [DOI] [PubMed] [Google Scholar]
- 61.Kolappan C, Subramani R. Association between biomass fuel and pulmonary tuberculosis: A nested case-control study. Thorax. 2009;64(8):705–708. doi: 10.1136/thx.2008.109405. [DOI] [PubMed] [Google Scholar]
- 62.Hu G, et al. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest. 2010;138(1):20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 63.Arslan M, Akkurt I, Egilmez H, Atalar M, Salk I. Biomass exposure and the high resolution computed tomographic and spirometric findings. Eur J Radiol. 2004;52(2):192–199. doi: 10.1016/j.ejrad.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Yeh JJ, Wang YC, Sung FC, Chou CYT, Kao CH. Nontuberculosis mycobacterium disease is a risk factor for chronic obstructive pulmonary disease: A nationwide cohort study. Lung. 2014;192(3):403–411. doi: 10.1007/s00408-014-9574-9. [DOI] [PubMed] [Google Scholar]
- 65.Rees D, Murray J. Silica, silicosis and tuberculosis. Int J Tuberc Lung Dis. 2007;11(5):474–484. [PubMed] [Google Scholar]
- 66.Churchyard GJ, et al. Silicosis prevalence and exposure-response relations in South African goldminers. Occup Environ Med. 2004;61(10):811–816. doi: 10.1136/oem.2003.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox MP, et al. Autosomal resequence data reveal Late Stone Age signals of population expansion in sub-Saharan African foraging and farming populations. PLoS One. 2009;4(7):e6366. doi: 10.1371/journal.pone.0006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalo-Asensio J, et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci USA. 2014;111(31):11491–11496. doi: 10.1073/pnas.1406693111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boritsch EC, et al. Pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. New Microbiol. 2016;1:15019. doi: 10.1038/nmicrobiol.2015.19. [DOI] [PubMed] [Google Scholar]
- 70.Herfst S, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berg S, et al. The burden of mycobacterial disease in ethiopian cattle: Implications for public health. PLoS One. 2009;4(4):e5068. doi: 10.1371/journal.pone.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol. 2012;176(5):409–422. doi: 10.1093/aje/kws007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starling AP, Stock JT. Dental indicators of health and stress in early Egyptian and Nubian agriculturalists: A difficult transition and gradual recovery. Am J Phys Anthropol. 2007;134(4):520–528. doi: 10.1002/ajpa.20700. [DOI] [PubMed] [Google Scholar]
- 74.Webb S. Palaeopathology of Aboriginal Australians: Health and Disease Across a Hunter-Gatherer Continent. Cambridge Univ Press; New York: 2009. [Google Scholar]
- 75.Barnes I, Duda A, Pybus OG, Thomas MG. Ancient urbanization predicts genetic resistance to tuberculosis. Evolution. 2011;65(3):842–848. doi: 10.1111/j.1558-5646.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 76.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model, as an example. Int Stat Rev. 1994;62(2):229–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.