Abstract
Benzo[a]pyrene (BaP) is a human carcinogen that covalently binds to DNA after activation by cytochrome P450 (P450). Here, we investigated whether NADH:cytochrome b5 reductase (CBR) in the presence of cytochrome b5 can act as sole electron donor to human P450 1A1 during BaP oxidation and replace the canonical NADPH:cytochrome P450 reductase (POR) system. We also studied the efficiencies of the coenzymes of these reductases, NADPH as a coenzyme of POR, and NADH as a coenzyme of CBR, to mediate BaP oxidation. Two systems containing human P450 1A1 were utilized: human recombinant P450 1A1 expressed with POR, CBR, epoxide hydrolase, and cytochrome b5 in Supersomes and human recombinant P450 1A1 reconstituted with POR and/or with CBR and cytochrome b5 in liposomes. BaP-9,10-dihydrodiol, BaP-7,8-dihydrodiol, BaP-1,6-dione, BaP-3,6-dione, BaP-9-ol, BaP-3-ol, a metabolite of unknown structure, and two BaP-DNA adducts were generated by the P450 1A1-Supersomes system, both in the presence of NADPH and in the presence of NADH. The major BaP-DNA adduct detected by 32P-postlabeling was characterized as 10-(deoxyguanosin-N2-yl)-7,8,9-trihydroxy-7,8,9,10-tetrahydro-BaP (assigned adduct 1), while the minor adduct is probably a guanine adduct derived from 9-hydroxy-BaP-4,5-epoxide (assigned adduct 2). BaP-3-ol as the major metabolite, BaP-9-ol, BaP-1,6-dione, BaP-3,6-dione, an unknown metabolite, and adduct 2 were observed in the system using P450 1A1 reconstituted with POR plus NADPH. When P450 1A1 was reconstituted with CBR and cytochrome b5 plus NADH, BaP-3-ol was the predominant metabolite too, and an adduct 2 was also generated. Our results demonstrate that the NADH/cytochrome b5/CBR system can act as the sole electron donor both for the first and second reduction of P450 1A1 during the oxidation of BaP in vitro. They suggest that NADH-dependent CBR can replace NADPH-dependent POR in the P450 1A1-catalyzed metabolism of BaP.
Introduction
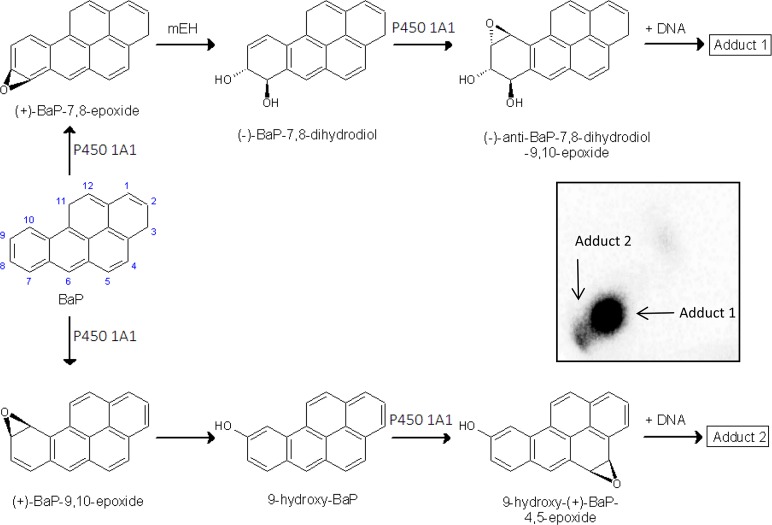
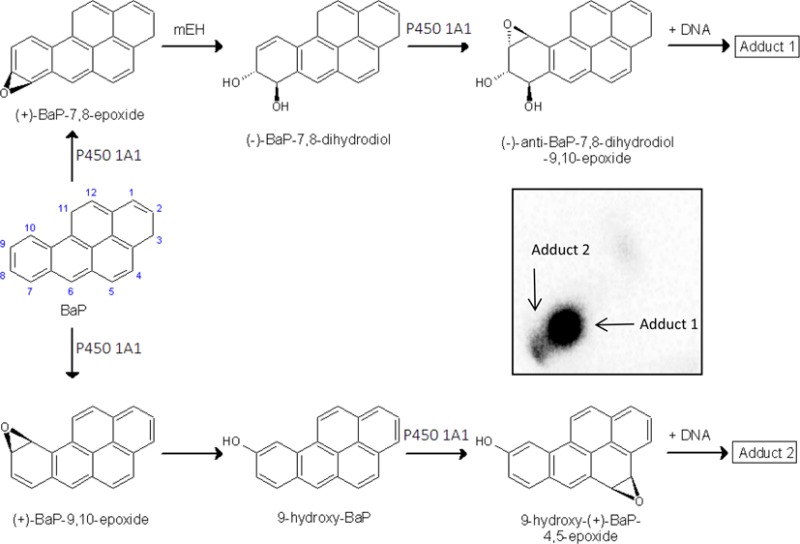
Benzo[a]pyrene (BaP) (Figure 1) is a polycyclic aromatic hydrocarbon (PAH) that has been classified as a human carcinogen (Group 1) by the International Agency for Research on Cancer.1 It is a pro-carcinogen requiring metabolic activation catalyzed by cytochrome P450 (P450) enzymes prior to reaction with DNA.2 Among the P450s, P450 1A1 is the most important enzyme, in combination with microsomal epoxide hydrolase (mEH), involved in the metabolic activation of BaP to species forming DNA adducts.2,3 First, P450 1A1 oxidizes BaP to an epoxide (i.e., BaP-7,8-epoxide) that is then converted to a dihydrodiol by mEH (i.e., BaP-7,8-dihydrodiol). Further bioactivation by P450 1A1 leads to the ultimate reactive species, BaP-7,8-dihydrodiol-9,10-epoxide (BPDE), that can react with DNA, forming adducts preferentially at guanine residues (Figure 1). The 10-(deoxyguanosin-N2-yl)-7,8,9-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (dG-N2-BPDE) adduct is the major product of the reaction of BPDE with DNA in vitro and in vivo.4−10 However, BaP is also oxidized to other metabolites such as other dihydrodiols, BaP-diones, and further hydroxylated metabolites.2,6,11−16 Although most of these metabolites are detoxification products, BaP-9-ol is the precursor of 9-hydroxy-BaP-4,5-epoxide that can form another adduct with deoxyguanosine in DNA (Figure 1).7,8,10,15,17−19
Figure 1.
Proposed pathways of biotransformation and DNA adduct formation of BaP catalyzed by P450 1A1 and mEH. Upper panel: the typical three-step activation process by P450 1A1 followed by hydrolysis by mEH leads to BPDE which forms dG-N2-BPDE (adduct 1). Lower panel: the two-step activation process by P450 1A1 leads to the formation of 9-hydroxy-BaP-4,5-epoxide that can react with deoxyguanosine in DNA (adduct 2). Insert: Autoradiographic profile of BaP-DNA adducts formed by human P450 1A1 in Supersomes with mEH in the presence of NADH and cytochrome b5 as evaluated by TLC 32P-postlabeling as described previously.19
The P450 enzymes, including P450 1A1, are components of a mixed-function oxidase (MFO) system located in the membrane of the endoplasmic reticulum (microsomes). This enzymatic system also contains other enzymes, the multidomain flavoprotein NADPH:cytochrome P450 oxidoreductase (POR) and cytochrome b5 accompanied by its NADH:cytochrome b5 reductase (CBR). Mammalian microsomal P450s function by catalyzing the insertion of one atom of molecular oxygen into a variety of xenobiotics, including BaP, while reducing the other atom to water, a reaction that requires two electrons.20 The oxygen is activated in the active center of P450s by two electrons. It is generally accepted that POR with NADPH serves as donor of electrons for both reductions of P450 in the MFO reaction cycle.20 However, the second electron may also be provided by CBR with cytochrome b5 and NADH, but cytochrome b5 seems to have also additional roles in the monooxygenase system.20−28
Although POR is considered an essential constituent of the electron transport chain toward P450,20 its exact role in the P450-mediated reaction cycle is still not clearly established. Recently, we used two mouse models in which the expression of POR has been permanently (the Hepatic P450 Reductase Null (HRN) line) or conditionally (the Reductase Conditional Null (RCN) line) deleted in hepatocytes leading to a lack of almost all hepatic POR activity. Despite this lack of POR, the levels of the P450-mediated dG-N2-BPDE adducts in the livers of mice of both lines exposed to BaP were higher than that in BaP-treated wild-type mice.7,8,19 These findings suggested BaP activation in other liver cells other than hepatocytes (e.g., Kupffer or endothelial cells)29 or bioactivation of BaP by non-P450 enzymes (e.g., prostaglandin H synthase and lipoxygenases),30,31 or that combinations of these mechanisms were operative. However, these phenomena might also indicate that another reductase such as microsomal CBR can contribute to the P450-mediated BaP oxidation in these animal models. The latter possibility is supported by experiments with rat P450s indicating the involvement of cytochrome b5 in the NADH-dependent hydroxylation of BaP in a reconstituted P450-containing system.32,33 Studies using cytochrome b5-knockout mouse lines, namely, HBN mice (hepatic cytochrome b5 null) with a conditional hepatic deletion of cytochrome b5, and HBRN mice (hepatic cytochrome b5/P450 reductase null), in which POR and cytochrome b5 are deleted specifically in the liver, also indicate the involvement of CBR in the P450-mediated metabolism of some other P450 substrates.27,34,35
We have recently demonstrated that the NADH/cytochrome b5/CBR system is indeed able to function as the sole electron donor for both reduction steps of rat P450 1A1 during the oxidation of BaP in vitro.15 Although this function of the NADH/cytochrome b5/CBR system is valid for rat P450 1A1, its role in the reaction cycle catalyzed by the human orthologue of P450 1A1 remained to be explored. To address this question, the present study was carried out, in which we utilized enzymatic systems containing recombinant human P450 1A1 overexpressed in Supersomes or pure human recombinant P450 1A1 reconstituted with CBR and cytochrome b5 in liposomes. Results presented here provide evidence that the NADH/cytochrome b5/CBR system can function as the sole electron donor to human P450 1A1 during BaP oxidation in vitro.
Experimental Procedures
Caution:
BaP is a human carcinogen and should be handled with care. Exposure to32P should be avoided, by working in a confined laboratory area, with protective clothing, plexiglass shielding, Geiger counters, and body dosimeters. Wastes must be discarded according to appropriate safety procedures.
Chemicals and Material
BaP (CAS no. 50-32-8; purity ≥96%), NADH (as disodium salt; purity ∼95%), NADPH (as tetrasodium salt; purity ∼98%), dilauroylphosphatidylcholine, glutathione, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), dimethyl sulfoxide (DMSO), 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS), and calf thymus DNA were purchased from Sigma-Aldrich (St. Louis, MO, USA) with ACS purity (purity meets the standards of the American Chemical Society), unless stated otherwise. P450 1A1-Supersomes, microsomes isolated from insect cells transfected with a baculovirus construct containing cDNA of human P450 1A1 and POR that are therefore overexpressed in these microsomes, were purchased from Gentest Corp. (Woburn, MI, USA). However, because they are microsomes (particles of broken endoplasmic reticulum), other enzymes (proteins) of the endoplasmic reticulum membrane [i.e., NADH:cytochrome b5 reductase (CBR), microsomal EH (mEH), and cytochrome b5] are also expressed at basal levels in these Supersomes (Gentest Corp., Woburn, MI, USA).
Preparation of Human Recombinant P450 1A1 and Rat Recombinant POR
Human recombinant P450 1A1 (EC 1.14.14.1) and rat POR (EC 1.6.2.4) were prepared by heterologous expression in E. coli by Jan Milichovsky and purified to apparent homogeneity (i.e., as single bands on sodium dodecyl sulfate–polyacrylamide gel electrophoresis) as described recently.36 The specific content of human recombinant CYP1A1 was 11.5 nmol/mg protein. The specific activity of rat recombinant POR measured with cytochrome c as a substrate was 49.7 μmol cytochrome c/min/mg protein. Both recombinant enzymes were utilized in the reconstitution experiments.37
Isolation of Rat NADH:Cytochrome b5 Reductase and Rabbit Cytochrome b5
Cytochrome b5 reductase (CBR) (E.C. 1.6.2.2) was isolated from rat liver microsomes by a procedure described by Perkins and Duncan.38 The specific activity of rat CBR measured as NADH-ferricyanide reductase was 49.2 μmol ferricyanide/min/mg protein. Cytochrome b5 was isolated from rabbit liver microsomes as described.39 Both proteins purified to apparent homogeneity38,39 were utilized in the reconstitution experiments.
Determination of P450 and Protein Content
The concentration of P450 was estimated according to Omura and Sato,40 based on the absorption of the complex of reduced P450 with carbon monoxide. The P450 1A1 content was calculated using an extinction coefficient of 91 mM–1 cm–1 and estimated to be 11.5 nmol per mg protein. Protein concentrations were determined using a bicinchonic acid assay (BCA; Thermo Fisher Scientific, Waltham, MA, USA) with bovine serum albumin as a standard.41
Reconstitution of Human P450 1A1 with POR or CBR in the Absence and Presence of Cytochrome b5 in Liposomes
Reconstitution of human P450 1A1 with POR, CBR, and/or cytochrome b5 in liposomes was carried out as described previously19,37 with small modifications. Briefly, dilauroylphosphatidylcholine was dissolved in chloroform (20 mg/mL), and a lipid film was obtained by evaporation of chloroform by a stream of nitrogen. The lipid film was dispersed with 50 mM HEPES/KOH buffer, pH 7.4, containing 3 mM reduced glutathione and 0.1 μM CHAPS, and sonicated twice at 20 °C for 3 min. The appropriate amounts of human recombinant P450 1A1 and rat recombinant POR or human recombinant P450 1A1 and purified rat CBR (200 pmol in a ratio of 1:1), without or with cytochrome b5 (at a ratio of P450 1A1 with reductase to cytochrome b5 of 1:5), were added to the prepared dispersion and incubated at 20 °C for 10 min. As shown in previous studies,19,25,37,42,43 the enzymatic activity of human P450 1A1 reconstituted with POR and cytochrome b5 from several animal models was the same as that of the enzyme reconstituted with the human orthologues of these enzymes.
Incubations to Study the Metabolism of BaP by Human Recombinant P450 1A1 in Supersomes or by Human Recombinant P450 1A1 Reconstituted with POR or CBR in the Presence or Absence of Cytochrome b5 in Liposomes
Incubation mixtures used to study BaP metabolism by human recombinant P450 1A1 in the presence of other components of the MFO-systems in Supersomes contained 100 mM potassium phosphate buffer (pH 7.4), 1 mM NADPH or NADH, 50 μM BaP (dissolved in 5 μL DMSO), and 100 nM human recombinant P450 1A1 in a final volume of 0.5 mL. The same amount of a solvent (DMSO) was used in control incubations without BaP. With this DMSO concentration (1% as a final concentration), no inhibition of the NADPH-dependent P450-catalyzed oxidation of several substrates has been found previously.7,8,19,25,28,44 The reaction was initiated by adding NADPH or NADH.
In separate experiments, 100 nM pure human recombinant P450 1A1 reconstituted individually with other components of the MFO-system was used instead of P450 1A1 in Supersomes. Negative control reactions lacked either P450 1A1, reductases, or BaP. After incubation (37 °C, 20 min), 5 μL of 1 mM phenacetin in methanol was added as an internal standard. BaP metabolism by the P450 1A1 systems has been shown to be linear up to 30 min of incubation.15,16,19 BaP metabolites were extracted twice with ethyl acetate (2 × 1 mL), the solvent was evaporated to dryness, the residues were dissolved in 25 μL of methanol, and subsequently BaP metabolites were separated by HPLC as reported.19,45 BaP metabolite peaks were analyzed by HPLC by comparison with metabolite standards whose structures were determined previously by NMR and/or mass spectrometry.19
Determination of BaP-DNA Adduct Formation by 32P-postlabeling
Incubation mixtures used to assess DNA adduct formation by BaP activated with all enzymatic systems containing human P450 1A1 consisted of 50 mM potassium phosphate buffer (pH 7.4), 1 mM NADPH or NADH, 100 nM human recombinant P450 1A1 plus other enzymes as indicated in the figures (or as described above), 0.1 mM BaP (dissolved in 7.5 μL of DMSO), and 0.5 mg of calf thymus DNA in a final volume of 0.75 mL as described previously.19 The reaction was initiated by adding 0.1 mM BaP, and incubations were carried out at 37 °C for 60 min. BaP-DNA adduct formation has been shown to be linear up to 90 min.7,19 Control incubations were carried out without P450 1A1, without reductases, without cytochrome b5, without NADPH (or NADH), without DNA, or without BaP. After the incubation, DNA was isolated from the residual water phase by standard phenol/chloroform extraction. DNA adduct formation was analyzed using the nuclease P1 version of the 32P-postlabeling technique.7,19 Resolution of the adducts by thin-layer chromatography (TLC) using polyethylenimine-cellulose plates (Macherey and Nagel, Düren, Germany) was carried out as described.7,19,46 DNA adduct levels (RAL, relative adduct labeling) were calculated as described.47
Statistical Analyses
Statistical analyses were carried out on the means ± standard deviations of three parallel experiments with Student’s t-test (UNISTAT Statistics Software v6, Unistat Ltd., Highgate, London N6 5UQ, UK), and p < 0.05 was considered significant. Statistical association between amounts of BaP metabolites formed by oxidation of BaP by the P450 reconstituted systems containing POR or CBR and levels of BaP-DNA adduct 2 formed by the same systems were determined by the Spearman correlation coefficients using Statistical Analysis System software, version 6.12. Spearman correlation coefficients were based on a sample size of 6. All Ps are two-tailed and considered significant at the 0.05 level.
Results
Oxidation of BaP by Human P450 1A1 Expressed in Supersomes and Pure Human Recombinant P450 1A1 Reconstituted with POR or CBR
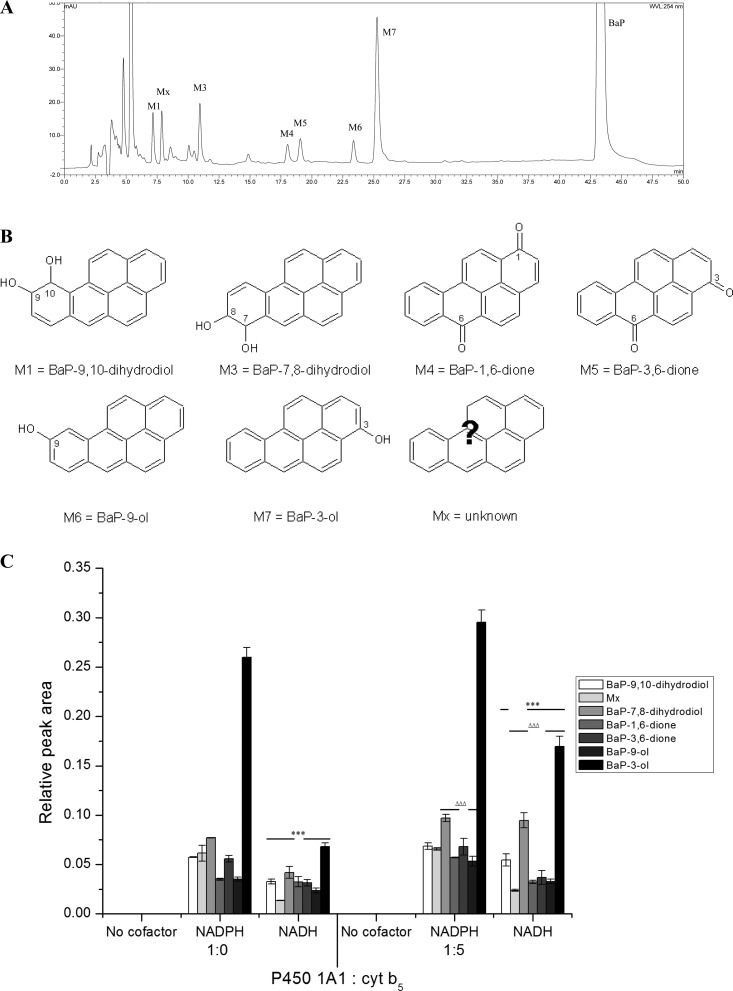
In order to evaluate the role of POR and CBR in the reduction of human P450 1A1 during BaP oxidation, two enzymatic systems containing this human P450 were utilized. The first system used Supersomes containing human recombinant P450 1A1 expressed with POR, CBR, mEH, and/or cytochrome b5. In the second system, pure human recombinant P450 1A1 was reconstituted in liposomes with either pure POR or CBR. The latter enzymatic system was utilized with or without cytochrome b5 to examine the function of both reductases as electron donors to P450 1A1 during BaP metabolism with NADPH (cofactor of POR) or NADH (cofactor of CBR). The BaP metabolites formed by human P450 1A1 in these enzyme systems were analyzed by HPLC (Figure 2A).
Figure 2.
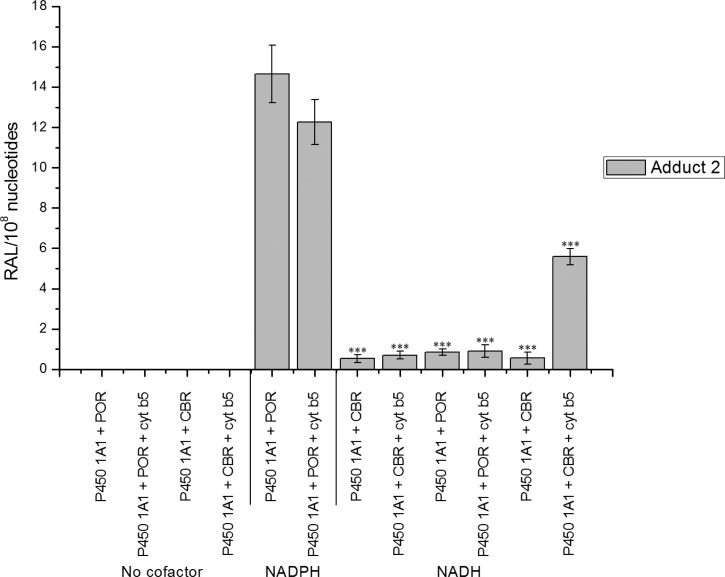
HPLC analysis of BaP metabolites formed by human recombinant P450 1A1 expressed in Supersomes in the presence of NADPH (A). Structures of BaP metabolites formed by human P450 1A1 (B). Amounts of BaP metabolites formed by human recombinant P450 1A1 expressed in Supersomes in the presence of either NADPH or NADH and the effect of cytochrome b5 (cyt b5) on this metabolism (C). Note that besides human P450 1A1 and POR overexpressed in Supersomes, this enzymatic system also contained mEH and CBR. Values represent the mean ± SD from three parallel measurements. ***P < 0.001 (Student’s t-test), significantly different from incubations using NADPH as cofactor; ΔΔΔP < 0.001 (Student’s t-test), significantly different from incubations without cytochrome b5.
Seven BaP metabolites were formed in Supersomes containing human P450 1A1, both in the presence of NADPH and NADH (Figure 2). They were structurally characterized previously16,19 as BaP-9,10-dihydrodiol (M1), BaP-7,8-dihydrodiol (M3), BaP-1,6-dione (M4), BaP-3,6-dione (M5), BaP-9-ol (M6), and BaP-3-ol (M7). The structures of these BaP metabolites are shown in Figure 2B. In addition, a metabolite of unknown structure (Mx) was detected. Essentially, no BaP metabolites were found when both NADPH and NADH were omitted from the incubation mixtures containing the P450 1A1-Supersomes (Figure 2C). NADH was less effective than NADPH to act as electron donor to human P450 1A1 in Supersomes (Figure 2C). Addition of cytochrome b5 to the incubation mixtures led to an increase in P450 1A1-mediated BaP oxidation both in the presence of NADPH and in the presence of NADH (Figure 2C).
In the second enzymatic system, where pure human recombinant P450 1A1 was reconstituted with POR with or without cytochrome b5 in liposomes, this P450 enzyme was also able to oxidize BaP in the presence of NADPH (Figure 3). Only five BaP metabolites were formed, which were BaP-3-ol (M7) as the major metabolite, BaP-9-ol (M6), BaP-1,6-dione (M4), BaP-3,6-dione (M5), and metabolite Mx (Figure S1). Because mEH was absent from the system, no dihydrodiols were formed. Addition of cytochrome b5 to this P450 1A1 system increased the formation of all BaP metabolites, in particular BaP-3-ol (Figure 3 and Figure S1). In contrast, NADH did not lead to significant BaP oxidation by P450 1A1 reconstituted with POR (Figure 3 and Figure S1), which confirms that NADH functions as a coenzyme of POR at a very slow rate that is negligible relative to NADPH, as we showed recently with cytochrome c as a substrate for POR.15 No BaP metabolites were found when both NADPH and NADH were omitted from the incubation mixtures containing the human recombinant P450 1A1 reconstituted with POR or CBR (Figure 3).
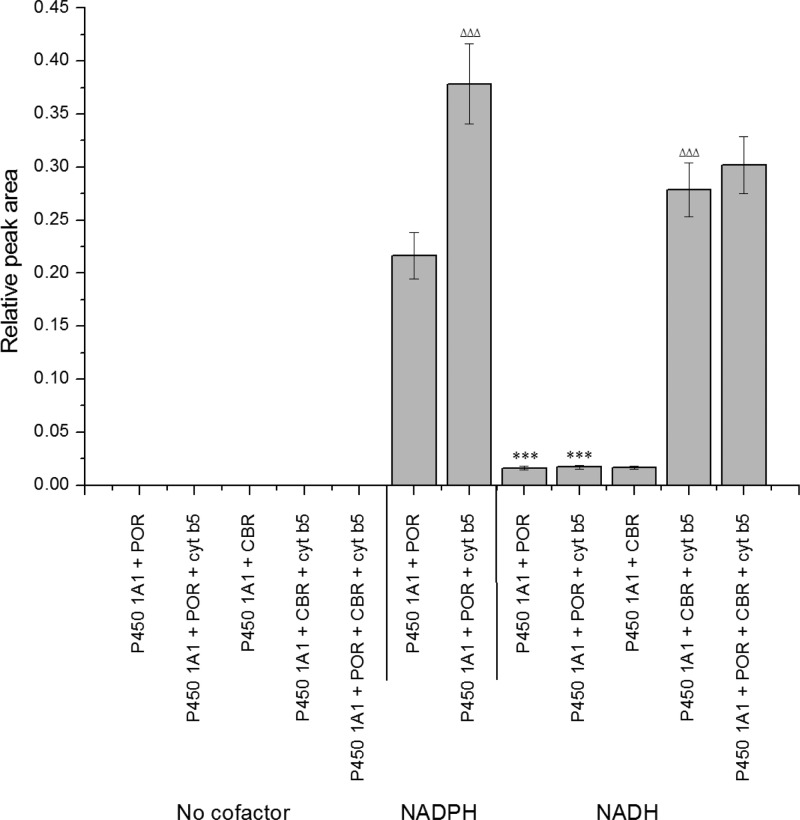
Figure 3.
BaP metabolism catalyzed by human P450 1A1 reconstituted with POR or CBR with or without cytochrome b5 (cyt b5). Columns show the sum of individual BaP metabolites formed by P450 1A1 (BaP-3-ol, BaP-9-ol, BaP-1,6-dione, BaP-3,6-dione, and the unknown metabolite Mx) Incubations were carried out in the presence of NADPH or NADH. ΔΔΔP < 0.001 (Student’s t-test), significantly different from incubations without cytochrome b5; ***P < 0.001 (Student’s t-test), significantly different from incubations with NADPH as cofactor.
In the enzymatic system where human P450 1A1 was reconstituted with CBR and cytochrome b5 in liposomes, BaP was predominantly oxidized to BaP-3-ol (M7) and to a lower extent to BaP-9-ol (M6), BaP-1,6-dione (M4), BaP-3,6-dione (M5), and a metabolite Mx (Figure S1). Cytochrome b5 as a substrate of CBR was necessary for BaP oxidation in the system of human P450 1A1 reconstituted with CBR with NADH as the cofactor. Without this protein, essentially no BaP oxidation was detectable by P450 1A1 reconstituted with CBR (Figure 3 and Figure S1). Addition of POR to the reconstituted system of human P450 1A1 with CBR and cytochrome b5 did not change BaP metabolite levels (Figure 3 and Figure S1).
Formation of BaP-DNA Adducts by Human P450 1A1 Expressed in Supersomes and by Human P450 1A1 Reconstituted with POR or CBR
In further experiments, the formation of DNA adducts by BaP incubated with human P450 1A1 expressed in Supersomes and human P450 1A1 reconstituted with POR or CBR was analyzed. First, we utilized human recombinant P450 1A1 expressed in Supersomes with other enzyme components of the microsomal monooxygenase system. Up to two DNA adducts (assigned adducts 1 and 2; see insert in Figure 1) were detected by 32P-postlabeling in incubations where BaP was activated with human P450 1A1 in Supersomes, in the presence of either NADPH or NADH. No such BaP adducts were found when both NADPH and NADH were omitted from the incubation mixtures containing the P450 1A1-Supersomes (Figure 4). Adduct 1 was the BaP-DNA adduct predominantly formed in this system (Figure 4). Comparison with previous 32P-postlabeling analyses10,19 showed that adduct 1 is the dG-N2-BPDE adduct.10,19 The other adduct, which was formed in this enzymatic system as only a minor product, if detectable at all (e.g., there was no formation of this adduct in Supersomes in the presence of NADPH), has similar chromatographic properties by TLC to a guanine adduct derived from the reaction with 9-hydroxy-BaP-4,5-epoxide (see adduct spot 2 in the insert of Figure 1 and Figure 5A). Addition of cytochrome b5 to P450 1A1 in Supersomes increased the levels of the dG-N2-BPDE adduct generated by human P450 1A1 in the presence of NADH (Figure 4), similar to the increase observed in BaP oxidation (Figure 2).
Figure 4.
DNA adduct formation (RAL, relative adduct labeling) by BaP activated with human recombinant P450 1A1 in Supersomes in the presence of either NADPH or NADH and the effect of cytochrome b5 (cyt b5) on this reaction. Note that besides P450 1A1 and POR overexpressed in Supersomes, this enzymatic system also contained mEH and CBR. Values represent the mean total RAL ± SD (n = 3; analyses of three independent in vitro incubations). ***P < 0.001 (Student’s t-test), levels of BaP-adduct 1 significantly different from incubations with NADH but without cytochrome b5.
Figure 5.
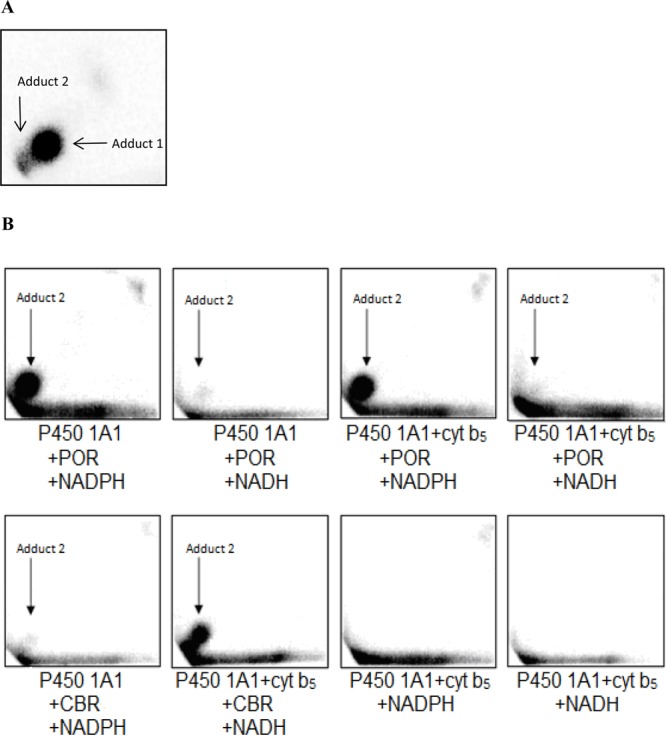
Autoradiographic profile of BaP-derived DNA adducts generated in DNA by BaP activated with human P450 1A1 in Supersomes with mEH in the presence of NADH and cytochrome b5 (A); with pure human P450 1A1 reconstituted with POR in liposomes in the presence of NADPH (B); P450 1A1 with POR in the presence of NADH; P450 1A1 with POR and cytochrome b5 in the presence of NADPH; P450 1A1 with POR and cytochrome b5 in the presence of NADH; P450 1A1 with CBR and cytochrome b5 in the presence of NADPH; P450 1A1 with CBR and cytochrome b5 in the presence of NADH; P450 1A1 with cytochrome b5 in the presence of NADPH; and P450 1A1 with cytochrome b5 in the presence of NADH.
Pure human recombinant P450 1A1 reconstituted with POR in the presence of NADPH formed only adduct 2 (Figures 5B and 6). Because of the absence of mEH, no adduct 1 (i.e., dG-N2-BPDE) was formed. Addition of cytochrome b5 to this P450 1A1 system decreased the levels of adduct 2, but this decrease was not statistically significant (Figure 6). NADH was essentially ineffective to mediate the activation of BaP by P450 1A1 reconstituted with POR (Figures 5B and 6), which again indicates that NADH functions as a coenzyme of POR at a very slow rate that is negligible relative to NADPH.
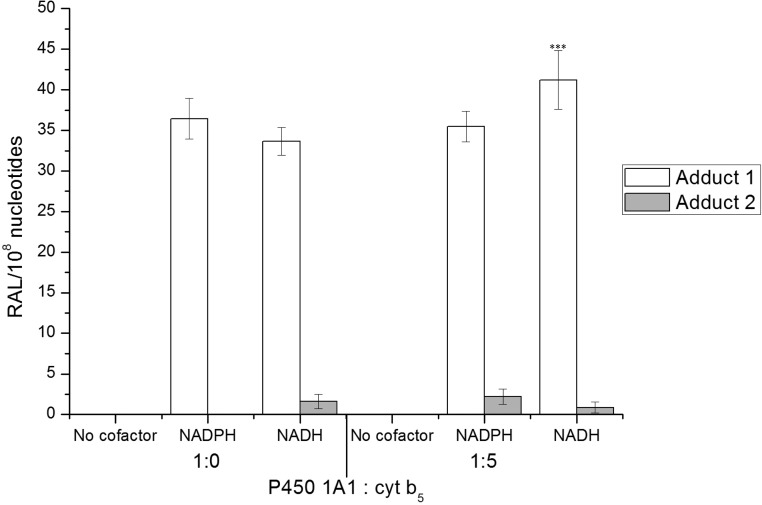
Figure 6.
Effect of NADPH and/or NADH on BaP-DNA adduct formation (RAL, relative adduct labeling) catalyzed by human recombinant P450 1A1 reconstituted with POR or CBR with or without cytochrome b5 in liposomes. Values are averages ± SD of three independent experiments. ***P < 0.001 (Student’s t-test), levels of BaP-adduct 2 significantly different from incubations with human P450 1A1, POR, and NADPH.
Human P450 1A1 reconstituted with CBR and cytochrome b5 also generated adduct 2. The presence of cytochrome b5 as a substrate of CBR and NADH as its cofactor was essential for this adduct formation; NADPH, the cofactor of POR, was ineffective (Figures 5B and 6). No BaP-DNA adduct 2 was found when both NADPH and NADH were omitted from the incubation mixtures containing human recombinant P450 1A1 reconstituted with POR or CBR (Figure 6). Levels of adduct 2 formed by human P450 1A1 in the presence of the NADH/cytochrome b5/CBR system were lower than those formed in the system of human P450 1A1 containing POR and NADPH (Figures 5 and 6). These results corresponded to lower levels of BaP-9-ol (a precursor of 9-hydroxy-BaP 4,5-epoxide generating this adduct) and other BaP metabolites formed in this system (see Figure S1). Significant correlations were found between levels of adduct 2 and BaP-9-ol (r = 0.943, P < 0.001, Spearman’s correlations) or BaP-3-ol (r = 0.943, P < 0.001), whereas no such correlations were found between levels of this adduct and BaP-1,6-dione, BaP-3,6-dione or a metabolite Mx (r < 0.5).
Discussion
The metabolism of BaP has been extensively studied over the past decades,2 and various studies have examined the role of P450 enzymes, particularly P450 1A1 of several species, to metabolize this carcinogen.2,6−8,15,16,19,28,44 However, the mechanism of the reaction cycle of BaP oxidation catalyzed by P450 1A1, particularly the roles of POR and CBR as electron donors to P450 1A1, has not yet been fully resolved.7,8,15,19 Enigmatic results have been found in two mouse models where deletion of POR in hepatocytes did not lead to an expected decrease in BaP-DNA adduct formation in the liver in vivo but instead to higher BaP-DNA binding.7,8,19 We also showed that in livers of HRN mice P450 1A1, cytochrome b5 and mEH can effectively activate BaP to DNA binding species, even in the presence of very low amounts of POR.19 Because this feature has biological significance, studying the role of the enzymes reducing P450 1A1 is important to better understand the mechanism(s) involved in BaP metabolism. Recently, we demonstrated that the NADH/cytochrome b5/CBR system is able to function as the sole donor of electrons for both reduction steps of rat P450 1A1 during BaP oxidation in vitro.(15) This finding indicates that CBR as an NADH-dependent reductase might substitute POR in the P450 1A1-mediated BaP metabolism and might help to explain our enigmatic results in the POR-knockout mouse models.7,8,19 However, the question whether this novel function of the NADH/cytochrome b5/CBR system as electron donor to rat P450 1A1 in BaP metabolism represents a general feature for the P450 1A1 reaction cycle in other species including humans remained to be answered. Therefore, the primary aim of this study was to determine whether the NADH/cytochrome b5/CBR system can be the exclusive donor of both electrons to the P450 1A1 human orthologue during BaP metabolism in vitro. To this end, two enzymatic systems containing this human P450 were utilized: (1) microsomes of baculovirus-infected insect cells (Supersomes) containing human recombinant P450 1A1 expressed with POR, CBR, mEH, and cytochrome b5 and (2) pure human P450 1A1 heterologously expressed in E. coli reconstituted either with pure POR or CBR with cytochrome b5 in liposomes.
Using the first system, human P450 1A1-Supersomes, we proved that NADH acts as electron donor for both reductions of the P450 1A1 human orthologue in BaP oxidation independent of NADPH and POR if CBR and cytochrome b5 is present. This conclusion is supported by the fact that NADH functions as a poor coenzyme of POR when cytochrome c is used as its substrate.15 Our results therefore confirm the novel feature of the mechanism of the catalytic cycle of human P450 1A1 during BaP oxidation previously described for rat P450 1A1. We demonstrated that the reaction cycle of BaP oxidation catalyzed by human P450 1A1 can proceed by ways that differ from the generally accepted mechanism, where the first reduction of P450 is considered to be catalyzed by POR without cytochrome b5.20,48−52 Considering the redox potentials of cytochrome b5 (+20 mV)53,54 and ferric substrate-bound P450 (−237 mV),27,53 it is thermodynamically impossible for cytochrome b5 to provide the first electron in the P450 catalytic cycle.55 Given that the redox potential of oxyferrous P450 is also approximately +20 mV, it is feasible that cytochrome b5 can supply the second electron into the catalytic cycle.27,53,55 However, based on the redox potential of CBR determined under the anaerobic conditions (−265 mV)56 it could provide the first electron in the P450 catalytic cycle.27,57 Moreover, considering the effect of the given Le Chatelier’s principle, the reduced P450 will rapidly bind dioxygen under aerobic conditions of the experiments. Indeed, in the present study we demonstrate that the first reduction of P450 1A1 which previously had been considered to be mediated exclusively by the NADPH/POR system can be substituted by the NADH/cytochrome b5/CBR system. This reaction was found not only in Supersomes containing human recombinant P450 1A1 but, even more importantly, in the system of pure human P450 1A1 reconstituted with CBR and cytochrome b5. Such NADH/cytochrome b5/CBR-mediated activity of the human P450 1A1 systems was proven by the formation of up to seven BaP metabolites [BaP-9,10-dihydrodiol (M1), BaP-7,8-dihydrodiol (M3), BaP-1,6-dione (M4), BaP-3,6-dione (M5), BaP-9-ol (M6), BaP-3-ol (M7), and a metabolite of unknown structure (Mx)]. In addition, these systems generated two BaP-DNA adducts, the dG-N2-BPDE adduct (adduct 1) and/or a guanine adduct derived from the reaction with 9-hydroxy-BaP-4,5-epoxide (adduct 2). The BaP metabolite profiles formed by P450 1A1 with the NADH/cytochrome b5/CBR system were the same as those in the system where NADPH and POR were used as electron donors. BaP-4,5-dihydrodiol (M2), which was previously found to be formed by rat P450 1A1,15,19 was not generated by the human P450 1A1 orthologue (Figure 2). This finding is in line with previous findings showing that this BaP metabolite was not formed in human bronchoalveolar H358 cells expressing P450 1A1 after BaP exposure.13
Interestingly, essentially no differences in the levels of dG-N2-BPDE adducts were seen when using either NADPH or NADH as cofactors in the P450 1A1-Supersomes system (see Figure 4). However, it should be noted that the oxidation reaction of BaP to its metabolites was catalyzed less efficiently by NADH than by NADPH in this system (see Figure 2). One reason might be the effects of the different experimental conditions used for the incubations utilized for BaP metabolite analysis and BaP-DNA adduct formation (i.e., incubation times, concentrations of BaP, and the presence or absence of DNA; see the Experimental Procedures section).
In summary, this study demonstrates for the first time that NADH, CBR, and cytochrome b5 can act as sole electron donors for both the first and second reduction of human P450 1A1 during the oxidative metabolism of BaP and formation of BaP-DNA adducts in vitro. These findings confirm our results of a recent study where rat P450 1A1 was utilized to study BaP metabolism.15 However, although the role of the NADH/cytochrome b5/CBR system in both reductions of rat15 and human P450 1A1present work is proven in our in vitro studies, further investigations are needed in the future. In this context, the mechanism of both the first and second reduction of P450 1A1 remains to be examined in detail. In addition, as shown by Guengerich and co-workers for the P450 3A4-mediated 6-hydroxylation of testosterone,57 the question as to whether the NADH/cytochrome b5/CBR system might also reduce other P450 enzymes needs to be addressed. This might be the case in rat hepatic microsomes where various P450s were induced by their specific inducers; in these microsomes, BaP was oxidized not only in the presence of NADPH but also in the presence of NADH.15 Another crucial question that remains to be addressed relates to the impact of the NADH/cytochrome b5/CBR system on BaP metabolism in vivo. Recent in vivo experiments using cytochrome b5-knockout mouse lines (i.e., HBN and HBRN) provide evidence that in the absence of POR, cytochrome b5/CBR are capable of supplying electrons for P450 catalytic function (i.e., in the metabolism of the P450 3A substrate midazolam).27 It is anticipated that these mouse lines will help to elucidate the different mechanisms of P450-catalyzed BaP biotransformation in vivo.
Glossary
Abbreviations
- BaP
benzo[a]pyrene
- BPDE
BaP-7,8-dihydrodiol-9,10-oxide
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CBR
NADH:cytochrome b5 reductase
- P450
cytochrome P450
- dG-N2-BPDE
10-(deoxyguanosin-N2-yl)-7,8,9-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene
- DMSO
dimethyl sulfoxide
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HBN
hepatic cytochrome b5 null
- HPLC
high performance liquid chromatography
- HBRN
hepatic cytochrome b5/P450 reductase null
- HRN
hepatic P450 reductase null
- mEH
microsomal epoxide hydrolase
- MFO
mixed-function oxidase
- POR
NADPH:P450 oxidoreductase
- RAL
relative adduct labeling
- RCN
reductase conditional null
- TLC
thin-layer chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.6b00143.
Amounts of BaP metabolites generated by human P450 1A1 reconstituted with POR or CBR with or without cytochrome b5 (cyt b5) (PDF)
Supported by GACR (grant 14-18344S in panel P301) and Charles University (grant UNCE 204025/2012). Work at King’s College London is supported by Cancer Research UK (grant number C313/A14329), the Wellcome Trust (Grants 101126/Z/13/Z and 101126/B/13/Z), the Natural Environmental Research Council (NE/L006782/1), and in part by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Health Impact of Environmental Hazards at King’s College London in partnership with Public Health England (PHE).
The authors declare no competing financial interest.
Supplementary Material
References
- IARC (International Agency for Research on Cancer) (2010) Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, in IARC Monogr. Eval. Carcinog. Risks Hum., Vol. 92, pp 1–853, IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Baird W. M.; Hooven L. A.; Mahadevan B. (2005) Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 45, 106–114. 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- Wohak L. E.; Krais A. M.; Kucab J. E.; Stertmann J.; Øvrebø S.; Seidel A.; Phillips D. H.; Arlt V. M. (2016) Carcinogenic polycyclic aromatic hydrocarbons induce CYP1A1 in human cells via a p53-dependent mechanism. Arch. Toxicol. 90, 291–304. 10.1007/s00204-014-1409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P.; Grover P. L.; Swaisland A.; Pal K.; Hewer A. (1974) Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature 252, 326–328. 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Wood A. W.; Levin W.; Lu A. Y.; Yagi H.; Hernandez O.; Jerina D. M.; Conney A. H. (1976) Metabolism of benzo(a)pyrene and benzo(a)pyrene derivatives to mutagenic products by highly purified hepatic microsomal enzymes. J. Biol. Chem. 251, 4882–4890. [PubMed] [Google Scholar]
- Bauer E.; Guo Z.; Ueng Y. F.; Bell L. C.; Zeldin D.; Guengerich F. P. (1995) Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 8, 136–142. 10.1021/tx00043a018. [DOI] [PubMed] [Google Scholar]
- Arlt V. M.; Stiborova M.; Henderson C. J.; Thiemann M.; Frei E.; Aimova D.; Singhs R.; Costa; da G. G.; Schmitz O. J.; Farmer P. D.; Wolf C. R.; Philips D. H. (2008) Metabolic activation of benzo[a]pyrene in vitro by hepatic cytochrome P450 contrasts with detoxification in vivo: experiments with hepatic cytochrome P450 reductase null mice. Carcinogenesis 29, 656–665. 10.1093/carcin/bgn002. [DOI] [PubMed] [Google Scholar]
- Arlt V. M.; Poirier M. C.; Sykes S. E.; John K.; Moserova M.; Stiborova M.; Wolf C. R.; Henderson C. J.; Phillips D. H. (2012) Exposure to benzo[a]pyrene of Hepatic Cytochrome P450 Reductase Null (HRN) and P450 Reductase Conditional Null (RCN) mice: Detection of benzo[a]pyrene diol epoxide-DNA adducts by immunohistochemistry and 32P-postlabelling. Toxicol. Lett. 213, 160–166. 10.1016/j.toxlet.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt V. M.; Krais A. M.; Godschalk R. W.; Riffo-Vasquez Y.; Mrizova I.; Roufosse C. A.; Corbin C.; Shi Q.; Frei E.; Stiborova M.; van Schooten F. J.; Phillips D. H.; Spina D. (2015) Pulmonary inflammation impacts on CYP1A1-mediated respiratory tract DNA damage induced by the carcinogenic air pollutant benzo[a]pyrene. Toxicol. Sci. 146, 213–225. 10.1093/toxsci/kfv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krais A. M.; Speksnijder E. N.; Melis J. P.; Indra R.; Moserova M.; Godschalk R. W.; van Schooten F. J.; Seidel A.; Kopka K.; Schmeiser H. H.; Stiborova M.; Phillips D. H.; Luijten M.; Arlt V. M. (2016) The impact of p53 on DNA damage and metabolic activation of the environmental carcinogen benzo[a]pyrene: effects in Trp53(+/+), Trp53(±) and Trp53(−/−) mice. Arch. Toxicol. 90, 839–851. 10.1007/s00204-015-1531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y. J.; Shimada T.; Guengerich F. P. (1996) Construction of a human cytochrome P450 1A1: rat NADPH-cytochrome P450 reductase fusion protein cDNA and expression in Escherichia coli, purification, and catalytic properties of the enzyme in bacterial cells and after purification. Arch. Biochem. Biophys. 330, 48–58. 10.1006/abbi.1996.0224. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Stansbury K. H.; Walker N. J.; Trush M. A.; Strickland P. T.; Sutter T. R. (1998) Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 19, 1847–1853. 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Gelhaus S. L.; Mangal D.; Harvey R. G.; Blair I. A.; Penning T. M. (2007) Metabolism of benzo[a]pyrene in human bronchoalveolar H358 cells using liquid chromatography-mass spectrometry. Chem. Res. Toxicol. 20, 1331–1341. 10.1021/tx700107z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Li L.; Thornton C.; Carvalho P.; Avery B. A.; Willett K. L. (2008) Simultaneous determination of benzo[a]pyrene and eight of its metabolites in Fundulus heteroclitus bile using ultra-performance liquid chromatography with mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 863, 141–149. 10.1016/j.jchromb.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiborová M.; Indra R.; Moserová M.; Šulc M.; Hodek P.; Frei E.; Schmeiser H. H.; Arlt V. M. (2016) NADPH- and NADH-dependent metabolism of and DNA adduct formation by benzo[a]pyrene catalyzed with rat hepatic microsomes and cytochrome P450 1A1. Monatsh. Chem. 147, 847–855. 10.1007/s00706-016-1713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šulc M.; Indra R.; Moserová M.; Schmeiser H. H.; Frei E.; Arlt V. M.; Stiborová M. (2016) The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo[a]pyrene in human livers. Environ. Mol. Mutagen. 57, 229–235. 10.1002/em.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H. W.; Thompson M. H.; Brookes P. (1976) The role of 9-hydroxybenzo(a)pyrene in the microsome mediated binding of benzo(a)pyrene to DNA. Int. J. Cancer 18, 339–344. 10.1002/ijc.2910180311. [DOI] [PubMed] [Google Scholar]
- Fang A. H.; Smith W. A.; Vouros P.; Gupta R. C. (2001) Identification and characterization of a novel benzo[a]pyrene-derived DNA adduct. Biochem. Biophys. Res. Commun. 281, 383–389. 10.1006/bbrc.2000.4161. [DOI] [PubMed] [Google Scholar]
- Stiborova M.; Moserova M.; Cerna V.; Indra R.; Dracinsky M.; Šulc M.; Henderson C. J.; Wolf C. R.; Schmeiser H. H.; Phillips D. H.; Frei E.; Arlt V. M. (2014) Cytochrome b5 and epoxide hydrolase contribute to benzo[a]pyrene-DNA adduct formation catalyzed by cytochrome P450 1A1 under low NADPH:P450 oxidoreductase conditions. Toxicology 318, 1–12. 10.1016/j.tox.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. (2008) Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 21, 70–83. 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- Porter T. D. (2002) The roles of cytochrome b5 in cytochrome P450 reactions. J. Biochem. Mol. Toxicol. 16, 311–316. 10.1002/jbt.10052. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B.; Jansson I. (2003) The many roles of cytochrome b5. Pharmacol. Ther. 97, 139–152. 10.1016/S0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. (2005) Reduction of cytochrome b5 by NADPH-cytochrome P450 reductase. Arch. Biochem. Biophys. 440, 204–211. 10.1016/j.abb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. A.; Ronseaux S.; Finn R. D.; Henderson C. J.; Wolf C. R. (2010) Deletion of microsomal cytochrome b5 profoundly affects hepatic and extrahepatic drug metabolism. Mol. Pharmacol. 78, 269–278. 10.1124/mol.110.064246. [DOI] [PubMed] [Google Scholar]
- Kotrbova V.; Mrazova M.; Moserova M.; Martinek V.; Hodek P.; Hudecek J.; Frei E.; Stiborova M. (2011) Cytochrome b5 shifts oxidation of the anticancer drug ellipticine by cytochromes P450 1A1 and 1A2 from its detoxication to activation, thereby modulating its pharmacological efficacy. Biochem. Pharmacol. 82, 669–680. 10.1016/j.bcp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Stiborova M.; Indra R.; Moserova M.; Cerná V.; Rupertová M.; Martínek V.; Eckschlager T.; Kizek R.; Frei E. (2012) Cytochrome b5 increases cytochrome P450 3A4-mediated activation of anticancer drug ellipticine to 13-hydroxyellipticine whose covalent binding to DNA is elevated by sulfotransferases and N,O-acetyltransferases. Chem. Res. Toxicol. 25, 1075–1085. 10.1021/tx3000335. [DOI] [PubMed] [Google Scholar]
- Henderson C. J.; McLaughlin L. A.; Wolf C. R. (2013) Evidence that cytochrome b5 and cytochrome b5 reductase can act as sole electron donors to the hepatic cytochrome P450 system. Mol. Pharmacol. 83, 1209–1217. 10.1124/mol.112.084616. [DOI] [PubMed] [Google Scholar]
- Indra R.; Moserova M.; Sulc M.; Frei E.; Stiborova M. (2013) Oxidation of carcinogenic benzo[a]pyrene by human and rat cytochrome P450 1A1 and its influencing by cytochrome b5 – a comparative study. Toxicol. Lett. 221, S72. 10.1016/j.toxlet.2013.05.057. [DOI] [PubMed] [Google Scholar]
- Steinberg P.; Schlemper B.; Molitor E.; Platt K. L.; Seidel A.; Oesch F. (1990) Rat liver endothelial and Kupffer cell-mediated mutagenicity of polycyclic aromatic hydrocarbons and aflatoxin B1. Environ. Health Perspect. 88, 71–76. 10.1289/ehp.908871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett L. J.; Bienkowski M. J. (1980) Hydroperoxide-dependent oxygenation of trans-7,8-dihydroxy-7,8-dihydro benzo[a]pyrene by ram seminal vesicle microsomes, Source of the oxygen. Biochem. Biophys. Res. Commun. 96, 639–647. 10.1016/0006-291X(80)91403-5. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. (1990) Prostaglandin synthase-mediated metabolism of carcinogens and a potential role for peroxyl radicals as reactive intermediates. Environ. Health Perspect. 88, 5–12. 10.1289/ehp.90885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. B.; Levin W.; Ryan D.; Vore M.; Lu A. Y. (1974) Liver microsomal electron transport systems. II. The involvement of cytochrome b5 in the NADH-dependent hydroxylation of 3,4-benzpyrene by a reconstituted cytochrome P-448-containing system. Biochem. Biophys. Res. Commun. 58, 516–522. 10.1016/0006-291X(74)90395-7. [DOI] [PubMed] [Google Scholar]
- Lu A. Y.; West S. B.; Vore M.; Ryan D.; Levin W. (1974) Role of cytochrome b5 in hydroxylation by a reconstituted cytochrome P-450-containing system. J. Biol. Chem. 249, 6701–6709. [PubMed] [Google Scholar]
- Finn R. D.; McLaughlin L. A.; Ronseaux S.; Rosewell I.; Houston J. B.; Henderson C. J.; Wolf C. R. (2008) Defining the in vivo role for cytochrome b5 in cytochrome P450 function through the conditional hepatic deletion of microsomal cytochrome b5. J. Biol. Chem. 283, 31385–31393. 10.1074/jbc.M803496200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. J.; McLaughlin L. A.; Scheer N.; Stanley L. A.; Wolf C. R. (2015) Cytochrome b5 is a major determinant of human cytochrome P450 CYP2D6 and CYP3A4 activity in vivo. Mol. Pharmacol. 87, 733–739. 10.1124/mol.114.097394. [DOI] [PubMed] [Google Scholar]
- Milichovský J.; Bárta F.; Schmeiser H. H.; Arlt V. M.; Frei E.; Stiborová M.; Martínek V. (2016) Active site mutations as a suitable tool contributing to explain a mechanism of aristolochic acid I nitroreduction by cytochromes P450 1A1, 1A2 and 1B1. Int. J. Mol. Sci. 17, 213. 10.3390/ijms17020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiborová M.; Martínek V.; Rýdlová H.; Hodek P.; Frei E. (2002) Sudan I is a potential carcinogen for humans: Evidence for its metabolic activation and detoxication by human recombinant cytochrome P450 1A1 and liver microsomes. Cancer Res. 62, 5678–5684. [PubMed] [Google Scholar]
- Perkins D. M.; Duncan J. R. (1987) Improved isolation of rat microsomal cytochrome b5 reductase. J. Chromatogr. 405, 319–325. 10.1016/S0021-9673(01)81773-8. [DOI] [PubMed] [Google Scholar]
- Roos P. H. (1996) Chromatographic separation and behavior of microsomal cytochrome P450 and cytochrome b5. J. Chromatogr., Biomed. Appl. 684, 107–131. 10.1016/0378-4347(96)00018-7. [DOI] [PubMed] [Google Scholar]
- Omura T.; Sato R. (1964) The carbon monoxide-binding pigment of liver microsomes: I Evidence for its hemoprotein nature. J. Biol. Chem. 239, 2370–2378. [PubMed] [Google Scholar]
- Wiechelman K. J.; Braun R. D.; Fitzpatrick J. D. (1988) Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal. Biochem. 175, 231–237. 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- Stiborová M.; Sejbal J.; Bořek-Dohalská L.; Aimová D.; Poljaková J.; Forsterová K.; Rupertová M.; Wiesner J.; Hudeček J.; Wiessler M.; Frei E. (2004) The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res. 64, 8374–8380. 10.1158/0008-5472.CAN-04-2202. [DOI] [PubMed] [Google Scholar]
- Kotrbová V.; Aimová D.; Březinová A.; Janouchová K.; Poljaková J.; Hodek P.; Frei E.; Stiborová M. (2006) Cytochromes P450 reconstituted with NADPH:P450 reductase mimic the activating and detoxicating metabolism of the anticancer drug ellipticine in microsomes. Neuro Endocrinol. Lett. 27 (Suppl. 2), 18–20. [PubMed] [Google Scholar]
- Indra R.; Moserova M.; Kroftova N.; Sulc M.; Martinkova M.; Adam V.; Eckschlager T.; Kizek R.; Arlt V. M.; Stiborova M. (2014) Modulation of human cytochrome P450 1A1-mediated oxidation of benzo[a]pyrene by NADPH:cytochrome P450 oxidoreductase and cytochrome b5. Neuro Endocrinol. Lett. 35 (Suppl. 2), 105–113. [PubMed] [Google Scholar]
- Moserová M.; Kotrbová V.; Aimová D.; Šulc M.; Frei E.; Stiborová M. (2009) Analysis of benzo[a]pyrene metabolites formed by rat hepatic microsomes using high pressure liquid chromatography: optimization of the method. Interdiscip. Toxicol. 2, 239–244. 10.2478/v10102-009-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodek P.; Koblihová J.; Kizek R.; Frei E.; Arlt V. M.; Stiborová M. (2013) The relationship between DNA adduct formation by benzo[a]pyrene and expression of its activation enzyme cytochrome P450 1A1 in rat. Environ. Toxicol. Pharmacol. 36, 989–996. 10.1016/j.etap.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Schmeiser H. H.; Stiborova M.; Arlt V. M. (2013) 32P-postlabeling analysis of DNA adducts. Methods Mol. Biol. 1044, 389–401. 10.1007/978-1-62703-529-3_21. [DOI] [PubMed] [Google Scholar]
- Sligar S. G.; Cinti D. L.; Gibson G. G.; Schenkman J. B. (1979) Spin state control of the hepatic cytochrome P450 redox potential. Biochem. Biophys. Res. Commun. 90, 925–932. 10.1016/0006-291X(79)91916-8. [DOI] [PubMed] [Google Scholar]
- Lewis D. F.; Hlavica P. (2000) Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects. Biochim. Biophys. Acta, Bioenerg. 1460, 353–374. 10.1016/S0005-2728(00)00202-4. [DOI] [PubMed] [Google Scholar]
- Hlavica P.; Schulze J.; Lewis D. F. (2003) Functional interaction of cytochrome P450 with its redox partners: a critical assessment and update of the topology of predicted contact regions. J. Inorg. Biochem. 96, 279–297. 10.1016/S0162-0134(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Meunier B.; de Visser S. P.; Shaik S. (2004) Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem. Rev. 104, 3947–3980. 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- Berka K.; Hendrychová T.; Anzenbacher P.; Otyepka M. (2011) Membrane position of ibuprofen agrees with suggested access path entrance to cytochrome P450 2C9 active site. J. Phys. Chem. A 115, 11248–11255. 10.1021/jp204488j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F. V.; Hlavica P. (2000) Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects. Biochim. Biophys. Acta, Bioenerg. 1460, 353–374. 10.1016/S0005-2728(00)00202-4. [DOI] [PubMed] [Google Scholar]
- Aono T.; Sakamoto Y.; Miura M.; Takeuchi F.; Hori H.; Tsubaki M. (2010) Direct electrochemical analyses of human cytochromes b5 with a mutated heme pocket showed a good correlation between their midpoint and half wave potentials. J. Biomed. Sci. 17, 90. 10.1186/1423-0127-17-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. C.; Waskell L. (2011) The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b5. Arch. Biochem. Biophys. 507, 144–153. 10.1016/j.abb.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyanagi T. (1977) Redox properties of microsomal reduced nicotinamide adenine dinucleotide-cytochrome bs reductase and cytochrome b5. Biochemistry 16, 2725–2730. 10.1021/bi00631a021. [DOI] [PubMed] [Google Scholar]
- Yamazaki H.; Johnson W. W.; Ueng Y. F.; Shimada T.; Guengerich F. P. (1996) Lack of electron transfer from cytochrome b5 in stimulation of catalytic activities of cytochrome P450 3A4. Characterization of a reconstituted cytochrome P450 3A4/NADPH-cytochrome P450 reductase system and studies with apo-cytochrome b5. J. Biol. Chem. 271, 27438–27444. 10.1074/jbc.271.44.27438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.