ABSTRACT
Recent studies have shown that inflammatory responses trigger and transmit senescence to neighboring cells and activate the senescence-associated secretory phenotype (SASP). Latent Epstein-Barr virus (EBV) infection induces increased secretion of several inflammatory factors, whereas lytic infections evade the antiviral inflammatory response. However, the changes in and roles of the inflammatory microenvironment during the switch between EBV life cycles remain unknown. In the present study, we demonstrate that latent EBV infection in EBV-positive cells triggers the SASP in neighboring epithelial cells. In contrast, lytic EBV infection abolishes this phenotype. BZLF1 attenuates the transmission of paracrine senescence during lytic EBV infection by downregulating tumor necrosis factor alpha (TNF-α) secretion. A mutant BZLF1 protein, BZLF1Δ207-210, that cannot inhibit TNF-α secretion while maintaining viral transcription, fails to block paracrine senescence, whereas a neutralizing antibody against TNF-α is sufficient to restore its inhibition. Furthermore, latent EBV infection induces oxidative stress in neighboring cells, while BZLF1-mediated downregulation of TNF-α reduces reactive oxygen species (ROS) levels in neighboring cells, and ROS scavengers alleviate paracrine senescence. These results suggest that lytic EBV infection attenuates the transmission of inflammatory paracrine senescence through BZLF1 downregulation of TNF-α secretion and alters the inflammatory microenvironment to allow virus propagation and persistence.
IMPORTANCE The senescence-associated secretory phenotype (SASP), an important tumorigenic process, is triggered and transmitted by inflammatory factors. The different life cycles of Epstein-Barr virus (EBV) infection in EBV-positive cells employ distinct strategies to modulate the inflammatory response and senescence. The elevation of inflammatory factors during latent EBV infection promotes the SASP in uninfected cells. In contrast, during the viral lytic cycle, BZLF1 suppresses the production of TNF-α, resulting in the attenuation of paracrine inflammatory senescence. This finding indicates that EBV evades inflammatory senescence during lytic infection and switches from facilitating tumor-promoting SASP to generating a virus-propagating microenvironment, thereby facilitating viral spread in EBV-associated diseases.
INTRODUCTION
Cellular senescence, an irreversible arrest of the cell cycle with major hallmarks of senescence-associated heterochromatic foci and DNA segments, is induced by genotoxic or oncogenic stress (1, 2). Oncogene-induced senescence (OIS) is triggered by excessive expression of oncogenes or oncogene-induced replicative stress and acts as an efficient barrier against malignancy (3, 4). However, tumors develop ways to evade OIS during early tumorigenesis (5). Interestingly, senescent cells also secrete proinflammatory factors that are important for tumor progression; this phenotype is called the senescence-associated secretory phenotype (SASP) (6). Recent studies have shown that inflammatory responses trigger and transmit cellular senescence to neighboring cells (7–9), indicating that profound cross talk and signal integration occur between senescent cells and the inflammatory microenvironment and that this communication may promote either tumor progression or suppression.
Herpesviruses produce few transcripts during latent infection. In contrast, during lytic infection, transcripts of the entire herpesvirus genome are produced and cellular machinery and multiple signaling pathways are exploited to facilitate replication and spread (10–12). Host defenses against viral infection include the activation of innate immune and inflammatory responses; however, herpesviruses employ multiple strategies and multiple viral products to evade host defenses (13–16). In addition to being involved in antiviral defenses during acute infection, inflammatory factors are also involved in the progression of persistent infection, cancers, and other inflammatory disorders (10, 17–19).
Studies have identified several inflammatory factors involved in infectious diseases caused by Epstein-Barr virus (EBV) infection that are mediated by both lytic and latent viral gene products (20–25). Levels of these inflammatory factors are elevated during EBV infection, and they elicit chronic inflammation, which leads to persistent EBV infection and disease (26, 27). Multiple oncogenes and immunomodulatory proteins encoded by EBV are involved in immune evasion and inflammation (13, 18). However, the expression levels of EBV oncogenes and the DNA damage response vary with the switch between latency and lytic infection (28, 29). In addition, the time course and function of autocrine and paracrine inflammatory factors in the latency and lytic replication remain elusive. It is also unknown whether neighboring cells and their microenvironments are influenced by inflammatory responses induced by either latent or lytic EBV infection.
Latent EBV infection immortalizes primary B cells and epithelial cells in part through the evasion of senescence (30, 31). In contrast, lytic infection causes cell cycle arrest and senescence via the expression of lytic viral proteins (32–34). However, paracrine senescence during latent and lytic EBV infection remains poorly understood. Recently we revealed that BZLF1 inhibited the expression of the proinflammatory factors tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) and consequently facilitated EBV lytic replication (35). In the present study, we demonstrate that lytic EBV infection attenuates the transmission of paracrine senescence of EBV-positive cells via a reduction in proinflammatory TNF-α secretion due to BZLF1. Consequently, the levels of inflammatory SASP and oxidative stress decrease in neighboring cells, indicating that lytic EBV replication induces a switch from a tumor-promoting to a virus-propagating microenvironment.
MATERIALS AND METHODS
Cells and antibodies.
EBV-negative Akata cells and EBV-positive P3HR-1 and Akata(+) cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin). Human nasopharyngeal carcinoma (NPC) CNE1 cells were cultured in RPMI 1640 supplemented with 5% FBS and antibiotics. Primary human tonsil epithelial cells (HTEpiCs [catalog no. 2560]) were purchased from ScienCell Research Laboratories, Carlsbad, CA, and maintained in tonsil epithelial cell medium (TEpiCM [catalog no. 2561]) containing growth factors (TepiCGs) and antibiotics. Antihemagglutinin (anti-HA), anti-poly(ADP-ribose) polymerase (anti-PARP), anti-p21CIP1, and anti-p16INK4a antibodies and recombinant human TNF-α and IFN-γ proteins were purchased from Cell Signaling Technology, Inc., Beverly, MA. Anti-BZLF1, anti-Ea-D (BMRF1), anti-VCA (viral capsid antigen), and anti-EBNA1 antibodies were purchased from Santa Cruz Biotechnology, Inc., Dallas, TX. Anti-p27Kip1, anti-cyclin D1, anti-cyclin E, anti-cyclin-dependent kinase 4 (anti-CDK4), and anti-CDK6 antibodies were purchased from ABclonal, Inc., College Park, MD. 12-O-Tetradecanoylphorbol-13-acetate (TPA), sodium butyrate (NaB), N-acetyl-l-cysteine (NAC), reduced glutathione (GSH), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and anti-β-actin were purchased from Sigma-Aldrich, Inc., St. Louis, MO. TNF-α, interleukin-6 (IL-6), and IL-8 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems, Inc., Minneapolis, MN. Fluorescein di-β-d-galactopyranoside (FDG), a membrane-permeable probe of β-galactosidase (β-Gal), was purchased from AAT Bioquest, Inc., Sunnyvale, CA (catalog no. 14001). Catalase (catalog no. S0082) was purchased from Beyotime Biotechnology, Shanghai, China. Anti-LMP2A antibody was a gift from Musheng Zeng's lab (Cancer Centre of Sun Yat-Sen University).
Plasmids and EBV BAC DNA.
Wild-type BZLF1, transcription domain deletion mutant BZLF1ΔTA, and mutant BZLF1Δ207-210 constructs were constructed and subcloned into pLXRN lentiviral vectors (Clontech Laboratories, Mountain View, CA), and TNF-α short hairpin RNA (shRNA) was subcloned into pLKO.1 lentiviral vectors as previously described (35). BRLF1-KO and BZLF1-KO EBV bacterial artificial chromosome (BAC) DNAs were a kind gift from Henri-Jacques Delecluse at the German Cancer Research Centre (DKFZ), Heidelberg, Germany, and have been previously described (36, 37). EBV BAC genomic DNA was nucleofected into Akata cells, and BAC-harboring cells were selected as described previously (35).
Lentivirus packaging and infection.
GP2-293 cells seeded in 10-cm dishes were cotransfected with 15 μg of the pLXRN lentiviral expression vector and 5 μg of pCMV-VSV-G. The supernatants were harvested 72 h posttransfection and concentrated by ultracentrifugation at 100,000 × g for 1 h to prepare 100-fold viral stocks. After preliminary tests and titration, lentiviral infections were performed following standard procedures.
Real-time PCR.
Total RNA was extracted, reverse transcribed to cDNA, and assayed by real-time SYBR green PCR. The primers for real-time PCR were designed using Primerbank (38); the sequences of the primer pairs are available upon request.
Conditioned medium culture.
Cultures containing 2 × 105/ml P3HR-1 cells were infected with wild-type or mutant BZLF1-expressing lentiviruses at a multiplicity of infection (MOI) of 50 in the presence of 4 μg/ml Polybrene. After 8 h, the lentivirus was removed, and the cells were washed twice with phosphate buffer and then incubated with fresh medium for an additional 24 h. The supernatants were then collected, filtered, and stored as conditioned medium. For CNE1 cells, the medium in conditioned medium cultures was replaced every 2 days. For HTEpiCs, the supernatants were mixed with 50% (vol/vol) TEpiCM without TepiCGs, and the medium was refreshed every 2 days.
Senescent β-Gal staining.
After incubation for 10 or 8 days, conditioned medium-cultured HTEpiCs or CNE1 cells, respectively, were washed with phosphate buffer and then fixed in fixing buffer (containing 4% paraformaldehyde, 1% glutaraldehyde, 0.01% NP-40, and 0.01% sodium deoxycholate) for 15 min and washed once with cold phosphate buffer. β-Gal activity was detected in staining buffer as described previously (39). After 48 to 72 h, the cells were washed twice with distilled water and visualized using an inverted microscope.
Senescent cell flow cytometry.
Detection of senescent cells using flow cytometry was performed as previously described (40). Briefly, 1 × 106 cells were digested, washed twice with phosphate-buffered saline (PBS), and resuspended with 200 μl of ice-cold PBS for 10 min. Then, 5 μl of 20 mM FDG was added, the mixture was incubated at 37°C for 1 min, and then 1.8 ml of cold PBS was immediately added to the cells. The cells were left on ice for an additional 1 h and then analyzed using the BD LSRFortessa fluorescence-activated cell sorter (FACS) system (BD Bioscience) at a 488-nm wavelength.
Antibody neutralization.
The conditioned medium was incubated with 10 μg/ml anti-TNF-α (catalog no. 7321S, rabbit monoclonal antibody [MAb]; Cell Signaling Technology) and/or anti-IFN-γ (catalog no. MAB286, mouse IgG2A; R&D Systems) neutralizing antibodies or an isotype-matched control IgG (eBioscience) for 1 h at 37°C before being directly added to the HTEpiCs. The pretreated medium containing neutralizing antibody was refreshed every 2 days.
Detection of oxidative agents.
DCFH-DA, a cell-permeable probe of reactive oxygen species (ROS), was used to detect endogenous ROS. Briefly, DCFH-DA was diluted with serum-free RPMI 1640 to a final concentration of 20 μM. Cells were washed twice with serum-free RPMI 1640 and then incubated with DCFH-DA for 20 min in 37°C. After incubation, the supernatants were discarded, and the cells were washed twice. Fluorescent intensity was analyzed using a TriStar2 multimode reader (Berthold) with an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The activities of NADH oxidase and malondialdehyde (MDA) were measured by following the standard procedure using the commercial kits purchased from Comin Biotechnology Co., Ltd. (catalog no. NOX-2-Y), and the Beyotime Institute of Biotechnology (catalog no. S0131), respectively.
EBV infection.
Human primary tonsil epithelial cells were infected with purified EBV virion at a very high MOI (≥1,000) by following a standard protocol as described previously (41). EBV infection in CNE1 cells was performed through coculture with lytically infected P3HR1 cells as described previously (42). Briefly, lytic replication of P3HR-1 cells was induced by treatment with 20 nM TPA and 0.3 mM sodium butyrate for 3 days. Following this treatment, cells were washed twice with fresh RPMI 1640 and added to CNE1 cells in a 12-well plate (5 × 105 cells/well). After 2 days, the supernatants and the floating cells were discarded. The CNE1 cells were then washed twice and cultured in RPMI 1640 medium for further analysis.
Statistical analysis.
Statistical analysis was performed using SPSS 13.0. The following methods were used for data analysis: one-way analysis of variance (ANOVA), Bonferroni's correction method, chi-square test, ANOVA for replicate measures, and linear correlation analysis.
RESULTS
Different paracrine senescence during latent and lytic EBV infections.
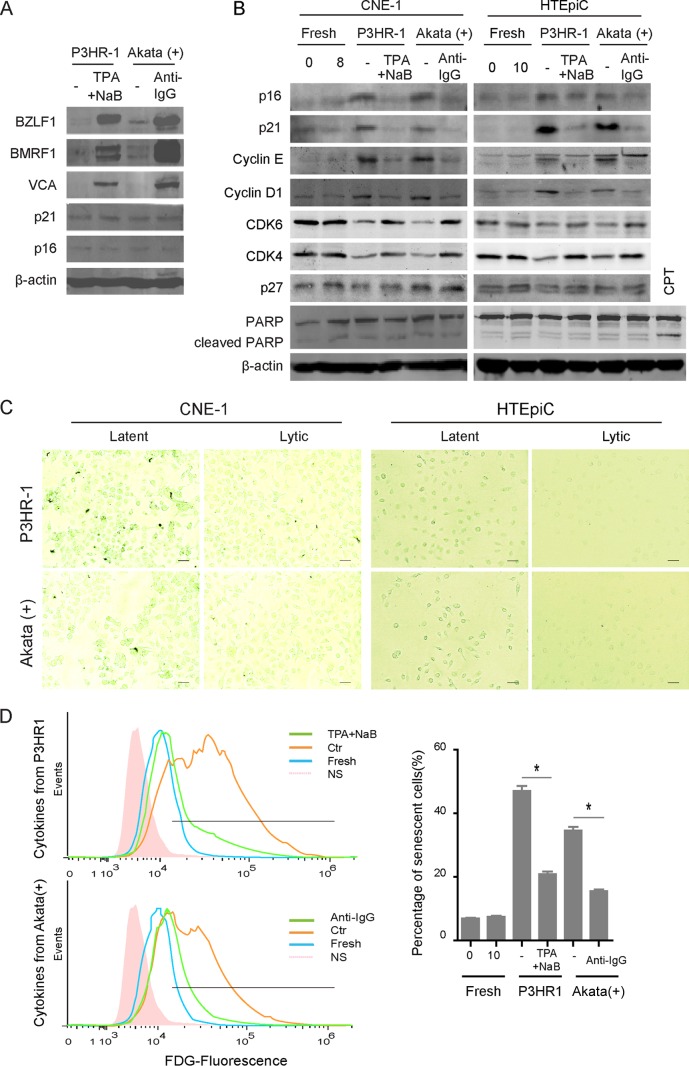
Distinct patterns of expression of EBV oncogenes and of EBV DNA replication during the switch from latency to lytic replication presumably result in different extents of oncogenic or replicative stress, followed by cellular senescence. To investigate autocrine and paracrine senescence during latent and lytic EBV infections, the levels of intrinsic and paracrine-induced expression of the senescence markers p16INK4a and p21CIP1 were measured in EBV-positive lymphoma cells or uninfected cells incubated with cytokines from EBV-infected cells, respectively. Elevated expression of p16INK4a and p21CIP1 was observed neither in latently infected P3HR-1 or Akata(+) cells nor in TPA-induced P3HR-1 or anti-IgG-induced Akata(+) cells, suggesting that intrinsic senescence is not induced by different EBV infections per se (Fig. 1A). Interestingly, the expression of p16INK4a and p21CIP1 was significantly increased in both CNE1 cells and HTEpiCs incubated with cytokines from latently EBV-infected cells but not in cells incubated with cytokines from lytically infected cells (Fig. 1B). The cell cycle regulators cyclin D1 and cyclin E were also induced in both epithelial cells by cytokines from latently EBV-infected cells but not by cytokines from lytically infected cells, and the levels of expression of positive regulators CDK4 and CDK6 were oppositely changed; however, the expression of p27Kip1 was unaffected. The pattern of PARP expression was not changed, and no cleavage of PARP was observed in any cells, compared with that in apoptotic cells induced by camptothecin (Fig. 1B). These changes indicated that cellular senescence, but not apoptosis, occurred in both groups of conditioned medium-cultured epithelial cells. Senescence-associated β-Gal staining was further used to visualize senescent cells in CNE1 and HTEpiCs incubated with cytokines from EBV-infected cells. A high proportion of senescent cells was observed among cells cultured with conditioned medium containing cytokines from both P3HR-1 and Akata(+) cells with latent EBV infection, whereas the level of senescent cells was much lower among cells cultured with conditioned medium containing cytokines from cells with lytic EBV infections (Fig. 1C). The senescent cells were measured by FDG fluorescent flow cytometry, and approximately 5-fold or 4-fold more senescent cells were present among HTEpiCs incubated with cytokines from latently infected P3HR-1 or Akata(+) cells, respectively. In contrast, no more than a 2.5- or 2-fold increase in the percentage of senescent cells was observed among cells incubated with cytokines from the two lytic cell types, respectively, compared with the basal percentage of senescent cells under normal culture with fresh media (Fig. 1D). These results suggested that latently EBV-infected cells transmit senescence to neighboring cells through cytokines, but lytic cells greatly abate it, indicating that EBV lytic replication switches the secretion of cytokines from a senescence-inducing to a senescence-attenuating phenotype.
FIG 1.
Different patterns of paracrine senescence were observed during latent and lytic EBV infections. (A) EBV-positive P3HR-1 or Akata(+) cells were left uninduced or induced with 20 ng/ml TPA plus 0.3 mM NaB or 0.8% (vol/vol) anti-IgG for 24 h, respectively. The cells were collected, lysed, and analyzed by Western blotting as indicated. (B) Twenty-four hours later, the cells were washed twice and incubated with fresh medium for additional 24 h, and the cytokines secreted from uninduced (latent) or induced (lytic) P3HR-1 or Akata(+) cells were collected and used to generate conditioned media for NPC epithelial cells (CNE1) and primary epithelial cells (HTEpiCs), as indicated. After 8 or 10 days of culture with conditioned media, CNE1 and HTEpiCs were collected and analyzed using Western blotting. CPT, camptothecin. (C) The CNE1 cells and HTEpiCs were fixed, and senescent cells were detected with senescent β-Gal staining. Representative images under 200-fold magnification are shown, and the size bars represent 100 μm under microscopy. (D) After 10 days of culture with conditioned media, the HTEpiCs were digested, stained using an FDG probe, and measured using fluorescence flow cytometry. Representative images are shown (left), and the percentage of senescent cells was calculated from three independent experiments (right). NS, no-FDG-staining control. *, P < 0.01.
EBV lytic infection alleviates senescence in neighboring cells.
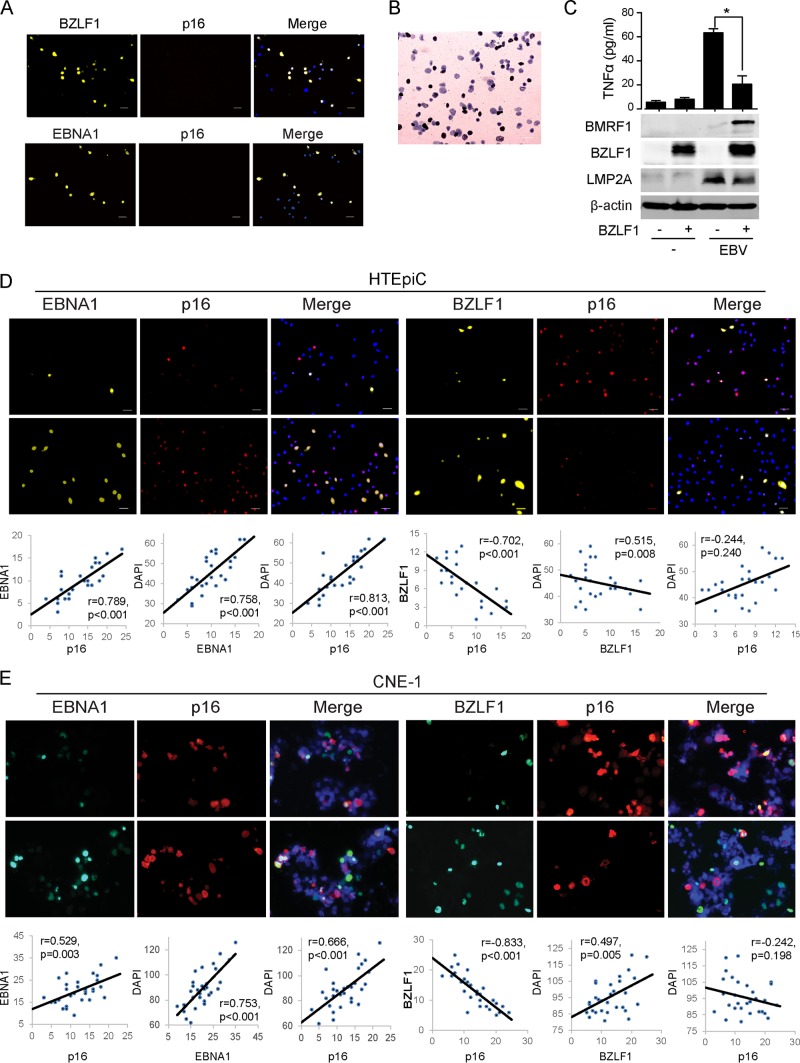
To investigate the transmission of inflammatory senescence by naturally EBV-infected cells, we observed senescence during primary EBV infection. A very high MOI and cell-to-cell contact are two efficient approaches for high efficiency of EBV infection in human epithelial cells (41, 42). HTEpiCs and CNE1 cells were infected by a high titer of purified virion (MOI of 1,000) or by coculture with lytic EBV-infected P3HR-1 cells, respectively. The efficiency of EBV infection varied from approximately 15% to 30%, as determined by in situ hybridization using an Epstein-Barr virus-encoded RNA (EBER) probe (Fig. 2B) and the EBNA1/DAPI (4′,6-diamidino-2-phenylindole) ratio (Fig. 2D and E), and 5 to 20% of the cells exhibited EBV lytic infection, as determined by the BZLF1/DAPI ratio (Fig. 2D and E). As expected, TNF-α production was induced by EBV infection, and BZLF1-mediated lytic replication inhibited TNF-α production (Fig. 2C). The senescence marker p16INK4a was rarely observed in EBNA1- or BZLF1-positive cells, but it was often detected in uninfected neighboring cells, indicating that EBV infection induces senescence in neighboring cells. A positive correlation between EBNA1- and p16INK4a-positive cell counts was observed (P < 0.01) (Fig. 2C and D, left panels). When EBNA1- and p16INK4a-positive cell counts were compared with total numbers of cells (labeled with DAPI), this correlation was positive. In contrast, a significant negative correlation (P < 0.001) was detected between BZLF1- and p16INK4a-positive cell counts during EBV lytic infection (Fig. 2C and D, right panels), and p16INK4a-positive cell counts were not related to cell number (P = 0.240 in HTEpiCs and P = 0.198 in CNE1 cells). However, neither ectopic EBNA1 nor BZLF1 expression alone was correlated with senescence in uninfected HTEpiCs or neighboring cells (Fig. 2A). These data indicate that the latent and lytic EBV life cycles induce distinct phenotypes of paracrine senescence in uninfected neighboring cells during primary infection in epithelial cells.
FIG 2.
EBV lytic infection reduced paracrine senescence during primary infection. (A) HTEpiCs were infected with BZLF1- or EBNA1-expressing lentiviruses and further cultured for an additional 10 days. Their expression and senescent marker p16INK4a were detected using immunofluorescent staining with anti-EBNA1, anti-BZLF1, and anti-p16INK4a antibodies. (B to D) HTEpiCs were infected with purified EBV virion (MOI of 1,000) and cultured for additional 10 days in alpha minimal essential medium (α-MEM) containing 0.9% methyl cellulose. (B) The EBV-positive cells were stained using in situ hybridization with an EBER probe. Black indicates the EBV-infected cells, and lavender indicates EBV-negative cells. (C) EBV-infected HTEpiCs were transfected with vector or BZLF1 for 2 days, and TNF-α production was detected using an ELISA. (E) CNE1 cells were infected with EBV by coculture with lytic P3HR-1 cells for 2 days, after which CNE1 cells were maintained for an additional 8 days in RPMI 1640 medium (containing 0.9% methyl cellulose). (D and E) Latent EBV infection, lytic replication, and cellular senescence were detected by immunofluorescent staining with anti-EBNA1, anti-BZLF1, and anti-p16INK4a antibodies, respectively. Two representative images with high or low expression of EBNA1 or BZLF1 are shown. Cells positive for EBNA1, BZLF1, or p16INK4a from 25 random fields were recorded, and linear correlations were calculated. Linear correlation analysis showed that EBNA1 and p16INK4a cell counts (first panel) were correlated, as were BZLF1 and p16INK4a cell counts (fourth panel). The results of correlation analyses for cell counts are also shown for EBNA1 and DAPI (second panel), BZLF1 and DAPI (fifth panel), and p16INK4a and DAPI (third and sixth panels). r, correlation coefficient; p, probability value.
BZLF1 attenuates the transmission of paracrine senescence.
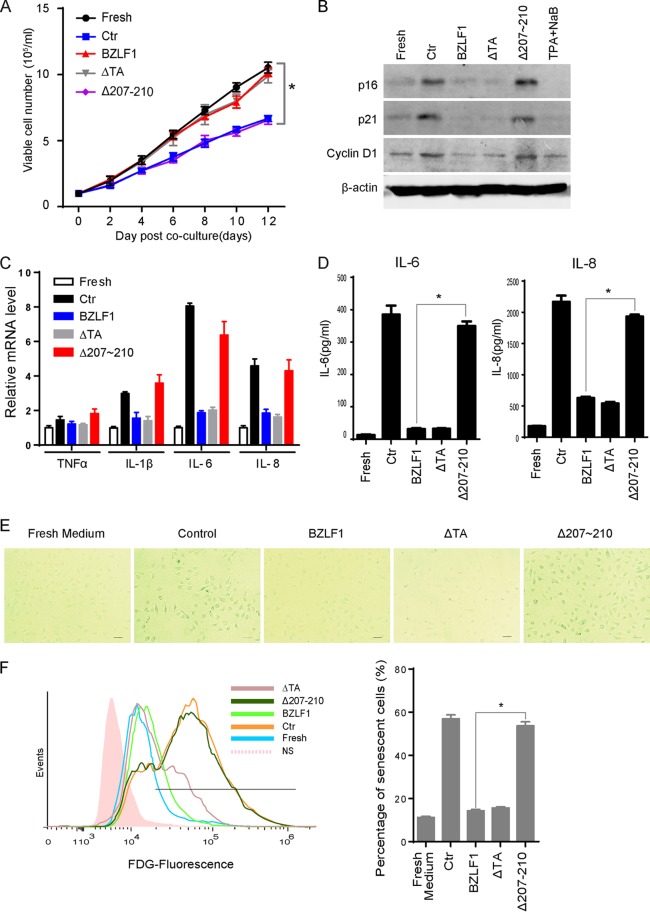
BZLF1, the EBV-encoded immediate early (IE) viral transactivator, initiates lytic replication during primary infection or reactivation from latency. BZLF1 exerts multiple functions through its different functional domains (43). We have developed a BZLF1Δ207-210 mutant with a deletion of 4 amino acids in the protein-protein binding domain, resulting in loss of the ability to inhibit TNF-α and IFN-γ production while maintaining normal transcriptional activity in EBV lytic gene expression (35). When HTEpiCs were cultured in conditioned medium containing cytokines secreted from control, BZLF1-, BZLF1ΔTA-, or BZLF1Δ207-210-transduced P3HR-1 cells, the growth of HTEpiCs was minimally affected by the presence of cytokines from lytic P3HR-1 cells expressing BZLF1. In contrast, cytokines from latent P3HR-1 cells significantly reduced HTEpiC growth (Fig. 3A). Interestingly, cell growth was dramatically suppressed when cells were cultured in conditioned medium containing cytokines from BZLF1Δ207-210-transduced P3HR-1 cells in which the lytic EBV cycle was reactivated. Cytokines from BZLF1ΔTA-transduced P3HR-1 cells failed to inhibit cell growth even though the EBV infection in these cells was latent, indicating that BZLF1, not other lytic viral proteins, favors the growth of epithelial cells in a paracrine manner. To investigate the role of BZLF1 in paracrine senescence during EBV infection, HTEpiCs were cultured for 10 days in conditioned medium in the presence of cytokines secreted from P3HR-1 cells expressing the control, BZLF1, BZLF1ΔTA, or BZLF1Δ207-210. Levels of the senescence markers p21CIP1, p16INK4a, and cyclin D1 were increased when cells were cultured with cytokines from control and BZLF1Δ207-210-transduced P3HR-1 cells but did not change when HTEpiCs were cultured in the presence of cytokines from BZLF1- and BZLF1ΔTA-transduced P3HR-1 cells (Fig. 3B). The expression of the senescence-associated inflammatory factors IL-1β, IL-6, and IL-8 was greatly elevated in HTEpiCs cultured in conditioned medium containing cytokines from BZLF1Δ207-210-transduced P3HR-1 cells, as well as cytokines from control P3HR-1 cells, compared with cytokines from wild-type BZLF1- and BZLF1ΔTA-transduced cells, whereas expression of TNF-α was unchanged (Fig. 3C). The secretion of IL-6 and IL-8 from HTEpiCs was similarly changed during conditioned medium culture (Fig. 3D). Furthermore, enhanced activity of senescence-associated-β galactosidase was observed in HTEpiCs cultured in conditioned medium containing cytokines from both control and BZLF1Δ207-210-transduced P3HR-1 cells compared with that from wild-type BZLF1- or BZLF1ΔTA-transduced cells (Fig. 3E). Transduction with BZLF1 or BZLF1ΔTA reduced the percentage of senescent cells to basal levels under normal conditions, whereas high percentages of senescent cells were induced by cytokines from BZLF1Δ207-210-transduced P3HR-1 cells, similar to those induced by cytokines from latently infected P3HR-1 cells transfected with the control vector (Fig. 3F). Because BZLF1ΔTA, which did not activate viral lytic transcription, inhibited cellular senescence to the same extent as BZLF1, the inhibitory function of paracrine senescence was considered to be independent of the transcription of lytic viral products. These results indicate that BZLF1 suppresses paracrine senescence independent of its transcriptional activity and of EBV lytic replication. Thus, we concluded that cells with latent EBV infections induce SASP in neighboring cells and that BZLF1 attenuates paracrine senescence during lytic replication.
FIG 3.
BZLF1 blocked paracrine-mediated senescence and SASP. (A) Different cell growth was mediated by cytokines from P3HR-1 cells expressing BZLF1 or different mutants. HTEpiCs were seeded at low density (1 × 104/ml) and then cultured with conditioned medium from control P3HR-1 cells or P3HR-1 cells overexpressing BZLF1, BZLF1ΔTA, or BZLF1Δ207-210. The conditioned medium was replaced every 2 days, and the living cells were digested and counted after trypan blue staining at different time points as indicated; the growth curves were recorded as the means of 5 random fields from three independent samples. Ctr, control. *, P < 0.01. (B) The cells were collected at 10 days after conditioned medium culture. p16INK4a, p21CIP1, and cyclin D1 expression levels were examined by Western blotting. (C) After 3 days of culture with conditioned medium, the cells were collected, and total RNA was extracted and analyzed by real-time PCR to detect expression of TNF-α, IL-1β, IL-6, and IL-8. (D) These cells were incubated with fresh FBS-free medium for 4 h, and then the titers of IL-6 and IL-8 in the medium were measured using ELISA kits. (E) After conditioned medium culture for 10 days, the cells were fixed, and β-Gal staining was used to detect activity of senescence-associated β-galactosidase. Representative images (200-fold magnification) are shown; the size bars represent 100 μm under light microscopy. (F) The senescent cells were determined using flow cytometry with FDG fluorescent staining, and the percentage of senescent cells was calculated from three independent experiments. *, P < 0.01.
BZLF1 inhibits paracrine senescence in BZLF1-KO or BRLF1-KO EBV-infected cells.
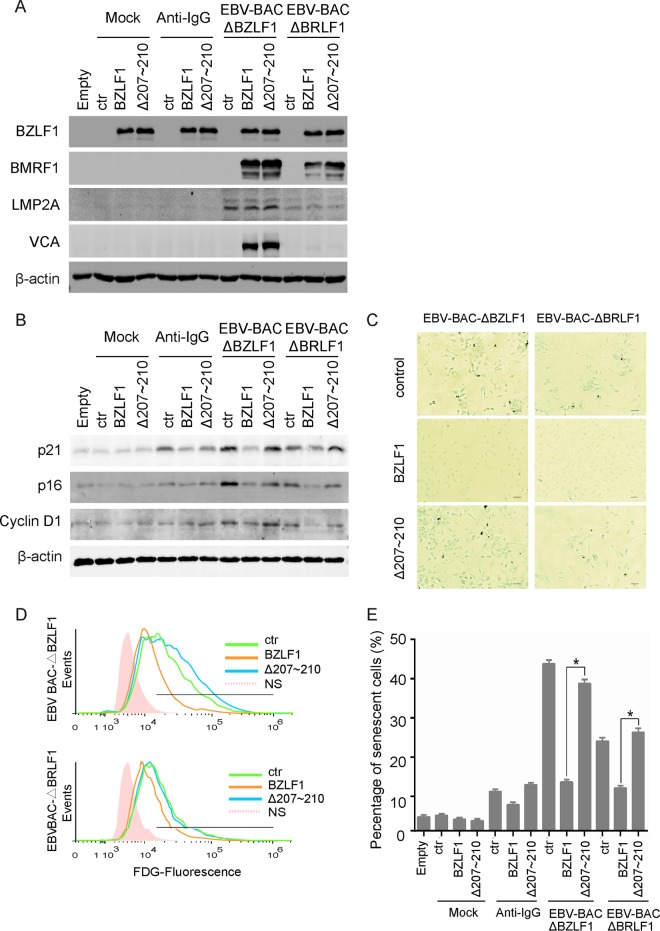
To exclude the effect of endogenous BZLF1 expression and to further investigate BZLF1 inhibition of paracrine senescence, BZLF1-KO or BRLF1-KO EBV BAC-harboring cells were established in EBV-negative Akata cells. More than 90% of cells were transduced with BZLF1 or BZLF1Δ207-210 when the cells were infected with BZLF1- or BZLF1Δ207-210-expressing lentiviruses at an MOI of >10, as described previously (35). A comparable level of ectopic BZLF1 expression was observed in EBV-negative cells and EBV BAC-harboring cells, followed by the induction of early gene BMRF1 expression in two types of EBV BAC-harboring cells and late gene VCA expression in BZLF1-KO cells but not in BRLF1-KO EBV BAC-harboring cells (Fig. 4A). The cytokines from EBV-negative Akata cells did not induce p16INK4a and cyclin D1 expression and paracrine senescence in HTEpiCs, though they did induce p21CIP1 expression after anti-IgG stimulation. In contrast, the cytokines from both types of EBV-infected cells induced p16INK4a and cyclin D1 expression and cellular senescence in epithelial cells (Fig. 4B and E), indicating that EBV-infected cells, but not uninfected cells, induced paracrine senescence. Notably, BZLF1 inhibits the induction of cyclin D1, p16INK4a, and p21CIP1 expression and the activity of senescence-associated β-Gal in both BZLF1-KO and BRLF1-KO EBV BAC-harboring cells, while BZLF1Δ207-210 completely lost the inhibition of senescent induction (Fig. 4B and C). The senescent cells were measured using FDG fluorescent flow cytometry, and the percentage of senescent cells was greatly decreased by BZLF1 expression, equal to the basal level during anti-IgG-stimulated Akata cell activation that only slightly increased senescence, whereas the percentage was unaffected by BZLF1Δ207-210 expression (Fig. 4D and E). Additionally, the ability of BRLF1-KO EBV BAC-harboring cells to induce senescence was slightly lower than that of BZLF1-KO EBV BAC-harboring cells, BRLF1-KO likely affected the viral genomic DNA load inside the cells and consequently reduced the expression of latent genes such as the gene coding for LMP2A (Fig. 4A). Because endogenous BZLF1 expression was abolished in BZLF1-KO EBV BAC-harboring cells and lytic replication was disrupted in BRLF1-KO EBV BAC-harboring cells, the inhibition of senescence induction in both cells by BZLF1 indicates that BZLF1 itself is sufficient to inhibit SASP and paracrine senescence during EBV infection.
FIG 4.
BZLF1 suppressed paracrine senescence in EBV BAC-harboring cells. (A) EBV-negative Akata cells were transfected with EBV-BAC-ΔBZLF1 and EBV-BAC-ΔBRLF1 genomic DNA and selected with 50 μg/ml hygromycin for 2 weeks. Akata, Akata-ΔBZLF1, and Akata-ΔBRLF1 cells were transduced with a lentivirus-based control vector, BZLF1, or BZLF1Δ207-210 for 24 h, and then Akata cells were left untreated or were treated with 0.8% (vol/vol) anti-IgG for 24 h. Cells were collected, and the cell extracts were subjected to Western blots as indicated. (B to E) HTEpiCs were conditionally cultured with the cytokines from different Akata cells as indicated. (B) Expression of p16INK4a, p21CIP1, and cyclin D1 was detected using Western blot analysis. (C) Senescent cells were detected using senescent β-Gal staining. (D) The senescent cells were determined using FDG fluorescent flow cytometry. (E) The percentage of senescent cells from three independent experiments is shown. *, P < 0.01.
TNF-α induces senescence in epithelial cells.
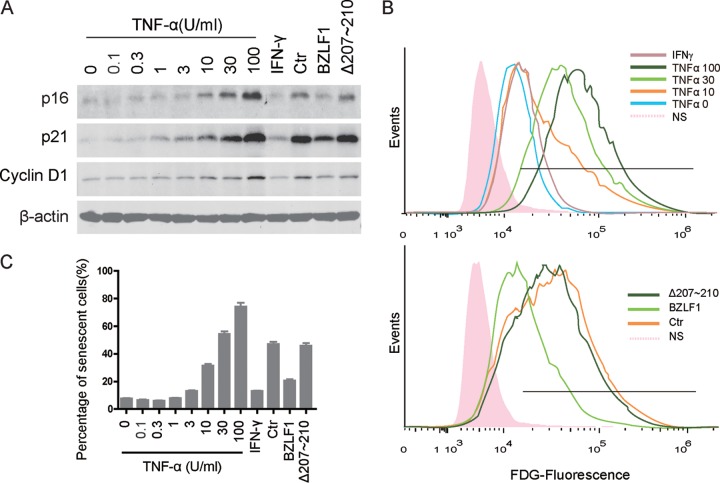
Previous studies have demonstrated that TNF-α production is elevated in EBV-infected cells (20–25, 44); in contrast, TNF-α and IFN-γ production was rapidly reduced during early EBV reactivation to facilitate optimal lytic replication (35). Because of the unique functions of inflammatory factors in the induction of the SASP phenotype, we hypothesized that TNF-α and IFN-γ have distinct roles in EBV-mediated paracrine senescence. To determine the roles of both proinflammatory factors in cellular senescence, recombinant human TNF-α or IFN-γ protein was added to HTEpiCs. Expression of cyclin D1, p16INK4a, and p21CIP1 was induced by TNF-α followed by an increased concentration, while a high titer of IFN-γ was incapable of inducing senescence markers (Fig. 5A). The percentage of senescent cells was elevated by different amounts of TNF-α, with a maximal percentage of approximately 80%, while IFN-γ barely increased the percentage of senescent cells (Fig. 5B and C). Compared to the induction of senescent cells by TNF-α, cytokines from cells with BZLF1Δ207-210-induced lytic EBV infection and from latently infected cells induced senescent cells equally to induction with approximately 25 U/ml TNF-α, while BZLF1 decreased senescence to the effect of induction with approximately 5 U/ml TNF-α (Fig. 5C). These results suggest that TNF-α is capable of inducing senescence in epithelial cells.
FIG 5.
TNF-α-induced senescence in epithelial cells. (A) HTEpiCs were cultured with fresh α-MEM containing different amounts of TNF-α or IFN-γ, as well as the cytokines from P3HR-1 cells expressing the control, BZLF1, or BZLF1Δ207-210. After culture for 10 days, the expression of p16INK4a, p21CIP1, and cyclin D1 was examined using Western blots (A), and the senescent cells were determined using fluorescent FDG-flow cytometry (B). (C) The percentage of senescent cells was calculated from three independent experiments.
Blockade of TNF-α abolishes paracrine senescence during BZLF1Δ207-210-mediated lytic replication.
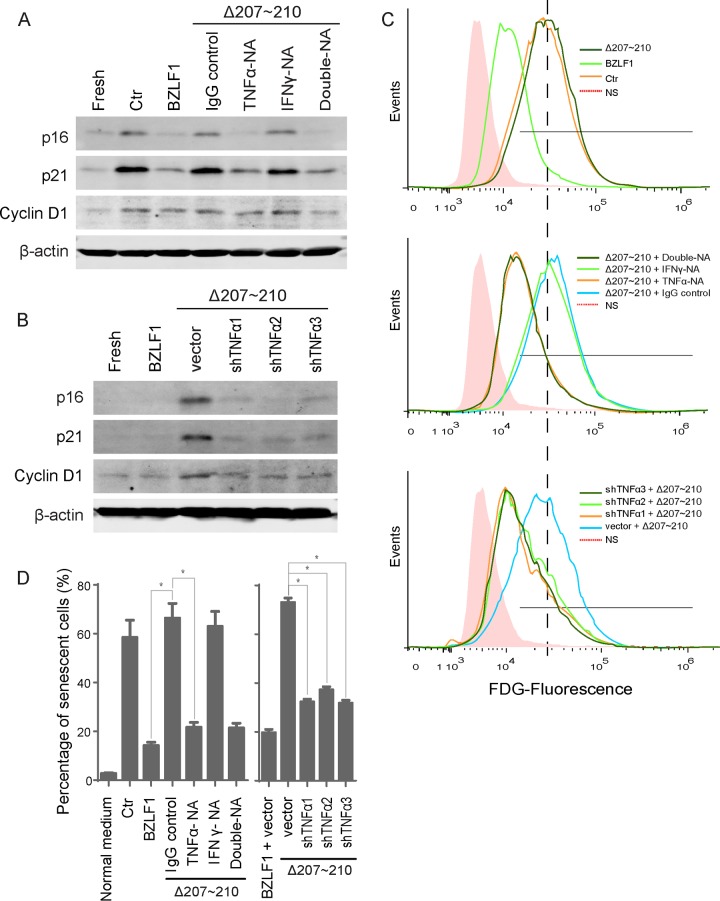
We have shown that BZLF1 significantly impaired TNF-α and IFN-γ production during the immediate early (IE) and early lytic stages, while BZLF1Δ207-210 showed minimal inhibition (35). To confirm that TNF-α is responsible for the senescence induced by cytokines derived from cells expressing BZLF1Δ207-210, anti-TNF-α and anti-IFN-γ neutralizing antibodies were used to deplete cytokines. Incubation with the anti-TNF-α neutralizing antibody alone inhibited the induction of cyclin D1, p16INK4a, and p21CIP1 expression in HTEpiCs cultured in conditioned medium containing cytokines derived from cells expressing BZLF1Δ207-210 (Fig. 6A). Remarkably, the anti-IFN-γ neutralizing antibody barely alleviated this induction, and the combination of both neutralizing antibodies resulted in inhibition of cyclin D1, p16INK4a, and p21CIP1 expression to levels similar to those observed with anti-TNF-α neutralizing antibody alone. The percentage of senescent cells clearly demonstrated that BZLF1- and BZLF1Δ207-210-derived cytokines resulted in low and high levels of cellular senescence, respectively. Anti-TNF-α neutralizing antibody alone reduced the induction of senescence by BZLF1Δ207-210 from 67% to 21.8%, and anti-IFN-γ neutralizing antibody resulted in minimal inhibition. The combination of neutralizing antibodies resulted in comparable inhibitory activity similar to that observed with anti-TNF-α alone and very near to that with BZLF1 transduction (Fig. 6C and D), indicating that TNF-α was mainly responsible for the induction of senescence by cytokines from BZLF1Δ207-210-transduced P3HR-1 cells. Furthermore, depletion of TNF-α by shRNA reduced the expression of three senescence markers in HTEpiCs incubated with cytokines from BZLF1Δ207-210-transduced P3HR-1 cells (Fig. 6B), and the percentage of senescent cells was reduced by TNF-α shRNA to half of their previous level (Fig. 6C and D). We therefore concluded that BZLF1 inhibits paracrine inflammatory senescence primarily through attenuation of TNF-α secretion.
FIG 6.
TNF-α depletion ablated paracrine senescence. (A) Conditioned medium from P3HR-1 cells was neutralized with the anti-TNF-α or anti-IFN-γ antibody or control IgG before the medium was added to HTEpiCs. (B) Conditioned medium from P3HR-1 cells expressing TNF-α shRNAs was added to HTEpiCs, and the medium containing neutralizing antibodies was refreshed every 2 days. (A and B) The expression of cyclin D1, p21CIP1, and p16INK4a was examined after 10 days. (C) Representative images of fluorescent FDG-flow cytometry are shown. (D) The percentage of senescent cells from three independent experiments is shown. *, P < 0.01. NA, neutralizing antibody.
ROS scavenger alleviates paracrine senescence during BZLF1Δ207-210-mediated lytic replication.
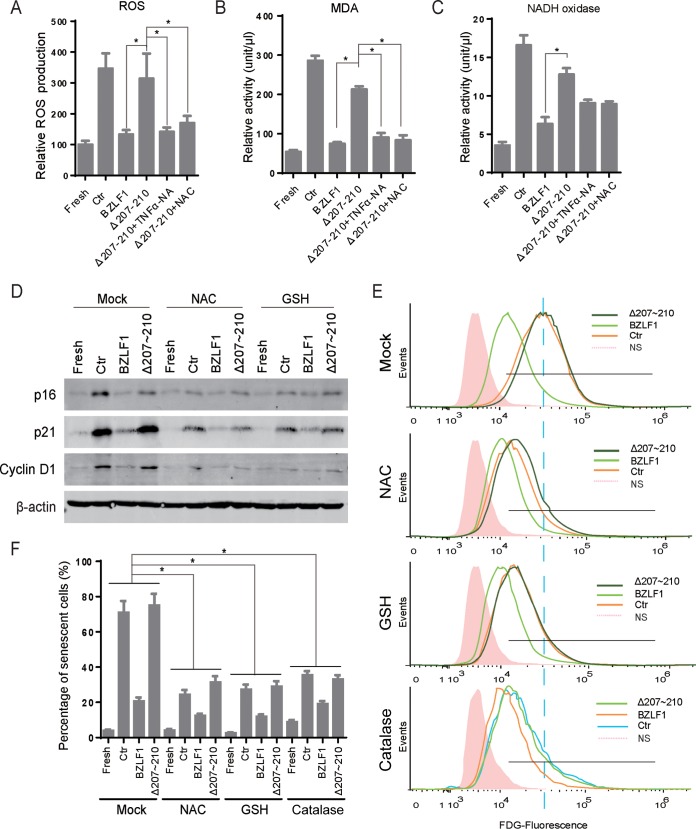
Oxidative stress is thought to be the factor responsible for cellular senescence, and the inactivation of reactive oxygen species (ROS) compromises OIS or replicative senescence (45–47). To further investigate paracrine senescence induced by EBV-infected cells, we measured the ROS and MDA in conditioned medium-cultured HTEpiCs in the presence of cytokines from P3HR-1 cells expressing different BZLF1 constructs. Elevated ROS and MDA production was induced by the addition of cytokines from cells with BZLF1Δ207-210-induced lytic EBV infection as well as from latently infected cells; however, this production was not increased by cytokines from BZLF1-transduced cells (Fig. 7A and B). Interestingly, ROS and MDA production was abolished when TNF-α was depleted by neutralizing antibodies as well as in the presence of the ROS scavenger NAC (Fig. 7A and B), suggesting that BZLF1 attenuates the induction of ROS production in neighboring cells primarily by inhibiting TNF-α secretion. One of the oxidase enzymes, NAD(P)H oxidase, which catalyzes the production of superoxide, was determined, and its activity was similarly altered following the production of reactive free radicals (Fig. 7C). Furthermore, the levels of expression of senescent markers cyclin D1, p16INK4a, and p21CIP1 in HTEpiCs were dramatically decreased when the ROS scavengers NAC and GSH were used to inactivate the ROS in HTEpiCs incubated with cytokines from latently infected P3HR-1 cells or from cells expressing BZLF1Δ207-210 (Fig. 7D). The percentage of senescent cells was reduced by more than half by NAC or GSH treatment, without a large reduction in the level of senescent cells in the presence of cytokines from cells expressing wild-type BZLF1 (Fig. 7E and F). Decomposition of hydrogen peroxide catalyzed by catalase also protected the cell from senescence (Fig. 7E and F). These results suggest that production of oxygen free radicals was required for paracrine senescence induced by EBV-infected cells and that BZLF1 attenuated the effects of the TNF-α–oxidative stress axis during these processes.
FIG 7.
The levels of oxidation were elevated in and required for paracrine senescence of EBV-infected cells. (A to C) HTEpiCs were incubated with the cytokines from P3HR-1 cells expressing the control, BZLF1, or BZLF1Δ207-210 in the absence or presence of anti-TNF-α neutralizing antibody or 5 mM NAC. Twenty-four hours later, ROS activity in living cells was measured using DCFH-DA (A), and MDA (B) and NADH oxidase (C) activities in cell lysates were measured with commercial kits according to the manufacturers' protocols. (D) HTEpiCs were cultured in conditioned media in the absence or presence of 5 mM NAC or 2 mM GSH for 10 days, and conditioned media containing NAC or GSH were refreshed every 2 days. The expression of cyclin D1, p21CIP1, and p16INK4a in HTEpiCs was examined. (E and F) HTEpiCs were cultured in conditioned medium in the absence or presence of 5 mM NAC, 2 mM GSH, or 500 U/ml catalase for 10 days; the medium was refreshed every 2 days. Senescent cells were determined using fluorescent FDG flow cytometry. (E) Representative images are shown. (F) The percentage of senescent cells was calculated from three independent experiments. *, P < 0.01.
DISCUSSION
During EBV infection and replication, replicative stress and the expression of multiple viral oncogenes trigger diverse inflammatory and DNA damage responses during different life cycles (28, 29), resulting in distinct patterns of replicative stress and oncogene-induced cellular senescence in EBV-harboring cells. Latent LMP1 attenuates senescence during latent EBV infection (30, 31), whereas during lytic EBV infection, BRLF1 induces intrinsic senescence in epithelial cells (32). Here, we reveal that latent EBV infection transmits inflammatory paracrine senescence to neighboring epithelial cells through TNF-α and elevated oxygen free radicals, whereas lytic EBV infection attenuates transmission via BZLF1-mediated reductions in both TNF-α secretion and consequent oxidative stress. These findings imply that both the latent and lytic EBV life cycles employ multiple ways to induce or escape cellular senescence in both EBV-infected cells and neighboring cells.
Although anti-IFN-γ neutralizing antibody exhibits a more important role in EBV lytic replication (35), IFN-γ plays a minimal role in the transmission of paracrine senescence from latently EBV-infected cells (Fig. 6). Our studies have revealed that TNF-α is responsible for the induction of paracrine senescence; it is essential for and sufficient to induce cellular senescence in epithelial cells (Fig. 5 and 6). This is consistent with the previous studies that show that TNF-α exposure induces cellular senescence in human T lymphocytes and leukemic cells as well as endothelial progenitor cells (48–50). Therefore, latently EBV-infected cells are presumably capable of transmitting cellular senescence to lymphocytes and epithelial cells and inducing SASP through high TNF-α secretion, which likely possesses a potential for the progression of chronic EBV-related diseases.
Changes in the microenvironment during EBV lytic replication result in a distinct senescent phenotype in neighboring cells. Latent EBV infection elevates the secretion of cytokines that facilitate the cancer-promoting inflammatory response and induce SASP in neighboring cells, along with the secretion of the proinflammatory factors IL-1β, IL-6, and IL-8 (Fig. 3B). However, these proinflammatory responses are not beneficial for persistent viral infection because TNF-α and IFN-γ drive the antiviral response and senescence (8, 51). Using the BZLF1ΔTA and BZLF1Δ207-210 constructs, we confirmed that the inhibition of TNF-α and inflammatory responses by BZLF1 during the EBV lytic life cycle is required to attenuate the transmission of paracrine senescence. The use of TNF-α- and IFN-γ-neutralizing antibodies revealed that BZLF1Δ207-210 is incapable of full inhibition of paracrine inflammatory senescence, indicating that the most important strategy for evasion of inflammatory senescence by BZLF1 is attenuation of both TNF-α and IFN-γ. Thus, in EBV-positive cells, latent infection promotes SASP through the secretion of cytokines onto neighboring epithelial cells; in contrast, lytic infection attenuates senescence-inducing secretion and promotes the growth of neighboring cells.
These phenotypes were confirmed during primary EBV infection. A positive linear correlation between senescent marker p16INK4a and EBNA1 expression levels reflects the induction of paracrine senescence by latent EBV infection (Fig. 2), whereas a negative linear correlation between p16INK4a and BZLF1 implies the attenuation of paracrine senescence by lytic EBV replication (Fig. 2). Consistent with the natural life cycle of EBV, the default latent EBV state, which results in the secretion of proinflammatory factors, promotes the SASP phenotype for malignancy. However, the EBV lytic life cycle suppresses and escapes from the inflammatory response and paracrine senescence to facilitate viral propagation. In addition to replicative stress and oncogene-induced senescence experienced during the EBV lytic cycle, BZLF1 and BRLF1 also induce the accumulation of p21CIP1 and G0/G1 arrest (32, 33, 52), which are components of the premature state of cellular senescence. These findings indicate that lytic EBV-replicating cells generate intrinsic senescent stress but prohibit transmitting it to neighboring cells via the inhibition of inflammatory factors by BZLF1.
Although the relationship between paracrine senescence and the progression of EBV-mediated diseases remains to be fully characterized, it likely involves the SASP for EBV pathogenesis and malignancy. Senescent cells act as a source of inflammatory factors (53): the SASP and proinflammatory factor secretion are induced by default latent infections and facilitate the expansion of a pool of EBV-infected B lymphocytes during the natural EBV life cycle in vivo. In contrast, as an irreversible arrest of cell cycle and growth, senescence is a reasonable antiviral defense (54) and should be attenuated during EBV lytic replication, which spontaneously occurs in epithelial cells and peripheral blood B lymphocytes to restore the EBV reservoir for EBV transmission and persistent infection (55–57). Further characterization of the unique inflammatory microenvironment and paracrine senescence in EBV-infected lymphoid tissue or tumor tissue will help to define the natural progression and pathogenesis of EBV infection in vivo.
In summary, we have demonstrated that latent EBV infection promotes and lytic infection attenuates the transmission of inflammatory senescence by inducing or reducing proinflammatory factors, respectively. BZLF1 inhibits the secretion of TNF-α and the transmission of paracrine senescence during the early stage of the lytic EBV life cycle. This finding may represent a switch in the microenvironment from the tumor-promoting SASP to a virus-propagating phenotype during lytic replication in EBV-transformed cells.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Science Foundation of China (81371792 and U1301121), the Science and Technology Program of Guangzhou (2014J4100164), and the Science and Technology Planning Project of Guangdong Province (2014A020210005) to E.K.
REFERENCES
- 1.Munoz-Espin D, Serrano M. 2014. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 2.Salama R, Sadaie M, Hoare M, Narita M. 2014. Cellular senescence and its effector programs. Genes Dev 28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieur A, Peeper DS. 2008. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Gorgoulis VG, Halazonetis TD. 2010. Oncogene-induced senescence: the bright and dark side of the response. Curr Opin Cell Biol 22:816–827. doi: 10.1016/j.ceb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 5.McDuff FK, Turner SD. 2011. Jailbreak: oncogene-induced senescence and its evasion. Cell Signal 23:6–13. doi: 10.1016/j.cellsig.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Haring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Rocken M. 2013. T-helper-1-cell cytokines drive cancer into senescence. Nature 494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 9.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. 2014. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesarman E. 2014. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol 9:349–372. doi: 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman PM. 2013. Keeping it quiet: chromatin control of gammaherpesvirus latency. Nat Rev Microbiol 11:863–875. doi: 10.1038/nrmicro3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. 2007. Lytic cycle switches of oncogenic human gammaherpesviruses. Adv Cancer Res 97:81–109. doi: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- 13.Hislop AD, Taylor GS, Sauce D, Rickinson AB. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol 25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 14.Chijioke O, Azzi T, Nadal D, Munz C. 2013. Innate immune responses against Epstein Barr virus infection. J Leukoc Biol 94:1185–1190. doi: 10.1189/jlb.0313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Usherwood EJ. 2014. Immune escape of γ-herpesviruses from adaptive immunity. Rev Med Virol 24:365–378. doi: 10.1002/rmv.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alibek K, Baiken Y, Kakpenova A, Mussabekova A, Zhussupbekova S, Akan M, Sultankulov B. 2014. Implication of human herpesviruses in oncogenesis through immune evasion and supression. Infect Agents Cancer 9:3. doi: 10.1186/1750-9378-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vischer HF, Siderius M, Leurs R, Smit MJ. 2014. Herpesvirus-encoded GPCRs: neglected players in inflammatory and proliferative diseases? Nat Rev Drug Discov 13:123–139. doi: 10.1038/nrd4189. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. 2006. Inflammation and cancer: how hot is the link? Biochem Pharmacol 72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 305:163–174. doi: 10.1016/j.canlet.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foss HD, Herbst H, Hummel M, Araujo I, Latza U, Rancso C, Dallenbach F, Stein H. 1994. Patterns of cytokine gene expression in infectious mononucleosis. Blood 83:707–712. [PubMed] [Google Scholar]
- 21.Klein SC, Kube D, Abts H, Diehl V, Tesch H. 1996. Promotion of IL8, IL10, TNF alpha and TNF beta production by EBV infection. Leuk Res 20:633–636. doi: 10.1016/0145-2126(96)00029-X. [DOI] [PubMed] [Google Scholar]
- 22.Lay JD, Tsao CJ, Chen JY, Kadin ME, Su IJ. 1997. Upregulation of tumor necrosis factor-alpha gene by Epstein-Barr virus and activation of macrophages in Epstein-Barr virus-infected T cells in the pathogenesis of hemophagocytic syndrome. J Clin Invest 100:1969–1979. doi: 10.1172/JCI119728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho JW, Liang RH, Srivastava G. 1999. Differential cytokine expression in EBV positive peripheral T cell lymphomas. Mol Pathol 52:269–274. doi: 10.1136/mp.52.5.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernadi K, Gyongyosi E, Meszaros B, Szakacs L, Szalmas A, Csoma E, Mogyorosi R, Czompa L, Veress G, Varga I, Marton IJ, Konya J. 2013. Elevated tumor necrosis factor-alpha expression in periapical lesions infected by Epstein-Barr virus. J Endod 39:456–460. doi: 10.1016/j.joen.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Wada T, Muraoka M, Yokoyama T, Toma T, Kanegane H, Yachie A. 2013. Cytokine profiles in children with primary Epstein-Barr virus infection. Pediatr Blood Cancer 60:E46–E48. doi: 10.1002/pbc.24480. [DOI] [PubMed] [Google Scholar]
- 26.Khan G. 2006. Epstein-Barr virus, cytokines, and inflammation: a cocktail for the pathogenesis of Hodgkin's lymphoma? Exp Hematol 34:399–406. doi: 10.1016/j.exphem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Dolcetti R, Dal Col J, Martorelli D, Carbone A, Klein E. 2013. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphomas. Semin Cancer Biol 23:441–456. doi: 10.1016/j.semcancer.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Leidal AM, Pringle ES, McCormick C. 2012. Evasion of oncogene-induced senescence by gammaherpesviruses. Curr Opin Virol 2:748–754. doi: 10.1016/j.coviro.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 29.McFadden K, Luftig MA. 2013. Interplay between DNA tumor viruses and the host DNA damage response. Curr Top Microbiol Immunol 371:229–257. doi: 10.1007/978-3-642-37765-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin B, He Z, Yang X, Chan CP, Ng MH, Cao L. 2001. TRADD domain of Epstein-Barr virus transforming protein LMP1 is essential for inducing immortalization and suppressing senescence of primary rodent fibroblasts. J Virol 75:3010–3015. doi: 10.1128/JVI.75.6.3010-3015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, He Z, Xin B, Cao L. 2000. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16INK4a expression. Oncogene 19:2002–2013. doi: 10.1038/sj.onc.1203515. [DOI] [PubMed] [Google Scholar]
- 32.Chen YL, Chen YJ, Tsai WH, Ko YC, Chen JY, Lin SF. 2009. The Epstein-Barr virus replication and transcription activator, Rta/BRLF1, induces cellular senescence in epithelial cells. Cell Cycle 8:58–65. doi: 10.4161/cc.8.1.7411. [DOI] [PubMed] [Google Scholar]
- 33.Wu FY, Chen H, Wang SE, apRhys CM, Liao G, Fujimuro M, Farrell CJ, Huang J, Hayward SD, Hayward GS. 2003. CCAAT/enhancer binding protein alpha interacts with ZTA and mediates ZTA-induced p21CIP-1 accumulation and G1 cell cycle arrest during the Epstein-Barr virus lytic cycle. J Virol 77:1481–1500. doi: 10.1128/JVI.77.2.1481-1500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez A, Armstrong M, Dwyer D, Flemington E. 1999. Genetic dissection of cell growth arrest functions mediated by the Epstein-Barr virus lytic gene product, Zta. J Virol 73:9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Long X, Huang L, Yang M, Yuan Y, Wang Y, Delecluse HJ, Kuang E. 2016. Epstein-Barr virus BZLF1-mediated downregulation of proinflammatory factors is essential for optimal lytic viral replication. J Virol 90:887–903. doi: 10.1128/JVI.01921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci U S A 95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse HJ. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J 19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Spandidos A, Wang H, Seed B. 2012. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 40:D1144–D1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. 2009. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 40.Nolan GP, Fiering S, Nicolas JF, Herzenberg LA. 1988. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-d-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A 85:2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HB, Zhang H, Zhang JP, Li Y, Zhao B, Feng GK, Du Y, Xiong D, Zhong Q, Liu WL, Du H, Li MZ, Huang WL, Tsao SW, Hutt-Fletcher L, Zeng YX, Kieff E, Zeng MS. 2015. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat Commun 6:6240. doi: 10.1038/ncomms7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang Y, Tung CH, Huang YT, Lu J, Chen JY, Tsai CH. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J Virol 73:8857–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Li D, Guo N. 2009. Regulation of cellular and viral protein expression by the Epstein-Barr virus transcriptional regulator Zta: implications for therapy of EBV associated tumors. Cancer Biol Ther 8:987–995. [DOI] [PubMed] [Google Scholar]
- 44.Gosselin J, Flamand L, D'Addario M, Hiscott J, Menezes J. 1992. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses. Differential induction of interleukin 6 and tumor necrosis factor-alpha. J Clin Invest 89:1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gambino V, De Michele G, Venezia O, Migliaccio P, Dall'Olio V, Bernard L, Minardi SP, Della Fazia MA, Bartoli D, Servillo G, Alcalay M, Luzi L, Giorgio M, Scrable H, Pelicci PG, Migliaccio E. 2013. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell 12:435–445. doi: 10.1111/acel.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M, Haase AD, Huang FK, Coulis G, Rivera KD, Dickinson BC, Chang CJ, Pappin DJ, Neubert TA, Hannon GJ, Boivin B, Tonks NK. 2014. Dephosphorylation of tyrosine 393 in Argonaute 2 by protein tyrosine phosphatase 1B regulates gene silencing in oncogenic RAS-induced senescence. Mol Cell 55:782–790. doi: 10.1016/j.molcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmon AB, Richardson A, Perez VI. 2010. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, Sabatier L, Mansat-De Mas V. 2004. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene 23:7507–7516. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- 49.Parish ST, Wu JE, Effros RB. 2009. Modulation of T lymphocyte replicative senescence via TNF-α inhibition: role of caspase-3. J Immunol 182:4237–4243. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. 2009. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J 23:1358–1365. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong GH, Goeddel DV. 1986. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature 323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 52.Cayrol C, Flemington EK. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J 15:2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 53.Davalos AR, Coppe JP, Campisi J, Desprez PY. 2010. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddel RR. 2010. Senescence: an antiviral defense that is tumor suppressive? Carcinogenesis 31:19–26. doi: 10.1093/carcin/bgp274. [DOI] [PubMed] [Google Scholar]
- 55.Tsai MH, Raykova A, Klinke O, Bernhardt K, Gartner K, Leung CS, Geletneky K, Sertel S, Munz C, Feederle R, Delecluse HJ. 2013. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep 5:458–470. doi: 10.1016/j.celrep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Laichalk LL, Thorley-Lawson DA. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prang NS, Hornef MW, Jager M, Wagner HJ, Wolf H, Schwarzmann FM. 1997. Lytic replication of Epstein-Barr virus in the peripheral blood: analysis of viral gene expression in B lymphocytes during infectious mononucleosis and in the normal carrier state. Blood 89:1665–1677. [PubMed] [Google Scholar]