Abstract
Background
Obsessive-compulsive disorder (OCD) is treated with exposure with response prevention (ERP) therapy, in which patients are repeatedly exposed to compulsive triggers but prevented from expressing their compulsions. Many compulsions are an attempt to avoid perceived dangers, and the intent of ERP is to extinguish compulsions. Patients failing ERP therapy are candidates for deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS), which facilitates patients’ response to ERP therapy. An animal model of ERP would be useful for understanding the neural mechanisms of extinction in OCD.
Methods
Using a platform-mediated signaled avoidance task, we developed a rodent model of ERP called “extinction with response prevention (“Ext-RP”), in which rats are given extinction trials while blocking access to the avoidance platform. Following 3 days of Ext-RP, rats were tested with the platform unblocked to evaluate persistent avoidance. We then assessed if pharmacological inactivation of lateral orbitofrontal cortex (lOFC) or DBS of the ventral striatum reduced persistent avoidance.
Results
Following Ext-RP training, most rats showed reduced avoidance at test (Ext-RP success), but a subset persisted in their avoidance (Ext-RP failure). Pharmacological inactivation of lOFC eliminated persistent avoidance, as did DBS applied to the VS during Ext-RP.
Conclusions
DBS of VS has been previously shown to inhibit lOFC activity. Thus lOFC, which is known to be hyperactive in OCD, may be responsible for impairing patients’ response to ERP therapy.
Keywords: obsessive compulsive disorder, orbitofrontal cortex, ventral striatum, deep brain stimulation, platform-mediated avoidance, rat
Introduction
Obsessive compulsive disorder (OCD) is a devastating illness affecting an estimated three million individuals in the U.S. alone (1). Many of the compulsive behaviors in OCD (e.g. hand washing, door lock checking) are viewed as protective against perceived threats (e.g. infection, intruders) (2). The standard behavioral therapy for OCD is “exposure with response prevention (ERP)”, in which patients are repeatedly exposed to triggers for their compulsions, but are prevented from expressing the compulsion (3). The goal of repeated sessions of ERP is to extinguish compulsive behaviors (2). ERP is effective in the majority of OCD patients, however, approximately 40% either drop out or fail ERP (4, 5). Little is known about the mechanisms of ERP therapy failure, or the interactions between extinction and compulsive behaviors, thereby necessitating an animal model.
Patients failing to respond to ERP as well as pharmacotherapies are candidates for deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS). DBS of VC/VS reduces OCD and anxiety symptoms (6), and facilitates patients’ response to ERP therapy (7, 8). OCD is associated with excessive activity in the orbitofrontal cortex (OFC) (for reviews see 9, 10-12), and DBS has been shown to reduce BOLD signaling in OFC together with compulsions (13-15). In rodents, DBS of dorsal-VS (a rodent homologue of VC/VS) has been shown to reduce the firing rate of neurons within the lateral OFC (lOFC) (16). However, the role of lOFC in ERP has not been studied. Therefore, to further explore the roles of lOFC and DBS in ERP, we developed a rodent model of ERP therapy using a platform mediated avoidance task (17). This allowed us to characterize persistent avoidance and its response to manipulations of OFC, both directly and indirectly via DBS of the ventral striatum.
Materials and Methods
Subjects
One hundred and ten male Sprague–Dawley rats (~325 g; Harlan Laboratories) were housed and handled as previously described (18). Rats were fed standard rat chow in a restricted manner (18 g/day) to facilitate pressing a bar for food on a variable interval schedule of reinforcement (VI-30). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico - School of Medicine in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Behavior
Rats were initially trained to press a bar to receive food pellets on a variable interval reinforcement schedule (VI-30) inside standard operant chambers (Coulbourn Instruments, Whitehall, PA) located in sound-attenuating cubicles (MED Associates, St. Albans, VT). Bar-pressing was used to maintain a constant level of activity against which avoidance and freezing could reliably be measured. Rats pressed for food throughout all phases of the experiment.
For platform mediated avoidance, rats were trained as previously described (17). Briefly, rats were conditioned with a pure tone (30 s, 4 kHz, 75 dB) co-terminating with a shock delivered through the floor grids (2 s, 0.4 mA). The inter-trial interval was variable, averaging 3 min. The platform is fixed to the floor and was present during all stages of training (including bar-press training). Rats were conditioned for 10 days, with 9 tone-shock pairings per day with the reinforcement schedule changed to a continuous schedule of reinforcement (FR-1) during the tone. The availability of food on the side opposite to the platform motivated rats to leave the platform during the inter-trial interval, facilitating trial-by-trial assessment of avoidance.
Once platform mediated avoidance was learned, rats underwent extinction (tones with no shock) in the presence of a transparent Plexiglas barrier which prevented access to the platform. During extinction-with-response prevention (Ext-RP), rats underwent sessions consisting of 15 tone-alone presentations across 3 consecutive days with a VI-30 food schedule. After three days of Ext-RP, the barrier was removed and rats were again exposed to the tone.
Surgery & histology
Rats were initially anesthetized with isofluorane inhalant gas (5 %) in an induction chamber and positioned in a stereotaxic frame. Isofluorane (2-3 %) was delivered through a facemask for anesthesia maintenance. For our inactivation experiment, rats were bilaterally implanted with 26-gauge guide cannulas (Plastics One, Roanoke, VA) in the lateral orbitofrontal cortex (lOFC) using the following coordinates: +3.20 mm AP; ±3.30 mm ML; +4.40 mm DV to bregma (19). Cannulas were fixed to the skull with anchoring screws and acrylic cement. After surgery, a topical triple antibiotic was applied around the surgery incision, and an analgesic (Ketoprofen, 5 mg/Kg) was injected intramuscularly. Stainless steel obdurators (33 gauge) were inserted into the guide cannulas to avoid obstructions until infusions were made. Rats were allowed 5-7 days to recover from surgery prior to behavioral testing.
For our deep brain stimulation (DBS) experiment, a similar surgical procedure as above was used, except that rats were implanted with concentric bipolar stimulating electrodes (NEX-100; Rhodes Medical Instruments, Santa Barbara, CA) as previously described (20). Electrodes were aimed at a dorsal-VS site (+1.2 mm AP, ±2.0 mm ML, and −6.5 mm DV to bregma). Rats were allowed 5 days to recover from surgery prior to behavioral testing.
After behavioral experiments, rats were deeply anesthetized with sodium pentobarbital (450 mg/kg i.p.) and transcardially perfused with 0.9 % saline followed by a 10 % formalin solution. Brains were removed from the skull and stored in 30 % sucrose for cryoprotection for at least 72 h before sectioning and Nissl staining. Histology was analyzed and correct placement of cannulas and stimulating electrodes were assessed.
Pharmacological inactivation
Fluorescent muscimol (0.2 μl; BODIPY TMR-X Conjugate; Sigma-Aldrich, St. Louis, MO) was infused to enhance GABA-A receptor activity, thereby temporally inactivating lOFC. On the day of infusion, 0.2 μl of MUS or saline (vehicle) was infused at a rate of 0.2 μl/min. Injector tips extended 1.0 mm beyond the guide cannula. After infusion, injectors were left in place for 1 min to allow the drug to diffuse. Eight rats were eliminated from our MUS experiment because the injections were located outside of our area of interest (lOFC).
Deep brain stimulation
Stimulation was monophasic, with the deeper contact as negative. We used DBS parameters similar to those used in humans (100μA, 0.1-ms pulse duration, 130 Hz), which have been used in previous rat models studying DBS-like stimulation (16, 20-22). Stimulation was generated with an S88X stimulator (Grass Instruments, Warwick, RI) and a constant-current unit (SIC-C Isolation Unit; Grass Instruments, Warwick, RI). Three rats were eliminated from DBS experiments because placements were not located in dorsal-VS, as previously described (20).
Data Collection and Analysis
Behavior was recorded with digital video cameras (Micro Video Products, Bobcaygeon, Ontario, Canada). Freezing was quantified from digitized video images using commercially available software (Freezescan, Clever Systems, Reston,VA). Platform avoidance was quantified by observers blind to experimental group, where avoidance was defined as the rat having at least two paws on the platform. The time spent avoiding during the tone (% time on platform) was used as our avoidance measure. We calculated percent suppression of bar pressing for each tone as previously described (17): (pretone rate - tone rate)/(pretone rate + tone rate) × (100). A value of 0 indicates no suppression, whereas a value of 100% indicates complete suppression. To calculate pretone rates, we used the 60 seconds prior to tone onset. Avoidance, freezing and suppression of bar pressing to the tone was expressed as a percentage of the 30 second tone presentation. Statistical significance was determined with Student's two-tailed t tests, Wilcoxon matched pairs test, one-way ANOVA, or repeated-measures ANOVA, followed by Tukey post hoc analysis, when appropriate (STATISTICA; Statsoft, Tulsa, OK).
Results
Extinction-with-response-prevention (Ext-RP) training reduces avoidance and freezing
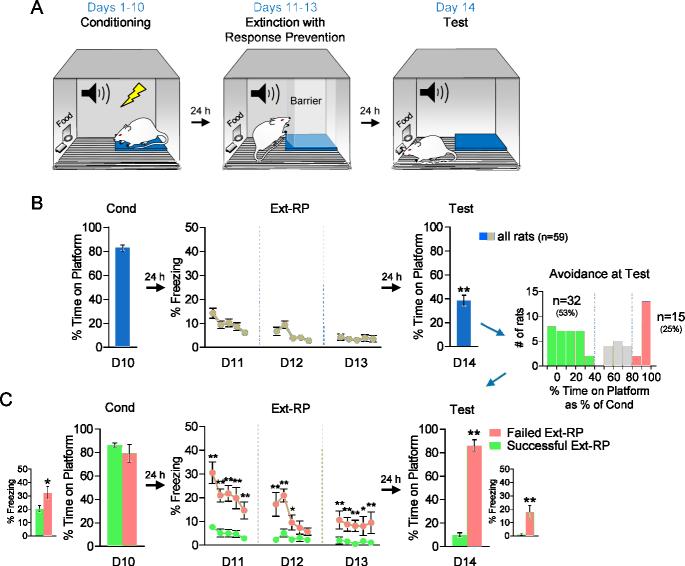
The extinction-with-response prevention (“Ext-RP”) task (Figure 1A) was a modification of the platform-mediated avoidance, in which rats avoid a tone-signaled shock by stepping onto a nearby platform (17). Following 10 days of avoidance training, access to the platform was blocked with a transparent Plexiglas barrier, and rats were given 3 days of tone-shock extinction. The following day (Day 14), the barrier to the platform was removed and rats were tested for avoidance. The time spent avoiding at test (% tone on platform) was significantly reduced compared to the last day of conditioning (Day 10: 83% vs. Day 14: 38%, Wilcoxon matched pairs test; t(58) = 98.5, p < 0.01; Figure 1B). Freezing to the tone was also reduced from Day 10 to Day 14 (Day 10: 25% vs. Day 14: 6%, Wilcoxon matched pairs test; t(58) = 47, p < 0.01). Thus, based on group averages, Ext-RP training successfully reduced both avoidance and freezing.
Figure 1.
Extinction with response prevention (Ext-RP) reduced avoidance in many, but not all, rats. A. Schematic of the behavioral protocol for Ext-RP. Rats were trained to avoid a tone signaled foot-shock by stepping onto a platform over 10 days. After conditioning, rats underwent 3 days of Ext-RP with platform access blocked by a Plexiglas barrier. A post Ext-RP test assessed if Ext-RP reduced the % time avoiding or not. B. Group data (N=59) showed that Ext-RP reduces the % time avoiding. Inset: Whereas 53% of rats reduced avoidance (Successful Ext-RP, n=32), 25% did not (Failed Ext-RP, n=15) C. Rats that failed Ext-RP showed elevated freezing throughout the task. Data during Ext-RP shown in blocks of three trials. All data are shown as mean ± SEM. *p<0.05, **p<0.01.
A subset of rats exhibited persistent avoidance following Ext-RP training
As can be observed in Figure 1B, the % reduction in avoidance behavior from day 10 to day 14 is not normally distributed. We therefore divided rats into different subgroups, based on the % reduction in avoidance from Day 10 to Day 14 (Figure 1B inset). We found that 53% of rats (n=32/59) showed a large reduction in avoidance (< 40% of conditioned), whereas 22% of rats (n=12/59) showed a partial reduction (40% - 80% of conditioned), and 25% of rats (n=15/59) showed almost no reduction (> 80% of conditioned). Rats showing partial reductions were eliminated from further analysis in order to compare Successful Ext-RP (< 40% of conditioned) vs. Failed Ext-RP (> 80% of conditioned) subgroups. At the end of conditioning on Day 10, successful and failed subgroups showed equivalent levels of avoidance (Failed Ext-RP: 79%, Successful Ext-RP: 86%, student t-test; t(45) = −0.98, p = 0.33), but the failed Ext-RP subgroup showed slightly higher freezing levels (student t-test; t(45) = 2.46, p = 0.02; Figure 1C). The failed subgroup continued to show higher freezing in all 3 sessions of Ext-RP (repeated measure ANOVA; all p's < 0.01), as well as in the test session following Ext-RP (student t-test; t(45) = 4.82, p < 0.01; Figure 1C). Thus, failed rats showed excessive fear to the tone throughout.
Further characterization of these two subgroups showed that the failed subgroup had higher bar-press suppression during the 3 sessions of Ext-RP (repeated measure ANOVA; all p's < 0.01), but not during the end of conditioning or the test session following Ext-RP (student t-test; all p's > 0.31; Supplementary Figure 1A). Subgroup differences in bar-press suppression do not reflect pre-existing differences in motivation to press for food because no differences were observed in pre-tone press rates prior to avoidance training or during the beginning of each Ext-RP session (student t-test; all p's >0.30; Supplementary Figure 1B). At the test session, both group showed a reduction in rates of pretone pressing (see Supplementary Figure 1B), suggesting that removal of the barrier may have been interpreted as a return of shock. However, the failed group was pressing significantly less than the successful group (student t-test; t(45) = −3.91, p < 0.01; Supplementary Figure 1B), which likely reflects the increased fear at test. Interestingly, we also found that rats that failed Ext-RP spent significantly more time looking in the direction of the blocked platform during the first minute of sessions 1 and 2 of Ext-RP (student t-test; p's < 0.05), but this behavior extinguished fully by session 3 (student t-test; t(45) = −0.12, p = 0.92; Supplementary Figure 1C).
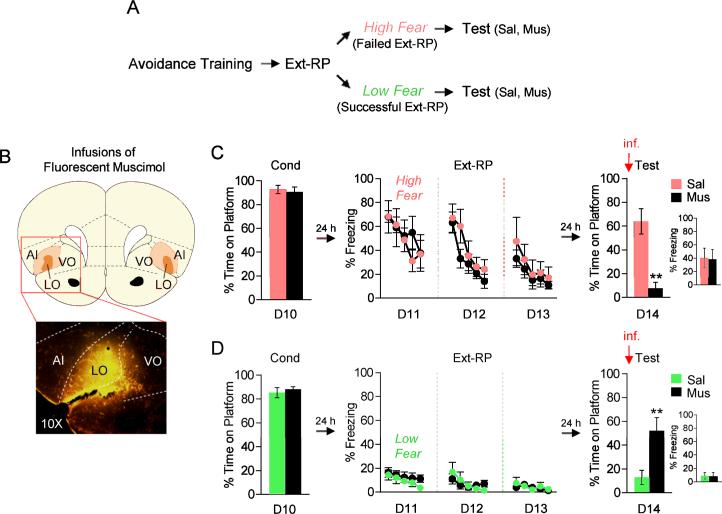
Persistent avoidance can be reduced by inactivation of lOFC
OCD is associated with a hyperactive OFC (for reviews see 9, 10, 11), suggesting that persistent avoidance may be due to an overactive OFC. In order to test this hypothesis in our task, we ran another group of rats through Ext-RP and subdivided them to into two subgroups: those showing high freezing levels during Ext-RP (presumed failure rats), and those showing low freezing levels during Ext-RP (presumed success rats). Rats showing high freezing levels were infused with either saline or muscimol (MUS) to pharmacologically inactivate lOFC just prior to test (Day 14, Figure 2A-B). We found that lOFC inactivation reduced avoidance at test (SAL: 64%, MUS: 8%, student t-test; t(10) = 5.22, p < 0.01), without decreasing freezing levels (SAL: 40%, MUS: 38%, student t-test; t(10) = 0.09, p = 0.93, Figure 2C) or bar-press suppression (SAL: 40%, MUS: 53%, student t-test; t(10) =−0.40, p = 0.69). Therefore, inhibition of lOFC reduces persistent avoidance in rats that would have otherwise shown Ext-RP failure.
Figure 2.
Pharmacological inactivation of lOFC bidirectionally modulated the expression of avoidance at test. A. Experimental protocol separating rats based on freezing levels at the start of Ext-RP. B. Top: Infusions of fluorescent muscimol (MUS) into lOFC. Orange areas represent the minimum (dark) and the maximum (light) spread of MUS. Bottom: Representative micrograph showing a MUS infusion into lOFC. C. In high freezing rats (likely to fail Ext-RP), inactivation of lOFC reduced persistent avoidance without reducing freezing levels (Sal, n=5; Mus, n=7). D. In low freezing rat (likely to succeed in Ext-RP), inactivation of lOFC induced persistent avoidance without increasing freezing levels (Sal, n=10; Mus, n=10). Ext-RP data shown in blocks of three trials. All data are shown as mean ± SEM. **p<0.01.
We also inactivated lOFC in rats showing low freezing levels during Ext-RP (presumed success rats, Figure 2A-B). In contrast to high freezing rats, lOFC inactivation in low freezing rats increased avoidance at test (SAL: 13%, MUS: 52%, student t-test; t(18) = −3.09, p < 0.01), without increasing freezing levels (SAL: 9%, MUS: 8%, student t-test; t(18) = 0.16, p = 0.89, Figure 2D) or bar-press suppression (SAL: 27%, MUS: 8%, student t-test; t(18) = 0.75, p = 0.46). Therefore, inhibition of lOFC induces persistent avoidance in rats that would have otherwise shown Ext-RP success.
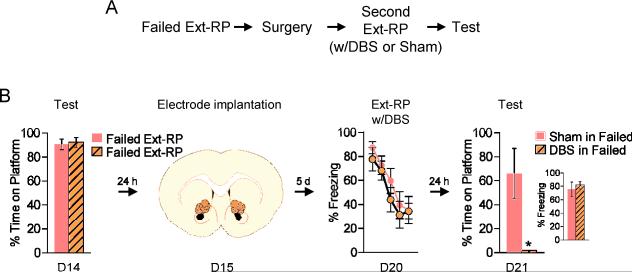
DBS-like stimulation of VS reduces persistent avoidance
One of the eligibility requirements for deep brain stimulation (DBS) is failing to respond to ERP therapy (7, 23). Furthermore, such OCD patients start responding to ERP when it is given together with DBS of the VC/VS (7, 8). We therefore assessed if DBS of the dorsal portion of VS (homologous to VC/VS; 22, 24, 25), given in combination with Ext-RP, would reduce avoidance in rats that previously failed Ext-RP (from Figure 1). Rats showing Ext-RP failure at test were subsequently implanted with bipolar stimulating electrodes in the dorsal-VS (Figure 3A). One week later, they were given DBS-like high frequency stimulation for 3h during an additional Ext-RP session on Day 20. A sham control group was implanted with electrodes but never stimulated. Both groups were matched for avoidance and freezing levels prior to surgery (all p's > 0.25, Figure 3B). DBS of dorsal-VS did not reduce freezing levels (repeated measure ANOVA; F(1,10) = 0.57, p = 0.47, Figure 3B), bar-press suppression (repeated measure ANOVA; F(1,10) = 0.11, p = 0.75), pre-tone bar pressing (Sham: 11%, DBS: 17%, student t-test; t(10) = −1.04, p = 0.32) or the time rats spent looking at the platform (Sham: 4%, DBS: 4%, student t-test; t(10) = −0.42, p = 0.68) during Ext-RP. However, the following day (Day 21), in the absence of DBS, persistent avoidance was abolished (Sham: 66%, DBS: 0%, student t-test; t(10) = 3.16, p = 0.01), with no reduction in freezing (Sham: 76%, DBS: 82%, student t-test; t(10) = −0.50, p = 0.63, Figure 3B) or bar-press suppression (Sham: 73%, DBS: 87%, student t-test; t(10) = −0.45, p = 0.66). Therefore, similar to inactivation of lOFC, DBS of dorsal-VS eliminated persistent avoidance in rats that had previously failed Ext-RP.
Figure 3.
DBS of Dorsal-VS eliminates persistent avoidance. A. Experimental protocol for delivery of DBS to rats that failed Ext-RP (from Figure 1). B. Compared to sham-operated controls, DBS of Dorsal-VS delivered during a second Ext-RP session had no effect on freezing, but eliminated persistent avoidance during Test (Sham Failed, n=6; DBS Failed, n=6). Ext-RP data shown in blocks of three trials. All data are shown as mean ± SEM. *p<0.05.
Discussion
We developed an avoidance-based rodent model of ERP therapy in which signaled avoidance behaviors are reduced following extinction of tone-shock associations. Ext-RP training reduced avoidance in the majority of rats; however, 25% persisted in their avoidance following Ext-RP. Persistent avoidance could be eliminated by inactivating lOFC, or by delivering DBS to the VS.
It is estimated that 26% of OCD patients suffer from the harm-avoidant type of OCD, because they believe that their compulsions protect them from danger (26). Many compulsions, therefore, constitute persistent avoidance responses which can be investigated with animal models of avoidance. Following Ext-RP training, the 25% of rats that exhibited persistent avoidance also expressed heightened freezing throughout Ext-RP, suggesting that excessive fear predicts Ext-RP failure. Interestingly, the majority of OCD patients who fail ERP therapy also show excessive fear to compulsive triggers (26, 27). While fear may be predictive, it is not clear if it is also a cause of Ext-RP failure. If so, reducing fear with pharmacological adjuncts such as the beta-blocker propranolol (28, 29) might reduce ERP failure. However, it is possible to observe persistent avoidance in the absence of elevated fear in animals (30), healthy humans (31), and OCD patients (32), suggesting that factors other than elevated fear can elicit persistent avoidance.
A prominent hypothesis of OCD is that it is due in part to a hyperactive OFC (9-11). Specifically, the lOFC plays a key role in assigning value to a given action (33-35). The inability of ERP therapy to decrease the value of avoidance in some individuals may result from heightened fear combined with excessive activity in lOFC. Consistent with this idea, rats showing persistent avoidance exhibited both elevated fear and heightened value as evidenced by the time spent looking towards the platform. While the latter fully extinguished by the end of Ext-RP, the former did not, suggesting that persistent avoidance may be driven by increased fear. In contrast, non-avoiders showed low freezing and spent little time looking towards the platform. Inactivation of lOFC had opposite effects in these groups, consistent with lOFC assignment of value to actions (33-35), depending on fear levels.
In a recent report, OCD patients with a hyperactive mOFC were unable to devalue avoidance responses (32). We previously showed that the mOFC in rodents is important for fear expression (22). Thus, failure to respond to ERP may stem from an interaction between elevated fear (mOFC) and deficient devaluation of avoidance (lOFC). Furthermore, our findings show that inactivation of lOFC can either induce or reduce persistent avoidance, suggesting that different neuronal populations within lOFC are responsible for driving or inhibiting persistent avoidance. Perhaps these distinct subpopulations receive inputs from different regions involved in fear regulation (e.g., infralimbic cortex, prelimbic cortex, amygdala, thalamus) (36), but this of course remains to be tested.
DBS of VC/VS reduces OCD symptoms in treatment-resistant patients (6), and facilitates their response to ERP therapy (7, 8). While the mechanisms of DBS action are unclear, reduced OFC activity by DBS has been reported (13-15). In the present study, we found that DBS-like stimulation of the dorsal-VS (a rodent homologue of VC/VS) eliminated persistent avoidance when DBS was conducted during Ext-RP. Recent reports showed that pathways from both mOFC and lOFC to VS regulate perseverative grooming behavior (37, 38), which has been used to model OCD. Furthermore, DBS at this VS site has been reported to reduce lOFC firing rate (16) and increase lOFC plasticity (20), suggesting that DBS may be acting by diminishing lOFC activity. It is important to note that DBS did not reduce heightened freezing, either during Ext-RP or at test the following day. This is similar to lOFC inactivation, which reduced persistent avoidance but not elevated freezing levels. This suggests that persistent avoidance stems from a hyperactive lOFC that exists downstream of fear circuits.
We previously showed that DBS of the VS reduced freezing during the extinction of conditioned fear (20), which may be due to inhibition of mOFC (22). In the present study, DBS of the same VS region reduced persistent avoidance, and this reduction may be due to inhibition of the lOFC. The benefits of DBS in VS may be due to an overall inhibition of cortical regions that project through this site, which include both mOFC and lOFC together with the prelimbic cortex (PL) (22). It is interesting to note that DBS in the present study did not reduce freezing, suggesting that PL and mOFC do not mediate freezing in our avoidance task, as previously observed for PL (17).
Our findings suggest that the effectiveness of clinical DBS may be due to enhancement of the extinction process occurring in ERP therapy. It is important to note, however, that clinical DBS electrodes are activated continuously after surgery, whereas the facilitation of Ext-RP we observed was during a DBS-Off phase. While our findings suggest that DBS during extinction is key for its beneficial effect, it remains to be determined if avoidance would still be reduced with DBS on at test. A recent behavioral task in humans mimics the experimental conditions of our rodent Ext-RP task (31). Future pre-clinical studies should model specific features of clinical ERP therapy in both humans and rodents, with the long-term goal of identifying markers predictive of OCD-like behavior and novel approaches for treatment.
Supplementary Material
Acknowledgments
We would like to thank Ricardo Rodríguez-Colón and Estefanía González-Araya for help with behavioral experiments and Carlos Rodríguez and Zarkalys Quintero for technical assistance. This work was supported by the Silvio O. Conte Center for Research in OCD P50-MH086400 to BDG, SAR and GJQ; R37 MH058883 and R01 MH081975 to GJQ, R36 MH105039 to JRR; and the University of Puerto Rico President's Office.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229–243. doi: 10.1146/annurev-clinpsy-032210-104533. [DOI] [PubMed] [Google Scholar]
- 3.Rachman S, Hodgson R, Marks IM. The treatment of chronic obsessive-compulsive neurosis. Behav Res Ther. 1971;9:237–247. doi: 10.1016/0005-7967(71)90009-x. [DOI] [PubMed] [Google Scholar]
- 4.Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- 5.Simpson HB, Foa EB, Liebowitz MR, Ledley DR, Huppert JD, Cahill S, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008;165:621–630. doi: 10.1176/appi.ajp.2007.07091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg BD, Gabriels LA, Malone DA, Jr., Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 8.Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychol Med. 2014;44:3515–3522. doi: 10.1017/S0033291714000956. [DOI] [PubMed] [Google Scholar]
- 9.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter LR, Jr., Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, et al. Brain Mediation of Obsessive-Compulsive Disorder Symptoms: Evidence From Functional Brain Imaging Studies in the Human and Nonhuman Primate. Semin Clin Neuropsychiatry. 1996;1:32–47. doi: 10.1053/SCNP00100032. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Le Jeune F, Verin M, N'Diaye K, Drapier D, Leray E, Du Montcel ST, et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68:1016–1022. doi: 10.1016/j.biopsych.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 16.McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. Neural structures mediating expression and extinction of platform-mediated avoidance. J Neurosci. 2014;34:9736–9742. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- 20.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Front Behav Neurosci. 2013;7:102. doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of Fear Extinction with Deep Brain Stimulation: Evidence for Medial Orbitofrontal Involvement. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 24.Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuire JF, Storch EA, Lewin AB, Price LH, Rasmussen SA, Goodman WK. The role of avoidance in the phenomenology of obsessive-compulsive disorder. Compr Psychiatry. 2012;53:187–194. doi: 10.1016/j.comppsych.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Foa EB. Failure in treating obsessive-compulsives. Behav Res Ther. 1979;17:169–176. doi: 10.1016/0005-7967(79)90031-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Bravo-Rivera C, Roman-Ortiz C, Montesinos-Cartagena M, Quirk GJ. Persistent active avoidance correlates with activity in prelimbic cortex and ventral striatum. Front Behav Neurosci. 2015;9:184. doi: 10.3389/fnbeh.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vervliet B, Indekeu E. Low-Cost Avoidance Behaviors are Resistant to Fear Extinction in Humans. Front Behav Neurosci. 2015;9:351. doi: 10.3389/fnbeh.2015.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillan CM, Apergis-Schoute AM, Morein-Zamir S, Urcelay GP, Sule A, Fineberg NA, et al. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry. 2015;172:284–293. doi: 10.1176/appi.ajp.2014.14040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 35.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.