Abstract
While the development of a protective HIV vaccine is the ultimate goal of HIV research, to date only one HIV vaccine trial, the RV144, has successfully induced a protective response. The 31% protection from infection achieved in the RV144 trial was linked to the induction of non-neutralizing antibodies, able to mediate ADCC, suggestive of an important role of Fc-mediated functions in protection. Likewise, Fc-mediated antiviral activity was recently shown to play a critical role in actively suppressing the viral reservoir, however, the Fc-effector mechanisms within tissues that provide protection from or after infection are largely unknown. Here we aimed to define the landscape of effector cells and Fc-receptors present within vulnerable tissues. We found negligible Fc-receptor expressing NK cells in the female reproductive and gastrointestinal mucosa. Conversely, Fc-receptor expressing macrophages were highly enriched in most tissues, but neutrophils mediated superior antibody-mediated phagocytosis. Modifications in Fc domain of VRC01 antibody increased phagocytic responses in both phagocytes. These data suggest that non-ADCC mediated mechanisms, such as phagocytosis and neutrophil activation, are more likely to play a role in preventative vaccine or reservoir-eliminating therapeutic approaches.
Introduction
While a vaccine able to induce broadly neutralizing antibodies (bnAbs) against HIV remains a top priority, to date no vaccine strategy has induced antibodies with appreciable neutralizing coverage of global viral quasispecies. However, a reduced risk of infection and/or enhanced viral control post-infection has been observed in both human 1 and non-human primate (NHP) studies, 2,3 both associated with the induction of non-neutralizing antibodies with potent Fc-mediated effector functions. Additionally, while the broadly neutralizing antibody b12 was able to provide sterilizing protection from infection, the protective efficacy of this monoclonal was partially lost upon the ablation of FcR binding activities 4.
Beyond their role in preventing infection, Fc-effector functions have also been implicated in post-infection control and clearance of viral infections including Ebola 5 and Influenza 6. Likewise, antibody-effector function has been linked to spontaneous and durable control of viral load in HIV infection 7,8. Importantly, control of the viral reservoir is critically dependent on the Fc effector functions of bnAbs, as loss of Fc-effector activity leads to the rapid loss of viral control despite potent neutralizing activity 9. Thus, while delivery of potent neutralizing antibodies drives a transient reduction in plasma viremia in both NHP 10 and humans, 11 the viral reservoir nearly always rebounds due to incomplete antibody-mediated eradication of reactivated cells suggesting that the enhanced Fc-mediated antibody effector functions, able to recruit innate immune killing, particularly at sites where the reservoir hides, may enhance the elimination of infected cells.
Antibodies are able to recruit a wide array of antiviral functions via interactions between the crystallizable Fc fragment and Fc receptors, complement, or lectin-like receptors found on innate immune cells 12. Fc receptors exist for each of the immunoglobulin isotypes and are involved in directing disparate innate immunological functions that range from direct antiviral activity via phagocytosis, cellular cytotoxicity, complement activation, cytokine secretion to indirect immune regulation via the direction of anti-inflammatory responses often aimed at dampening inflammation 13. Thus, Fc receptors link the specificity of the adaptive immune system to the powerful effector functions of the innate immune system. However, because both the distribution of innate immune cells 14 as well as the range of Fc-mediated effector functions they can mediate 15 differs from tissue compartment to compartment, it is likely that the potency of any given monoclonal antibody will depend on the distribution of FcRs on distinct subsets of innate immune cells within target tissues.
Because HIV infections largely occur through mucosal membranes, 16 and persistent reservoirs likely hide in lymphoid tissues 17 and the intestinal tract, 18 in this study, we aimed to define the antibody effector functions available within mucosal and lymphoid tissues that can be harnessed in the context of future prophylactic/therapeutic strategies. Surprisingly, tissue-resident NK cells expressed negligible levels of Fc receptors, suggesting that tissue-resident NK cells are unlikely to contribute to antibody-mediated protective immunity at the site of infection. However, FcγRII- and FcγRIII-expressing macrophages and neutrophils, were present in tissues collected from both HIV-seronegative and -seropositive subjects. While tissue-resident neutrophils were less abundant, they mediated more effective phagocytic clearance of immune complexes. Neither macrophage- nor neutrophil-mediated clearance was affected by inflammatory cytokines associated with enhanced risk of HIV acquisition 19. Moreover, Fc-engineered VRC01 antibodies drove improved mucosal phagocytic activity, offering a means to further increase innate immune antiviral activity aimed at preventing or clearing infection. These data strongly argue that antibody-driven functional activities mediated by cells other than NK cells are more likely to afford protection from infection as well as have therapeutic activity within mucosal and lymphoid tissues.
Methods
Subjects
Formalin-fixed, paraffin-embedded tissue slides from normal human colon, rectum, mesenteric lymph node, vagina, cervix and uterus were obtained from the Massachusetts General Hospital tissue repository. Fresh HIV-negative tissue samples from colon and cervix, collected from healthy regions, outside of cancerous lesions, were obtained through the Ragon Institute Tissue Platform. Biopsy samples from rectum, ileum and inguinal lymph nodes, from HIV+ donors and HIV− controls were obtained from the University of Minnesota. Tissue samples were unmatched (i.e. rectum, ileum, lymph node were collected from independent donors) and therefore each tissue type was treated independently in the analysis. All HIV+ patients were treated with anti-retroviral therapy (ART) and were biopsied at different stages of infection (acute, early, chronic or AIDS). 13 rectal biopsies (all from HIV+ subjects), 42 ileal biopsies (15 uninfected HIV− controls, 27 HIV+ subjects) and 35 inguinal lymph node biopsies (11 uninfected HIV− controls, 24 HIV+ subjects) were analyzed. Viral Load (VL) and CD4 counts were measured at the time of biopsy. All tissue donors were adults, between 20 to 63 years of age (median= 40). All samples were analyzed in a blinded fashion.
Immunohistochemistry
Antigen-retrieval and signal detection were performed as previously described 20. The following primary Abs were used: goat polyclonal anti-NKp46 (R&D Systems), mouse anti-CD56 (clone 123C3, Dako), mouse anti-CD68 (clone KP1, Dako), mouse anti-neutrophil defensins (clone D21, Leica Biosystems), mouse anti-CD16 (clone 2H7, Leica Biosystems), rabbit anti-CD32 (clone EPR6657(2), Abcam), mouse anti-CD20 (clone L26, Dako), rabbit polyclonal anti-CD3 (Dako). The following control Abs were used: mouse IgG1 (Dako), goat IgG (R&D Systems), mouse IgG2a (Abcam) and rabbit IgG (Abcam).
Immunofluorescence
Antigen-retrieval and autofluorescence masking were performed as previously described 20. The primary and control Abs were used as above. The following detection Abs were used: goat anti-mouse IgG1 Alexa Fluor 488, goat anti-rabbit IgG Alexa Fluor 594, and goat anti-mouse IgG2a Alexa Fluor 647 (Life Technologies).
Microscopy
Slides were scanned using a Zeiss MIRAX MIDI slide scanner in the brightfield mode or a TissueFAXS (Tissue Gnostics, based on a Zeiss Axio Imager Z2 microscope) in the fluorescence mode.
Image Analysis
Cell counts were performed using the TissueQuest analysis software (TissueGnostics) on fluorescence images.
Flow cytometry
Flow cytometric evaluation of Fc receptor expression was performed on freshly isolated cells from enzymatically digested colon and cervical tissues. 1x106 colon or 1x105 cervical cells were stained in two panels with anti-CD3 Alexa Fluor 700 (clone UCHT1), anti-CD56 PE-Cy7 (clone NCAM16.2), anti-CD64 FITC (clone 10.1), anti-CD89 PE (clone A59), anti-CD45 PE-Cy5 (clone HI30), anti-CD15 PacificBlue (clone W6D3), anti-CD16 BV510 (clone 3G8), anti-CD32 APC (clone FUN-2), anti-CD64 R-PE (clone 10.1, Dako), blue viability dye (Life Technologies). Cells were washed and fixed with Perm A buffer (Life Technologies). Intracellular staining with anti-CD68 FITC (clone KP1, Dako) was performed in the presence of permeabilization buffer (Perm B, Life Technologies). The data was acquired using an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo and SPICE version 5.1.
Generation of VRC01 variants
Antibody variants were expressed as previously described 21. In brief, CMV/R mammalian expression vectors for the VRC- 01 IgG1 light and heavy chains were obtained from the NIH AIDS Reagent Program. Following sequence verification, DNA was isolated via maxi-prep (Qiagen), and used to transfect suspension cultures of human embryonic kidney (HEK) 293F cells grown in Freestyle media (Invitrogen) using 25 kD branched PEI (PolySciences). Antibodies were transiently expressed for 7 days at 37 °C, 5% CO2. Fc domain amino acid point mutations were incorporated via QuikChange PCR (Stratagene, La Jolla, CA). Sequences for each construct were previously described 21.
Tissue phagocytosis assay
A tissue phagocytosis assay was developed based on a previously described THP1-based phagocytosis assay 22. In brief, biotinylated gp120 antigen was incubated with 1 μm diameter red fluorescent neuravidin beads (Life Technologies) at a 4:1 protein: bead ratio (4μg protein:1μl bead suspension), overnight at 4°C. Beads were subsequently washed twice in PBS- 1% BSA in order to remove excess unbound antigen, and then resuspended at a final dilution of 1:100 in PBS-BSA. Immune complexes were formed by incubating gp120-coated beads with antibodies for 2hrs at 37°C: different doses of HIV-IG (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Catalog #3957, HIV-IG from NABI and NHLBI), VRC01 wild type and VRC01 Fc mutants: FcRγIIa enhancing- S239D/I332E/G236A (SDIEGA), 23 complement enhancing- S267E/H268F/S324T (SEHFST), 24 two FcRγIIIa enhancing- S239D/I332E (SDIE) 23 and S239D/I332E/A330L (SDIEAL) 23. Immune complexes were then co-cultured with 1x106 colonic or 1x105 cervical cells for 2 hrs at 37°C. Additionally, inflammatory cytokines and chemokines (recombinant human MIP1α, recombinant human MIP1β, recombinant human IL-8, recombinant human IP-10 -R&D systems), each at 200 ng/ml, were added to cervical mucosa, in a subset of experiments, to induce an inflammatory environment prior to co-culture with immune complexes. All cells were washed and stained with anti-CD45 BV510 (clone HI30), anti-CD3 Alexa Fluor 700 (clone UCHT1), anti-CD56-PE-Cy7 (clone NCAM16.2), anti-66b Pacific Blue (clone G10F5), blue viability dye, anti-CD68 FITC (clone KP1). The data were acquired using a BD LSRII flow cytometer. Bead uptake was evaluated by FlowJo analysis and phagocytic score (% cells taking up beads x mean fluorescent intensity (MFI)/1000) was calculated.
Statistics
Descriptive measures (median, inter quartile range, frequency and percent) were used to summarize the data. Logarithmic transformation was performed to facilitate normalization of the data distribution. Two-way analysis of variance (ANOVA) with Dunn’s post hoc analyses were used to compare phagocytosis between cell types stimulated by different conditions (gp120-beads alone or immune complexes) and to compare the effect of cytokine treatment on cells stimulated by different conditions. One-way ANOVA with Dunn’s post hoc analyses were used to compare groups of: uninfected and HIV-infected individuals with controlled or uncontrolled viremia and subjects at different stages of HIV disease (acute, early, chronic, AIDS and uninfected controls) to test differences in cellular counts. Spearman’s rank correlations were used to examine bivariate associations between cellular counts and viral load or CD4 count. Paired t-tests were used to compare phagocytic activity induced by gp120-beads and immune complexes formed by VRC01 antibody and different VRC01 Fc mutants. Reported p-values are two sided and values of p ≤ 0.05 were considered significant. Statistical analysis and graphing were performed using GraphPad Prism Software.
Results
Differential NK cell, macrophage and neutrophil distribution in mucosal tissues
Given that the distribution of innate immune cells may vary among different tissues, 14,25 the biological activity of functional antibodies is highly dependent on the availability of each effector cell type at the site of transmission or viral replication. Thus, we first aimed to define the frequency and localization of these cells within healthy tissues, as this would represent the landscape of effector cells available to antibodies induced by a prophylactic vaccine or passive monoclonal therapeutic prevention strategy. Specifically, healthy samples from rectum, colon, vagina, cervix, uterus, and lymph node were stained for NKp46 or CD56 (NK cell markers), CD68 (macrophage marker) and 1-, 2-, 3- defensins (resting neutrophil marker, with its expression limited to inactivated mature neutrophils) to determine the location of these innate effector cells.
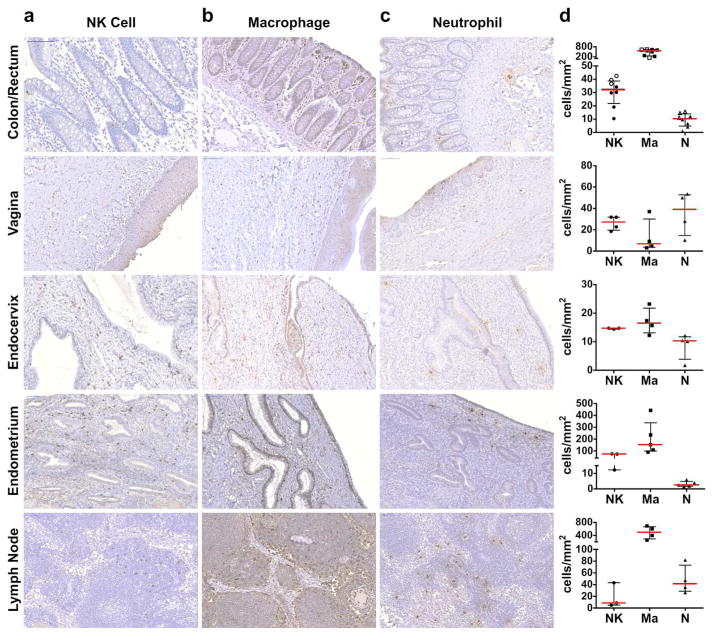
Overall, the distribution of tissue-resident NK cells varied widely depending on the tissue type (Figure 1a). Rectum-, colon-, and vaginal mucosal–resident NK cells were found primarily in intraepithelial spaces, while NK cells in the endocervix and uterus were largely found in the mucosal lamina propria. Lymph node–resident NK cells were found sparsely distributed in the lymph node cortex, outside of follicles. Overall, the frequency of tissue-resident NK cells was variable (median cells per mm2: colon = 29, vagina = 27, ectocervix = 8, endocervix = 15, uterine endometrium = 74, lymph node = 9), relatively low in intestine and lymph node, with the greatest number in the uterine endometrium, where NK cells are known to be enriched and play an important role in fertilization and pregnancy 26.
Figure 1. Localization of innate immune cells in tissues.
NK cells (a), macrophages (b) and neutrophils (c) in colorectal mucosa, vagina, endocervix, uterus endometrium and lymph node. Magnification 200x. Quantification of the cells is depicted in (d). NK cells, circles, macrophages, squares, neutrophils, triangles. Open shape, rectum; closed shape, colon.
In contrast, macrophages were more prevalent in the gastrointestinal tract lamina propria, densely localized directly beneath the epithelium, and in the lymph node (median cells per mm2: colon= 560, lymph node = 500, Figure 1b). Other tissue compartments, including the upper genital tract, also showed slightly greater numbers of macrophages (median cells per mm2: uterus endometrium = 153, Figure 1d), with the exception of the lower female reproductive tract, where macrophages were less frequently observed (median cells per mm2: vagina = 7, ectocervix = 12, endocervix = 17).
Lastly, we examined the frequency and location of neutrophils within the same tissues (Figure 1c). In general, neutrophils were the least abundant cell type in the rectal and colonic mucosa and in the upper female genital tract (median cells per mm2: colon= 7, endocervix= 10, uterus= 3); however, in vaginal tissue, often seen in epithelium, neutrophils were present at similar levels to NK cells and slightly outnumbered macrophages (median cells per mm2= 39, Figure 1d). Lymph node neutrophils were found to be more abundant than NK cells, but less frequent than macrophages (median= 110).
Taken together, these data suggest distinct tissue-specific distribution profiles for each innate immune cell subset. Specifically, the data argue that macrophages are the most abundant innate immune cell-type within intestine and lymph node, while comparable levels of NK cells and neutrophils are observed in vaginal tissue, suggestive that distinct antibody-dependent mechanisms may play a dominant role at distinct portals of HIV entry as well as in curative strategies.
Different FcR expression profiles in tissues
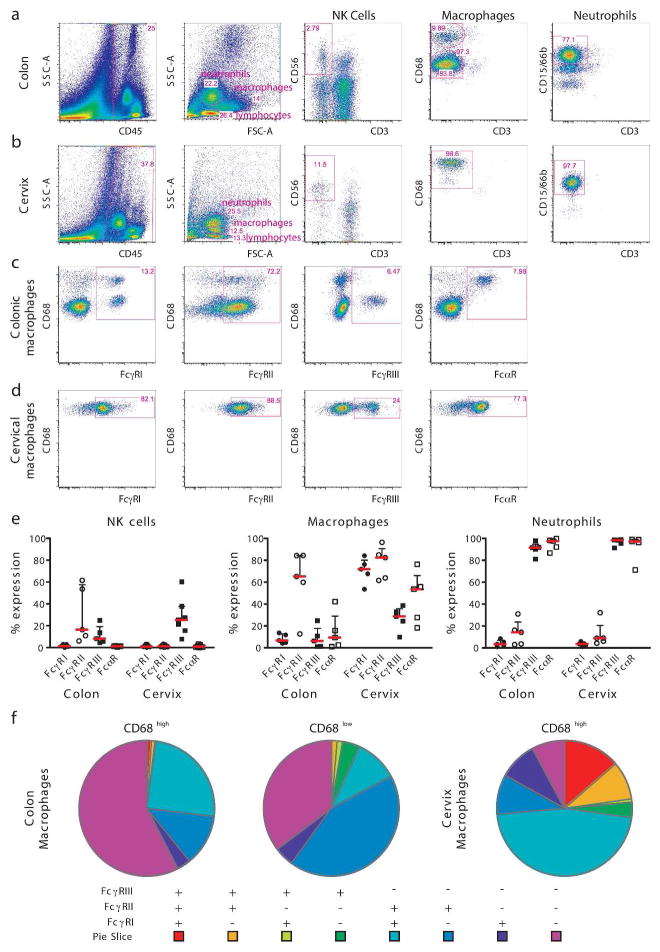
Fc receptor expression varies both depending on the maturation state of the cell as well as on its tissue localization 14. Thus, to gain deeper insights into the Fc receptor distribution on innate immune cells within tissue compartments, fresh colon and cervix specimens were collected from HIV-seronegative subjects. The tissues were mechanically disrupted and enzymatically digested to generate a cell suspension for flow cytometric interrogation using a panel of antibodies to detect FcγRI (CD64), FcγRII (CD32), FcγRIII (CD16), and FcαR (CD89) on NK cells, macrophages and neutrophils.
NK cells were defined as FSClow, SSClow, CD45+ CD3− CD56+ lymphocytes (Figure 2a, b). In contrast to blood NK cells, very few tissue-resident NK cells expressed FcγRIII with slightly more FcγRIII+ NK cells observed in cervical tissues compared to colon. As expected, no FcγRI+ or FcαR+ colonic or cervical NK cells were detected; yet, variable, but always detectable levels of FcγRII+ NK cells were observed in the gastrointestinal tract. Expression of functional CD32 molecules on human NK cells has been shown to be linked to an allelic polymorphism of the FCGRIIC gene that can drive the expression of functional FcRγIIc isoforms 27. This polymorphism may account for the high levels of FcRγII+ NK cells observed in the two donor samples.
Figure 2. FcR expression profile on colonic and cervical innate immune cells.
Gating strategy for NK cells, macrophages and neutrophils is depicted in (a) for colon and (b) for cervix and a representative flow plot for each FcR is presented on colonic (c) and cervical (d) macrophages. Percent of FcR expression on NK cells, macrophages and neutrophils (e) in colon and cervix. The pie charts depict the co-expression profile of all Fcγ receptors in colonic CD68high and CD68low and cervical CD68high macrophages (f).
Mucosal macrophages were larger and more granular and were defined as FSCintermed, SSCintermed, CD45+ CD3−CD68+ (Figure 2a, b). Two distinct populations of macrophages were consistently observed in colonic samples: CD68high and CD68low (Figure 2a). The majority of dominant CD68low macrophages expressed a single FcγRII or no FcγR, while ~40% of the CD68high macrophages co-expressed FcγRI/FcγRII or expressed FcγRII alone (Figure 2f). FcγRIII and FcRα expression on total colonic macrophages was negligible (Figure 2c, e) with FcγRIII restricted to CD68low and FcαR restricted to CD68high macrophages (Figure 2c). In contrast, macrophages in the female reproductive tract exhibited a very different Fc-receptor profile. Only CD68high macrophages were observed in cervical tissues (Figure 2b). Moreover, these macrophages expressed all Fc-receptors frequently with a dominant expression of FcRγII, followed by FcRγI detected on the majority (70%) of cervical macrophages. FcRγIII and FcRα expressing macrophages were also more frequent in the female reproductive tract compared to gastrointestinal macrophages (Figure 2d, e). Notably, higher frequencies of FcγR co-expressing macrophages were found in the cervix compared to the gut, including dominant double positive FcγRI+ FcγRII+ macrophages and up to 20% of cervical macrophages expressing all three Fc-receptors (FcγRI+FcγRII+FcγRIII+), a population that was never observed in the intestinal tract. In summary, these data highlight the remarkable diversity in FcR expression on macrophages at distinct mucosal sites, as has been previously reported for lymphoid tissues 15.
Neutrophils were defined as small, highly granular, FSClow, SSChigh, CD45+, CD3−, CD15+ cells (Figure 2a, b). Both colonic and cervical neutrophils expressed FcRγIII and FcRα but negligible levels of FcRγI and FcRγII. Interestingly, while previous studies suggest that all neutrophils constitutively express FcγRIIa, albeit at 4- to 5-fold lower levels compared to FcγRIIIb 28, we only observed FcγRII expression on ~14% of colon resident neutrophils and ~9% of cervical neutrophils. Thus, here we report a dominant FcγRIII and FcαR profile on tissue-resident neutrophils.
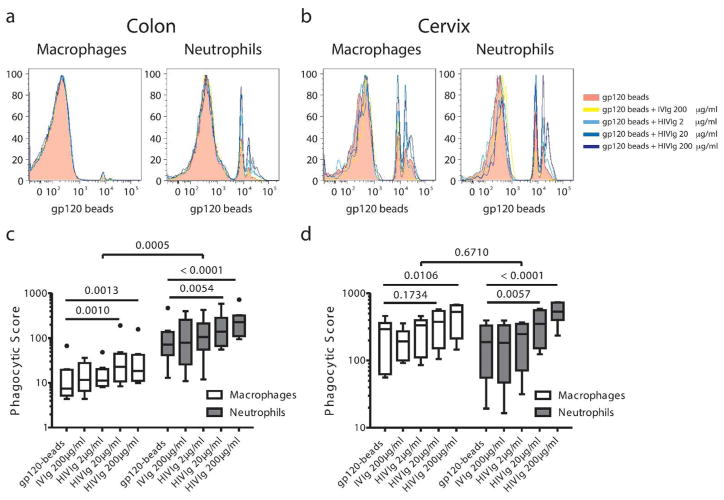
Distinct mucosal phagocytic activity profiles
As tissue-resident NK cells very rarely expressed Fc receptors (Figure 2e), suggesting poor antibody-directed functionality, we aimed to define the functional capacity of mucosal macrophages and neutrophils, which frequently expressed Fc receptors. As both innate effector cell types are critically involved in the rapid phagocytic clearance of immune complexes in the blood, we evaluated the phagocytic potential of these cells from the different mucosal sites using a tissue phagocytosis assay. Gp120-coated fluorescent beads, complexed with a pool of HIV specific polyclonal antibodies (HIVIG), no antibodies, or a pool of non-specific polyclonal antibodies from healthy individuals (IVIG), were added to mucosal cell suspensions (colon or cervical cells). Both neutrophil and macrophage phagocytic activity demonstrated a dose dependent increase in phagocytic uptake in the presence of increasing HIV-specific antibodies and unaltered phagocytosis from the baseline level, in the presence of non-specific antibodies (Figure 3a, b, c, d). Interestingly, despite their lower frequencies, colonic neutrophils showed more robust phagocytic activity, significantly outperforming colonic macrophages (Figure 3a, c). By contrast, cervical macrophages exhibited identical phagocytic potentials as cervical neutrophils (Figure 3b, d), highlighting striking differences in macrophage, but not neutrophil activity, isolated from different mucosal sites. Given that neutrophils represent a much smaller fraction of total innate effector cells within the gut in healthy individuals (Figure 1), the contribution of both neutrophils and macrophages to absolute phagocytosis would likely be similar at the moment of transmission, where poor macrophage responsiveness will be counterbalanced by their sheer abundance.
Figure 3. Phagocytic activity profiles in colon and cervix.
Representative histograms of phagocytic activity of macrophages and neutrophils to gp120-coated beads and gp120-directed immune complexes in colon (a) and cervix (b). Phagocytic activity is represented as a phagocytic score =% of cells taking-up beads x MFI/1000 in colon (c) and cervix (d). Colon, N=8; cervix, N=6.
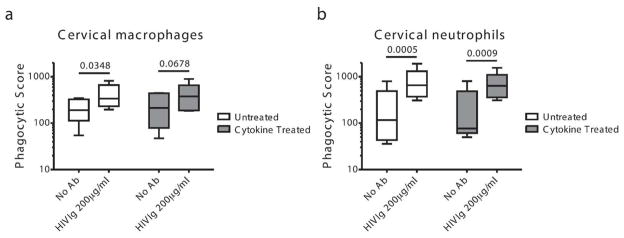
Unaltered cervical phagocytic activity in the presence of inflammatory cytokines associated with increased risk of HIV infection
Epidemiological studies in young women in Africa suggest that sexually transmitted infections that cause inflammation are strongly associated with increased susceptibility to HIV infection 29. Specifically, elevated levels of macrophage inflammatory protein (MIP)-1α, MIP-1β, interleukin (IL)-8 and interferon-γ inducible protein-10 (IP-10) in the cervico-vaginal lavage of women prior to infection have all been linked to increased risk of HIV infection 19. Moreover, MIP-1α, MIP-1β, IL-8 are essential for the establishment of productive simian immunodeficiency virus (SIV) infection in rhesus macaques 30. Thus, we next sought to examine whether alterations in the inflammatory milieu might alter the phagocytic activity of mucosal-resident macrophages and neutrophils or whether vaccine or monoclonal therapeutic antibodies would continue to provide protection irrespective of inflammation. Cell suspensions from cervical mucosa were treated overnight with MIP-1α, MIP-1β, IL-8 and IP-10, after which their phagocytic activity was determined. No differences in phagocytosis were observed between untreated and cytokine treated cervical macrophages (Figure 4a) or cervical neutrophils (Figure 4b). These data suggest that cytokine exposure associated with an increased risk of HIV acquisition does not impair the phagocytic potential of tissue-resident phagocytes, rendering phagocytic activity an attractive mechanism to drive preventative immunity or therapeutic clearance in the setting of existing inflammation.
Figure 4. Phagocytic activity in cervix in the setting of inflammation.
The phagocytic activity of cervical macrophages (a) and neutrophils (b) treated with inflammatory cytokines: MIP1α, MIP1β, IL-8 and IP-10 was compared to untreated cells. Responses to gp120-beads and immune complexes (gp120-beads + HIV-Ig at 200μg/ml) are depicted. N=5.
Alterations in Fc-effector cells in HIV infection
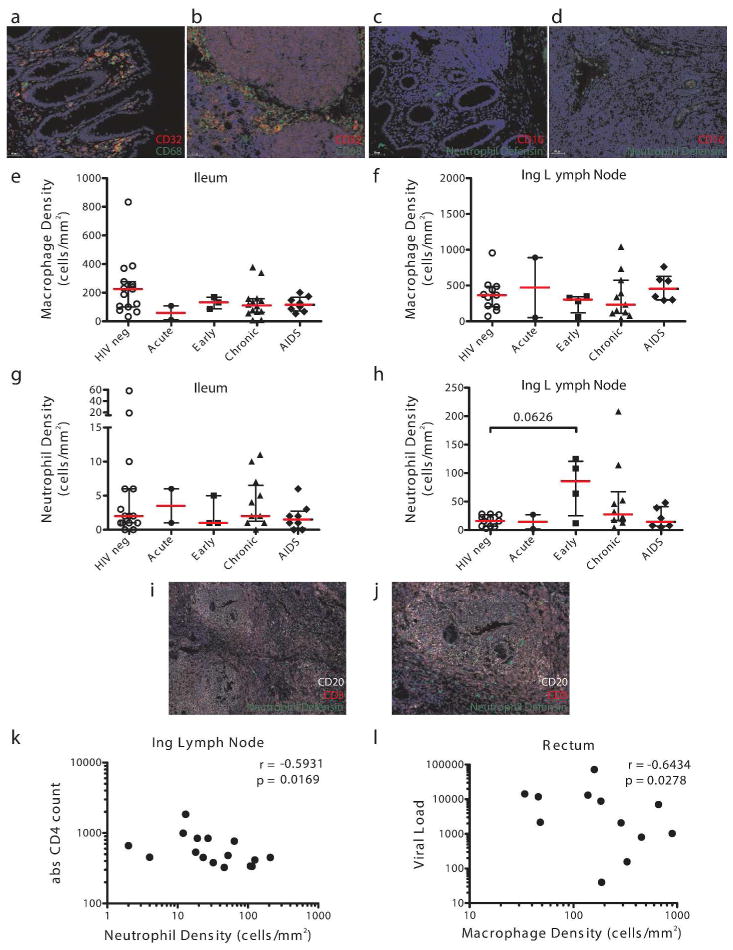
In addition to their role in protection from infection, 5,6 growing interest lies in the potential application of monoclonal therapeutics in HIV eradication. Importantly, the viral reservoir likely persists within the gut 18 and the lymph nodes, specifically the germinal centers 17. Therefore, optimized monoclonal therapeutic strategies able to recruit local innate effector killing may drive more effective reservoir elimination. Given that previous studies have shown altered distribution of mucosal NK cell subsets in the gut in HIV infection, 20 it is possible that other innate immune cells and Fc-receptors may change during infection. Thus, we quantified the frequency of FcγRII+ macrophages (Figure 5a, b) and FcγRIII+ neutrophils (Figure 5c, d) in gastrointestinal and lymph node sections from 27 HIV-infected subjects at different stages of disease and 15 seronegative controls. In lymph node and ileum, stable levels of macrophages were observed during HIV-infection (Figure 5e, f). Likewise, neutrophil numbers were also constant in these compartments (Figure 5g, h), however, infiltrating neutrophils were observed in the lymph nodes in the early phase of HIV infection (documented seroconversion within the last 6 months), and several subjects in the chronic stage of the disease (Figure 5h). Notably, these infiltrating neutrophils were observed in both the T-cell zone and B-cell follicles (Figure 5i, j), suggesting that these innate effector cells may gain access to the immunologic sanctuaries where the reservoir is thought to persist. Interestingly, this neutrophil expansion was strongly correlated with viremia (data not shown) and negatively correlated with absolute CD4 counts in acute, early and chronic patients (Figure 5k), arguing that active viral replication, due to incomplete reservoir control, may drive the recruitment of neutrophils to the lymph node. In contrast to the associations observed for neutrophils, no link was observed between macrophage numbers and viremia in lymph nodes (data not shown). However macrophage numbers were inversely associated with viremia in the rectum (Figure 5l) and ileum as compared to uninfected controls (data not shown).
Figure 5. Changes in Fc-effector expressing innate immune cells in tissues during HIV infection.
Immunofluorescence staining of CD32+ macrophages in colon (a) and lymph node (b) and CD16+ neutrophils in colon (c) and lymph node (d). Quantification of macrophages (e, f) and neutrophils (g, h) in ileum and inguinal lymph node in subjects at different stages of HIV disease (HIV neg, open circle; acute, closed circle; early, closed square; chronic, closed triangle; AIDS, closed diamond). Infiltrating neutrophils into T-cell zone and B-cell follicles in HIV infected lymph nodes (i, j). Neutrophil density in lymph nodes correlated with absolute CD4 counts (k) in acute, early and chronically infected subjects. Macrophage density in rectal biopsies correlated negatively with peripheral viral load (l).
Thus, given the selective recruitment of neutrophils to the germinal centers and stable, albeit low levels in intestine, the application of Fc-enhancing monoclonal therapeutics that could selectively direct clearance of infected/reactivated cells may represent a promising novel means by which to eradicate the viral reservoir to attain functional cure. Further, this study demonstrates that Fc-receptor expressing macrophages decline in the gastrointestinal mucosa but not in lymph nodes, suggesting their compromised effector cell potential for reservoir eradication at specific sites.
Enhancing Fc-effector activity to drive more effective antiviral activity
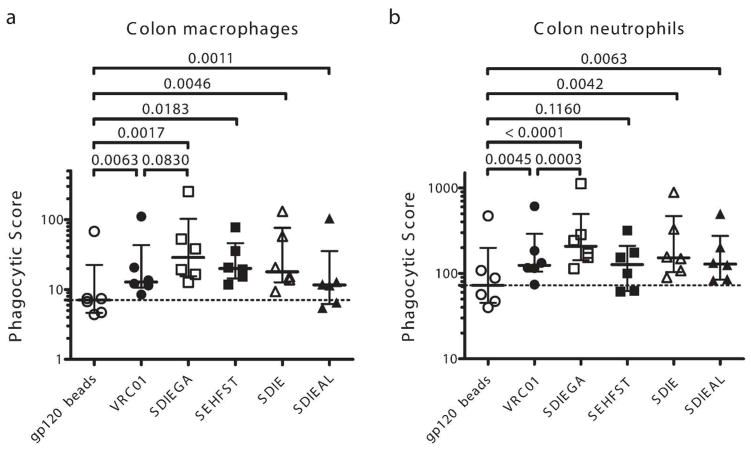
The use of some of the most potent neutralizing antibodies to target and eliminate the viral reservoir is gaining momentum 31. However, while these antibodies clearly diminish viral loads transiently, 11 they have demonstrated limited success in eliminating latently infected cells. Conversely, efforts in the monoclonal therapeutics community have shown that Fc-effector function can be improved via Fc-modifications. Many Fc modifications have now been identified, and each of these modifications can drive differential Fc-effector activities 32,33. Thus, we aimed to exploit these Fc modifications to identify Fc enhancements that could augment the uptake of immune complexes by one of the most potent bNAbs, VRC01 34. The tissue phagocytosis assay was employed using immune complexes generated with wild-type VRC01 (WT) or the following mutants: the S239D/I332E/G236A (SDIEGA) variant with enhanced FcRγIIA affinity, 23 the S267E/H268F/S324T (SEHFST) variant with enhanced C1q affinity, 24 and the S239D/I332E (SDIE) and S239D/I332E/A330L (SDIEAL) variants with enhanced FcRγIIIA affinity 23. All antibodies successfully boosted macrophage phagocytosis, and nearly all (with an exception of SEHFST variant) boosted neutrophil phagocytosis, however the FcRγIIa enhanced monoclonal variant, SDIEGA, was the most potent in enhancing phagocytosis of both innate immune cells (Figure 6a, b). Interestingly, the complement enhancing-variant, SEHFST, improved macrophage, but not neutrophil phagocytosis, possibly due to expression of membrane C1q in gut macrophages 35 or an increased affinity for FcγRIIa and FcγRIIb 36 of the S267E mutation. Nevertheless, none of the Fc enhancement strategies could improve colonic macrophage responsiveness to the levels observed in neutrophils, confirming the attenuated nature of macrophage potency in the gut environment and suggesting that harnessing the alternate innate immune effector cells may be critical for effective killing of virally infected cells in the gut.
Figure 6. Enhancement of phagocytic activity via Fc-engineering.
Phagocytic activity of macrophages (a) and neutrophils (b) to immune complexes was evaluated using a VRC01 WT and its Fc-enhanced variants at 4μg/ml (open circle, no antibody; closed circle, wild type VRC01; open square, SDIEGA, enhanced FcRγIIa affinity; closed square, SEHFST, enhanced C1q (complement) affinity; open triangle, SDIE, enhanced FcRγIIIa affinity; closed triangle, SDIEAL, enhanced FcRγIIIa affinity). Phagocytic score is depicted.
Overall, the data presented here suggest that clearance of reactivated latent cells may be improved following the delivery of Fc-enhanced monoclonal therapeutics, specifically targeting phagocytes as effector cells in anti-HIV-1 prevention or cure strategies.
Discussion
While mounting evidence points to a critical role for antibody Fc-effector function in protection from infection 37 and eradication, 10,11 knowledge about the principal tissue FcR+ effector cells, their Fc receptor profile, functional activity and their changes in infection is critical to maximize the effectiveness of antibody-mediated functions, capable of containing the infection at the site of HIV transmission and persistence. Here we found that while FcR+ NK cells were present at low frequencies in tissues, major differences in the distribution of FcR+ macrophages and neutrophils were noticeable among the compartments. Specifically, we observed a dominant population of macrophages in the intestinal tract, lymph node, endocervix in addition to large proportions of neutrophils in female genital tract, especially in the vaginal mucosa. Conversely, HIV-infection was associated with reduced macrophage frequencies in intestinal tissues and increased neutrophil numbers in lymph nodes in the setting of detectable viremia. Additionally, while neutrophil phagocytic activity was similar between the FRT and GI tract, unaltered in the presence of inflammatory cytokines, colonic macrophages exhibited an attenuated functional profile compared to FRT macrophages. Promisingly, Fc-engineered monoclonal variants were able to improve both macrophage and neutrophil phagocytosis, providing a means to selectively promote these innate immune effector responses both in the setting of preventative and curative strategies. Overall, the study provides the first insights related to the specific innate immune Fc-effector mechanisms in tissues that may confer the greatest level of protective or therapeutic immunity for future rationally designed interventions.
Accumulating data point to a role for ADCC in post-infection control of viremia in both humans 38 and NHP 39,40. By contrast, the role of Fc-mediated functions in blocking HIV acquisition is indirect and more controversial 2,4,41 with the most provocative evidence emerging from the passive immunization studies employing neutralizing monoclonal antibodies, 4,42 where the abrogation of FcR binding resulted in compromised protection from infection 4,43. More recently, FcγR-mediated effector function was shown to contribute substantially to the in vivo capacity of several bNAbs to block viral entry, suppress viremia, and confer therapeutic activity 9. However, directed Fc-receptor enhancing strategies aimed at increasing protection from infection via ADCC, including the generation of nonfucosylated b12, resulting in improved affinity for FcRγIIIa and more effective in vitro NK cell mediated-killing, did not increase protection from HIV acquisition in an NHP low dose challenge model 41. These results suggest that enhanced NK cell ADCC may not improve protective immunity, but instead other innate Fc-effector mechanisms may be more critical. Along these lines, here we demonstrate that FcγRIII+ NK cells are nearly absent from most vulnerable tissues (Figure 2), supporting the notion that ADCC activity will depend heavily on the recruitment of peripheral blood NK cells to the site of viral invasion. Conversely, the employment of highly phagocytic cells, that represent the first line of innate FcR activity, may provide a rational means by which the first infectious virions and/or infected cells may be readily cleared prior to the establishment of a latent reservoir.
To date, little is known about the potential role of phagocytosis in blocking HIV acquisition. However, in vitro studies have demonstrated that HIV specific antibodies are able to inhibit HIV in the presence of monocyte-derived macrophages 44. Moreover, Fc-mediated phagocytosis, but not ADCC, was directly associated with protection from a SHIV162P3 challenge following Ad26 vaccination of NHP 3. Additionally, phagocytosis was also indirectly implicated in post-infection viral control by non-neutralizing antibodies in NHPs challenged vaginally with SHIV162p3 2. Here we report highly enriched resident phagocytes at the sites of vulnerability (Figure 1). While neutrophils were relatively infrequent within the gut lamina propria, these cells exhibited remarkably high phagocytosis (Figure 3), were often seen in vaginal epithelium, and were highly abundant in the venules perfusing all the tissues (Figure 1), potentially allowing for the rapid migration into the tissues upon infection.
Intriguingly, despite their high abundance in the gut, intestinal macrophages exhibited a log lower phagocytic activity than cervical macrophages, likely explained by distinct FcR expression profiles, where all FcRs were expressed on cervical macrophages, and gut macrophages predominantly expressed FcγRII (Figure 2). These substantially different profiles reflect well established phenotypic and functional heterogeneity of tissue resident macrophages, reflective of their anatomic origin 45 and essential for their niche-specific functions. For example, intestinal macrophages play a critical role in the maintenance of gut homeostasis and the regulation of immune response to commensals 46. Thus under steady state conditions, lamina propria macrophages display an anergic phenotype, demonstrated by their inability to produce pro-inflammatory cytokines, 47 that may account for their poor responsiveness to phagocytosis. In contrast, FRT macrophages may be poised to respond to pathogens due to chronic exposure to non-self antigens, bacteria, and viruses strongly pointing to their critical role as an innate effector that may confer enhanced protection in heterosexual exposure. Furthermore, these inherent differences in phagocytic activity observed in two distinct mucosal compartments could be directly linked to permeability of mucosal barriers to infection, where rectal mucosa is more permissive to viral transmission and systemic infection than vaginal mucosa as described in atraumatic SHIV infection model 48.
Despite their low tissue-resident frequencies, neutrophils mediated the highest phagocytic activity (Figure 3), implicating these cells as potentially critical Fc-effectors for both prevention and therapeutic strategies. Neutrophils are able to phagocytose bacteria, fungi and virions. Yet most of the work performed on neutrophils in HIV infection has focused on their role in viral pathogenesis. Circulating neutrophils constitutively express FcγRIIa and 4- to 5- fold higher levels of FcγRIIIb 28. FcγRIIIb, the only glycosylphosphatidylinositol (GPI)-anchored Fc-receptor, is 97% homologous in its extracellular domain to FcγRIIIa, however, FcγRIIIb differs from FcγRIIIa in its ability to drive phagocytosis 49. While some studies suggest that FcγRIIa drives the bulk of neutrophil phagocytic activity, 50 the functional interaction between FcγRIIIb and FcγRIIa on neutrophils is unclear but likely collaborate to tune effector activity 51. Furthermore, neutrophils have been linked to vaccine induced antiviral control of human papilloma virus in the FRT 52. Given that neutrophils are stable during the menstrual cycle and retain unaltered activity in inflammatory conditions (Figure 4), they may prove especially important in preventing heterosexual HIV-transmission in women. Yet, because neutrophil activity was also potent in gut tissue (Figure 3), it is plausible that this innate immune effector cell may also contribute to the prevention of rectal HIV transmission.
To date, only one case of sterilizing cure has been reported, however an array of different strategies are being explored to rapidly eliminate reactivated cells. 53 Among them, efforts to utilize monoclonals have been tested 31. Specifically, combinations of 2G12, 2F5, and 4E10 mAbs transiently controlled viremia in humans prior to viral escape, 54 similar to the effect observed with 3BNC117 in NHPs 55 and humans 11. Nevertheless, studies in HIV–infected humanized mice demonstrate the clear need for Fc-effector functions in reservoir control 56. Furthermore, recent data shows that tissue myeloid cells, particularly within mesenteric lymph nodes and spleen, play a critical role in the phagocytosis of infected T cells 57, thus, particular Fc-modifications that can enhance myeloid clearance mechanisms at the sites of virus persistence, including germinal centers, 17 may enhance the elimination of the viral reservoir size. Moreover, FcR+ neutrophils were present within the GCs (Figure 5) where they have been shown to collaborate in the induction of potent humoral immune responses 58. Along these lines, the Fc- mutant SDIEGA, known to exhibit enhanced affinity for FcγRII, drove enhanced macrophage- and neutrophil-mediated phagocytosis compared to the wild type bNAb (Figure 6), pointing to specific Fc-modifications that could enhance reservoir eradication.
In conclusion, our data demonstrate that unlike NK cells, phagocytic cells represent our major mucosal tissue Fc-receptor bearing effectors prepared to respond to antibodies for the rapid control and clearance of HIV. Additionally, mucosal macrophages and neutrophils express FcαR, pointing to further opportunities to harness both IgG and IgA molecules to harness the full innate armamentarium within vulnerable mucosal tissues.
Acknowledgments
We thank Dr Joseph Misdraji, Joelle Brown and Eric Safai for their help with healthy control tissue collection. Further, we thank summer students Fernanda Cerqueira and Madelyn O’Kelley-Bangsberg for help with optimization of immunohistochemistry stainings and Ciprian Husanu for help with the Tissue Gnostics software. This work was supported by the National Institute of Health (R01 AI080289, R21 AI110165 and AI102660), the Bill and Melinda Gates Foundation CAVD (OPP1032817: Leveraging Antibody Effector Function) and the Ragon Institute of MGH, MIT and Harvard.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moog C, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–9. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 5.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A. 2011;108:20690–4. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–51. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forthal DN, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180:1338–41. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer LA, et al. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990;6:341–56. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 9.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–99. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–8. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015 doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5:2684–90. doi: 10.1096/fasebj.5.12.1916092. [DOI] [PubMed] [Google Scholar]
- 13.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–13. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 14.Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front Immunol. 2012;3:347. doi: 10.3389/fimmu.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuijnman WB, Van Wichen DF, Schuurman HJ. Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: an immunohistochemical study. APMIS. 1993;101:319–29. doi: 10.1111/j.1699-0463.1993.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 16.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 U.S. dependent areas - 2012. CDC: HIV Surveillance Supplemental Report 2014. 2014;19(3) [Google Scholar]
- 17.Fukazawa Y, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–9. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun TW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 19.Masson L, et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin Infect Dis. 2015;61:260–9. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sips M, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boesch AW, et al. Highly parallel characterization of IgG Fc binding interactions. MAbs. 2014;6:915–27. doi: 10.4161/mabs.28808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackerman ME, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards JO, et al. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–27. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 24.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2:181–9. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leno-Duran E, Munoz-Fernandez R, Olivares EG, Tirado-Gonzalez I. Liaison between natural killer cells and dendritic cells in human gestation. Cell Mol Immunol. 2014;11:449–55. doi: 10.1038/cmi.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metes D, et al. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcgammaRIIC gene. Blood. 1998;91:2369–80. [PubMed] [Google Scholar]
- 28.Selvaraj P, Rosse WF, Silber R, Springer TA. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988;333:565–7. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- 29.Masson L, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90:580–7. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31:13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jefferis R, Lund J, Pound JD. IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev. 1998;163:59–76. doi: 10.1111/j.1600-065x.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–10. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, et al. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol. 2011;85:8954–67. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul M, Loos M. Expression of membrane C1q in human monocyte-derived macrophages is developmentally regulated and enhanced by interferon-gamma. FEBS Lett. 2001;500:91–8. doi: 10.1016/s0014-5793(01)02592-3. [DOI] [PubMed] [Google Scholar]
- 36.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109:6181–6. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung AW, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 38.Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142:46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrantelli F, et al. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS. 2003;17:301–9. doi: 10.1097/00002030-200302140-00003. [DOI] [PubMed] [Google Scholar]
- 40.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses. 2002;18:1197–205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 41.Moldt B, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86:6189–96. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bournazos S, et al. Broadly Neutralizing Anti-HIV-1 Antibodies Require Fc Effector Functions for In Vivo Activity. Cell. 2014;158:1243–53. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holl V, et al. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J Immunol. 2004;173:6274–83. doi: 10.4049/jimmunol.173.10.6274. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz AM, DiNapoli SR, Brenchley JM. Macrophages Are Phenotypically and Functionally Diverse across Tissues in Simian Immunodeficiency Virus-Infected and Uninfected Asian Macaques. J Virol. 2015;89:5883–94. doi: 10.1128/JVI.00005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162–8. doi: 10.1016/j.it.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chenine AL, et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J Infect Dis. 2010;201:1155–63. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagarajan S, et al. Ligand binding and phagocytosis by CD16 (Fc gamma receptor III) isoforms. Phagocytic signaling by associated zeta and gamma subunits in Chinese hamster ovary cells. J Biol Chem. 1995;270:25762–70. doi: 10.1074/jbc.270.43.25762. [DOI] [PubMed] [Google Scholar]
- 50.Rivas-Fuentes S, Garcia-Garcia E, Nieto-Castaneda G, Rosales C. Fcgamma receptors exhibit different phagocytosis potential in human neutrophils. Cell Immunol. 2010;263:114–21. doi: 10.1016/j.cellimm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, et al. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood. 2012;120:4421–31. doi: 10.1182/blood-2011-12-401133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day PM, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–70. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deeks SG, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–14. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–22. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 55.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–80. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–22. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calantone N, et al. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity. 2014;41:493–502. doi: 10.1016/j.immuni.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]