Abstract
Study Objectives:
School night total sleep time decreases across adolescence (9–18 years) by 10 min/year. This decline is comprised entirely of a selective decrease in NREM sleep; REM sleep actually increases slightly. Decreasing sleep duration across adolescence is often attributed to insufficient time in bed. Here we tested whether sleep restriction in early adolescence produces the same sleep stage changes observed on school nights across adolescence.
Methods:
All-night sleep EEG was recorded in 76 children ranging in age from 9.9 to 14.0 years. Each participant kept 3 different sleep schedules that consisted of 3 nights of 8.5 h in bed followed by 4 nights of either 7, 8.5, or 10 h in bed. Sleep stage durations and NREM delta EEG activity were compared across the 3 time in bed conditions.
Results:
Shortening time in bed from 10 to 7 hours reduced sleep duration by approximately 2 hours, roughly equal to the decrease in sleep duration we recorded longitudinally across adolescence. However, sleep restriction significantly reduced both NREM (by 83 min) and REM (by 47 min) sleep. Sleep restriction did not affect NREM delta EEG activity.
Conclusions:
Our findings suggest that the selective NREM reduction and the small increase in REM we observed longitudinally across 9–18 years are not produced by sleep restriction. We hypothesize that the selective NREM decline reflects adolescent brain maturation (synaptic elimination) that reduces the need for the restorative processes of NREM sleep.
Citation:
Campbell IG, Kraus AM, Burright CS, Feinberg I. Restricting time in bed in early adolescence reduces both NREM and REM sleep but does not increase slow wave EEG. SLEEP 2016;39(9):1663–1670.
Keywords: adolescent, brain maturation, delta, sleep deprivation
Significance.
The decline in sleep duration across adolescence is commonly attributed to reduced time in bed due to progressively later bedtimes. We previously found that this decline is entirely produced by decreasing non-rapid eye movement (NREM) sleep. Here we show that reducing time in bed in adolescence decreases both NREM and REM sleep. Therefore, sleep restriction cannot explain the EEG pattern of decreasing adolescent sleep. We propose that adolescent brain maturation driven by synaptic pruning reduces the need for the recuperation provided by NREM sleep, i.e. is maturational rather than environmentally produced. Future research should further elucidate the bio-behavioral causes and functional significance of changes in sleep across adolescence. This information is needed for evidence-based recommendations of adolescent sleep durations.
INTRODUCTION
Sleep schedules change substantially across the teenage years. A survey of Australian children found that sleep duration decreases by 12 minutes per year between ages 9 and 18 years.1 Cross-sectional survey studies from industrialized nations have found that, across adolescence, bedtime becomes later (reviewed in Gradisar2). Delayed bedtimes are attributed to multiple environmental factors including scholastic and social pressures, technology use, and reduced parental control. Changes in circadian and homeostatic regulation of sleep have also been implicated.3 A circadian phase delay is evident in adolescents' propensity to delay bedtime and rise times on weekends or on vacation when the restrictions of school schedules are removed.2–4 During the school year, a delay in bedtimes, combined with rise times that remain relatively unchanged or become even earlier as children progress through adolescence,1 would reduce time in bed. The adolescent decline in sleep duration is commonly attributed to this reduced time in bed. One additional factor and/or alternative explanation for declining sleep duration is that adolescent brain maturation decreases the need for the restorative processes of NREM sleep. We have hypothesized that synaptic elimination during adolescence produces a profound reorganization of the human brain and accounts for changes in sleep, brain metabolic rate, and cognitive development.5
In addition to raising this basic neuroscience issue, the adolescent decline in sleep duration bears on social policy. The American Academy of Pediatrics has proposed later school start times to mitigate the harm caused by sleep loss,6 and some school districts have responded by delaying school start times. In order for the sleep research community to make the best public policy recommendations, we must fully understand how sleep and sleep need change across childhood and adolescence.
In a recent large-scale longitudinal study, we measured sleep duration across adolescence with EEG recording.7 All-night sleep EEG was obtained twice yearly in subjects sleeping at home on their current school-night sleep schedules. These direct measurements confirmed the survey reports of declining sleep duration across adolescence. Between ages 9 and 18 years, bedtime was delayed by about 13 min per year, but rise time did not change.7 From 515 minutes at age 9 years, recorded sleep durations decreased by about 10 min per year. This decrease was composed entirely of a 12 min per year decline in NREM sleep. REM sleep duration actually increased, by about 2 min per year producing the net decline of ∼10 min/ year in total sleep. These EEG patterns deviate markedly from those produced by experimental restriction of time in bed in young adults which significantly reduces both NREM and REM sleep,8–11 with the reduction in REM proportionally greater.
Since most previous EEG studies of sleep restriction were conducted in adults, it remains conceivable that the sleep EEG of adolescents would respond differently to sleep restriction, i.e., by selectively reducing NREM and increasing REM durations. We were able to test this possibility in our current longitudinal study that varies sleep schedules across adolescence. This study is aimed at measuring changes in sleep need across adolescence: we systematically vary time in bed and determine the effects on sleep EEG, daytime sleepiness, and cognitive function. The goal of the analyses presented here is more limited: to determine whether an experimental restriction of time in bed (TIB) in adolescents produces changes in sleep durations and sleep architecture similar to those we observed across adolescence. If it does not, it would argue that the NREM decrease and the REM increase observed in our longitudinal study are not due to sleep restriction.
Our study also provided the opportunity to further examine slow wave EEG regulation in adolescents. Delta EEG activity during NREM sleep is a marker of a recuperative process of sleep. Delta activity is elevated following total sleep deprivation12 and reduced following daytime naps.13–15 Our current study enables us to determine whether a reduction of TIB from 10 to 7 hours in young adolescents increases delta (1–4 Hz) EEG power (average delta activity) and/or delta energy (total delta activity). A reduction of sleep time (and increase in waking) similar to that proposed here does not stimulate delta production in young adults.9–11 However, since young adolescents have very high levels of delta EEG activity, they might respond differently.
METHODS
Subjects
Seventy-seven children (41 male, 36 female) are enrolled in the study. Data presented here are from the first year of this multi-year longitudinal study. At the time of the first recording, participants' ages ranged from 9.9 to 14.0 years (mean 12.2, SD 1.2). Data from one participant were omitted from the analyses because he failed to adhere to the prescribed sleep schedules (see below). Despite our attempts to recruit a diverse participant pool, the subjects were primarily (75%) white.
Subjects were screened in an interview with a parent for the following exclusion criteria: diagnosed psychiatric or behavioral disorder, epilepsy, head injury resulting in loss of consciousness and symptoms persisting longer than 24 h, diagnosed sleep disorder, a Sleep Disturbance Scale for Children t-score > 70, visual problems that could not be overcome with corrective lenses, manual dexterity problems that would interfere with daytime performance testing, and use of medication affecting the central nervous system. For logistical reasons, participants were selected from an area within 20 miles of the UC Davis Sleep Lab and had to plan to live in this area for the duration of the study. Parents provided informed consent, and children older than 12 years provided informed assent. Participants received monetary compensation for each study component they completed.
Experiment Design
Annually, participants complete 3 weeks of study, each with a different assigned sleep schedule. All weeks begin with 3 nights with 8.5 h in bed followed by 4 consecutive nights of either 7, 8.5, or 10 h in bed. Sleep opportunity can be restricted by either delaying bedtime or advancing rise time. We wished to mimic the habitual sleep schedule changes of adolescence; therefore, participants maintain their habitual schoolday rise times, and shift their bedtime in order to achieve the assigned time in bed. Annually, participants complete each of the 3 schedules within a 3-month period. Accommodating the participants' scholastic, athletic, and social demands prevented us from randomizing the order that participants completed each TIB schedule. Adherence to the assigned schedule is confirmed with actigraphy watches. If participants fail to maintain the sleep schedule, the week is rescheduled. Technical problems with the actigraphy watches allowed one participant's deviation from the assigned schedule to go unnoticed until it was too late for him to repeat the condition. This subject's year 1 data were not included in the analyses presented here.
EEG Recording and Analysis
All night sleep EEG was recorded on the 2nd and 4th night of the 4 consecutive nights on the assigned sleep schedule. Recordings were made at the participants' homes in their habitual sleep environment. Trained technicians traveled to the participants' homes and applied electrodes at the following locations: F3, F4, C3, C4, P3, P4, O1, O2, A1, A2, LOC, ROC, forehead, and 2 chin locations. Reference and ground electrodes were also applied on the scalp and face. Signals were recorded at 400 Hz on Grass Aura ambulatory EEG recorders. The Aura amplifiers have single pole low frequency filters with a −3 dB point at 0.5 Hz and a 6 dB/octave slope and 3 pole high frequency filters with a −3 dB point at 100 Hz and an 18 dB/octave slope. All signals were recorded versus reference. EEG signals versus contralateral mastoid were obtained via subtraction as were EOG signals versus forehead. Chin EMG was recorded bipolarly. The Auras store all night data on a flash card for later downloading to a laboratory computer. Technicians left the house after starting the recording, i.e., lab personnel did not monitor the all-night recordings.
Sleep stage scoring of all night EEG was done using PASS PLUS (Delta Software, St. Louis) to visually display the digitized data. Time cues on this display prevented the scorer from being blind to time in bed condition. Each 20-s epoch was scored as wake, NREM 1, NREM 2, NREM 3, or REM according to 2007 AASM visual scoring standards.16 Prior to scoring, the entire night's record was briefly examined to determine which central channel, C3-A2 or C4-A1, had fewer artifacts. At the time of visual stage scoring, epochs containing artifacts were also identified. All scoring was completed by 3 trained scorers. All records were either scored or checked by a senior scientist (IGC). These data are being compared to previously published data on the adolescent maturation of NREM and REM duration. We scored 20-s epochs to match the previous study. In that study NREM 2 and NREM 3 were combined into a single NREM stage. We similarly summed NREM 2 and NREM 3 of the current data. Stage N1 was not included in total NREM sleep duration. As in the previous study, total sleep time was the sum of NREM and REM sleep durations with N1 excluded.
We also examined the effect of sleep schedule on slow wave activity in the central EEG signal (C3-A2 or C4-A1) that contained fewer artifacts. Both total delta energy and average delta power were evaluated for NREM sleep in the entire night, in the first 5 h of NREM sleep, and in the first NREM period (NREMP1). The entire night provides a measure of all delta accumulated. The first 5 h of NREM sleep provides a duration common to all 3 sleep conditions. NREMP1 has been shown to be particularly sensitive to age17 and to homeostatic manipulation such as napping.13 All artifact free epochs were analyzed with the fast Fourier transform component of PASS PLUS using 5.12-s Welch tapered windows with a 2.62-s overlap resulting in 8 windows per 20-s epoch.
Statistical Analysis
Data were analyzed with SAS mixed effect analysis. Mixed effect analysis is appropriate for repeated measures on the same subject because it accounts for the inherent correlation between these multiple observations.18,19 The main hypotheses of effects of sleep schedule on sleep stage durations and delta EEG activity were tested on night 4 sleep durations because the largest effects would be expected following more nights of sleep restriction. However, we also tested for differences between night 2 and night 4.
In order to fully understand the data, we ran analyses in addition to those that addressed the main hypotheses. Specifically, we tested the effects of the 3 TIB schedules on sleep latency and wake after sleep onset (WASO). We also tested for sex differences and age effects using age from birth to the first week on the sleep schedule, and we tested whether TIB effects differed by sex or changed with age.
RESULTS
Effect of Time in Bed on Sleep Duration
As shown in Figure 1A, restricting time in bed significantly (F1,118 = 1,498, P < 0.0001) shortened total sleep time. From an intercept of 535 min at 10 h in bed, total sleep time decreased by 43.2 min for each hour reduction in time in bed. The average sleep duration on night 4 of the 7-h TIB condition (405 ± 2 min, mean ± SE) was more than 2 hours shorter than the average sleep duration on night 4 of the 10-h TIB condition (534 ± 3 min).
Figure 1.
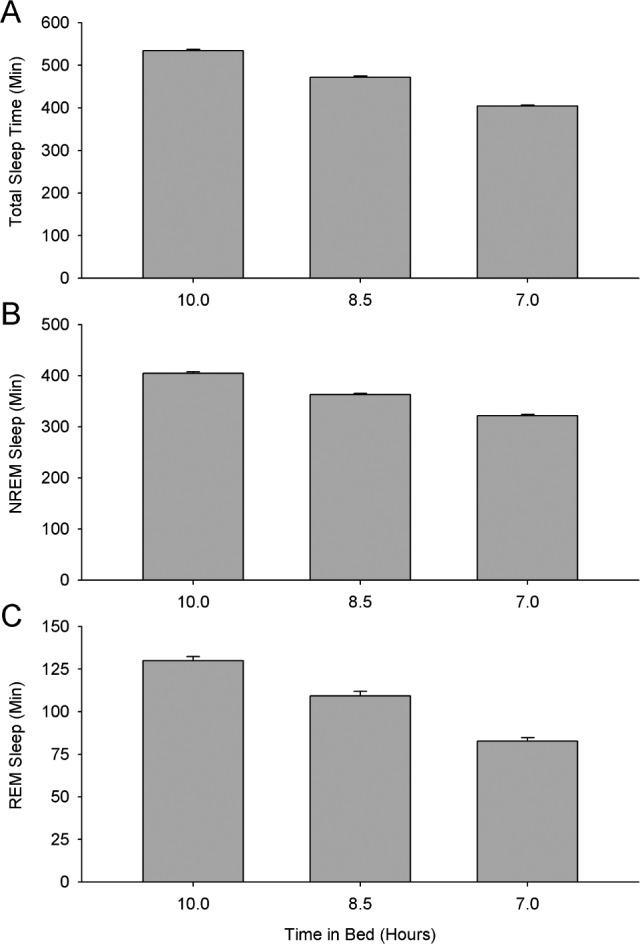
Bar graph comparing average (± standard error) sleep duration for each of the 3 sleep schedules. Total sleep time (A), NREM sleep time (B), and REM sleep time (C) all declined significantly with decreasing time in bed.
This total sleep time decrease was comprised of reductions in both NREM and REM sleep durations (Figures 1B and 1C). Average NREM duration on night 4 declined from 405 ± 3 min with 10 h in bed to 322 min ± 2 min with 7 h in bed. Average REM sleep duration declined from 130 ± 2 min with 10 h in bed to 83 ± 2 min with 7 h in bed. Linear mixed effect analysis revealed that from an intercept of 404 min at 10 h in bed, NREM sleep duration decreased significantly (F1,118 = 699, P < 0.0001) by 27.5 min for each hour of TIB reduction. This NREM sleep decrease was comprised entirely of an N2 decrease (F1,118 = 532, P < 0.0001); N3 duration (Table 1) was unaffected by TIB reduction (F1,118 = 0.02, P = 0.90). REM sleep duration decreased significantly (F1,118 = 244, P < 0.0001) from an intercept of 131 min at 10 h in bed by 15.7 min for each hour of TIB reduction. The REM decrease was proportionally greater than the NREM decrease; consequently, REM as a percentage of total sleep time decreased significantly (F1,118 = 44.5, P < 0.0001) as TIB was reduced. REM sleep latency was unaffected (F1,98 = 0.75, P = 0.39) by TIB reduction.
Table 1.
Sleep stage durations and latencies for each time in bed schedule and number of nights on the schedule.

As noted above, when TIB was 10 h, TST was 129 min longer than at 7 h. The 51 min difference between the increased TIB and increased TST (i.e., 180 min - 129 min) consisted of increased sleep onset latency, increased WASO, increased time in N1, and slight deviations from the assigned sleep schedules. Sleep onset latency (Figure 2A) increased by 4.4 min per additional hour TIB (F1,38 = 69.1, P < 0.0001). WASO (Figure 2B) increased by 4.3 min per additional hour TIB (F1,118 = 39.6, P < 0.0001), and N1 sleep duration increased by 1.7 min per additional hour TIB (F1,118 = 60.6, P < 0.0001). The remainder of the difference between increased TIB and increased TST was made up of small deviations from the assigned sleep schedules. On the 7-h TIB condition, participants went to bed on average about 1 min prior to the assigned bedtime and arose about 5 min after their assigned rise time. On the 10-h TIB condition, participants went to bed on average about 6 min after their assigned bedtime and arose about 4 min prior to the assigned rise time.
Figure 2.

Bar graphs comparing average (± SE) sleep onset latency (A) and wake after sleep onset (B) for each of the 3 sleep schedules. Both sleep onset latency and WASO declined significantly with decreasing time in bed.
Differences between Night 2 and Night 4
With all conditions combined, total sleep time did not differ (F1,303 = 1.48, P = 0.23) between night 2 and night 4 (Table 1), nor did NREM duration (F1,303 = 0.88, P = 0.35), N2 duration (F1,303 = 0.55, P = 0.46), N3 duration (F1,303 = 0.02, P = 0.88), REM duration (F1,303 = 1.96, P = 0.16), WASO (F1,303 = 2.6, P = 0.11), or sleep onset latency (F1,132 = 0.98, P = 0.32). Although none of these measured showed a night by TIB interaction (P > 0.05 for all), we tested whether sleep duration increased between nights 2 and 4 on the 7-h TIB condition and whether sleep duration decreased between nights 2 and 4 on the 10-h TIB condition. For the 7-h TIB condition, participants slept 7 min longer on night 4 (F1,55 = 11.1, P = 0.0016). For the 10-h TIB condition, sleep duration did not differ between nights 2 and 4 (F1,52 = 0.43, P = 0.52).
TIB Restriction Effects on NREM EEG Delta Activity
No delta activity measures showed a night effect, and no measures showed a significant night by TIB interaction. Therefore, we used night 4 data to test the effects of TIB on delta EEG activity (Figure 3). All night delta energy did not differ (F1,112 = 2.33, P = 0.13) across the 3 TIB conditions. From 841 μV2 at 10 h TIB, average all night delta power increased by 55 μV2 per hour of TIB reduction (F = 35, P < 0.0001). For the first 5 h of NREM sleep and for NREM period 1, delta energy and delta power were not significantly affected by TIB restriction (P > 0.1 for all).
Figure 3.
Bar graphs comparing average (± SE) delta energy (left column) or power (right column) for each of the 3 sleep schedules for all night NREM sleep (top row), in the first 5 hours of NREM sleep (middle row), and NREM period 1 (bottom row). Delta energy was not significantly affected by reducing time in bed. Reduced time in bed increased only all night delta power, but this increase was a result of decreased NREM duration (see text).
Age and Sex Effects on Sleep Duration and Delta Power
Controlling for differences due to TIB condition and the interaction of age and TIB, mixed effect analysis showed no age effect on total sleep time (F1,74 = 0.0, P = 0.97), NREM duration (F1,74 = 1.84, P = 0.18), REM duration (F1,74 = 2.28, P = 0.14), REM percentage (F1,74 = 1.98, P = 0.16), WASO (F1,74 = 0.03, P = 0.86), sleep latency (F1,57 = 2.37, P = 0.13), or REM latency (F1,73 = 0.54, P = 0.51), nor were there any significant interactions between TIB and age (P > 0.3 for all). N3 duration decreased by 9 min/year (F1,74 = 18.1, P < 0.0001), and N2 duration increased by 6 min/year (F1,74 = 5.83, P = 0.018). TIB effects on N2 and N3 did not differ by age (P > 0.4 for both). Stage N1 sleep decreased slightly (1.1 min/year, F1,74 = 8.06, P = 0.0058) with increasing age, and the N1 decrease with decreasing TIB trended toward becoming greater with age (F1,74 = 3.60, P = 0.060). Even over the limited 10 to 14-year age range, all measures of delta activity declined significantly (P < 0.0001 for all). For example, delta power in the first 5 hours of NREM declined steeply at 227 μV2/year (F1,73 = 27.6, P < 0.0001), from an age 10 intercept of 1,559 μV2. No measure of delta showed an age by TIB interaction (P > 0.2 for all).
In analyses that controlled for differences due to TIB condition and the interaction of sex and TIB effects, boys and girls did not differ in TST, NREM duration, N3 duration, REM sleep duration, WASO, and sleep onset latency (P > 0.1 for all). Furthermore, none of these measures showed a sex by TIB interaction (P > 0.45 for all). Girls did show a trend toward greater N2 duration (F1,74 = 3.22, P = 0.077), greater N1 duration (F1,74 = 5.86, P = 0.018) and a greater decrease in N1 with decreasing TIB (F1,74 = 4.40, P = 0.038). Boys and girls did not differ in REM latency (F1,73 = 0.0, P = 0.99), but the TIB effect differed between the sexes (F1,96 = 8.32, P = 0.0049), such that reducing TIB slightly increased REM latency in boys and slightly decreased REM latency in girls. Girls and boys did not differ in the amount of delta nor was there an interaction between sex and TIB effects; however, girls did show a trend toward a greater age-related decline in delta (F1,71 = 2.79, P = 0.099).
DISCUSSION
This study tested whether sleep restriction in young adolescents produces the same pattern of sleep state changes seen across adolescence and found that it does not. Thus, the decreasing NREM and increasing REM durations across ages 9–18 years observed in our previous longitudinal study7 are not attributable to sleep restriction and are more likely due to other factors. We hypothesize that brain maturation is mainly responsible and discuss this interpretation below. Our findings here also show that restricting sleep from 10 to 7 hours in bed does not increase delta EEG activity. This finding is consistent with published data in young adults, but it raises issues of sleep theory that have not been fully considered.
Effect of Sleep Restriction on Sleep Stage Durations and Latencies
Although our adolescent subjects adhered well to their prescribed sleep schedules, they did deviate by slightly extending their time in bed on the 7-hour schedule and slightly shortening it on the 10-hour sleep schedule. Their adherence makes the experimental time in bed restriction from 10 to 7 hours a reasonable approximation of the sleep schedule changes across adolescence. Sleep restriction in the current study reduced total sleep time by over two hours, an amount somewhat greater than the adolescent decrease of 90 min between 9 and 18 years.7
Our experimental sleep restriction reduced both NREM and REM sleep durations. These results contrast starkly with the adolescent decline in self-selected total sleep time, which was composed entirely of a reduction in NREM sleep duration. While sleep restriction may contribute to sleep loss in adolescents, it does not appear to be the main factor driving the adolescent reduction in sleep time. As we discuss in detail below, one alternative possibility is that brain maturational changes during adolescence reduce the need for the recuperation provided by NREM sleep.
With time in bed restricted to 7 hours, the REM reduction was proportionally greater than that of NREM sleep, so that REM as a percent of total sleep time decreased. Normally, REM percentage increases across the night due to a combination of longer REM and shorter NREM periods.20 Increased REM percentage reflects both a decreased need for NREM recuperation17 and a circadian factor that increases REM at the time of the body temperature minimum.21 Our study design restricts time in bed by delaying bedtime, thereby preserving the morning hours when the circadian propensity for REM is higher. Our 7 hour TIB data showing a decreased REM as a percent of TST indicate that in children 10–14 years of age, the interaction with NREM outweighs the morning circadian drive for REM sleep.
The reduction in NREM duration with TIB restriction was comprised entirely of a reduction of N2 duration; N3 duration was unaffected. Voderholzer's study of sleep restriction effects on memory in adolescents also found a reduction in both stage 2 and REM sleep but no change in slow wave sleep duration.22 The normal pattern of sleep across the night demonstrates that N3 is concentrated in the first part of the night, and N2 percentage increases across the night. Very little N3 is lost when the night is truncated. The fact that N3 has temporal priority has long been recognized as an indicator of its biological importance.17
The main goal of our ongoing study is to measure changes in sleep need across adolescence. In spite of the limited age range studied thus far, the available data offer some clues regarding sleep need. The short sleep onset latency and minimal wake after sleep onset for the 7-hour condition indicate that this duration of time in bed does not provide sufficient sleep for children in this age range. The significant increase in sleep duration between nights 2 and 4 of the 7-hour sleep schedule further indicates that 7 hours is insufficient. Examining the data more closely revealed that the 7-minute increase in sleep time from night 2 to 4 was not due to increased sleep efficiency; instead, the participants sought more sleep by going to bed slightly earlier than scheduled.
The age range of cross-sectional data presented here covers only early adolescence, ages 10 to 14 years. We did not find the age-related decreased NREM duration and increased REM duration that we previously found across 9–18 years.7 Furthermore, we did not find age-related difference in how TIB altered TST, NREM, or REM duration. We did find that N3 duration decreased with age, a reduction that is likely related to the delta decline that we discuss below. We also did not find sex differences in duration of TST, NREM sleep, REM sleep, or TIB effects on these sleep duration measures. Age and sex differences may be revealed as we continue this longitudinal study that will eventually cover ages 10 to 17 years.
Brain Maturation May Explain Adolescent Changes in Sleep Stage Durations
If it is not due to sleep restriction, what accounts for the selective decrease in NREM duration across adolescence that we found in our previous study? One alternative explanation is that this change is a manifestation of adolescent brain maturation. In childhood the brain over-produces connections between neurons, and during adolescence the brain removes unnecessary synapses.23 This synaptic pruning streamlines neural circuits, making them more efficient, and thereby reduces waking brain activity, as shown in the decline in cerebral metabolic rate.24 NREM delta power reflects the intensity of a recuperative process that occurs during NREM sleep.17,25 As demonstrated by the steep decline in delta power over the teenage years,26,27 adolescent brain maturation reduces the need for this recuperative process. The strikingly parallel trajectories of synaptic density,23 cerebral metabolic rate,24 and high-amplitude NREM delta EEG across the first two decades of life provide support for this interpretation.28 We propose that the adolescent decline in NREM duration and NREM delta activity are components of the same maturational process. If true, NREM delta activity and NREM duration should have similar age trajectories. Figure 4, taken from the data of our previous longitudinal study (but not previously published), reveals that the age trajectories of delta power and NREM duration are parallel.
Figure 4.

Roughly parallel maturational trends for delta power (open symbols) and NREM sleep duration (filled symbols). Average (± SE) data are from our previous longitudinal study of changes in the sleep EEG in two cohorts: one studied from age 9 to 16 years (circles) and the other studied from age 12 to 18 years (triangles).
This working model could explain the decline in NREM duration across 9–18 years. But what accounts for the small but highly significant increase in REM sleep duration? The function of REM sleep is unknown, but we believe that its physiological and behavioral differences from NREM offer important clues. Whereas NREM duration and intensity increase with increasing duration of prior waking, REM duration and %REM increase with increasing prior sleep. While NREM duration and intensity are conserved across naps and post-nap sleep, REM duration is not. In spite of evidence that the quantity of REM (i.e., its duration) is not biologically protected, periodic episodes of REM sleep seem to be necessary: thus, selective REM deprivation with repeated awakenings produces rapid increases in attempts to enter REM sleep.29,30 We have interpreted this set of facts as indicating that some amount of REM is required for NREM sleep to continue but that absolute REM durations can vary widely.17 This pattern could indicate that REM provides a cofactor or neuromodulator required for NREM sleep.17 One possibility is that the intense neuronal firing in hard-wired systems that characterizes REM sleep (which we interpret as escape from a NREM inhibition) produces the hypothetical cofactor needed for NREM.31 Whatever the underlying mechanisms, we propose that as NREM sleep becomes less intense, REM “escape” occurs earlier, increasing REM duration and percentage across adolescence (i.e., that the increase in REM across adolescence is secondary to NREM changes.)
Effect of Sleep Restriction on Delta EEG Activity
The high levels of delta power in young adolescents raised the question of whether their high delta need would produce a stronger response to sleep restriction than has been found in young adults. In the present study, reducing time in bed by 3 hours failed to increase delta energy, i.e., the total delta accumulated across the night. All night average delta power was significantly increased with shorter time in bed, but this was simply a result of averaging over a smaller NREM duration. Delta power was unaffected by time in bed duration when comparing the 5-hour NREM duration common to all three conditions. Nor did delta power increase from the 2nd to 4th night on the 7-hour TIB condition. Thus, the response of these young adolescents is similar to that of adults. In adults, reducing time in bed by 3.5 hours or 4 hours for 1 night does not increase delta EEG activity on the recovery night.9,10 Even reducing time in bed to 4 hours for 14 consecutive days does not increase delta power above baseline.11 In children, a single night of sleep restriction to 4 hours does not increase slow wave sleep on the recovery night.32
Delta activity is, on average, greatest in the first sleep cycle when the need for recuperation is greatest.17 Previous studies have shown that NREMP1 delta is particularly sensitive to age17 and to homeostatic manipulation such as napping.13 In the current study, TIB restriction did not affect NREMP1 delta energy or power. Nor did TIB restriction affect the duration of NREMP1 as shown by the lack of an effect on REM latency.
We have been comparing the effects of 3 hours of TIB restriction in children to 3 to 4 hours of TIB restriction in adults, but, admittedly, the percent TIB reduction was greater in the adult studies. Furthermore, some of these adult studies reduced TIB by advancing rise time rather than delaying bed time. It would be interesting to establish a dose-response relation between sleep restriction and delta power across childhood and adolescence and into young adulthood to determine whether this aspect of delta regulation changes with development. In young adults, sleep must be restricted to two hours or less to produce an increase in recovery night delta power above baseline levels.33 This finding has important implications for restorative models of delta,17,25 for it shows that the delta increase following total sleep deprivation is not simply due to increased waking but requires deprivation of delta in the first hours of sleep (see discussion in Feinberg33).
Slow wave EEG declines steeply across the teenage years. Delta power decreases by about two-thirds between 11 and 16.5 years.26 This decline was evident in the current study even though the age range was limited to 10 to 14 years. We previously found that the delta decline occurred earlier in girls.34 In the 10- to 14-year age range studied here, we found no sex difference in delta power, but girls did show a trend toward a steeper age related decline.
Our laboratory has long argued that changes in sleep EEG can index brain maturation and aging.35 The finding here that the adolescent decline in NREM sleep duration is not likely due to sleep restriction but is more likely the result of brain maturation encourages further research into late brain development using sleep EEG. Such studies could provide insight into the genetically controlled mechanisms that underlie the emergence of adult cognition and behavior. If these changes go awry they may give rise to mental illness.5,36
DISCLOSURE STATEMENT
This was not an industry supported study. United States Public Health Service grant R01HL116490 supported this research. The authors have indicated no financial conflicts of interest. All work performed at the University of California, Davis, Davis, CA.
ACKNOWLEDGMENTS
The authors greatly appreciate the work of the undergraduate research assistants who helped collect and analyze data. We also thank the study participants and their families.
REFERENCES
- 1.Olds T, Maher C, Blunden S, Matricciani L. Normative data on the sleep habits of Australian children and adolescents. Sleep. 2010;33:1381–8. doi: 10.1093/sleep/33.10.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–47. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bei B, Allen NB, Nicholas CL, Dudgeon P, Murray G, Trinder J. Actigraphy-assessed sleep during school and vacation periods: a naturalistic study of restricted and extended sleep opportunities in adolescents. J Sleep Res. 2014;23:107–17. doi: 10.1111/jsr.12080. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982/1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 6.Owens JA Adolescent Sleep Working Group, Committee on Adolescence, Council on School Health. School start times for adolescents. Pediatrics. 2014;134:642–9. doi: 10.1542/peds.2014-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302:R533–40. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–13. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg I, Floyd TC, March JD. Acute deprivation of the terminal 3.5 hours of sleep does not increase delta (0-3-Hz) electroencephalograms in recovery sleep. Sleep. 1991;14:316–9. doi: 10.1093/sleep/14.4.316. [DOI] [PubMed] [Google Scholar]
- 10.Travis F, Maloney T, Means M, March JD, Feinberg I. Acute deprivation of the terminal four hours of sleep does not increase delta (0-3-Hz) electroencephalograms: a replication. Sleep. 1991;14:320–4. doi: 10.1093/sleep/14.4.320. [DOI] [PubMed] [Google Scholar]
- 11.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 12.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg I, Maloney T, March JD. Precise conservation of NREM Period 1 (NREMP1) delta across naps and nocturnal sleep: implications for REM latency and NREM/REM alternation. Sleep. 1992;15:400–3. doi: 10.1093/sleep/15.5.400. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalogr Clin Neurophysiol. 1985;61:134–7. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- 15.Werth E, Dijk D-J, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 16.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 17.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–55. [Google Scholar]
- 19.Twisk JWR. Cambridge: Cambridge University Press; 2003. Applied longitudinal data analysis for epidemiology. [Google Scholar]
- 20.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 21.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voderholzer U, Piosczyk H, Holz J, et al. Sleep restriction over several days does not affect long-term recall of declarative and procedural memories in adolescents. Sleep Med. 2011;12:170–8. doi: 10.1016/j.sleep.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Huttenlocher PR. Synaptic density in human frontal cortex -developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 24.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 25.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 26.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304:R296–303. doi: 10.1152/ajpregu.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinberg I, Thode HC, Jr., Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 29.Dement W. The effect of dream deprivation. Science. 1960;131:1705–7. doi: 10.1126/science.131.3415.1705. [DOI] [PubMed] [Google Scholar]
- 30.Kales A, Hoedemaker FS, Jacobson A, Lichtenstein EL. Dream deprivation: an experimental reappraisal. Nature. 1964;204:1337–8. doi: 10.1038/2041337a0. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg I, March JD. Observations on delta homeostasis, the one-stimulus model of NREM-REM alternation and the neurobiologic implications of experimental dream studies. Behav Brain Res. 1995;69:97–108. doi: 10.1016/0166-4328(95)00010-q. [DOI] [PubMed] [Google Scholar]
- 32.Carskadon MA, Harvey K, Dement WC. Acute restriction of nocturnal sleep in children. Percept Mot Skills. 1981;53:103–12. [Google Scholar]
- 33.Feinberg I, Baker T, Leder R, March JD. Response of delta (0-3 Hz) EEG and eye movement density to a night with 100 minutes of sleep. Sleep. 1988;11:473–87. [PubMed] [Google Scholar]
- 34.Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci U S A. 2012;109:5740–3. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]



