Abstract
Study Objectives:
The rise in obesity has been paralleled by a decline in sleep duration in epidemiological studies. However, the potential mechanisms linking energy balance and the sleep/wake cycle are not well understood. We aimed to examine the effects of manipulating energy balance on the sleep/wake cycle.
Methods:
Twelve healthy normal weight men were housed in a clinical research facility and studied at three time points: baseline, after energy balance was disrupted by 2 days of caloric restriction to 10% of energy requirements, and after energy balance was restored by 2 days of ad libitum/free feeding. Sleep architecture, duration of sleep stages, and sleep-associated respiratory parameters were measured by polysomnography.
Results:
Two days of caloric restriction significantly increased the duration of deep (stage 4) sleep (16.8% to 21.7% of total sleep time; P = 0.03); an effect which was entirely reversed upon free feeding (P = 0.01). Although the apnea-hypopnea index stayed within the reference range (< 5 events per hour), it decreased significantly from caloric restriction to free feeding (P = 0.03). Caloric restriction was associated with a marked fall in leptin (P < 0.001) and insulin levels (P = 0.002). The fall in orexin levels from baseline to caloric restriction correlated positively with duration of stage 4 sleep (Spearman rho = 0.83, P = 0.01) and negatively with the number of awakenings in caloric restriction (Spearman rho = -0.79, P = 0.01).
Conclusions:
We demonstrate that changes in energy homeostasis directly and reversibly impact on the sleep/wake cycle. These findings provide a mechanistic framework for investigating the association between sleep duration and obesity risk.
Citation:
Collet TH, van der Klaauw AA, Henning E, Keogh JM, Suddaby D, Dachi SV, Dunbar S, Kelway S, Dickson SL, Farooqi IS, Schmid SM. The sleep/ wake cycle is directly modulated by changes in energy balance. SLEEP 2016;39(9):1691–1700.
Keywords: sleep, caloric restriction, leptin, orexin
Significance.
Acute manipulation of energy balance without change in body weight affects the sleep/wake cycle by increasing the duration of the deepest stage of sleep, which was normalized with restoration of energy balance. Our results are in line with a study in the early 1970s in which the duration of slow wave sleep increased after 4 days of complete starvation associated with weight loss. Taken together, these studies and previous studies of sleep deprivation provide a mechanistic framework for investigating the well-recognized associations between obesity and sleep disorders and between sleep debt and obesity risk.
INTRODUCTION
The rising prevalence of obesity and associated disorders such as type 2 diabetes is associated with significant morbidity and mortality and represents a major public health concern. Reduced levels of physical activity and the increased consumption of highly palatable energy dense foods are major contributors to the rise in body mass index (BMI). Another factor that has been associated with an increased risk of obesity is an increase in sleep debt.1,2 Surveys of secular trends in sleeping habits have reported a marked decrease in sleep duration over the past 30 y.3 Multiple cross-sectional and longitudinal studies have reported a positive correlation between short sleep duration (by self-report and measured objectively by actigraphy) and increased susceptibility to obesity.4 It is unclear why sleep debt and obesity risk appear to be associated, but potentially causal mechanisms have been suggested by experimental clinical studies in which moderate sleep restriction has been shown to reduce energy expenditure,5 increase hunger ratings and food intake,6,7 and decrease insulin sensitivity.8,9 However, surprisingly little is known about the reverse relationship, namely the effect of changes in energy balance on the sleep/wake cycle.
To directly examine the effects of manipulating energy balance on the sleep/wake cycle, we studied 12 normal weight men before and after 2 days of caloric restriction (CR) to 10% of their normal energy requirements. CR was followed by a period of free feeding (FF) to allow for energy homeostasis to be reset. We measured ad libitum food intake to quantify changes in energy balance during this experimental paradigm. We assessed sleep architecture and sleep-associated respiratory parameters in the baseline state, after CR, and upon FF using polysomnography (PSG), which combines overnight electroencephalographic recording with measurements of chest wall movements, eye movements, and peripheral oxygen saturation. We measured fasting levels of peripheral hormones, which might mediate the effects of changes in energy balance on the sleep/wake cycle (leptin, insulin, and total ghrelin), and the neuropeptide orexin A, which plays a critical role in arousal. In response to physiological stresses such as CR, hypothalamic pathways activate autonomic, neuroendocrine, and behavioral responses to maintain homeostasis. Therefore, we measured heart rate (autonomic nervous system activity), the overnight pulsatile secretion of thyroid-stimulating hormone (TSH), growth hormone (GH), and cortisol release, as well as cognitive parameters and mood-related symptom scores.
METHODS
The study was approved by the Cambridge local research ethics committee and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was received from each participant prior to inclusion in the study. All clinical studies were conducted at the NIHR-Wellcome Trust Clinical Research Facility, Adden-brooke's Hospital, Cambridge, United Kingdom.
We recruited 17 normal weight adult male volunteers (BMI 20–25 kg/m2). After screening, 12 volunteers satisfied the following inclusion criteria: normal glucose tolerance measured by a 75-g oral glucose tolerance test, no evidence of renal, liver, or thyroid disease, average alcohol intake < 2 units/day, not participating in an organized exercise program, not treated with anorectic agents or medications known to affect carbohydrate and/or lipid metabolism, or blood pressure. Shift workers were excluded from the study and all participants had a normal sleep/wake pattern as determined by PSG at screening and self-reported quality of sleep scores (Table S1 in the supplemental material). Weight and height were measured barefoot in light clothing and BMI calculated (weight in kg/height in meters squared).
Participants were residents on the Clinical Research Facility for the duration of the study under direct observation. At baseline, volunteers consumed a balanced diet (50% carbohydrate, 30% fat, 20% protein) matching their daily energy requirement calculated by basal metabolic rate multiplied by a physical activity level of 1.25 using the Schofield equation.10 To manipulate energy balance, baseline day 1 was followed by CR to 10% of normal energy requirement (mean of 222 ± standard error of the mean [SEM] 4 kcal/day) for 2 days. After CR, participants were offered three substantial ad libitum buffet meals per day (20 MJ = 4,777 kcal) and additional snacks (16 MJ = 3,821 kcal) between meals for 2 days. They were invited to eat freely; food consumption was covertly measured. Seven volunteers continued to an additional day of FF (Figure S1 in the supplemental material). We performed PSG and measured metabolic, neuroendocrine, autonomic, and cognitive parameters at baseline, after CR, and FF, as detailed in the following paragraphs.
Polysomnography
PSG for the assessment of sleep was performed during all nights using a SomnoScreen Plus device (SOMNOmedics GmbH, Randesacker, Germany). Electrodes were attached to the scalp (Cz, C3, C4, O1, O2, A1, A2, Gnd) for electroencephalographic (EEG) recordings, above, below, and beside the eyes for horizontal and vertical electrooculogram, and on the chin for electromyogram. Recordings were scored offline by one investigator (S.M.S.) according to standard criteria by Rechtschaffen and Kales,11 and independently assessed by a second sleep laboratory analyst unaware of the study design and hypothesis. The following sleep parameters were determined: sleep period time (SPT, i.e., time interval between sleep onset and morning awakening), wake after sleep onset (WASO, i.e., duration of wake during SPT), total sleep time (TST, i.e., SPT minus WASO), time spent in sleep stages 1, 2, 3, 4, and rapid eye movement (REM) sleep (all in minutes and % of TST), as well as sustained sleep efficiency (TST divided by [time in bed minus sleep latency S1]). Furthermore, respiratory function as assessed by nasal air flow, chest excursions, and blood oxygen saturation (% SpO2) were analyzed for measures of apnea-hypopnea index (AHI, i.e., number of apnea + hypopnea per hour of TST), number of central apnea episodes during TST, central apnea index (i.e., number of central apnea episodes per hour of SPT), mean SpO2 (i.e., average value of complete SpO2 curve during TST), minimal SpO2 (minimum SpO2 during TST), and number of oxygen desaturations (i.e., a minimum decrease of 4% SpO2). All participants attended a prestudy overnight recording session with PSG to ensure that they had normal sleep architecture.
Analytical Methods
Plasma glucose, insulin, leptin, serum lipids, TSH, free thyroxin, GH, and cortisol, as well as routine biochemical and hematological assays were performed using standard commercially available assays. Concentrations of both total ghrelin and plasma orexin A were assessed using commercially available enzyme-linked immunosorbent assay kits for humans (EZGRT-89K; Millipore, Billerica, MA and Uscn Life Science Inc., Wuhan, Hubei, China, respectively). The detection limit was 50 pg/mL for total ghrelin and 4.83pg/mL for orexin A.
Pulsatility Analysis
For overnight pulsatility analysis, we collected serum samples every 10 min from 24:00 to 06:00, via a long line running from the participants to the adjacent room to avoid any interference with their sleep. Cluster analysis was used for the detection of discrete TSH, GH, and cortisol peaks.12 This computerized pulse algorithm is largely model free and identifies statistically significant pulses in relation to dose-dependent measurement error in the hormone time series. For the current analysis a 2 × 1 test cluster configuration was used, two data points for the test nadir and one for the test peak, and a t-statistic of 2.0 for the upstrokes and downstrokes, which minimizes both false-positive and false-negative peaks. The locations and widths of all significant concentration peaks were identified, the total number of peaks was counted, and the mean peak interval was calculated in minutes as well as peak height, width, and area. In addition, valley mean and nadir, area under the curve, and total average value were calculated.
Measurement of Blood Pressure and Autonomic Nervous System Activation
Blood pressure was measured using a wrist-type blood pressure monitor (OMRON Healthcare, Hamburg, Germany). Heart rate was measured continuously using a wireless sensor applied to the chest wall (Actiheart, CamNtech Ltd, Cambridge, UK). This digitalizes the electrocardiogram signal and stores the R-R interval time-series from which heart rate can be calculated. Heart rate data was exported to a spreadsheet via Actiheart software (version 4.0.116, CamNtech Ltd, Cambridge, UK). Sleep data collected by the PSG device was examined to determine a window of time (240 min) between 00:00 and 05:00 during which each participant was asleep. Average heart rate while sleeping and on waking was calculated, and the difference between average asleep and average waking heart rate for each participant on each day was recorded.
Mood, Fatigue, and Cognition
Using validated questionnaires we collected data on neuroglycopenia and autonomic symptoms,13 mood,14 and sleepiness.15,16 As adequate sleep is necessary for the consolidation of memory,17 we tested whether concentration and the ability to retain information were affected by the study intervention. We measured alertness by reaction times and error rates in a computer-based vigilance performance test during the three study phases.18 Procedural memory formation was measured by finger tapping test19 and declarative memory formation by associate word learning paradigm.20
Statistical Analyses
Unless specified otherwise, data are expressed as mean and SEM. Data were tested for normality using graphical and numerical methods (Shapiro-Wilk test). Data were compared by analysis of variance (ANOVA) with repeated measures to test for within-subjects changes. The within-subjects P value was adjusted using the Greenhouse-Geisser correction factor for lack of sphericity. Pairwise comparisons of the study phases were performed by two-sided Student t-test when appropriate. A value of P = 0.05 was considered significant after Bonferroni correction for multiple comparisons, i.e. by multiplying the uncorrected P value by the number of comparisons. For analyses of correlation between fasting hormones and sleep parameters, the nonparametric Spearman correlation test was used and repeated in sensitivity analyses excluding outliers. Data were analyzed using Stata software package (version 13.1, Stata Corp, College Station, TX).
RESULTS
Rebound Hyperphagia in Response to Caloric Restriction
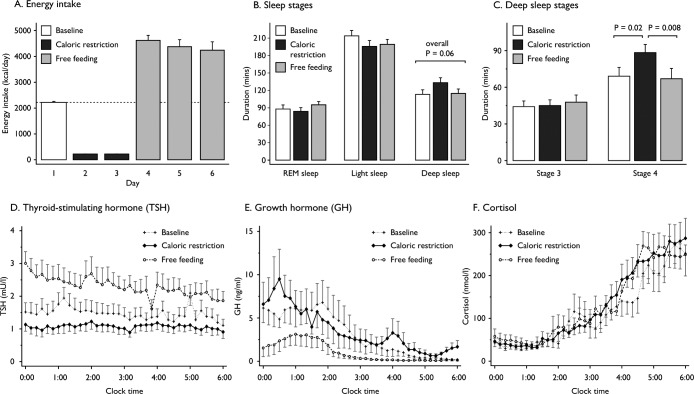
Twelve adult males (mean age 24.2 ± SEM 1.3 y; mean BMI 23.1 ± 0.4 kg/m2) were studied. Blood pressure, body composition, baseline biochemical and hematological parameters, and self-reported quality of sleep scores were within normal ranges (Table S1 in the supplemental material). Participants overconsumed when allowed to eat freely after 2 days of CR (mean 4,500 ± 165 kcal/day), to an extent that fully compensated for their energy deficit after 2 days of FF (Figure 1A). However, those individuals provided with ad libitum meals for a third day continued to overeat, eating 2,000 kcal in excess on the third day (Figure 1A).
Figure 1.
Barcharts of changes in the duration of sleep stages at baseline, during caloric restriction, and free feeding; and scatterplots of overnight pulsatile secretion of thyroid-stimulating hormone, growth hormone, and cortisol. (A) Energy intake was fixed to calculated 24-h energy requirement on day 1 (baseline), was reduced to 10% of energy requirement on days 2 and 3 (caloric restriction) and free feeding was allowed on days 4 and 5, with an additional day as part of an extended protocol in seven individuals; to convert kilocalories (kcal) to mega-Joules (MJ), multiply by 0.0041868. (B,C) The duration of rapid eye movement (REM) sleep, light sleep (stages 1 + 2), and deep sleep (stages 3 + 4) was recorded using polysomnography at baseline, after 2 days of CR and after 2 days of FF. The 18% increase in the duration of deep sleep after CR (P = 0.06) was entirely due to an increase in the duration of stage 4 sleep while stage 3 sleep was unaffected (C). Vertical bars represent the standard error of the mean (n = 12 participants). Durations of all sleep stages were analyzed using analysis of variance (ANOVA) with repeated measures to test for within-subject changes. The within-subjects P value was adjusted using the Greenhouse-Geisser correction factor for lack of sphericity. Pairwise comparisons of the three study phases were performed by two-sided Student t-test when appropriate. A P value of 0.05 was considered significant after Bonferroni correction for multiple comparisons. (D–F) Pulsatile secretion of thyroid-stimulating hormone (D), growth hormone (E) and cortisol secretion (F) was measured in blood samples taken every 10 min from midnight until 06:00 at baseline, after 2 days of caloric restriction and after 2 days of free feeding. Vertical bars represent the standard error of the mean (n = 8 participants).
Sleep Architecture and Sleep-Associated Respiratory Parameters
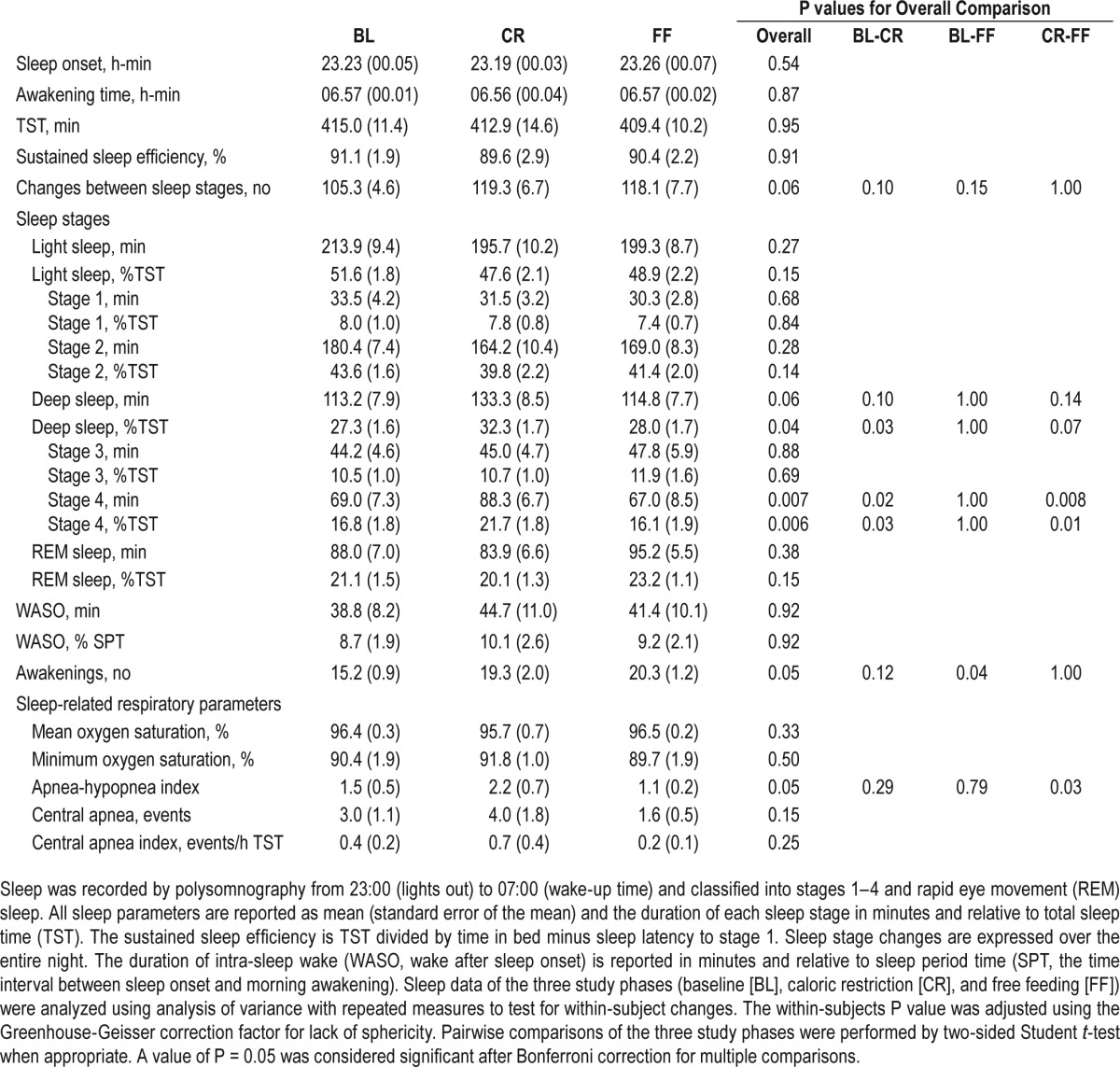
PSG recordings were performed at baseline, after CR and FF, and were visually scored by investigators blinded to the study design.11 At baseline, participants' sleep architecture displayed a normal pattern when compared to reference data21 with approximately 50% of the night spent in stages 1 and 2, 25%–30% spent in stages 3 and 4, and 20%–25% spent in REM sleep. TST and sustained sleep efficiency were not affected by changes in energy balance (Table 1). Whilst there was no significant change in light sleep (stage 1 and 2) or REM sleep (Figure 1B), the duration of deep sleep (stage 3 and 4, or slow wave sleep [SWS]) increased by 18% in CR (Table 1). This change in deep sleep was entirely due to a marked increase in the duration of stage 4 sleep (P = 0.02), which was fully reversed to baseline levels upon FF (P = 0.008; Figure 1C). Although there was no significant difference in the number of awakenings with CR, the number of transitions between sleep stages was increased with borderline significance (105 at baseline versus 119 in CR, P = 0.06, Table 1). Changes in energy balance were followed by modest changes of the AHI, a marker of hypoventilation (P = 0.05, Table 1), but the AHI stayed below the threshold of sleep-disordered breathing (≥ 5 events/h) throughout.
Table 1.
Sleep parameters.
Disordered sleep has been associated with impaired memory retention. Alertness, as measured by reaction times and error rates in a vigilance performance test, did not change during the study (data not shown). Sleep-dependent consolidation of procedural and declarative memory tested by a standard finger tapping task and paired associate word learning task were preserved during all study phases (Figure S2 in the supplemental material) and not modified by changes in energy balance. There was a discrete improvement in overall mood score as assessed by the Profile Of Mood States (POMS) questionnaire immediately upon FF compared to CR, but no significant changes in mood subdomains (Table S2 in the supplemental material).
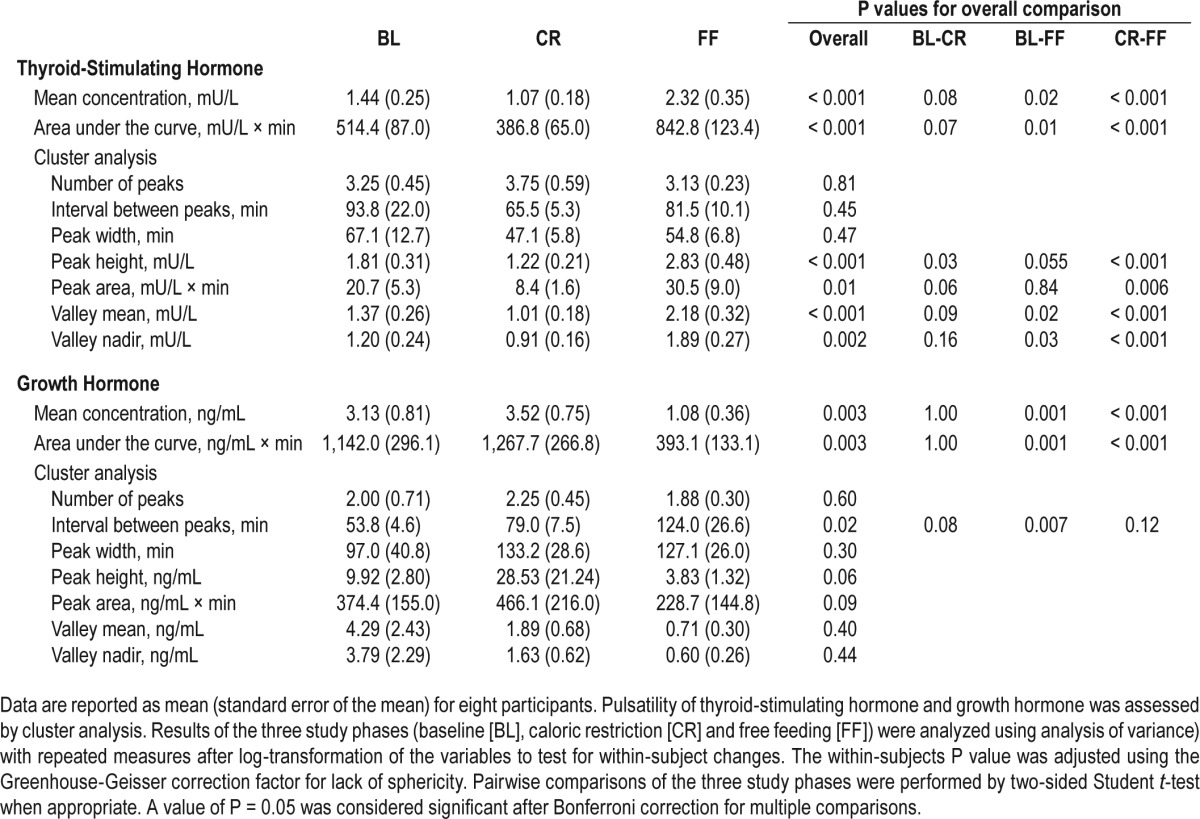
Pulsatile Secretion of TSH, GH, and Cortisol
Changes in energy balance can affect the hypothalamic regulation of pituitary hormone synthesis and secretion, which may in turn influence sleep architecture. We measured serum TSH, GH, and cortisol release (a marker of hypothalamopituitary adrenal axis activation) every 10 min for 6 h overnight when participants were asleep as confirmed by PSG recordings. Mean hormone concentrations and parameters of pulsatile secretion were analyzed at baseline, after CR and FF using the pulse detection cluster algorithm (Table 2; Table S3 in the supplemental material). Compared to baseline values, mean TSH concentrations, integrated total area under the curve (AUC), the peak pulse height and area, as well as valley means and nadirs, were reduced after 48 h of CR and increased to approximately 60% above baseline levels on FF (Figure 1D; Table 2). There were no differences in the number of pulses and pulse width. There was no change in the pulsatile secretion of GH from baseline to CR, whereas FF was associated with a decrease in mean GH concentrations and integrated total AUC compared to baseline and CR values (Figure 1E; Table 2). In conjunction, the interval between peaks was longer during FF compared to baseline. No differences in cortisol secretion were seen as result of changes in energy balance (Figure 1F; Table S3 in the supplemental material).
Table 2.
Analysis of pulsatile thyroid-stimulating hormone and growth hormone secretion.
Autonomic Nervous System Activity
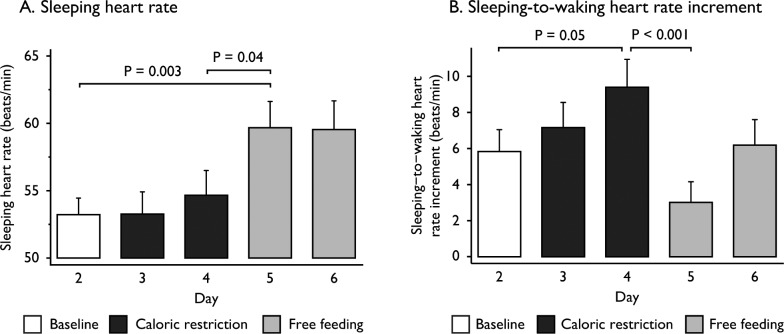
To examine activation of the autonomic nervous system, we measured heart rate continuously throughout the study. The mean sleeping heart rate (predominantly influenced by para-sympathetic tone) was unchanged after CR but increased by 5.0 beats/min with FF (P = 0.04, Figure 2A). The increase in heart rate on waking (sleeping-to-waking heart rate increment; predominantly due to sympathetic nervous system activation) increased from 5.8 to 9.4 beats/min in response to CR (P = 0.05) and was reduced by 6.3 beats/min after 24 h of FF (P < 0.001, Figure 2B). Autonomic symptoms (predominantly adrenergic) were more prominent upon CR and decreased in FF (Table S4 in the supplemental material).
Figure 2.
Barcharts of changes in heart rate at baseline, during caloric restriction, and free feeding. Mean sleeping heart rate (A) and the sleeping-to-waking heart rate increment (B) were measured every night in all 12 participants at baseline, during caloric restriction and free feeding. Vertical bars represent the standard error of the mean. Measurements were compared using analysis of variance (ANOVA) with repeated measures to test for within-subject changes. The within-subjects P value was adjusted using the Greenhouse-Geisser correction factor for lack of sphericity. Pairwise comparisons of the three study phases were performed by two-sided Student t-test when appropriate. A P value of 0.05 was considered significant after Bonferroni correction for multiple comparisons.
Peripheral Hormones and Orexin
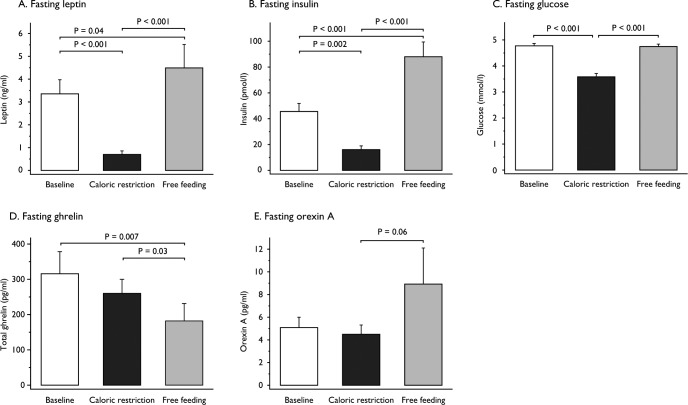
Fasting plasma leptin decreased to 20% of baseline levels after 48 hours of CR (P < 0.001), increasing to higher than baseline levels in FF (126%; P < 0.001; Figure 3A). Fasting plasma insulin also decreased in CR (35%) and increased in FF (203% of baseline levels; both P ≤ 0.002; Figure 3B). Fasting plasma glucose decreased by 1.2 mmol/L during CR and normalized upon FF (both P < 0.001; Figure 3C). Glucose AUC over daytime (08:00 to 22:00) and over 24 h (08:00 to 08:00) significantly decreased in CR compared to baseline and increased above baseline values in FF (all comparisons: P < 0.001; data not shown). Plasma ghrelin levels exhibit diurnal variation, act as a short-term hunger signal peaking before meal initiation, and are affected by sleep restriction.22 Fasting total ghrelin did not change significantly with CR but decreased with FF in this study (P = 0.03; Figure 3D); changes in ghrelin levels over 24 h were not measured in our study. Plasma orexin increased in FF although this change was not statistically significant (P = 0.06; Figure 3E).
Figure 3.
Barcharts of changes in peripheral hormones and orexin at baseline, during caloric restriction, and free feeding. Fasting plasma levels of leptin (A) (n = 11), insulin (B) (n = 10), glucose (C) (n = 10), total ghrelin (D) (n = 9) and orexin A (E) (n = 10) were measured at baseline, after 48 h of caloric restriction and after 48 h of free feeding. Vertical bars represent the standard error of the mean. Hormone levels were compared using analysis of variance (ANOVA) with repeated measures to test for within-subject changes. The within-subjects P value was adjusted using the Greenhouse-Geisser correction factor for lack of sphericity. Pairwise comparisons of the three study phases were performed by two-sided Student t-test when appropriate. A P value of 0.05 was considered significant after Bonferroni correction for multiple comparisons.
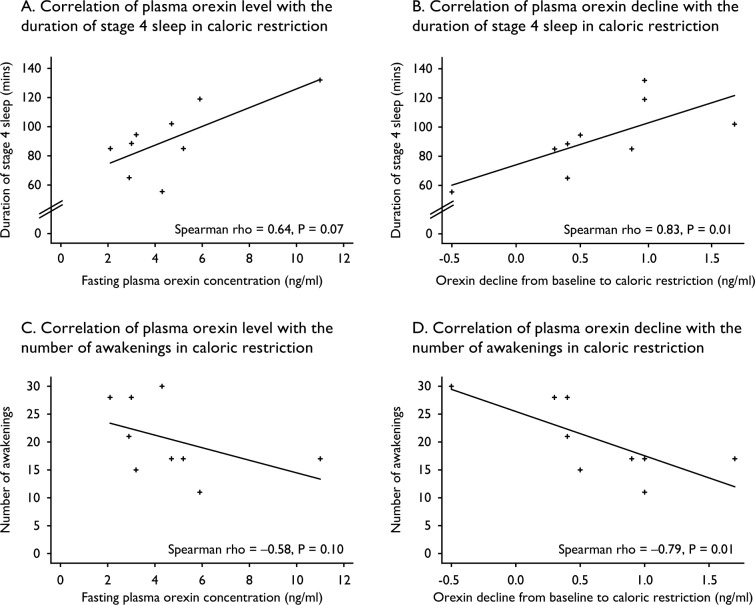
We hypothesized that changes in peripheral hormones or in orexin might mediate the change in duration of stage 4 sleep seen with CR. Although there was no correlation between fasting leptin, insulin, or total ghrelin and the duration of stage 4 sleep in CR (data not shown), plasma orexin levels correlated with specific sleep parameters after 48 h of CR (Figure 4A). The duration of stage 4 sleep correlated positively with orexin decline from baseline to CR (Spearman rho = 0.83, P = 0.01; Figure 4B). Although the number of awakenings in CR did not correlate with plasma orexin (Figure 4C), they correlated negatively with orexin decline from baseline to CR (Spearman rho = −0.79, P = 0.01; Figure 4D). A sensitivity analysis ex -cluding one outlier confirmed the correlation of orexin decline in 48 h from baseline to CR with the duration of stage 4 sleep in CR (Spearman rho = 0.75, P = 0.03) and the number of awakenings in CR (Spearman rho = −0.70, P = 0.05; Figure S3 in the supplemental material).
Figure 4.
Scatterplots of correlation of plasma orexin A levels with sleep parameters after 48 h of caloric restriction (CR) among nine participants. The duration of stage 4 sleep correlated positively with orexin level in CR (A), as well as orexin decline from baseline to CR (B). There was no correlation between the number of awakenings and the absolute level of orexin in CR (C). The number of awakenings in CR correlated negatively with orexin decline from baseline to CR (D). A sensitivity analysis (SA) excluding one outlier confirmed the correlation of orexin decline in 48 h from baseline to CR with the duration of stage 4 sleep in CR (SA of (B), Spearman rho = 0.75, P = 0.03) and the number of awakenings in CR (SA of (D), Spearman rho = −0.70, P = 0.05). In this SA, there was no correlation between the plasma concentration of orexin in CR and the duration of sleep stage 4 (SA of (A), Spearman rho = 0.48, P = 0.23) or the number of awakenings in CR (SA of (C), Spearman rho = −0.59, P = 0.12; Figure S3 in the supplemental material).
DISCUSSION
In this study we found that acute CR for 2 days significantly increased the duration of the deepest stage of sleep, stage 4 sleep. The effect of CR on stage 4 sleep was normalized with FF, which restored energy balance. Our findings provide direct evidence that energy balance and the sleep/wake cycle are tightly coupled in humans. Our findings align with a study from the 1970s that observed an increased duration of SWS (stages 3 and 4 together) and reduced REM sleep in males studied before and after four days of complete starvation associated with weight loss, with reversal of these changes in refeeding characterized by weight regain.23
Why might changes in energy balance lead to changes in the sleep/wake cycle? One possibility is that increasing the time spent in the deepest stage of sleep may allow for the conservation of energy resources in response to acute CR. Interestingly, positron emission tomography studies have found that cerebral glucose utilization rates decrease by ∼11% during non-REM sleep24 and even further (by ∼44%) in SWS compared to wakefulness.25 The effect of CR on stage 4 sleep in humans is consistent with experiments in mammals and birds, where acute starvation can induce shallow torpor by almost-continuous sleep.26 As animals mostly enter torpor and hibernation through SWS,27 an increase in SWS as seen in our study may represent part of the evolutionarily conserved physiological response to conserve energy in response to negative energy balance and the threat of starvation.
Possible mechanisms linking energy balance and the regulation of the sleep/wake cycle may involve the adipocyte-derived hormone leptin, which plays a pivotal role in mediating the physiological response to fasting/starvation.28 In our study, 48 hours of CR led to a marked decrease in leptin levels that rebounded in FF above baseline levels. Although a decline in leptin has not previously been associated with changes in the sleep/wake cycle, direct evidence for the role of leptin in the regulation of the sleep/wake cycle comes from genetic disruption of leptin and the leptin receptor in rodents,29,30 which leads to increased TST due to an increase in non-REM sleep, sleep fragmentation characterized by an elevated number of arousals and increased number of transitions between sleep stages. To date, very little is known about sleep architecture in rare severely obese patients with congenital leptin deficiency, a disorder that is often complicated by marked central and obstructive sleep apneas (own observations).
Leptin and other peripheral signals of nutritional status may mediate effects on the sleep/wake cycle in part by acting on orexin neurons in the lateral hypothalamus, an important center for feeding and arousal. Targeted disruption of orexin and orexin receptors in mice leads to severely defective sleep/ wake cycles.31 Furthermore, narcolepsy is characterized by low levels of orexin in the cerebrospinal fluid (CSF).32 For ethical reasons, we were unable to obtain CSF and measured plasma orexin A instead. We found that the decline in plasma orexin from baseline to CR was positively correlated with the duration of stage 4 sleep in CR and inversely correlated with the number of awakenings. This finding is intriguing but will require further investigation. We do not know whether, or how far, plasma orexin levels reflect orexin-mediated signaling in the brain. However, Strawn et al.,33 who performed simultaneous measurements of CSF and plasma orexin, found a strong correlation between CSF and plasma orexin levels (Spearman rho = 0.81, P < 0.0001), suggesting that plasma orexin levels may be used as an index of CSF orexin concentrations.
In addition to the effects of CR on the sleep/wake cycle, we were able to demonstrate a trend towards reduced pulsatile secretion of TSH and impaired sympathetic nervous system activation. These observations in healthy volunteers are entirely consistent with studies in patients with genetic disruption of leptin signaling34,35 and in individuals with obesity following weight loss36 (a state of partial leptin deficiency). These physiological changes were predominantly mediated by falling leptin concentrations and could be reversed by concomitant leptin administration in previous studies.34,36 We would have expected therefore, that 2 days of FF, which restored energy balance, would restore leptin levels, pulsatile TSH secretion, and autonomic function to baseline levels. However, intriguingly, we found that these parameters exceeded baseline values after 2 days of FF. The explanation for these findings is unclear. Such changes could contribute to an exaggerated compensatory response to CR, for example, by overeating. Some participants were studied during a third day of FF as we hypothesized that their food intake would return to baseline levels. Although ad libitum access to food may have promoted higher energy intake relative to energy requirement on this day, it is notable that energy intake on this third day remained excessive (mean 4,293 ± 325 kcal/day), comparable to the first day of FF (P = 0.29). These findings warrant further investigation and, if replicated, may shed light on the physiological response to weight loss and the mechanisms that promote weight regain.
In this study, we did not observe a significant change in GH pulses with CR in contrast to some, but not all, previous studies.37 As overnight sampling started at midnight in our study and the major GH pulse occurs within 30 min of sleep onset, changes in the sleep-onset GH pulse may not have been captured in some participants. Notably, we found that mean GH concentrations and integrated total AUC were significantly reduced during FF compared to baseline and CR. The pulsatile secretion of GH is predominantly the product of stimulatory GH-releasing hormone (GHRH)-expressing neurons and inhibitory somatostatin-expressing neurons in the hypothalamus. Leptin treatment of rats food deprived for 48 h increases somatostatin messenger RNA levels,38 which would result in suppression of pulsatile GH secretion as seen in this study. It is recognized that pulsatile GH secretion is suppressed in obesity, but it is striking that we observed comparable levels of GH suppression after 2 days of FF when participants were consuming excess calories but had restored energy balance. Variations in pulsatile release define the physiological actions of GH, which is a critical mediator of insulin action and glucose homeostasis. We postulate that the suppression of GH secretion as seen in this study may reflect the physiological response to maintain glucose homeostasis in light of excess caloric consumption. This hypothesis requires further testing in experimental studies.
In conclusion, we have demonstrated for the first time in humans that acute manipulation of energy balance without change in body weight affects the sleep/wake cycle by specifically increasing the duration of the deepest stage of sleep, stage 4 sleep. Interestingly, previous studies have shown that the duration of stage 4 sleep is reduced in individuals with obesity without obstructive sleep apnea39 and that bidirectional changes in energy balance in mice can alter the sleep/wake cycle.40
A number of investigators have examined the effects of changes in the sleep/wake cycle induced by sleep deprivation on energy homeostasis,2,9 leptin levels, insulin sensitivity, and weight gain.41 Although the magnitude of metabolic effects seen varies depending on the duration of sleep deprivation, cumulatively these studies and ours demonstrate that energy balance and the sleep/wake cycle are tightly coupled in humans. These studies provide a mechanistic framework for investigating the well-recognized associations between obesity and sleep disorders and between sleep debt and obesity risk.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Wellcome Trust (to Dr. van der Klaauw and Dr. Farooqi), the National Institute for Health Research Cambridge Biomedical Research Centre, the European Research Council, the Bernard Wolfe Health Neuroscience Fund (all to Dr. Farooqi), the Swiss National Science Foundation (PBLAP3-145870, P3SMP3-155318, to Dr. Collet), the European Society of Endocrinology (IESP grant, to Dr. Schmid) and the German Research Foundation (TR-SFB 654, B01, to Dr. Schmid). This work was supported by the NeuroFAST consortium which is funded by the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement no 245009. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the volunteers who took part in the study, as well as Dr. Keith Burling and Dr. Peter Barker who performed the biochemical assays (NIHR Cambridge Biomedical Research Centre Core Biochemical Assay Laboratory).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- AUC
area under the curve
- BL
baseline
- BMI
body mass index
- CR
caloric restriction
- CSF
cerebrospinal fluid
- EEG
electroencephalographic
- FF
free feeding
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- mRNA
messenger ribonucleic acid
- PET
positron emission tomography
- POMS
profile of mood states questionnaire
- PSG
polysomnography
- REM
rapid eye movement
- SA
sensitivity analysis
- SEM
standard error of the mean
- SNS
sympathetic nervous system
- SpO2
blood oxygen saturation
- SPT
sleep period time
- SWS
slow wave sleep
- TIB
time in bed
- TSH
thyroid-stimulating hormone
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–13. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales A. National Institute of Health Publications 204. Washingtion, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 12.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–93. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 13.Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 14.McNair DM, Lorr M, Droppleman LF. San Diego, CA: Education and Industrial Testing Service; 1971. Manual for the profile of mood states. [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 18.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–59. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–20. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 20.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 21.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 23.MacFadyen UM, Oswald I, Lewis SA. Starvation and human slow-wave sleep. J Appl Physiol. 1973;35:391–4. doi: 10.1152/jappl.1973.35.3.391. [DOI] [PubMed] [Google Scholar]
- 24.Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125:1105–15. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- 25.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–43. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 26.Walker LE, Walker JM, Palca JW, Berger RJ. A continuum of sleep and shallow torpor in fasting doves. Science. 1983;221:194–5. doi: 10.1126/science.221.4606.194. [DOI] [PubMed] [Google Scholar]
- 27.Heller HC, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol. 2004;66:275–89. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- 28.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 30.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2059–66. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 32.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 33.Strawn JR, Pyne-Geithman GJ, Ekhator NN, et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–7. doi: 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez P, Isidro L, Leal-Cerro A, Casanueva FF, Dieguez C, Cordido F. Effect of withdrawal of somatostatin plus GH-releasing hormone as a stimulus of GH secretion in obesity. Clin Endocrinol. 2002;56:487–92. doi: 10.1046/j.1365-2265.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 38.Carro E, Senaris RM, Seoane LM, et al. Role of growth hormone (GH)-releasing hormone and somatostatin on leptin-induced GH secretion. Neuroendocrinology. 1999;69:3–10. doi: 10.1159/000054397. [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 40.Perron IJ, Pack AI, Veasey S. Diet/Energy Balance Affect Sleep and Wakefulness Independent of Body Weight. Sleep. 2015;38:1893–903. doi: 10.5665/sleep.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.