Abstract
Objective
To measure long-term changes in resting metabolic rate (RMR) and body composition in participants of The Biggest Loser competition.
Methods
Body composition was measured by dual energy X-ray absorptiometry and RMR was determined by indirect calorimetry at baseline, at the end of the 30 week competition, and 6 years later. Metabolic adaptation was defined as the residual RMR after adjusting for changes in body composition and age.
Results
Of the 16 Biggest Loser competitors originally investigated, 14 participated in this follow-up study. Weight loss at the end of the competition was (mean±SD) 58.3±24.9 kg (p<0.0001) and RMR decreased by 610±483 kcal/d (p=0.0004). After 6 years, 41.0±31.3 kg of the lost weight was regained (p=0.0002) while RMR was 704±427 kcal/d below baseline (p<0.0001) and metabolic adaptation was −499±207 kcal/d (p<0.0001). Weight regain was not significantly correlated with metabolic adaptation at the competition’s end (r=−0.1, p=0.75) but those subjects maintaining greater weight loss at 6 years also experienced greater concurrent metabolic slowing (r=0.59, p=0.025).
Conclusions
Metabolic adaptation persists over time and is likely a proportional, but incomplete, response to contemporaneous efforts to reduce body weight.
Introduction
Weight loss is accompanied by a slowing of resting metabolic rate (RMR) that is often greater than would be expected based on the measured changes in body composition. This phenomenon is called “metabolic adaptation” or “adaptive thermogenesis” and acts to counter weight loss and is thought to contribute to weight regain (1, 2). Several years ago, we investigated the body composition and RMR changes in 16 people with class III obesity undergoing an intensive diet and exercise intervention as part of The Biggest Loser televised weight loss competition (3). The participants rapidly lost massive amounts of weight, primarily from body fat mass (FM) with relative preservation of fat-free mass (FFM) likely due to the intensive exercise training. RMR was substantially reduced at the end of the competition indicating a large degree of metabolic adaptation.
Because metabolic adaptation has been suggested to persist for many years following weight loss (4), we hypothesized that the former Biggest Loser participants continued to experience metabolic adaptation years after the competition. We also hypothesized that the degree of metabolic adaptation would be correlated with weight regain. To test these hypotheses, we recruited 14 of the 16 originally studied Biggest Loser competitors and measured RMR and body composition changes 6 years after the end of the weight loss competition.
Methods
The study protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (ClinicalTrials.gov Identifier: NCT02544009). Fourteen of the 16 subjects who were studied previously (3) provided informed consent via telephone and visited the NIH Clinical Center for follow-up testing, all within a time span of 6 weeks.
Body weight and composition
For 2 weeks prior to being admitted to the NIH Clinical Center for the follow-up measurements, body weights were monitored daily via a scale (model UC-352BLE, A&D Medical, San Jose, CA) connected via Bluetooth to an iPad mini (Apple Inc., Cupertino, CA) that transmitted the data back to the study team using a remote patient monitoring system (Tactio RPM Platform, Tactio Health Group, Montreal, Canada). Subjects were then admitted to the NIH Clinical Center for a 3 day inpatient stay to conduct the RMR and body composition measurements. Body composition was determined by dual energy x-ray absorptiometry using the same model of scanner used to make the original measurements during the weight loss competition (iDXA; GE Lunar, Madison, WI). Body FFM and FM were calculated from weight and whole-body percent fat using the thick scan mode. All participants’ whose supine body width exceeded the dimensions of the scan window and were analyzed using the iDXA MirrorImage™ application (5).
Resting metabolic rate
The RMR measurements were performed using indirect calorimetry (TrueOne metabolic cart, ParvoMedics, Sandy, UT) following a 12-hour overnight fast. Participants rested supine in a quiet, darkened room for 30 minutes before making measurements of VO2 and VCO2 for 20 minutes with the last 15 minutes used to determine RMR according to:
which assumes that protein oxidation contributes 15% to the energy expenditure (6).
Total energy expenditure
After returning home from the NIH Clinical Center, subjects drank from a stock solution of 10% 18O enriched H2O and 99% enriched 2H2O at a dose of 1.5 g/kg body weight followed by 100–200 mL tap water to rinse the dose container. Spot urine samples were collected at 1.5, 3, 4.5, and 6 hours after administration and once daily over the next 13 days when the subjects were instructed not change their usual routine. Isotopic enrichments of urine samples were measured by dual inlet chromium reduction and continuous flow CO2 equilibration isotope ratio mass spectrometry. An aliquot of the stock solution was saved for dilution to be analyzed along with each set of urine samples. The average CO2 production rate (rCO2) over the 14 day period was estimated from the rate constants describing the exponential disappearance of the labeled 18O and D water isotopes (kO and kD) in repeated spot urine samples collected over several days. We used the parameters of Racette et al. (7) with the poolsize, N, determined as 73% of the FFM:
The average total energy expenditure (TEE) from the doubly labeled water measurement of rCO2 was calculated as:
where the respiratory quotient, RQ, was assumed to be 0.86 representative of the food quotient of a typical diet.
Physical activity energy expenditure
Physical activity energy expenditure was calculated as the non-resting energy expenditure (TEE-RMR) minus the estimated thermic effect of food which was assumed to be 10% of energy intake and was calculated as 0.1×TEE at baseline and 6 years. At the end of the 30 week competition we assumed the thermic effect of food was 0.1×TEEbaseline − 180 kcal/d since energy intake was estimated to have decreased by ~1800 kcal/d compared to baseline at the end of the competition (8). Since most physical activities involve locomotion and therefore have an energy cost that is proportional to body weight for a given intensity and duration (9), we normalized the physical activity energy expenditure by dividing by body weight.
Biochemical assays
Blood samples from overnight fasted participants were analyzed by a commercial laboratory (West Coast Clinical Laboratories, Van Nuys, CA). The chemistry panel was measured on a Beckman Synchron CX5CE or CX9PRO. Insulin was determined by radioimmunoassay, and leptin and adiponectin concentrations were measured using a commercially available kit (Millipore, St. Charles, MO). Triglycerides and total, HDL, and LDL cholesterol were assayed with ACE reagents and instrumentation (Alfa Wassermann, Caldwell, NJ). Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) using fasting measurements of glucose and insulin (10). Thyroid panel (T3, T4, TSH) was measured by immunoassay with chemiluminescent detection (Millipore Corporation, Billerica, MA).
Statistical Analysis
The pre-specified primary aim of the study was to measure body composition and RMR several years after the end of The Biggest Loser competition and the study was powered to detect a metabolic adaptation ≥ 220 kcal/d in 12 subjects using an endpoint analysis with probability (power) 0.8 assuming a 250 kcal/d standard deviation and a two-sided test with Type I error probability of 0.05. We chose to power the study for 12 subjects since we did not expect to recruit the entire 16 subject original cohort and the 220 kcal/d effect size was considered to be physiologically significant.
Baseline data from all 16 subjects were used to generate a least squares best-fit linear regression equation for RMR as a function of FFM, FM, age, and sex (R2=0.84):
We calculated the predicted RMR using this equation along with the corresponding FFM, FM, and age at each time point for every individual. Differences between the measured and predicted RMR defined the magnitude of metabolic adaptation which was considered to be present if the RMR residuals were significantly different from zero (3).
Despite all of our subjects having class III obesity at baseline, the coefficients of the best-fit RMR regression equation above were similar to those previously published using data from subjects that were not as obese (11, 12, 13). Furthermore, the baseline RMR measurements in our subjects were not significantly different (p=0.34) from those predicted using a standard equation as a function of height, weight, age, and sex (12).
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Data are expressed as mean ± SD and were analyzed by analysis of variance (PROC GLM, SAS) with each subject as a fixed block effect. Associations were examined using Pearson correlation (PROC CORR, SAS). Significance was declared at p < 0.05.
Results
Body weight and composition
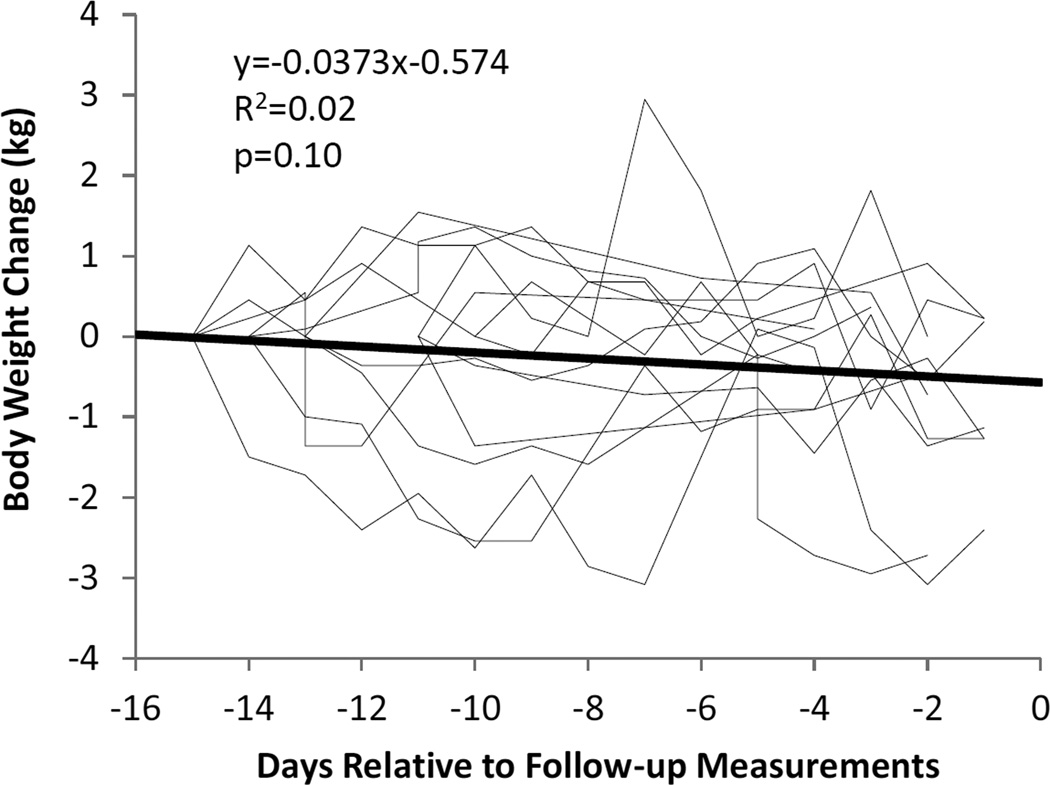
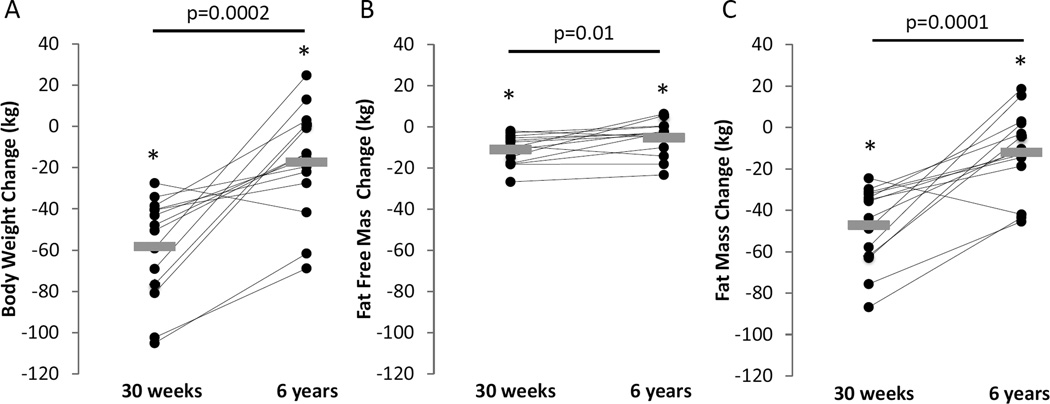
Of the original 16 Biggest Loser competitors, 6 males and 8 females agreed to participate in the follow-up study. These 14 subjects weighed (mean±SD) 148.9±40.5 kg at baseline and lost 58.3 ±24.9 kg at the end of the 30 week competition (Table 1). Body weight was relatively stable in the weeks prior to the follow-up measurements (Figure 1) with a mean rate of weight change of −37.3 ± 84.6 g/d that was not significantly different from zero (p=0.1). Figure 2 and Table 1 show the changes in body weight, FM, and FFM at the end of the 30 week competition and at the 6 year follow-up compared to baseline.
Table 1.
Anthropometric and energy expenditure variables in 14 of the original 16 study subjects who participated in the 30 week Biggest Loser weight loss competition. The predicted RMR was obtained using a linear regression equation developed using baseline data on body composition, age, and sex in the full 16 subject cohort. The p values were not adjusted for multiple comparisons.
| Baseline | End of competition at 30 weeks |
Follow-up at 6 years |
p-value Baseline vs. 30 weeks |
p-value Baseline vs. 6 years |
p-value 30 weeks vs. 6 years |
|
|---|---|---|---|---|---|---|
| Age (y) | 34.9±10.3 | 35.4±10.3 | 41.3±10.3 | <.0001 | <.0001 | <.0001 |
| Weight (kg) | 148.9±40.5 | 90.6±24.5 | 131.6±45.3 | <.0001 | 0.0294 | 0.0002 |
| BMI (kg/m2) | 49.5±10.1 | 30.2±6.7 | 43.8±13.4 | <.0001 | 0.0243 | 0.0002 |
| % Body fat | 49.3±5.2 | 28.1±8.9 | 44.7±10 | <.0001 | 0.0894 | 0.0003 |
| FM (kg) | 73.4±22.6 | 26.2±13.6 | 61.4±30 | <.0001 | 0.0448 | 0.0001 |
| FFM (kg) | 75.5±21.1 | 64.4±15.5 | 70.2±18.3 | <.0001 | 0.0354 | 0.0101 |
| RQ | 0.77±0.05 | 0.75±0.03 | 0.81±0.02 | 0.272 | 0.0312 | <.0001 |
| RMR measured (kcal/d) |
2607±649 | 1996±358 | 1903±466 | 0.0004 | <.0001 | 0.3481 |
| RMR predicted (kcal/d) |
2577±574 | 2272±435 | 2403±507 | <.0001 | 0.0058 | 0.0168 |
| Metabolic adaptation (kcal/d) |
29±206 | −275±207 | −499±207 | 0.0061 | <.0001 | 0.0075 |
| TEE (kcal/d) |
3804±926 | 3002±573 | 3429±581 | 0.0014 | 0.0189 | 0.0034 |
| Physical Activity (kcal/kg/d) |
5.6±1.8 | 10.0±4.6 | 10.1±4.0 | 0.0027 | 0.001 | 0.8219 |
Figure 1.
Daily body weight changes in the individual subjects (thin lines) and the mean weight change (thick line) over the 2 weeks prior to the follow-up measurements 6 years after the Biggest Loser competition.
Figure 2.
Individual (●) and mean (gray rectangles) changes in body weight (A), fat-free mass (B), and fat mass (C) at the end of the 30 week Biggest Loser weight loss competition and after 6 years. Horizontal bars and corresponding p values indicate comparisons between 30 weeks and 6 years. * indicates p<0.05 compared to baseline.
After 6 years, most subjects regained a significant amount of the weight lost during the competition, but there was a wide degree of individual variation and a mean weight loss of 11.9 ± 16.8 % (p=0.02) compared to baseline. All but one subject regained some of the weight lost during the competition and 5 subjects were within 1% of their baseline weight or above. Mean FM and FFM significantly increased in the 6 years since the competition but remained significantly below baseline.
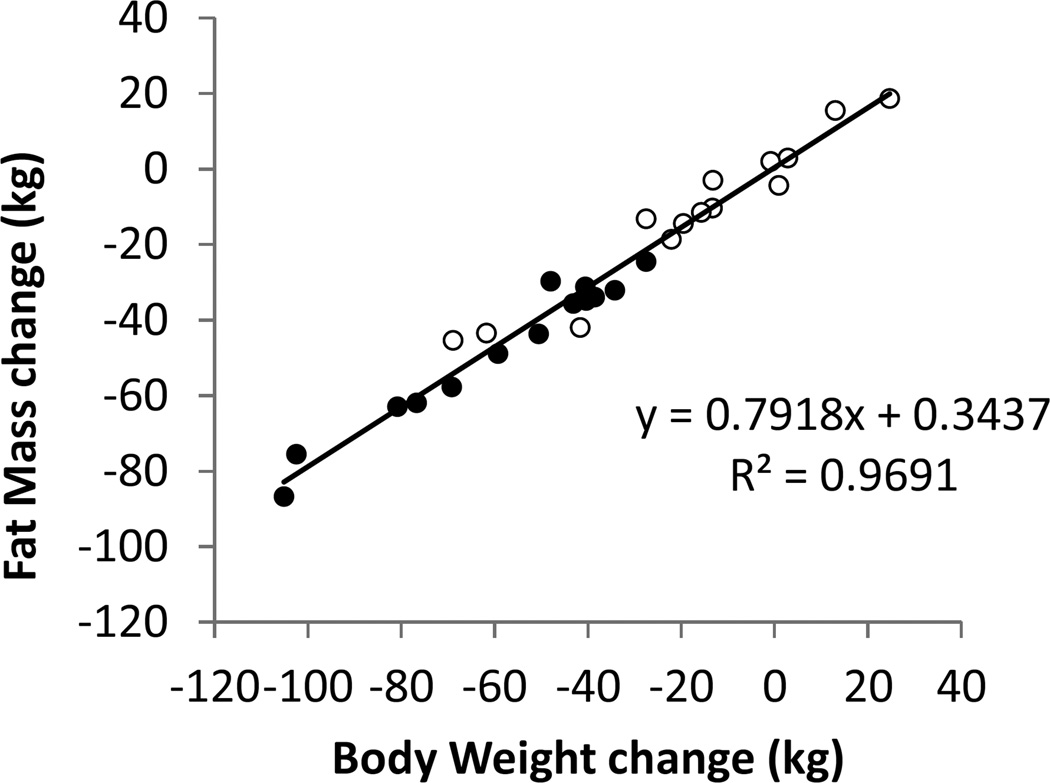
Figure 3 illustrates the relationship between body weight and FM changes and shows that ~80% of the weight changes at both 30 weeks and 6 years were attributable to FM. Since the data points all fell on the same curve, there was no evidence for a disproportionate regain of FM.
Figure 3.
Body fat mass changes accounted for the vast majority of weight loss at both the end of the 30 week competition (●) and 6 years later (○). All data points fell on the same curve indicating that there was no evidence for preferential body fat regain.
Total energy expenditure and physical activity
TEE decreased at the end of the 30 week competition despite a significant increase in physical activity expenditure (Table 1). After 6 years, TEE increased but remained below baseline while physical activity was not significantly changed since the end of the competition.
Fasting plasma hormones and metabolites
Table 2 presents the fasting hormone and metabolite data at baseline, the end of the 30 week competition, and 6 years later. After 6 years, plasma leptin, thyroxin (T4), and triglyceride (TG) remained lower than baseline while HDL and adiponectin were increased. Interestingly, insulin sensitivity was not significantly improved 6 years after the competition compared to baseline despite significant sustained weight loss.
Table 2.
Plasma hormone and metabolite concentrations in the overnight fasted state. The p values were not adjusted for multiple comparisons.
| Baseline | End of competition at 30 weeks |
Follow-up at 6 years |
p-value Baseline vs. 30 weeks |
p-value Baseline vs. 6 years |
p-value 30 weeks vs. 6 years |
|
|---|---|---|---|---|---|---|
| Glucose (mg/dl) |
95.7±16.3 | 70.2±21.9 | 104.9±48.7 | 0.0042 | 0.4759 | 0.0264 |
| Insulin (µU/ml) |
10.4±8.5 | 3.9±1.9 | 12.1±7.5 | 0.0126 | 0.3204 | 0.0013 |
| C-peptide (ng/ml) |
3±1.4 | 1.3±0.9 | 2.7±1.1 | 0.0019 | 0.4241 | 0.0016 |
| HOMA-IR | 2.5±2.2 | 0.7±0.4 | 3.6±4.6 | 0.0134 | 0.1892 | 0.0431 |
| TG (mg/dl) | 128.5±76.3 | 57.4±22.3 | 92.9±43.9 | 0.0019 | 0.053 | 0.0082 |
| Cholesterol (mg/dl) |
174±41.2 | 192.4±52.8 | 180.9±45.9 | 0.2115 | 0.5945 | 0.3549 |
| LDL (mg/dl) | 105±30 | 126±46 | 108±35 | 0.132 | 0.8343 | 0.1083 |
| HDL (mg/dl) | 42.5±17.6 | 54.6±14.9 | 54.5±21.2 | 0.0036 | 0.001 | 0.9751 |
| Adiponectin (mg/ml) |
2.46±1.28 | 4.69±2.05 | 7.29±4.71 | 0.0003 | 0.0025 | 0.0164 |
| T3 (ng/dl) | 9.42±2.78 | 5.31±1.45 | 11.15±1.81 | 0.0006 | 0.0623 | <.0001 |
| T4 (µg/dl) | 7.3±1.58 | 6.95±1.43 | 6.18±1.12 | 0.3814 | 0.0486 | 0.0828 |
| TSH (µIU/ml) | 1.52±1.26 | 1.42±0.73 | 1.93±0.9 | 0.7175 | 0.1933 | 0.0641 |
| Leptin (ng/ml) | 41.14±16.91 | 2.56±2.19 | 27.68±17.48 | <.0001 | 0.013 | 0.0001 |
Resting metabolic rate and metabolic adaptation
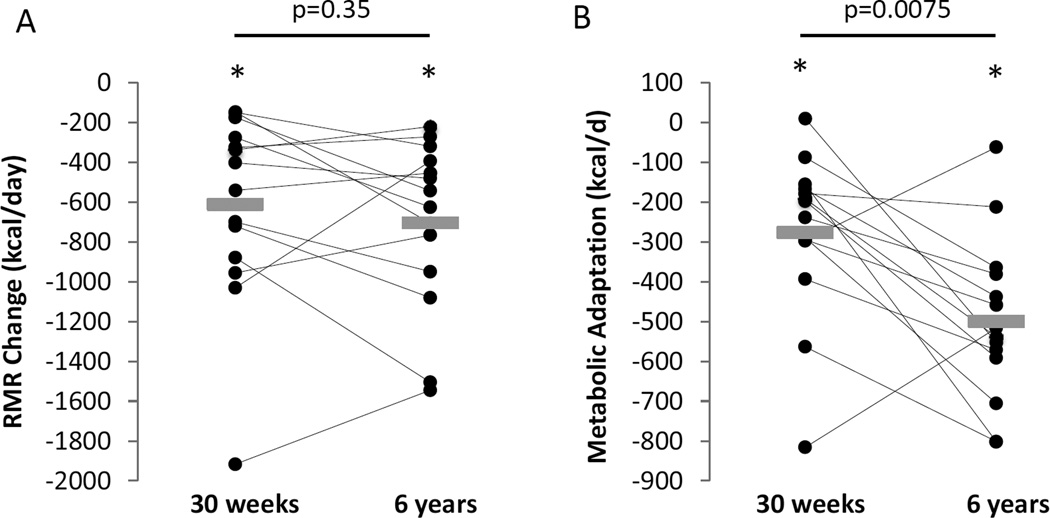
Table 1 shows that the RMR at baseline was 2607±649 kcal/d which fell to 1996±358 kcal/d at the end of the 30 week competition (p=0.0004). Despite a significant amount of weight regain 6 years later, the mean RMR was 1903±466 kcal/d which was not significantly different from the end of the competition (p=0.35). Figure 4A shows that RMR was decreased by 610±483 kcal/d at the end of the competition (p=0.0004) and was 704±427 kcal/d below baseline 6 years later (p<0.0001) which was not significantly different from the end of the competition (p=0.35).
Figure 4.
Individual (●) and mean (gray rectangles) changes in resting metabolic rate (A), and metabolic adaptation (B) at the end of the 30 week Biggest Loser weight loss competition and after 6 years. Horizontal bars and corresponding p values indicate comparisons between 30 weeks and 6 years. * indicates p<0.001 compared to baseline.
We previously showed in the full 16 subject cohort that the magnitude of metabolic adaptation at the end of the competition was significantly correlated with the amount of weight lost (3), and this trend continued with the 14 subjects of the current study but did not reach statistical significance (r=0.48, p=0.08). Figure 4B shows that at the end of the 30 week competition there was a significant metabolic adaptation of −275±207 kcal/d (p=0.00025) that increased in magnitude to −499±207 kcal/d after 6 years (p<0.0001). Metabolic adaptation at 6 years was not significantly correlated with metabolic adaptation at the end of the competition (r=0.18, p=0.54).
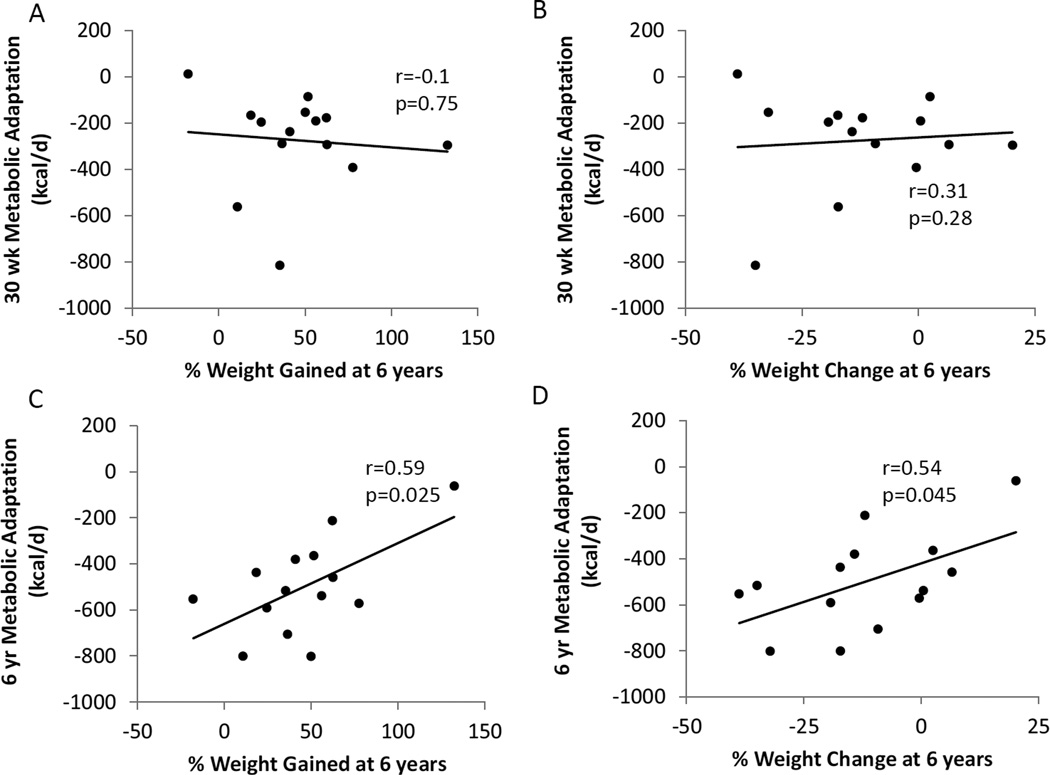
Figure5A and 5B show that metabolic adaptation measured at the end of the 30 week competition was not significantly related to weight gained (r=−0.1, p=0.75) or the percent weight change from baseline (r=0.31, p=0.28) at the 6 year follow-up. However, metabolic adaptation at follow-up was significantly related to both weight gain (r=0.59, p=0.025) and the percent weight change from baseline (r=0.54, p=0.045) such that those with greater weight loss at 6 years continued to experience greater metabolic slowing. Metabolic adaptation at 6 years was not significantly correlated with changes in fasting plasma leptin (r=0.204, p=0.48), T3 (r=0.34, p=0.23), TSH (r=0.42, p=0.88), or T4 (r=0.044, p=0.88).
Figure 5.
No significant associations were detected between metabolic adaptation at the end of the 30 week competition with percent weight gained since 30 weeks (A) or percent weight change versus baseline (B) at 6 years. Metabolic adaptation at 6 years was significantly associated with the percent weight gained since 30 weeks (C) or percent weight change versus baseline (D) at 6 years.
Assessing metabolic cart bias
Different metabolic carts were used to make the RMR measurements at the 6 year follow-up compared to the cart used at both baseline and 30 weeks. To investigate the potential bias of using different instruments, we tested the original Max II cart in comparison to the Parvo cart used to make the 6 year follow-up measurements as described in the Supplementary Materials. We found that the Max II cart had no significant energy expenditure bias compared to the Parvo cart (1.96±8.45%, p=0.82). Supplementary Figure 1A illustrates that the Max II cart was more variable and Supplementary Figure 1B demonstrates that there was no significant trend compared to the Parvo cart as a function of energy expenditure (r=0.45, p=0.32). We cannot rule out the possibility that the original Max II cart became more accurate at the time of testing compared to 6 years earlier, but this seems unlikely.
We also performed a sensitivity analysis to investigate how the metabolic adaptation measurements varied in response to an assumed % bias of the RMR measurements during the competition versus at the 6 year follow-up. Supplementary Figure 2 illustrates that a metabolic cart bias >16% would be required to eliminate the statistical significance of the measured metabolic adaptation at the 6 year follow-up. A bias ≥4% would be required to nullify the statistical significance of the measured increase in metabolic adaptation observed at the 6 year follow-up compared to the end of the 30 week competition. Therefore, we found no evidence that the use of different metabolic carts could explain our observations in the absence of a real effect on resting metabolic rate.
Discussion
To the best of our knowledge, this study is the longest follow-up investigation of the changes in metabolic adaptation and body composition subsequent to weight loss and regain. We found that despite substantial weight regain in the 6 years following participation in The Biggest Loser, RMR remained suppressed at the same average level as at the end of the weight loss competition. Mean RMR after 6 years was ~500 kcal/d lower than expected based on the measured body composition changes and the increased age of the subjects.
Metabolic adaptation acts to decrease energy expenditure and thereby impedes the rate of weight loss during an intervention. However, the Biggest Loser participants with the greatest weight loss at the end of the competition also experienced the greatest slowing of RMR at that time (3). Similarly, those who were most successful at maintaining lost weight after 6 years also experienced greater ongoing metabolic slowing. These observations suggest that metabolic adaptation is a proportional, but incomplete, response to contemporaneous efforts to reduce body weight from its defended baseline or “set point” value (14).
The magnitude of metabolic adaptation increased 6 years after the Biggest Loser competition. This was surprising given the relative stability of body weight prior to the follow-up measurements compared to the substantial negative energy balance at the end of the competition which is known to further suppress RMR (15, 16). In contrast, a matched group of Roux-en-Y gastric bypass surgery patients who experienced significant metabolic adaptation 6 months after the surgery had no detectable metabolic adaptation after 1 year despite continued weight loss (17). It is intriguing to speculate that the lack of long-term metabolic adaptation following bariatric surgery may reflect a permanent resetting of the body weight set-point (18).
We found no significant correlations between the degree of metabolic adaptation at 6 years and the changes in fasting metabolites and hormones. However, the study was not powered to detect such correlations and it is possible that other unmeasured variables, such as changes in circulating organic pollutants (19), might be more strongly related to metabolic adaptation.
A meta-analysis of previous cross-sectional studies found that subjects who had lost weight exhibited a 3–5% lower RMR compared to control subjects that had not lost weight (20). While metabolic adaptation has been suggested to persist over the long-term with sustained weight loss (4), few studies have measured the same subjects at baseline as well as multiple occasions after weight loss (21, 22, 23, 24, 25, 26, 27, 28). Two short term studies investigating the effects of small weight loss and regain cycles in overweight women yielded conflicting data on whether RMR was significantly altered after weight regain (23, 24). Energy expenditure was found to be decreased in two male polar explorers following weight cycling (26) and a recent study reported a sustained RMR reduction 6 months after weight loss in subjects who were classified as weight regainers in comparison to weight maintainers who recovered their expected RMR (21). However, when all subjects were considered together, no significant metabolic adaptation was found after the weight regain period and there was no significant association between weight regain and metabolic adaptation (A. Bosy-Westphal, personal communication).
Longer term studies in women found no significant sustained reductions in RMR following weight regain (22, 28). However, the classic Minnesota semi-starvation experiment (25) demonstrated a sustained suppression of RMR during a period of weight regain with controlled re-feeding when subjects were prevented from eating above baseline levels (29, 30). Interestingly, increased hunger has been associated with metabolic adaptation (31) and when the Minnesota experiment subjects were allowed to eat ad libitum they consumed calories substantially above baseline levels and the suppression of RMR rapidly reversed (25). Similarly, a recent study demonstrated an elevated RMR during a period of enforced overfeeding following a period of underfeeding that was coincident with significant metabolic adaptation and weight loss (27). The concurrent state of energy balance at the time of the RMR measurements can therefore have a profound impact on whether metabolic adaptation is detected.
Unlike previous studies where the metabolic adaptation measurements may have been confounded by ongoing positive or negative energy balance in the period immediately prior to the measurements, we monitored body weight changes of the subjects for 2 weeks prior to admission to ensure that they were relatively weight stable and the mean rate of weight change was not significantly different from zero.
While most subjects experienced substantial weight regain in the 6 years since the Biggest Loser competition, the mean weight loss was 11.9 ± 16.8% compared to baseline and 57% of the participants maintained at least 10% weight loss. In comparison, it has been estimated that ~20% of overweight individuals maintain at least 10% weight loss after 1 year of a weight loss program (32). Only 37% of the lifestyle intervention arm of the Diabetes Prevention Program maintained at least 7% weight loss after 3 years (33), and 27% of the intensive lifestyle intervention arm of the Look AHEAD trial maintained 10% weight loss after 8 years (34).
Rapid weight loss, such as that experienced by the Biggest Loser participants, is sometimes claimed to increase the risk of weight regain, but recent studies have failed to support this idea since weight loss rate per se was not observed to affect long-term weight regain (35, 36). The relatively greater success at maintaining lost weight in the Biggest Loser participants may have been due to the massive weight loss experienced during the competition since the magnitude of early weight loss is the best predictor of long-term weight loss (37, 38). In addition, it is likely that the public nature of The Biggest Loser competition may have subjected its former participants to a degree of external accountability that contributed to their relative success at maintaining significant weight loss over the long term. Of course, the extreme and public nature of this weight loss intervention makes it difficult to translate our results to more typical weight loss programs.
In conclusion, we found that The Biggest Loser participants regained a substantial amount of their lost weight in the 6 years since the competition, but overall were quite successful at long-term weight loss compared to other lifestyle interventions. Despite substantial weight regain, a large persistent metabolic adaptation was detected. Contrary to expectations, the degree of metabolic adaptation at the end of the competition was not associated with weight regain, but those with greater long-term weight loss also had greater ongoing metabolic slowing. Therefore, long term weight loss requires vigilant combat against persistent metabolic adaptation that acts to proportionally counter ongoing efforts to reduce body weight.
Supplementary Material
What is already known about this subject
Weight loss results in a suppression of resting metabolic rate (RMR) that is often beyond what is expected due to changes in body composition – a phenomenon called “metabolic adaptation”.
Metabolic adaptation to weight loss has been suggested to persist over the long-term and predispose to weight regain, but longitudinal data supporting these contentions are lacking.
What this study adds
Six years following massive weight loss during The Biggest Loser competition, a large persistent metabolic adaptation was observed despite substantial weight regain.
Metabolic adaptation at the end of the weight loss competition was not significantly correlated with weight regain.
Subjects maintaining the greatest long-term weight loss also experienced greater ongoing metabolic adaptation.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes & Digestive & Kidney Diseases.
We thank the former Biggest Loser contestants for volunteering to participate in this follow-up study. Eric Ravussin and Brian Gilmore assisted in obtaining the original Max II metabolic cart for testing and Alison Baskin, Courtney Duckworth, and Brooks P. Leitner helped conduct the follow-up measurements.
Footnotes
ClinicalTrials.gov Identifier: NCT02544009
Disclosure: No authors have conflicting interests.
References
- 1.Muller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring) 2013;21:218–228. doi: 10.1002/oby.20027. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johanssen DL, Knuth ND, Huizenga R, Rood J, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [Corrigendum. J Clin Endocrinol Metab 101(5), 2016.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 5.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17:1281–1286. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015;22:427–436. doi: 10.1016/j.cmet.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267:E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 8.Hall KD. Diet versus exercise in "the biggest loser" weight loss competition. Obesity (Silver Spring) 2013;21:957–959. doi: 10.1002/oby.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoeller DA, Jefford G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes (Lond) 2002;26:97–101. doi: 10.1038/sj.ijo.0801851. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–969. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 12.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 13.Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 14.Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Disease models & mechanisms. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, Foster GD, Letizia KA, Mullen JL. Long-term effects of dieting on resting metabolic rate in obese outpatients. Jama. 1990;264:707–711. [PubMed] [Google Scholar]
- 16.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 17.Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring) 2014;22:2563–2569. doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Z, Mumphrey MB, Townsend RL, Morrison CD, Munzberg H, Ye J, et al. Reprogramming of defended body weight after Roux-En-Y gastric bypass surgery in diet-induced obese mice. Obesity (Silver Spring) 2016;24:654–660. doi: 10.1002/oby.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay A, Pelletier C, Doucet E, Imbeault P. Thermogenesis and weight loss in obese individuals: a primary association with organochlorine pollution. Int J Obes Relat Metab Disord. 2004;28:936–939. doi: 10.1038/sj.ijo.0802527. [DOI] [PubMed] [Google Scholar]
- 20.Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69:1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 21.Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond) 2013;37:1371–1377. doi: 10.1038/ijo.2013.1. [DOI] [PubMed] [Google Scholar]
- 22.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Jebb SA, Goldberg GR, Coward WA, Murgatroyd PR, Prentice AM. Effects of weight cycling caused by intermittent dieting on metabolic rate and body composition in obese women. Int J Obes. 1991;15:367–374. [PubMed] [Google Scholar]
- 24.Kajioka T, Tsuzuku S, Shimokata H, Sato Y. Effects of intentional weight cycling on non-obese young women. Metabolism. 2002;51:149–154. doi: 10.1053/meta.2002.29976. [DOI] [PubMed] [Google Scholar]
- 25.Keys A. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 26.Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 2007;31:204–212. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 27.Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.109173. [DOI] [PubMed] [Google Scholar]
- 28.Wadden TA, Foster GD, Stunkard AJ, Conill AM. Effects of weight cycling on the resting energy expenditure and body composition of obese women. The International journal of eating disorders. 1996;19:5–12. doi: 10.1002/(SICI)1098-108X(199601)19:1<5::AID-EAT2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr. 1998;68:599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- 30.Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord. 1996;20:393–405. [PubMed] [Google Scholar]
- 31.Tremblay A, Royer MM, Chaput JP, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond) 2013;37:759–764. doi: 10.1038/ijo.2012.124. [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:954–962. doi: 10.1016/S2213-8587(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 36.Vink RG, Roumans NJ, Arkenbosch LA, Mariman EC, van Baak MA. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity (Silver Spring) 2016;24:321–327. doi: 10.1002/oby.21346. [DOI] [PubMed] [Google Scholar]
- 37.Astrup A, Rossner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17–19. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 38.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? International journal of behavioral medicine. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.