Abstract
The emergence of Zika virus (ZiV), a mosquito borne Flavivirus like dengue (DEN) and chikungunya (CHIK), in Brazil in 2014 and its spread to various countries have led to a global health emergency. Aedes aegypti is the major vector for ZiV. Fast dissemination of this virus in different geographical areas posses a major threat especially to regions where the population lacks herd immunity against the ZiV and there is abundance of Aedes mosquitoes. In this review, we focus on current global scenario, epidemiology, biology, diagnostic challenges and remedial measures for ZiVconsidering the Indian perspective.
Keywords: Aedes, microcephaly, mosquito, vector, vertical transmission, Zika virus
Introduction
Aedes mosquito borne diseases have become one of the major threats to human population. Zika virus infection is a mosquito-borne illness like dengue (DEN) and chikungunya (CHIK) viruses. A lot about the biology of vectors and its role in disease transmission is known but Aedes mosquito-borne infections continue to be the major cause of mortality in many subtropical and tropical countries.
The introduction of any emerging infection and its rapid spread to other parts of the world draws global attention. The changing climate also results in a boom in vector population and their accelerated dispersal. The Aedes vector species borne infections like Zika are a potential threat, especially in urban settings where Ae. aegypti is abundant. The growing population in urban settings also increased the need for potable water, which necessitated storage practices in households, making ideal breeding habitats for Ae. aegypti mosquitoes, that also increase dengue and CHIKV infections. Due to this mosquito-borne viral diseases are going to be a major threat in the 21st century. We present here an overview of Zika virus (ZiV) in the context to India.
Till today, in India its presence has not been reported, however, several points need to be addressed on a priority: (i) What is the risk for India, if it appears?; (ii) What is our capacity on preparedness in term of diagnostics and vaccine?; (iii) What is the preparedness keeping in mind about our existing health infrastructure and surveillance programme? [Wider surveillance in terms of human and vectors and bringing the Virus Research Diagnostic Laboratories (VRDLs) component into the surveillance programme, is the need of today]; (iv) What can be added for the better surveillance in human and vectors and what are the specific research components needed?; (v) Evidence of ZiV infections in the mothers of children with microcephaly; and (vi) What is the effective message to our scientific community on certain policy related issues?
Historical perspective
ZiV was first isolated from a Rhesus monkey (Rhesus# 766) in the Zika Forest, Uganda, in April 1947 during the Rockefeller Foundation's initiative for research on yellow fever. This monkey was placed in a cage on a tree platform in the forest, and developed fever1. The serum of the same monkey was inoculated intracerebrally into mice and virus was isolated from the inoculated mouse brain after 10 days. The virus was later named as ZiV due to its origin in Zika forest. This virus was also isolated from the Ae. africanus mosquitoes trapped from the same forest in early 19482. Studies based on serology first indicated that humans could also get infected which was proved by the first isolation from humans in 1952, in Tanzania and Uganda3. The presence of antibodies has been reported from Africa to Asia. The virus lived a ubiquitous life for decades in the narrow tropical and equatorial zones predominately sylvatic with arboreal Aedes mosquitoes as transmitting vector. Until 2007, the first documented large natural human infections/outbreak occurred in the Yap state of Micronesia followed by French Polynesia and Cambodia4.
Since then this virus has been considered emergent as a few cases have been reported from the time including a major epidemic in French Polynesia (FP) in October 2013 (Direction de la santé, PF, 2013), and in New Caledonia in January 20145. In 2014, the virus started spreading eastward across the Pacific Ocean, to some part of French Polynesia and then to Easter Island. Presently, the ZiV outbreak has reached to a pandemic level after its spread to Mexico, Central America, the Caribbean and South Americas5.
Interestingly, from a mild self-limiting febrile illness similar to dengue fever, the ZiV outbreak in French Polynesia presented with highly unusual clusters of Guillain-Barré syndrome. However, overall morbidity was still low with low/no mortality. In 2015 Campos et al6 of the Federal University of Bahia, Salvador, reported detection of ZiV RNA in serum of patients (predominately females) with dengue-like illness in the background of dengue and CHIK virus infection in that region; the major clinical presentation in these cases being a maculoexantematic illness6.
Subsequently, between May and November 2015 approximately 1.3 million confirmed ZiV infections were reported from different parts of South America with 4000 cases of ZiV associated microcephaly7. European Centre for Disease Prevention and Control (ECDC): rapid risk assessment mentioned that international travel played an important role in the dissemination of ZiV to newer niches (ecdc.europa.eu/) and WHO sounded an international alert on ZiV8. Which factors played a role in the “split personality” of this rather ubiquitous virus from a benign eco-cycle towards threatening to become a global pandemic agent? The answers will take time to come. While full genome sequence analysis on limited isolates of the virus has not shown any dramatic “evolutionary” mutations, vector biology and disease pathogenesis of ZiVis far from being clear.
The whole world is concerned over spread of ZiV virus in tropical and subtropical areas of many geographical regions of all continents for its possible association with clusters of birth and neurological conditions. The WHO declared a Public Health Emergency of International Concern on January 28, 2016, keeping in view the lesson learnt from the African Ebola outbreak9. Fast dissemination of this virus in different geographical areas is a major concern for non-endemic regions where the population does not possess herd immunity to ZiV virus and abundant presence of the vector Aedes mosquitoes. Till now the only report on the possible presence of ZiV in the Indian subcontinent is the detection of antibodies against ZiV(16.8% prevalence) mostly in the Bharuch district of the then Bombay State, Gujarat and Nagpur in 1954, which could be a result of cross-reactivity with other flaviviruses as dengue was found prevalent in these areas10. The major concern is that, once endemicity is established, ZiV can exist in its natural eco-cycle for a significant period with a potential to emerge as a pathogenic human agent. However, determinant complex factors are still poorly understood. In the absence of recent serological data from India or virus isolations, it is difficult to predict the impact of ZiV in India. Most people infected with ZiV do not show any pathogenomic/specific symptoms. Only, one in five people infected may develop a mild disease, with symptoms lasting from several days to a week. The most common symptoms are fever, rash, joint pain and conjunctivitis.
The virus
ZiV is transmitted by arthropod vectors thus called arbovirus. This virus belongs to the family Flaviviridae. It is placed under the genus Flavivirus and is closely related to yellow fever, Japanese encephalitis, dengue, and West Nile viruses10. It also has a close relationship with Ilheus, Rocio and Saint Louis encephalitis viruses11,12,13. ZiV is an enveloped virus, having icosahedral symmetry and has a non-segmented, single stranded, positive sense RNA genome containing 10,794 nucleotides encoding 3,419 amino acids. The virus can be inactivated by potassium permanganate, ether, and temperature >60°C but neutralization with 10 per cent ethanol is not effective1.
Phylogeny
ZiV is one of the two viruses in the Spondweni virus clade and is very much similar to the Spondweni virus, which was first identified in South Africa12,13. Various sub-clades have been revealed by genomic comparisons indicating two major lineages, Asian and African14 and phylogenetic studies have shown that the virus spreading in the Americas is most closely related to the Asian strain, the same strain that has been circulating since the 2013 outbreak in French Polynesia14,15. ZiV complete genome sequence has been published16 and genetic analyses have revealed that ZiV has evolved into three distinct genotypes. Genetically, it was postulated that the virus originated in East Africa (East African or MR766 prototype cluster), and then spread to West Africa (West African or Nigerian cluster) and Asia (Asian genotype) approximately some 50-100 years ago17,18. Recent preliminary findings from sequences in the public domain have shown that increase in viral replication rate in humans may be due to a possible change in non-structural protein 1 (NS1) codon usage19.
The host range and transmission pattern
Human and non-human primates most likely serve as the main reservoirs for the virus but some authors have also reported anti-ZiV antibodies in various mammals (such as, Orang-outang, zebras, elephants, etc.) and rodents20,21. Human-vector-human cycle of transmission occurs during outbreaks, which are rare events where arboviruses become established as a cause of human disease, spread in a mosquito-human-mosquito cycle, instead of enzootic mosquito-monkey-mosquito cycle. ZiV is transmitted by Aedes mosquitoes, which are daytime active and aggressive biters. The virus has been isolated from Ae. aegypti, Ae. albopictus, Ae. africanus, Ae. apicoargenteus, Ae. furcifer, Ae. hensilli, Ae. luteocephalus and Ae. vitattus having extrinsic incubation period of about 10 days11. There are also some reports of sexual transmission of ZiV. The non-infected partner showed symptoms of ZiV infection and laboratory tests found ZiV antibodies in both the partners’ blood22,23,24. Detection of ZiV RNA in the amniotic fluid of foetuses and a possible link between ZiV fever and microcephaly in newborn babies25 indicate that it can cross the placenta and cause vertical transmission.
Epidemiology
The first isolation of virus from humans was carried out in 1952, in Uganda and in Tanzania1. In a study conducted in Nigeria in 1968 and during 1971-1975, ZiVwas also isolated from humans and in another study it was evident that 40 per cent of the tested persons had neutralizing antibody to ZiV26,27. From various studies viz., virological, serological and case reports of human ZiV infection, the virus was identified and reported from various other African countries (Uganda, Senegal, Ivory Coast, Nigeria, Gabon, Egypt, Tanzania, Sierra Leone, Central African Republic), Asian countries (Cambodia, Indonesia, Malaysia, India, Pakistan, Singapore, Thailand, Philippines and Vietnam), Pacific islands (Micronesia/Yap, FP, Cook islands and New Caledonia) and Oceania28. The outbreak on Yap Island in 2007 was evidence of ZiV illness that has been detected outside of Africa and Asia28.
Pathogenesis and clinical manifestation
Most of the flavivirus replication is thought to occur in cellular cytoplasm but perhaps it might not be the case with the ZiV as one of the studies detected antigens in infected cell nuclei29. Only a few facts are known about the ZiV pathogenesis but most of the mosquito-borne flaviviruses are thought to replicate initially in dendritic cells near the site of inoculation. The spread of virus takes place via lymph nodes and then through bloodstream30. Still there are insufficient data regarding incubation period and very limited data are available regarding its appearance in body fluids and the duration to which it persists in the body. ZiV can be detected as early as onset of the illness begins and even after 11 days of onset of illness in human blood31,32. In an experiment, even after nine days of experimental inoculation, virus was isolated from the serum of a monkey1. Symptoms of infection with the virus begin with mild headache followed by maculopapular rash (neck, face, trunk, and upper arms, and spread to palms and soles), fever, malaise, conjunctivitis and joint pains. Other manifestations include diarrhoea, constipation, abdominal pain, anorexia, dizziness and conjunctivitis. Rashes are not the consistent feature of this disease31. Some less frequent manifestations are myalgia, vomiting, oedema, and retro-orbital pain32. Only one in five persons develop symptoms with no fatalities and thus it is a relatively mild disease. Research has shown some link between ZiV fever and microcephaly in newborn babies by mother-to-child transmission25 and neurologic conditions in infected adults, including cases of the Guillain-Barré syndrome.
Current scenario of ZiV outbreaks
In October, 2013, a ZiV epidemic was reported for the first time by healthcare authorities, on the Society, Tuamotu, and Marquesas islands, which later spread to all the islands of the archipelagos33. From October 2013 to February 14, 2014, about 10 per cent of the population consulted for a probable ZiV infection. This was the first time when such a large epidemic was reported and cases imported from FP were reported in New Caledonia, Japan, Norway, Easter Island and continental France. Ae. aegypti and Ae. polynesiensis were presented as the vectors of ZiV for this epidemic in an entomological study34. Forty patients with Guillain-Barré syndrome were diagnosed in three months among 72 severe cases with neurological symptoms35. The direct involvement of the ZiV leading to these severities of symptoms needs to be investigated. The first indigenous case of the Americas was found in February 2015, in Isla de Pascua (Chile). Since April 2015, a large outbreak of ZiV has spread across much of South and Central America and the Caribbean36. Then it began in Brazil and in May 2015, Pan American Health Organization confirmed the first 16 cases of ZiV infection36. On June 4, 2015, the first case of ZIV was presented in Dominican Republic37. In January 2016, a travel alert was issued by the CDC (U.S.) for people travelling to regions where ZiV was prevalent and suggested that women should consult with their physicians before travelling if they are thinking about becoming pregnant in near future38. Similar travel warnings are also issued from health agencies of different countries like Ireland, United Kingdom, Canada, New Zealand, and the European Union. Health ministries of some countries like Colombia, El Salvador, Ecuador, and Jamaica have issued recommendation to avoid pregnancy for eight months. ZiV associated outcomes with infection during pregnancy are not yet fully understood and before giving any concluding remarks research in this particular direction has to go a long run.
Diagnosis
The symptoms of ZiV are similar to those in case of dengue and chikungunya; therefore, the diagnostic molecular techniques are employed on the acute samples and serological tests are applied on samples 5-6 days after onset of symptoms. Reverse transcription (RT)-PCR of acute-phase serum samples is the test of choice. It is evident that the viruria can last longer than viraemia and hence the RT-PCR based detection of viral RNA in patients’ urine samples could be an alternative method in case genetic material disappears from the serum11,30,39. An alternative to the RT-PCR is the “pan flavivirus” amplification technique combined with sequencing data11,18,40,41. Real time RT-PCR can also be very useful for diagnostic purpose32.
Virus isolation from samples collected upto five days after the onset of symptoms can be beneficial. The plaque reduction neutralization test (PRNT) by neutralizing antibody that appears as early as five days after onset of illness has greater specificity with respect to immunoassays, but the drawback is that it may give cross-reactive results in case of secondary flavivirus infections. Immunoglobulin (Ig) M to ZiV can be detected as early as three days after onset of illness by ELISA31.
Issue of high cross-reactivity with dengue viruses
Cross-reactivity is a major problem and is frequently observed with dengue virus than with yellow fever, Murray Valley encephalitis (MVE), Japanese encephalitis or West Nile viruses. In this context, an investigation into the composition of envelope glycoprotein (E-protein) of ZiV with other flaviviruses may be necessary as E-protein is the main target for host antibodies.
Hitherto unpublished data from the authors’ laboratory reveal similarity between E protein of ZiV and dengue viruses. ZiV E-protein amino acid sequence has been compared (using bioinformatics tools) with those from other flaviviruses viz. dengue type 2 (DEN2), MVEV, Japanese encephalitis (JEV), West Nile (WNV), Kyasanur forest disease (KFDV), Tick-borne encephalitis (TBEV), yellow fever (YFV) and St. Louis encephalitis (StLu). The results of pair-wise comparison of all possible pairs of sequences (as obtained from ISHAN package)42 are summarized in Table I. It should be noted that among flaviviruses ZiV is closest to DEN2 with 54.2 per cent identity, and 81 per cent similarity, whereas it is least homologous to KFDV with 36.3 per cent identity and 67.2 per cent similarity. Comparison of ZiV E-protein with those from all serotypes of dengue virus revealed that ZiV was 46, 54.2, 55.3 and 54.2 per cent identical to DEN1, DEN2, DEN3 and DEN4, respectively in terms of amino acid composition (Table II). Phylogenetic analyses show that ZiV and DEN2 are most closely related as these appear in the same sub-cluster within the bigger clade formed by mosquito-borne flaviviruses (Fig. 1). E-proteins from Tick-borne flaviviruses formed a different cluster.
Table I.
Per cent identity of amino acid composition of flavivirus E-proteins
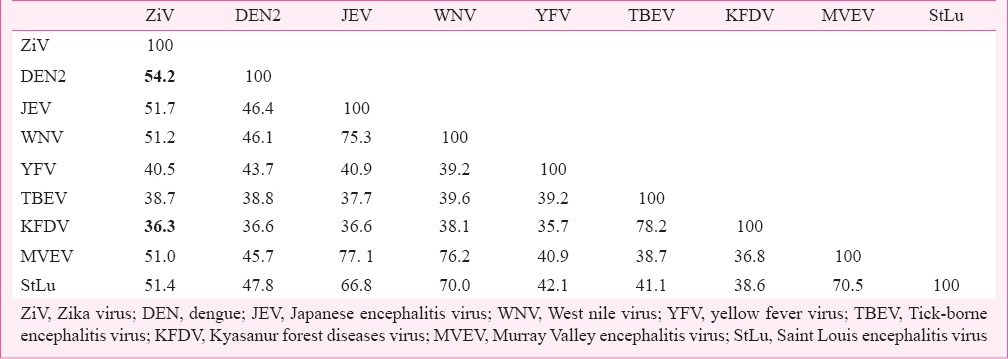
Table II.
Per cent identity of amino acid composition between ZiV virus and dengue E-proteins
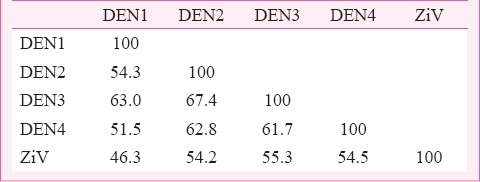
Fig. 1.
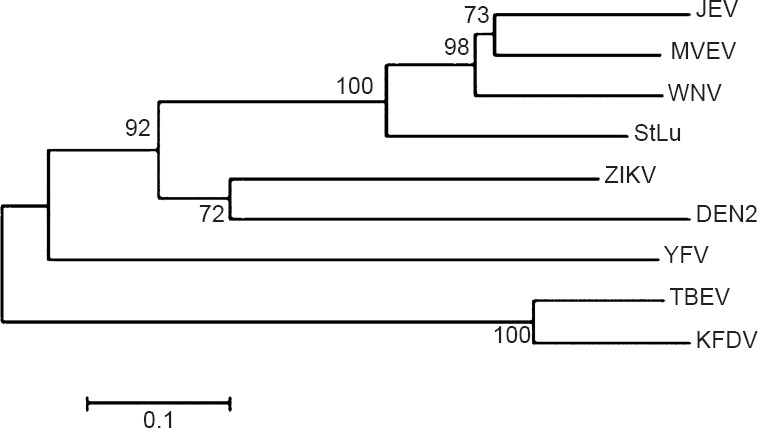
Phylogenetic tree (Neighbor-Joining) of E-protein amino acid sequences from flaviviruses generated from MEGA 6.0 using 10000 bootstraps. (Note: ZiV marked as ZIKV). ZIKV- Zika virus; DEN, dengue; JEV-Japanese encephalitis virus; WNV, West Nile virus; YFV, yellow fever virus; TBEV, Tick-borne encephalitis virus; KFDV, Kyasanur forest diseases virus; MVEV, Murray Valley encephalitis virus; StLu, Saint Louis encephalitis virus.
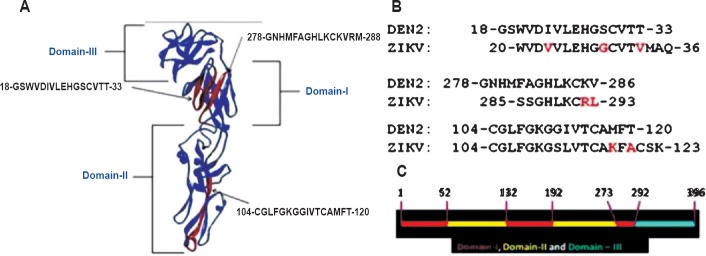
Analysis of the predicted B-cell epitope43 revealed the occurrence of three antigenic regions with >80 per cent identity of amino acid composition with dengue virus sequences. Of these, the epitope 20-WVDVVLEHGGCVTVMAQ-36 in ZiV corresponds to the epitope 18-GSWVDIVLEHGSCVTT-33 in DEN2 and highly conserved in other dengue serotypes (unpublished observation from in silico results comparing dengue E protein sequences). This epitope occurs in domain I of 3D structure of flavivirus E-protein (DEN2, 1OAN.pdb numbering)43. The epitope 285-SSGHLKCRLKM-295 in ZiV corresponds to the epitope 278-GNHMFAGHLKCKVRM-288 in DEN2, and occurs in domain I extending towards the hinge region44, which connects the domain I with domain III (Fig. 2). The epitope 104-GCGLFGKGSLVTCAKFACSK-123 in ZiV corresponds to 104-CGLFGKGGIVTCAMFT-120 in DEN2 and is located in the domain II. The occurrence of these B-cell epitopes on ZiV having ≥80 per cent identity of composition with dengue virus may be the reason for the reported cross-reactivity in serological tests. In the natural dimeric arrangement of E-protein on viral membrane, these epitopes are exposed for interaction with host immune system.
Fig. 2.
(A). Positions of three most similar predicted B-cell epitopes on 3D structure of DEN2 E- protein (1OAN.pdb). 3D structure visualization and rendering of images was performed in Discovery Studio viewer 3.1. (B). Comparison of epitope compositions, DEN2 verses ZiV, with differences marked in Red font. (C). Domain classification of dengue virus E-protein (1OAN.pdb) with numbers denoting amino acid order in the sequence. (Note: ZiV marked as ZIKV).
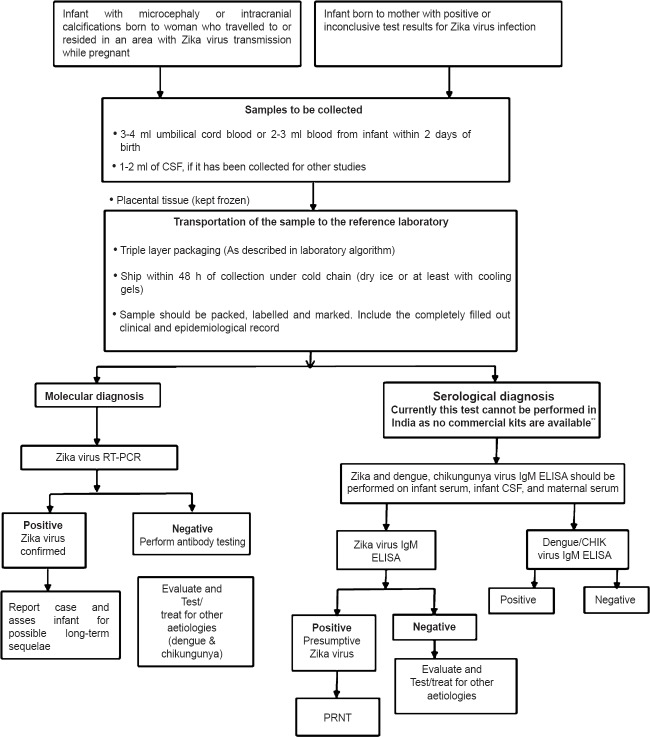
Fig. 3 shows the flowchart for Zika diagnostic protocol as adapted by many countries45.
Fig. 3.
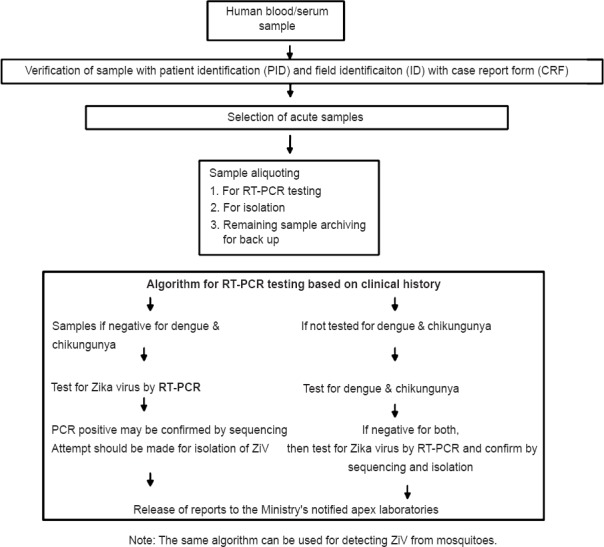
Flowchart for Zika diagnostic protocol.
Diagnostic RT-PCR for ZiV detection
Blood samples should be collected from febrile illness cases and must be tested for common viral diseases; DENV and CHIKV and then ZiV. Blood samples negative for DENV and CHIKV should be processed for detection of Zika virus aetiology. The early detection of ZiV cases in community would help for quicker prevention and control of the disease. The primary means of diagnosis is nucleic acid detection by RT-PCR targeting the non-structural protein 5 (NS-5) genomic regions. Standard RT-PCR and quantitative RT-PCR usually provide a rapid, specific and sensitive method for early detection of ZiV. ZiVRNA also has been detected in saliva or urine samples. However, it is recommended that the serum sample be taken during the first five days after the onset of symptom46.
Detection of IgM antibodies to ZiV by diagnostic ELISA
To date there are no commercially available kits (approved or validated) for the serological determination of ZiV. Further, it is a known fact that performing ELISA for Zika virus would be further challenging in countries like India due to endemicity of other flaviviruses like dengue, JE, WN and KFD.
Virus isolation
Viral isolation is not regarded as a diagnostic tool and can be used only for supplemental research studies in public health surveillance. However, till date the prevalence of this virus in India has not been reported, therefore, it should be considered exotic and isolation procedures should be followed only in apex laboratories that are equipped with good high containment facilities.
Treatment and control
Presently there is no vaccine or antiviral drug available to combat the ZiV outbreak situation. Symptomatic treatment is rest, fluids (to prevent dehydration) and paracetamol (acetaminophen) to relieve fever and pain. If the patient has dengue virus infection also, aspirin and other non-steroidal anti-inflammatory drugs should be used cautiously because of the risk of bleeding/haemorrhages, hence, dengue should be ruled out before such medication47.
Prevention and control relies on controlling mosquito population through effective removal of breeding sites and also reducing mosquito-human interactions. Reducing the breeding sites of Aedes mosquitoes is the best preventive public health measure. The other measures include use of insect repellent, use of physical barriers such as screens, sleeping under mosquito nets, wearing clothes that cover maximum parts of the body. Young children, sick, elderly and those who may not be able to protect themselves should be given special attention. Buckets, flowerpots or tyres that can hold water need cleaning or covering with suitable materials so that such places are not available for mosquito breeding. Doors and windows should remain closed for most of the time whenever there is an alert by the competent authority. Travellers and tourists should take the basic precautions to protect themselves from mosquito bites.
In India those States where dengue transmission is a major problem must be under vigilance. The guidelines for the integrated vector control should be released on vector surveillance and vector management. There must be provision for personal protection, biological and chemical control at household, community and institutional levels. Individuals, especially the pregnant women or women planning/attempting pregnancy should avoid travelling to the affected countries and in places where at present outbreak is in progress. Women must strictly follow individual protective measures to prevent mosquito bites like use of electronic mosquito repellents, mosquito repellent creams, use of bed nets and dress that covers most of the body parts. Persons with diabetes, hypertension, chronic respiratory illness, immune disorders, should seek advice prior to travel to an affected country. Persons having febrile illness within two weeks of return from an affected country should report to the nearest health facility so that suitable measures to combat the situation can be taken.
Vector biology of mosquitoes in relation to ZiV
ZiV is mainly transmitted by infected Ae. aegypti mosquitoes. Ae albopictus, a highly invasive mosquito, also has the potential to transmit the virus. Both the mosquitoes are highly prevalent in India and play a major role in the transmission of DENV and CHIKV. It is imperative to determine vector competence of Indian strains of Ae. aegypti and Ae. albopictus to ZiV in relation to DEN and CHIK viruses. Since the vector potential of these mosquito species to ZiV is not known, there is need of studies to determine the susceptibility, replication potential and different modes of transmission in Ae. aegypti and Ae. albopictus. If this virus emerges in India, the understanding of the phenomenon of transovarial transmission will give information about its possible maintenance in nature during non-mosquitogenic periods. India has known endemic areas for DEN and CHIK viruses that are also transmitted by these Aedes mosquito vectors, thus understanding the possible interaction on multiplication of ZiV, DENV and CHIKV in concomitantly infected vector mosquitoes is essential. There is a need to understand whether presence of ZiV may play any role in altering the susceptibility of these vector mosquitoes to other viruses (DEN and CHIK viruses).
Newborn screening for possible congenital ZiV infection
In Brazil, a precipitous surge in infants born with microcephaly and the detection of ZiV RNA in the amniotic fluid of affected newborns has been reported48. Identification of high-risk international pathways for the dispersion of ZiV and global geographies conducive to transmission49 is necessary. There have been many hypotheses about possible association of microcephaly6 due to this virus and Guillain Barré syndrome as well as contradictory hypothesis.
Considering the possibility of ZiV related congenital infection, it is important to evaluate neonates clinically presenting with suspected congenital infection, especially microcephaly. The testing algorithm for infants with possible congenital ZiV infection is given in Fig. 446,50. Obtaining blood samples from neonates is practically difficult and can cause complications like venous thrombosis and iatrogenic anaemia. Non-invasive sampling of saliva/urine ensures better compliance and larger volume of sample available for testing. Newborns presenting with microcephaly should be identified. Infants presenting, with neural tube defects and congenital cataract /ophthalmological findings should also be included. There are no data on birth prevalence of ZiV and its possible relation with congenital infection from India. In view of the present public health emergency, such data can provide vital information on this issue and help to understand the association, if any, of ZiV and congenital infection.
Fig. 4.
Testing algorithm for infants with possible congenital Zika virus infection. Source: Adapted from Refs. 46, 51. **Commercial kits not manufactured or available in markets in India.
Zika virus disease has the potential for further international spread, since the potential mosquito vector Ae. aegypti has wide geographical distribution and due to lack of immunity (cross-reactive herd immunity) among population in newly affected areas. As of now, the disease is not reported in India but preparedness is required to identify ZiV aetiology among the reported febrile outbreaks/unusual rise of febrile syndrome cases in the community.
The presence of ZiV in Senegal has been detected by RT-PCR in ten species of mosquitoes belonging to the genus; Aedes, Mansonia, Anopheles and Culex51. However, it is difficult to say whether several species represented in above four genuses may act as vector. Mere presence of virus in a mosquito sample does not incriminate it as a vector. Laboratory experiments are required to prove that the mosquito species is able to acquire the pathogen through bloodmeal, virus propagates in the vector and after incubation period it gets transmited to other susceptible hosts.
It is anticipated that international spread of ZiV from endemic to non-endemic areas is through air traffic. Studies based on mathematical modelling on Americas, estimated 3-4 million likely cases of ZiV infection (including asymptomatic cases) in the next 12 months52. Interestingly, same vector species involvement caused about two million dengue cases in the Americas in 2015, which were in circulation in the region since the 1980s)53. However, ZiV is another flavivirus and new to this region circulating at a very high intensity. This suggests that cross-reactive genus specific antibodies may not be effective to contain its spread in known dengue endemic areas.
Only a few complete genomes are available for ZiV. Recently, viral sequencing was done directly from the urine samples of four viraemic patients54. Complete coding of the ZiV sequence was obtained for one patient and envelope protein coding sequences for the three others. As reported earlier14, phylogenetic analyses were conducted for NS-5 protein coding region, envelope protein coding region and complete coding region, against the sequences available in databases which showed the same topology. The Suriname strains belong to the Asian genotype and seem to be most closely related to the strain that was circulating in French Polynesia in 2013, with which they share more than 99.7 and 99.9 per cent identity14.
When any pathogen is recognized as emerging in any part of the world the alert is sent across the world and search begins to detect its presence in other areas. Today due to very high and fast movement of people across the world, this is considered as main route of spread of emerging infection. However, there is always a possibility that the infection might have reached to a certain country/region long back but remained unnoticed. Due to recent notification if detected in such areas, it is considered as newly spread to this region. Majority of emerging infections are either result of mutation(s) (mutation, shift or/and drift kind of situations) or recent recognition as part of improved/newly added part of surveillance system. These infections might have been existing in such areas but recognized recently.
Emerging arboviruses in India
In India, Chandipura virus (CHPV) was first recognized in 1950s in central parts of India, but later recognized as a disease of public health importance in 2000. CHPV is a Rhabdovirus, that caused febrile illness in humans in 1965, has emerged as an encephalitis-causing virus with a high case fatality rate in children in Central India during 2003-200455. Similarly, Crimean-Congo haemorrhagic fever (CCHF) was a known infection in a large part of the world but its presence was identified in 2011 and follow up studies showed that it existed in India since a long time56. Similarly, Kyasanur forest disease (KFD) was considered to be restricted only to five districts of Karnataka State in India, but with the availability of newer molecular and serological assays and awareness among the health personnel the presence of this disease was recognized in many States in India56,57,58.
Therefore, with such examples from the history for arboviruses it is difficult to predict with certainty, what would happen if ZiV virus is introduced into a new region and new ecosystem. No antiviral or vaccine is available for this viral infection. Thus, option for control is only the management of mosquitoes, which currently relies on either insecticides or the destruction of larval breeding sites.
Conclusion and recommendations
Though the presence of Zika virus has not been detected yet in India and serious mortality and morbidity is also not associated with this virus but the possible association of this virus infection with microcephaly and other neurological symptoms is revealed. Therefore, the preparedness for Zika virus has to be there in our country. This disease has been notified recently internationally and requires very rigorous surveillance programme including detection of ZiV in vector mosquitoes.
Footnotes
Conflicts of Interest: None.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. ZiV. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Macnamara FN. ZiV: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–45. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 3.Dick GW. ZiV. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–34. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 4.Grard G. ZiV in Gabon (Central Africa) - 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenna M. ZiV: A new threat and a new kind of pandemic. Germination. [accessed on May 20, 2016]. Available from: http://phenomena.nationalgeographic.com/2016/01/13/zika-2/
- 6.Compos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–6. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation. Zika virus and complications. [accessed on May 20, 2016]. Available from http://www.who.int/emergencies/zika-virus/en .
- 8.Zika virus infection. [accessed on May 20, 2016]. Available from: http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/Pages/index.aspx .
- 9.World Health Organisation. WHO Director General summarizes outcome of emergency committee meeting on Zika virus. [accessed on May 20, 2016]. Available from: www.who.int/mediacentre/news/statements/2016/emergency-committee-zika/en/1 .
- 10.Smithburn KC, Kerr JA, Gatne PB. Neutralizing antibodies against certain viruses in the sera of residents of India. J Immunol. 1954;72:248–57. [PubMed] [Google Scholar]
- 11.Hayes E. ZiV outside Africa. Emerg Infect Dis. 2009;15:1347–60. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and ZiV infections–an unprecedented epidemic wave of mosquito borne viruses in the Pacific 2012–2014. Euro Surveill. 2014;19:pii–20929. doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 13.Dupont-Rouzeyrol M, O’Connor O, Calvez E, Daures M, John M, Grangeon JP, et al. Co-infection with ZiV and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21:381–2. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faye O, Freire CCM, Iamarino A, Faye O, de Oliveira JV, Diallo M, et al. Molecular evolution of ZiV during Its emergence in the 20th century. PLoS Negl Trop Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields BN, Knipe DM, Howley PM, editors. Fields’ virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1156–99. [Google Scholar]
- 16.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. ZiV genome from the Americas. Lancet. 2016;387:227–8. doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 17.Zanluca C, deMelo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K, et al. First report of autochthonous transmission of ZiV in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–72. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuno G, Chang GJJ. Full length sequencing and genomic characterization of Bagaza, Kedougou, and ZiVes. Arch Virol. 2007;152:687–96. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 19.Freire CCM, Iamarino A, Neto DFL, Sall AA, Zanotto PMA. Spread of the pandemic Zika virus lineage is associated with NS1 codon usage adaptation in humans. bioRxiv. 2015 Nov 25; doi: http://dx.doi.org/10.1101/0328-39. [Google Scholar]
- 20.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77:442–5. doi: 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 21.Fagbami AH. ZiV infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–9. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, Travassos Da Rosa A, Haddow AD, et al. Probable non-vector borne transmission of ZiV, Colorado, USA. Emerg Infect Dis. 2011;17:880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enserink M. Sex After a Field Trip Yields Scientific First. Science News. [accessed on January 12, 2016]. Available from: http://www.sciencemag.org/news/2011/04/sex-after-field-trip-yields-scientific-first .
- 24.Maron DF. First Case of U.S. Transmission in Ongoing ZiV Outbreak Announced in Texas. Scientific American. [accessed on May 20, 2016]. Available from: http://www.scientificamerican.com/article/first-case-of-u-s-transmission-in-ongoing-zika-outbreak-announced-in-texas/
- 25.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM, et al. ZiV intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 26.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, et al. Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol. 1975;69:49–64. doi: 10.1080/00034983.1975.11686983. [DOI] [PubMed] [Google Scholar]
- 27.Fagbami A. Epidemiological investigations on arbovirus infections at Igbo-Ora, Nigeria. Trop Geogr Med. 1977;29:187–91. [PubMed] [Google Scholar]
- 28.Heang V. ZiV infection, Cambodia, 2010. Emerg Infect Dis. 2012;18:349–51. doi: 10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley A, Gould EA. Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with ZiV or Langat virus. J Gen Virol. 1988;69:1913–20. doi: 10.1099/0022-1317-69-8-1913. [DOI] [PubMed] [Google Scholar]
- 30.Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–78. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 31.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of ZiV associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipe AR, Martins CM, Rocha H. Laboratory infection with ZiV after vaccination against yellow fever. Arch Gesamte Virusforsch. 1973;43:315–9. doi: 10.1007/BF01556147. [DOI] [PubMed] [Google Scholar]
- 33.proMEDmail.org. International Society for Infectious Disease. Zika virus - Pacific: French Polynesia. [accessed on May 20, 2016]. Available from: http://www.promedmail.org/post/201311062041959 .
- 34.Mosquitocatalogue.org. The 1963 world-wide mosquito situation. [accessed on May 20, 2016]. Available from: www.mosquitocatalog.org/files/pdfs/124199-7.pdf .
- 35.Centre for Disease Control, Atlanta, United States. Zika and Guillain-Barre syndrome. [accessed on May 20, 2016]. Available from: http://www.cdc.gov/zika/about/gbs-qa.html .
- 36.Pan American Health Organisation. Zika virus infection – Epidemiological updates. [accessed on May 20, 2016]. available from: www.paho.org/
- 37.Duffy MR, Chen T, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. ZiV outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 38.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VA, et al. Potential sexual transmission of ZiV. Emerg Infect Dis. 2015;21:359–61. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowes L, Clark TS, Noritz G. Factors associated with caregiver experience in families with a child with cerebral palsi. J Pediatr Rehabil Med. 2016;9:65–72. doi: 10.3233/PRM-160362. [DOI] [PubMed] [Google Scholar]
- 40.Kutsuna S, Kato Y, Takasaki T, Moi M, Kotaki A, Uemura H, et al. Two cases of ZiV fever imported from French Polynesia to Japan, December to January 2013. Euro Surveill. 2014;19:20683. doi: 10.2807/1560-7917.es2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe ND. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310–6. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 42.Shil P, Dudani N, Vidyasagar PB. ISHAN: sequence homology analysis package. In Silico Biol. 2006;6:373–7. [PubMed] [Google Scholar]
- 43.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–4. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 44.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100:6986–91. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Center for Disease Control, Atlanta, United States. Revised testing algorithm for Zika, chikungunya and dengue viruses in US public health laboratories. [accessed on May 20, 2016]. Available from: www.cdc.gov/zika/pdfs/denvchikvzikv-testing-algorithm.pdf .
- 46.Pan American Health Organization. 2015. Epidemiological alert. Zika virus infection. 7 May 2015. [accessed on May 20, 2016]. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=30176 &Itemid=270&gid=30075 .
- 47.World Health Organization. Dengue control - The Human. [accessed on May 20, 2016]. Available from: www.who.int/denguecontrol/human/en/
- 48.Musso D, Gubler DJ. Zike virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan K, Boqochi I, Brownstein JS, Miniota J, Nicolucci A, Hu W, et al. Assessing the origin of and potential for international spread of chikungunya virus from the Caribbean. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.2134a0a7bf37fd8d388181539fea2da5. pii:ecurrent.outbreaks.2134aOa7bf37fd8d388181539fea2da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report. [accessed on May 1, 2016]. Available from: www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm .
- 51.Constância FJA. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016. [Published Online February 4, 2016]. Available from: http://dx.doi.org/101016/ S1473-3099(16)00073-6 . [DOI] [PubMed]
- 52.Statnews. What's behind the prediction of 3-4 million of Zika virus this year? [accessed on May 20, 2016]. Available from: https://www.statnews.com/2016/01/29/zika-case-estimate-4-million/
- 53.Bogoch II, Brady OJ, Kraemer MU, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–6. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyro M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–6. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatt PN, Rodrigues FM. Chandipura: a new arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967;55:1295–305. [PubMed] [Google Scholar]
- 56.Mourya DT, Yadav PD, Shete AM, Sathe PS, Sarkale PC, Pattnaik B, et al. Cross-sectional serosurvey of Crimean-congo hemorrhagic fever virus IgG in livestock, India, 2013-2014. Emerg Infect Dis. 2015;21:1837–9. doi: 10.3201/eid2110.141961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mourya DT, Yadav PD, Mehla R, Barde PV, Yergolkar PN, Kumar S, et al. Diagnosis of Kyasanur forest disease by nested RT-PCR, real-time RT-PCR and IgM capture ELISA. J Methods Virol. 2012;186:49–54. doi: 10.1016/j.jviromet.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Mourya DT, Yadav PD, Patil DY. Highly infectious Tick-borne viral diseases: Kyasanur Forest Disease and Crimean-Congo hemorrhagic fever in India. WHO South-East Asia J Public Health. 2014;3:8–21. doi: 10.4103/2224-3151.206890. [DOI] [PubMed] [Google Scholar]