Abstract
Monocarboxylate transporters (MCTs) constitute a family of 14 members among which MCT1–4 facilitate the passive transport of monocarboxylates such as lactate, pyruvate and ketone bodies together with protons across cell membranes. Their anchorage and activity at the plasma membrane requires interaction with chaperon protein such as basigin/CD147 and embigin/gp70. MCT1–4 are expressed in different tissues where they play important roles in physiological and pathological processes. This review focuses on the brain and on cancer. In the brain, MCTs control the delivery of lactate, produced by astrocytes, to neurons, where it is used as an oxidative fuel. Consequently, MCT dysfunctions are associated with pathologies of the central nervous system encompassing neurodegeneration and cognitive defects, epilepsy and metabolic disorders. In tumors, MCTs control the exchange of lactate and other monocarboxylates between glycolytic and oxidative cancer cells, between stromal and cancer cells and between glycolytic cells and endothelial cells. Lactate is not only a metabolic waste for glycolytic cells and a metabolic fuel for oxidative cells, but it also behaves as a signaling agent that promotes angiogenesis and as an immunosuppressive metabolite. Because MCTs gate the activities of lactate, drugs targeting these transporters have been developed that could constitute new anticancer treatments. This article is part of a Special Issue entitled: Mitochondrial Channels edited by Pierre Sonveaux, Pierre Maechler and Jean-Claude Martinou.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; CA, carbonic anhydrase; CAF, cancer-associated fibroblast; CHC, α-cyano-4-hydroxycinnamate; CN, calcineurin; CTL, cytolytic T lymphocyte; DBDS, 4,4′-dibenzamidostilbene-2,2′-disulphonate; DC, dendritic cell; DIDS, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid; EAAT1, excitatory amino acid transporter 1; glpT, glycerol phosphate transporter; IGF1, insulin-like growth factor 1; IkBα, inhibitor of NF-kB α; Iκκβ, inhibitor of NF-κB kinase β; LDH, lactate dehydrogenase; MCP, monocarboxylate porter; MCT, monocarboxylate transporter; MDSC, myeloid-derived suppressor cell; MFS, major facilitator superfamily; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NFAT, nuclear factor of activated T cells; NF-kB, nuclear factor-kB; NK, natural killer (cell); NMDA, N-methyl-D-aspartate; NSCLC, non-small cell lung cancer; OXPHOS, oxidative phosphorylation; pCMBS, p-chloromercuribenzene sulphonate; PHD, prolylhydroxylase; ROS, reactive oxygen species; TM, transmembrane (domain); VEGF, vascular endothelial growth factor
Keywords: Metabolic cooperation, Lactate shuttle, Neurons, Astrocytes, Tumor cells, Tumor microenvironment
1. Introduction
Monocarboxylate transporters (MCTs) constitute a family of 14 transmembrane proteins encoded by the SLC16A family of genes. According to the transporter classification system of Milton Saier (http://www.tcdb.org), MCTs belong to the monocarboxylate porter (MCP) family (2.A.1.13), itself a member of the major facilitator superfamily (MFS). MCTs have been identified in all eukaryotic organisms of which genomes have been sequenced to date. They can transport a wide variety of substrates (Table 1). Four members of the MCT family, MCT1, MCT2, MCT3 and MCT4 are monocarboxylate transporters responsible for the proton-linked transport of several monocarboxylate metabolites, such as pyruvate, l-lactate and ketone bodies (acetoacetate and D-β-hydroxybutyrate) across the plasma membrane [1], [2]. Other best characterized MCTs are MCT8 that has a high affinity for thyroid hormones T3 and T4, and MCT10/TAT1, a transporter of aromatic amino acids [2], [3]. MCT6 has been reported to facilitate the proton-linked transport of bumetanide, but its natural substrate is unknown [4]. MCT7 has been implicated in the export of ketone bodies by hepatocytes fueled with circulating fatty acids upon fasting [5]. Accordingly, loss of MCT7 expression resulted in hepatic steatosis in Zebrafishes, which was prevented by the introduction of human MCT7. MCT9 has been clearly identified as a sodium- and pH-independent carnitine efflux transporter when it was expressed in Xenopus oocytes injected with [3H]-carnitine [6]. The substrates and functions of the five other MCT family members are currently unknown.
Table 1.
The MCT family of transporters.§
| Human gene name | Protein name | Sequence accession ID | Main substrates | Tissue distribution |
|---|---|---|---|---|
| SLC16A1 | MCT1 | NM_003051 | Lactate, pyruvate, ketone bodies | Ubiquitous except β cells of the endocrine pancreas |
| SLC16A2 | MCT8 | NM_006517 | T2, rT3, T3, T4 | Ubiquitous |
| SLC16A3 | MCT4 | NM_004207 | Lactate, ketone bodies | Skeletal muscle, chondrocytes, leucocytes, testis, lung, brain, ovary, placenta, heart |
| SLC16A4 | MCT5 | NM_004696 | – | Brain, muscle, liver, kidney, lung, ovary, placenta, heart |
| SLC16A5 | MCT6 | NM_004695 | Bumetanide probenecid nateglinide | Kidney, muscle, brain, heart, pancreas, prostate, lung, placenta |
| SLC16A6 | MCT7 | NM_004694 | Ketone bodies | Liver, brain, pancreas, muscle, prostate |
| SLC16A7 | MCT2 | NM_004731 | Pyruvate, lactate, ketone bodies | High expression in testis, moderate to low in spleen, heart, kidney, pancreas, skeletal muscle, brain and leucocytes |
| SLC16A8 | MCT3 | NM_013356 | Lactate | Retinal pigment epithelium, choroid plexus |
| SLC16A9 | MCT9 | NM_194298 | Carnitine | Endometrium, testis, ovary, breast, brain, kidney, spleen, retina |
| SLC16A10 | MCT10/TAT1 | NM_018593 | Aromatic amino acids, T3,T4 | Kidney (basolateral), intestine, muscle, placenta, heart |
| SLC16A11 | MCT11 | NM_153357 | – | Skin, lung, ovary, breast, lung, pancreas, retinal pigment epithelium, choroid plexus |
| SLC16A12 | MCT12 | NM_213606 | – | Kidney, retina, lung testis |
| SLC16A13 | MCT13 | NM_201566 | – | Breast, bone marrow stem cells |
| SLC16A14 | MCT14 | NM_152257 | – | Brain, heart, muscle, ovary, prostate, breast, lung, pancreas liver, spleen, thymus |
MCTs have numerous physiological functions as they are expressed in a wide range of tissues (such as brain, skeletal muscle, heart, bowel and liver) where they control, among others, the central metabolism of glucose, gluconeogenesis, activation of T-lymphocytes, spermatogenesis, pancreatic β cell activity, thyroid hormone metabolism and drug transport. In addition to their implication in various physiological processes, they play significant roles in pathological situations as can be exemplified by the case of tumors. This review will summarize the current knowledge pertaining to their functions in the brain, where their physiological roles are starting to emerge in greater details, and in cancer, which represents a pathology in which MCTs are clearly occupying an important position.
2. General description of monocarboxylate transporters
2.1. The MCT family: structural and functional characteristics
2.1.1. Structure
Theoretical predictions and biochemical assays with MCT1 as a model protein indicate that all the members of the MCT family share a common topology. Hydropathy plots predict the presence of 12-transmembrane (TM) helices with intracellular C- and N-termini, a large cytosolic loop between TM helices 6 and 7, and two highly conserved sequences in TM1 and TM5 [7]. A common feature shared with MFS members is a better sequence conservation in TM regions compared to loops and C-termini. To date, no crystallographic characterization of MCTs has been reported, but Halestrap et al. [7], [8], [9] have proposed 3D models based on molecular modeling, the structure of the E. coli glycerol phosphate transporter GlpT, site-directed mutagenesis and the binding sites for 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS), a MCT1 inhibitor. These models suggest that the structure of MCT1 at the plasma membrane may swing between two states: a closed conformation where the substrate-binding site is cytosolic and an open conformation where this site is extracellular (for a graphical representation, see Fig. 3 in reference [7]).
Fig. 3.
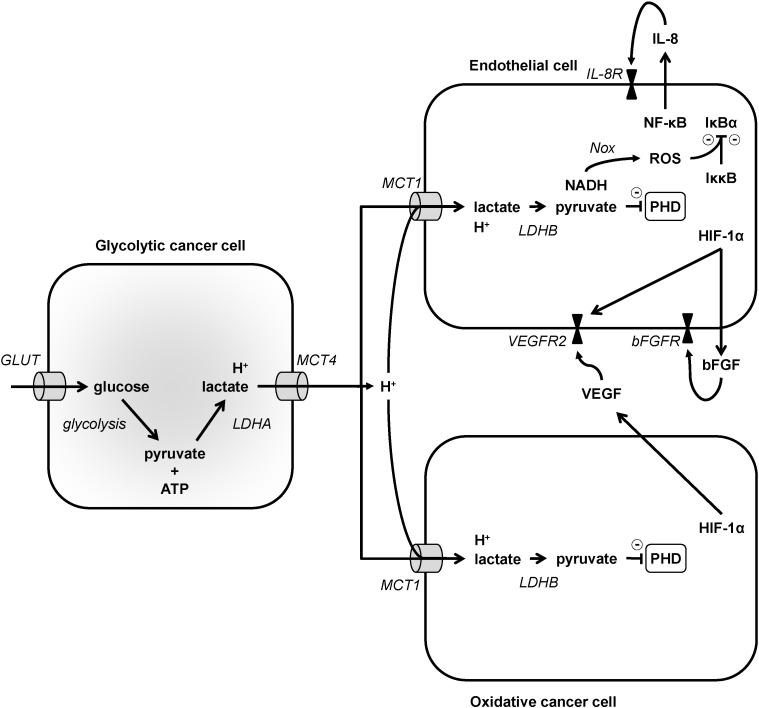
Model depicting proangiogenic lactate signaling in cancer. In the model, glycolytic cancer cells, depicted on the left, import glucose via glucose transporters (GLUT) and then sequentially convert glucose to pyruvate and ATP using glycolysis, and pyruvate to lactate using lactate dehydrogenase A (LDHA). Lactate is exported together with protons via MCT4. MCT1 is expressed in endothelial cells and in oxidative cancer cells, depicted on the left, and catalyzes the uptake of lactate together with protons. In these cells, lactate is oxidized to pyruvate by LDHB, producing NADH as a byproduct. Pyruvate inhibits prolylhydroxylases (PHDs). In endothelial cells, PHD inhibition stabilizes hypoxia-inducible factor (HIF-1) subunit α, triggering HIF-1 activation and the transcriptional upregulation of vascular endothelial growth factor receptor 2 (VEGFR2). HIF-1 also indirectly increases bFGF, which is secreted and activates the proangiogenic bFGF receptor (bFGFR). PHD inhibition further stabilizes inhibitor of NF-κB kinase β (Iκκβ), an inhibitor of inhibitor of NF-kB α (IκBα). NADH aliments NAD(P)H oxidases (Nox) that produce reactive oxygen species (ROS) to further inhibit IκBα. Consequently, transcription factor NF-κB is activated and upregulates IL-8, which is secreted and activates the proangiogenic IL-8 receptor (IL-8R). Oxidative cancer cells share with endothelial cells the lactate-HIF-1 pathway, but not the lactate-NF-κB pathway. HIF-1 activation by lactate in these cells transcriptionally upregulates VEGF, which upon secretion can activate proangiogenic VEGFR2 in endothelial cells. Lactate signaling as a whole can thus trigger angiogenesis independently of hypoxia.
2.1.2. Mechanism of activity
The predicted open and closed conformations of MCTs and kinetic analyses of proton-linked transport of lactate into erythrocytes are the basis for the proposed translocation mechanism of lactic acid transport by human MCT1 through the plasma membrane. MCT1 preferentially facilitates the uptake of lactic acid and operates in an ordered process that starts when a proton binds to K38 at the extracellular surface of MCT1, providing a positive charge to the lysine [8], [9], [11]. Proton binding is followed by the binding of one molecule of lactate to form an ionic pair, which promotes a conformational change from closed to open state. It follows that the proton is transferred to D302 and lactate to R306 (both residues are localized at the inner surface of the channel), thus deprotonating K38, which induces the return to the closed conformation and exposure of the D302/R306 site to the cytosol. The pair H+/lac− is released into the cytoplasm. Another essential residue for MCT1 activity is F360, protruding into the channel of the transporter where it controls substrate selectivity by steric hindrance. According to this mechanism, the transport of lactic acid by MCT1 is passive and bidirectional: import and export depend on the intra- and extracellular concentrations of lactate and protons [2], [7]. This molecular model highlights the importance of three residues, which are conserved in the four members of the MCT family that transport monocarboxylates (MCT1, MCT2, MCT3 and MCT4) and in MCT7, where there is a conservative substitution of D302 by E302.
2.1.3. Substrates
MCT1, MCT2, MCT3 and MCT4 are responsible for the bidirectional proton-linked transport of monocarboxylates across the plasma membrane, and will be the focus of this review. These MCT isoforms show preference for short chain monocarboxylates, including those substituted on positions two and three, such as pyruvate, l-lactate, D-β-hydroxybutyrate and acetoacetate. Quantitatively, lactate is one of the most important metabolites for these transporters, with a stereoselectivity for l- over d-lactate [7] consistent with the fundamental role of the l stereoisomer in eukaryotic cell metabolism. With a Km of 22–28 mM, MCT4 has the lowest affinity for lactic acid [12] (Table 2). However, it has a high turnover rate [13], making it particularly well adapted for the export of lactate by glycolytic cells where it helps to control intracellular pH homeostasis [12], [14]. Comparatively, MCT1 has an intermediate affinity for lactate (Kmlactate = 3.5–10 mM) and is widely expressed in healthy and cancer tissues [2], [15]. MCT2 (Kmlactate = 0.5–0.75 mM) and MCT3 (Kmlactate = 5–6 mM) show the highest affinity for lactate, but their expression is restricted to very specific tissues (see Section 2.1.5) [16], [17], [18]. Differences in the Km of the transporters for pyruvate are more pronounced, with Kmpyruvate values of 1.0, 0.1 and 153 mM for MCT1, MCT2 and MCT4, respectively [7]. MCT2 has the highest affinity for ketone bodies, with KmD-β-hydroxybutyrate = 1.2 mM and Kmacetoacetate = 0.8 mM determined in Xenopus oocytes [19]. For MCT1, these values are KmD-β-hydroxybutyrate = 12.5 mM and Kmacetoacetate = 5.5 mM [20]. The transport of ketone bodies by MCT3 has not been evaluated, and is not likely to occur through MCT4 due to the very low affinity of the transport for these substrates [7]. Beyond natural substrates, recent evidence also shows that MCT1 is controlling the uptake of 3-bromopyruvate [21] (an alkylating agent developed for cancer therapy) and of dichloroacetate [22] (a pyruvate dehydrogenase kinase inhibitor that restores the mitochondrial metabolism of pyruvate in glycolytic cancer cells and is currently undergoing clinical trials for several types of cancers).
Table 2.
Km values of MCT1, MCT2, MCT3 and MCT4.
Lactate is one of the main substrates of MCT1–4. This metabolite is generated from pyruvate (produced from glycolysis and glutaminolysis) during lactic fermentation. In most normal tissues where lactate is produced, MCT1 is responsible for its export across the plasma membrane to the extracellular space [2], [23]. However, in glycolytic cancer cells and other specific tissues such as white muscle fibers and astrocytes, MCT4 predominates over MCT1 for lactate export (see details in 3.1, 4.1) [15], [24], [25], [26]. The low affinity of MCT4 for pyruvate could prevent the release of pyruvate that is essential for the conversion of NADH into NAD+ for the maintenance of a high glycolytic flux [2], [23].
In metabolic pathways comprising lipogenesis, gluconeogenesis and oxidative phosphorylation (OXPHOS), lactate can be used as a substrate taken up from the extracellular space. Cells that consume lactate may express MCT1, MCT2 or both depending on tissues and species [2], [27]. In many cancer cells with an oxidative metabolic fingerprint, MCT1 is the major MCT isoform to be expressed [2], [25], [28]. It promotes the import of lactate that then fuels the TCA cycle and OXPHOS [25], [29]. Accordingly, there is increasing evidence pointing towards shuttling of this metabolite between cells with different metabolic behaviors in a same tissue. Such phenomenon has been described in the skeletal muscle where glycolytic/white fibers export lactate through MCT4 and oxidative/red fibers express MCT1 to import lactate to be used as a fuel for the TCA cycle [30], [31]. A similar mechanism has been proposed to account for a metabolic symbiosis between glycolytic/hypoxic cancer cells and oxidative/oxygenated cancer cells in tumors [25]. In the brain, glycolytic oligodendrocytes and astrocytes export lactate through MCT1 and MCT4 to fuel oxidative neurons expressing MCT2 [32], [33], [34]. The roles of MCTs in brain physiology and in tumor pathology are detailed in 3, 4, respectively.
2.1.4. Inhibitors
Several MCT inhibitors have been identified, but none are specific for a given MCT isoform and most of them present a high affinity for other proteins (Table 3). Oldest described inhibitors are phloretin, flavonoids such as quercetin, stilbene disulphonates (including DIDS and 4,4′-dibenzamidostilbene-2,2′-disulphonate [DBDS]), and α-cyano-4-hydroxycinnamate (CHC) and its analogs [1], [7]. All have off-target effects. In particular, CHC has the capacity to inhibit the mitochondrial pyruvate carrier (MPC, Ki ~ 2 μM), hence the mitochondrial uptake of pyruvate, much more efficiently than MCT1 (Ki 166 μM) [35]. Other inhibitors have been more recently developed that exhibit a high affinity for MCTs [36]. Compound AZD3965 is a dual MCT1 and MCT2 inhibitor currently evaluated as an anticancer agent in Phase I clinical trials for patients with prostate cancer, gastric cancer or diffuse large B cell lymphoma (ClinicalTrials.gov NCT01791595). Importantly, the related compound AR-C155858 is a dual MCT1/2 inhibitor that inhibits MCT2 when it is bound to ancillary protein basigin but not when it is bound to its preferred chaperone protein embigin [36] (see also Section 2.2.3). Draoui et al. [37], [38] have recently identified several original compounds belonging to the 7-aminocarboxycoumarine family that potently inhibit MCTs. Measurements of the inhibition of lactate flux revealed that the most active compounds (IC50 = 10–50 nM) are three log orders more active than CHC. Interestingly, 7-(N-benzyl-N-methylamino)-2-oxo-2H-chromene-3-carboxylic acid (7ACC2) was further reported to be an inhibitor of lactate uptake (IC50 = 11 nM on 14C-lactate flux inhibition) that does not inhibit lactate export [38]. Subtle allosteric regulation of MCT conformation was proposed to account for unidirectional flux inhibition. Another possibility that remains to be investigated would be an inhibitory effect on the MPC [35].
Table 3.
Comparison of inhibitor selectivity for MCT1, MCT2 and MCT4.
| Inhibitor | Inhibition index | MCT1 | MCT2 | MCT4 | Other targets | References |
|---|---|---|---|---|---|---|
| Phloretin | Ki (μM) | 5 | 14 | 41 | GLUT | [14], [19], [39], [40], [41] |
| Quercetin | % inhibition | 89 | 83 | – | Many | [19], [42] |
| DIDS | Ki (μM) | 434 | – | NI | Aquaporin, Cl−/HCO3− exchanger | [14], [39], [43], [44] |
| DBDS | % inhibition at 0.1 mM | 0 | 56 | – | [19], [39] | |
| NPPB | Ki (μM) | 9 | – | 240 | [14], [19], [39] | |
| CHC | Ki (μM) | 166 | 24 | 991 | Mitochondrial pyruvate carrier | [14], [19], [35], [39] |
| AR-C155858 | Ki (nM) | 1.2 (2.3)⁎ | < 10⁎, $ | – | [45], [46] | |
| AZD3965 | Binding affinity (nM) | 1.6 | 9.6 | – | [47] | |
| 7ACC2 | IC50 for lactate uptake (nM) | 11 | – | – | [37], [38] |
Abbreviations: CHC, α-cyano-4-hydroxycinnamate; DBDS, 4,4′-dibenzamidostilbene-2,2′-disulphonate; DIDS, 4,4′-diisothiocyanostilbene-2,2′-disulphonate; GLUT, glucose transporter; MCT, monocarboxylate transporter; NI, no inhibition; NPPB, 5-nitro-2-(3-phenylpropylamino)-benzoate.
Determined on rat MCT isoforms.
No significant inhibitory effect when MCT2 is associated with embigin instead of basigin.
2.1.5. Cell-specific distribution
Information exists about the cellular distribution of MCT1–4 (Table 1), although it cannot be considered exhaustive. Expression of MCT1 is viewed as being rather ubiquitous, since it is found in several tissues and organs. It is encountered in all muscle cells, although it is particularly enriched in cells of oxidative (type I) fibers [48], [49], [50], [51], [52]. In the heart, it is found in cardiomyocytes [16], [53], [54]. In the liver, hepatocytes exhibit strong MCT1 expression [16], [22], [53], [55]. Different levels of MCT1 expression are found along the gastrointestinal tract in the stomach, duodenum, jejunum, ileum, cecum, colon and rectum, associated with epithelial cells (including enterocytes) on both basolateral and apical membranes, suggesting that the transporter is involved in both the absorption of monocarboxylates from the lumen and their efflux towards blood [56], [57], [58], [59], [60], [61], [62]. In the blood, erythrocytes [53], [63], [64], [65] and immune cells including lymphocytes and monocytes [66] do express MCT1. The transporter is also present in adipocytes of white adipose tissue [18], [67]. In the placenta, MCT1 was found to be expressed in syncytiotrophoblasts in a polarized manner, but with a different orientation in human versus mouse [68], [69]. The transporter is also present in cells of exocrine glands, such as sebaceous glands of the skin and mammary glands [70]. The cellular distribution of MCT1 in the nervous system has been studied and described in more details. MCT1 was found to be expressed in the brain by endothelial cells [71], [72], [73], astrocytes [74], [75], [76], [77], oligodendrocytes [78], [79] and microglial cells [80], [81]. In addition, some subsets of neurons express MCT1 [75], [82], [83], notably in specific brain areas such as the hypothalamus [84], [85]. Tanycytes, a glial cell type found in the hypothalamus, do also express MCT1 [86]. In the peripheral nervous system, MCT1 was found in Schwann cells and dorsal root ganglia neurons within the endoneurium [87], [88], in addition to the epineurium [89].
MCT2 has a more restricted tissular and cellular distribution than MCT1. It was first described in liver hepatocytes and in the kidney in collecting ducts [16]. MCT2 is also expressed by the spermatid tail in the testis [16], [90]. In the central nervous system, it was shown to be the major neuronal MCT isoform [77], [83], [91]. Some data further suggest that MCT2 could be expressed by endothelial cells delineating cerebral blood vessels [73], [92], by some rat astrocytes [76], [93] and by tanycytes in the hypothalamus [86].
MCT3 is expressed by only two cell populations in the entire organism. It is found on retinal pigmented epithelial cells [94], [95] as well as on the choroid plexus epithelium [17].
MCT4 was shown to be expressed by a subset of muscle cells composing glycolytic (type II) fibers [49], [50], [96]. It is also present in some types of white blood cells, including macrophages [66], [97], [98]. In the central nervous system, the transporter is found exclusively in astrocytes [83], [99], with the exception of tanycytes in the hypothalamus [86]. In the peripheral nervous system, Schwann cells have been recently found to express MCT4 [87].
2.1.6. Subcellular distribution
Since MCTs play an essential role in the exchange of metabolites between the cytoplasm and the extracellular environment, a great majority of reports have described them to be localized at the subcellular level on the plasma membrane of every cell type in which they were described. There are, however, a few examples for which further details about the subcellular distribution have been reported. In muscle cells, both MCT1 and MCT4 were found to be associated with intracellular structures such as triads, T tubules, sarcoplasmic reticulum and intracellular membranes, but to a different degree for each transporter [52], [96]. Similarly in neurons, which are highly polarized cells, a specific subcellular distribution of MCT2 was evidenced and described: MCT2 expression is particularly enriched in dendritic spines, associated with the postsynaptic density [91], [100]. MCT2 was found not only to colocalize with several postsynaptic density proteins (e.g. PSD95), but also to be associated with a subset of proteins specifically enriched in the postsynaptic density including PICK1 and GluR2/3, a subunit of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [101], [102]. Moreover, MCT2 was detected within spines in a vesicular-like compartment [101], [102], suggesting that it could undergo some trafficking between this intracellular compartment and the plasma membrane. Indeed, it was shown that neuronal stimulation with neuroactive substances such as glutamate (in the presence of glycine) or brain-derived neurotrophic factor (BDNF) can induce the translocation of the intracellular MCT2 pool to the plasma membrane and, thereby, enhance lactate uptake [102]. Likewise, in several types of cancers, MCT2, MCT3 and MCT4 have been found to be expressed at the membrane of intracellular substructures [103], [104], which could indicate intracellular trafficking also in tumors.
In addition to its association with vesicle-like structures in neuronal spines, MCT2 can also be found on the intracellular membrane of mitochondria in neurons [105], [106]. Such an expression in mitochondria, in association with the presence of the lactate dehydrogenase B (LDHB) isoform, was proposed as an evidence for direct lactate oxidation within mitochondria [105], [107]. A similar observation was reported for MCT1 in neurons [105] and in cardiac and skeletal muscle cells [108], [109]. These observations, however, remain highly controversial, notably due to the non-specific binding of antibodies and contamination of mitochondrial membrane preparations, as extensively reviewed and discussed previously [35].
2.2. Regulation
Most of the studies pertaining to MCT regulation focused on MCT1, so most available information is related to this protein. Known regulatory processes can be divided in 3 categories: transcriptional, expressional and regulation through interactions with proteins.
2.2.1. Transcriptional and epigenetic regulations
SLC16A1/MCT1 gene expression can be regulated in different ways depending on cell and tissue types and on metabolic needs. In the skeletal muscle, increases in Ca2 + and AMP levels due to chronic stimulation or exercise increase MCT1 transcription and promote the activity of the Ca2 +-dependent protein phosphatase calcineurin (CN) and of AMP-activated protein kinase (AMPK) [110], [111]. CN is responsible for the dephosphorylation and activation of nuclear factor of activated T cells (NFAT), a transcription factor that recognizes several NFAT-binding consensus sequences on the MCT1 promoter [111]. In the skeletal muscle, inhibition of CN by cyclosporine A and FK506 induced a phenotypic reshaping of red oxidative fibers rich in MCT1 into white slow oxidative fibers with low MCT1 and higher MCT4 expression [111], [112]. In myocytes, cyclosporine A can further prevent hypertrophy [112], a process related to upregulation of MCT1 [111]. Thus, although it was not yet formally demonstrated, the control of the expression of MCT1 in the exercising muscle probably depends on the binding of NFAT to the gene promoter. When AMP levels rise, AMPK stimulates PGC1α, a transcriptional coactivator that upregulates MCT1 expression and promotes the formation of highly oxidative muscle fibers [113]. Accordingly, exercise stimulation of rats resulted in a transient increase in both MCT1 and MCT4 mRNA levels, with evidence for AMPK involvement as an upstream intermediate [114], [115]. In another report, however, direct AMPK activation by 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside (AICAR) has been shown to stimulate MCT1 promoter activity in both L6 myoblasts and HepG2 hepatoma cells, but it significantly reduced the activity of the MCT4 promoter by more than 50% [23]. AICAR has also been reported to decrease the activity of the MCT1 promoter in Sertoli cells [116]. It has further been reported that thyroid hormone T3 is able to increase the levels of MCT1 and MCT4 mRNAs, although in the skeletal muscle only MCT4 protein expression increased [117]. In cancer, MCT1 expression has been found to be repressed in human breast cancer cells through hypermethylation of CpG islands in the promoter region of the gene [118]. Still in cancer, MCT1 protein expression has also been reported to be regulated by hypoxia in a p53-dependent manner [119]. A direct interaction between p53 and the MCT1 gene promoter was proposed to decrease MCT1 mRNA stability.
MCT2 expression is mainly controlled posttranscriptionally, although some authors have described that food deprivation can induce the SLC16A7/MCT2 mRNA expression in rat brain stem cells for the use of ketone bodies as respiratory fuels [120]. In the brain, stimulation of the PI3K-Akt–mTOR pathway by insulin, insulin-like growth factor 1 (IGF1) or noradrenaline induces a translational activation of MCT2 in a way similar to the increment seen in cultured cortical murine neurons treated with BDNF [121], [122], [123]. An epigenetic regulation of SLC16A7/MCT2 was also evidenced in prostate cancer cells [124]. A selective demethylation of an internal promoter and a reciprocal hypermethylation of an upstream promoter region led to a switch in MCT2 isoform expression and a distinct regulation of MCT2 translation.
The promoter activity of SLC16A3/MCT4 is stimulated by hypoxia following the binding of transcription factor hypoxia-inducible factor-1 (HIF-1) to two hypoxia-responsive elements (HRE) located upstream to the transcription start [23], [24]. Consequently, hypoxia increases MCT4 expression in several cell types, including cancer cells. An epigenetic regulation of SLC16A3/MCT4 was also evidenced in clear cell renal cell carcinoma [125]. Indeed, lower DNA methylation at specific CpG sites resulted in higher MCT4 mRNA expression and correlated with worst outcome for patients.
Information regarding other MCT isoforms is very limited, though there is a study connecting MCT8 expression changes with development and disease [3].
2.2.2. Expressional regulation
Mechanisms implicated in the regulation of MCTs have not been deeply investigated, but some authors have pointed at the importance of some specific sequences and secondary structures in the 5′ and 3′ untranslated region (UTR) of MCTs mRNAs. In particular, the 3′ UTR of MCT1 is much longer than the ones of MCT2 and MCT4, and it contains a potential cytosolic polyadenylation element and hexanucleotide repeats [23]. These elements might be critical for polyadenylation at the exit of the mitotic phase during cell cycle and for the regulation of initiation factor eIF4E, which could be linked with the increase in MCT1 expression observed at protein but not mRNA level [23], [111]. In addition, MCT1 bears in its C-terminus a LL motif and two acidic clusters of amino acids that could be involved in protein targeting [111], for example in the translocation of the protein to the sarcolemma in cisternae close to the t-tubules in cardiomyocytes [126]. Sequence analysis suggests that MCTs are not targets for posttranslational modifications such as glycosylation, and to date no evidence of other posttranslational modifications has been reported.
2.2.3. Regulatory proteins
The correct expression, localization and activity of MCTs depend on interactions with ancillary proteins, primarily basigin/CD147 and embigin/gp70 [8], [127], [128]. These proteins are anchored to the plasma membrane through a single transmembrane domain containing a conserved glutamate residue. They also possess a short intracellular C-terminus and a large glycosylated extracellular domain with two or three immunoglobulin domains depending on the splice variant [129]. In addition to its chaperone role, basigin presents a huge variety of ligands, such as cyclophilins and integrins, taking part in several functions including the induction of metalloproteinases, spermatogenesis and responsiveness to lymphocytes [130].
Several groups have demonstrated with different techniques (e.g. immunofluorescence, co-immunoprecipitation, cross-linking and fluorescence resonance energy transfer) the crucial role of ancillary proteins for MCTs to be localized and active at the plasma membrane [36], [128], [131], [132], [133]. In their absence, MCTs are not able to reach their final position and accumulate in the Golgi apparatus [128], [132]. Basigin is more widely expressed in tissues than embigin, and appears more frequently as the preferential partner for MCT1, MCT3 and MCT4, whereas MCT2 preferentially interacts with embigin [36], [128]. Experiments of site-directed mutagenesis have further revealed that the transmembrane domain of the ancillary protein lies adjacent to TM3 and TM6 of MCTs [8], [36] and that disruption of the interaction between MCT1 and basigin by organomercurial p-chloromercuribenzene sulphonate (pCMBS) induces inhibition of MCT1 activity [128]. Besides MCT1–4, the necessity of the other members of the MCT family to interact with ancillary proteins remains unclear. MCT8 does not require such interaction for its correct plasma membrane localization and activity [134].
Other proteins have been suggested to bind to MCTs. The receptor for hyaluronic acid CD44 can interact with MCT1 and MCT4 in lung and prostate cancer cells [103]. Carbonic anhydrase II (CAII, a cytosolic CA isoform) and CAIV (an extracellular CA isoform) can also establish interactions with MCT/basigin complexes via MCTs on the cytosolic side and basigin on the extracellular side, enhancing the activity of the transporters [135], [136]. Increased MCT activity by CAs could be due to a process whereby CAs supply MCTs with protons, or to faster transport. Nonetheless, improved MCT activity was also seen with catalytically inactive CAs [135], [136], a phenomenon that is still incompletely understood. MCT1 has also been reported to colocalize with LDH in the mitochondria of L6 rat skeletal muscle cells and to co-immunoprecipitate in the mitochondrial fraction of the protein extract in absence of detergent, suggesting a weak interaction between both proteins at least at the mitochondrial level in these cells [137]. The authors proposed a model of “intracellular lactate shuttle/lactate mitochondrial oxidation complex” in which lactate would be reduced in pyruvate by LDH and further imported into the mitochondrial stroma by MCT1 for further oxidation. Because LDHB and LDHA have different affinities for lactate and pyruvate and as MCT isoforms have different Km and Vmax values depending on their substrate, potential interactions of LDHs with MCTs could represent a mechanism to fine-tune monocarboxylate transport orientation across membranes. As mentioned earlier, however, the concept of intracellular lactate shuttle and lactate mitochondrial oxidation is highly disputed for several reasons [35]. Finally, in neurons, MCT2 binds to the AMPA receptor subunit GluR2, of which it controls the plasma membrane localization [102], [138]. Moreover, it was shown that two adhesion molecules, the neuroplastins, can also serve as accessory proteins for MCT2 in the central nervous system and regulate its exposure at the cell surface [139].
3. MCTs in the central nervous system
3.1. MCTs and the astrocyte-neuron lactate shuttle
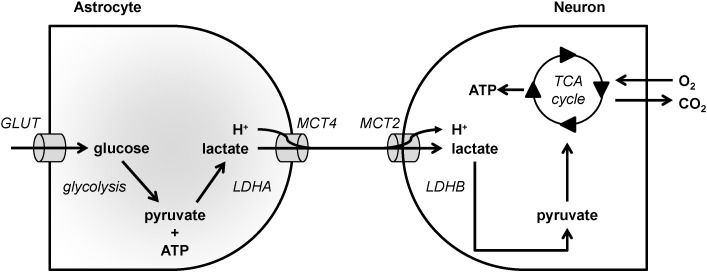
The cellular distribution of MCTs in the central nervous system associated with the distribution of other essential metabolic components (such as the LDH isoenzymes [140], [141]) has suggested a key role of these carriers in the shuttling of energy metabolites between brain cells. Indeed, the expression of MCT4 on astrocytes that produce large amounts of lactate and the expression of MCT2 on neurons using lactate as an efficient oxidative energy substrate have contributed to the emergence and establishment of the “astrocyte-neuron lactate shuttle” concept [32], [141], [142], [143] (Fig. 1).
Fig. 1.
Model depicting MCT-mediated lactate exchanges between astrocytes and neurons in the brain. In the model, astrocytes, depicted on the left, have a high glycolytic metabolism. They import glucose via glucose transporters (GLUT) and then sequentially convert glucose to pyruvate and ATP using glycolysis, and pyruvate to lactate using lactate dehydrogenase A (LDHA). Lactate is exported together with protons via MCT4. Neurons, depicted on the right, import lactate and protons via MCT2. Lactate is oxidized to pyruvate by lactate dehydrogenase B (LDHB) and pyruvate fuels the TCA cycle to support ATP production through oxidative phosphorylation.
Glutamate uptake by astrocytes via the high affinity, Na+-dependent glutamate transporters GLAST and GLT1 was initially reported to cause a rise in intracellular Na+ that would activate the Na+/K+ ATPase [144], [145], [146], [147]. As a consequence, glucose uptake would be enhanced and would result in a greater lactate production and release [148], [149]. Both MCT1 and MCT4 can convey lactate release by astrocytes: MCT1 appears to be involved in basal lactate release, whereas MCT4 would be required for enhanced lactate export [150], [151]. In addition to glutamate, K+ can also enhance glycolysis in neurons [152], [153], [154]. Moreover, it was recently demonstrated that NH4+ constitutes a signal released by neurons that favors lactate release by astrocytes, most likely via MCTs [155]. Lactate constitutes not only a suitable oxidative energy substrate for neurons, but it can even be preferred over glucose both in vitro and in vivo [156], [157], [158], [159], [160], [161]. The expression of MCT2 by neurons, together with LDHB, provides an ideal condition for lactate uptake and utilization as an energy substrate by these cells. It has further been shown that the level of MCT2 expression at the plasma membrane of these cells can be modulated by specific neuroactive substances, including BDNF, via a translocation mechanism allowing for a rapid regulation and control of energy substrate supply [162]. In addition, MCT2 expression can also be regulated at the translational level in cultured cortical neurons by various neuroactive substances including BDNF, insulin, IGF-1 and noradrenaline [121], [122], [123], [162]. Such a relatively rapid mechanism to enhance MCT2 expression occurs at the level of neuronal processes and does not require the cell body, suggesting a synapse-specific regulation [123]. A similar enhancement of MCT2 expression, together with the synaptic proteins PSD95 and the AMPA receptor subunit GluR2, was demonstrated in mice in vivo after injection of BDNF into the hippocampus [163].
3.2. MCTs, learning and memory
As mentioned in Section 3.1, the neuronal expression of MCT2 is regulated in parallel with other proteins that are also regulated in a synapse-specific manner (such as PSD95), whereas MCT2 is even associated physically with GluR2, a specific subunit of AMPA receptors with which it undergoes trafficking either by being inserted together into the plasma membrane or being internalized from the plasma membrane into the endosomal system [102], [138]. Moreover, MCT2 expression in vivo is regulated by the same mechanism that activates some immediate early genes, i.e., Arc and Zif268, known to be involved in synaptic plasticity [163]. These observations strongly suggest that MCT2 plays a key role in the supply of energy substrates to neurons in order to sustain changes involved in synaptic plasticity. Indeed, Alberini et al. [164] have shown that downregulating the expression of not only MCT2 (the neuronal transporter) but also of the two glial transporters MCT1 and MCT4 in the rat hippocampus prevented learning and memory. Remarkably, while exogenously supplied lactate could overcome the deficit when either of the glial transporters, MCT1 or MCT4, were downregulated, it was not the case for MCT2, further demonstrating the importance of lactate release from astrocytes and its use by neurons via MCT-mediated transfer. These results were confirmed in a different learning task involving spatial memory [165]. Interestingly, exposure of neurons to lactate can lead to the modification of N-methyl-D-aspartate (NMDA) receptor-mediated currents and to enhancement of plasticity gene expression [166]. These effects require the presence of neuronal MCTs. Lactate indeed acts by modifying the level of intracellular NADH (which requires lactate to be metabolized intracellularly) and, thus, the redox state of the cell.
3.3. MCTs in brain diseases
3.3.1. Neurodegenerative diseases
Considering the important emerging roles of MCTs in metabolic interactions between the different brain cell types, it is not surprising to discover that alterations of MCT expression are implicated in a variety of diseases involving the central or the peripheral nervous systems. It is specifically the case for neurodegenerative diseases. Thus, together with the discovery that oligodendrocytes express MCT1 and are involved in a lactate shuttle to provide energetic support to axons [33], [167], it was also shown that a reduction of MCT1 expression in a transgenic mouse model leads to axonal degeneration, notably in the spinal cord [79]. A similar reduction of MCT1 expression was evidenced in the spinal cord of amyotrophic lateral sclerosis patients [79]. Similarly, in parallel with the finding that MCT1 and MCT4 are expressed by Schwann cells, a reduction of the expression of MCT1 in cultured cells was reported to alter myelination [87], which was associated with a delay of regeneration following nerve crush in a transgenic mouse model [88]. Accordingly, in brain tissues of multiple sclerosis patients, astrocytes exhibit enhanced MCT1 expression, whereas demyelinated axons have lower MCT2 expression in inactive lesions [81].
In a rat model of Alzheimer's disease where rat received bilateral intrahippocampal injections of the Aβ25–35 fragment, MCT2 expression was reduced in parallel with decreased lactate content and cognitive deficits in spatial learning and memory, as assessed by the Morris Water Maze test [168]. In contrast, no alteration of the expression and localization of MCT1 and MCT2 was found in the substantia nigra and striatum of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease [169].
3.3.2. Epilepsy
Epilepsy represents a condition for which several studies have reported changes in the expression and/or localization of the different MCTs in the brain. In the human epileptogenic hippocampus, MCT1 expression is decreased in microvessels [170], whereas MCT2 is redistributed from astrocytic end-feet to plasma membranes facing synapses [171]. In an immature and an adult lithium-pilocarpine rat model of epilepsy, MCT1 mRNA was found to be commonly enhanced, but more in the adult during the acute phase of status epilepticus [172]. MCT2 mRNA increased mainly in adults within the circuit of seizures. In three other rat models of epilepsy, one using the drug methionine sulfoximine and two using episodes of perforant path stimulation, MCT1 expression was reduced in microvessels, whereas it was enhanced in astrocytes of the hippocampus [173]. The expression of MCT4 was also investigated in patients with temporal lobe epilepsy and in a pilocarpine rat model of temporal lobe epilepsy. A decrease in MCT4 expression was detected in the cortex of temporal lobe epilepsy patients, and a similar decrease was observed in the pilocarpine rat model 12 to 14 h after drug administration [174]. The reduction of the abundance of MCT4 (which is essentially an astrocytic transporter) was linked to a parallel reduction of the glial glutamate transporter excitatory amino acid transporter 1 (EAAT1), an observation that could explain neuronal hyperexcitability and epileptogenesis.
3.3.3. Brain ischemia
Changes in MCT expression have been reported following brain ischemia. In a transient global rat ischemia model, intense MCT1 labeling was found in CA1 pyramidal neurons, astrocytes, endothelial cells and ependymocytes [175]. An increase in the expression of MCT1 and MCT2 associated with microglial cells was found 48 h after focal ischemia induced by compression in rats [80]. In addition, modulation of MCT4 expression by hypoxia, as demonstrated in cultured astrocytes [151], could provide a mechanism by which neuronal death can be preserved under ischemic conditions [176] and could be exploited as a neuroprotective strategy in intermittent hypoxia preconditioning-induced epileptic tolerance [177].
3.3.4. Metabolic diseases
A distinct class of pathologies in which modulation of brain MCT expression could play a role is represented by certain metabolic diseases. Indeed, repeated hypoglycemia was reported to have an impact on MCT expression in various brain regions. This is the case in the hypothalamus, an important region for nutrient sensing and metabolic homeostasis. Thus, an injection of insulin to induce an hypoglycemic episode altered MCT2 expression of the vagal complex in opposite direction between male rats and estrogen-treated oviarectomized female rats [178]. Recurrent insulin-induced hypoglycemia enhanced MCT2 expression in the dorsal vagus complex, whereas acute hypoglycemia suppressed MCT2 expression in the ventromedial hypothalamus [179]. Conversely, catecholaminergic A2 neurons in the dorsal vagal complex exhibited reduced MCT2 expression following insulin-induced hypoglycemia [180].
In contrast to hypoglycemia, hyperglycemia was found to enhance MCT1 expression globally in the brain, most likely in endothelial cells and astrocytes [181], while having no effect on MCT1, MCT2 and MCT4 expression in the medio-basal hypothalamus [182]. Exposure of mice to a high fat, high sugar diet for several weeks induced the cortical expression of MCT1, MCT2 and MCT4 that occurred preferentially in neurons throughout the cortex [183].
While these data suggest the possible involvement of MCTs in metabolic homeostasis and its possible dysregulations, clear evidence was provided by the phenotypical characterization of haploinsufficient MCT1 transgenic mice [184]. These mice were shown to resist to the development of diet-induced obesity and its associated metabolic dysfunctions, including insulin resistance and hepatic steatosis. Interestingly, under a high fat, high sugar diet, haploinsufficient MCT1 mice had reduced food intake, suggesting that MCTs could be key factors implicated in nutrient sensing and subsequent food intake regulation and, eventually, dysregulation. This would certainly constitute an interesting topic for future investigation.
4. MCTs in cancer
4.1. Expression of MCTs in different cancer types
MCT1 and MCT4 are the most widely MCT isoforms expressed in cancer cells. MCT1 expression has been reported in a variety of human cancers including head and neck, breast, lung, stomach, colon, bladder, prostate and cervix cancers, as well as gliomas [25], [26], [185], [186], [187], [188]. MCT1 has also been proposed to be the most important isoform responsible for lactate transport across the plasma membrane in breast cancer, bladder cancer, non-small cell lung carcinomas (NSCLC) and ovarian carcinomas [103], [188]. MCT4 is also widely distributed in different cancer types. Its expression has indeed been reported in breast, colon, bladder, and prostate cancers, as well as in cancers of the gynecologic tract and gliomas [103], [186], [187], [188]. Despite a low expression at the plasma membrane, MCT4 is further present intracellularly in NSCLC [104]. Comparatively to MCT1 and MCT4, MCT2 and MCT3 have been less studied in cancer. MCT2 has been found in the cytoplasm of breast carcinoma, NSCLC, colon adenocarcinoma and ovarian adenocarcinoma cancer cells [103], [104], and MCT3 has also been reported to be expressed intracellularly in cancer and stromal cells of NSCLC [104]. With respect to MCT2 and MCT4, some authors have postulated that a high intracellular expression could reflect the localization of these transporters on the membranes of intracellular vesicles or organelles where they could potentially transport lactate and/or pyruvate [26], [188].
The expression of the chaperon proteins of MCTs in cancer cells is an important factor to take into account because they behave as key mediators in oncogenesis. CD147 and CD44 have been associated to MCT1 and MCT4 in different tumor types. MCT1 and CD147 expression have been significantly correlated in bladder [188] and ovarian cancers [103], and CD44 was reported to be associated with MCT1 expression in lung cancer [103]. CD147-MCT1 double-positive bladder tumors showed poor prognosis and unfavorable clinical pathological parameters [188]. In addition, experimentally induced CD147 downregulation increased the chemosensitivity of bladder cancer cell lines to cisplatin chemotherapy [188]. MCT4 expression has also been correlated to CD147 expression in prostate, breast and lung cancers [103], [188]. Recently, Pertega-Gomes and Baltazar [189] further reported a correlation between the expression of MCT1, MCT2 and MCT4 and the different stages of prostate cancer progression. MCT1 was present in both normal epithelium and malignant glands, MCT2 was expressed from benign to prostate intraepithelial neoplasia lesions and in malignant glands, and MCT4 was expressed only in malignant glands associated to poor prognosis. Therefore, the authors proposed that MCT1 and MCT2 would play a role in tumor maintenance, whereas MCT4 would increase tumor aggressiveness. MCT2 was also proposed as an interesting biomarker for prostate cancer [189]. In another study in NSCLC [104], Eilertsen et al. proposed MCT1 as a biomarker for prognostic and survival. The co-expression of GLUT1 and MCT1 and of GLUT1 and MCT4 was found as a negative prognostic factor associated with poor disease-specific survival.
4.2. MCTs in the metabolic relationships between glycolytic and oxidative cancer cells
From a metabolic standpoint, established tumors contain different types of cancer cells. Oxidative cancer cells can use different metabolic substrates to fuel OXPHOS, including glucose, glutamine, lipids and lactate [15]. Glycolytic cancer cells result from environmental selection (e.g. by hypoxia) or from metabolic adaptation in order to sustain biosynthesis for proliferation (the ‘Warburg effect’) [190], [191], [192], [193], [194]. The switch from an oxidative to a glycolytic metabolism formally corresponds to unframing glycolysis from the TCA cycle and OXPHOS in order for glycolysis to preferentially fuel lactic fermentation. The conversion of glycolytic end-products pyruvate, NADH and H+ into lactate and NAD+ indeed serves to regenerate the NAD+ pool necessary to maintain glycolysis at a high rate [195]. In the glycolytic compartment of tumors, metabolism accounts for elevated lactate production and for an extracellular lactate accumulation up to concentrations that can reach 10 to 40 mM [196]. These high levels of lactate have been shown to correlate with tumor aggressiveness and poor prognosis in several types of human cancers [15], [196], [197], [198], [199], [200], [201], [202]. MCTs play an essential role in the metabolic homeostasis of the tumor microenvironment: they ensure the maintenance of glycolytic and acid-resistant phenotypes [15], [203], thus contributing to the malignant behavior of cancer cells. MCTs allow glycolysis to operate at high speed by facilitating lactate export, and they are also involved in pH regulation by the co-transport of protons.
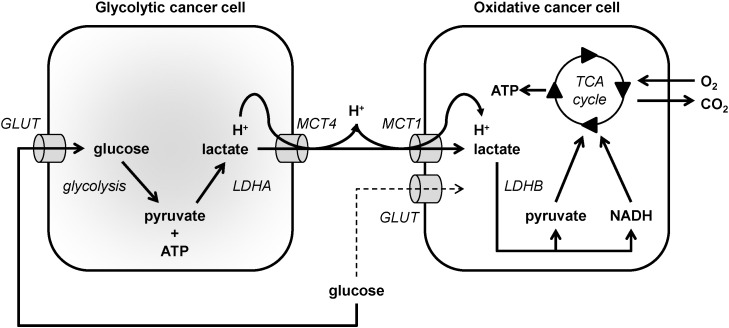
Lactate is not a metabolic dead-end product of glycolysis but is rather a tumor growth-promoting factor. In a striking similarity with brain metabolism, lactate can be used as a metabolic substrate by a variety of oxidative cancer cells. Lactate can indeed generally fuel the oxidative metabolism of oxygenated cancer cells [25], [29]. According to the ‘metabolic symbiosis’ hypothesis initially proposed by Sonveaux et al. in cancer [25], lactate produced by glycolytic cancer cells that rely on glucose as a main fuel is imported by oxidative cancer cells that use lactate in mitochondrial metabolism as a main fuel compared to glucose, thus sparing glucose for glycolytic cancer cells [204], [205] (Fig. 2). Indeed, this concept seems to also apply for brain tumors (gliomas) in which the occurrence of an intercellular lactate shuttle akin to the one normally operating to fuel neurons would take place, subverting the normal cellular metabolic interactions to sustain the survival and growth of the tumor [206]. MCT4, which has a low affinity for lactate but a high turnover rate, is particularly well adapted to facilitate lactate export by glycolytic cancer cells [12], [13]. Its expression is up-regulated by hypoxia: when the O2 supply is compromised, cancer cells activate transcription factor HIF-1, of which MCT4 is a target gene [24]. MCT4 also shows a higher Km for pyruvate compared to lactate, which prevents the efflux of pyruvate that would be detrimental for the maintenance of a high glycolytic flux. Conversely, MCT1 has a high affinity for lactate and is preferentially expressed in oxidative cancer cells that take up lactate [25], [207]. The use of pharmacological inhibitors and RNA interference experiments have revealed that MCT1 is the main transporter responsible for lactate uptake by these cells [25], [119], [207], [208]. Inside oxidative cancer cells, lactate reacts with NAD+ to yield pyruvate, NADH and H+ (the LDHB reaction), and both pyruvate and NADH can fuel the TCA cycle and OXPHOS in mitochondria, which depends on the malate–aspartate shuttle for the mitochondrial import of NADH [29].
Fig. 2.
Model depicting a metabolic symbiosis based on the exchange of lactate between glycolytic and oxidative cancer cells. In the model, glycolytic cancer cells, depicted on the left, import glucose via glucose transporters (GLUT) and then sequentially convert glucose to pyruvate and ATP using glycolysis, and pyruvate to lactate using lactate dehydrogenase A (LDHA). Lactate is exported together with protons via MCT4. Oxidative cancer cells, depicted on the right, import lactate and protons via MCT1. Lactate is oxidized to pyruvate by lactate dehydrogenase B (LDHB) generating NADH as a byproduct, and both pyruvate and NADH fuel the TCA cycle to support ATP production through oxidative phosphorylation.
In addition to its function as a metabolic substrate, lactate can also act as a signaling agent in oxidative cancer cells. In two seminal papers [209], [210], Lu et al. initially showed that lactate can behave is a hypoxia mimetic factor capable of activating transcription factor HIF-1 in normoxic cancer cells. In the process, lactate must be taken up by cancer cells through a MCT1-facilitated process, oxidized to pyruvate by LDHB, and pyruvate competes with 2-oxoglutarate to inhibit the prolylhydroxylases (PHDs) of HIF-1 [207], [209], [210] (Fig. 3), i.e., oxygen- and 2-oxoglutarate-dependent enzymes that catalyze the hydroxylation of HIF-1 subunit α on two proline residues to initiate the proteasome-dependent degradation pathway of HIF-1α [211]. Thus, by interfering with the normal degradation pathway of HIF-1α, lactate can trigger HIF-1 activity independently of hypoxia in oxidative cancer cells. An established consequence of such signaling is an increased transcription and release of vascular endothelial growth factor (VEGF) by oxidative cancer cells, which can stimulate endothelial cells to initiate and angiogenic response [194], [207] (Fig. 3, see also Section 4.3). Because MCTs are equilibrative lactate/H+ transporters and glycolytic cancer cells produce large amounts of lactate and protons, these cells cannot abundantly take up exogenous lactate and, therefore, do not activate HIF-1 upon exposure to lower levels of exogenous lactate [195], [207].
MCTs also promote migration and invasion processes in cancer. Izumi et al. [212] first showed that MCT1 and MCT4 expression correlates with invasion in human lung cancer cells. In agreement with their findings, others further showed that the MCT inhibitor CHC decreases the migration and invasion of glioma cells [187]. Moreover, in conditions of glucose deprivation, MCT1-CD147 complexes were found to be stabilized and MCT1 actively promoted the migration of oxidative SiHa and HeLa human cervix cancer cells towards glucose through a mechanism that was blocked using specific shRNAs targeting MCT1, or CHC to inhibit MCT1 activity [208]. However, whether and how MCT1 could function as a glucose sensor is still unknown.
4.3. MCTs in the crosstalk between cancer and endothelial cells
Endothelial cells are responsive to lactate stimulation in a way that promotes tumor progression. Indeed, similar to oxidative cancer cells, endothelial cells can take up lactate in a MCT1-facilitated process, although, contrary to oxidative cancer cells, they do not efficiently use lactate as a fuel [213]. In normoxic endothelial cells, lactate was found to stimulate HIF-1 activity in a manner similar to what was described for oxidative cancer cells, i.e., by sequentially serving as a precursor of pyruvate, inhibiting HIF PHDs, stabilizing HIF-1α and activating HIF-1 [213] (Fig. 3). However, the final proangiogenic effectors identified are different. Indeed, in endothelial cells, lactate did not stimulate VEGF production but rather increased the production of basic fibroblast growth factor (bFGF) and the expression of the prototypical VEGF receptor VEGFR2. Taking into account the fact that lactate can stimulate VEGF production independently of hypoxia in cancer cells [207], [209], [210], both paracrine VEGF signaling and autocrine bFGF signaling on endothelial cells could thus account for the proangiogenic effects of lactate in tumors.
Independently of HIF-1, lactate has also been show to sequentially activate NF-κB, trigger proangiogenic IL-8 production and activate the IL-8 pathway in an autocrine manner in endothelial cells [214], [215] (Fig. 3). This pathway shares with the lactate-HIF-1 pathway MCT1-facilitated lactate uptake, lactate oxidation to pyruvate (the LDHB reaction) and pyruvate-mediated PHD inhibition. PHD inhibition then rescues inhibitor of NF-κB kinase β (Iκκβ) from degradation, resulting in inhibition of inhibitor of NF-kB α (IκBα). NF-κB activation was found to further mandatorily require NADH, the byproduct of the LDHB reaction, to be available to sequentially fuel NAD(P)H oxidases, trigger the production of reactive oxygen species (ROS), and fully inactivate IκBα [214]. Accordingly, the mitochondrial use of NADH by oxidative cancer cells but not by endothelial cells that are more glycolytic explains why the oxidative pathway of lactate can activate NF-kB in endothelial cells but not in oxidative cancer cells [29].
The proangiogenic properties of lactate and MCT1 are also supported by observations in ischemic wound healing. Indeed, chronic lactate delivery to the wound site from a poly-D,L-lactide-co-glycolide polymer was shown to accelerate revascularization, hence wound healing [216]. Conversely, pharmacological inhibition of MCT1 by CHC blocked reparative angiogenesis and the healing activity of lactate.
4.4. MCTs in the crosstalk between cancer and stromal cells
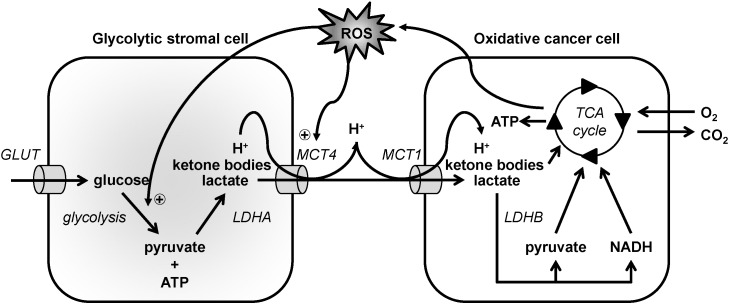
Over the past decades, studies on cancer metabolism highlighted the importance of the interaction between cancer cells and their microenvironment, and particular attention has been paid to interactions between cancer cells and fibroblasts. Lisanti et al. [217] proposed the existence of a metabolic relationship between stromal fibroblasts and oxidative epithelial cancer cells involving MCTs, which they coined ‘Reverse Warburg Effect’. In this process, oxidative cancer cells induce an oxidative stress in fibroblasts that triggers a glycolytic switch, MCT4 expression and the secretion of lactate, pyruvate and ketone bodies (Fig. 4). These metabolites are then imported in a MCT1-dependent manner and used oxidatively by the cancer cells. Experimentally, co-culturing breast cancer cells with normal fibroblasts upregulated MCT4 expression in the fibroblasts, which was prevented by the use of anti-oxidants such as N-acetyl-L-cysteine [218]. The co-culture also stimulated MCT1 expression in the cancer cells. In another experiment, HaCaT human epithelial keratinocytes were co-cultured with fibroblasts, resulting in MCT4 induction in the fibroblasts [219]. Mechanistically, ROS production following activation of the RAS oncogene and transcription factor NF-kB in cancer cells was reported to be responsible for metabolic reprogramming and enhanced MCT4 expression in fibroblasts [219].
Fig. 4.
Model depicting metabolic commensalism of oxidative cancer cells for stromal cells. In the model, oxidative cancer cells, depicted on the right, produce reactive oxygen species (ROS) that promote glycolysis and MCT4 expression in stromal cells. Glycolytic stromal cells, depicted on the left, import glucose via glucose transporters (GLUT) and then sequentially convert glucose to pyruvate and ATP using glycolysis, and pyruvate to lactate using lactate dehydrogenase A (LDHA). They also produce ketone bodies. Lactate and ketone bodies are exported together with protons via MCT4. Oxidative cancer cells import lactate, ketone bodies and protons via MCT1. Lactate is oxidized to pyruvate by lactate dehydrogenase B (LDHB) generating NADH as a byproduct, and both pyruvate and NADH fuel the TCA cycle to support ATP production through oxidative phosphorylation. Ketone bodies can also be catabolized.
In vivo observations support, to some extent, the existence of a metabolic commensalism whereby oxidative cancer cells exploit the metabolic activities of stromal cells. In biopsies of human breast cancer, MCT1 expression was found to predominate in epithelial cancer cells, whereas stromal cells preferential expressed MCT4 [218]. Tissue analysis of positive lymph nodes revealed amplified mitochondrial mass and metabolism in metastatic breast cancer cells and a lack of detectable mitochondria together with high MCT4 expression in stromal cells, including cancer-associated fibroblasts (CAFs), adipocytes and inflammatory cells [220]. Frozen sections of primary tumors were further screened for functional mitochondria. Analyses showed higher expression and activity of succinate dehydrogenase and cytochrome C oxidase, as well as higher levels of NADH, in epithelial cancer cells compared to adjacent stromal cells, thus convincingly indicating strong OXPHOS activity in the cancer cells [221]. High MCT4 expression in the stromal tissue has been proposed to have an important prognostic role in breast cancer, which could improve the prognostic value of other biomarkers: stromal MCT4 expression is normally inversely correlated to the expression of caveolin-1, and the combination of the two markers could be helpful to identify intermediate risk groups [222], [223].
In human head and neck cancer, MCT1 expression was reported to be associated to cancer cells [224]. Cancer-associated fibroblasts (CAFs) had high MCT4 expression.
In human prostate cancer, MCT1 was present in cancer cells and MCT4 in CAFs [189]. CAFs also had high CAIX expression, suggesting a glycolytic metabolism. Interestingly, the concomitant presence of elevated levels of MCT4 in CAFs and MCT1 in cancer cells was related to a poor clinical outcome [189]. An independent study confirmed MCT1 expression in prostate cancer cells [225], but it was associated to a strong cytoplasmic reactivity for LDHA, which would indicate a glycolytic rather than an oxidative metabolism of the cancer cells. In the same study, MCT1 was found in CAFs and tumor-associated myoblasts, with LDHB upregulation (compared to cancer cells) indicative of an oxidative metabolism of the stroma in 40% of cases. Similar findings have been made in lung cancer, with, comparatively, MCT1 and LDHA overexpression in cancer cells and no MCT but LDHB expression in CAFs [226].
Collectively, these data support the general existence of MCT-dependent relationships between cancer cells and stromal cells that could encompass metabolic commensalism of cancer cells for stromal cells but also the opposite situation where stromal cells would metabolically use lactate and other monocarboxylates exported by cancer cells.
4.5. MCTs in the crosstalk between cancer and immune cells
Cancer cells and immune cells interact in a broad range of manners. As recently reviewed by Hirt et al. [227], the in vitro study of those interactions in 3D models replicates better in vivo observations than standard 2D co-cultures. Among protumoral immune influences on cancer cells, a well-known observation is that chronic inflammation can promote oncogenesis, as illustrated for example by the higher incidence of colorectal cancer in inflammatory bowel disease patients. With respect to metabolism, various studies point the high intratumoral lactic acid concentration as a unifying mechanism of the immunosuppressive effects of cancer cells [228].
Using monocyte-derived dendritic cells (DC), Puig-Kroger et al. [229] first observed that lactate can decrease the DNA binding activity NF-κB in a dose-dependent manner, thus impairing DC maturation. They also showed that TNFα release was decreased, although this occurred only at higher lactate concentrations (40 mM), i.e., the highest dose of lactate that can be found in human tumors. Nasi et al. [230] analyzed the defect of DC differentiation in dense cell cultures, and identified lactic acid as being responsible for this. In their experiments, DC induced to differentiate in high density cultures developed an anti-inflammatory phenotype (with IL-10 production) and displayed a preserved osteoclast differentiation potential, whereas low density cultures resulted in IL-12- and TNFα-producing cells capable to migrate towards lymphoid tissue chemokine. While acidic pH alone had no effect and lactate alone had only a weak effect, lactic acid reproduced the effects of high density culture, underlying the potential role of MCTs in DC differentiation [230].
Activated T cells depend on glycolysis for energy production and therefore need to release lactate, which depends on MCT1 in these cells [231], [232], [233] (Fig. 5). In tumors, they are in competition with cancer cells for nutrients, which, in addition to low pH, promotes T cell anergy [227]. In this context, Fischer et al. [234] observed that lactic acid inhibits the proliferation of human cytolytic T lymphocytes (CTLs) in a dose-dependent manner. Neither sodium lactate, lactic acid in a solution buffered at pH 7.4 nor acidification alone had antiproliferative effects, indicating a specific activity of lactic acid. Upon lactic acid treatment, CTLs showed decreased IL-12 and IFN-γ production and a lower cytotoxic activity linked to a decrease in granzyme and perforin B content [234]. Because lactic acid blocked the release of lactate by CTLs and pharmacological MCT1 inhibition had a similar antiproliferative effect than lactic acid, immunosuppression of T cells by lactic acid most likely depends on lactate efflux inhibition [234] (Fig. 5). Consistently, others independently showed that CTL stimulation in a lactic acidosis medium, without prior exposure to lactic acid, decreased IFN-γ, TNFα and IL-12 production, a response that was concentration-dependent [235]. While cytokine production was completely inhibited at 20 mM of lactic acid, exocytosis of lytic granules was reduced by only 50% at this concentration, and CTL viability was not affected. This rapid effect of lactic acid is concordant with the fast uptake of lactate. The differential manifestation of the two types of responses was explained by a rapid inhibition of the MAPK-p38-JNK pathway by lactic acid, whereas ERK and Akt pathways were preserved [235]. In these assays, similar to what had been observed by Fischer et al., lactate alone or acidic pH alone did not recapitulate the effects of lactic acid.
Fig. 5.
Model showing the immunosuppressive activity of lactic acid on T cells. In the model, glycolytic cells, depicted on the left, import glucose via glucose transporters (GLUT) and then sequentially convert glucose to pyruvate and ATP using glycolysis, and pyruvate to lactate using lactate dehydrogenase A (LDHA). Lactate is exported together with protons via MCT4. Activated T lymphocytes, depicted on the right, also depend on a glycolytic metabolism and must release lactate and protons, which in these cells is facilitated by MCT1. MCT1, however, is a passive transporter that is antagonized by the high microenvironmental levels of lactic acid that can be found in glycolytic tissues.
Lactate is also involved in tumor immune escape. In vivo, knocking-down LDHA in cancer cells (and thus decreasing their glycolytic rate and lactate secretion) resulted in natural killer (NK) cell activation, reduced accumulation of myeloid-derived suppressor cells (MDSCs), reduced immunosuppressive activity and smaller tumor sizes [236]. Direct effects of lactate were demonstrated by the same authors in vitro, by showing that lactate, per se, decreases NK cell activity and increases the differentiation of peripheral blood mononuclear cells to MDSCs and their immunosuppressive activity. In vivo, a ketogenic diet, used as a surrogate of LDHA knock-down, improved antitumor immunity, although to a lesser extent [236]. Regulatory T cells and MDSCs were less present than with a normal diet, and the number of immune effector cells was increased.
In tumors, myeloid cells may polarize into M1 tumor-suppressive macrophages or into M2 tumor-associated macrophages that secrete proliferation- and survival-stimulating cytokines and proangiogenic factors. Lactate can influence this process. Colegio et al. [237] indeed showed that lactate can activate HIF-1 in macrophages, driving their polarization to the M2 phenotype with a high VEGF secretion that would promote tumor angiogenesis. Importantly, lactate needed the presence of free protons to exert its effect, but an acidic pH alone was not sufficient to stabilize HIF-1α and activate HIF-1. When HIF-1α was silenced, the effects of lactate were absent.
5. Conclusions
MCTs represent the major path for inward and outward monocarboxylate fluxes from cells and are key regulators of intracellular and extracellular pH.
The description of the cell-specific distribution of MCT1–4 has contributed to the emergence of the physiological concept of intercellular lactate shuttle [238], [239]. First described in muscles by George Brooks [240], this concept has seen its scope enlarged by its description in other organs and tissues like the testis [90], [241]. In the brain, the debated but now largely supported establishment of the ‘astrocyte-neuron lactate shuttle’ concept is also expanding by the description of similar lactate exchanges taking place between oligodendrocytes and axons [34], [167], as well as between Schwann cells and peripheral nerves [87]. Considering the importance of such a mechanism of metabolic cooperation between cells under physiological activity, it may come as no surprise that under pathological conditions such as cancer, a similar mechanism operates or is used for the benefit of cancer cells. Cancer cells being opportunistic, they have developed strategies to survive in their environment, and the expression of appropriate MCTs contribute to their adaptation to many situations, as described in Section 4 by many examples.
In the brain, the type of metabolic cooperation between cells is less opportunistic but more homeostatic. It is for the functional benefit of the organ that these metabolic exchanges have developed, and they rely on subtle equilibria. Any imbalances may lead on the long run to deleterious consequences. Indeed, there is more and more evidence that metabolic dysfunctions underlie neurodegenerative diseases rather than being mere consequences [242]. Thus, it has been proposed in many instances that bolstering neuroenergetics (including the capacity of brain cells to produce and/or utilize monocarboxylates, which also include ketone bodies) might be a good neuroprotective strategy [243], [244]. This possibility remains to be fully tested, but we lack for the moment a proper strategy to be able to enhance these aspects of energy metabolism. This is why we need to gather more information about the mechanisms regulating the expression of MCTs in the various brain cell types. A better characterization of these mechanisms might provide the necessary insights to develop an appropriate therapeutic approach based on this knowledge.
Due to their important activities and their general overexpression in cancers compared to healthy tissues, MCTs are interesting targets for cancer therapy. In tumors, MCT1 gates lactate uptake by oxidative cancer cells and by endothelial cells, thus simultaneously controlling the cataplerotic (Fig. 2, Fig. 3), proangiogenic (Fig. 4) and pro-migratory activities of lactate. Consequently, MCT1 inhibition can have antimetabolic, anti-angiogenic and anti-migratory effects that repress tumor growth [25], [119], [207], [213], [214] and, potentially, progression to the metastatic state [208]. In fact, in vitro MCT1 inhibition has been found to decrease intracellular pH [25], elicit cell death [25], [245] and decrease cancer cell migration [208]. MCT4 also represents an interesting anticancer target, as can notably be illustrated by the fact that MCT4 inhibition by siRNAs reduced the migration of breast cancer cells [246].
Developing MCT inhibitors for anticancer therapy is currently an ongoing task. While historical inhibitors are poorly selective for MCTs, CHC in particular has been the focus of a vast number of in vitro and in vivo studies showing its therapeutic effects [26]. However, this compound has several drawbacks. It is poorly specific for a given MCT isoform and can also inhibit the mitochondrial pyruvate carrier and anion exchanger 1 [26]. Moreover, it is usually described to be active in the upper micromolar range. More recently, AR-C155858, a highly potent MCT1/2 inhibitor, and AZD3965, a second-generation MCT1/2 inhibitor, have been disclosed by AstraZeneca [46], [233]. AZD3695 is currently in Phase I/II clinical trials for advanced solid tumors and diffused B cell lymphomas [247]. However, these molecules present a limited therapeutic action due to the compensatory effect of MCT4, which they do not inhibit [248]. A new family of inhibitors, of which 7ACC2 is the leading compound, has been recently disclosed and described to selectively inhibit lactate uptake (versus export) by cancer cells expressing both MCT1 and MCT4 [37], [38]. The future in vivo evaluation of these compounds will provide key information about their therapeutic activity and suitability for clinical evaluation.
Important issues still remain to be addressed for the clinical exploitation of MCT inhibitors. First, the physiological role of these transporters is still poorly characterized in many tissues. Systematic characterization could benefit from the generation of tissue-specific MCT-deficient mice that would further potentially reveal so far unsuspected toxicities linked to MCT inhibition. Second, little is currently known about the regulation of MCT expression and activity. A better characterization of endogenous and exogenous factors, including conventional therapies, capable of influencing MCTs could eventually allow to identify efficient treatment combinations. Interestingly, MCTs can transport anticancer agents 3-bromopyruvate, dichloroacetate and iodoacetate across cancer cell membranes [26]. The study of MCT substrates that can act as anticancer compounds is an interesting new field that should be further explored. Finally, the preclinical and clinical development of MCT inhibitors would benefit from the identification and validation of biomarkers of MCT activity that would allow to predict and to document a therapeutic response.
Acknowledgments
Work in Luc Pellerin's lab was supported by the Swiss Fonds National de la Recherche grant n° 3100A3_140957. Works in Pierre Sonveaux's lab are supported by a Starting Grant from the European Research Council (ERC No. 243188 TUMETABO), EU Horizon2020 Marie Skłodowska-Curie Innovative Training Networks (ITN No. 642623 RADIATE), Interuniversity Attraction Pole (IAP) grant #UP7-03 from the Belgian Science Policy Office (Belspo), an Action de Recherche Concertée from the Communauté Française de Belgique (ARC 14/19-058), the Belgian Fonds National de la Recherche Scientifique (F.R.S.-FNRS), the Télévie, the Belgian Fondation contre le Cancer (2012-186), the Belgian Federal Agency for Nuclear Control (FANC-AFCN), the Joseph Maisin Fund, the Louvain Foundation and the UCL Fonds Spéciaux de la Recherche (FSR). Pierre Sonveaux is a Research Associate and Valéry L. Payen a PhD Fellow of the F.R.S.-FNRS. Vincent F. Van Hée and Martina Sboarina are Télévie PhD Fellows.
Footnotes
This article is part of a Special Issue entitled: Mitochondrial Channels edited by Jean-Claude Martinou.
Contributor Information
Luc Pellerin, Email: luc.pellerin@unil.ch.
Pierre Sonveaux, Email: pierre.sonveaux@uclouvain.be.
References
- 1.Halestrap A.P., Price N.T. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 1999;343(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 2.Halestrap A.P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 3.Visser W.E., Friesema E.C., Visser T.J. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol. Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami Y., Kohyama N., Kobayashi Y., Ohbayashi M., Ohtani H., Sawada Y., Yamamoto T. Functional characterization of human monocarboxylate transporter 6 (SLC16A5) Drug Metab. Dispos. 2005;33:1845–1851. doi: 10.1124/dmd.105.005264. [DOI] [PubMed] [Google Scholar]
- 5.Hugo S.E., Cruz-Garcia L., Karanth S., Anderson R.M., Stainier D.Y., Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 2012;26:282–293. doi: 10.1101/gad.180968.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suhre K., Shin S.Y., Petersen A.K., Mohney R.P., Meredith D., Wagele B., Altmaier E., Deloukas P., Erdmann J., Grundberg E., Hammond C.J., de Angelis M.H., Kastenmuller G., Kottgen A., Kronenberg F., Mangino M., Meisinger C., Meitinger T., Mewes H.W., Milburn M.V., Prehn C., Raffler J., Ried J.S., Romisch-Margl W., Samani N.J., Small K.S., Wichmann H.E., Zhai G., Illig T., Spector T.D., Adamski J., Soranzo N., Gieger C. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halestrap A.P. The monocarboxylate transporter family—structure and functional characterization. IUBMB. Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 8.Manoharan C., Wilson M.C., Sessions R.B., Halestrap A.P. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol. Membr. Biol. 2006;23:486–498. doi: 10.1080/09687860600841967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson M.C., Meredith D., Bunnun C., Sessions R.B., Halestrap A.P. Studies on the DIDS-binding site of monocarboxylate transporter 1 suggest a homology model of the open conformation and a plausible translocation cycle. J. Biol. Chem. 2009;284:20011–20021. doi: 10.1074/jbc.M109.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halestrap A.P. The SLC16 gene family—structure, role and regulation in health and disease. Mol. Asp. Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Galic S., Schneider H.P., Broer A., Deitmer J.W., Broer S. The loop between helix 4 and helix 5 in the monocarboxylate transporter MCT1 is important for substrate selection and protein stability. Biochem. J. 2003;376:413–422. doi: 10.1042/BJ20030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimmer K.S., Friedrich B., Lang F., Deitmer J.W., Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000;350(Pt 1):219–227. [PMC free article] [PubMed] [Google Scholar]
- 13.Chiche J., Fur Y.L., Vilmen C., Frassineti F., Daniel L., Halestrap A.P., Cozzone P.J., Pouyssegur J., Lutz N.W. In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int. J. Cancer. 2012;130:1511–1520. doi: 10.1002/ijc.26125. [DOI] [PubMed] [Google Scholar]
- 14.Manning Fox J.E., Meredith D., Halestrap A.P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J. Physiol. 2000;529(Pt 2):285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhup S., Dadhich R.K., Porporato P.E., Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr. Pharm. Des. 2012;18:1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 16.Garcia C.K., Brown M.S., Pathak R.K., Goldstein J.L. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J. Biol. Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 17.Philp N.J., Yoon H., Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am. J. Physiol. Cell Physiol. 2001;280:C1319–C1326. doi: 10.1152/ajpcell.2001.280.5.C1319. [DOI] [PubMed] [Google Scholar]
- 18.Pérez de Heredia F., Wood I.S., Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch. 2010;459:509–518. doi: 10.1007/s00424-009-0750-3. [DOI] [PubMed] [Google Scholar]
- 19.Broer S., Broer A., Schneider H.P., Stegen C., Halestrap A.P., Deitmer J.W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J. 1999;341(Pt 3):529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broer S., Schneider H.P., Broer A., Rahman B., Hamprecht B., Deitmer J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998;333(Pt 1):167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birsoy K., Wang T., Possemato R., Yilmaz O.H., Koch C.E., Chen W.W., Hutchins A.W., Gultekin Y., Peterson T.R., Carette J.E., Brummelkamp T.R., Clish C.B., Sabatini D.M. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 2013;45:104–108. doi: 10.1038/ng.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson V.N., Halestrap A.P. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J. Biol. Chem. 1996;271:861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- 23.Halestrap A.P., Wilson M.C. The monocarboxylate transporter family—role and regulation. IUBMB Life. 2012;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 24.Ullah M.S., Davies A.J., Halestrap A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 25.Sonveaux P., Végran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F., Kelley M.J., Gallez B., Wahl M.L., Feron O., Dewhirst M.W. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltazar F., Pinheiro C., Morais-Santos F., Azevedo-Silva J., Queiros O., Preto A., Casal M. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol. Histopathol. 2014;29:1511–1524. doi: 10.14670/HH-29.1511. [DOI] [PubMed] [Google Scholar]
- 27.Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 2001;86:6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy K.M., Scarbrough P.M., Ribeiro A., Richardson R., Yuan H., Sonveaux P., Landon C.D., Chi J.T., Pizzo S., Schroeder T., Dewhirst M.W. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Hée V.F., Pérez-Escuredo J., Cacace A., Copetti T., Sonveaux P. Lactate does not activate NF-kB in oxidative tumor cells. Front. Pharmacol. 2015;6:228. doi: 10.3389/fphar.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juel C., Halestrap A.P. Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J. Physiol. 1999;517(Pt 3):633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks G.A. Cell–cell and intracellular lactate shuttles. J. Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellerin L., Magistretti P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funfschilling U., Supplie L.M., Mahad D., Boretius S., Saab A.S., Edgar J., Brinkmann B.G., Kassmann C.M., Tzvetanova I.D., Mobius W., Diaz F., Meijer D., Suter U., Hamprecht B., Sereda M.W., Moraes C.T., Frahm J., Goebbels S., Nave K.A. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saab A.S., Tzvetanova I.D., Nave K.A. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr. Opin. Neurobiol. 2013;23:1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Halestrap A.P. Monocarboxylic acid transport. Compr. Physiol. 2013;3:1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 36.Ovens M.J., Manoharan C., Wilson M.C., Murray C.M., Halestrap A.P. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem. J. 2010;431:217–225. doi: 10.1042/BJ20100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Draoui N., Schicke O., Fernandes A., Drozak X., Nahra F., Dumont A., Douxfils J., Hermans E., Dogne J.M., Corbau R., Marchand A., Chaltin P., Sonveaux P., Feron O., Riant O. Synthesis and pharmacological evaluation of carboxycoumarins as a new antitumor treatment targeting lactate transport in cancer cells. Bioorg. Med. Chem. 2013;21:7107–7117. doi: 10.1016/j.bmc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Draoui N., Schicke O., Seront E., Bouzin C., Sonveaux P., Riant O., Feron O. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol. Cancer Ther. 2014;13:1410–1418. doi: 10.1158/1535-7163.MCT-13-0653. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter L., Halestrap A.P. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochem. J. 1994;304(Pt 3):751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenton R.A., Chou C.L., Stewart G.S., Smith C.P., Knepper M.A. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7469–7474. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson J.A., Falk R.E. Phloridzin and phloretin inhibition of 2-deoxy-d-glucose uptake by tumor cells in vitro and in vivo. Anticancer Res. 1993;13:2293–2299. [PubMed] [Google Scholar]
- 42.Moskaug J.O., Carlsen H., Myhrstad M., Blomhoff R. Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech. Ageing Dev. 2004;125:315–324. doi: 10.1016/j.mad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Endeward V., Cartron J.P., Ripoche P., Gros G. Red cell membrane CO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus. Clin. Biol. 2006;13:123–127. doi: 10.1016/j.tracli.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Reinertsen K.V., Tonnessen T.I., Jacobsen J., Sandvig K., Olsnes S. Role of chloride/bicarbonate antiport in the control of cytosolic pH. Cell-line differences in activity and regulation of antiport. J. Biol. Chem. 1988;263:11117–11125. [PubMed] [Google Scholar]
- 45.Guile S.D., Bantick J.R., Cooper M.E., Donald D.K., Eyssade C., Ingall A.H., Lewis R.J., Martin B.P., Mohammed R.T., Potter T.J., Reynolds R.H., St-Gallay S.A., Wright A.D. Optimization of monocarboxylate transporter 1 blockers through analysis and modulation of atropisomer interconversion properties. J. Med. Chem. 2007;50:254–263. doi: 10.1021/jm060995h. [DOI] [PubMed] [Google Scholar]
- 46.Ovens M.J., Davies A.J., Wilson M.C., Murray C.M., Halestrap A.P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem. J. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Critchlow S.E., Hopcroft L., Curtis L.N., Whalley N., Zhong H., Logie A., Revill M., Xie L., Zhang J., Yu D.H., Murray C., Smith P.D. Abstract 3224: pre-clinical targeting of the metabolic phenotype of lymphoma by AZD3965, a selective inhibitor of monocarboxylate transporter 1 (MCT1) Cancer Res. 2012;72:3224. [Google Scholar]
- 48.McCullagh K.J., Poole R.C., Halestrap A.P., O'Brien M., Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am. J. Phys. 1996;271:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- 49.Pilegaard H., Terzis G., Halestrap A., Juel C. Distribution of the lactate/H + transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am. J. Phys. 1999;276:E843–E848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- 50.Fishbein W.N., Merezhinskaya N., Foellmer J.W. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve. 2002;26:101–112. doi: 10.1002/mus.10168. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi M. Fiber type-specific localization of monocarboxylate transporters MCT1 and MCT4 in rat skeletal muscle. Kurume Med. J. 2004;51:253–261. doi: 10.2739/kurumemedj.51.253. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto T., Masuda S., Taguchi S., Brooks G.A. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J. Physiol. 2005;567:121–129. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia C.K., Goldstein J.L., Pathak R.K., Anderson R.G., Brown M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 54.Johannsson E., Nagelhus E.A., McCullagh K.J., Sejersted O.M., Blackstad T.W., Bonen A., Ottersen O.P. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart. A high-resolution immunogold analysis. Circ. Res. 1997;80:400–407. [PubMed] [Google Scholar]
- 55.Kirat D., Inoue H., Iwano H., Yokota H., Taniyama H., Kato S. Monocarboxylate transporter 1 (MCT1) in the liver of pre-ruminant and adult bovines. Vet. J. 2007;173:124–130. doi: 10.1016/j.tvjl.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Tamai I., Takanaga H., Maeda H., Sai Y., Ogihara T., Higashida H., Tsuji A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem. Biophys. Res. Commun. 1995;214:482–489. doi: 10.1006/bbrc.1995.2312. [DOI] [PubMed] [Google Scholar]
- 57.Ritzhaupt A., Wood I.S., Ellis A., Hosie K.B., Shirazi-Beechey S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport l-lactate as well as butyrate. J. Physiol. 1998;513(Pt 3):719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orsenigo M.N., Tosco M., Bazzini C., Laforenza U., Faelli A. A monocarboxylate transporter MCT1 is located at the basolateral pole of rat jejunum. Exp. Physiol. 1999;84:1033–1042. [PubMed] [Google Scholar]
- 59.Kirat D., Inoue H., Iwano H., Hirayama K., Yokota H., Taniyama H., Kato S. Monocarboxylate transporter 1 gene expression in the ovine gastrointestinal tract. Vet. J. 2006;171:462–467. doi: 10.1016/j.tvjl.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Iwanaga T., Takebe K., Kato I., Karaki S., Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed. Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- 61.Shimoyama Y., Kirat D., Akihara Y., Kawasako K., Komine M., Hirayama K., Matsuda K., Okamoto M., Iwano H., Kato S., Taniyama H. Expression of monocarboxylate transporter 1 (MCT1) in the dog intestine. J. Vet. Med. Sci. 2007;69:599–604. doi: 10.1292/jvms.69.599. [DOI] [PubMed] [Google Scholar]
- 62.Welter H., Claus R. Expression of the monocarboxylate transporter 1 (MCT1) in cells of the porcine intestine. Cell Biol. Int. 2008;32:638–645. doi: 10.1016/j.cellbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Poole R.C., Halestrap A.P. N-terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem. J. 1994;303(Pt 3):755–759. doi: 10.1042/bj3030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koho N.M., Vaihkonen L.K., Poso A.R. Lactate transport in red blood cells by monocarboxylate transporters. Equine Vet. J. Suppl. 2002;34:555–559. doi: 10.1111/j.2042-3306.2002.tb05482.x. [DOI] [PubMed] [Google Scholar]
- 65.Koho N.M., Raekallio M., Kuusela E., Vuolle J., Poso A.R. Lactate transport in canine red blood cells. Am. J. Vet. Res. 2008;69:1091–1096. doi: 10.2460/ajvr.69.8.1091. [DOI] [PubMed] [Google Scholar]
- 66.Merezhinskaya N., Ogunwuyi S.A., Mullick F.G., Fishbein W.N. Presence and localization of three lactic acid transporters (MCT1, − 2, and − 4) in separated human granulocytes, lymphocytes, and monocytes. J. Histochem. Cytochem. 2004;52:1483–1493. doi: 10.1369/jhc.4A6306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajduch E., Heyes R.R., Watt P.W., Hundal H.S. Lactate transport in rat adipocytes: identification of monocarboxylate transporter 1 (MCT1) and its modulation during streptozotocin-induced diabetes. FEBS Lett. 2000;479:89–92. doi: 10.1016/s0014-5793(00)01889-5. [DOI] [PubMed] [Google Scholar]
- 68.Settle P., Mynett K., Speake P., Champion E., Doughty I.M., Sibley C.P., D'Souza S.W., Glazier J. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta. 2004;25:496–504. doi: 10.1016/j.placenta.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Nagai A., Takebe K., Nio-Kobayashi J., Takahashi-Iwanaga H., Iwanaga T. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta. 2010;31:126–133. doi: 10.1016/j.placenta.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Takebe K., Nio-Kobayashi J., Takahashi-Iwanaga H., Yajima T., Iwanaga T. Cellular expression of a monocarboxylate transporter (MCT1) in the mammary gland and sebaceous gland of mice. Histochem. Cell Biol. 2009;131:401–409. doi: 10.1007/s00418-008-0543-3. [DOI] [PubMed] [Google Scholar]
- 71.Gerhart D.Z., Enerson B.E., Zhdankina O.Y., Leino R.L., Drewes L.R. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Phys. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- 72.Pellerin L., Pellegri G., Martin J.L., Magistretti P.J. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mac M., Nalecz K.A. Expression of monocarboxylic acid transporters (MCT) in brain cells. Implication for branched chain alpha-ketoacids transport in neurons. Neurochem. Int. 2003;43:305–309. doi: 10.1016/s0197-0186(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 74.Broer S., Rahman B., Pellegri G., Pellerin L., Martin J.L., Verleysdonk S., Hamprecht B., Magistretti P.J. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 75.Leino R.L., Gerhart D.Z., Drewes L.R. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res. Dev. Brain Res. 1999;113:47–54. doi: 10.1016/s0165-3806(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 76.Hanu R., McKenna M., O'Neill A., Resneck W.G., Bloch R.J. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Cell Physiol. 2000;278:C921–C930. doi: 10.1152/ajpcell.2000.278.5.C921. [DOI] [PubMed] [Google Scholar]
- 77.Pierre K., Pellerin L., Debernardi R., Riederer B.M., Magistretti P.J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–627. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- 78.Rinholm J.E., Hamilton N.B., Kessaris N., Richardson W.D., Bergersen L.H., Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W., Pellerin L., Magistretti P.J., Rothstein J.D. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreira T.J., Pierre K., Maekawa F., Repond C., Cebere A., Liljequist S., Pellerin L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J. Cereb. Blood Flow Metab. 2009;29:1273–1283. doi: 10.1038/jcbfm.2009.50. [DOI] [PubMed] [Google Scholar]
- 81.Nijland P.G., Michailidou I., Witte M.E., Mizee M.R., van der Pol S.M., van Het H.B., Reijerkerk A., Pellerin L., van V d., de Vries H.E., van H.J. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia. 2014;62:1125–1141. doi: 10.1002/glia.22667. [DOI] [PubMed] [Google Scholar]
- 82.Debernardi R., Pierre K., Lengacher S., Magistretti P.J., Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J. Neurosci. Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- 83.Pellerin L., Bergersen L.H., Halestrap A.P., Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 84.Ainscow E.K., Mirshamsi S., Tang T., Ashford M.L., Rutter G.A. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J. Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carneiro L., Geller S., Fioramonti X., Hébert A., Repond C., Leloup C., Pellerin L. Evidence for hypothalamic ketone bodies sensing: impact on food intake and peripheral metabolic responses in mice. Am. J. Phys. 2016;310:E103–E115. doi: 10.1152/ajpendo.00282.2015. [DOI] [PubMed] [Google Scholar]
- 86.Cortes-Campos C., Elizondo R., Llanos P., Uranga R.M., Nualart F., Garcia M.A. MCT expression and lactate influx/efflux in tanycytes involved in glia–neuron metabolic interaction. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domenech-Estevez E., Baloui H., Repond C., Rosafio K., Medard J.J., Tricaud N., Pellerin L., Chrast R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015;35:4151–4156. doi: 10.1523/JNEUROSCI.3534-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morrison B.M., Tsingalia A., Vidensky S., Lee Y., Jin L., Farah M.H., Lengacher S., Magistretti P.J., Pellerin L., Rothstein J.D. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp. Neurol. 2015;263:325–338. doi: 10.1016/j.expneurol.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takebe K., Nio-Kobayashi J., Takahashi-Iwanaga H., Iwanaga T. Histochemical demonstration of a monocarboxylate transporter in the mouse perineurium with special reference to GLUT1. Biomed. Res. 2008;29:297–306. doi: 10.2220/biomedres.29.297. [DOI] [PubMed] [Google Scholar]
- 90.Boussouar F., Mauduit C., Tabone E., Pellerin L., Magistretti P.J., Benahmed M. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol. Reprod. 2003;69:1069–1078. doi: 10.1095/biolreprod.102.010074. [DOI] [PubMed] [Google Scholar]
- 91.Pierre K., Magistretti P.J., Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Balmaceda-Aguilera C., Cortes-Campos C., Cifuentes M., Peruzzo B., Mack L., Tapia J.C., Oyarce K., Garcia M.A., Nualart F. Glucose transporter 1 and monocarboxylate transporters 1, 2, and 4 localization within the glial cells of shark blood–brain-barriers. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerhart D.Z., Enerson B.E., Zhdankina O.Y., Leino R.L., Drewes L.R. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22:272–281. [PubMed] [Google Scholar]
- 94.Yoon H., Fanelli A., Grollman E.F., Philp N.J. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem. Biophys. Res. Commun. 1997;234:90–94. doi: 10.1006/bbrc.1997.6588. [DOI] [PubMed] [Google Scholar]
- 95.Philp N.J., Yoon H., Grollman E.F. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am. J. Phys. 1998;274:R1824–R1828. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- 96.Bonen A., Miskovic D., Tonouchi M., Lemieux K., Wilson M.C., Marette A., Halestrap A.P. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. Am. J. Physiol. Endocrinol. Metab. 2000;278:E1067–E1077. doi: 10.1152/ajpendo.2000.278.6.E1067. [DOI] [PubMed] [Google Scholar]
- 97.Merezhinskaya N., Ogunwuyi S.A., Fishbein W.N. Expression of monocarboxylate transporter 4 in human platelets, leukocytes, and tissues assessed by antibodies raised against terminal versus pre-terminal peptides. Mol. Genet. Metab. 2006;87:152–161. doi: 10.1016/j.ymgme.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 98.Tan Z., Xie N., Banerjee S., Cui H., Fu M., Thannickal V.J., Liu G. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J. Biol. Chem. 2015;290:46–55. doi: 10.1074/jbc.M114.603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rafiki A., Boulland J.L., Halestrap A.P., Ottersen O.P., Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 100.Bergersen L., Waerhaug O., Helm J., Thomas M., Laake P., Davies A.J., Wilson M.C., Halestrap A.P., Ottersen O.P. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp. Brain Res. 2001;136:523–534. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- 101.Bergersen L.H., Magistretti P.J., Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb. Cortex. 2005;15:361–370. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- 102.Pierre K., Chatton J.Y., Parent A., Repond C., Gardoni F., Di L.M., Pellerin L. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur. J. Neurosci. 2009;29:1951–1963. doi: 10.1111/j.1460-9568.2009.06756.x. [DOI] [PubMed] [Google Scholar]
- 103.Pinheiro C., Reis R.M., Ricardo S., Longatto-Filho A., Schmitt F., Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010;2010:427694. doi: 10.1155/2010/427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eilertsen M., Andersen S., Al-Saad S., Kiselev Y., Donnem T., Stenvold H., Pettersen I., Al-Shibli K., Richardsen E., Busund L.T., Bremnes R.M. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashimoto T., Hussien R., Cho H.S., Kaufer D., Brooks G.A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tescarollo F., Covolan L., Pellerin L. Glutamate reduces glucose utilization while concomitantly enhancing AQP9 and MCT2 expression in cultured rat hippocampal neurons. Front. Neurosci. 2014;8:246. doi: 10.3389/fnins.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hashimoto T., Brooks G.A. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med. Sci. Sports Exerc. 2008;40:486–494. doi: 10.1249/MSS.0b013e31815fcb04. [DOI] [PubMed] [Google Scholar]
- 108.Brooks G.A., Brown M.A., Butz C.E., Sicurello J.P., Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J. Appl. Physiol. 1999;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- 109.Butz C.E., McClelland G.B., Brooks G.A. MCT1 confirmed in rat striated muscle mitochondria. J. Appl. Physiol. 2004;97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- 110.Lee W.J., Kim M., Park H.S., Kim H.S., Jeon M.J., Oh K.S., Koh E.H., Won J.C., Kim M.S., Oh G.T., Yoon M., Lee K.U., Park J.Y. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Halestrap A.P. UCSD Nature Molecule Pages. 2009. Monocarboxylate transporter 1. [Google Scholar]
- 112.Olson E.N., Williams R.S. Calcineurin signaling and muscle remodeling. Cell. 2000;101:689–692. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- 113.Benton C.R., Yoshida Y., Lally J., Han X.X., Hatta H., Bonen A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol. Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- 114.Takimoto M., Takeyama M., Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism. 2013;62:1633–1640. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Kitaoka Y., Takahashi Y., Machida M., Takeda K., Takemasa T., Hatta H. Effect of AMPK activation on monocarboxylate transporter (MCT)1 and MCT4 in denervated muscle. J. Physiol. Sci. 2014;64:59–64. doi: 10.1007/s12576-013-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galardo M.N., Riera M.F., Pellizzari E.H., Cigorraga S.B., Meroni S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-d-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 2007;39:279–288. doi: 10.1677/JME-07-0054. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y., Tonouchi M., Miskovic D., Hatta H., Bonen A. T3 increases lactate transport and the expression of MCT4, but not MCT1, in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003;285:E622–E628. doi: 10.1152/ajpendo.00069.2003. [DOI] [PubMed] [Google Scholar]
- 118.Asada K., Miyamoto K., Fukutomi T., Tsuda H., Yagi Y., Wakazono K., Oishi S., Fukui H., Sugimura T., Ushijima T. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology. 2003;64:380–388. doi: 10.1159/000070297. [DOI] [PubMed] [Google Scholar]
- 119.Boidot R., Végran F., Meulle A., Le Breton A., Dessy C., Sonveaux P., Lizard-Nacol S., Feron O. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72:939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 120.Matsuyama S., Ohkura S., Iwata K., Uenoyama Y., Tsukamura H., Maeda K., Kimura K. Food deprivation induces monocarboxylate transporter 2 expression in the brainstem of female rat. J. Reprod. Dev. 2009;55:256–261. doi: 10.1262/jrd.20214. [DOI] [PubMed] [Google Scholar]
- 121.Chenal J., Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J. Neurochem. 2007;102:389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- 122.Chenal J., Pierre K., Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur. J. Neurosci. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- 123.Robinet C., Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J. Cereb. Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pertega-Gomes N., Vizcaino J.R., Felisbino S., Warren A.Y., Shaw G., Kay J., Whitaker H., Lynch A.G., Fryer L., Neal D.E., Massie C.E. Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over-expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget. 2015;6:21675–21684. doi: 10.18632/oncotarget.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fisel P., Kruck S., Winter S., Bedke J., Hennenlotter J., Nies A.T., Scharpf M., Fend F., Stenzl A., Schwab M., Schaeffeler E. DNA methylation of the SLC16A3 promoter regulates expression of the human lactate transporter MCT4 in renal cancer with consequences for clinical outcome. Clin. Cancer Res. 2013;19:5170–5181. doi: 10.1158/1078-0432.CCR-13-1180. [DOI] [PubMed] [Google Scholar]
- 126.Johannsson E., Lunde P.K., Heddle C., Sjaastad I., Thomas M.J., Bergersen L., Halestrap A.P., Blackstad T.W., Ottersen O.P., Sejersted O.M. Upregulation of the cardiac monocarboxylate transporter MCT1 in a rat model of congestive heart failure. Circulation. 2001;104:729–734. doi: 10.1161/hc3201.092286. [DOI] [PubMed] [Google Scholar]
- 127.Poole R.C., Halestrap A.P. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J. Biol. Chem. 1997;272:14624–14628. doi: 10.1074/jbc.272.23.14624. [DOI] [PubMed] [Google Scholar]
- 128.Wilson M.C., Meredith D., Fox J.E., Manoharan C., Davies A.J., Halestrap A.P. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) J. Biol. Chem. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 129.Iacono K.T., Brown A.L., Greene M.I., Saouaf S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yurchenko V., Constant S., Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117:301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Philp N.J., Ochrietor J.D., Rudoy C., Muramatsu T., Linser P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5 A11/basigin-null mouse. Invest. Ophthalmol. Vis. Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 132.Kirk P., Wilson M.C., Heddle C., Brown M.H., Barclay A.N., Halestrap A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson M.C., Meredith D., Halestrap A.P. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 2002;277:3666–3672. doi: 10.1074/jbc.M109658200. [DOI] [PubMed] [Google Scholar]
- 134.Visser W.E., Philp N.J., van Dijk T.B., Klootwijk W., Friesema E.C., Jansen J., Beesley P.W., Ianculescu A.G., Visser T.J. Evidence for a homodimeric structure of human monocarboxylate transporter 8. Endocrinology. 2009;150:5163–5170. doi: 10.1210/en.2009-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Klier M., Schuler C., Halestrap A.P., Sly W.S., Deitmer J.W., Becker H.M. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J. Biol. Chem. 2011;286:27781–27791. doi: 10.1074/jbc.M111.255331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klier M., Andes F.T., Deitmer J.W., Becker H.M. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J. Biol. Chem. 2014;289:2765–2775. doi: 10.1074/jbc.M113.537043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hashimoto T., Hussien R., Brooks G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 138.Maekawa F., Tsuboi T., Fukuda M., Pellerin L. Regulation of the intracellular distribution, cell surface expression, and protein levels of AMPA receptor GluR2 subunits by the monocarboxylate transporter MCT2 in neuronal cells. J. Neurochem. 2009;109:1767–1778. doi: 10.1111/j.1471-4159.2009.06100.x. [DOI] [PubMed] [Google Scholar]
- 139.Wilson M.C., Kraus M., Marzban H., Sarna J.R., Wang Y., Hawkes R., Halestrap A.P., Beesley P.W. The neuroplastin adhesion molecules are accessory proteins that chaperone the monocarboxylate transporter MCT2 to the neuronal cell surface. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bittar P.G., Charnay Y., Pellerin L., Bouras C., Magistretti P.J. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J. Cereb. Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 141.Pellerin L., Pellegri G., Bittar P.G., Charnay Y., Bouras C., Martin J.L., Stella N., Magistretti P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 142.Pellerin L., Bouzier-Sore A.K., Aubert A., Serres S., Merle M., Costalat R., Magistretti P.J. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 143.Pellerin L. Brain energetics (thought needs food) Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:701–705. doi: 10.1097/MCO.0b013e328312c368. [DOI] [PubMed] [Google Scholar]
- 144.Pellerin L., Magistretti P.J. Glutamate uptake stimulates Na +,K + -ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J. Neurochem. 1997;69:2132–2137. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- 145.Abe K., Saito H. Involvement of Na + -K + pump in l-glutamate clearance by cultured rat cortical astrocytes. Biol. Pharm. Bull. 2000;23:1051–1054. doi: 10.1248/bpb.23.1051. [DOI] [PubMed] [Google Scholar]
- 146.Chatton J.Y., Marquet P., Magistretti P.J. A quantitative analysis of l-glutamate-regulated Na + dynamics in mouse cortical astrocytes: implications for cellular bioenergetics. Eur. J. Neurosci. 2000;12:3843–3853. doi: 10.1046/j.1460-9568.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- 147.Magistretti P.J., Chatton J.Y. Relationship between l-glutamate-regulated intracellular Na + dynamics and ATP hydrolysis in astrocytes. J. Neural Transm. 2005;112:77–85. doi: 10.1007/s00702-004-0171-6. [DOI] [PubMed] [Google Scholar]
- 148.Pellerin L., Magistretti P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pellerin L., Magistretti P.J. Excitatory amino acids stimulate aerobic glycolysis in astrocytes via an activation of the Na +/K + ATPase. Dev. Neurosci. 1996;18:336–342. doi: 10.1159/000111426. [DOI] [PubMed] [Google Scholar]
- 150.Maekawa F., Minehira K., Kadomatsu K., Pellerin L. Basal and stimulated lactate fluxes in primary cultures of astrocytes are differentially controlled by distinct proteins. J. Neurochem. 2008;107:789–798. doi: 10.1111/j.1471-4159.2008.05650.x. [DOI] [PubMed] [Google Scholar]
- 151.Rosafio K., Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1alpha-mediated transcriptional regulation. Glia. 2014;62:477–490. doi: 10.1002/glia.22618. [DOI] [PubMed] [Google Scholar]
- 152.Bittner C.X., Loaiza A., Ruminot I., Larenas V., Sotelo-Hitschfeld T., Gutierrez R., Cordova A., Valdebenito R., Frommer W.B., Barros L.F. High resolution measurement of the glycolytic rate. Front. Neuroenerg. 2010;2:26. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bittner C.X., Valdebenito R., Ruminot I., Loaiza A., Larenas V., Sotelo-Hitschfeld T., Moldenhauer H., San M.A., Gutierrez R., Zambrano M., Barros L.F. Fast and reversible stimulation of astrocytic glycolysis by K + and a delayed and persistent effect of glutamate. J. Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruminot I., Gutierrez R., Pena-Munzenmayer G., Anazco C., Sotelo-Hitschfeld T., Lerchundi R., Niemeyer M.I., Shull G.E., Barros L.F. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K + J. Neurosci. 2011;31:14264–14271. doi: 10.1523/JNEUROSCI.2310-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lerchundi R., Fernandez-Moncada I., Contreras-Baeza Y., Sotelo-Hitschfeld T., Machler P., Wyss M.T., Stobart J., Baeza-Lehnert F., Alegria K., Weber B., Barros L.F. NH4 + triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proc. Natl. Acad. Sci. U. S. A. 2015;112:11090–11095. doi: 10.1073/pnas.1508259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bouzier-Sore A.K., Voisin P., Canioni P., Magistretti P.J., Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J. Cereb. Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 157.Bouzier-Sore A.K., Voisin P., Bouchaud V., Bezancon E., Franconi J.M., Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur. J. Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 158.Serres S., Bouyer J.J., Bezancon E., Canioni P., Merle M. Involvement of brain lactate in neuronal metabolism. NMR Biomed. 2003;16:430–439. doi: 10.1002/nbm.838. [DOI] [PubMed] [Google Scholar]
- 159.Serres S., Bezancon E., Franconi J.M., Merle M. Ex vivo analysis of lactate and glucose metabolism in the rat brain under different states of depressed activity. J. Biol. Chem. 2004;279:47881–47889. doi: 10.1074/jbc.M409429200. [DOI] [PubMed] [Google Scholar]
- 160.Serres S., Bezancon E., Franconi J.M., Merle M. Ex vivo NMR study of lactate metabolism in rat brain under various depressed states. J. Neurosci. Res. 2005;79:19–25. doi: 10.1002/jnr.20277. [DOI] [PubMed] [Google Scholar]
- 161.Wyss M.T., Jolivet R., Buck A., Magistretti P.J., Weber B. In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pierre K., Debernardi R., Magistretti P.J., Pellerin L. Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. J. Neurochem. 2003;86:1468–1476. doi: 10.1046/j.1471-4159.2003.01964.x. [DOI] [PubMed] [Google Scholar]
- 163.Robinet C., Pellerin L. Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience. 2011;192:155–163. doi: 10.1016/j.neuroscience.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 164.Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J., Alberini C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Newman L.A., Korol D.L., Gold P.E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang J., Ruchti E., Petit J.M., Jourdain P., Grenningloh G., Allaman I., Magistretti P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morrison B.M., Lee Y., Rothstein J.D. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 2013;23:644–651. doi: 10.1016/j.tcb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu W., Huang J., Sun S., Huang S., Gan S., Xu J., Yang M., Xu S., Jiang X. Changes in lactate content and monocarboxylate transporter 2 expression in Abeta(2)(5)(−)(3)(5)-treated rat model of Alzheimer's disease. Neurol. Sci. 2015;36:871–876. doi: 10.1007/s10072-015-2087-3. [DOI] [PubMed] [Google Scholar]
- 169.Puchades M., Sogn C.J., Maehlen J., Bergersen L.H., Gundersen V. Unaltered lactate and glucose transporter levels in the MPTP mouse model of Parkinson's disease. J. Parkinsons Dis. 2013;3:371–385. doi: 10.3233/JPD-130190. [DOI] [PubMed] [Google Scholar]
- 170.Lauritzen F., de Lanerolle N.C., Lee T.S., Spencer D.D., Kim J.H., Bergersen L.H., Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol. Dis. 2011;41:577–584. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lauritzen F., Heuser K., de Lanerolle N.C., Lee T.S., Spencer D.D., Kim J.H., Gjedde A., Eid T., Bergersen L.H. Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia. 2012;60:1172–1181. doi: 10.1002/glia.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Leroy C., Pierre K., Simpson I.A., Pellerin L., Vannucci S.J., Nehlig A. Temporal changes in mRNA expression of the brain nutrient transporters in the lithium-pilocarpine model of epilepsy in the immature and adult rat. Neurobiol. Dis. 2011;43:588–597. doi: 10.1016/j.nbd.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lauritzen F., Perez E.L., Melillo E.R., Roh J.M., Zaveri H.P., Lee T.S., Wang Y., Bergersen L.H., Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol. Dis. 2012;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu B., Niu L., Shen M.Z., Gao L., Wang C., Li J., Song L.J., Tao Y., Meng Q., Yang Q.L., Gao G.D., Zhang H. Decreased astroglial monocarboxylate transporter 4 expression in temporal lobe epilepsy. Mol. Neurobiol. 2014;50:327–338. doi: 10.1007/s12035-013-8619-z. [DOI] [PubMed] [Google Scholar]
- 175.Tseng M.T., Chan S.A., Schurr A. Ischemia-induced changes in monocarboxylate transporter 1 reactive cells in rat hippocampus. Neurol. Res. 2003;25:83–86. doi: 10.1179/016164103101200978. [DOI] [PubMed] [Google Scholar]
- 176.Gao C., Zhou L., Zhu W., Wang H., Wang R., He Y., Li Z. Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures. Neurosci. Lett. 2015;594:99–104. doi: 10.1016/j.neulet.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 177.Gao C., Wang C., Liu B., Wu H., Yang Q., Jin J., Li H., Dong S., Gao G., Zhang H. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem. Res. 2014;39:2160–2169. doi: 10.1007/s11064-014-1411-2. [DOI] [PubMed] [Google Scholar]
- 178.Vavaiya K.V., Paranjape S.A., Patil G.D., Briski K.P. Vagal complex monocarboxylate transporter-2 expression during hypoglycemia. Neuroreport. 2006;17:1023–1026. doi: 10.1097/01.wnr.0000224766.07702.51. [DOI] [PubMed] [Google Scholar]
- 179.Vavaiya K.V., Paranjape S.A., Briski K.P. Testicular regulation of neuronal glucose and monocarboxylate transporter gene expression profiles in CNS metabolic sensing sites during acute and recurrent insulin-induced hypoglycemia. J. Mol. Neurosci. 2007;31:37–46. doi: 10.1007/BF02686116. [DOI] [PubMed] [Google Scholar]
- 180.Briski K.P., Cherian A.K., Genabai N.K., Vavaiya K.V. In situ coexpression of glucose and monocarboxylate transporter mRNAs in metabolic-sensitive caudal dorsal vagal complex catecholaminergic neurons: transcriptional reactivity to insulin-induced hypoglycemia and caudal hindbrain glucose or lactate repletion during insulin-induced hypoglycemia. Neuroscience. 2009;164:1152–1160. doi: 10.1016/j.neuroscience.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 181.Canis M., Maurer M.H., Kuschinsky W., Duembgen L., Duelli R. Increased densities of monocarboxylate transporter MCT1 after chronic hyperglycemia in rat brain. Brain Res. 2009;1257:32–39. doi: 10.1016/j.brainres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Allard C., Carneiro L., Collins S.C., Chretien C., Grall S., Penicaud L., Leloup C. Alteration of hypothalamic glucose and lactate sensing in 48 h hyperglycemic rats. Neurosci. Lett. 2013;534:75–79. doi: 10.1016/j.neulet.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 183.Pierre K., Parent A., Jayet P.Y., Halestrap A.P., Scherrer U., Pellerin L. Enhanced expression of three monocarboxylate transporter isoforms in the brain of obese mice. J. Physiol. 2007;583:469–486. doi: 10.1113/jphysiol.2007.138594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lengacher S., Nehiri-Sitayeb T., Steiner N., Carneiro L., Favrod C., Preitner F., Thorens B., Stehle J.C., Dix L., Pralong F., Magistretti P.J., Pellerin L. Resistance to diet-induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kennedy K.M., Dewhirst M.W. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–148. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pinheiro C., Longatto-Filho A., Azevedo-Silva J., Casal M., Schmitt F.C., Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J. Bioenerg. Biomembr. 2012;44:127–139. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 187.Miranda-Goncalves V., Honavar M., Pinheiro C., Martinho O., Pires M.M., Pinheiro C., Cordeiro M., Bebiano G., Costa P., Palmeirim I., Reis R.M., Baltazar F. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro-Oncol. 2013;15:172–188. doi: 10.1093/neuonc/nos298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Afonso J., Santos L.L., Miranda-Goncalves V., Morais A., Amaro T., Longatto-Filho A., Baltazar F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol. Carcinog. 2015;54:1451–1466. doi: 10.1002/mc.22222. [DOI] [PubMed] [Google Scholar]
- 189.Pertega-Gomes N., Baltazar F. Lactate transporters in the context of prostate cancer metabolism: what do we know? Int. J. Mol. Sci. 2014;15:18333–18348. doi: 10.3390/ijms151018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 191.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 193.Porporato P.E., Dhup S., Dadhich R.K., Copetti T., Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Payen V.L., Brisson L., Dewhirst M.W., Sonveaux P. Common responses of tumors and wounds to hypoxia. Cancer J. 2015;21:75–87. doi: 10.1097/PPO.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 195.Spugnini E.P., Sonveaux P., Stock C., Perez-Sayans M., De M.A., Avnet S., Garcia A.G., Harguindey S., Fais S. Proton channels and exchangers in cancer. Biochim. Biophys. Acta. 2015;1848:2715–2726. doi: 10.1016/j.bbamem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 196.Brizel D.M., Schroeder T., Scher R.L., Walenta S., Clough R.W., Dewhirst M.W., Mueller-Klieser W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 197.Schwickert G., Walenta S., Sundfor K., Rofstad E.K., Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55:4757–4759. [PubMed] [Google Scholar]
- 198.Walenta S., Salameh A., Lyng H., Evensen J.F., Mitze M., Rofstad E.K., Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am. J. Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- 199.Walenta S., Snyder S., Haroon Z.A., Braun R.D., Amin K., Brizel D., Mueller-Klieser W., Chance B., Dewhirst M.W. Tissue gradients of energy metabolites mirror oxygen tension gradients in a rat mammary carcinoma model. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:840–848. doi: 10.1016/s0360-3016(01)01700-x. [DOI] [PubMed] [Google Scholar]
- 200.Walenta S., Chau T.V., Schroeder T., Lehr H.A., Kunz-Schughart L.A., Fuerst A., Mueller-Klieser W. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J. Cancer Res. Clin. Oncol. 2003;129:321–326. doi: 10.1007/s00432-003-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walenta S., Schroeder T., Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr. Med. Chem. 2004;11:2195–2204. doi: 10.2174/0929867043364711. [DOI] [PubMed] [Google Scholar]
- 202.Walenta S., Mueller-Klieser W.F. Lactate: mirror and motor of tumor malignancy. Semin. Radiat. Oncol. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 203.Corbet C., Draoui N., Polet F., Pinto A., Drozak X., Riant O., Feron O. The SIRT1/HIF2alpha axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014;74:5507–5519. doi: 10.1158/0008-5472.CAN-14-0705. [DOI] [PubMed] [Google Scholar]
- 204.Semenza G.L. Tumor metabolism: cancer cells give and take lactate. J. Clin. Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 206.Galeffi F., Turner D.A. Exploiting metabolic differences in glioma therapy. Curr. Drug Discov. Technol. 2012;9:280–293. doi: 10.2174/157016312803305906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.De Saedeleer C.J., Copetti T., Porporato P.E., Verrax J., Feron O., Sonveaux P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.De Saedeleer C.J., Porporato P.E., Copetti T., Perez-Escuredo J., Payen V.L., Brisson L., Feron O., Sonveaux P. Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene. 2014;33:4060–4068. doi: 10.1038/onc.2013.454. [DOI] [PubMed] [Google Scholar]
- 209.Lu H., Forbes R.A., Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 210.Lu H., Dalgard C.L., Mohyeldin A., McFate T., Tait A.S., Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 211.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Izumi H., Takahashi M., Uramoto H., Nakayama Y., Oyama T., Wang K.Y., Sasaguri Y., Nishizawa S., Kohno K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102:1007–1013. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 213.Sonveaux P., Copetti T., De Saedeleer C.J., Végran F., Verrax J., Kennedy K.M., Moon E.J., Dhup S., Danhier P., Frérart F., Gallez B., Ribeiro A., Michiels C., Dewhirst M.W., Feron O. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Végran F., Boidot R., Michiels C., Sonveaux P., Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 215.Végran F., Seront E., Sonveaux P., Feron O. Lactate-induced IL-8 pathway in endothelial cells—response. Cancer Res. 2012;72:1903–1904. doi: 10.1158/0008-5472.CAN-11-1540. [DOI] [PubMed] [Google Scholar]
- 216.Porporato P.E., Payen V.L., De Saedeleer C.J., Préat V., Thissen J.P., Feron O., Sonveaux P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15:581–592. doi: 10.1007/s10456-012-9282-0. [DOI] [PubMed] [Google Scholar]
- 217.Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A.K., Frank P.G., Casimiro M.C., Wang C., Fortina P., Addya S., Pestell R.G., Martinez-Outschoorn U.E., Sotgia F., Lisanti M.P. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 218.Whitaker-Menezes D., Martinez-Outschoorn U.E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A.K., Birbe R.C., Howell A., Pavlides S., Gandara R., Pestell R.G., Sotgia F., Philp N.J., Lisanti M.P. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Martinez-Outschoorn U.E., Curry J.M., Ko Y.H., Lin Z., Tuluc M., Cognetti D., Birbe R.C., Pribitkin E., Bombonati A., Pestell R.G., Howell A., Sotgia F., Lisanti M.P. Oncogenes and inflammation rewire host energy metabolism in the tumor microenvironment: RAS and NFkappaB target stromal MCT4. Cell Cycle. 2013;12:2580–2597. doi: 10.4161/cc.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U.E., Flomenberg N., Birbe R.C., Witkiewicz A.K., Howell A., Philp N.J., Pestell R.G., Lisanti M.P. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11:1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Whitaker-Menezes D., Martinez-Outschoorn U.E., Flomenberg N., Birbe R.C., Witkiewicz A.K., Howell A., Pavlides S., Tsirigos A., Ertel A., Pestell R.G., Broda P., Minetti C., Lisanti M.P., Sotgia F. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10:4047–4064. doi: 10.4161/cc.10.23.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Witkiewicz A.K., Dasgupta A., Sotgia F., Mercier I., Pestell R.G., Sabel M., Kleer C.G., Brody J.R., Lisanti M.P. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am. J. Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Witkiewicz A.K., Whitaker-Menezes D., Dasgupta A., Philp N.J., Lin Z., Gandara R., Sneddon S., Martinez-Outschoorn U.E., Sotgia F., Lisanti M.P. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–1117. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Curry J.M., Tuluc M., Whitaker-Menezes D., Ames J.A., Anantharaman A., Butera A., Leiby B., Cognetti D.M., Sotgia F., Lisanti M.P., Martinez-Outschoorn U.E. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–1384. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Giatromanolaki A., Koukourakis M.I., Koutsopoulos A., Mendrinos S., Sivridis E. The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. Cancer Biol. Ther. 2012;13:1284–1289. doi: 10.4161/cbt.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Koukourakis M.I., Giatromanolaki A., Bougioukas G., Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biol. Ther. 2007;6:1476–1479. doi: 10.4161/cbt.6.9.4635. [DOI] [PubMed] [Google Scholar]
- 227.Hirt C., Papadimitropoulos A., Mele V., Muraro M.G., Mengus C., Iezzi G., Terracciano L., Martin I., Spagnoli G.C. “In vitro” 3D models of tumor-immune system interaction. Adv. Drug Deliv. Rev. 2014;79-80:145–154. doi: 10.1016/j.addr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 228.Singer K., Gottfried E., Kreutz M., Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol. Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Puig-Kroger A., Pello O.M., Selgas R., Criado G., Bajo M.A., Sanchez-Tomero J.A., Alvarez V., del Peso G., Sanchez-Mateos P., Holmes C., Faict D., Lopez-Cabrera M., Madrenas J., Corbi A.L. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J. Leukoc. Biol. 2003;73:482–492. doi: 10.1189/jlb.0902451. [DOI] [PubMed] [Google Scholar]
- 230.Nasi A., Fekete T., Krishnamurthy A., Snowden S., Rajnavolgyi E., Catrina A.I., Wheelock C.E., Vivar N., Rethi B. Dendritic cell reprogramming by endogenously produced lactic acid. J. Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 231.Murray C.M., Hutchinson R., Bantick J.R., Belfield G.P., Benjamin A.D., Brazma D., Bundick R.V., Cook I.D., Craggs R.I., Edwards S., Evans L.R., Harrison R., Holness E., Jackson A.P., Jackson C.G., Kingston L.P., Perry M.W., Ross A.R., Rugman P.A., Sidhu S.S., Sullivan M., Taylor-Fishwick D.A., Walker P.C., Whitehead Y.M., Wilkinson D.J., Wright A., Donald D.K. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 232.Bueno V., Binet I., Steger U., Bundick R., Ferguson D., Murray C., Donald D., Wood K. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 233.Ekberg H., Qi Z., Pahlman C., Veress B., Bundick R.V., Craggs R.I., Holness E., Edwards S., Murray C.M., Ferguson D., Kerry P.J., Wilson E., Donald D.K. The specific monocarboxylate transporter-1 (MCT-1) inhibitor, AR-C117977, induces donor-specific suppression, reducing acute and chronic allograft rejection in the rat. Transplantation. 2007;84:1191–1199. doi: 10.1097/01.tp.0000287541.53389.be. [DOI] [PubMed] [Google Scholar]
- 234.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., Renner K., Timischl B., Mackensen A., Kunz-Schughart L., Andreesen R., Krause S.W., Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 235.Mendler A.N., Hu B., Prinz P.U., Kreutz M., Gottfried E., Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 236.Husain Z., Huang Y., Seth P., Sukhatme V.P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 237.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., Cline G.W., Phillips A.J., Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brooks G.A. Lactate shuttles in nature. Biochem. Soc. Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 239.Gladden L.B. Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Brooks G.A. The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc. 1986;18:360–368. doi: 10.1249/00005768-198606000-00019. [DOI] [PubMed] [Google Scholar]
- 241.Mita M., Hall P.F. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol. Reprod. 1982;26:445–455. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- 242.Mosconi L. Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin. Transl. Imaging. 2013;1 doi: 10.1007/s40336-013-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sapolsky R.M. Neuroprotective gene therapy against acute neurological insults. Nat. Rev. Neurosci. 2003;4:61–69. doi: 10.1038/nrn1006. [DOI] [PubMed] [Google Scholar]
- 244.Demetrius L.A., Magistretti P.J., Pellerin L. Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect. Front. Physiol. 2014;5:522. doi: 10.3389/fphys.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mathupala S.P., Parajuli P., Sloan A.E. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. [DOI] [PubMed] [Google Scholar]
- 246.Gallagher S.M., Castorino J.J., Wang D., Philp N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 247.Doherty J.R., Yang C., Scott K.E., Cameron M.D., Fallahi M., Li W., Hall M.A., Amelio A.L., Mishra J.K., Li F., Tortosa M., Genau H.M., Rounbehler R.J., Lu Y., Dang C.V., Kumar K.G., Butler A.A., Bannister T.D., Hooper A.T., Unsal-Kacmaz K., Roush W.R., Cleveland J.L. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014;74:908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Le Floch R., Chiche J., Marchiq I., Naiken T., Ilk K., Murray C.M., Critchlow S.E., Roux D., Simon M.P., Pouyssegur J. CD147 subunit of lactate/H + symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]