Abstract
Cannabis use has been increasingly accepted legally and in public opinion. However, cannabis has the potential to produce adverse physical and mental health effects and can result in cannabis use disorder (CUD) in a substantial percentage of both occasional and daily cannabis users. Many people have difficulty discontinuing use. Therefore, it would be beneficial to develop safe and effective medications for treating CUD. To achieve this, methods have been developed for screening and evaluating potential medications using animal models and controlled experimental protocols in human volunteers. In this chapter we describe: 1) animal models available for assessing the effect of potential medications on specific aspects of CUD; 2) the main findings obtained so far with these animal models; 3) the approaches used to assess potential medications in humans in laboratory experiments and clinical trials; and 4) the effectiveness of several potential pharmacotherapies on the particular aspects of CUD modeled in these human studies.
Keywords: THC, marijuana, cannabinoids, spice drugs, withdrawal, relapse, animal models, clinical trials
1. Introduction
Cannabis has long been used for medicinal and recreational purposes, and both of these uses are becoming increasingly accepted legally and in public opinion. However, these societal trends should not obscure the fact that —like other medicinal/recreational drugs— cannabis has the potential to produce adverse effects on physical and mental health. It should also be recognized that currently-available strains of high-potency cannabis and synthetic cannabinoid “spice” drugs can have adverse effects that are substantially worse than those associated with traditional strains of marijuana.
Cannabis-derived materials such as marijuana and hashish contain a large number of chemical constituents, but the abuse-related euphorigenic effects result from the actions of THC on CB1 receptors in the brain (Le Foll & Goldberg, 2005; Williams and Kirkham, 2002). Like other drugs of abuse including opioids and psychostimulants, THC and synthetic cannabinoids increase dopamine signaling in neurons projecting from the ventral tegmental area to the nucleus accumbens and prefrontal cortex (Oleson & Cheer, 2012). These effects on dopamine are believed to underlie the rewarding/reinforcing effects that induce users to repeatedly seek out and take the drug.
Cannabis, THC and synthetic cannabinoids can also produce adverse emotional effects (anxiety, panic), cognitive impairment (amnesia, difficulty concentrating), schizophrenia-like effects (paranoia, delusions) and cardiovascular effects (hypertension, tachycardia) (Hall, 2015; Karila et al., 2014; Panlilio, Goldberg, & Justinova, 2015). Cannabis smoking also has adverse effects on the respiratory system (Gates, Jaffe, & Copeland, 2014). Synthetic cannabinoids are typically more potent than THC (Gates et al., 2014) and have stronger effects at the CB1 receptor because they are full agonists, unlike THC, which is only a partial agonist (Fantegrossi, Moran, Radominska-Pandya, & Prather, 2014). Furthermore, the ingredients and doses in spice drug preparations are highly variable, and these preparations have been associated with large numbers of emergency room visits.
In addition to these direct adverse effects, cannabis can have indirect adverse effects associated with its illegality and the fact that many employers do not tolerate its use. These many direct and indirect adverse effects exert a limiting effect on cannabis use, and in many cases may be sufficient to lead to abstinence. As with other drugs of abuse (Heyman, 2009), it is likely that most users either control or discontinue their cannabis use without the aid of psychosocial or pharmacologic intervention. However, many individuals have difficulty controlling their use. Cannabis use disorder (CUD) is recognized in DSM-5 (American Psychiatric Association, 2013) and diagnosed in terms that mirror those of other substance use disorders, including inability to curtail use despite a desire to do so, continued use despite the consequent loss of important social, occupational and recreational activities, and showing symptoms of drug craving, tolerance and withdrawal. As of 2010, over 760,000 people per year were being treated specifically for cannabis use in the United States (Batts et al., 2014). On average, individuals seek treatment after more than 10 years of daily use and more than 6 serious attempts to quit (Budney, Roffman, Stephens, & Walker, 2007).
There are currently no medications approved specifically for treating CUD. The purpose of this chapter is to review basic methods that are used to evaluate compounds that might be developed into such medications. Animal models are useful for understanding the basic neurobiology that underlies the effect of cannabinoid, and for screening novel drugs for potentially therapeutic effects. Controlled studies in human volunteers are critical for determining whether a medication can be effective. So far, most of this research in humans has involved testing medications that are already approved for other purposes, including pharmaceutical formulations of THC, which might function as a replacement therapy analogous to methadone as a treatment for heroin addiction. The goals of this human and animal research are to provide treatments that can help decrease or stop cannabis use, reduce withdrawal symptoms, and prevent craving and relapse.
2. Animal models
Most animal research relevant to medications development for the treatment of CUD has been conducted in rats, mice and squirrel monkeys. In general, the basic mechanisms and functions of the central nervous system in rodents and nonhuman primates are similar enough to humans to provide useful information concerning the effects of drugs on human behavior. Consequently, animal models have been indispensable to the fields of psychopharmacology and behavioral neuroscience. However, there are obviously differences in behavior across species, and the differences between rodents and primates happen to be more striking with respect to the rewarding effects of cannabinoids that they are with other drugs of abuse. That is, rats will self-administer most of the drugs that are abused by humans, but they do not show robust self-administration of THC. The reasons for this difference are not clear, but rodents still play an important role in cannabinoid research because they do self-administer the synthetic cannabinoid WIN 55,212-2 (Fattore, Cossu, Martellotta, & Fratta, 2001; Lefever, Marusich, Antonazzo, & Wiley, 2014; Mendizabal, Zimmer, & Maldonado, 2006), and they also display other abuse-relevant effects of THC. In contrast with rats, squirrel monkeys readily self-administer THC and other cannabinoids (Justinova, Solinas, Tanda, Redhi, & Goldberg, 2005; Justinova, Tanda, Redhi, & Goldberg, 2003; Justinova, Yasar, Redhi, & Goldberg, 2011; Tanda, Munzar, & Goldberg, 2000), and we believe this behavior represents the best available model of human cannabis use for testing potential medications (Panlilio, Justinova, & Goldberg, 2010).
Drug discrimination procedures are used to model the subjective, perceived effects of drugs (Solinas, Panlilio, Justinova, Yasar, & Goldberg, 2006). In a typical procedure, animals (usually rats) are trained to detect the effects of a drug. They are given an injection before each daily training session, during which they can obtain food pellets by pressing one of two levers. Some days the injection contains THC, and some days it contains only vehicle (placebo), and the type of injection signals which of the two levers produces food on that day. For example, a rat might always receive food for pressing the left lever on THC days and the right lever on vehicle days. Once this discrimination has been established, tests can be performed with novel compounds to determine whether they block, enhance, or mimic the effects of THC. Although it cannot be determined exactly what aspects of the drug effect the rat is responsive to, this type of procedure can provide reliable information about the pharmacological specificity of a drug for receptor classes and subtypes, and whether a test compound can block or mimic the effects of THC. Training for this procedure can take weeks or months, but well-trained rats can be tested repeatedly, which is ideal for screening novel compounds.
Place-conditioning procedures are typically used as a relatively simple means of studying the rewarding effects of drugs in rodents. Training takes place in an apparatus with two distinctive compartments. During training, one of the compartments is paired with the effects of THC or another cannabinoid agonist by injecting the rat before confining it to the compartment. The other compartment is paired with vehicle in a similar manner. During a test, the rat is given access to both compartments, and the relative amount of time spent in each compartment is measured. If the drug has a rewarding effect, this typically imparts a conditioned rewarding effect to the drug-paired compartment through Pavlovian conditioning, and the rat spends more time there (i.e., exhibits a conditioned place preference). In contrast, if the drug has aversive effects, the rat avoids the drug-paired compartment and spends more time in the vehicle-paired compartment. In most studies with cannabinoid agonists, rats have shown either conditioned place avoidance or no clear preference for either compartment (Cheer, Kendall, & Marsden, 2000; Klein et al., 2011; Polissidis et al., 2009; Robinson, Hinder, Pertwee, & Riedel, 2003; Vlachou, Nomikos, Stephens, & Panagis, 2007). This suggests that cannabinoid reward is weak in rats, or that it is masked by aversive effects of THC (e.g., anxiety).
Drug self-administration procedures provide the most direct evidence of rewarding effects of a drug (Panlilio & Goldberg, 2007). Typically, a rat or monkey is given an intravenous catheter and allowed to press a lever that activates a syringe pump that delivers a drug through the catheter. Squirrel monkeys self-administer THC at rates similar to the rates at which they self-administer cocaine, methamphetamine and nicotine (Justinova, Ferre, et al., 2011; Justinova et al., 2015; Panlilio et al., 2015; Schindler et al., 2010). In many ways, the basic drug self-administration procedure is a close analog of human drug use, modeling the contingencies that critically influence drug-seeking behavior. For example, in both the drug abuse environment and the animal model, drug-seeking can be induced by environmental cues that signal the availability of a drug, and it can be maintained over time by presenting cues that have been associated with the effects of the drug. Thus, the basic drug self-administration procedure can be modified to study treatments that might have therapeutic treatment on specific phases of addiction. In the study of potential treatments for CUD, four such procedures have been used with THC self-administration in squirrel monkeys: maintenance tests, drug-induced reinstatement, cue-induced reinstatement and second-order schedules.
Maintenance tests involve establishing a stable baseline level of THC self-administration, then testing the effects of a treatment drug on this baseline. Except for the treatment, all other aspects of the baseline and test sessions are held constant, including response requirements, drug delivery and cue presentations. Typically, baseline behavior is recorded for at least a week, then the treatment is given for five consecutive sessions, followed by a return to baseline. This procedure is designed to assess the potential of a treatment for decreasing ongoing cannabis use, and to determine whether the effects are consistent over time (i.e., whether an effect is immediately apparent or builds over time, and whether it is continues to occur with extended treatment).
Drug-induced reinstatement and cue-induced reinstatement procedures are used to model the effects of a treatment on relapse to drug use after a period of abstinence (Bossert, Marchant, Calu, & Shaham, 2013). In humans, relapse can be triggered by re-exposure to the drug, to cues that signal the availability of the drug, or to cues that have been paired with effects of the drug. In both the drug-induced and cue-induced and reinstatement procedures used with squirrel monkeys, extinction is used to impose abstinence; that is, drug delivery is discontinued. However, the two procedures differ with respect to the effect of lever responding during the extinction phase. When imposing abstinence prior to a drug-induced reinstatement test, vehicle is simply substituted for THC (i.e., the usual cue signals the availability of an injection, and responding produces the usual visual cues as well as any interoceptive cues from intravenous injection). In contrast, when imposing abstinence prior to a cue-induced reinstatement test, the visual cue previously associated with drug availability is not presented, and responding has no programmed effect (i.e., it does not produce cues or injections). Under both of these extinction procedures, responding decreases to a very low level after a few sessions, at which point a reinstatement test can conducted. For drug-induced reinstatement, the only procedural difference between the extinction phase and the test session is that the monkey is given an automatic intravenous injection of THC just before the test session. This re-exposure to THC typically has a priming effect, causing lever responding to increase substantially. For cue-induced reinstatement, no THC injection is given before the test, but the normal cues are presented and responding produces vehicle injections during the session. Like drug priming, re-exposure to the cues that were previously associated with THC typically causes a relapse-like increase in the drug-seeking response. To screen potential medications for CUD, the test drug is given before the drug-induced or cue-induced reinstatement session to see if it will prevent the effects of drug or cue re-exposure. Potential medications are also tested alone to determine whether they might have effects of their own that are liable to induce relapse.
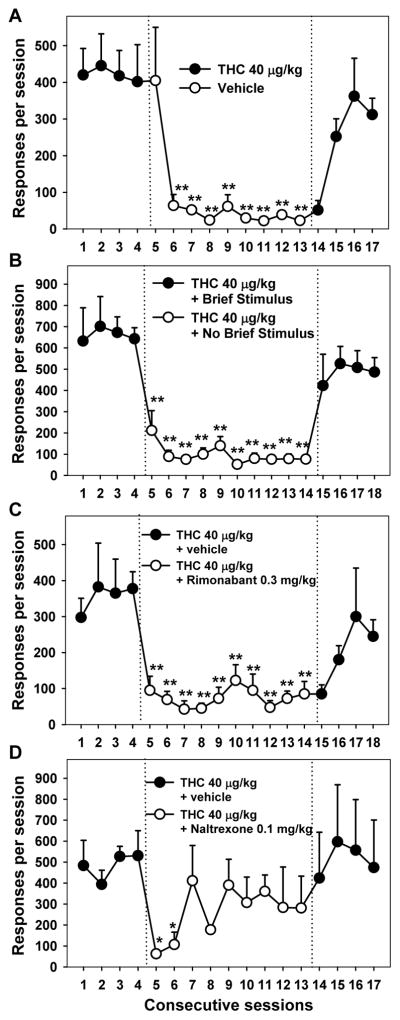
Second-order schedules of drug self-administration are used to model the long sequences of behavior that are involved in obtaining, preparing and ingesting drugs (Schindler, Katz, & Goldberg, 1988). In the second-order schedule that has been used with THC self-administration (Justinova et al., 2008), squirrel monkeys press a lever that produces brief (2 second) presentations of a cue light for every tenth response. After 30 minutes has elapsed since the beginning of the session, the next cue light that is presented is extended to 90 seconds, during which 10 injections of THC are delivered intravenously. Thus, all THC is delivered at the end of the session, and responding during the session is not directly influenced by the pharmacological effects of THC, and responding during the session can be described specifically as THC seeking. This drug seeking is maintained at high rates because it produces the brief stimulus, and the stimulus has this effect because it is associated with the drug. The effects of this end-of-session THC delivery are clear because: 1) when the drug is not delivered, responding decreases substantially in the next session; and 2) when THC delivery is resumed, responding resumes in the next session (see Figure 1A). The powerful effects of the brief stimulus are clear from the fact that response rates are about six times higher under baseline conditions compared to when the brief stimulus is not presented (see Figure 1B). Thus, with the monkey receiving no THC until to the end of the session, it can be determined whether a pretreatment drug specifically affects drug seeking (i.e., the motivation to receive the drug), as opposed to altering the effects of THC after it is received, such as in a maintenance test.
Figure 1.
Effects of pharmacological treatments and contingency-based environmental manipulations on THC seeking in squirrel monkeys. A second-order schedule was used to model the environmental cues that maintain cannabis-seeking behavior in humans. Under baseline conditions (the first 4 days in each panel), lever responding produced a brief stimulus presentation (2 s of amber cue light) for every 30th response. The first stimulus presentation that occurred at least 30 min after the start of the session was accompanied by THC (40 mcg/kg, IP), after which the session ended. (A) When vehicle was substituted for THC (sessions 5–13), the rate of the THC-seeking response decreased to a low level, and when THC delivery was reinstituted (sessions 14–17), responding resumed at a high rate. Note that the effects of these manipulations were evident in the second session of each condition, because injection occurred only at the very end of the session. (B) When presentation of the brief stimulus was discontinued (sessions 5–14), there was an immediate and sustained decrease in the THC-seeking response, even though THC was still delivered at the end of the session. This demonstrates the important role of environmental cues in maintaining THC-seeking behavior when long sequences of responding are required to obtain the drug. (C) When the CB1-receptor antagonist/inverse-agonist rimonabant (0.3 mg/kg, IM) was given prior to the daily session (with cues presented and THC delivered as under the baseline conditions), there was an immediate and sustained decrease in THC seeking (sessions 5–14). The immediacy of this effect suggests that rimonabant decreased the effectiveness of the brief stimulus in addition to blocking the rewarding effects of THC. Drug seeking gradually resumed when rimonabant treatment was discontinued (sessions 15–18). (D) When the μ-opioid antagonist naltrexone (0.1 mg/kg, IM) was given before each daily session under maintenance conditions, THC seeking was immediately decreased, but this effect was diminished after the second day. This suggests that manipulation of opioid receptors can affect THC seeking, but that the effects might be less robust than those of direct manipulation of CB1 receptors. Asterisks indicate statistically significant differences between treatment and baseline (*p < 0.05, ** p < 0.01). Figure adapted from Justinova, Z., Munzar, P., Panlilio, L. V., Yasar, S., Redhi, G. H., Tanda, G., & Goldberg, S. R. (2008). Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology, 33(12), 2870–2877. doi:10.1038/npp.2008.21 with permission.
Withdrawal symptoms can occur when heavy, prolonged cannabis use is discontinued (Lee et al., 2014). DSM-5 diagnostic criteria for cannabis withdrawal include symptoms such as irritability, anxiety, insomnia, decreased appetite and depressed mood. Avoidance of such symptoms might be one factor that contributes to the persistence of chronic cannabis use, and medications that relieve such symptoms might be helpful for people attempting to quit cannabis smoking. In animals, cannabis withdrawal is usually studied in rodents. However, simply discontinuing regular THC administration in animals does not produce prominent, easily observable symptoms. Therefore, withdrawal is usually precipitated by administering a cannabinoid antagonist, which rapidly blocks CB1 receptors even if there is still THC in the system (Lichtman, Fisher, & Martin, 2001; Tai et al., 2015). Precipitated withdrawal in rodents produces symptoms such as scratching, face rubbing, licking and wet-dog shakes.
3. Findings from research with animal models
The fact that there are large numbers of cannabis users who have difficulty controlling their use and who seek treatment is not well-recognized by the public. Even among researchers, the possibility of developing medications to treat CUD has received little attention compared to medications for psychostimulant, opioid, nicotine or alcohol use disorders. This lack of attention stems at least in part from the fact that the adverse effects of these other drugs tend to be more devastating than those of cannabis, to both the user and to society. But, it also stems from the fact that cannabinoid self-administration procedures are relatively difficult to implement and have only been established in a few laboratories. Nonetheless, the studies conducted so far have identified some pharmacologic strategies that might eventually lead to approved medications.
The most straightforward approach to preventing the rewarding effects of cannabis would be to administer a CB1 receptor antagonist. This would be analogous to opioid antagonist therapy as a treatment for heroin addiction. The CB1 antagonist rimonabant blocks THC self-administration in squirrel monkeys in maintenance tests and second-order schedules (see Figure 1C) (Justinova et al., 2008; Tanda et al., 2000), and it also blocks the rewarding effects of WIN 55,212-2 in rodents (Fattore et al., 2001; Lefever et al., 2014; Martellotta, Cossu, Fattore, Gessa, & Fratta, 1998). CB1 antagonist treatment also blocks the effects of THC in drug discrimination procedures with rats and monkeys (Wiley, Lowe, Balster, & Martin, 1995). Another potentially advantage of CB1 antagonists is that they decrease both drug-induced and cue-induced reinstatement in squirrel monkeys (Justinova et al., 2008), which could be a highly valuable property given that relapse to drug use is a substantial impediment to most therapeutic interventions. Cannabinoid antagonists also decrease self-administration and cue-induced reinstatement of drug seeking for some non-cannabinoid drugs (De Vries & Schoffelmeer, 2005; Maccioni, Colombo, & Carai, 2010; Schindler et al., 2010) and might therefore be beneficial for the treatment of polydrug abuse. One drawback to this treatment strategy is that CB1 antagonists could precipitate withdrawal if taken while a person is cannabinoid dependent. It should be noted that rimonabant has not only antagonist effects (blocking the effects of THC at the CB1 receptor), but also inverse agonist effects (producing effects opposite to those of THC). These inverse agonist properties might be responsible for the depression-like side effects that have been associated with rimonabant (Le Foll, Gorelick, & Goldberg, 2009). Neutral antagonists of CB1 can presumably block the effects of THC effectively and precipitate withdrawal (Tai et al., 2015), but might not produce depression-like effects in humans (Bergman et al., 2008).
Another general approach to treating CUD involves manipulating non-cannabinoid transmitter systems that interact with the endocannabinoid system. This strategy has been applied with squirrel monkey THC self-administration procedures to investigate drugs that affect opioid, adenosine, and acetylcholine receptors. It is well established that the cannabinoid and opioid systems of the brain interact, and there is evidence from animal models suggesting that mu-opioid receptor antagonists might decrease the rewarding effects of cannabis. In squirrel monkeys, the opioid antagonist naltrexone decreased THC self-administration in maintenance tests with a simple fixed-ratio schedule, in which THC was delivered for every 30th response (Justinova, Tanda, Munzar, & Goldberg, 2004), and under the second-order schedule described in the section above (Justinova et al., 2008). However, even though this effect of naltrexone was consistent when tested for five full days of testing under the simple fixed-ratio schedule, the effect only lasted for about two days under the second-order schedule, in which high rates of drug seeking are maintained by the presentation of environmental cues (see Figure 1D),. These findings suggest that an opioid antagonist would probably not be as effective as a cannabinoid antagonist at decreasing cannabinoid use, perhaps because they decrease the rewarding effects of THC but do not alter the effects of THC-associated cues.
The endogenous cannabinoid system is also influenced by adenosine A2A receptors, which are known to form heteromeric complexes with CB1 receptors. Two studies were conducted looking at the effects of A2A antagonists on THC self-administration in squirrel monkeys ((Justinova, Ferre, et al., 2011) (Justinova, Redhi, Goldberg, & Ferre, 2014). These studies showed that selective antagonism of presynaptic A2A receptors can block the rewarding effects of THC, while selective antagonism of postsynaptic receptors (or nonselective antagonism) has the opposite effect, increasing the rewarding effects of THC. These findings suggest that selectively presynaptic A2A receptors should be further developed as candidate medications for CUD.
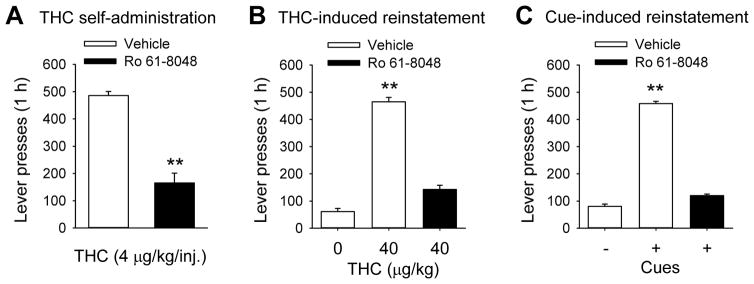
Like A2A antagonists, α7 nicotinic acetylcholine receptor antagonists such as methyllycaconitine can decrease corticostriatal glutamate signaling, which might be an important component of cannabinoid reward circuitry (Solinas et al., 2007). Methyllycaconitine blocked the interoceptive effects of THC in a drug discrimination procedure and blocked THC’s ability to increase dopamine levels in the shell of the nucleus accumbens in rats (Solinas et al., 2007). Since direct orthosteric α7 antagonists, such as methyllycaconitine, tend to have adverse side effects, Justinova et al. (2013) studied the effects of Ro 61-8048, a negative allosteric modulator of α7 receptors which increases endogenous levels of kynurenic acid. Negative allosteric modulators differ from orthosteric antagonists in that they do not directly affect the receptor, but alter the effects of receptor ligands when they bind to the receptor. Thus, kynurenic acid decreases the effects of acetylcholine on the neuron. Ro 61-8048 showed promising effects in several models of cannabinoid reward, including maintenance tests with THC self-administration in squirrel monkeys (Figure 2A), cue-induced reinstatement of WIN 55,212-2 seeking in rats, drug-induced and cue-induce reinstatement of THC seeking in squirrel monkeys (Figures 2B and 2C), and THC-induced elevation of dopamine levels in the nucleus accumbens of rats. Drug discrimination procedures indicated that KMO inhibition blocked the subjective effects of THC in monkeys but not in rats, perhaps consistent with THC discrimination not being indicative of non-reward-related effects in rats. Overall, Ro 61-8048 was highly effective in behavioral, neurochemical and electrophysiological models of cannabinoid reward and reward-related effects. These findings suggest that enhancing endogenous levels of kynurenic acid might be an effective and safe strategy for treating CUD.
Figure 2.
Effects of treatment with Ro 61-8048 in squirrel monkey models of relapse to cannabis seeking after a period of abstinence. Ro 61-8048 increases endogenous levels of kynurenic acid, a negative allosteric modulator of the α-7 nicotinic receptor. Monkeys had extensive experience self-administering THC under a schedule in which a green light indicated that THC (4 mcg/kg, IV) could be obtained by pressing a lever 10 times (fixed-ratio 10). Under these training conditions (maintenance), sessions were 1 h long, and each THC delivery was accompanied by a brief stimulus (amber light), followed by a 60-s timeout period (lights off) before the green light signaled that another injection could be obtained. (A) Treatment with Ro 61-8048 (20 mg/kg, IM) decreased THC self-administration under these maintenance conditions, with all cues and THC delivery maintained. (B) To model relapse induced by reexposure to THC, abstinence was first imposed by simply substituting vehicle for THC (but continuing to present cues) for several sessions prior to and including the test session. Responding decreased to low levels, but increased substantially when THC (40 mcg/kg, IV) was given automatically prior to the test session. Ro 61-8048 blocked this THC-induced reinstatement effect. (C) To model relapse induced by reexposure to cannabis-associated cues, abstinence was first induced by discontinuing all intravenous injections and all visual cue presentations. Then, to test for cue-induced reinstatement, the green light was presented, and every 10th response produced the amber light and an intravenous vehicle injection (to provide interoceptive injection-related cues), but no THC was given. These cues reinstated THC seeking, but their effect was blocked when monkeys were pretreated with Ro 61-8048. Thus, Ro 61-8048 blocked the direct reinforcing effects of THC, and it also blocked reinstatement by reexposure to THC or THC-related cues. These findings suggest that enhancing kynurenic acid levels might be a useful strategy for decreasing cannabis smoking and also for preventing relapse when users who have achieved abstinence are re-exposed to the drug or to environmental cues associated with the drug. Double asterisks indicate statistically significant differences between treatment and baseline (p < 0.01). Figure adapted from Justinova, Z., Mascia, P., Wu, H. Q., Secci, M. E., Redhi, G. H., Panlilio, L. V., … Goldberg, S. R. (2013). Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nature Neuroscience, 16(11), 1652–1661. doi:10.1038/nn.3540 with permission.
Depression is known to be associated with heavy use of cannabis and other drugs, and cannabis might serve a self-medicating function in depressed users (Degenhardt, Hall, & Lynskey, 2003). Consistent with this hypothesis, THC shows positive effects in some animal models of depression, including olfactory bulbectomy in rats (Rodriguez-Gaztelumendi, Rojo, Pazos, & Diaz, 2009). Amchova et al. (2014) tested the effects of bulbectomy on self-administration of the synthetic cannabinoid WIN55-212 in rats, finding that it caused rats to self-administered about twice as much drug as control rats. Because serotonin 5-HT1B receptors has been implicated in depression and has also been found to decrease the self-administration of some drugs, Amchova et al. tested the effects of a 5-HT1 agonist on WIN55-212 self-administration in bulbectomized and control rats. However, the agonist did not decrease cannabinoid self-administration in either group of rats.
Administration of THC or synthetic CB1 agonists in rodents increases the synthesis of pregnenolone, the precursor of all steroid hormones, including neurosteroids synthesized directly in the brain (Vallee et al., 2014). Using the tetrad test (THC-induced hypolocomotion, hypothermia, catalepsy and analgesia) and neurochemical and electrophysiological measures of dopamine activity in the nucleus accumbens, Vallee et al. (2014) obtained results suggesting that pregnenolone decreases the effects of THC through negative feedback, acting as an allosteric modulator of CB1 (altering the effects of THC when it binds to the receptor). When pregnenolone was given to mice trained to self-administer the CB1 agonist WIN55-212, it decreased cannabinoid intake, and it decreased the number of responses mice performed to obtain the cannabinoid when the requirement was progressively increased during the session. These results suggest that pregnenolone decreases the motivation to receive cannabinoid reward. Allosteric modulators also have two advantages over orthosteric antagonists such as rimonabant: they should not inhibit all CB1 receptor activities (and might therefore be less prone to side effects), and their effects should not be surmountable even if cannabis intake is increased. Thus, treatment with pregnenolone or other allosteric modulators of CB1 represent another new strategy that should be further developed and assessed for treating CUD.
Noting that some of the symptoms of cannabis withdrawal resemble bipolar mood disorder, which is treated with the mood stabilizer lithium, Cui et al. (2001) studied the effects of lithium on precipitated withdrawal from a synthetic cannabinoid agonist in rats. They found that lithium prevented withdrawal symptoms, that this effect was blocked when lithium was combined with an oxytocin antagonist, and that administration of oxytocin could also prevent precipitated withdrawal. However, when lithium was subsequently tested during withdrawal in treatment-seeking cannabis users, it prevented certain symptoms (loss of appetite, stomach aches, and nightmares/strange dreams), but it did not reduce overall withdrawal scores or increase (Johnston et al., 2014).
Another potential strategy for treating withdrawal is to increase levels of endogenous cannabinoid ligands, such as anandamide and 2-arachidonoylglycerol. For example, levels of anandamide can be increased by administering an inhibitor of fatty acid amide hydrolase (FAAH), the main enzyme responsible for the inactivation of anandamide. The FAAH inhibitor URB597 increases anandamide levels in the brain, which could potentially prevent withdrawal symptoms when cannabis use is discontinued. Importantly, URB597 does not produce adverse THC-like effects in animal models, suggesting that it would not be liable to be abused, to reinstate cannabis use, or have cannabis-like side effects. Stewart and McMahon (2011) tested URB597 in a withdrawal-related drug discrimination procedure. Rhesus monkeys were chronically treated with the CB1 agonist WIN55-212 and trained to detect the effects of the CB1 antagonist rimonabant, essentially combining drug discrimination and precipitated withdrawal procedures. They found that URB597 did not block the interoceptive effects of rimonabant in this model, which suggests that it would not block all the subjective effects of cannabis withdrawal in humans. However, the strategy of relieving withdrawal by increasing endogenous cannabinoid levels should be further investigated, because other FAAH inhibitors and monoacylglycerol lipase inhibitors (which increase levels of 2-arachidonoylglycerol) can have different effects than URB597, and because the results might also be different with precipitated withdrawal versus simple discontinuation of cannabinoid administration. Other experiments with this discrimination procedure showed that treatment with the CB1 agonist WIN55-212 or the alpha(2)-adrenergic agonist clonidine partially blocked withdrawal symptoms ((Stewart and McMahon (2010); see also Lichtman et al. (2001)).
4. Human laboratory approaches
Recent systematic reviews indicate a growing interest to explore different therapeutic options for the treatment of CUD (Laprevote, Schwan, Schwitzer, Rolland, & Thome, 2015; Marshall, Gowing, Ali, & Le Foll, 2014). In this section we will describe two different approaches that have been used to study a variety of potentially therapeutic options for the treatment of CUD: human laboratory studies and randomized clinical trials. Detailed results obtained using different pharmacotherapies on human studies will be described in more detail in the next section.
Human laboratory studies have allowed for advances in drug abuse research in a variety of ways. They are an invaluable tool for the study of potential pharmacotherapies for drug dependence, and they also provide a means of investigating the mechanisms underlying drugs’ addictive effects and the ways that experimental treatments alter these effects. Human laboratory studies can be used to test the effects of different doses of the same medication, to compare the effectiveness of different medications and even to assess preference for medication vs. placebo (e.g. self-administration studies, see below), often using a single sample of participants (e.g. within-subject designs) and short-term treatments (e.g. 5 or 7 days). Laboratory studies using within-subject designs usually control for possible carryover effects of the different doses or medications by administering the different treatments on a counter-balanced order and by including inter-treatment clearance phases in which participants can often consume drugs as usual (i.e. smoke cannabis as usual). Due the intrinsic nature of these studies only those participants not seeking for treatment are included.
The number of human laboratory studies for CUD has increased over the past decade. Several studies have investigated the effects of pharmacological interventions involving diverse neurotransmitter systems, including GABA (agonist: baclofen), norepinephrine/serotonin (e.g. antidepressants; mirtazapine, nefazodone), norepinephrine/dopamine (e.g., bupropion), α2A-adrenergic receptors (e.g. agonist; lofexidine), CB1 agonists (e.g. THC, Dronabinol, nabilone) and histamine (e.g., antagonist: quetiapine). However, so far, the majority of human laboratory studies have been focused on studying the effects of CB1 agonists on cannabis withdrawal symptoms (Budney, Vandrey, Hughes, Moore, & Bahrenburg, 2007; Haney et al., 2013; Haney et al., 2008; Haney et al., 2004). Succeeding studies in laboratory settings exploring the effects of Sativex (a ~1:1 combination of THC and cannabidiol) on withdrawal are expected to provide further input in this respect (Trigo et al., 2016). Several human laboratory studies by Haney et al., have also explored the ability of a variety of compounds including THC, baclofen, mirtazapine and quetiapine (Cooper et al., 2013; Haney et al., 2013; Haney et al., 2010) on preventing marijuana relapse. Therefore, laboratory studies can be used to study the effects of pharmacotherapy, in participants who are not seeking treatment, on different aspects of the addictive process including drug taking, withdrawal and relapse.
Drug self-administration studies are a subtype of human laboratory studies that allows participants who are not seeking treatment to consume drugs under controlled conditions. Self-administration procedures have been used to study preferences for different concentrations of addictive substances (e.g. nicotine or THC), or whether certain treatments are able to modify drug self-administration (Haney, Ramesh, et al., 2015; Hart, Haney, Ward, Fischman, & Foltin, 2002). Drug self-administration studies have been also useful for evaluating motivation for different types of reinforcers (i.e. if participants would prefer the drug to alternative reinforcers as food or money), for assessing whether the decision to take a drug can be affected by monetary contingencies (Haney, Comer, Ward, Foltin, & Fischman, 1997; Ward, Comer, Haney, Foltin, & Fischman, 1997), or for determining whether a treatment affects how much participants are willing to pay (i.e. using study earnings) to self-administer marijuana (Haney et al., 2008). Laboratory conditions on drug self-administration studies have allowed assessment of marijuana dose-response function for both subjective ratings (i.e. “high” or “good effect”) and relevant functioning parameters such as psychomotor activity, attention, cardiovascular effects, etc. (Ramesh, Haney, & Cooper, 2013). This type of experimental design can also be used to compare the effectiveness of fixed vs. self-administered doses of a particular pharmacotherapy on CUD or to assess preferences in medication dosage (e.g. if, when given the opportunity, participants choose lower doses as compared with an imposed high fixed dose) (Trigo et al., 2016).
Cannabis withdrawal syndrome has been included, after years of debate, in DSM-5. The relevance of including cannabis withdrawal syndrome as a mental disorder is underlined by the fact that relapse to cannabis use is associated with greater severity of withdrawal symptoms (Allsop et al., 2012) and that 65% of treatment-seekers reports report using marijuana to alleviate withdrawal symptoms (Budney, Novy, & Hughes, 1999; Vandrey, Budney, Kamon, & Stanger, 2005). Before this inclusion, several laboratory studies modeled cannabis withdrawal under controlled conditions (Haney et al., 2004; Haney, Ward, Comer, Foltin, & Fischman, 1999a, 1999b; Hart, Ward, et al., 2002; Jones, Benowitz, & Bachman, 1976; Jones, Benowitz, & Herning, 1981). These studies showed the existence of “negative” effects (e.g. anxiety, sleep difficulty, feeling “irritable” “depressed” or “miserable”) during abstinence (e.g. 4–5 days with no marijuana or only placebo preparations). These “negative” effects were evident even following low daily doses of THC (Haney et al., 1999a; Hart, Haney, et al., 2002) or short time administration of THC (e.g. 3–7 days). In a typical laboratory study modeling cannabis withdrawal, marijuana smokers will be alternatively exposed to active marijuana (oral or smoked preparations) followed by placebo. The effects of both marijuana and the absence of it will be then evaluated using subjective/objective measures (e.g. feeling “high”, “irritable” / food intake, psychomotor performance).
Randomized clinical trials can provide the strongest evidence for the efficacy of preventive and therapeutic procedures and are an essential step towards approval for clinical use. Clinical trials for CUD typically test the effectiveness of a particular medication, in combination with counseling (see below), in reducing craving/withdrawal and cannabis use as compared with a placebo control group of treatment seekers.
Recent systematic reviews have shown an increasing incidence of double-blind randomized placebo-controlled trials evaluating treatments for CUD (vs. the total number of relevant studies on the literature, ~ 1:3 ratio) (Laprevote et al., 2015; Marshall et al., 2014). Clinical trials have evaluated the effectiveness of both psychotherapy and pharmacotherapy (alone or in combination) for CUD. Therefore, several clinical trials have evaluated the effects of brief/extended cognitive-behavioral interventions, motivational enhancement therapy plus cognitive behavioral therapy and adolescent community reinforcement approach, on CUD with, in general, better outcomes for extended (vs. brief) treatments (Copeland, Swift, Roffman, & Stephens, 2001; Dennis et al., 2004; Marijuana Treatment Project Research, 2004; Stephens, Roffman, & Curtin, 2000). On the other hand, randomized clinical trials have been also used to test the effects of different pharmacotherapies including antidepressants (e.g. escitalopram, nefazodone and bupropion), anticonvulsants (e.g. divalproex sodium and gabapentin) and CB1 agonists (e.g. dronabinol and Sativex), usually in combination with cognitive-behavior therapy or other type of counseling to increase coping skills (Allsop et al., 2014; Carpenter, McDowell, Brooks, Cheng, & Levin, 2009; Levin et al., 2011; Levin et al., 2004; Mason et al., 2012; Weinstein et al., 2014).
5. Findings from clinical trials and laboratory studies in human volunteers
Currently used medications with wide variety of mechanisms have been assessed for treatment of CUD in humans. However, most of them have failed to decrease cannabis use, withdrawal symptoms or laboratory measures of relapse (Balter, Cooper, & Haney, 2014; Marshall et al., 2014). Among the ineffective medications were antidepressants, anxiolytics, and antipsychotics, including fluoxetine, venlafaxine, escitalopram, atomoxetine, buspirone, bupropion, nefazodone, mirtazapine, and quetiapine (Balter et al., 2014). Here, we will concentrate on medications that showed some effectiveness in human studies or are currently in clinical testing, particularly cannabinoid CB1 agonists and antagonists and opioid ligands.
The CB1 agonists that were studied as a replacement therapy for CUD are dronabinol and nabilone. Dronabinol (Marinol) is a synthetic form of THC that has been approved for treatment of chemotherapy-associated nausea and for appetite stimulation in patients with AIDS or cancer. Nabilone is a synthetic, highly-bioavailable THC analogue with clinical indications similar to those of dronabinol. Dronabinol was tested in several small within-subject, placebo-controlled human-laboratory studies for its effect on withdrawal symptoms. Several studies found a dose-dependent reduction of withdrawal symptoms and reversal of the anorexia and weight loss associated with withdrawal while observing minimal adverse effects (Budney, Vandrey, et al., 2007; Haney et al., 2008; Haney et al., 2004; Vandrey et al., 2013) (Figure 3). However, dronabinol did not reduce cannabis self-administration or relapse (Haney et al., 2008; Hart, Haney, et al., 2002). Levin and colleagues (Levin et al., 2011) conducted the first randomized, double-blind, placebo-controlled clinical trial that evaluated the safety and efficacy of dronabinol in combination with behavioral therapies in treating cannabis dependence. Both placebo and dronabinol groups showed a reduction in marijuana use over time, but there were no differences in cannabis use outcomes. Although it failed to improve abstinence, agonist substitution pharmacotherapy with dronabinol reduced withdrawal symptoms and improved retention in treatment with few adverse events. Nabilone, which has higher bioavailability, clearer dose-linearity, and longer duration of action than dronabinol, also showed promise as a potential treatment medication for marijuana dependence (Bedi, Cooper, & Haney, 2013; Haney et al., 2013). Nabilone maintenance produced a robust attenuation of marijuana withdrawal symptoms and a laboratory measure of relapse in a small within-subject study (Haney et al., 2013). Another clinical study is currently evaluating nabilone as a treatment for cannabis dependence and aims to assess the correlation of neuropsychological performance to brain changes using functional MRI brain scans (ClinicalTrials.gov).
Figure 3.
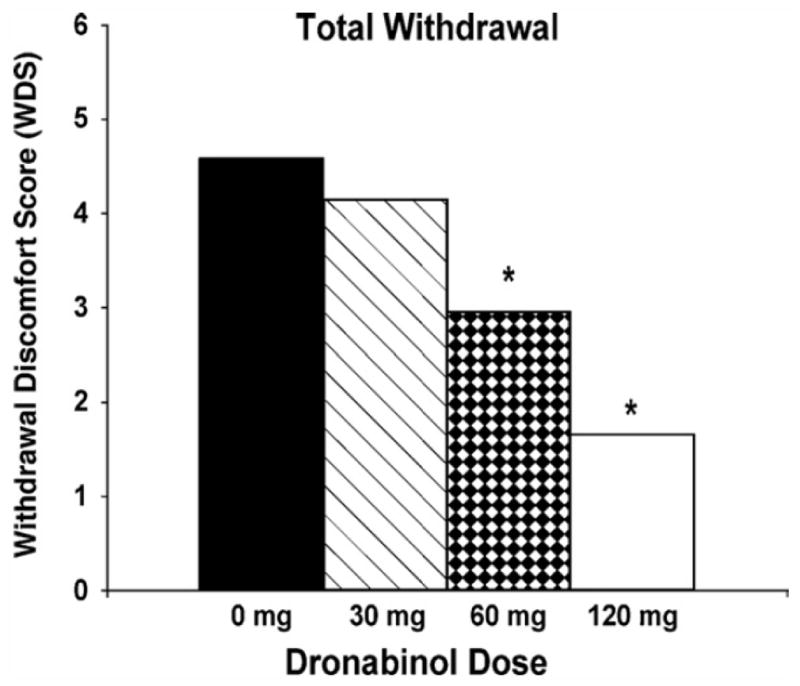
Effects of treatment with dronabinol on cannabis withdrawal symptoms in daily cannabis users. Significant withdrawal symptoms were observed during the placebo maintenance phase for subjective ratings of decreased appetite, diarrhea, nausea, stomach pain, irritability, sleep difficulty, total sleep time, subjective sleep quality, mood at morning awakening, alertness at morning awakening, restlessness, nervousness/anxiety, chills, increased aggression, increased anger, headaches, difficulty concentrating, and total withdrawal discomfort score (WDS). Significant dose-dependent effects of dronabinol were observed for each withdrawal item except “sleep difficulty” and “nervousness/anxiety.” Mean subjective withdrawal ratings (WDS) are presented as a function of dronabinol maintenance dose. Asterisks indicate statistically significant differences between dronabinol dose conditions and placebo. These results suggest that replacement therapy with a CB1 receptor agonist can decrease withdrawal symptoms, analogous to nicotine replacement therapy for tobacco smokers. Figure from Vandrey, R., Stitzer, M. L., Mintzer, M. Z., Huestis, M. A., Murray, J. A., & Lee, D. (2013). The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug and Alcohol Dependence, 128(1–2), 64–70. doi:10.1016/j.drugalcdep.2012.08.001 with permission.
The activation of CB1 receptors can also be achieved by pharmacological inhibition of the enzymes that degrade the endogenous CB1 agonists anandamide and 2-arachidonoylglycerol (2-AG). The inhibitors of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase enhance and prolong the effects of endocannabinoids at the sites of their release in the brain, and might therefore be effective as a replacement therapy or for attenuation of withdrawal symptoms. The FAAH inhibitor PF-04457845 was well tolerated in previous clinical trials in healthy subjects and patients with osteoarthritis of the knee (Huggins, Smart, Langman, Taylor, & Young, 2012; Li et al., 2012), in which no cannabis-like adverse effects were reported. Currently, PF-04457845 is being tested in a clinical trial as a treatment for marijuana withdrawal (ClinicalTrials.gov).
Cannabidiol is a major non-psychoactive ingredient in marijuana that has been shown to exert multiple pharmacological effects, mediated by multiple mechanisms (Devinsky et al., 2014; Izzo, Borrelli, Capasso, Di Marzo, & Mechoulam, 2009). For example, cannabidiol has a low affinity for CB1 and CB2 receptors, increases anandamide level via FAAH inhibition, acts as an agonist at TRPV1,2 and 5HT1A receptors, and acts as an antagonist at the orphan GPR55 receptor and nicotinic alpha 7 receptors (Grotenhermen, 2005; Izzo et al., 2009; Mahgoub et al., 2013; Mechoulam, Parker, & Gallily, 2002; Pertwee et al., 2010; Ryberg et al., 2007). Cannabidiol has been shown to have anticonvulsant, neuroprotective and anti-inflammatory effects in animal models and anxiolytic and antipsychotic effects in humans (Bergamaschi, Queiroz, Zuardi, & Crippa, 2011; Crippa et al., 2011; Devinsky et al., 2014; Leweke et al., 2012). Cannabidiol alone or in combination with THC (nabiximols, Sativex) has been investigated in clinical trials for management of CUD (Allsop et al., 2014; Haney, Malcolm, et al., 2015). It is hypothesized that when administered in combination with THC, cannabidiol likely adds anxiolytic, antidepressant, and antipsychotic effects to the simple agonist THC substitution approach, and facilitates the safe delivery of the THC doses needed to control cannabis cravings (Allsop, Lintzeris, Copeland, Dunlop, & McGregor, 2015). In a double-blind, randomized, placebo-controlled, inpatient clinical trial (Allsop et al., 2014), 6-day treatment with nabiximols attenuated cannabis withdrawal symptoms and improved patient retention in treatment, but was no more effective than placebo in encouraging long-term reductions in cannabis use. Recently, Haney and colleagues (Haney, Malcolm, et al., 2015) conducted double-blind, randomized within-subject laboratory study and showed that acute oral administration of cannabidiol alone does not reduce the reinforcing, physiological or positive subjective effects of smoked cannabis. There are several clinical trials currently underway or have recently been completed that will hopefully shed more light on effectiveness of cannabidiol or nabiximols on cannabis use outcomes.
Drugs that prevent activation of cannabinoid CB1 receptors may represent an alternative approach to the treatment of cannabis addiction, by directly blocking the subjective and reinforcing effects of THC. Huestis and colleagues showed that acute and repeated administration of CB1 antagonists/inverse agonist rimonabant attenuated the subjective and physiological effects of smoked marijuana (Gorelick et al., 2006; Huestis et al., 2007; Huestis et al., 2001). CB1 antagonists/inverse agonists (like rimonabant, taranabant, and surinabant) showed effectiveness in treatment of obesity and in smoking cessation, but were eventually pulled from clinical use and further development due to reports of psychiatric side effects (anxiety, depression, suicidality) (Le Foll et al., 2009). It is possible that CB1 antagonists that lack inverse-agonist effects (neutral antagonists) would effectively block the abuse-related effects of THC, but without alteration of endocannabinoid activity and thus be safer than rimonabant (Janero & Makriyannis, 2009). Prototypical and best characterized neutral CB1 antagonists (e.g., AM4113) have not yet been tested clinically, but a natural cannabinoid tetrahydrocannabivarin was shown to be acting as a neutral CB1 antagonist at lower doses (McPartland, Duncan, Di Marzo, & Pertwee, 2015). In healthy subjects, tetrahydrocannabivarin was found to increase neural responding to rewarding and aversive stimuli (Tudge, Williams, Cowen, & McCabe, 2015) and when administered in combination with THC, it significantly inhibited THC-induced impairment to delayed recall as well as THC-induced increase of heart rate (Englund et al., 2016). Although tetrahydrocannabivarin was well tolerated among the participants in these studies, and no serious adverse effects were observed, a larger study is needed to confirm the findings and safety profile of tetrahydrocannabivarin.
So far, we described compounds that bind to the orthosteric site of the CB1 receptor, where endogenous cannabinoid ligands also bind. Cannabinoid receptors can also contain allosteric binding sites that are discrete from the orthosteric site (Ross, 2007). Allosteric modulators are ligands that bind to the allosteric site and induce conformational changes that lead to alteration of affinity and/or efficacy of orthosteric ligands (Bosier, Muccioli, Hermans, & Lambert, 2010). Newly developed CB1 allosteric modulators can have complex effects and unclear mechanism of action (Keov, Sexton, & Christopoulos, 2011), but can also potentially be signaling-specific and regulate only some of the functions of the receptor, which could lead to a more targeted action compared to orthosteric compounds (Ross, 2007). Thus, allosteric modulation of CB1 receptors represents a new approach for development of medications modulating function of cannabinoid system that could potentially avoid the adverse effects associated with orthosteric agonism or antagonism of these receptors. Recently, inactive precursor of steroid hormones, pregnenolone that acts as negative allosteric modulator of CB1 receptors, was shown to block behavioral and neurobiological effects of cannabinoid drugs in rodents (Vallee et al., 2014). Based on these and other preclinical results, a clinical study has been initiated that will investigate the effect of acute administration of pregnenolone on cue-related craving in individuals with CUD (ClinicalTrials.gov).
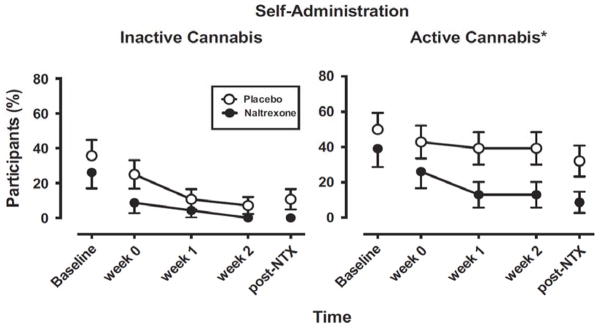
A wealth of preclinical evidence indicates an interplay between the opioid (μ-opioid) and cannabinoid receptor systems in the mutual modulation of the addictive behavior and suggested that opioid antagonists block the reinforcing effects of cannabinoids (Fattore et al., 2004). Treatment with mu-opioid antagonists such as naltrexone represents a possible approach to reducing the reinforcing effects of THC (Justinova et al., 2004). In humans, acute naltrexone administration was shown to increase the positive subjective effects of oral THC or cannabis in marijuana smokers (Cooper & Haney, 2010; Haney, Bisaga, & Foltin, 2003). In another study, acute naltrexone administration did not attenuate the effects of intravenous THC in healthy humans (Ranganathan et al., 2012). However, the drug history of the person might be an important determinant of the effects of naltrexone, as THC intoxication was blunted by naltrexone in marijuana users but not in naïve subjects (Haney, 2007). A recent human laboratory study showed that chronic administration of naltrexone attenuates reinforcing and positive subjective effects in daily cannabis smokers (Haney, Ramesh, et al., 2015), which is encouraging for further testing in clinical trials (Figure 4). In fact, a clinical trial studying effectiveness of long-acting injectable naltrexone (Vivitrol) in patients with cannabis dependence is currently recruiting participants (ClinicalTrials.gov).
Figure 4.
Effects of naltrexone (NTX) or placebo treatment on cannabis self-administration by chronic cannabis users in a laboratory study. Points show the percentage of participants self-administering inactive (0.0% THC) or active (5.5% THC) smoked cannabis as a function of time and naltrexone dose. Baseline refers to the session before naltrexone administration commenced. Post-NTX refers to the session that occurred at least 1 week following termination of naltrexone administration. All participants received placebo capsules during baseline and post-NTX sessions. Self-administration of inactive cannabis was low and not significantly influenced by naltrexone (left panel), but naltrexone significantly decreased self-administration of active cannabis relative to placebo (right panel). Asterisks indicate significant difference between the naltrexone and placebo groups. These findings suggest that opioid antagonists might be useful as medications for decreasing cannabis smoking. Figure from Haney, M., Malcolm, R. J., Babalonis, S., Nuzzo, P. A., Cooper, Z. D., Bedi, G.,…Walsh, S. L. (2015). Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked Cannabis. Neuropsychopharmacology. doi:10.1038/npp.2015.367; Haney, M., Ramesh, D., Glass, A., Pavlicova, M., Bedi, G., & Cooper, Z. D. (2015). Naltrexone maintenance decreases Cannabis self-administration and subjective effects in daily Cannabis smokers. Neuropsychopharmacology, 40(11), 2489–2498. doi:10.1038/npp.2015.108 with permission.
There are also several drugs from different pharmacological groups that showed some promise in humans. A study by Haney and colleagues (Haney et al., 2008) showed that combined treatment with the α2-adrenergic receptor agonist lofexidine and dronabinol decreased marijuana withdrawal, craving, and relapse in a small group of daily marijuana smokers. Recently concluded, randomized, double-blind, placebo-controlled trial did not find the combination of dronabinol and lofexidine effective in promoting abstinence among a larger sample of cannabis-dependent patients (Levin et al., 2016). However, there is currently another clinical study underway that evaluates effectiveness of combination of dronabinol and another α2-adrenergic receptor agonist, clonidine, in cannabis-dependent schizophrenics (ClinicalTrials.gov). Based on the hypothesis of the involvement of glutamatergic pathways in the pathophysiology of addiction, an over-the-counter supplement N-acetylcysteine (NAC) has been suggested for the treatment of addiction. NAC restores extracellular glutamate levels and increases tonic activation of type 2 and 3 metabotropic glutamate receptors (mGluR2/3), thus inhibiting glutamate transmission and excitotoxicity (Asevedo, Mendes, Berk, & Brietzke, 2014). In young marijuana users, an open-label pilot study showed decreases in self-report measures of marijuana use and craving during 4 weeks of daily administration of NAC (Gray, Watson, Carpenter, & Larowe, 2010). This study was followed by a double-blind randomized placebo-controlled trial in cannabis-dependent adolescents. The treatment with NAC, when added to contingency management and brief cessation counseling, yielded improved cannabis abstinence during treatment compared to placebo (Gray et al., 2012). Currently, the National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) is conducting a study to test the efficacy of NAC versus placebo, added to contingency management, for cannabis cessation in adults (McClure et al., 2014). Chronic cannabis use and withdrawal is associated with neural dysregulation in stress systems and the calcium channel/GABA modulating antiepileptic gabapentin can restore homeostasis in brain stress systems. In a proof-of-concept study in adults, gabapentin significantly decreased withdrawal severity and reduced cannabis use in (Mason et al., 2012). Another randomized, double-blind, placebo-controlled clinical trial clinical trial in a larger sample is currently underway (ClinicalTrials.gov).
6. Conclusion
Current prevalence of cannabis use worldwide, together with the estimated prospect for developing dependence in occasional and daily cannabis users (7–9% and 10–20%, respectively), underline the necessity of efficacious tools to treat CUD. This chapter summarizes and provides an update on the different pre-clinical and clinical models for the screening and evaluation of medications for treating CUD.
Pre-clinical research is an early stage phase in pharmacotherapy development, and many of the aspects involved in drug dependence can be studied by using specific animal models. Among these models, the self-administration paradigm provides the most direct evidence of drug’s reinforcing properties. However, the infeasibility of modeling THC self-administration in rodents has (so far) constituted a drawback in this area. Encouragingly, non-human primates readily self-administer THC, and do so reliably over long periods of time, providing a model of chronic use in humans. Therefore, THC self-administration in monkeys, together with other paradigms in rodents (e.g. drug discrimination), have allowed testing of the efficacy of several compounds for modifying the reinforcing/perceived effects of THC. In this respect, is interesting that findings in squirrel monkeys showing the ability of naltrexone to reduce THC’s reinforcing properties have been confirmed in human self-administration studies, which supports the validity of this animal model. In addition to nonhuman primate research, it is also possible to take advantage of the fact that rodents will self-administer synthetic cannabinoids such as WIN55-212. In fact, synthetic cannabinoids (e.g. agonists, FAAH inhibitors, antagonists) have are a useful tool in animal models of CUD and withdrawal, allowing manipulation of many different components of the endocannabinoid system.
Clinical research on CUD has been relatively scarce compared to other addictions, but there is a growing interest in this area, and human studies have become increasingly prevalent. Importantly, these human studies have modeled and established the existence of the cannabis withdrawal syndrome that is now included on DSM-5. Human laboratory studies and clinical trials have also produced relevant information regarding the effectiveness of different pharmacotherapies (and counseling). In this regard, THC analogues such as dronabinol and nabilone have been shown to be effective in reducing withdrawal symptoms, with no apparent changes on cannabis self-administration or relapse. Similarly, studies using a combination of THC/CBD (Sativex) showed reduced withdrawal with no changes in cannabis use. On the other hand, new pharmacological tools such as FAAH inhibitors, neutral CB1 antagonists, allosteric modulators of CB1 receptors, and drugs from different pharmacological groups as α2-adrenergic receptor agonists, NMDA/AMPA or GABA ligands have shown promising results in small or proof-of-concept studies and might yield relevant findings in ongoing clinical trials.
Acknowledgments
The preparation of this manuscript was supported in part by Intramural Research Program of National Institute on Drug Abuse, National Institutes of Health and by CAMH.
Abbreviations
- α7
nicotinic acetylcholine type alpha 1
- AM4113
a neutral CB1 antagonist
- A2A
adenosine receptor type A2A
- 5-HT1 and 5-HT1B
serotonin receptor type 1A and 1B
- CUD
Cannabis Use Disorder
- CB1
cannabinoid receptor type 1
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (2013)
- FAAH
fatty acid amide hydrolase
- GABA
gamma-aminobutyric acid
- GPR55
G protein-coupled receptor 55
- PF-04457845
a FAAH inhibitor
- NAC
N-acetylcysteine
- THC
Δ9-tetrahydrocannabinol
- TRPV1
2, transient receptor potential channels 1 and 2
- URB597
a FAAH inhibitor
- WIN55-212
a synthetic CB1 agonist
References
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, … McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71(3):281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, Budney AJ. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Lintzeris N, Copeland J, Dunlop A, McGregor IS. Cannabinoid replacement therapy (CRT): Nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Ther. 2015;97(6):571–574. doi: 10.1002/cpt.109. [DOI] [PubMed] [Google Scholar]
- Amchova P, Kucerova J, Giugliano V, Babinska Z, Zanda MT, Scherma M, … Fattore L. Enhanced self-administration of the CB1 receptor agonist WIN55,212-2 in olfactory bulbectomized rats: evaluation of possible serotonergic and dopaminergic underlying mechanisms. Front Pharmacol. 2014;5:44. doi: 10.3389/fphar.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asevedo E, Mendes AC, Berk M, Brietzke E. Systematic review of N-acetylcysteine in the treatment of addictions. Rev Bras Psiquiatr. 2014;36(2):168–175. doi: 10.1590/1516-4446-2013-1244. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24676047. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Balter RE, Cooper ZD, Haney M. Novel Pharmacologic Approaches to Treating Cannabis Use Disorder. Curr Addict Rep. 2014;1(2):137–143. doi: 10.1007/s40429-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts K, Pemberton M, Bose J, Weimer B, Henderson L, Penne M, … Strashny H. Comparing and Evaluating Substance Use Treatment Utilization Estimates from the National Survey on Drug Use and Health and Other Data Sources. CBHSQ DATA REVIEW. 2014:1–120. [PubMed] [Google Scholar]
- Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. 2013;18(5):872–881. doi: 10.1111/j.1369-1600.2011.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22129319. [DOI] [PubMed] [Google Scholar]
- Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4–5):666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80(1):1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10615717. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4(1):4–16. doi: 10.1151/ascp07414. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2797098/pdf/ascp-04-1-4.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86(1):22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18(1):53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151(1):25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2013;18(6):993–1002. doi: 10.1111/j.1369-1600.2012.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology (Berl) 2010;211(2):141–148. doi: 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21(2):55–64. doi: 10.1016/s0740-5472(01)00179-9. discussion 65–56. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11551733. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, … Hallak JE. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- Cui SS, Bowen RC, Gu GB, Hannesson DK, Yu PH, Zhang X. Prevention of cannabinoid withdrawal syndrome by lithium: involvement of oxytocinergic neuronal activation. J Neurosci. 2001;21(24):9867–9876. doi: 10.1523/JNEUROSCI.21-24-09867.2001. Retrieved from http://www.jneurosci.org/content/21/24/9867.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26(8):420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. Retrieved from http://onlinelibrary.wiley.com/store/10.1046/j.1360-0443.2003.00437.x/asset/j.1360-0443.2003.00437.x.pdf?v=1&t=ijyh6b60&s=d1560f9fd2425d9c2d709d5f6e828478a126ab64. [DOI] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, … Funk R. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, … Friedman D. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A, Atakan Z, Kralj A, Tunstall N, Murray R, Morrison P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol. 2016;30(2):140–151. doi: 10.1177/0269881115615104. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Delta(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97(1):45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156(4):410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Spano MS, Deiana S, Fadda P, Scherma M, Fratta W. Cannabinoids and reward: interactions with the opioid system. Crit Rev Neurobiol. 2004;16(1–2):147–158. doi: 10.1615/critrevneurobiol.v16.i12.160. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15581410. [DOI] [PubMed] [Google Scholar]
- Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: consideration of the literature. Respirology. 2014;19(5):655–662. doi: 10.1111/resp.12298. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J. 2006;151(3):754 e751–754 e755. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, … Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, Larowe SD. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187–189. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. Cannabinoids. Curr Drug Targets CNS Neurol Disord. 2005;4(5):507–530. doi: 10.2174/156800705774322111. [DOI] [PubMed] [Google Scholar]
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32(6):1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 2003;166(1):77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8(2–3):101–112. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9833006. [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38(8):1557–1565. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, Foltin RW. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2010;211(2):233–244. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197(1):157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, … Walsh SL. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone Maintenance Decreases Cannabis Self-Administration and Subjective Effects in Daily Cannabis Smokers. Neuropsychopharmacology. 2015;40(11):2489–2498. doi: 10.1038/npp.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999a;141(4):385–394. doi: 10.1007/s002130050848. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10090646. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999b;141(4):395–404. doi: 10.1007/s002130050849. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10090647. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67(3):301–309. doi: 10.1016/s0376-8716(02)00084-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12127201. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002;164(4):407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction: A disorder of choice. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194(4):505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58(4):322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14(1):43–65. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, … McGregor IS. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient setting. Psychopharmacology (Berl) 2014;231(24):4623–4636. doi: 10.1007/s00213-014-3611-5. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz N, Bachman J. Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci. 1976;282:221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/798533. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6271820. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, … Goldberg SR. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict Biol. 2011;16(3):405–415. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, … Goldberg SR. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci. 2013;16(11):1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33(12):2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, … Goldberg SR. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology. 2015;40(9):2185–2197. doi: 10.1038/npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Redhi GH, Goldberg SR, Ferre S. Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Delta9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys. J Neurosci. 2014;34(19):6480–6484. doi: 10.1523/jneurosci.5073-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25(23):5645–5650. doi: 10.1523/jneurosci.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173(1–2):186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169(2):135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. 2011;31(19):7043–7048. doi: 10.1523/jneurosci.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, Lancon C. Acute and long-term effects of cannabis use: a review. Curr Pharm Des. 2014;20(25):4112–4118. doi: 10.2174/13816128113199990620. [DOI] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology. 2011;60(1):24–35. doi: 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, … McGregor IS. Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218(2):443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Laprevote V, Schwan R, Schwitzer T, Rolland B, Thome J. Is There a Place for Off-Label Pharmacotherapy in Cannabis Use Disorder? A Review on Efficacy and Safety. Curr Pharm Des. 2015;21(23):3298–3305. doi: 10.2174/1381612821666150619093940. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26088109. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312(3):875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205(1):171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Schroeder JR, Karschner EL, Goodwin RS, Hirvonen J, Gorelick DA, Huestis MA. Cannabis withdrawal in chronic, frequent cannabis smokers during sustained abstinence within a closed residential environment. Am J Addict. 2014;23(3):234–242. doi: 10.1111/j.1521-0391.2014.12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav. 2014;118:30–35. doi: 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, … Choi JC. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;159:53–60. doi: 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13(1):21–32. doi: 10.1080/10550490490265280. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14766435. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, … Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Winter H, Arends R, Jay GW, Le V, Young T, Huggins JP. Assessment of the pharmacology and tolerability of PF-04457845, an irreversible inhibitor of fatty acid amide hydrolase-1, in healthy subjects. Br J Clin Pharmacol. 2012;73(5):706–716. doi: 10.1111/j.1365-2125.2011.04137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69(1–2):181–188. doi: 10.1016/s0091-3057(01)00514-7. Retrieved from http://ac.els-cdn.com/S0091305701005147/1-s2.0-S0091305701005147-main.pdf?_tid=ff38e0b2-c5d2-11e5-a683-00000aab0f6c&acdnat=1453994752_1d124e1e739c2e05d8db3cf5152650f0. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Colombo G, Carai MA. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets. 2010;9(1):55–59. doi: 10.2174/187152710790966623. [DOI] [PubMed] [Google Scholar]
- Mahgoub M, Keun-Hang SY, Sydorenko V, Ashoor A, Kabbani N, Al Kury L, … Oz M. Effects of cannabidiol on the function of alpha7-nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;720(1–3):310–319. doi: 10.1016/j.ejphar.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research G. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72(3):455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst Rev. 2014;12:Cd008940. doi: 10.1002/14651858.CD008940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85(2):327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, … Rao S. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Sonne SC, Winhusen T, Carroll KM, Ghitza UE, McRae-Clark AL, … Gray KM. Achieving cannabis cessation -- evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211–223. doi: 10.1016/j.cct.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42(11 Suppl):11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12412831. [DOI] [PubMed] [Google Scholar]
- Mendizabal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology. 2006;31(9):1957–1966. doi: 10.1038/sj.npp.1300957. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102(12):1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Justinova Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin Pharmacol Ther. 2015;97(6):616–627. doi: 10.1002/cpt.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. Animal models of cannabinoid reward. Br J Pharmacol. 2010;160(3):499–510. doi: 10.1111/j.1476-5381.2010.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, … Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissidis A, Chouliara O, Galanopoulos A, Marselos M, Papadopoulou-Daifoti Z, Antoniou K. Behavioural and dopaminergic alterations induced by a low dose of WIN 55,212-2 in a conditioned place preference procedure. Life Sci. 2009;85(5–6):248–254. doi: 10.1016/j.lfs.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Haney M, Cooper ZD. Marijuana’s dose-dependent effects in daily marijuana smokers. Exp Clin Psychopharmacol. 2013;21(4):287–293. doi: 10.1037/a0033661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Carbuto M, Braley G, Elander J, Perry E, Pittman B, … D’Souza DC. Naltrexone does not attenuate the effects of intravenous Delta9-tetrahydrocannabinol in healthy humans. Int J Neuropsychopharmacol. 2012;15(9):1251–1264. doi: 10.1017/s1461145711001830. [DOI] [PubMed] [Google Scholar]
- Robinson L, Hinder L, Pertwee RG, Riedel G. Effects of delta9-THC and WIN-55,212-2 on place preference in the water maze in rats. Psychopharmacology (Berl) 2003;166(1):40–50. doi: 10.1007/s00213-002-1302-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gaztelumendi A, Rojo ML, Pazos A, Diaz A. Altered CB receptor-signaling in prefrontal cortex from an animal model of depression is reversed by chronic fluoxetine. J Neurochem. 2009;108(6):1423–1433. doi: 10.1111/j.1471-4159.2009.05898.x. [DOI] [PubMed] [Google Scholar]
- Ross RA. Allosterism and cannabinoid CB(1) receptors: the shape of things to come. Trends Pharmacol Sci. 2007;28(11):567–572. doi: 10.1016/j.tips.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, … Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Katz JL, Goldberg SR. The use of second-order schedules to study the influence of environmental stimuli on drug-seeking behavior. NIDA Res Monogr. 1988;84:180–195. [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Gilman JP, Justinova Z, Vemuri VK, Makriyannis A, Goldberg SR. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633(1–3):44–49. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1(3):1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, … Goldberg SR. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27(21):5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68(5):898–908. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11068976. [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334(1):347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol. 2011;164(2b):655–666. doi: 10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S, Nikas SP, Shukla VG, Vemuri K, Makriyannis A, Jarbe TU. Cannabinoid withdrawal in mice: inverse agonist vs neutral antagonist. Psychopharmacology (Berl) 2015;232(15):2751–2761. doi: 10.1007/s00213-015-3907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, … Le Foll B. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug and Alcohol Dependence. 2016 doi: 10.1016/j.drugalcdep.2016.02.020. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudge L, Williams C, Cowen PJ, McCabe C. Neural effects of cannabinoid CB1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. Int J Neuropsychopharmacol. 2015;18(6) doi: 10.1093/ijnp/pyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, … Piazza PV. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343(6166):94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005;78(2):205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2013;128(1–2):64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol. 2007;18(4):311–319. doi: 10.1097/FBP.0b013e3282186cf2. [DOI] [PubMed] [Google Scholar]
- Ward AS, Comer SD, Haney M, Foltin RW, Fischman MW. The effects of a monetary alternative on marijuana self-administration. Behav Pharmacol. 1997;8(4):275–286. doi: 10.1097/00008877-199708000-00001. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9832987. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, Bloch M. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. Am J Drug Alcohol Abuse. 2014;40(1):16–22. doi: 10.3109/00952990.2013.819362. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275(1):1–6. Retrieved from http://jpet.aspetjournals.org/content/275/1/1.full.pdf. [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of delta 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002;71(1–2):333–340. doi: 10.1016/s0091-3057(01)00694-3. Retrieved from http://ac.els-cdn.com/S0091305701006943/1-s2.0-S0091305701006943-main.pdf?_tid=a490694c-c5c2-11e5-a09e-00000aab0f01&acdnat=1453987728_0eb2493988e2293683f8fcf8a02067db. [DOI] [PubMed] [Google Scholar]