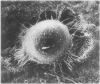
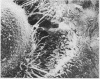
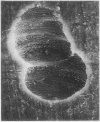
Abstract
This article reviews recent information concerning the origin of osteoclasts and the local and systemic regulation of their activity. It appears that much of the environmental responsiveness of osteoclasts is mediated by cells of the osteoblastic lineage, which exert a major influence on the localisation, induction, stimulation, and inhibition of osteoclastic bone resorption. Some of the mechanisms by which osteoclast function may be disturbed by inflammatory and neoplastic diseases are discussed, and it is suggested that many pathological disturbances of osteoclastic bone resorption may be explicable as mimicry of physiological regulatory mechanisms by local hormones introduced into bone as the local regulators of the diseased tissue.
Full text
PDF

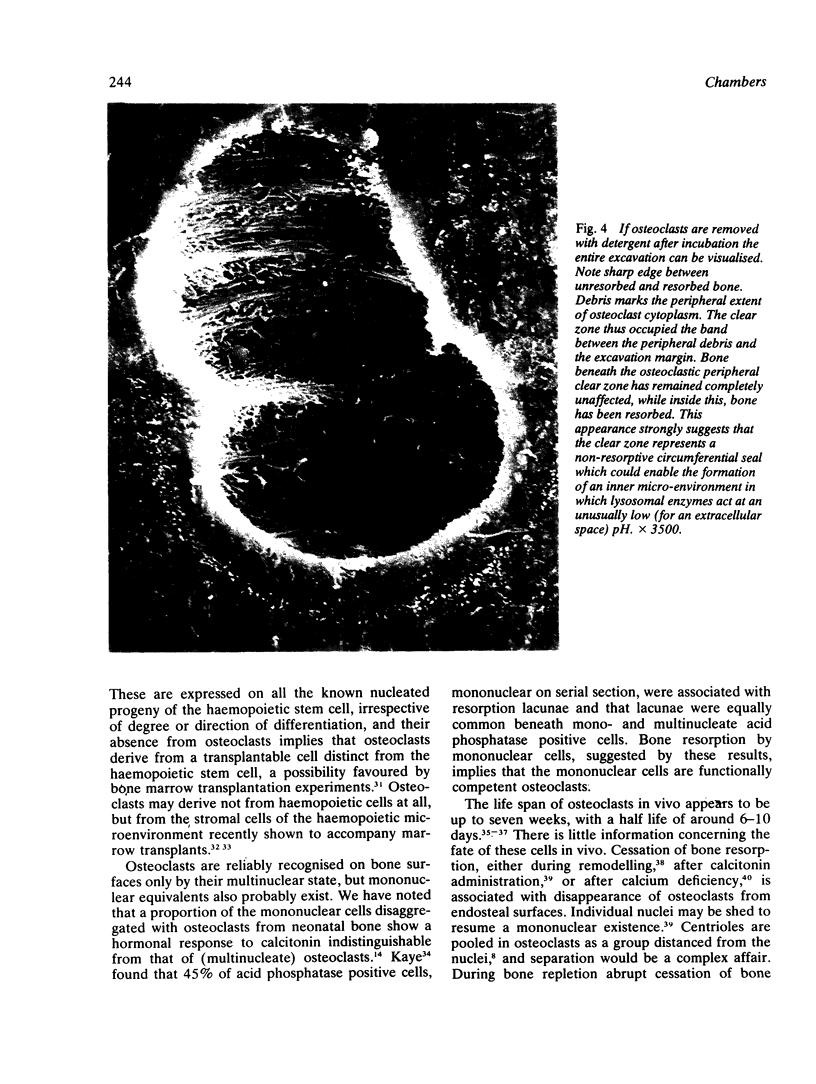


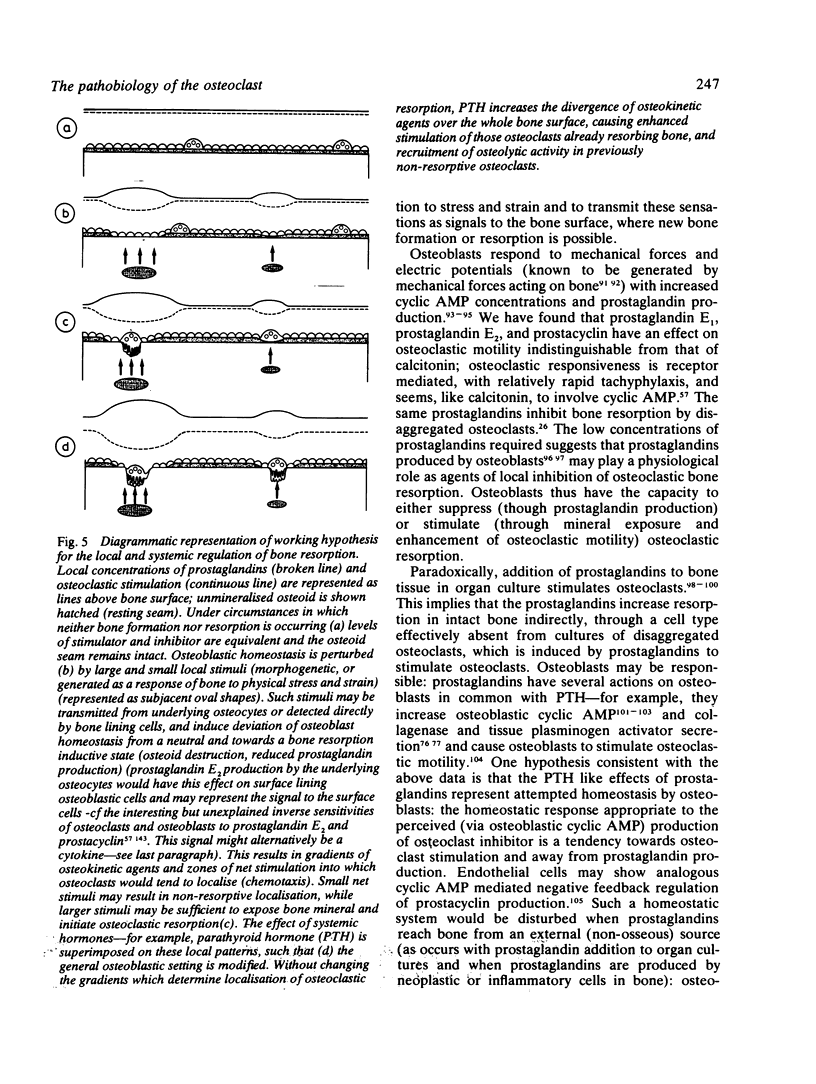





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. D., Colyer R. A., Riley L. H., Jr Skeletal changes during prolonged external irradiation: alterations in marrow, growth plate and osteoclast populations. Johns Hopkins Med J. 1979 Sep;145(3):73–83. [PubMed] [Google Scholar]
- Austin L. A., Heath H., 3rd Calcitonin: physiology and pathophysiology. N Engl J Med. 1981 Jan 29;304(5):269–278. doi: 10.1056/NEJM198101293040505. [DOI] [PubMed] [Google Scholar]
- Avioli L. V., Haddad J. G. The vitamin D family revisited. N Engl J Med. 1984 Jul 5;311(1):47–49. doi: 10.1056/NEJM198407053110109. [DOI] [PubMed] [Google Scholar]
- BASSETT C. A., BECKER R. O. Generation of electric potentials by bone in response to mechanical stress. Science. 1962 Sep 28;137(3535):1063–1064. doi: 10.1126/science.137.3535.1063. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett C. A. Pulsing electromagnetic fields: a new method to modify cell behavior in calcified and noncalcified tissues. Calcif Tissue Int. 1982 Jan;34(1):1–8. doi: 10.1007/BF02411199. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Carter R. L., Stamford I. F., Tanner N. S. Prostaglandin-like material extracted from squamous carcinomas of the head and neck. Br J Cancer. 1980 Feb;41(2):204–208. doi: 10.1038/bjc.1980.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. J., Brazell I. A., Owen M. The effect of parathyroid extract on cellular activity and plasma calcium levels in vivo. J Endocrinol. 1969 Nov;45(3):387–400. doi: 10.1677/joe.0.0450387. [DOI] [PubMed] [Google Scholar]
- Bonucci E. New knowledge on the origin, function and fate of osteoclasts. Clin Orthop Relat Res. 1981 Jul-Aug;(158):252–269. [PubMed] [Google Scholar]
- Brenner D. E., Harvey H. A., Lipton A., Demers L. A study of prostaglandin E2, parathormone, and response to indomethacin in patients with hypercalcemia of malignancy. Cancer. 1982 Feb 1;49(3):556–561. doi: 10.1002/1097-0142(19820201)49:3<556::aid-cncr2820490327>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Ali N. N. Inhibition of osteoclastic motility by prostaglandins I2, E1, E2 and 6-oxo-E1. J Pathol. 1983 Mar;139(3):383–397. doi: 10.1002/path.1711390313. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Athanasou N. A., Fuller K. Effect of parathyroid hormone and calcitonin on the cytoplasmic spreading of isolated osteoclasts. J Endocrinol. 1984 Sep;102(3):281–286. doi: 10.1677/joe.0.1020281. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Dunn C. J. Pharmacological control of osteoclastic motility. Calcif Tissue Int. 1983 Jul;35(4-5):566–570. doi: 10.1007/BF02405095. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Moore A. The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab. 1983 Oct;57(4):819–824. doi: 10.1210/jcem-57-4-819. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Phagocytosis and trypsin-resistant glass adhesion by osteoclasts in culture. J Pathol. 1979 Feb;127(2):55–60. doi: 10.1002/path.1711270202. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Revell P. A., Fuller K., Athanasou N. A. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984 Mar;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. The cellular basis of bone resorption. Clin Orthop Relat Res. 1980 Sep;(151):283–293. [PubMed] [Google Scholar]
- Doty S. B., Schofield B. H. Electron microscopic localization of hydrolytic enzymes in osteoclasts. Histochem J. 1972 May;4(3):245–258. doi: 10.1007/BF01890996. [DOI] [PubMed] [Google Scholar]
- Drezner M. K., Harrelson J. M. Newer knowledge of vitamin D and its metabolites in health and disease. Clin Orthop Relat Res. 1979 Mar-Apr;(139):206–231. [PubMed] [Google Scholar]
- Eggert F. M. Stable acid phosphatase: II. Effects of pH and inhibitors. Histochemistry. 1980;66(3):319–329. doi: 10.1007/BF00495745. [DOI] [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Association of increased cyclic adenosine 3':5'-monophosphate content in cultured human breast cancer cells and release of hydrolytic enzymes and bone-resorbing activity. Cancer Res. 1983 Dec;43(12 Pt 1):5792–5794. [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978 Dec 14;276(5689):726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- Faccini J. M. The mode of growth of experimental metastases in rabbit femora. Virchows Arch A Pathol Anat Histol. 1974;364(3):249–263. doi: 10.1007/BF00433077. [DOI] [PubMed] [Google Scholar]
- Fallon M. D., Teitelbaum S. L., Kahn A. J. Multinucleation enhances macrophage-mediated bone resorption. Lab Invest. 1983 Aug;49(2):159–164. [PubMed] [Google Scholar]
- Galasko C. S., Bennett A. Relationship of bone destruction in skeletal metastases to osteoclast activation and prostaglandins. Nature. 1976 Oct 7;263(5577):508–510. doi: 10.1038/263508a0. [DOI] [PubMed] [Google Scholar]
- Galasko C. S. Mechanisms of bone destruction in the development of skeletal metastases. Nature. 1976 Oct 7;263(5577):507–508. doi: 10.1038/263507a0. [DOI] [PubMed] [Google Scholar]
- Galasko C. S., Samuel A. W., Rushton S., Lacey E. The effect of prostaglandin synthesis inhibitors and diphosphonates on tumour-mediated osteolysis. Br J Surg. 1980 Jul;67(7):493–496. doi: 10.1002/bjs.1800670714. [DOI] [PubMed] [Google Scholar]
- Galasko C. S. The pathological basis for skeletal scintigraphy. J Bone Joint Surg Br. 1975 Aug;57(3):353–359. [PubMed] [Google Scholar]
- Gingell D. Computed surface potential changes with membrane interaction. J Theor Biol. 1968 Jun;19(3):340–344. doi: 10.1016/0022-5193(68)90147-1. [DOI] [PubMed] [Google Scholar]
- Goodson J. M., Dewhirst F. E., Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974 Apr 10;6(1):81–85. doi: 10.1016/s0090-6980(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Grossman B., Schechter G. P., Horton J. E., Pierce L., Jaffe E., Wahl L. Hypercalcemia associated with T-cell lymphoma-leukemia. Am J Clin Pathol. 1981 Feb;75(2):149–155. doi: 10.1093/ajcp/75.2.149. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Lingelbach S. R., Partridge N. C., Martin T. J. Stimulation of plasminogen activator in osteoblast-like cells by bone-resorbing hormones. Biochem Biophys Res Commun. 1984 Jul 18;122(1):230–236. doi: 10.1016/0006-291x(84)90464-9. [DOI] [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981 Mar 19;290(5803):249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Harris M., Jenkins M. V., Bennett A., Wills M. R. Prostaglandin production and bone resorption by dental cysts. Nature. 1973 Sep 28;245(5422):213–215. doi: 10.1038/245213a0. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Meikle M. C., Atkinson S. J., Reynolds J. J. A factor synthesized by rabbit periosteal fibroblasts stimulates bone resorption and collagenase production by connective tissue cells in vitro. Biochim Biophys Acta. 1984 Aug 21;800(3):301–305. doi: 10.1016/0304-4165(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Hogg N., Shapiro I. M., Jones S. J., Slusarenko M., Boyde A. Lack of Fc receptors on osteoclasts. Cell Tissue Res. 1980;212(3):509–516. doi: 10.1007/BF00236514. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Mergenhagen S. E., Raisz L. G. Macrophage-lymphocyte synergy in the production of osteoclast activating factor. J Immunol. 1974 Oct;113(4):1278–1287. [PubMed] [Google Scholar]
- Hume D. A., Loutit J. F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984 Mar;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Jaworski Z. F., Duck B., Sekaly G. Kinetics of osteoclasts and their nuclei in evolving secondary Haversian systems. J Anat. 1981 Oct;133(Pt 3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Jilka R. L., Cohn D. V. A collagenolytic response to parathormone, 1,25-dihydroxycholecalciferol D3, and prostaglandin E2 in bone of osteopetrotic (mi/mi) mice. Endocrinology. 1983 Mar;112(3):945–950. doi: 10.1210/endo-112-3-945. [DOI] [PubMed] [Google Scholar]
- Josse R. G., Murray T. M., Mundy G. R., Jez D., Heersche J. N. Observations on the mechanism of bone resorption induced by multiple myeloma marrow culture fluids and partially purified osteoclast-activating factor. J Clin Invest. 1981 May;67(5):1472–1481. doi: 10.1172/JCI110177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye M. When is it an osteoclast? J Clin Pathol. 1984 Apr;37(4):398–400. doi: 10.1136/jcp.37.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating A., Singer J. W., Killen P. D., Striker G. E., Salo A. C., Sanders J., Thomas E. D., Thorning D., Fialkow P. J. Donor origin of the in vitro haematopoietic microenvironment after marrow transplantation in man. Nature. 1982 Jul 15;298(5871):280–283. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Raisz L. G. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970 Jun;86(6):1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- Ko J. S., Bernard G. W. Osteoclast formation in vitro from bone marrow mononuclear cells in osteoclast-free bone. Am J Anat. 1981 Aug;161(4):415–425. doi: 10.1002/aja.1001610407. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983 Oct;62(4):709–721. [PubMed] [Google Scholar]
- Krane S. M. Heberden Oration 1980: aspects of the cell biology of the rheumatoid synovial lesion. Ann Rheum Dis. 1981 Oct;40(5):433–448. doi: 10.1136/ard.40.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Rader J. I., Gruber H., Baylink D. J. Acute reduction in osteoclast number during bone repletion. Metab Bone Dis Relat Res. 1982;4(3):201–209. doi: 10.1016/0221-8747(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Raisz L. G., Hock J. M. DNA synthesis is not necessary for osteoclastic responses to parathyroid hormone in cultured fetal rat long bones. J Clin Invest. 1983 Dec;72(6):1924–1929. doi: 10.1172/JCI111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. F., Nisbet N. W. Resorption of bone. Lancet. 1979 Jul 7;2(8132):26–27. doi: 10.1016/s0140-6736(79)90186-7. [DOI] [PubMed] [Google Scholar]
- Loutit J. F., Nisbet N. W. The origin of osteoclasts. Immunobiology. 1982 Apr;161(3-4):193–203. doi: 10.1016/S0171-2985(82)80074-0. [DOI] [PubMed] [Google Scholar]
- Loutit J. F., Townsend K. M. Longevity of osteoclasts in radiation chimaeras of osteopetrotic beige and normal mice. Br J Exp Pathol. 1982 Apr;63(2):221–223. [PMC free article] [PubMed] [Google Scholar]
- Luben R. A., Cain C. D., Chen M. C., Rosen D. M., Adey W. R. Effects of electromagnetic stimuli on bone and bone cells in vitro: inhibition of responses to parathyroid hormone by low-energy low-frequency fields. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4180–4184. doi: 10.1073/pnas.79.13.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luben R. A., Chen M. C., Rosen D. M., Mohler M. A. Effects of osteoclast activating factor from human lymphocytes on cyclic AMP concentrations in isolated mouse bone and bone cells. Calcif Tissue Int. 1979 Aug 24;28(1):23–32. doi: 10.1007/BF02441214. [DOI] [PubMed] [Google Scholar]
- MILCH R. A., CHANGUS G. W. Response of bone to tumor invasion. Cancer. 1956 Mar-Apr;9(2):340–351. doi: 10.1002/1097-0142(195603/04)9:2<340::aid-cncr2820090221>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Schneider G. B. Transformations of osteoclast phenotype in ia rats cured of congenital osteopetrosis. J Morphol. 1982 Nov;174(2):141–147. doi: 10.1002/jmor.1051740203. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr The origin of osteoclasts: evidence, clinical implications and investigative challenges of an extra-skeletal source. J Oral Pathol. 1983 Aug;12(4):226–256. doi: 10.1111/j.1600-0714.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Marshall M. J., Nisbet N. W., Evans S. Donor origin of the in vitro hematopoietic microenvironment after marrow transplantation in mice. Experientia. 1984 Apr 15;40(4):385–386. doi: 10.1007/BF01952566. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- McDonnell G. D., Dunstan C. R., Evans R. A., Carter J. N., Hills E., Wong S. Y., McNeil D. R. Quantitative bone histology in the hypercalcemia of malignant disease. J Clin Endocrinol Metab. 1982 Dec;55(6):1066–1072. doi: 10.1210/jcem-55-6-1066. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Bowman B. M., Myers R. L. Morphological and ultrastructural aspects of the activation of avian medullary bone osteoclasts by parathyroid hormone. Anat Rec. 1984 Feb;208(2):223–231. doi: 10.1002/ar.1092080209. [DOI] [PubMed] [Google Scholar]
- Miller S. C. Rapid activation of the medullary bone osteoclast cell surface by parathyroid hormone. J Cell Biol. 1978 Mar;76(3):615–618. doi: 10.1083/jcb.76.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Wolf A. M., Arnaud C. D. Bone cells in culture: morphologic transformation by hormones. Science. 1976 Jun 25;192(4246):1340–1343. doi: 10.1126/science.1273593. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Martin T. J. The hypercalcemia of malignancy: pathogenesis and management. Metabolism. 1982 Dec;31(12):1247–1277. doi: 10.1016/0026-0495(82)90012-9. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Raisz L. G., Cooper R. A., Schechter G. P., Salmon S. E. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974 Nov 14;291(20):1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Murad F., Brewer H. B., Jr, Vaughan M. Effect of thyrocalcitonin on adenosine 3':5'-cyclic phosphate formation by rat kidney and bone. Proc Natl Acad Sci U S A. 1970 Feb;65(2):446–453. doi: 10.1073/pnas.65.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbaitz R., Stumpf W. E., Sar M., Huang S., DeLuca H. F. Autoradiographic localization of target cells for 1 alpha, 25-dihydroxyvitamin D3 in bones from fetal rats. Calcif Tissue Int. 1983;35(2):177–182. doi: 10.1007/BF02405028. [DOI] [PubMed] [Google Scholar]
- Nimberg R. B., Humphries D. E., Lloyd W. S., Badger A. M., Cooperband S. R., Wells H., Schmid K. Isolation of a bone-resorptive factor from human cancer ascites fluid. Cancer Res. 1978 Jul;38(7):1983–1989. [PubMed] [Google Scholar]
- Partridge N. C., Kemp B. E., Livesey S. A., Martin T. J. Activity ratio measurements reflect intracellular activation of adenosine 3',5'-monophosphate-dependent protein kinase in osteoblasts. Endocrinology. 1982 Jul;111(1):178–183. doi: 10.1210/endo-111-1-178. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Kemp B. E., Veroni M. C., Martin T. J. Activation of adenosine 3',5'-monophosphate-dependent protein kinase in normal and malignant bone cells by parathyroid hormone, prostaglandin E2, and prostacyclin. Endocrinology. 1981 Jan;108(1):220–225. doi: 10.1210/endo-108-1-220. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion reaction: a theory. J Theor Biol. 1971 Jul;32(1):165–184. doi: 10.1016/0022-5193(71)90144-5. [DOI] [PubMed] [Google Scholar]
- Powles T. J., Dowsett M., Easty G. C., Easty D. M., Neville A. M. Breast-cancer osteolysis, bone metastases, and anti-osteolytic effect of aspirin. Lancet. 1976 Mar 20;1(7960):608–610. doi: 10.1016/s0140-6736(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Puzas J. E., Brand J. S. Parathyroid hormone stimulation of collagenase secretion by isolated bone cells. Endocrinology. 1979 Feb;104(2):559–562. doi: 10.1210/endo-104-2-559. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. Stimulation of bone resorption by parathyroid hormone in tissue culture. Nature. 1963 Mar 9;197:1015–1016. doi: 10.1038/1971015a0. [DOI] [PubMed] [Google Scholar]
- Raina V. Normal osteoid tissue. J Clin Pathol. 1972 Mar;25(3):229–232. doi: 10.1136/jcp.25.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Ralston S., Fogelman I., Gardner M. D., Boyle I. T. Hypercalcaemia and metastatic bone disease: is there a causal link? Lancet. 1982 Oct 23;2(8304):903–905. doi: 10.1016/s0140-6736(82)90868-6. [DOI] [PubMed] [Google Scholar]
- Roberts W. E. Cell population dynamics of periodontal ligament stimulated with parathyroid extract. Am J Anat. 1975 Jul;143(3):363–370. doi: 10.1002/aja.1001430307. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Tashjian A. H., Jr, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975 Nov;56(5):1181–1188. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan G. A., Bourret L. A., Harvey A., Mensi T. Cyclic AMP and cyclic GMP: mediators of the mechanical effects on bone remodeling. Science. 1975 Aug 8;189(4201):467–469. doi: 10.1126/science.168639. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Rude R. K., Sharp C. F., Jr, Fredericks R. S., Oldham S. B., Elbaum N., Link J., Irwin L., Singer F. R. Urinary and nephrogenous adenosine 3',5'-monophosphate in the hypercalcemia of malignancy. J Clin Endocrinol Metab. 1981 Apr;52(4):765–771. doi: 10.1210/jcem-52-4-765. [DOI] [PubMed] [Google Scholar]
- SHIVAS A. A., BLACK J. W., FINLAYSON N. D. THE GROWTH OF BROWN-PEARCE CARCINOMA IN THE MEDULLARY CAVITY OF THE RABBIT FEMUR. Br J Cancer. 1963 Dec;17:711–714. doi: 10.1038/bjc.1963.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M. Biochemical and immunohistochemical studies on collagenase in resorbing bone in tissue culture. A novel hypothesis for the mechanism of bone resorption. J Periodontal Res. 1982 Sep;17(5):523–526. doi: 10.1111/j.1600-0765.1982.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Scherft J. P. The lamina limitans of the organic matrix of calcified cartilage and bone. J Ultrastruct Res. 1972 Feb;38(3):318–331. doi: 10.1016/s0022-5320(72)90008-1. [DOI] [PubMed] [Google Scholar]
- Schneider G. B., Byrnes J. E. Cellular specificity of the cure for neonatal osteopetrosis in the ia rat. Exp Cell Biol. 1983;51(1):44–50. doi: 10.1159/000163172. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Segre G. V., Morgan J. L., Sweetman B. J., Potts J. T., Jr, Oates J. A. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N Engl J Med. 1975 Dec 18;293(25):1278–1283. doi: 10.1056/NEJM197512182932502. [DOI] [PubMed] [Google Scholar]
- Silve C. M., Hradek G. T., Jones A. L., Arnaud C. D. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol. 1982 Aug;94(2):379–386. doi: 10.1083/jcb.94.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen D., Binderman I., Berger E., Harell A. Bone remodelling induced by physical stress is prostaglandin E2 mediated. Biochim Biophys Acta. 1980 Jan 3;627(1):91–100. doi: 10.1016/0304-4165(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Stern P. H. A monolog on analogs: in vitro effects of vitamin D metabolites and consideration of the mineralization question. Calcif Tissue Int. 1981;33(1):1–4. doi: 10.1007/BF02409404. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Horst R., Deftos L. J., Cadman E. C., Lang R., Broadus A. E. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. N Engl J Med. 1980 Dec 11;303(24):1377–1383. doi: 10.1056/NEJM198012113032401. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Vignery A., Silverglate A., Ravin N. D., LiVolsi V., Broadus A. E., Baron R. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982 Aug;55(2):219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- TONNA E. A. Periosteal osteoclasts, skeletal development and ageing. Nature. 1960 Feb 6;185:405–407. doi: 10.1038/185405a0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Role of prostaglandins in the production of hypercalcemia by tumors. Cancer Res. 1978 Nov;38(11 Pt 2):4138–4141. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Goldhaber P., Levine L. Successful treatment of hypercalcemia by indomethacin in mice bearing a prostaglandin-producing fibrosarcoma. Prostaglandins. 1973 Apr;3(4):515–524. doi: 10.1016/0090-6980(73)90161-5. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- Tran Van P. T., Vignery A., Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982 Apr;202(4):445–451. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- Tsao S. W., Burman J. F., Easty D. M., Easty G. C., Carter R. L. Some mechanisms of local bone destruction by squamous carcinomas of the head and neck. Br J Cancer. 1981 Mar;43(3):392–401. doi: 10.1038/bjc.1981.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J. L., DeLuca H. F. Vitamin D is not directly necessary for bone growth and mineralization. Am J Physiol. 1984 Jun;246(6 Pt 1):E493–E498. doi: 10.1152/ajpendo.1984.246.6.E493. [DOI] [PubMed] [Google Scholar]
- Vaes G. Cartilage and bone tissue damage in arthritis: cellular co-operation and enzymatic mechanisms. Scand J Rheumatol Suppl. 1981;40:65–71. doi: 10.3109/03009748109102881. [DOI] [PubMed] [Google Scholar]
- Valentin-Opran A., Charhon S. A., Meunier P. J., Edouard C. M., Arlot M. E. Quantitative histology of myeloma-induced bone changes. Br J Haematol. 1982 Dec;52(4):601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Warshawsky H., Goltzman D., Rouleau M. F., Bergeron J. J. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980 Jun;85(3):682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. L., Luben R. A., Cohn D. V. 1,25-dihydroxycholecalciferol and parathormone: effects on isolated osteoclast-like and osteoblast-like cells. Science. 1977 Aug 12;197(4304):663–665. doi: 10.1126/science.195343. [DOI] [PubMed] [Google Scholar]
- Yu J. H., Wells H., Ryan W. J., Lloyd W. S. Effects of prostaglandins and other drugs on the cyclic AMP content of cultured bone cells. Prostaglandins. 1976 Oct;12(4):501–513. doi: 10.1016/0090-6980(76)90031-9. [DOI] [PubMed] [Google Scholar]