Abstract
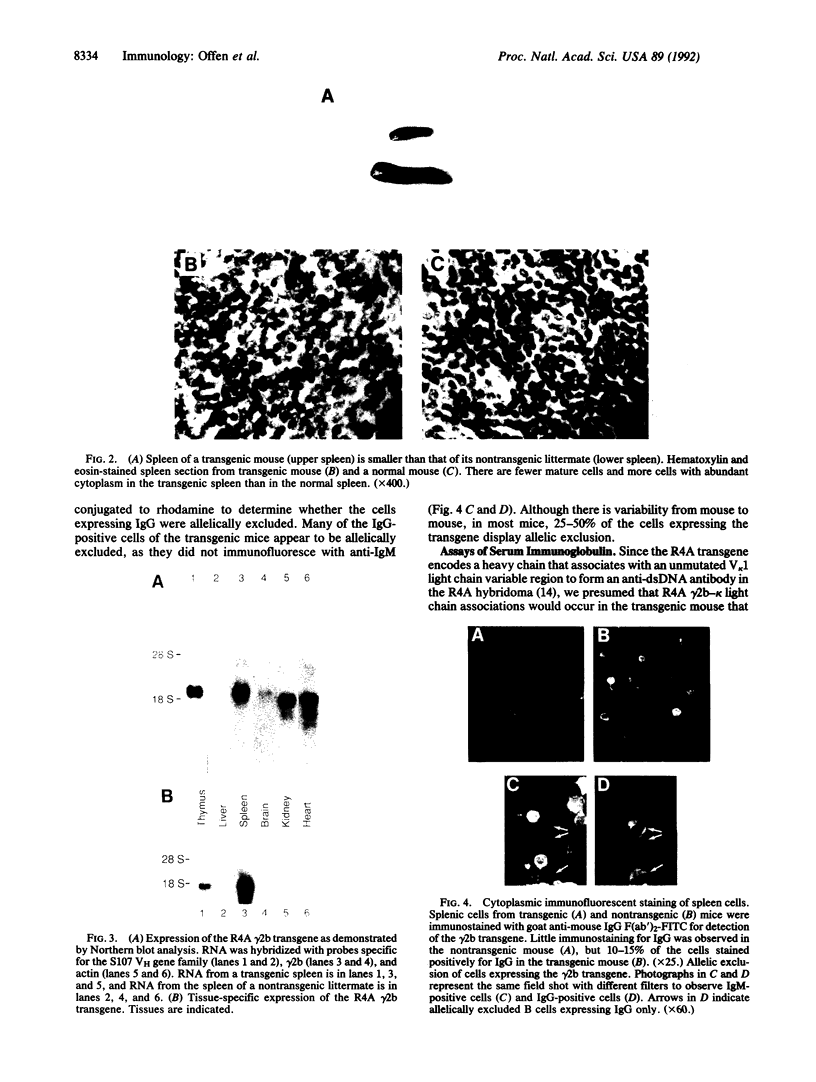
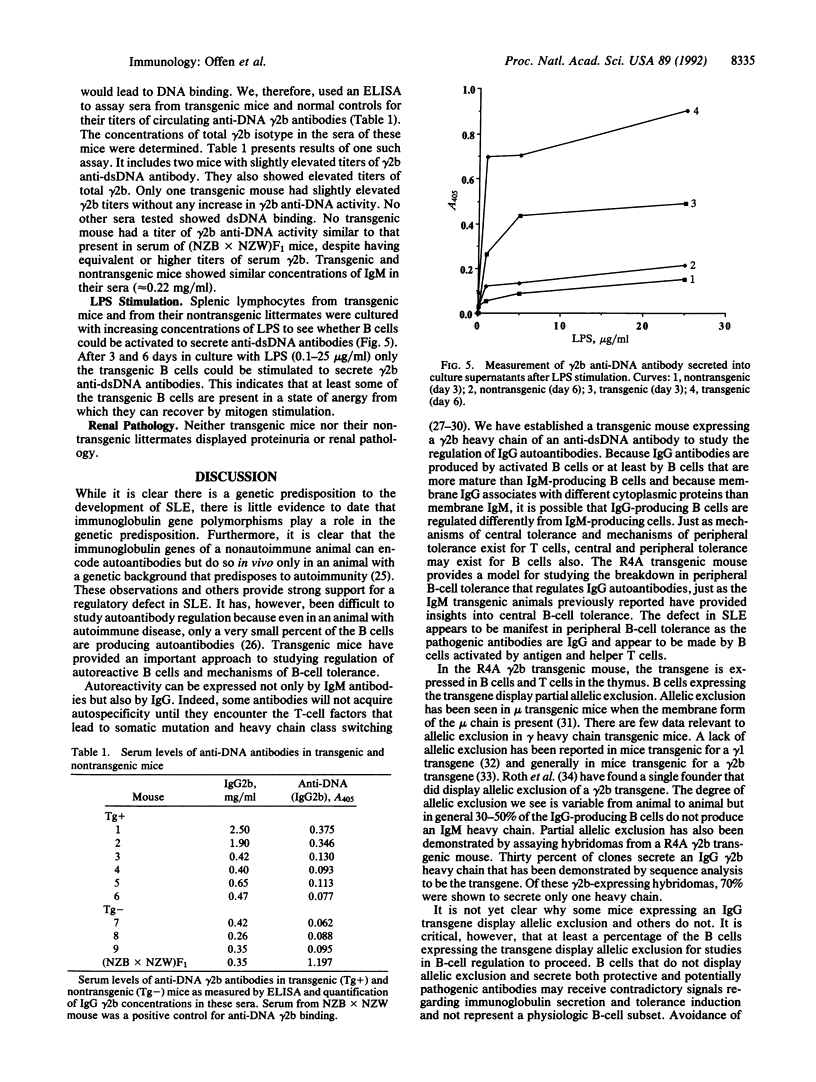
Nonautoimmune mice transgenic for the heavy chain of an IgG2b anti-double-stranded-DNA antibody express the transgene in lymphoid organs and display partial allelic exclusion of this gamma 2b transgene. The spleens of these mice are characterized by marked B-cell depletion. Although there are B cells in these mice that express the transgene and recognize double-stranded DNA, they are anergic in vivo. Recovery from the state of anergy occurs in vitro after lipopolysaccharide stimulation. Thus this transgenic model demonstrates the induction of self tolerance to an IgG autoantibody.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando D. G., Ebling F. M., Hahn B. H. Detection of native and denatured DNA antibody forming cells by the enzyme-linked immunospot assay. A clinical study of (New Zealand black x New Zealand white)F1 mice. Arthritis Rheum. 1986 Sep;29(9):1139–1146. doi: 10.1002/art.1780290912. [DOI] [PubMed] [Google Scholar]
- Behar S. M., Scharff M. D. Somatic diversification of the S107 (T15) VH11 germ-line gene that encodes the heavy-chain variable region of antibodies to double-stranded DNA in (NZB x NZW)F1 mice. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3970–3974. doi: 10.1073/pnas.85.11.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Page D. M., Blum J. H., Gold M. R. Signal transduction by the antigen receptor of B lymphocytes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):733–740. doi: 10.1101/sqb.1989.054.01.086. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Webster D. M., Rees A. R. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988 Sep 1;141(5):1745–1753. [PubMed] [Google Scholar]
- Erikson J., Radic M. Z., Camper S. A., Hardy R. R., Carmack C., Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991 Jan 24;349(6307):331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Gavalchin J., Nicklas J. A., Eastcott J. W., Madaio M. P., Stollar B. D., Schwartz R. S., Datta S. K. Lupus prone (SWR x NZB)F1 mice produce potentially nephritogenic autoantibodies inherited from the normal SWR parent. J Immunol. 1985 Feb;134(2):885–894. [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991 Oct 24;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Murakami M., Shimizu A., Ozaki S., Tsubata T., Kumagai S., Honjo T. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992 Jan 1;175(1):71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radic M. Z., Mascelli M. A., Erikson J., Shan H., Weigert M. Ig H and L chain contributions to autoimmune specificities. J Immunol. 1991 Jan 1;146(1):176–182. [PubMed] [Google Scholar]
- Scott D. W., Alés-Martínez J. E., Chace J. H., LoCascio N. J., Silver L., Warner G. L. Models of B-cell unresponsiveness. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):899–905. doi: 10.1101/sqb.1989.054.01.105. [DOI] [PubMed] [Google Scholar]
- Shefner R., Kleiner G., Turken A., Papazian L., Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991 Feb 1;173(2):287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Honjo T. Immunoglobulin class switching. Cell. 1984 Apr;36(4):801–803. doi: 10.1016/0092-8674(84)90029-1. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storb U., Engler P., Manz J., Gollahon K., Denis K., Lo D., Brinster R. Expression of immunoglobulin genes in transgenic mice and transfected cells. Ann N Y Acad Sci. 1988;546:51–56. doi: 10.1111/j.1749-6632.1988.tb21618.x. [DOI] [PubMed] [Google Scholar]
- Storb U. Transgenic mice with immunoglobulin genes. Annu Rev Immunol. 1987;5:151–174. doi: 10.1146/annurev.iy.05.040187.001055. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepicchio W., Jr, Barrett K. J. Eleven MRL-lpr/lpr anti-DNA autoantibodies are encoded by genes from four VH gene families: a potentially biased usage of VH genes. J Immunol. 1987 Apr 1;138(7):2323–2331. [PubMed] [Google Scholar]
- Tsang H., Pinkert C., Hagman J., Lostrum M., Brinster R. L., Storb U. Cloning of a gamma 2b gene encoding anti-Pseudomonas aeruginosa H chains and its introduction into the germ line of mice. J Immunol. 1988 Jul 1;141(1):308–314. [PubMed] [Google Scholar]
- Tucker P. W., Marcu K. B., Newell N., Richards J., Blattner F. R. Sequence of the cloned gene for the constant region of murine gamma 2b immunoglobulin heavy chain. Science. 1979 Dec 14;206(4424):1303–1306. doi: 10.1126/science.117549. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- Yamamura K., Kudo A., Ebihara T., Kamino K., Araki K., Kumahara Y., Watanabe T. Cell-type-specific and regulated expression of a human gamma 1 heavy-chain immunoglobulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2152–2156. doi: 10.1073/pnas.83.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]