Abstract
Wheat is one of the most important crops in the world, and osmotic stress has become one of the main factors affecting wheat production. Understanding the mechanism of the response of wheat to osmotic stress would be greatly significant. In the present study, isobaric tag for relative and absolute quantification (iTRAQ) was used to analyze the changes of protein expression in the wheat roots exposed to different osmotic stresses. A total of 2,228 expressed proteins, including 81 differentially expressed proteins, between osmotic stress and control, were found. The comprehensive analysis of these differentially expressed proteins revealed that osmotic stress increased the variety of expressed proteins and suppressed the quantity of expressed proteins in wheat roots. Furthermore, the proteins for detoxifying and reactive oxygen species scavenging, especially the glutathione system, played important roles in maintaining organism balance in response to osmotic stress in wheat roots. Thus, the present study comprehensively describes the protein expression changes in wheat roots in response to osmotic stress, providing firmer foundation to further study the mechanism of osmotic resistance in wheat.
Keywords: Triticum aestivum L., Root, iTRAQ, Osmotic stress, Glutathione
Osmotic stress, primarily resulting from drought or excessive salt in water, refers to insufficient water availability that limits plant growth and development (Zhu et al., 1997). Osmotic stress has become one of the major abiotic stresses affecting crop growth and production. For high-yield and high-quality production, it is imperative to improve the osmotic tolerance of crops, and some methods had been developed to alleviate osmotic stress through cultural practices, conventional breeding, exogenous regulators and molecular breeding. However, this situation has not substantially changed. To further improve osmotic tolerance, it is necessary to understand the responding mechanism of osmotic resistance in plants. Previous studies in rice, Arabidopsis, and other plants have been performed, including molecular cloning, transgenic studies and high throughput analyses.
Previous studies have primarily focused on molecular cloning and functional analysis of osmotic resistance genes. Many functional genes with high osmotic resistance have been identified. The water loss rate of transgenic Arabidopsis with AtMYB15 over-expression was significantly reduced compared with that in wild-type under drought conditions (Ding et al., 2009). Using cDNA microarray analysis, Hu et al. (2006) observed that SNAC1 was up-regulated in rice under drought stress and the over-expression of SNAC1 enhanced drought tolerance in transgenic rice. Subsequently, Liu et al. (2014) achieved SNAC1 over-expression in cotton and found that the tolerance to drought and salt stresses was significantly improved in these transgenic plants. In addition, many other genes, such as the WRKY (Qiu & Yu, 2009; Ma et al., 2014) transcription factor, DREB (Liu et al., 1998) and AtGAMT1 (Arabidopsis thaliana GA methyl transferase 1) (Nir, Moshelion & Weiss, 2014; Qin & Zeevaart, 2002; Shou, Bordallo & Wang, 2004; Pasquali et al., 2008), have also been implicated in drought or salt tolerance in plants. Based on these osmotic tolerance genes, some gene regulatory networks in response to osmotic stress were also identified in plants, indicating that the mechanism of osmotic resistance is complex with multigenic control (Shinozaki, Yamaguchi-Shinozakiy & Sekiz, 2003; Valliyodan & Nguyen, 2006; Krasensky & Jonak, 2012).
In recent years, high throughput screening platforms have been rapidly developed, providing more comprehensive insights into the cellular and molecular mechanisms of the response to osmotic stress. In Arabidopsis, a gene microarray was performed under drought, high-salinity and cold stresses in 2008, and thousands of stress-related genes were identified, many of which had been previously reported (Matsui et al., 2008). Lenka et al. (2011) performed a transcriptome analysis of drought-tolerant and drought-sensitive rice cultivars, and found that the up-regulation of the α-linolenic acid metabolic pathway was closely associated with drought responses. Many studies on plant responses to osmotic stress have also been performed using RNA-seq or microarray analysis (Zheng et al., 2010; Le et al., 2012; Li et al., 2012). The results of these studies provided a platform for understanding the responses of osmotic stress at the level of gene expression.
However, proteins directly participate in the activities of organisms, and proteomic analysis has become the best strategy for studying the response of organisms to osmotic stress. Many studies have already been performed in this area. Mirzaei et al. (2012) conducted a quantitative label-free shotgun proteomic analysis using the root tissues of rice plants under four different drought treatments, and 1,487 differentially expressed proteins (DEPs) were identified. After further analysis of the DEPs, Mirzaei et al. (2012) found that the proteins involved in transport and reactive oxygen species (ROS) were highly dependent on drought signals. In cotton, Deeba et al. (2012) identified 22 drought-related proteins through two-dimensional gel electrophoresis (2-DE) analysis. In wheat, many studies on osmotic stress were also performed using 2-DE, and some osmotic-related proteins and processes were also identified (Peng et al., 2009; Caruso et al., 2009; Ge et al., 2012). However, these studies could not comprehensively describe the protein expression changes under osmotic stress due to the limitations of the technology.
The isobaric tag for relative and absolute quantification (iTRAQ) system, which uses isotope labeling combined with multidimensional liquid chromatography and tandem mass spectrometry (MS) (Fan et al., 2011), simultaneously identified and quantitatively compared proteins expressed in an organism by analyzing the peak intensities of reporter ions (Lan et al., 2011). It can provide more global information of proteins expression for proteomic analysis. In the present study, we performed proteomic analysis using iTRAQ to analyze the osmotic response in the root of wheat seedlings. A total of 2,228 proteins were identified, among which 81 proteins were found to be related to osmotic stress in wheat.
Materials and Methods
Plant materials and the measurement of relative water content (RWC)
Seeds of Aikang58 were sterilized using 0.1% HgCl2 for 7 min and washed eight times with sterile distilled water. Subsequently, the seeds were cultured in Petri dishes in a chamber under the same conditions according to Li et al. (2013). At the two-leaf stage, the wheat seedlings were transferred into Hoagland solution containing 0%, 5%, 10%, 15% and 20% PEG-6000 to simulate osmotic stress. After cultivation for 24 h, the root tissues from seedlings exposed to the five treatments were collected and frozen in −80 °C for subsequent experiments. RWC was measured according to Gao et al. (2011).
Protein extraction
Frozen root samples were thoroughly ground into powder in liquid nitrogen. Lysis buffer (pH 8.5), containing 2 M thiourea, 7 M urea and 4% CHAPS with protease inhibitor (Sigma, USA), was added to the powder at 1:10 (w/v). The mixture was sonicated for 60 s and extracted for 30 min at room temperature. Subsequently, the mixture was centrifuged at 40,000 g for 1 h at 10 °C, and the supernatant was transferred to a 50 mL tube containing four volumes of 10% (w/v) TAC/acetone. After mixing, the mixture was stored at −20 °C overnight, and the supernatant was removed after centrifugation at 40,000 g for 10 min at 4 °C. The protein was washed three times with acetone and then dried through lyophilization to form a protein powder, and suspended in lysis buffer (2 M thiourea, 7 M urea and 4% CHAPS). The protein concentration was determined using the Bradford assay with BSA as a standard. The remaining samples were stored at −80 °C until further use.
Trypsin digestion and iTRAQ labeling
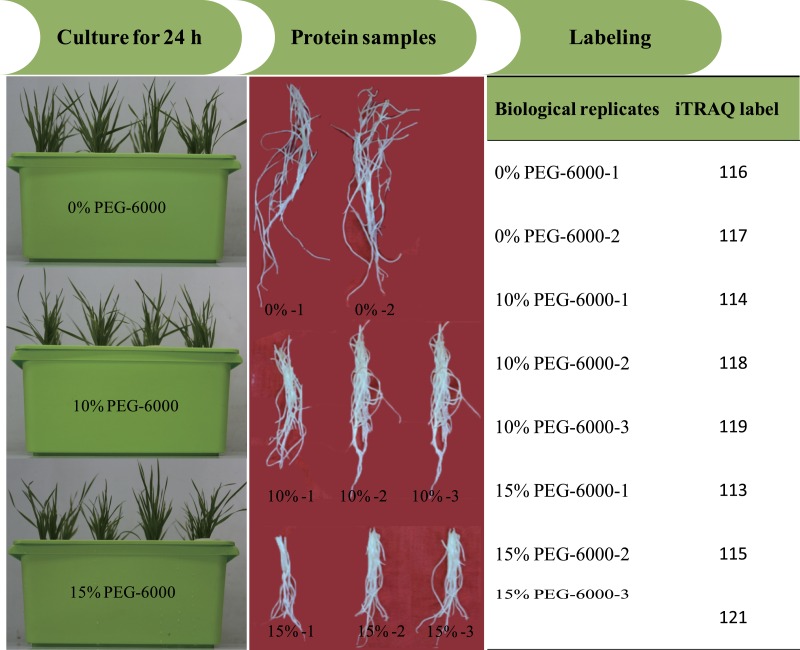
All reagents and buffers for iTRAQ labeling and cleaning were purchased from Applied Biosystems (Foster City, CA, USA). iTRAQ labeling was performed according to the manufacturer’s instructions. The proteins were dissolved, denatured, alkylated and digested with trypsin at 37 °C overnight. And 100 µg of the digestion product were thawed and reconstituted in 150 µL of isopropanol, and subsequently labelled with iTRAQ reagent (Applied Biosystems). The iTRAQ experiment just contained two experiment settings of four-plex and eight-plex, which could only analyze four or eight samples once time respectively. In previous studies, researchers had performed the eight-plex iTRAQ experiments using the setting of 3:3:2 and 3:2 (Longworth et al., 2012; Ge et al., 2014). In the present study, the experiment setting of 3:3:2 (eight-plex) was selected for this analysis. The three biological replicates of roots exposed to 15% PEG-6000 treatment were labeled with 113, 115 and 121 tags, the three biological replicates of roots exposed to 10% PEG-6000 treatment were labeled with 114, 118 and 119 tags, and the two biological replicates of control (0% PEG-6000) were labeled with 116 and 117 tags (Fig. 1). Subsequently, the labeled samples were pooled in equal ratios. The labeled peptide mixture was dissolved in 100 µL mobile of phase A (2% (v/v) acetonitrile, 98% (v/v) ddH2O, pH 10) and subsequently centrifuged at 14,000 g for 20 min. The supernatant was carefully collected and further loaded onto the column for stepwise elution through the injection of mobile phase B (98% acetonitrile, 2% ddH2O, pH 10) with a 700 µl/min flow rate. The fractions were eluted (1.8 min each) and collected using step gradients of mobile phase B.
Figure 1. The illustration of the experimental design.
Analysis using Q-Exactive mass spectrometer
The fractionated peptides were analyzed using a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) fitted with a nano-liquid chromatography system (Thermo Scientific EASY-nLC 1000 System). A binary solvent system comprising 99.9% H2O, 0.1% formic acid (phase A) and 99.9% ACN, 0.1% formic acid (phase B) were used to elute the peptides. The following linear gradient was used: 4–8% B in 5 min, 8–35% B in 35 min, 35–90% B in 5 min, washed at 95% B for 6 min, and equilibrated with 4% B for 8 min at a 350 nL/min flow rate. The eluent was further introduced to a Q-Exactive mass spectrometer via an EASY-Spray ion source. The following source ionization parameters were used: 2.1 kV spray voltage, capillary temperature 250 °C and 100 V declustering potential.
A Top 20 data-dependent mode with automatic switching between MS and MS/MS was used in mass spectrometer. Full-scan MS mode (350–1,800 m/z) was performed at a resolution of 70,000 with 1 × 106 ions automatic gain control (AGC) target and a maximum ion transfer (IT) of 60 ms. The precursor ions were fragmented using high-energy collisional dissociation (HCD) and subjected to MS/MS scans with the following parameters: 17,500 resolution, AGC with 5 × 106 ions, maximum IT with 70 ms, 5,000 intensity threshold and 29% normalized collision energy.
Sequence database searching and data analysis
Mascot 2.2 (Matrix Science, London, UK) and Proteome Discoverer 1.4 (Thermo Electron, San Jose, CA) were used for processing the raw data of MS/MS spectra and completing database search and a quantitative analysis against a non-redundant protein database of hexaploid wheat genome, which had been generated by Mayer et al. (2014) and provided as File S1. For database searching, the following parameters were used: trypsin enzyme, two missed cleavages at maximum, 20 ppm of peptide mass tolerance, 0.1 Da of fragment mass tolerance, carbamidomethylation of cysteine as fixed modification, methionine oxidation and iTRAQ 8 plex labels at the N-termini and at lysine side chains as dynamic modification. For protein identification, only peptides with significant scores (iron score ≥ 35) at 99% confidence interval were used, and 2,228 proteins were finally got, of which 1,391 proteins with two or more peptides were considered for further analysis. The protein fold-change was obtained based on the quantity comparison between each treatment sample and the average level of control. For statistical analysis, the average fold-change ≥ 95% confidence interval and P-values ≤ 0.05, which was got by the t-test with different repeat times in two groups, were considered significant. The sequence data of the DEPs was searched against the UniProt database for protein function, and the BlastKOALA website (http://www.kegg.jp/blastkoala/) was used for the KEGG analysis with an E-value of 1 × 10−5.
The mass spectrometry data have been deposited to the iProx database with the accession number: IPX00075800.
Phylogenetic analysis of glutathione S-transferases (GSTs)
Multiple amino acid sequence alignment of GSTs was performed using ClastalW. An unrooted phylogenetic tree of these GST protein sequences was constructed using the neighbor-joining method with MEGA 5.10 software, and a bootstrap analysis with 1,000 replicates was performed to assess the significance of each node.
Results and Discussion
The effects of osmotic stress on wheat seedlings
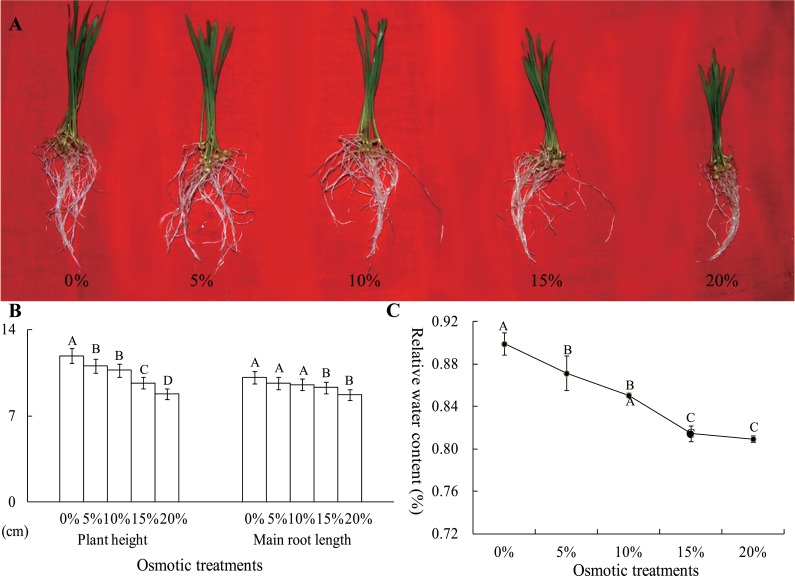
To analyze the effects of osmotic stress, five different osmotic treatments (0%, 5%, 10%, 15% and 20% PEG-6000) were performed on wheat seedlings at the two-leaf stage. After cultivation for 24 h, the plant height and main root length were severely restrained by osmotic stress, declining to 8.76 and 8.74 cm from 11.88 and 10.13 cm, respectively (Figs. 2A and 2B). The RWC of whole plants was measured, and this value was significantly different between the control and osmotic treatment samples. The RWC was 89.92% after a 0% PEG-6000 treatment and decreased to 81.44% after a 15% PEG-6000 treatment. However, when the PEG-6000 treatment increased to 20% from 15%, the RWC only decreased 0.48%, and this difference was not statistically significant (Fig. 2C). Based on these results, we found that treatment with 10% PEG-6000 for 24 h should be considered as mild osmotic stress (MOS), while treatment with 15% PEG-6000 for 24 h should be considered as severe osmotic stress (SOS).
Figure 2. The plant height, main root length (B) and RWC of wheat seedling at the two-leaf stage, which were exposed to five osmotic stresses, were measured to assess the effects of osmotic.
The data was analyzed by one-way ANOVA analysis, and the LSD method was used for multiple comparisons. The significant difference is represented by capital letters at 0.01 level.
Identification of root proteins under osmotic stress using iTRAQ
As roots directly sense osmotic stress, total protein was extracted from the root samples of wheat plants under control, MOS and SOS conditions (two, three and three replicates, respectively). The protein expression profiles of these eight root samples were analyzed in one 8-plex iTRAQ experiment. A total of 150,440 triggered MS/MS spectra were identified, and 2,228 proteins were identified by 7,392 peptides (File S2), and about 45.17% of the identified proteins included at least two unique peptides.
The DEPs between osmotic stress and control
The 95% confidence interval of each group distribution was constructed to analyze the maximum scope of difference within group, and the results showed that the maximum scope is 0.855–1.17 (File S2). To insure the difference between groups larger than the difference within group, a fold-change of more than 1.2 (more than 1.2 or less than 0.833) was selected as one of the parameters for DEPs selection. To enhance the confidence of DEPs, the following parameters were also considered: each protein with two or more peptides, at least two times differential expression among three repetition and a significance level of p < 0.05. Based on these four parameters, a total of 81 DEPs were identified, including 34 DEPs between the MOS and control samples and 64 DEPs between the SOS and control samples. Among these DEPs, 17 DEPs were common in the MOS and SOS samples compared with the control samples, 30 DEPs were down-regulated, and 51 DEPs were up-regulated under osmotic stress.
Analysis of the DEPs between osmotic stress and control
Among these DEPs, the molecular function information for 69 DEPs was identified. Many proteins had functions in processes, such as carbohydrate metabolism, protein metabolism, phytohormones responsive etc., and the plant protection system played important roles in the wheat roots response to osmotic stress (Tables 1 and 2).
Table 1. The differentially expressed proteins between MOS and control.
| Protein name | Protein function | Peptides | Coverage | Fold-change between MOS and control | P-value |
|---|---|---|---|---|---|
| Protein Metabolism | |||||
| Traes_2BS_7700613D4 | 40S ribosomal protein | 2 | 22.31 | 1.24 ± 0.05 | 0.012 |
| Traes_7AL_65F481DB9 | 40S ribosomal protein | 2 | 13.82 | 1.40 ± 0.15 | 0.044 |
| Traes_5DL_9D4164773 | 60S ribosomal protein | 2 | 8.20 | 1.21 ± 0.04 | 0.010 |
| Traes_1AL_D20D648FD | 60S ribosomal protein | 4 | 36.17 | 1.29 ± 0.11 | 0.047 |
| Traes_2AL_396E0F5A3 | 60S ribosomal protein | 2 | 15.03 | 1.51 ± 0.14 | 0.025 |
| Histone Protein | |||||
| Traes_1AS_4CA1A835D1 | Histone H2B | 4 | 50.00 | 1.41 ± 0.09 | 0.015 |
| Traes_4BL_96E367077 | Histone H2A | 3 | 21.33 | 1.46 ± 0.17 | 0.042 |
| CarhohydrateMetabolism | |||||
| Traes_7DS_529BAB150 | Sucrose synthase | 15 | 22.00 | 1.27 ± 0.10 | 0.040 |
| Traes_3B_BC152C5D7 | Glycosyltransferase | 2 | 6.18 | 1.23 ± 0.05 | 0.016 |
| Traes_2AL_AEB11A672 | Beta-glucosidase | 7 | 15.93 | 1.26 ± 0.09 | 0.036 |
| Traes_4AL_8845F411B | UDP-glucose 6-dehydrogenase | 12 | 31.03 | 0.70 ± 0.07 | 0.016 |
| Traes_7BS_DE33B2B49 | Fructokinase | 2 | 8.79 | 1.27 ± 0.09 | 0.037 |
| Traes_4AL_4B09F91AE | Alcohol dehydrogenase | 6 | 24.74 | 0.77 ± 0.02 | 0.003 |
| Phytohormones | |||||
| Traes_4DS_E2055C83D | Abscisic stress-ripening protein | 3 | 56.52 | 2.53 ± 0.26 | 0.009 |
| Antioxidant Protection Proteins | |||||
| Traes_1AL_46245C5D5 | Peroxidase | 2 | 42.47 | 0.79 ± 0.04 | 0.010 |
| Traes_2BS_9C71D6F5F | Peroxidase | 2 | 7.81 | 0.80 ± 0.03 | 0.007 |
| Traes_1DL_7BCE5B151 | Glutathione S-transferase | 3 | 20.37 | 1.60 ± 0.16 | 0.023 |
| Traes_1AL_CC4CF4E71 | Glutathione S-transferase | 2 | 11.86 | 1.59 ± 0.15 | 0.020 |
| Traes_4AS_36CB7931F | Glutathione S-transferase | 2 | 14.75 | 1.38 ± 0.08 | 0.015 |
| Traes_XX_BEAB3FB5A | Glutathione S-transferase | 2 | 7.12 | 1.26 ± 0.06 | 0.018 |
| Traes_6BL_8360C77EF | Glutathione peroxidase | 2 | 16.28 | 1.21 ± 0.03 | 0.006 |
| Other drought resistance proteins | |||||
| Traes_XX_B7AF82F34 | ATP synthase subunit beta | 6 | 20.00 | 1.32 ± 0.08 | 0.019 |
| Traes_2AL_B7FC2C090 | Wali7 protein | 3 | 15.81 | 1.24 ± 0.01 | 0.001 |
| Traes_4AL_198AD99FF | Clathrin heavy chain | 9 | 15.15 | 1.27 ± 0.10 | 0.045 |
| Traes_1AL_1538AC680 | Nucleoside diphosphate kinase | 3 | 11.98 | 1.27 ± 0.04 | 0.009 |
| Traes_4BL_B5BF83119 | Hemoglobin Hb1 | 6 | 46.01 | 1.58 ± 0.08 | 0.006 |
| Traes_2DL_47E335BA6 | NAD(P)H-dependent 6′-deoxychalcone synthase | 2 | 24.19 | 1.34 ± 0.10 | 0.028 |
| Traes_XX_F9BB1AA7A | 6-phosphogluconate dehydrogenase | 6 | 21.31 | 0.79 ± 0.06 | 0.031 |
| Traes_1BL_BEEBE83B7 | Cysteine proteinase inhibitor | 2 | 22.06 | 0.69 ± 0.02 | 0.001 |
| Traes_5DL_A6B7B0525 | Peptidyl-prolyl cis-trans isomerase | 2 | 16.07 | 0.81 ± 0.04 | 0.012 |
| Traes_7AS_8E6B88A80 | Pathogenesis-related protein | 4 | 37.27 | 0.80 ± 0.06 | 0.033 |
| Traes_2AL_800303D8D | Pathogenesis-related protein | 5 | 42.24 | 0.80 ± 0.07 | 0.042 |
| Traes_3DL_3D1319ECF | Acyl-(Acyl-carrier-protein) desaturase | 2 | 10.38 | 0.58 ± 0.07 | 0.010 |
| Uncharacterized proteins | |||||
| Traes_7BL_BE36675C8 | Uncharacterized protein | 2 | 8.82 | 0.74 ± 0.01 | 0.001 |
Table 2. The differentially expressed proteins between SOS and control.
| Protein name | Protein function | Peptides | Coverage | Fold-change between SOS and control | P-value |
|---|---|---|---|---|---|
| Protein Metabolism | |||||
| Traes_2BS_7700613D4 | 40S ribosomal protein | 2 | 22.31 | 1.25 ± 0.05 | 0.013 |
| Traes_7AL_65F481DB9 | 40S ribosomal protein | 2 | 13.82 | 1.45 ± 0.14 | 0.031 |
| Traes_3AL_0A1239316 | Glycine dehydrogenase | 2 | 5.82 | 0.83 ± 0.01 | 0.001 |
| Traes_2DS_64EC7E533 | Eukaryotic translation initiation factor 3 | 3 | 10.53 | 0.82 ± 0.07 | 0.045 |
| Traes_XX_B2924FB2E | Eukaryotic translation initiation factor 3 | 2 | 10.71 | 0.81 ± 0.01 | 0.001 |
| Traes_4BL_E2E2C4E1D | Adenylate kinase 1 | 2 | 13.85 | 0.81 ± 0.03 | 0.006 |
| Traes_XX_7DC2CED29 | E3 ubiquitin-protein ligase | 2 | 13.75 | 1.23 ± 0.07 | 0.031 |
| Histone Protein | |||||
| Traes_4BL_96E367077 | Histone H2A | 3 | 21.33 | 1.49 ± 0.14 | 0.024 |
| CarhohydrateMetabolism | |||||
| Traes_3B_BC152C5D7 | Glycosyltransferase | 2 | 6.18 | 1.38 ± 010 | 0.021 |
| Traes_4AL_82AB2E772 | Beta-fructofuranosidase | 7 | 18.10 | 0.83 ± 0.02 | 0.004 |
| Traes_4DS_084803084 | Beta-glucosidase | 2 | 5.52 | 0.80 ± 0.02 | 0.004 |
| Traes_3B_B8697F82E | Glucan endo-1,3-beta-glucosidase | 4 | 16.87 | 0.82 ± 0.06 | 0.034 |
| Traes_4AL_8845F411B | UDP-glucose 6-dehydrogenase | 12 | 31.03 | 0.73 ± 0.04 | 0.006 |
| Traes_4BS_11DDF29B31 | Xylanase inhibitor protein | 2 | 9.02 | 0.70 ± 0.06 | 0.014 |
| Traes_4DL_A80B33149 | Beta-amylase | 3 | 10.89 | 0.74 ± 0.07 | 0.025 |
| Traes_1DL_FDF182BF9 | Hexokinase | 6 | 20.88 | 1.37 ± 0.07 | 0.012 |
| Traes_4AL_E6D679339 | Alcohol dehydrogenase | 3 | 10.09 | 1.23 ± 0.08 | 0.035 |
| Traes_4AL_4B09F91AE | Alcohol dehydrogenase | 6 | 24.74 | 0.80 ± 0.07 | 0.041 |
| Phytohormones | |||||
| Traes_4BS_BB26E5EE1 | Abscisic stress-ripening protein | 3 | 56.12 | 2.39 ± 0.14 | 0.003 |
| Traes_4DS_E2055C83D | Abscisic stress-ripening protein | 3 | 56.52 | 2.37 ± 0.24 | 0.010 |
| Antioxidant Protection Proteins | |||||
| Traes_2DS_E3F0742FF | Peroxidase | 2 | 24.36 | 0.82 ± 0.04 | 0.018 |
| Traes_2DS_2CCCA54C1 | Peroxidase | 13 | 56.83 | 0.78 ± 0.07 | 0.034 |
| Traes_6AS_621A7A571 | Peroxidase | 4 | 25.71 | 0.78 ± 0.03 | 0.008 |
| Traes_7DL_D99ED7064 | Peroxidase | 7 | 26.39 | 0.77 ± 0.07 | 0.032 |
| Traes_2DS_090AF6B73 | Peroxidase | 3 | 14.86 | 0.78 ± 0.01 | 0.001 |
| Traes_2AL_520618712 | Peroxidase | 7 | 24.85 | 1.20 ± 0.05 | 0.018 |
| Traes_1DL_7BCE5B151 | Glutathione S-transferase | 3 | 20.37 | 2.04 ± 0.02 | 0.000 |
| Traes_1AS_D25875432 | Glutathione S-transferase | 4 | 20.44 | 1.32 ± 0.03 | 0.002 |
| Traes_1AL_CC4CF4E71 | Glutathione S-transferase | 2 | 11.86 | 1.92 ± 0.33 | 0.040 |
| Traes_1DS_FD8511876 | Glutathione S-transferase | 5 | 28.64 | 1.23 ± 0.08 | 0.039 |
| Traes_4AS_36CB7931F | Glutathione S-transferase | 2 | 14.75 | 2.30 ± 0.10 | 0.002 |
| Traes_6AS_A2A2B273C | Glutathione S-transferase | 3 | 12.55 | 1.43 ± 0.07 | 0.009 |
| Traes_1BL_3765A51EC | Glutathione S-transferase | 2 | 10.27 | 1.50 ± 0.15 | 0.030 |
| Traes_XX_BEAB3FB5A | Glutathione S-transferase | 2 | 7.12 | 1.57 ± 0.06 | 0.004 |
| Traes_1DS_EFDF9CB72 | Glutamate-cysteine ligase | 5 | 12.45 | 1.23 ± 0.01 | 0.001 |
| Traes_XX_52CBB24F1 | glutathione reductase (GR) | 6 | 23.10 | 1.25 ± 0.03 | 0.04 |
| Traes_5BL_34593C7D1 | Aldehyde oxidase 3 | 2 | 2.23 | 1.29 ± 0.09 | 0.032 |
| Traes_1AL_5A7E85C4E | Sulfite reductase | 6 | 12.58 | 1.28 ± 0.10 | 0.038 |
| Traes_3B_1962330BB | Oxalate oxidase 2 | 3 | 33.98 | 1.40 ± 0.15 | 0.043 |
| Traes_XX_3D56A9D19 | Monodehydroascorbate reductase | 3 | 11.90 | 0.82 ± 0.01 | 0.001 |
| Other drought resistance proteins | |||||
| Traes_5BL_B92355534 | Germin-like protein | 3 | 26.41 | 1.27 ± 0.03 | 0.004 |
| Traes_4AL_198AD99FF | Clathrin heavy chain | 9 | 15.15 | 1.28 ± 0.11 | 0.049 |
| Traes_4BL_B5BF83119 | Hemoglobin Hb1 | 6 | 46.01 | 1.89 ± 0.11 | 0.005 |
| Traes_2DL_47E335BA6 | NAD(P)H-dependent 6′-deoxychalcone synthase | 2 | 24.19 | 1.46 ± 0.12 | 0.020 |
| Traes_2AL_141C6B5E4 | ATP synthase subunit alpha | 6 | 10.59 | 1.36 ± 0.03 | 0.003 |
| Traes_XX_175EF4A84 | Deoxymugineic acid synthase1 | 2 | 10.49 | 1.28 ± 0.06 | 0.013 |
| Traes_XX_6A9FEF618 | ATP sulfurylase | 5 | 16.17 | 1.28 ± 0.06 | 0.016 |
| Traes_5BL_17F1F28B6 | Wali7 protein | 2 | 13.08 | 1.34 ± 0.09 | 0.024 |
| Traes_XX_F9BB1AA7A | 6-phosphogluconate dehydrogenase | 6 | 21.31 | 0.82 ± 0.03 | 0.010 |
| Traes_1BL_BEEBE83B7 | Cysteine proteinase inhibitor | 2 | 22.06 | 0.82 ± 0.04 | 0.017 |
| Traes_4BS_9F3A928B7 | Low temperature-responsive RNA-binding protein | 2 | 53.03 | 0.76 ± 0.03 | 0.007 |
| Traes_XX_903D8ADBC | Fasciclin-like protein FLA15 | 2 | 14.48 | 0.81 ± 0.04 | 0.013 |
| Uncharacterized proteins | |||||
| Traes_7BL_BE36675C8 | Uncharacterized protein | 2 | 8.82 | 0.75 ± 0.02 | 0.002 |
| Traes_5DL_43046228D | Uncharacterized protein | 5 | 15.97 | 1.22 ± 0.00 | 0.000 |
| Traes_3B_67E790B47 | Uncharacterized protein | 2 | 3.17 | 1.20 ± 0.04 | 0.010 |
| Traes_6BS_4EED05084 | Uncharacterized protein | 3 | 14.91 | 1.74 ± 0.19 | 0.022 |
| Traes_4AL_6A515079C | Uncharacterized protein | 2 | 9.52 | 0.78 ± 0.03 | 0.008 |
| Traes_1BL_BF3813A4B | Uncharacterized protein | 2 | 12.86 | 1.24 ± 0.02 | 0.003 |
| Traes_2BL_B09F6D195 | Uncharacterized protein | 5 | 11.76 | 1.22 ± 0.02 | 0.004 |
| Traes_3B_4490CECAF | Uncharacterized protein | 2 | 6.74 | 1.21 ± 0.03 | 0.006 |
| Traes_7AS_C87C2FF27 | Uncharacterized protein | 3 | 10.40 | 1.24 ± 0.05 | 0.017 |
| Traes_6BS_E96E17B28 | Uncharacterized protein | 2 | 30.00 | 1.47 ± 0.14 | 0.026 |
| Traes_XX_6DDA59584 | Uncharacterized protein | 2 | 5.94 | 1.34 ± 0.03 | 0.050 |
| Traes_XX_7F3775F4C | Uncharacterized protein | 3 | 8.97 | 0.77 ± 0.09 | 0.048 |
Protein metabolism
Osmotic stress greatly impacted the variety and quantity of expressed proteins in plants. The ribosome is a large complex comprising 40S subunit and 60S subunits, and this complex is responsible for protein synthesis from mRNA. Proteome analysis of the wheat roots found three 60S ribosomal proteins and two 40S ribosomal proteins, which are important components of the ribosome, were up-regulated in the roots under osmotic stress compared with control. In addition, the glycine dehydrogenase, which degrade the glycine, showed down-regulate. These results indicate that the process of translation is more active under osmotic stress in response to the adverse environment.
However, two eukaryotic translation initiation factors, which promote the assembly of the ribosome and initiation code for further translation (You, Coghill & Brown, 2013), were down-regulated, and one E3 ubiquitin-protein ligase, which is involved in ubiquitin mediated proteolysis, was up-regulated under drought stress. These factors do not support functional proteins formation.
Based on the identification of DEPs involved in protein metabolism and the dry weight changes under osmotic stress, we speculated that protein synthesis is more active under osmotic stress, thereby produce a greater variety of proteins to increasing the environmental adaptability in wheat, but the protein quantity is inhibited under osmotic stress.
Histone proteins
The nucleosome is the basic unit of chromatin, comprising approximately 147 bp of DNA and a histone octamer composed involving a (Histone3-Histone4)2 tetramer and two (Histone2A-Histone2B) dimmers (Luger et al., 1997). The results of the iTRAQ analysis revealed the up-regulation of two histone proteins in the roots under osmotic stresses. This finding indicates a high level of chromatin condensation in the roots under osmotic stress, generating transcriptional inertness and a significant decrease in total protein. It consistent with the results of the dry weight result and protein metabolism analysis, which speculate the protein variety is increased and the protein quantity is inhibited under osmotic stress.
Carbohydrate metabolism
Carbohydrates are the primary energy resources for organisms and act as small signaling molecules. Under osmotic stress, water-soluble carbohydrates, such as glucose, fructose, sucrose and fructans, are increased in the stems (Foulkes, Scott & Sylvester-Bradley, 2002; Asseng & Herwaarden, 2003; Ruuska et al., 2006), leaves (Roover et al., 2000) and roots (Roover et al., 2000) to impede water loss in plants. Herein, we also found that the enzymes that catalyze the production of small carbohydrate osmolytes, such as sucrose synthase, glucosidase and glycosyltransferase, were up-regulated except Traes_4DS_084803084 and Traes_3B_B8697F82E, and the enzymes that inhibit the formation of small carbohydrate osmolytes, such as UDP-glucose 6-dehydrogenase and xylanase inhibitor protein, was down-regulated under osmotic stress. This finding indicates that small molecular carbohydrates are produced at significant levels to increase osmotic potential in the roots of wheat under osmotic stress.
Glycolysis is an important metabolic pathway, which would produce energy and carbon skeletons for the primary and secondary metabolites biosynthesis (Cramer et al., 2013). And some previous studies had found that the genes or proteins, involved in glycolysis, would be induced (Rizhsky, Liang & Mittler, 2002; Oh & Komatsu, 2015). In this study, fructokinase, hexokinase and alcohol dehydrogenase, which take part in the pathway of glycolysis, were found to be up-regulated for more energy production under osmotic stress.
Phytohormones responsive
Phytohormones play important roles in the adaption of plants to abiotic stresses. The abscisic acid (ABA)-dependent signaling pathway is one of the most important pathways in the resistance to drought stress in plants, and many important drought- or osmotic-related genes, such as AREB1, AREB2, ABF3, SnRK2, and ABF1, are involved in this pathway (Yoshida, Mogami & Yamaguchi-Shinozaki, 2014). In the present study, two abscisic stress-ripening proteins, which can be induced by ABA and abiotic stress (Golan et al., 2014), were up-regulated in the roots under osmotic stress, suggesting that the ABA signaling pathway is important in the resistance of wheat to osmotic stress.
Plant protection system
Many studies have demonstrated that ROS and cytotoxin would significantly increase under osmotic conditions, which would induce cellular damage in plants. To prevent the damages, plants have generated many plant protection systems to remove ROS and cytotoxins. Here, we found that the proteins involved in ROS scavenging and detoxifying were up-regulated, except some peroxidase.
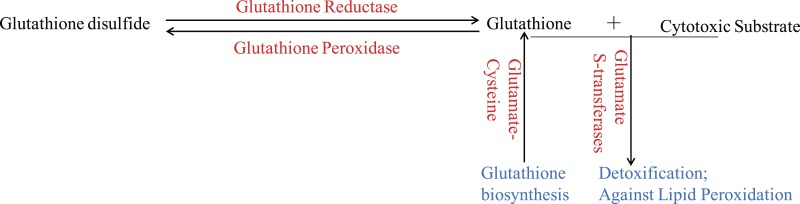
Glutathione (GSH) has multiple functions, such as antioxidant and detoxification, in plants (Noctor et al., 2012). The ROS would be continuous eliminated by GSH-GSSH (Glutathione disulfide) cycle in organism, which depends on glutathione reductase and glutathione peroxidase. And GSTs, which could be induced through different biotic and abiotic stresses, would protect organisms against oxidative damage and lipid peroxidation, and catalyze the conjugation of electrophilic substrates and glutathione to eliminate cytotoxic substrates (Marrs, 1996; Chen et al., 2012; Yang et al., 2001). In the present study, one glutathione reductase and one glutathione peroxidase were found to be up-regulated (Fig. 3), indicating that GSH-GSSH cycle was more active to maintain ROS balance, under osmotic stress. And eight GSTs were also up-regulated to detoxify harmful materials and maintain cell redox homeostasis in plants under osmotic stress (Fig. 3). In addition, one glutamate-cysteine ligase, which catalyzes the first and rate-limiting step of glutathione biosynthesis, was up-regulated (Fig. 3). All these results showed that glutathione system played important roles in protecting organism from damage caused by osmotic stress in wheat roots.
Figure 3. The glutathione system in wheat roots under osmotic stress.
The up-regulated proteins were denoted with red color, and the functions of proteins were denoted with blue color.
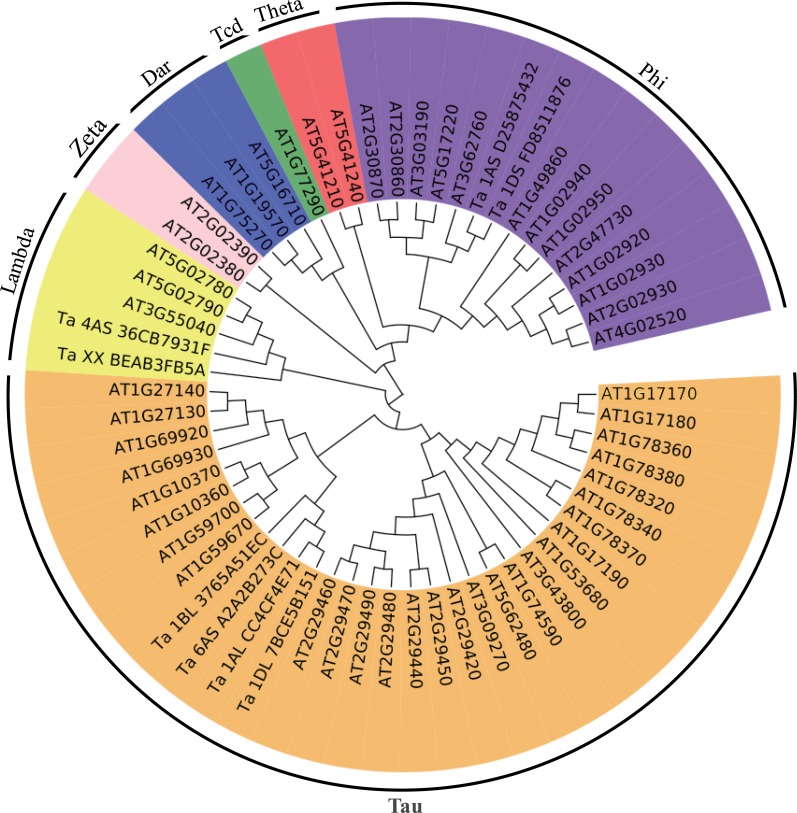
To better understand the evolutionary relationships of these GSTs, an unrooted phylogenetic tree, including AtGSTs and these eight GSTs, was constructed. We identified two GSTs belonging to the Phi family, two GSTs belonging to the Lambda family and four GSTs belonging to the Tau family (Fig. 4). Most GSTs are Phi or Tau, which are plant-specific GSTs and the major phase II enzymes in a common detoxification pathway (Frova, 2003). Transgenic plants over-expressing Tau or Phi GSTs showed high tolerance to herbicides, salt and UV radiation (Karavangeli et al., 2005; Benekos et al., 2010; Jha, Sharma & Mishra, 2011). These results indicate that glutathione play an important role in the detoxification of cytotoxin under osmotic stress in wheat.
Figure 4. Phylogenetic tree of the GSTs.
The unrooted phylogenetic tree of GSTs from Arabidopsis and the eight differentially expressed GSTs in the current proteome analysis was constructed by the neighbor-joining method using MEGA 5.10 software. The subgroups of the GSTs are distinguished with different colors.
In addition, many other DEPs associated with redox reactions, such as reductase and oxidase, were observed under osmotic stress.
Other osmotic resistance proteins
In addition to the proteins mentioned above, twenty DEPs with known functions were also found in this proteome analysis. nine of these DEPs were down-regulated under osmotic stress, including cysteine proteinase inhibitor, adenylate kinase etc. Eleven of these DEPs were up-regulated under osmotic stress, including ATP synthase subunit alpha, Wali7 protein etc.
Conclusions
In the present study, we used iTRAQ to comprehensively study the protein expression profile in the root of wheat under osmotic stress. A total of 2,228 expressed proteins were identified. Among these, 81 were DEPs associated with protein metabolism, carbohydrate metabolism, phytohormones, plant protection system and other functions. These findings help clarify the response to osmotic stress in wheat and provide additional information for future studies of the mechanism of osmotic resistance in wheat.
Supplemental Information
Funding Statement
This work was financially supported by the National Key Technology Support Program of China (2013BAD07B14, 2012BAD14B08 and 2013BAD07B07-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Jianhui Ma, Email: cricaas@163.com.
Lina Jiang, Email: jianglina73@aliyun.com.
Chunxi Li, Email: wheatlab@163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jianhui Ma conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Wen Dong performed the experiments, prepared figures and/or tables.
Daijing Zhang performed the experiments, analyzed the data, prepared figures and/or tables.
Xiaolong Gao analyzed the data, contributed reagents/materials/analysis tools.
Lina Jiang and Chunxi Li conceived and designed the experiments, reviewed drafts of the paper.
Yun Shao analyzed the data.
Doudou Tong contributed reagents/materials/analysis tools.
Data Availability
The following information was supplied regarding data availability:
The raw data is provided as Supplemental Files. The mass spectrometry data are deposited in the iProx database (http://www.iprox.cn/index) with the accession number: IPX00075800.
References
- Asseng & Herwaarden (2003).Asseng S, Herwaarden AFV. Analysis of the benefits to wheat yield from assimilates stored prior to grain filling in a range of environments. Plant and Soil. 2003;256(1):217–229. doi: 10.1023/A:1026231904221. [DOI] [Google Scholar]
- Benekos et al. (2010).Benekos K, Kissoudis C, Nianiou-Obeidat I, Labrou N, Madesis P, Kalamaki M, Makris A, Tsaftaris A. Overexpression of a specific soybean GmGSTU4 isoenzyme improves diphenyl ether and chloroacetanilide herbicide tolerance of transgenic tobacco plants. Journal of Biotechnology. 2010;150(1):195–201. doi: 10.1016/j.jbiotec.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Caruso et al. (2009).Caruso G, Cavaliere C, Foglia P, Gubbiotti R, Samperi R, Laganà A. Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGEand MALDI-TOF mass spectrometry. Plant Science. 2009;177(6):570–576. doi: 10.1016/j.plantsci.2009.08.007. [DOI] [Google Scholar]
- Chen et al. (2012).Chen J-H, Jiang H-W, Hsieh E-J, Chen H-Y, Chien C-T, Hsieh H-L, Lin T-P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiology. 2012;158(1):340–351. doi: 10.1104/pp.111.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer et al. (2013).Cramer GR, Van Sluyter SC, Hopper DW, Pascovici D, Keighley T, Haynes PA. Proteomic analysis indicates massive changes in metabolism prior to the inhibition of growth and photosynthesis of grapevine (Vitis vinifera L.) in response to water deficit. BMC Plant Biology. 2013;13(1):49. doi: 10.1186/1471-2229-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeba et al. (2012).Deeba F, Pandey AK, Ranjan S, Mishra A, Singh R, Sharma YK, Shirke PA, Pandey V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiology and Biochemistry. 2012;53:6–18. doi: 10.1016/j.plaphy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2009).Ding Z, Li S, An X, Liu X, Qin H, Wang D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. Journal of Genetics and Genomics. 2009;36(1):17–29. doi: 10.1016/S1673-8527(09)60003-5. [DOI] [PubMed] [Google Scholar]
- Fan et al. (2011).Fan J, Chen C, Yu Q, Brlansky RH, Li Z-G, Gmitter FG., Jr Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by “Candidatus Liberibacter asiaticus”. Physiologia Plantarum. 2011;143(3):235–245. doi: 10.1111/j.1399-3054.2011.01502.x. [DOI] [PubMed] [Google Scholar]
- Foulkes, Scott & Sylvester-Bradley (2002).Foulkes MJ, Scott TLRK, Sylvester-Bradley R. The ability of wheat cultivars to withstand drought in UK conditions: formation of grain yield. The Journal of Agricultural Science. 2002;138(02):153–169. [Google Scholar]
- Frova (2003).Frova C. The plant glutathione transferase gene family: genomic structure, functions, expression and evolution. Physiologia Plantarum. 2003;119(4):469–479. doi: 10.1046/j.1399-3054.2003.00183.x. [DOI] [Google Scholar]
- Gao et al. (2011).Gao L, Yan X, Li X, Guo G, Hu Y, Ma W, Yan Y. Proteome analysis of wheat leaf under salt stress by two-dimensional difference gel electrophoresis (2D-DIGE) Phytochemistry. 2011;72(10):1180–1191. doi: 10.1016/j.phytochem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2012).Ge P, Ma C, Wang S, Gao L, Li X, Guo G, Ma W, Yan Y. Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress. Analytical and Bioanalytical Chemistry. 2012;402(3):1297–1313. doi: 10.1021/pr500688g. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2014).Ge X, Zhang C, Wang Q, Yang Z, Wang Y, Zhang X, Wu Z, Hou Y, Wu J, Li F. iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. Journal of Proteome Research. 2014;14(1):268–278. doi: 10.1021/pr500688g. [DOI] [PubMed] [Google Scholar]
- Golan et al. (2014).Golan I, Dominguez PG, Konrad Z, Shkolnik-Inbar D, Carrari F, Bar-Zvi D. Tomato ABSCISIC ACID STRESS RIPENING (ASR) gene family revisited. PLoS ONE. 2014;9(10):e2334. doi: 10.1371/journal.pone.0107117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2006).Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, Sharma & Mishra (2011).Jha B, Sharma A, Mishra A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Molecular Biology Reports. 2011;38(7):4823–4832. doi: 10.1007/s11033-010-0625-x. [DOI] [PubMed] [Google Scholar]
- Karavangeli et al. (2005).Karavangeli M, Labrou NE, Clonis YD, Tsaftaris A. Development of transgenic tobacco plants overexpressing maize glutathione S-transferase I for chloroacetanilide herbicides phytoremediation. Biomolecular Engineering. 2005;22(4):121–128. doi: 10.1016/j.bioeng.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Krasensky & Jonak (2012).Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany. 2012;63(4):1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan et al. (2011).Lan P, Li W, Wen T-N, Shiau J-Y, Wu Y-C, Lin W, Schmidt W. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiology. 2011;155(2):821–834. doi: 10.1104/pp.110.169508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le et al. (2012).Le DT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Ham le H, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS ONE. 2012;7(11):e2334. doi: 10.1371/journal.pone.0049522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka et al. (2011).Lenka SK, Katiyar A, Chinnusamy V, Bansal KC. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnology Journal. 2011;9(3):315–327. doi: 10.1111/j.1467-7652.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2013).Li C, Li T, Zhang D, Jiang L, Shao Y. Exogenous nitric oxide effect on fructan accumulation and FBEs expression in chilling-sensitive and chilling-resistant wheat. Environmental and Experimental Botany. 2013;86:2–8. doi: 10.1016/j.envexpbot.2011.12.032. [DOI] [Google Scholar]
- Li et al. (2012).Li Y, Meng F, Zhang C, Zhang N, Sun M, Ren J, Niu H, Wang X, Yin J. Comparative analysis of water stress-responsive transcriptomes in drought-susceptible and -tolerant wheat (Triticum aestivum L.) Journal of Plant Biology. 2012;55(5):349–360. doi: 10.1007/s12374-011-0032-4. [DOI] [Google Scholar]
- Liu et al. (1998).Liu Q, Kasuga M, Sakuma Y, Abea H, Miuraa S, Yamaguchi-Shinozakia K, Shinozakib K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell. 1998;10(8):1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu G, Li X, Jin S, Liu X, Zhu L, Nie Y, Zhang X. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS ONE. 2014;9(1):e2334. doi: 10.1371/journal.pone.0086895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth et al. (2012).Longworth J, Noirel J, Pandhal J, Wright PC, Vaidyanathan S. HILIC-and SCX-based quantitative proteomics of Chlamydomonas reinhardtii during nitrogen starvation induced lipid and carbohydrate accumulation. Journal of Proteome Research. 2012;11(12):5959–5971. doi: 10.1021/pr300692t. [DOI] [PubMed] [Google Scholar]
- Luger et al. (1997).Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2014).Ma J, Zhang D, Shao Y, Liu P, Jiang L, Li C. Genome-wide analysis of the WRKY transcription factors in Aegilops tauschii. Cytogenetic and Genome Research. 2014;144(3):240–250. doi: 10.1159/000370172. [DOI] [PubMed] [Google Scholar]
- Marrs (1996).Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47(1):127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Matsui et al. (2008).Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, Satou M, Kim JM, Kobayashi N, Toyoda T, Shinozaki K, Seki M. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant and Cell Physiology. 2008;49(8):1135–1149. doi: 10.1093/pcp/pcn101. [DOI] [PubMed] [Google Scholar]
- Mayer et al. (2014).Mayer KFX, Rogers J, Doležel J, Pozniak C, Eversole K, Feuillet C, Gill B, Friebe B, Lukaszewski AJ, Sourdille P, Endo TR, Kubaláková M, Cíhalíková J, Dubská Z, Vrána J, Sperková R, Simková H, Febrer M, Clissold L, McLay K, Singh K, Chhuneja P, Singh NK, Khurana J, Akhunov E, Choulet F, Alberti A, Barbe V, Wincker P, Kanamori H, Kobayashi F, Itoh T, Matsumoto T, Sakai H, Tanaka T, Wu J, Ogihara Y, Handa H, Maclachlan PR, Sharpe A, Klassen D, Edwards D, Batley J, Olsen OA, Sandve SR, Lien S, Steuernagel B, Wulff B, Caccamo M, Ayling S, Ramirez-Gonzalez RH, Clavijo BJ, Wright J, Pfeifer M, Spannagl M, Martis MM, Mascher M, Chapman J, Poland JA, Scholz U, Barry K, Waugh R, Rokhsar DS, Muehlbauer GJ, Stein N, Gundlach H, Zytnicki M, Jamilloux V, Quesneville H, Wicker T, Faccioli P, Colaiacovo M, Stanca AM, Budak H, Cattivelli L, Glover N, Pingault L, Paux E, Sharma S, Appels R, Bellgard M, Chapman B, Nussbaumer T, Bader KC, Rimbert H, Wang S, Knox R, Kilian A, Alaux M, Alfama F, Couderc L, Guilhot N, Viseux C, Loaec M, Keller B, Praud S. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345(6194):1251788. doi: 10.1126/science.1251788. [DOI] [PubMed] [Google Scholar]
- Mirzaei et al. (2012).Mirzaei M, Soltani N, Sarhadi E, Pascovici D, Keighley T, Salekdeh GH, Haynes PA, Atwell BJ. Shotgun proteomic analysis of long-distance drought signaling in rice roots. Journal of Proteome Research. 2012;11(1):348–358. doi: 10.1021/pr2008779. [DOI] [PubMed] [Google Scholar]
- Nir, Moshelion & Weiss (2014).Nir I, Moshelion M, Weiss D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant, Cell and Environment. 2014;37(1):113–123. doi: 10.1111/pce.12135. [DOI] [PubMed] [Google Scholar]
- Noctor et al. (2012).Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH. Glutathione in plants: an integrated overview. Plant, Cell and Environment. 2012;35(2):454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Oh & Komatsu (2015).Oh MW, Komatsu S. Characterization of proteins in soybean roots under flooding and drought stresses. Journal of Proteomics. 2015;114:161–181. doi: 10.1016/j.jprot.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Pasquali et al. (2008).Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Reports. 2008;27(10):1677–1686. doi: 10.1007/s00299-008-0587-9. [DOI] [PubMed] [Google Scholar]
- Peng et al. (2009).Peng Z, Wang M, Li F, Lv H, Li C, Xia G. A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Molecular & Cellular Proteomics. 2009;8(12):2676–2687. doi: 10.1074/mcp.M900052-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin & Zeevaart (2002).Qin X, Zeevaart JAD. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiology. 2002;128(2):544–551. doi: 10.1104/pp.010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu & Yu (2009).Qiu Y, Yu D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environmental and Experimental Botany. 2009;65(1):35–47. doi: 10.1016/j.envexpbot.2008.07.002. [DOI] [Google Scholar]
- Rizhsky, Liang & Mittler (2002).Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology. 2002;130(3):1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roover et al. (2000).Roover JD, Vandenbranden K, Laere AV, Ende WVD. Drought induces fructan synthesis and 1-SST (sucrose: sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.) Planta. 2000;210(5):808–814. doi: 10.1007/s004250050683. [DOI] [PubMed] [Google Scholar]
- Ruuska et al. (2006).Ruuska SA, Rebetzke GJ, Herwaarden AFV, Richards RA, Fettell NA, Tabe L, Jenkins CLD. Genotypic variation in water-soluble carbohydrate accumulation in wheat. Functional Plant Biology. 2006;33(9):799–809. doi: 10.1071/FP06062. [DOI] [PubMed] [Google Scholar]
- Shinozaki, Yamaguchi-Shinozakiy & Sekiz (2003).Shinozaki K, Yamaguchi-Shinozakiy K, Sekiz M. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology. 2003;6(5):410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- Shou, Bordallo & Wang (2004).Shou H, Bordallo P, Wang K. Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. Journal of Experimental Botany. 2004;55(399):1013–1019. doi: 10.1093/jxb/erh129. [DOI] [PubMed] [Google Scholar]
- Valliyodan & Nguyen (2006).Valliyodan B, Nguyen HT. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Current Opinion in Plant Biology. 2006;9(2):189–195. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2001).Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, Awasth YC. Role of glutathione S-transferases in protection against lipid peroxidation. Journal of Biological Chemistry. 2001;276(22):19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- Yoshida, Mogami & Yamaguchi-Shinozaki (2014).Yoshida T, Mogami J, Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Current Opinion in Plant Biology. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- You, Coghill & Brown (2013).You T, Coghill GM, Brown AJP. Encyclopedia of systems biology. New York: Springer; 2013. Eukaryotic translation initiation factor interactions; pp. 675–678. [Google Scholar]
- Zheng et al. (2010).Zheng J, Fu J, Gou M, Huai J, Liu Y, Jian M, Huang Q, Guo X, Dong Z, Wang H, Wang G. Genome-wide transcriptome analysis of two maize inbred lines under drought stress. Plant Molecular Biology. 2010;72(4–5):407–421. doi: 10.1007/s11103-009-9579-6. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (1997).Zhu JK, Hasegawa PM, Bressan RA, Bohnert HJ. Molecular aspects of osmotic stress in plants. Critical Reviews in Plant Sciences. 1997;16(3):253–277. doi: 10.1080/07352689709701950. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is provided as Supplemental Files. The mass spectrometry data are deposited in the iProx database (http://www.iprox.cn/index) with the accession number: IPX00075800.