Abstract
Background
Race-based survival in children and adolescents with hematologic malignancies has been a national challenge for decades. Large-scale investigations of age- and race-based survival trends over time in these patients have not previously been reported.
Objective
To investigate whether race- and age-related differences in pediatric and adolescent and young adult (AYA) leukemia and lymphoma survival persist and to what extent these differences have changed over time.
Methods
Using the Surveillance, Epidemiology and End Results (SEER) Program we investigated the outcomes of black and white (1975–2012; N=27,369) and white and Hispanic (1992–2012; N=20,574) children (0–14 years old) and AYAs (15–39 years old) with acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML) and Hodgkin lymphoma (HL). Five- and 10-year relative survival estimates were compared over time.
Results
Trends showed convergence of survival in white and black children with ALL, but divergence in survival in AYA patients. Hispanic children and AYAs both suffer inferior outcomes. Trends for AML revealed persistent survival differences between black and white children and suggested worsening disparities for AYAs. Survival trends in HL revealed sustained survival differences between black and white AYA patients whereas no differences were found in Hispanic vs. white patient outcomes for AML or HL.
Conclusion
Although survival in children and AYAs with ALL, AML and HL has improved over the past four decades, differences persist between black, white, and Hispanic children and AYAs; Survival disparities between black and white children with ALL has been nearly eliminated. Strategies aimed at identifying causality and reducing disparities are warranted.
Keywords: Leukemia, Lymphoma, SEER, Adolescent, AYA, Pediatric, Survival, Race, Disparities
INTRODUCTION
Advances made in the treatment of acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML) and Hodgkin lymphoma (HL) over the last quarter-century are among the most dramatic and successful in modern medicine. Despite successes, patients of racial/ethnic minorities and adolescent and young adults (AYAs) continue to suffer reduced survival.1,2 The cancer burden facing AYA patients has been underestimated. Cancer incidence is more than five times higher among 15- to 39-year-olds than in younger patients and the rate of progress in prolonging survival and reducing mortality in the AYA group has been approximately half.3 Studies report disparities in ALL outcomes, with black and Hispanic patients having considerably lower 5-year survival than non-Hispanic whites.4,5 Similarly, survival differences based on age and race hold true in pediatric and AYA AML and HL.6,7,8
While the epidemiology of ALL, AML and HL in pediatric and AYA patients has been investigated, survival trends over time have not been fully described. We investigated whether black and Hispanic patients with ALL, AML and HL in the pediatric and AYA populations continue to experience poorer survival when compared with non-Hispanic white patients. Here we present the results of a retrospective analysis of Surveillance, Epidemiology and End Results (SEER) data investigating relative survival trends of black and white children (0–14 years) and AYAs (15–39 years) with ALL, AML and HL from 1975 to present, and Hispanic and non-Hispanic white children and AYAs from 1992 to present.
METHODS
Study Population
Using the SEER database we identified 27,369 black and white children and AYAs diagnosed with a ALL, AML or HL between 1975 and 2012 inclusive (International Classification of Diseases-Oncology, 3rd edition [ICD-O-3] morphology codes 9811–9818, 9826, 9835–9837 for ALL, 9840, 9861, 9865–9867, 9869, 9871–9874, 9895–9897, 9898, 9910–9911, 9920 for AML, and 9650–9667 for HL). Patients from nine original SEER sites including Connecticut, Iowa, New Mexico, Utah, Hawaii, metropolitan areas of Detroit, San Francisco-Oakland, Atlanta and 13 counties of the Seattle-Puget Sound region (SEER-9) were included. Data from 1973 and 1974 were excluded because of inconsistent and incomplete entries. Our study of SEER-9 consisted of black and white races only; insufficient cases were available for analysis of other racial/ethnic groups before 1992. We excluded 103 patients who were diagnosed by death certificate/autopsy only or who had no reported survival time. The final study population was 27,266. We obtained information routinely recorded at diagnosis for each patient including age, sex, race and year of diagnosis. Patients were divided into two age groups: children (0–14 years) and AYAs (15–39 years) and into black and white cohorts for subgroup analyses. Non-Hodgkin lymphoma (NHL) was not included in this study because the human immunodeficiency virus /acquired immunodeficiency syndrome (HIV/AIDS) epidemic during the 1980s and early 1990s resulted in a temporary increase in incidence of a poor-prognosis type of NHL in AYAs and we lacked information on which patients had HIV/AIDS;.9 As a result, the overall survival of AYAs with NHL dropped as much as 25% and the effect lasted for 20–25 years, from 1985 to 2000–2005.
In 1992, SEER expanded its registries with four additional regions: Los Angeles, San Jose-Monterey, the rest of the state of Georgia, and Alaska native (SEER-13) and included data on whether patients identified as Hispanic-Spanish-Latino. Using the SEER-13 database we identified 14,188 non-Hispanic white and 6,386 Hispanic children or AYA patients. Trends over time using the SEER-13 database were limited to a relatively short interval.
Statistical Analysis
Relative survival was used to assess cancer mortality changes over time. Relative survival accounts for competing causes of death and is the ratio of observed survival among cancer patients to expected survival in the overall population as computed from life tables of mortality in the general population.10 We obtained 5- and 10-year relative survival estimates with corresponding 95% confidence intervals (95% CI) by age, race, and primary site of the cancer in the body pooled over multiple years by using SEER*Stat software program (version 4.2.0.2; National Cancer Institute [NCI], Bethesda, MD)11 Average percent change (APC) in annual survival was determined by converting 5- and 10-year survival estimates to log values, applying the linear estimate regression, and the exponential of the linear regression. If the survival estimate was 0% (zero), the rate was estimated as the average of the prior and succeeding years for which the estimates were non-zero. The test of APC= 0 was tested with the F-test for regression. P-values ≥0.05 were considered not significant (NS).
RESULTS
Five-year relative survival was calculated for successive intervals children and AYAs with ALL, AML and HL, by race (Figure 1). In general, all subgroups have improved over the 32 calendar years depicted. Among 8,201 ALL patients, 92% were white and 28% were AYAs. Among 3,958 AML patients, 87% were white and 77% were AYAs. Among 15,107 HL patients, 89% were white and 93% were AYAs (Table 1). Five- and 10-year relative survival rates with 95% CI among black and white children and AYAs for ALL, AML, and HL are presented in Table 2. A comparison of Hispanic and non-Hispanic cohorts with ALL, AML, and HL are presented in Table 3.
Figure 1.
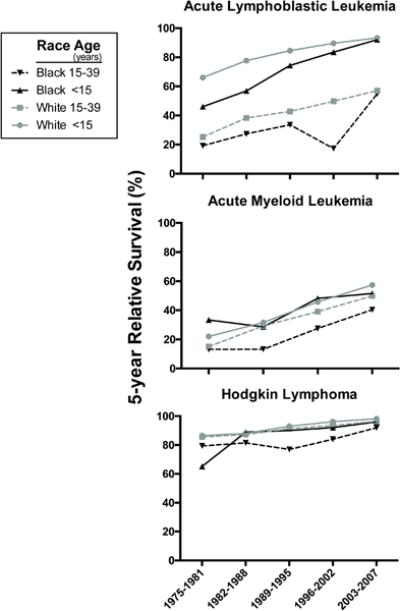
Five-year relative survival by 5-year period of diagnosis and cancer site, SEER-9, 1975–2012. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia, HL. Due to insufficient data, survival estimate for black children with HL for the time period 1989–1995 is not reported.
Table 1.
Number and proportion of children and AYAs with race, gender and distribution of acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML) and Hodgkin lymphoma (HL) in children, adolescents and young adults by race, sex, age and era of diagnosis, SEER 9, 1975–2012
| ALL | AML | HL | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Sex | ||||||
| Female | 4777 | 58.2% | 2015 | 50.9% | 8001 | 53.0% |
| Male | 3424 | 41.8% | 1943 | 49.1% | 7106 | 47.0% |
|
| ||||||
| Race | ||||||
| White | 7500 | 91.5% | 3390 | 85.6% | 13494 | 89.3% |
| Black | 701 | 8.5% | 568 | 14.4% | 1613 | 10.7% |
|
| ||||||
| Era of diagnosis | ||||||
| 1975–1981 | 82 | 11.7% | 72 | 12.7% | 159 | 9.9% |
| 1982–1988 | 103 | 14.7% | 98 | 17.3% | 217 | 13.5% |
| 1989–1995 | 130 | 18.5% | 89 | 15.7% | 319 | 19.8% |
| 1996–2002 | 143 | 20.4% | 119 | 21.0% | 353 | 21.9% |
| 2003–2007 | 119 | 17.0% | 89 | 15.7% | 277 | 17.2% |
| 2008–2012 | 124 | 17.7% | 101 | 17.8% | 288 | 17.9% |
Table 2.
Five- and 10-year relative survival (%) and annual percentage change (APC) among children and AYAs with acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML) and Hodgkin lymphoma (HL) by race, age and era, SEER-9, 1975–2012
| Age and era of diagnosis | White | Black | ||
|---|---|---|---|---|
|
| ||||
| 5-year (95% CI) | 10-year (95% CI) | 5-year (95% CI) | 10-year (95% CI) | |
| ALL, 0–14 years | N = 5497 | N = 517 | ||
| 1975–1981 | 66.0 (62.6–69.1) | 57.7 (54.2–61.0) | 46.0 (33.2–57.9) | 37.9 (25.8–49.8) |
| 1982–1988 | 77.7 (74.9–80.3) | 72.2 (69.1–75.1) | 56.9 (45.4–66.9) | 53.3 (41.9–63.5) |
| 1989–1995 | 84.5 (82.1–86.6) | 80.6 (78.0–82.9) | 74.4 (64.4–81.9) | 68.3 (58.0–76.6) |
| 1996–2002 | 89.6 (87.7–91.2) | 86.8 (84.7–88.7) | 83.5 (75.0–89.3) | 78.1 (68.9–84.8) |
| 2003–2007 | 93.3 (91.3–94.9) | – | 92.0 (82.9–96.3) | |
| APC (95% CI) | 1.37 * (1.08–1.66) | 2.15* (1.59–2.71) | 3.01* (2.09–3.93) | 3.91* (2.29–5.55) |
|
| ||||
| ALL, 15–39 years | N = 2003 | N = 184 | ||
| 1975–1981 | 25.2 (20.2–30.5) | 22.7 (17.9–27.9) | 19.2 (6.0–38.0) | 14.6 (3.6–32.7) |
| 1982–1988 | 38.3 (33.3–43.4) | 33.4 (28.5–38.3) | 27.4 (11.2–46.6) | 18.4 (5.7–36.7) |
| 1989–1995 | 42.8 (37.6–47.8) | 38.5 (33.4–43.5) | 33.6 (18.3–49.6) | 33.6 (18.3–49.6) |
| 1996–2002 | 49.8 (44.7–54.7) | 46.9 (41.7–51.8) | 17.3 (7.0–31.4) | 11.6 (3.7–24.5) |
| 2003–2007 | 57.0 (51.5–62.2) | – | 54.9 (39.1–68.2) | – |
| APC (95% CI) | 3.13* (2.18–4.08) | 3.74* (2.56–4.94) | 1.25 (−0.68–3.22) | −1.26 (−3.19–0.71) |
|
| ||||
| AML, 0–14 years | N = 729 | N = 158 | ||
| 1975–1981 | 22 (15.2–29.6) | 18.7 (12.4–26.0) | 33.4 (13.7–54.6) | 33.4 (13.7–54.6) |
| 1982–1988 | 31.6 (22.9–40.5) | 30.6 (22.1–39.6) | 28.6 (11.7–48.2) | 28.6 (11.7–48.2) |
| 1989–1995 | 45.8 (37.4–53.8) | 44.4 (36.1–52.4) | 48.2 (28.7–65.3) | 44.5 (25.6–61.9) |
| 1996–2002 | 57.4 (49.2–64.8) | 54.8 (46.6–62.3) | 51.5 (34.0–66.5) | 48.9 (31.6–64.1) |
| 2003–2007 | 71.2 (61.2–79.0) | – | 53.6 (33.8–69.9) | – |
| APC (95% CI) | 4.58* (3.69–5.48) | 5.47* (3.83–7.14) | 0.31 (−0.86–1.49) | 0.01 (−1.31–1.35) |
|
| ||||
| AML, 15–39 years | N = 2661 | N = 410 | ||
| 1975–1981 | 15.0 (12.0–18.3) | 12.8 (10.0–16.0) | 13.1 (5.7–23.5) | 11.3 (4.6–21.4) |
| 1982–1988 | 29.3 (25.1–33.5) | 25.4 (21.5–29.5) | 13.2 (6.8–21.8) | 11.9 (5.9–20.4) |
| 1989–1995 | 39.1 (34.6–43.5) | 35.3 (31–39.7) | 27.5 (17.0–39.0) | 27.5 (17.0–39.0) |
| 1996–2002 | 49.9 (45.5–54.1) | 46.7 (42.4–51) | 40.4 (29.7–50.7) | 38 (27.5–48.4) |
| 2003–2007 | 55.1 (49.7–60.2) | – | 37.6 (25.5–49.6) | – |
| APC (95% CI) | 5.10* (4.15–6.06) | 6.67* (5.13–8.22) | 2.07* (0.41–3.75) | 2.55* (0.12–5.04) |
|
| ||||
| HL, 0–14 years | N = 973 | N = 146 | ||
| 1975–1981 | 86.5 (81.2–90.4) | 81.7 (75.8–86.3) | 65.2 (40.4–81.8) | 55.4 (31.5–74.0) |
| 1982–1988 | 88.0 (82.2–92.1) | 84.9 (78.5–89.5) | 88.9 (62.4–97.1) | 88.9 (62.4–97.1) |
| 1989–1995 | 93.0 (87.8–96.0) | 91.5 (85.9–94.9) | ~ | ~ |
| 1996–2002 | 96.2 (92.0–98.2) | 94.8 (90.1–97.3) | 92.1 (71.5–98.0) | 92.1 (71.5–98.0) |
| 2003–2007 | 98.2 (92.7–99.6) | – | 96.0 (74.8–99.4) | – |
| APC (95% CI) | 0.55* (0.29–0.80) | 0.81* (0.39–1.23) | 0.78 (−0.08–1.66) | 2.40* (1.16–3.66) |
|
| ||||
| HL, 15–39 years | N = 12521 | N = 1467 | ||
| 1975–1981 | 85.5 (83.9–87.0) | 78.3 (76.4–80.1) | 79.4 (71.4–85.4) | 66.2 (57.3–73.8) |
| 1982–1988 | 87.3 (85.9–88.6) | 81.9 (80.3–83.4) | 81.5 (75.1–86.5) | 77.3 (70.3–82.9) |
| 1989–1995 | 91.3 (90.1–92.4) | 87.5 (86.0–88.8) | 76.9 (71.5–81.4) | 69.1 (63.2–74.2) |
| 1996–2002 | 93.5 (92.4–94.5) | 91.1 (89.7–92.3) | 84.0 (79.4–87.7) | 80.5 (75.5–84.6) |
| 2003–2007 | 96.4 (95.3–97.3) | – | 92.0 (87.6–94.9) | – |
| APC (95% CI) | 0.44* (0.37–0.51) | 0.73* (0.61–0.86) | 0.38* (0.13–0.63) | 0.53 (−0.06–1.12) |
, APC is significant at P< 0.05; -, Survival estimate not available due to insufficient number of patients
APC for 5-year survival was calculated based on data from 1975–2007; APC for 10-year survival was calculated based on data from 1975–2002.
Table 3.
Five-and ten-year relative survival (%) with 95% confidence intervals (CI) among children and AYAs with acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML) and Hodgkin lymphoma (HL) by race, age, and era, SEER-13, 1992–2007
| Age and era of diagnosis | Non-Hispanic White | Hispanic | ||
|---|---|---|---|---|
| ALL, 0–14 years | N = 3180 | N = 2343 | ||
| 1992–1995 | 87.3 (84.4–89.7) | 84.4 (81.2–87.1) | 78.4 (73.8–82.3) | 76.5 (71.7–80.5) |
| 1996–1999 | 90.1 (87.6–92.2) | 86.8 (84–89.2) | 83.1 (79.1–86.5) | 78.4 (74.1–82.2) |
| 2000–2003 | 93.3 (91–95.1) | 90.9 (88.2–93) | 84.5 (80.8–87.6) | 79.1 (74.9–82.7) |
| 2004–2007 | 92.9 (90.5–94.7) | – | 88.3 (85–90.9) | – |
|
| ||||
| ALL, 15–39 years | N=1100 | N=1060 | ||
| 1992–1995 | 47.5 (40.7–54) | 3.1 (36.4–49.6) | 37.8 (29.3–46.3) | 33.7 (25.4–42.2) |
| 1996–1999 | 56.1 (49–62.7) | 53 (45.7–59.7) | 38.9 (31–46.6) | 35.5 (27.8–43.2) |
| 2000–2003 | 51.5 (44.9–57.8) | 50.3 (43.6–56.7) | 42 (34.7–49.2) | 34.4 (27.3–41.6) |
| 2004–2007 | 62.4 (55.4–68.7) | – | 46.9 (40.8–52.8) | – |
|
| ||||
| AML, 0–14 years | N=425 | N=292 | ||
| 1992–1995 | 47.6 (36.8–57.6) | 45.3 (34.7–55.4) | 46.8 (32.3–59.9) | 46.8 (32.3–59.9) |
| 1996–1999 | 57.5 (47.2–66.4) | 56.6 (46.3–65.6) | 44.5 (31–57.1) | 44.5 (31–57.1) |
| 2000–2003 | 58.8 (47.2–68.7) | 56.3 (44.7–66.3) | 59.4 (44–71.9) | 54.4 (38.8–67.6) |
| 2004–2007 | 80.6 (69.3–88.0) | – | 63.5 (49.7–74.5) | – |
|
| ||||
| AML, 15–39 years | N=1504 | N=810 | ||
| 1992–1995 | 43 (37.5–48.4) | 39.4 (33.9–44.8) | 36.7 (27.9–45.6) | 34.9 (26.2–43.8) |
| 1996–1999 | 49.3 (43.2–55.2) | 46.2 (40.1–52.1) | 47.9 (39.7–55.6) | 45.1 (36.8–53) |
| 2000–2003 | 56.2 (50.3–61.7) | 53.7 (47.8–59.3) | 46.1 (38.1–53.7) | 41.5 (33.4–49.3) |
| 2004–2007 | 54.7 (48.5–60.6) | – | 51.8 (43.6–59.3) | – |
|
| ||||
| HL, 0–14 years | N=498 | N=286 | ||
| 1992–1995 | 90.9(82.3–95.4) | 89.7 (81–94.6) | 92.2 (80.2–97.1) | 88.4 (75.5–94.7) |
| 1996–1999 | 94.8(88.6–97.7) | 92.5 (85.5–96.2) | 98.1 (87–99.7) | 98.1 (87–99.7) |
| 2000–2003 | 98 (92–99.5) | 96.2 (89.3–98.7) | 87.7 (75.7–94) | 85.8 (73.3–92.7) |
| 2004–2007 | 97.7(90.8–99.5) | – | – | – |
|
| ||||
| HL, 15–39 years | N=7481 | N=1595 | ||
| 1992–1995 | 92.3(90.9–93.6) | 89.4 (87.6–90.8) | 86.3 (81.1–90.1) | 79.8 (73.9–84.5) |
| 1996–1999 | 93.4(91.9–94.6) | 91.1 (89.4–92.5) | 88.8 (84.4–92) | 83.3 (78.2–87.3) |
| 2000–2003 | 94.6(93.2–95.7) | 92.3 (90.6–93.7) | 92.2 (88.2–94.9) | 87.8 (82.8–91.4) |
| 2004–2007 | 96.6(95.5–97.5) | – | 93.9 (90.5–96.1) | – |
Acute Lymphoblastic Leukemia
Children with ALL
From 1975–1981, the 10-year survival in white children was 57.7% (95% CI 54.2–61.0) vs. 37.9% (95% CI 25.8–49.8) in black children. Improvements in survival for black children with ALL have been greater than improvements in white children (APC=3.01% vs. 1.37%), resulting in a narrowing of the survival gap between these cohorts. From 2003–2007, 10-year survival rates were 86.8% (95% CI 84.7–88.7) in white children and 78.1% (95% CI 68.9–84.8) in black children.
AYAs with ALL
From 1975–1981, 10-year relative survival in white AYAs was 22.7% (95% CI 17.9–27.9) vs. 14.6% (95% CI 3.6–32.7) in black AYAs. There was no significant improvement in 5- or 10-year survival of black AYAs (5-year APC=1.25%,10-year APC=−1.26%) over the study periods. In contrast, white AYAs had significant improvements in 5- and 10-year survival (5-year APC=3.13%, 10-year APC=3.74%). For Black AYAs, there is a decrease in relative survival between 1996 and 2002 (Figure 1, top panel) in an otherwise increasing trend over time, which may be a result of a small number of patients (Table 1) rather than a true survival nadir.
Comparison of children and AYAs with ALL
From 2003–2007 there was a difference of 36 percentage-points in 5-year survival between white children and AYAs (93.3%, 95% [CI 91.3–94.9] vs. 57.0%, [95% CI 51.5–62.2]). Similarly, there was a 37 percentage-point difference between black children and black AYAs (54.9%, [95% CI 39.1–68.2] vs. 92%, [95% CI 82.9–96.3]).
Comparison of Hispanic and non-Hispanic children and AYAs with ALL
Differences in 10-year survival between Hispanic and non-Hispanic white children with ALL have persisted since 1992. From 2000–2003, survival in Hispanic children with ALL was 11 percentage-points lower than in non-Hispanic white children (79.1%, [95% CI 74.9–82.7] vs. 90.9%, [95% CI 88.2–93.0]). Similarly, 10-year relative survival in Hispanic AYAs was 16 percentage-points lower than in non-Hispanic white AYAs, (34.4%, [95% CI 27.3–41.6] vs. 50.3%, [95% CI 43.6–56.7]). From 2004–2007, 5-year survival in Hispanic AYAs was 46.9% (95% CI 40.8–52.8) vs. 88.3% (95% CI 85–90.9) in Hispanic children. There remained a 35 percentage-point difference in 10-year survival between Hispanic AYAs and Hispanic children (34.4%, [95% CI 27.3–41.6] vs. 79.1%, [95% CI 74.9–82.7]) from 2000–2003.
Acute Myeloid Leukemia
Children with AML
From 1975–1981, 10-year relative survival in white children with AML was 18.7% (95% CI 12.4–26.0) vs. 33.4% (95% CI 13.7–54.6) in black children. Survival improvements in black children have been lower than in white children (5-year APC=0.31% vs. 4.58%). Ten-year APCs revealed no significant improvement in survival for black children with AML vs. significant improvement for white children (APC=0.01% vs. 5.47%).
AYAs with AML
From 1975–1981, the 5-year survival in white AYAs was 15% (95% CI 12–18.3) vs. 13% (95% CI 5.7–23.5) in black AYAs. Improvements in 5- and 10-year survival in white AYAs were greater than for black AYAs (APC=5.10% vs. 2.07%). From 2003–2007 there remained an 18 percentage-point difference in 5-year relative survival between black and white AYAs (37.6%, [95% CI 25.5–49.6] vs. 55.1%, [95% CI 53.2–63.6]).
Comparison of children and AYAs with AML
From 2003 to 2007, 5-year relative survival for white children remained higher than for white AYAs (71%, [95% CI 61.2–79.0] vs. 55%, [95% CI 49.7–60.2]) and both cohorts had significant improvements in APC over time. Black children did not have a significant improvement in 10-year survival rates (APC=0.01%) while black AYAs did (APC=2.55%).
Comparison of Hispanic and non-Hispanic children and AYAs with AML
Relative survival estimates in Hispanic vs. non-Hispanic white children and AYAs with AML were comparable between all groups in the most recent evaluable time period.
Hodgkin Lymphoma
Children with HL
From 1975 to 1981, 10-year relative survival in white children was 81.7% (95% CI 75.8–86.3) vs. 55.4% (95% CI 31.5–74.0) in black children. Improvements in 10-year survival of black children have been greater than in white children (APC=2.4 vs. 0.81). As a result, the survival gap has decreased, with 5- and 10-year survival averaging between 96–98% and 92–95% in white and black patients, respectively.
AYAs with HL
From 1975 to 1981, 10-year survival in white AYAs was 78.3% (95% CI 76.4–80.1) vs. 66.2% (95% CI 57.3–73.8) in black AYAs. Over the study period white AYAs had significant improvements in 10-year survival (APC=0.73%) vs. black AYAs who did not (APC=0.53%). From 2003–2007 there remained a four percentage-point difference in 5-year survival between white and black AYA patients (96%, [95% CI 95.3–97.3] vs. 92%, [95% CI 87.6–94.9]) and a 9 percentage-point difference in 10-year survival (80.5%, [95% CI 75.5–84.6] vs. 91.1%, [95% CI 89.7–92.3]).
Comparison of children and AYA with HL
Over the study period, 10-year relative survival improved significantly for both white children and white AYAs (APC=0.81 vs. 0.73). In contrast, black children had significant improvements in 10-year survival (APC=2.4) but black AYAs did not (APC=0.53).
Comparison of Hispanic and non-Hispanic children and AYAs with HL
Relative survival estimates in Hispanic vs. non-Hispanic white children and AYAs with HL were similar in the most recent evaluable time period.
DISCUSSION
Relative survival in children and AYAs with ALL, AML and HL have improved over the last four decades, albeit to varying degrees, which has resulted in persistent age- and race-related survival differences since 1975. Improved survival in all patients likely reflects diagnostic and therapeutic advances, such as improvements in cellular and molecular diagnostics, staging, targeted therapies, hematopoietic cell transplantation (HCT), supportive care, and expansion of pediatric cooperative group trials.4,6 Unequal access to these advances may potentially underlie survival disparities between groups as well as both biologic and non-biologic factors (e.g. medication adherence, disease biology and pharmacogenomics).12
Clinical trial enrollment
Survival improvements in pediatric ALL and AML patients are largely a result of national efforts aimed at enrolling children on cooperative group clinical trials. In general, AYA patients are not enrolled on clinical trials as often as children.13–15 As of 2008, 90–95% of children <15 years were treated at Children’s Oncology Group (COG) institutions and approximately 50–60% enrolled on clinical trials.16 In contrast, 21% of 15 to 19 year-olds and 8% of 20 to 29 year-olds were treated at institutions with NCI-sponsored clinical trials.16 Identification of the AYA gap in clinical trial participation has fueled a collaborative initiative between the COG and the adult groups aimed at expanding eligibility criteria for trial enrollment.17,18 While this program represents a major effort to improve AYA outcomes, its survival impact will likely not be appreciable for decades.
Biologic basis for survival differences
Age-related differences in disease-specific prognostic factors may contribute to observed survival differences between children and AYAs with ALL and AML. AYAs with ALL more often have high-risk disease characteristics such as L2 morphology or pro-T cell immunophenotype.19,20 The BCR-ABL genotype occurs in <3% of children and in up to 26% of AYAs with ALL.21 Nearly 50% of children with ALL have favorable genotypes such as TEL-AML translocation vs. approximately 10% of AYAs.22 In patients with AML, FLT3-ITD mutations increase in frequency with age and are associated with poorer prognosis in all age groups.21 Additionally, certain genetic factors in patients with ALL and AML may be associated with drug-resistant phenotypes and may partly explain the reduced survival observed in certain ethnic groups.23,24
Treatment-related mortality
Historically, the risk of treatment-related mortality in AYA patients is higher than in children.25 Truong et al identified adolescent age as an independent risk factor for tumor lysis syndrome, a potentially life-threatening condition at initiation of leukemia or lymphoma treatment.6,26,27 Patients receiving ALL and AML therapy are at risk for serious bacterial infections and higher rates of infection-associated mortality in ALL patients over age 10.6 Multiple clinical trials have identified a correlation between infection-associated mortality and older patient age during AML therapy.28,29 In recent years, children with ALL and AML who are treated on clinical trials receive prophylactic antibiotics during times of prolonged immune-suppression. This supportive care measure has resulted in significant reduction in infection-related mortality rates in both populations.
Oral chemotherapy adherence
Non-adherence to prescribed treatment regimens is a challenge in both pediatric and adult oncology.30 Oral corticosteroids are a key component of therapeutic regimens in both HL and ALL. In children with ALL, Bhatia and colleagues identified that <90% adherence to oral mercaptopurine over the course of therapy was associated with a 3.9-fold increased risk of relapse.31,32 In a follow up study, non-adherence rates were quoted as high as 33% and were higher in Hispanics, blacks and teenagers.32,33 In our ALL populations, 5-year relative survival rates were comparable across age and race, but 10-year relative survival diverged, with black and Hispanic patients having reduced long-term survival. This difference may, in part, be a reflection of more frequent late-relapses associated with non-adherence in the black and Hispanic cohorts.
Access to hematopoietic cell transplantation
Allogeneic HCT is a key treatment option for with AML and Philadelphia chromosome positive ALL, for patients with relapsed ALL and HL, and at some centers in AYAs with high-risk Philadelphia chromosome negative ALL.29 Historically, Hispanic and African American patients are under-represented in national marrow donor registries and as a result, are less likely to undergo transplantation. In a 2006 report, Aplenc et al investigated the treatment and outcomes of black and white children with AML on a series of Children’s Cancer Group (CCG) studies. Investigators noted that because of fewer available donors, black children with high-risk disease were less likely to receive HCT as consolidation therapy than white children.7 As the field of HCT moves forward, the use of alternative donor sources may expand the donor pool for minority patients with relapsed or high-risk hematologic malignancies.34
Study strengths and limitations
This study includes a large number of patients from a population-based setting thus eliminating biases related to treatment/referral patterns observed in single-institution or smaller consortium-based clinical studies. Moreover, survival trends were calculated over many decades informing the kinetics of population-based survival metrics. There are important limitations in the SEER registry such as minimal disease-, treatment- and relapse-specific data. We could not assess socioeconomic determinants and indicators of access-to-care (e.g., health insurance status and treatment facility) and medication adherence, which are potential contributing factors to observed survival outcomes.
Conclusion
To date, this is the largest longitudinal analysis of race and age-related survival trends in pediatric and AYA patients with ALL, AML and HL. Our findings clearly identify a major and persistent public health disparity. Particular attention should be paid to Hispanic and black children with ALL and AML as these patients continue to suffer significantly poorer outcomes when compared to non-Hispanic white children. Similarly, characteristics that distinguish the unique cancer burden of AYAs with ALL and AML should be investigated and interventions aimed at improving awareness, access and quality cancer care to these patient populations should be implemented.
Acknowledgments
Supported in part by a fellowship from the NCI (R25 CA094061, JMK), an ASCO Young Investigator Award (ADV) and a William Raveis Charitable Fund Fellow of the Damon Runyon Cancer Research Foundation (DRG-117-15, ADV). This work is unfunded.
Footnotes
There are no conflicts of interest to disclose.
Author Contributions: Conceptualization, JMK, THMK, LT, RA, AB, ADV; Methodology, LT, RA, AB; Investigation, JMK, THMK, AB, ADV; Writing – Original Draft, JMK, THMK; Writing – Review & Editing, JMK, THMK, LT, RA, AB, ADV; Funding Acquisition, This work is unfunded; Resources, AB, ADV; Supervision, AB, ADV
References
- 1.Goggins WB, Lo FF. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database 1988–2008. Cancer causes & control:CCC. 2012;23(5):737–743. doi: 10.1007/s10552-012-9943-8. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Pei D, Pappo AS, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(16):2005–2012. doi: 10.1200/JCO.2011.40.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults With Cancer: Report of the Adolescent and Young Adult Oncology Progress Review Group. Bethesda, MD: National Institutes of Health, National Cancer Institute, LiveStrong Young Adult Alliance; 2006. (NIH publication 06-6067). [Google Scholar]
- 4.Pui CH, Boyett JM, Hancock ML, Pratt CB, Meyer WH, Crist WM. Outcome of treatment for childhood cancer in black as compared with white children. The St Jude Children’s Research Hospital experience, 1962 through 1992. Jama. 1995;273(8):633–637. [PubMed] [Google Scholar]
- 5.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Lensing S, Razzouk BI, Pounds S, Pui CH, Ribeiro RC. Effect of race on outcome of white and black children with acute myeloid leukemia: The St. Jude experience. Pediatr Blood Cancer. 2007;48(1):10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 7.Children’s Oncology G. Aplenc R, Alonzo TA, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108(1):74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: the past, present, and future. Nature clinical practice. Oncology. 2008;5(9):543–556. doi: 10.1038/ncponc1186. [DOI] [PubMed] [Google Scholar]
- 9.Chow KU, Mitrou PS, Geduldig K, Helm EB, Hoelzer D, Brodt HR. Changing incidence and survival in patients with aids-related non-Hodgkin’s lymphomas in the era of highly active antiretroviral therapy (HAART) Leukemia & lymphoma. 2001;41(1–2):105–116. doi: 10.3109/10428190109057959. [DOI] [PubMed] [Google Scholar]
- 10.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. National Cancer Institute monograph. 1961;6:101–121. [PubMed] [Google Scholar]
- 11.Joinpoint Regression Program. Statistical Research and Applications Branch. National Cancer Institute; Aug, 2014. Version 4.1.1. http://surveillance.cancer.gov/joinpoint. Accessed May, 5, 2015. [Google Scholar]
- 12.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatric blood & cancer. 2011;56(6):994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 14.Unger JCE, Bleyer A. Clinical Trial Participation: What are the Barriers and Why It’s Important. American Society of Clinical Oncology Educational Book. 2016 doi: 10.14694/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleyer A. Adolescents and young adult cancer trial participation: Will the National Community Oncology Research Program also fail and what about the rest of us? J Oncol Pract. 2016 doi: 10.1200/JOP.2016.011114. [DOI] [PubMed] [Google Scholar]
- 16.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 Suppl):1645–1655. doi: 10.1002/cncr.22102. [DOI] [PubMed] [Google Scholar]
- 17.Freyer DR, Felgenhauer J, Perentesis J, Adolescent COG, Young Adult Oncology Discipline C Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatric blood & cancer. 2013;60(6):1055–1058. doi: 10.1002/pbc.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss AR, Nichols CR, Freyer DR. Enhancing Adolescent and Young Adult Oncology Research Within the National Clinical Trials Network: Rationale, Progress, and Emerging Strategies. Seminars in oncology. 2015;42(5):740–747. doi: 10.1053/j.seminoncol.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinney PA, Alexander FE, Cartwright RA, Scott CS, Staines A. Acute lymphoblastic leukaemia incidence in the UK by immunophenotype. Leukemia. 1993;7(10):1630–1634. [PubMed] [Google Scholar]
- 20.Catovsky D. Symposium: classification of leukemia. 1. The classification of acute leukemia. Pathology. 1982;14(3):277–281. doi: 10.3109/00313028209061377. [DOI] [PubMed] [Google Scholar]
- 21.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nature reviews. Cancer. 2008;8(4):288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 22.Bleyer A, Siegel SE, Coccia PF, Stock W, Seibel NL. Children, adolescents, and young adults with leukemia: the empty half of the glass is growing. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(32):4037–4038. doi: 10.1200/JCO.2012.44.7466. author reply 4038–4039. [DOI] [PubMed] [Google Scholar]
- 23.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. The New England journal of medicine. 2004;350(15):1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 24.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: A population-based observational study. Pediatric blood & cancer. 2015;62(10):1819–1825. doi: 10.1002/pbc.25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb DK, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98(6):1714–1720. doi: 10.1182/blood.v98.6.1714. [DOI] [PubMed] [Google Scholar]
- 26.Inaba H, Fan Y, Pounds S, et al. Clinical and biologic features and treatment outcome of children with newly diagnosed acute myeloid leukemia and hyperleukocytosis. Cancer. 2008;113(3):522–529. doi: 10.1002/cncr.23581. [DOI] [PubMed] [Google Scholar]
- 27.Truong TH, Beyene J, Hitzler J, et al. Features at presentation predict children with acute lymphoblastic leukemia at low risk for tumor lysis syndrome. Cancer. 2007;110(8):1832–1839. doi: 10.1002/cncr.22990. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro RC, Razzouk BI, Pounds S, Hijiya N, Pui CH, Rubnitz JE. Successive clinical trials for childhood acute myeloid leukemia at St Jude Children’s Research Hospital, from 1980 to 2000. Leukemia. 2005;19(12):2125–2129. doi: 10.1038/sj.leu.2403872. [DOI] [PubMed] [Google Scholar]
- 29.Razzouk BI, Estey E, Pounds S, et al. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106(11):2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- 30.Landier W. Age span challenges: adherence in pediatric oncology. Seminars in oncology nursing. 2011;27(2):142–153. doi: 10.1016/j.soncn.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(9):1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(17):2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. The New England journal of medicine. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]


